Abstract
Aims
To identify promising intervention components intended to help smokers to attain and maintain abstinence in their quit smoking attempts.
Design
A fully crossed, 6-factor randomized fractional factorial experiment.
Setting
Eleven primary care clinics in southern Wisconsin, USA.
Participants
637 adult smokers (55% women, 88% White) motivated to quit smoking who visited primary care clinics.
Interventions
Six intervention components designed to prepare smokers to quit, and achieve and maintain abstinence (i.e., for the preparation, cessation, and maintenance phases of smoking treatment): 1) preparation nicotine patch vs. none; 2) preparation nicotine gum vs. none; 3) preparation counseling vs. none; 4) intensive cessation in-person counseling vs. minimal; 5) intensive cessation phone counseling vs. minimal; and 6) 16 vs. 8 weeks of combination nicotine replacement therapy (nicotine patch + nicotine gum).
Measurements
7-day self-reported point-prevalence abstinence at 16 weeks.
Findings
Preparation counseling significantly improved week 16 abstinence rates (p<.05), while both forms of preparation nicotine replacement therapy interacted synergistically with intensive cessation in-person counseling (p<.05). Conversely, intensive cessation phone counseling and intensive cessation in-person counseling interacted antagonistically (P<.05)—these components produced higher abstinence rates by themselves than in combination.
Conclusions
Preparation counseling and the combination of intensive cessation in-person counseling with preparation nicotine gum or patch are promising intervention components for smoking and should be evaluated as an integrated treatment package.
Keywords: Smoking cessation, Multiphase Optimization Strategy (MOST), Phase-Based Model of smoking treatment, tobacco dependence, nicotine replacement therapy, chronic care smoking treatment, comparative effectiveness, primary care, factorial experiment
Introduction
The health, economic, and human costs of tobacco use are profound [1], and while there are effective smoking treatments, long-term abstinence rates have increased only modestly over the past two decades [2–6] and remain disappointing (15–35%; [2]). This slow progress may be due, in part, to reliance on randomized controlled trials (RCTs), to the exclusion of other experimental designs. RCTs often compare two multicomponent treatments with one another (skill training, support, relapse prevention counseling+active medication versus the same counseling interventions+placebo medication) and therefore do not reveal the effects of individual intervention components or their interactions with one another. Such information would permit treatment development on a methodologically principled basis [7, 8].
The present research, which is based on the Multiphase Optimization Strategy (MOST) [7–10], uses factorial designs to simultaneously screen multiple intervention components and identify the most promising ones based on main effects and interactions. This is one of four linked articles. One reviews the theory and methods behind this research [11]; the others report factorial experiments of intervention components for the Motivation [12] and Maintenance [13] phases of smoking treatment.
This research experimentally evaluated intervention components designed for three phases of the cessation process: Preparation, Cessation, and Maintenance [14, 15]. These phases, as described in the Phase-Based Model of smoking treatment [11, 14, 15], present distinct challenges and opportunities that can be addressed with different types of intervention components delivered at different times in the cessation process. The Preparation phase prepares smokers for a quit attempt and comprises the ~3 weeks prior to the quit day. The goal of the Cessation phase is to establish abstinence and comprises the first ~2–4 weeks postquit, when withdrawal symptoms tend to peak and most lapses occur [16–19]. The goal of the subsequent Maintenance phase, lasting ~1–12 months, is to support abstinence and prevent relapse during a time when withdrawal typically diminishes but other risks are present—treatment nonadherence [20, 21], decreased self-efficacy (especially after lapses [22, 23]), and exacerbations of craving [24].
The intervention components tested in the current experiment were selected to address phase-specific challenges, designed for translation into real-world healthcare settings (with low staff and patient burden, and cost [25–27]), and implemented and tested in primary care clinics. For the Preparation phase, we tested Preparation Nicotine Patch, Preparation Nicotine Gum, and Preparation Counseling. Preparation nicotine replacement therapy (NRT) may prepare smokers for cessation by blunting the pharmacologic effects of smoking, allowing practice of NRT self-administration, reducing smoking, and degrading the smoking-reward contingency [28–30]. Preparation Counseling, which included practice quit attempts, may inculcate relevant skills (coping, medication use [2, 31, 32]), increase self-efficacy [33, 34], provide intratreatment social support [2, 31], reduce cue-smoking contingencies [35–37], and reduce smoking contexts and smoking rate [35, 36, 38–42].
Challenges in the Cessation phase include withdrawal [43, 44], exposure to smoking cues [45–47], and lapse occurrences [16]. In-Person Counseling and Phone Counseling were designed to: 1) promote avoidance of smoking triggers, 2) train coping responses to address withdrawal and lapsing, and 3) provide intratreatment social support to buffer withdrawal distress.
Finally, we tested 16 vs. 8 weeks of postquit combination NRT to address Maintenance-phase threats such as withdrawal exacerbation [17, 24] and late lapses [5, 22, 23, 48–50]. Longer-term medication may reduce both lapse-relapse progression [51, 52] and the likelihood or severity of prolonged or recurrent withdrawal (e.g., anhedonia, craving [53]). We used combination NRT as the post-target quit date (TQD) medication for all participants based on its efficacy [2, 54], ability to suppress withdrawal [55–57], cost, and translatability into real-world healthcare settings [2].
In sum, using state-of-the-art theory and methods, this research used a factorial experiment to screen multiple intervention components that were selected to be effective for the Preparation, Cessation, and Maintenance phases of smoking treatment and that had high translation potential. We examined their main and interactive effects to identify effects on initial (2-week), end-of-treatment (16-week), and long-term (6-month) abstinence. The 16-week time point was the primary outcome because of its hypothesized sensitivity, occurring shortly after the delivery of all treatment but before encounters with relapse precipitants unrelated to treatment that could introduce error [14]. Thus, this research yields valuable comparative effectiveness data on multiple intervention components, which should help guide future treatment development (e.g., additional factorial experiments, an RCT that evaluates a multicomponent treatment).
Methods
Procedure
This experiment was conducted from June, 2010 through October, 2013. Participants were recruited from 11 primary care clinics in two health systems in southern Wisconsin. During clinic visits, clinical care staff (i.e., medical assistants) were prompted by electronic health record technology to invite identified smokers to participate in a research program to help them quit smoking [58, 59]. Interested patients were electronically referred by clinic staff and then contacted by research staff to assess their eligibility. The inclusion criteria were: ≥18 years old; ≥5 cigarettes/day for the previous 6 months; motivation to quit; ability to read, write, and speak English; no plan to move from the area for at least 12 months; not currently taking bupropion or varenicline; agreement to use only study medication for the duration of the study (e.g., discontinuing on-going NRT use); no medical contraindications to NRT use; and, for women of childbearing potential, agreement to use an approved method of birth control during treatment. Participants interested in quitting were randomly assigned to either this experiment or the cessation experiment described in [13] in this issue. It should be noted that although there were three related experiments (this experiment, [12], and [13]), each used an independent sample.
Eligible patients were invited to return to their primary care clinic to hear more about the study, provide written informed consent, and complete initial assessments. A research database then created intervention and assessment schedules, based on randomly assigned treatment conditions, which guided delivery of interventions by bachelor’s level case managers supervised by licensed clinical psychologists.
Experimental Design
This experiment used a balanced fractional factorial design with six factors: 1) Preparation Nicotine Patch vs. None; 2) Preparation Nicotine Gum vs. None; 3) Preparation Counseling vs. None; 4) Intensive Cessation In-Person Counseling vs. Minimal; 5) Intensive Cessation Phone Counseling vs. Minimal; and 6) 16 vs. 8 Weeks of Combination NRT. The fractional nature of the design calls for delivery of half of the experimental component combinations that would have been delivered in a full factorial design (32 versus 64), making the research more logistically manageable. However, this design allows for the estimation of only main effects and two-way interactions (see Supplemental Materials for additional detail [60]).
Participants were randomized to treatment conditions via a database that used stratified permuted block randomization; we stratified by gender and clinic with a fixed block size of 32 based on the 32 unique treatment conditions (in random order within each block). Staff were blinded to randomization until eligibility was confirmed; participants were blinded until consent was provided.
Experimental Factors
The intervention components were designed to be consistent with the 2008 US Public Health Service Clinical Practice Guideline recommendations [2], address phase-specific cessation challenges and opportunities, and be feasible for real-world healthcare. See Supplemental Materials for counseling protocol summaries and fidelity assessments.
Preparation-Phase Intervention Components
Preparation Nicotine Patch
Participants assigned to the active level (i.e., the active condition) received 14-mg patches for the 3 weeks prior to the TQD while the other half did not receive prequit patches.
Preparation Nicotine Gum
Participants in the active condition received 2-mg nicotine gum for the 3 weeks prior to the TQD (≥9/day, 1 piece/1–2 hours); the other half did not receive gum. Participants who received both Preparation Patch and Gum were told to use at least 5 pieces/day of gum, unless such use produced adverse effects.
Preparation Counseling
Participants in the active condition received 20-minute counseling sessions 1 (in-person), 2 (phone) and 3 (in-person) weeks prior to the TQD. The counseling focused on smoking reduction, withdrawal coping, environmental restrictions on smoking, intra-treatment social support, and autonomous motivation. Participants were also asked to engage in two 8-hour practice quit attempts. The other half of participants did not receive this counseling.
Cessation-Phase Intervention Components
Cessation In-Person Counseling
Participants in the intensive condition received three 20-minute face-to-face counseling sessions: one week pre-TQD (Week −1), TQD, and Week 1. The counseling emphasized intra-treatment social support and skill-building [2]. Participants assigned to the minimal level received one 3-minute in-person session at Week −1 [2].
Cessation Phone Counseling
Participants in the intensive condition received three 15-minute phone sessions (TQD, Days 2 and 10). These calls emphasized intra-treatment social support, skill execution, and avoidance of danger situations [61–64]. Participants assigned to the minimal condition received one 10-minute session on the TQD that provided support and addressed motivation to quit, strategies for coping with craving, and medication use. Thus, all participants received some TQD phone counseling.
Cessation and Maintenance-Phase Intervention Component
Extended Medication
All participants received Cessation- and Maintenance-phase combination NRT (nicotine patch + nicotine gum) starting on their TQD. Half were assigned to receive 8 weeks of patches (>9 cigarettes/day=4 weeks of 21-mg, 2 weeks of 14-mg, and 2 weeks of 7-mg nicotine patches; 5–9 cigarettes/day=4 weeks of 14-mg and 4 weeks of 7-mg nicotine patches) and 8 weeks of nicotine gum (smoke within 30 minutes of waking=4-mg; smoke more than 30 minutes after waking=2-mg). The other half received 16 weeks of patches (>9 cigarettes/day=21-mg for 12 weeks, 14-mg for 2 weeks, and 7-mg for 2 weeks; 5–9 cigarettes/day=14-mg for 12 weeks and 7-mg for 4 weeks) and 16 weeks of gum. Participants were advised to use one piece of gum every 1–2 hours until 2 weeks before treatment termination [2], and at least 5 pieces/day unless such use produced adverse effects. Participants were instructed to decrease gum use over the final 2 weeks of medication treatment.
Combinations of Intervention Components
The intervention components were designed to be distinct but also complementary (e.g., when a participant received phone and in-person counseling, the case manager would integrate information across the two types of contacts). Even the timing was complementary, i.e., contacts were shifted slightly to prevent conflicts. Thus, intervention components were independent, but integrated when offered together, as would occur in real-world use.
Assessments
All participants had 3 study visits (Weeks −3, −1, and 4); those assigned to Intensive In-Person Cessation Counseling also had two counseling-only visits (TQD and Week 1). Participants completed baseline assessments of vital signs, exhaled carbon monoxide using the Bedfont Smokerlyzer (Bedfont Scientific, Rochester, England), demographics, smoking history, and tobacco dependence (Fagerström Test of Nicotine Dependence; FTND [65]). At subsequent study contacts (visits at Week −1 and 4 and calls at Weeks 8, 16, and 26) participants were asked about medication adverse events and about their smoking since last contact and in the last 7 days, using the validated timeline follow-back method [66]. These data were used to establish self-reported 7-day point-prevalence abstinence at 2, 16, and 26 weeks post-TQD. Medication adherence was assessed during automated calls that occurred every other evening from Week −3 to Week 2.
Outcome Measures
The primary outcome was self-reported 7-day point-prevalence abstinence (PPA) at 16 weeks, with secondary outcomes at 2 and 26 weeks, assessed by staff who were not involved in treatment, but were not blind to treatment assignment1. These time points were selected to index sensitively the effects of intervention components that were delivered at different treatment phases [14]. The 16-week outcome was deemed to be an early, sensitive index of treatment effects, occurring shortly after treatment completion. The 2-week outcome reflects the effects of the Preparation and Cessation-phase components on early cessation, and Week 26 reflects Maintenance-phase effects, permitting comparison with other treatment research.
Analytic Plan
Initial analyses characterized the study population and examined treatment engagement and safety. We examined the likelihood of participant dropout in relation to treatment components to inform missingness analyses. Logistic regression (SPSS [67]) modeled the 6 main effects and 15 2-way interactions using effect coding (levels are coded −1 and +1 [11]), to analyze self-reported PPA at each time point. Analyses were conducted with and without adjusting for a predetermined set of demographic and tobacco dependence covariates: gender, race (White vs. non-White), age, education (up to high school/GED vs. at least some college), the Heaviness of Smoking Index [68], baseline exhaled carbon monoxide, and healthcare system (A vs. B). Reported results reflect intent-to-treat analyses assuming that missing=smoking. These analyses were supplemented with multiple imputation (MI)/sensitivity analyses (see Supplemental Materials [69]). The results of the missing=smoking and MI/sensitivity analyses were similar, therefore, we present only the results of the former.
Results
Participants
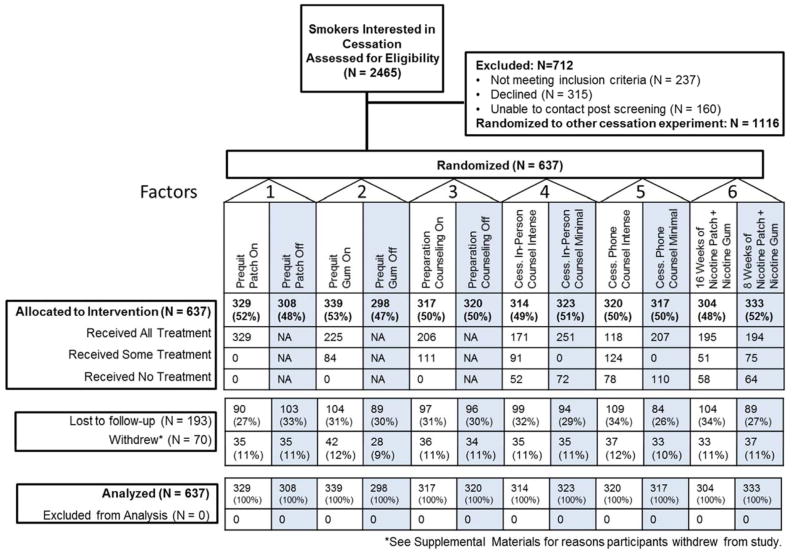
Of the smokers who were interested in quitting, 1349 were referred to this experiment, and 637 consented (see Figure 1 for the CONSORT diagram and Supplemental Materials for sample size justification). Table 1 describes the demographic and tobacco dependence characteristics of the sample. The 11 clinics recruited 23–89 participants each.
Figure 1.
CONSORT Diagram
Table 1.
Demographic and smoking history characteristics
| Total Sample |
Preparation Gum |
Preparation Patch |
Preparation Counseling |
In-Person Counseling |
Phone Counseling |
Medication Duration |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| On | Off | On | Off | On | Off | On | Off | On | Off | On | Off | ||
| Women (%) | 54.6 | 54.3 | 55.0 | 55.3 | 53.9 | 54.6 | 54.7 | 55.4 | 53.9 | 53.8 | 55.5 | 56.3 | 53.2 |
| Age (mean, SD) | 45.8 (12.0) | 45.3 (11.9) | 46.2 (12.2) | 45.2 (11.8) | 46.3 (12.3) | 46.2 (12.2) | 45.3 (11.9) | 45.8 (12.2) | 45.7 (11.9) | 45.1 (12.0) | 46.4 (12.0) | 45.1 (12.2) | 46.4 (11.6) |
| High School diploma or GED only (%) | 31.4 | 32.0 | 30.7 | 31.4 | 31.5 | 34.5 | 28.4 | 30.1 | 32.7 | 32.5 | 30.4 | 30.6 | 32.2 |
| At least some college (%) | 58.7 | 57.2 | 60.2 | 57.6 | 59.7 | 55.7 | 61.5 | 58.7 | 58.2 | 58.1 | 59.1 | 60.1 | 57.3 |
| White (%) | 87.8 | 87.8 | 87.8 | 87.8 | 87.7 | 88.5 | 87.0 | 87.8 | 87.7 | 89.5 | 86.0 | 87.0 | 88.4 |
| African-American (%) | 7.8 | 9.0 | 6.5 | 8.6 | 7.0 | 6.7 | 8.9 | 8.4 | 7.2 | 7.0 | 8.6 | 9.3 | 6.4 |
| Hispanic (%) | 3.9 | 4.5 | 3.1 | 3.1 | 4.7 | 4.2 | 3.5 | 4.2 | 3.5 | 4.5 | 3.2 | 5.1 | 2.8 |
| Health System A (%) | 57.3 | 55.5 | 59.4 | 59.9 | 54.5 | 56.8 | 57.8 | 61.1 | 53.6 | 59.1 | 55.5 | 60.9 | 54.1 |
| Cigs/day (mean, SD) | 17.7 (8.2) | 17.5 (8.4) | 17.9 (7.9) | 18.1 (8.0) | 17.2 (8.4) | 17.8 (8.4) | 17.5 (8.1) | 17.9 (8.2) | 17.4 (8.2) | 18.1 (8.4) | 17.3 (7.9) | 17.8 (7.8) | 17.6 (8.5) |
| Baseline carbon monoxide (mean, SD) | 20.3 (11.4) | 20.3 (11.7) | 20.4 (11.0) | 20.6 (10.7) | 20.0 (12.1) | 20.6 (11.3) | 20.0 (11.4) | 20.3 (11.6) | 20.4 (11.1) | 20.2 (11.4) | 20.4 (11.3) | 19.6 (10.8) | 21.0 (11.9) |
| FTND (mean, SD) | 4.8 (2.2) | 4.8 (2.1) | 4.8 (2.3) | 4.9 (2.2) | 4.7 (2.1) | 4.9 (2.2) | 4.7 (2.1) | 4.9 (2.1) | 4.7 (2.1) | 4.9 (2.2) | 4.8 (2.1) | 4.8 (2.2) | 4.8 (2.1) |
| Heaviness of Smoking Index (mean, SD) | 3.1 (1.4) | 3.1 (1.4) | 3.1 (1.4) | 3.2 (1.4) | 3.0 (1.4) | 3.1 (1.5) | 3.0 (1.4) | 3.2 (1.4) | 3.0 (1.5) | 3.2 (1.4) | 3.0 (1.4) | 3.1 (1.4) | 3.1 (1.4) |
Note. On = Factor was present or at the intensive level or longest duration (e.g., intensive counseling, 16 weeks of medication). Off = Factor was not present or was at the minimal level or shortest duration (e.g., minimal counseling, 8 weeks of medication). The study was conducted in two healthcare systems (A and B). FTND = Fagerstrom Test of Nicotine Dependence.
Treatment Engagement
On average, participants in the Preparation Counseling condition attended 2.50 (SD=.74) out of 3 counseling sessions and 69% reported making a practice quit attempt. Participants in the Intensive Cessation In-Person Counseling condition completed 2.13 (SD=1.13) out of 3 sessions, significantly more than those in the Intensive Cessation Phone Counseling condition (M=1.74 out of 3 sessions, SD=1.19, p<.01). Participants in the Preparation Patch condition used an average of 6.24 patches/week (SD=3.97) and those in the Preparation Gum condition used an average of 3.19 pieces of gum/day (SD=2.37). Participants in the 16 vs. 8 week medication duration conditions did not differ in postquit patches used/week (M=4.59, SD=2.87 vs. M=4.98, SD=2.77) or postquit pieces of gum/day (M=4.02, SD=3.31 vs. M=3.81, SD=3.34).
Safety
Reports of adverse events were low (e.g., 10% of those who received Preparation Patch or Gum reported vivid dreams, skin rash occurred in 8% of participants while on combination NRT post-quit) and there were no serious adverse events related to study participation or study medications.
Missing Data
Rates of missing PPA data went from 15.1% at Week 2 to 23.7% at Week 16 to 30.0% at Week 26. Missingness was significantly more likely amongst participants receiving no Preparation Patch versus those receiving Preparation Patch (28.6% vs. 19.1%; Week 16) and those receiving 16 weeks of combination NRT versus 8 weeks (33.9% vs. 26.4%; Week 26).
Cessation Outcome
Table 2 presents the self-reported 7-day PPA rates for each main effect at 2, 16, and 26 weeks postquit. Table 3 presents the logistic regression results for the 2-, 16-, and 26-week outcomes. The patterns of statistical significance were consistent between the adjusted and unadjusted models. The only significant main effect on the Week 16 primary outcome was that participants who received Preparation Counseling had higher abstinence rates.
Table 2.
Main Effects Self-Reported Point-Prevalence Abstinence Rates at 2, 16 and 26 Weeks Postquit
| Percent Abstinent at 2 Weeks | Percent Abstinent at 16 Weeks | Percent Abstinent at 26 Weeks | ||||
|---|---|---|---|---|---|---|
| Factor | On | Off | On | Off | On | Off |
| Preparation Patch | 44.1 | 43.2 | 36.5 | 32.5 | 29.8 | 26.0 |
| Preparation Gum | 43.4 | 44.0 | 36.3 | 32.6 | 29.8 | 25.8 |
| Preparation Counseling | 44.5 | 42.8 | 38.2 | 30.9 | 30.3 | 25.6 |
| Cessation In-Person Counseling | 47.5 | 39.9 | 36.3 | 32.8 | 27.7 | 28.2 |
| Cessation Phone Counseling | 43.1 | 44.2 | 35.6 | 33.4 | 30.3 | 25.6 |
| Medication Duration | 44.1 | 43.2 | 36.2 | 33.0 | 28.5 | 27.3 |
Note. On = Factor was present or at the intensive level or longest duration (e.g., intensive counseling, 16 weeks of medication). Off = Factor was not present or was at the minimal level or shortest duration (e.g., minimal counseling, 8 weeks of medication).
Table 3.
Logistic Regression Models for 2, 16 and 26 Week Point-Prevalence Outcome
| 2 Weeks Post-TQD | 16 Weeks Post-TQD | 26 Weeks Post-TQD | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted** | Unadjusted | Adjusted** | Unadjusted | Adjusted** | |||||||
| Variable | b | p-value | b | p-value | b | p-value | b | p-value | b | p-value | b | p-value |
| Intercept | −.26 | .001 | .43 | .45 | −.70 | <.001 | −.69 | .25 | −1.04 | <.001 | −.34 | .59 |
| Preparation Patch | .01 | .87 | .04 | .66 | .08 | .34 | .11 | .22 | .09 | .34 | .11 | .24 |
| Preparation Gum | −.01 | .95 | −.00 | .96 | .11 | .21 | .12 | .19 | .12 | .20 | .12 | .23 |
| Preparation Counseling | .03 | .69 | .04 | .60 | .18 | .04 | .19 | .03 | .11 | .23 | .11 | .23 |
| Cessation In-Person Counseling | .14 | .10 | .15 | .07 | .05 | .54 | .06 | .48 | −.04 | .68 | −.03 | .80 |
| Cessation Phone Counseling | −.03 | .72 | −.03 | .75 | .05 | .59 | .06 | .54 | .12 | .21 | .12 | .20 |
| Medication Duration | .02 | .78 | .01 | .91 | .08 | .39 | .07 | .46 | −.02 | .80 | −.03 | .73 |
| Preparation Patch x Preparation Gum | .05 | .56 | .05 | .54 | .04 | .68 | .04 | .65 | .06 | .54 | .06 | .53 |
| Preparation Patch x Preparation Counseling | .16 | .050 | .18 | .04 | .15 | .10 | .17 | .07 | .18 | .07 | .19 | .06 |
| Preparation Patch x Cessation In-Person Counseling | .14 | .09 | .15 | .08 | .19 | .03 | .20 | .03 | .22 | .02 | .22 | .02 |
| Preparation Patch x Cessation Phone Counseling | .20 | .01 | .23 | .01 | .18 | .047 | .20 | .03 | .13 | .18 | .15 | .14 |
| Preparation Patch x Medication Duration | −.06 | .50 | −.07 | .43 | .00 | .97 | −.00 | .96 | −.01 | .95 | −.02 | .84 |
| Preparation Gum x Preparation Counseling | .10 | .22 | .05 | .59 | −.06 | .52 | −.11 | .22 | −.05 | .61 | −.09 | .35 |
| Preparation Gum x Cessation In-Person Counseling | .11 | .18 | .12 | .15 | .20 | .02 | .21 | .02 | .22 | .02 | .22 | .02 |
| Preparation Gum x Cessation Phone Counseling | −.07 | .37 | −.08 | .37 | −.01 | .96 | .01 | .93 | −.06 | .51 | −.06 | .52 |
| Preparation Gum x Medication Duration | −.00 | .99 | −.00 | .97 | .01 | .89 | −.00 | .96 | −.04 | .67 | −.05 | .60 |
| Preparation Counseling x Cessation In-Person Counseling | −.04 | .59 | −.03 | .68 | −.10 | .26 | −.10 | .26 | −.17 | .08 | −.16 | .09 |
| Preparation Counseling x Cessation Phone Counseling | −.01 | .95 | .00 | 1.00 | −.18 | .04 | −.19 | .04 | −.14 | .15 | −.14 | .14 |
| Preparation Counseling x Medication Duration | .05 | .58 | .06 | .48 | .06 | .52 | .06 | .48 | −.11 | .29 | −.11 | .27 |
| Cessation In-Person Counseling x Cessation Phone Counseling | −.05 | .56 | −.06 | .47 | −.28 | .002 | −.28 | .002 | −.23 | .02 | −.23 | .02 |
| Cessation In-Person Counseling x Medication Duration | −.06 | .46 | −.04 | .65 | −.13 | .15 | −.10 | .27 | −.08 | .40 | −.05 | .61 |
| Cessation Phone Counseling x Medication Duration | −.06 | .51 | −.06 | .52 | .03 | .78 | .04 | .66 | .02 | .87 | .02 | .81 |
Note. Bold indicates p < .05.
Models were adjusted for gender, race (White vs. non-White), age, education (high school or less vs. at least some college), healthcare system, Heaviness of Smoking Index, and baseline carbon monoxide (N = 631 due to missing covariates).
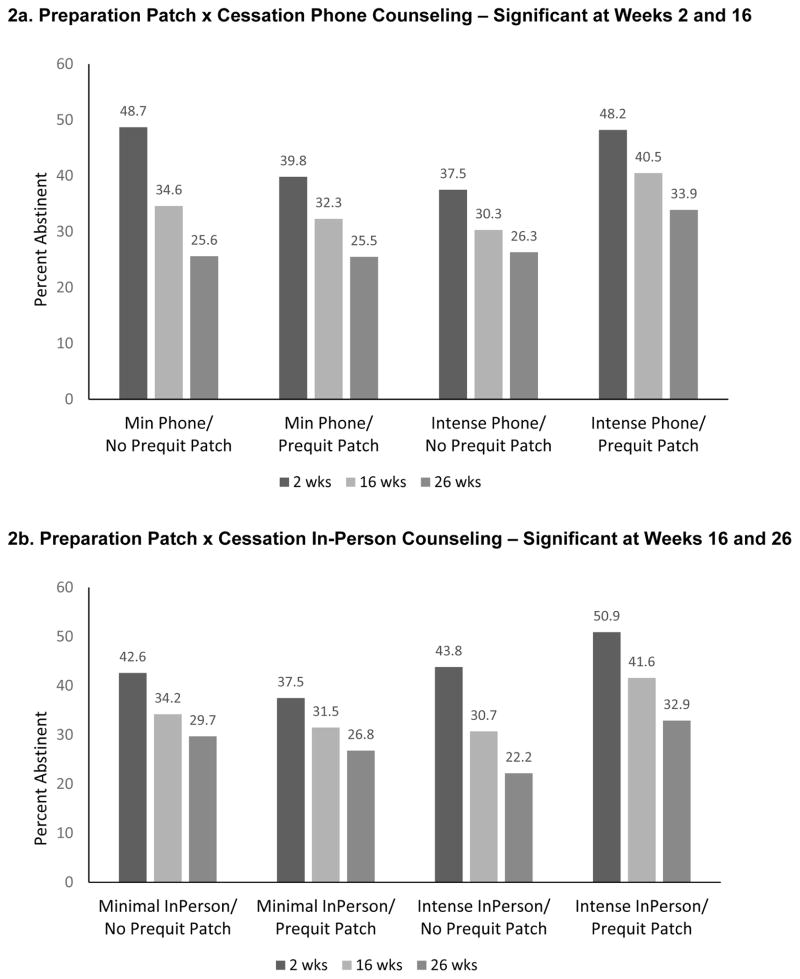
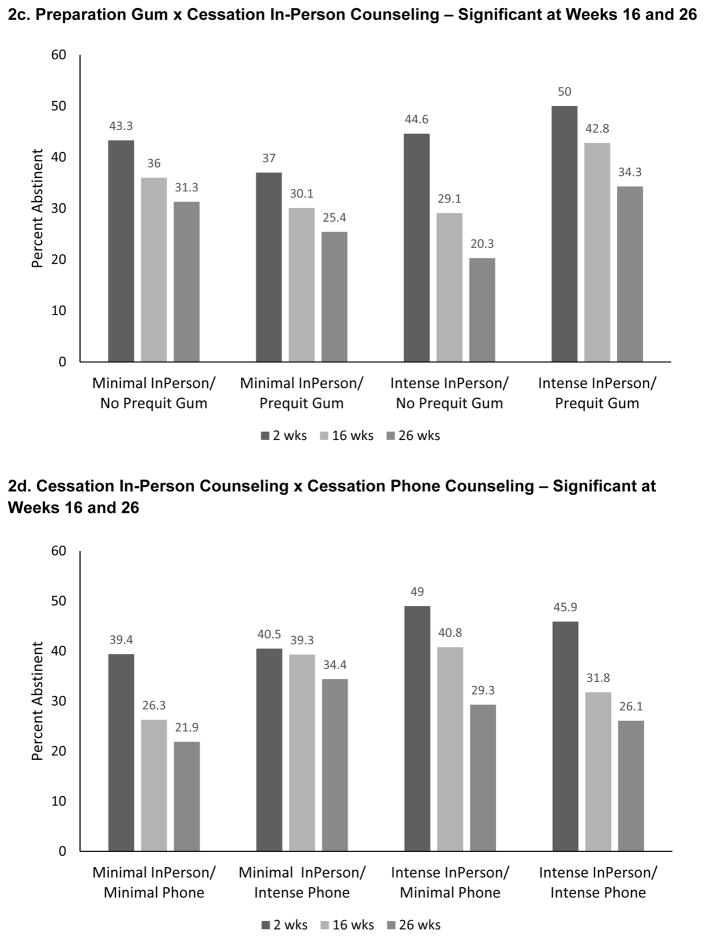
There were five significant 2-way interactions at Week 16: Preparation Patch x Cessation Phone Counseling, Preparation Patch x Cessation In-Person Counseling, Preparation Gum x Cessation In-Person Counseling, Cessation In-Person Counseling x Cessation Phone Counseling, and Preparation Counseling x Cessation Phone Counseling. The three interactions involving Preparation NRT and Cessation-phase counseling interventions were synergistic; i.e., the combination of Preparation NRT and Cessation counseling yielded better 16-week abstinence rates than would be expected based upon summing the main effects (Figures 2a–c). As Figure 2c shows, participants who received Preparation Gum and Intensive Cessation In-Person Counseling had a higher 16-week PPA rate (42.8%), than did participants who received only one of these components (31.1% or 29.1%) or neither (36%). Similar patterns were found at Week 26; differences tended to be less pronounced at Week 2 (Figure 2c).
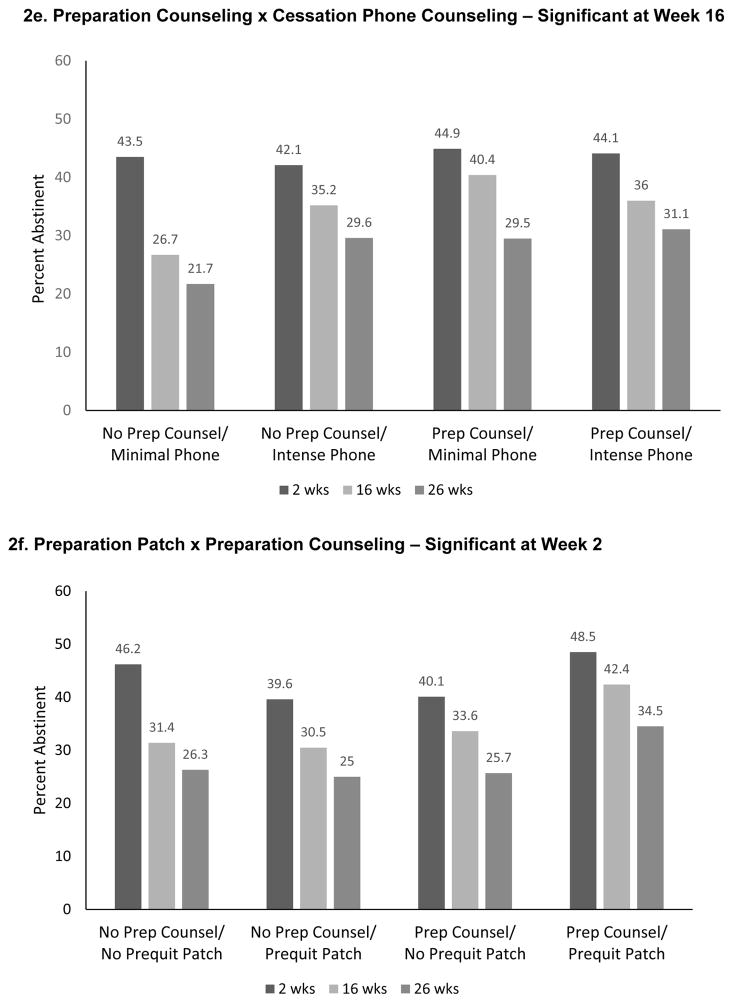
Figure 2.
Significant Interactions from the 7-Day Point-Prevalence Abstinence Models
The Cessation In-Person Counseling x Cessation Phone Counseling interaction was antagonistic—participants receiving either of those interventions without the other had higher abstinence rates at Weeks 16 and 26 than did participants receiving both (Figure 2d). The Week 16 Preparation Counseling x Phone Counseling interaction was also antagonistic (Figure 2e). The Preparation Patch x Preparation Counseling interaction was significant only at Week 2. Participants receiving either of those components, without the other, actually had lower abstinence rates than those receiving both components or neither (Figure 2f).
Discussion
The goal of this screening experiment was to identify Preparation, Cessation, and Maintenance-phase intervention components that yield patterns of promising effects on smoking abstinence when used in a primary care setting. In keeping with MOST, after these components are identified, they would then undergo further research evaluation such as an RCT that would determine their effects when they are used together as an integrated treatment (see [11] for more detail about subsequent experiments). This research also provides important comparative effectiveness data that suggest that Preparation-phase treatment can indeed enhance abstinence rates (cf. [30]) and that combining in-person and phone counseling might constitute ineffective duplication. Finally, the results provide insight into how intervention components work together (i.e., interact).
The only significant main effect was that Preparation Counseling improved abstinence rates at Week 16. However, interaction effects revealed meaningful differences in component effectiveness depending on the levels of other components. In particular, the effects of Cessation-phase counseling were enhanced by the use of Precessation NRT (Figures 2a–c). That this pattern appeared with regard to both the patch and gum, and manifested at two time points, suggests the robustness of this relation. Thus, while prior data have yielded a mixed picture of the effectiveness of Preparation pharmacotherapy [28–30], the current results suggest that NRT pretreatment can be helpful, but its benefit depends on the nature of the cessation counseling that is provided, with intensive Cessation-phase counseling providing more benefit than minimal counseling.
Conversely, some intervention components appeared to undermine each other’s effects. For instance, there was evidence that the two intensive levels of counseling used together produced lower abstinence rates than when either was used without the other (i.e., at Weeks 16 and 26, Intensive Cessation In-Person and Intensive Cessation Phone Counseling produced lower abstinence rates when used together than when used by themselves: Figure 2d). This may be due to redundancy in treatment mechanism or to participant burden—the content of the two counseling types were similar and were designed to last 15 (phone) to 20 (in-person) minutes.
Three types of intervention components yielded promising effects—Preparation NRT, Preparation Counseling, and Intensive Cessation In-Person counseling—based on patterns of effects observed across the three time points. Preparation Counseling produced a significant main effect at 16 weeks, and a significant synergistic interaction with Preparation Patch at 2 weeks (Table 3). Preparation NRT (either Patch or Gum) and Intensive Cessation In-Person Counseling interacted synergistically at both 16- and 26-weeks. Cessation In-Person Counseling appears more promising than Cessation Phone Counseling because it produced a somewhat stronger main effect at 2 weeks (although not significant: Table 3), and it uniquely participated in the synergistic interactions with Preparation NRT at Weeks 16 and 26. The data do not permit a clear-cut decision as to whether Preparation Patch or Gum would be superior. They produced similar synergistic interactions with Cessation In-Person Counseling at both 16- and 26-weeks, and the two could not be distinguished based on their main effects (Tables 2 & 3).
However, the evidence supporting these three components is not wholly compelling. The main effect for Preparation Counseling occurred at only one time point, and the promise of the other components is supported by interaction effects, which show that a component can be effective, but its effects are conditional on the presence of another component [70]. Another concern with interactions is that the cause of the interaction is unknown—does it occur because of antagonistic effects on change mechanisms, or because of some other factor such as perceived burden? These interactions involve factors that have been experimentally manipulated in a controlled fashion, which increases the likelihood of replicability. However, future research is required to identify the extent to which such interactions replicate, especially when they are not stipulated a priori.
The number of interaction effects, and the fact that they reflect both synergistic and antagonistic effects amongst components, illustrates the importance of evaluating intervention components with factorial designs before combining components into treatment packages [8]. One cannot confidently extrapolate the joint actions of intervention components based upon their individual effects or on their effects as elements of unvaried combinations of components as occurs in standard RCTs [7, 9, 10]. This highlights a potential value of factorial designs (as per MOST [8]), which uniquely permit the modeling of interaction effects.
The Phase-Based Model emphasizes the importance of examining component effectiveness over time. In fact, Preparation NRT and Cessation-phase counseling interactions were present at the 16 and 26 weeks but not at 2 weeks. This suggests that some treatment effects take time to appear—they may “incubate”. Thus, there may be no simple relation between temporal propinquity and sensitivity to treatment effects [14]; more research is needed to characterize the main and interactive effects of intervention components over time and to elucidate the mechanisms that account for observed patterns.
Additional research is also needed to confirm which intervention components are most effective at the three treatment phases targeted in this research and to assess the effects of components on other outcome criteria, both general (e.g., cost) and phase-relevant (e.g., does Preparation-phase intervention reduce prequit smoking? [14]) criteria. In addition, our use of a fractional factorial design precluded the estimation of higher-order interactions; such interactions are assumed to be negligible relative to main effects and two-factor interactions but we were unable to test this empirically. Further, this research examined intervention components that function primarily during the Preparation and Cessation phases; it is possible that a longer duration of medication use would produce stronger Maintenance-phase effects [13, 71]. Finally, consistent with this experiment’s goal of hypothesis generation, it was not powered for simple effects tests; therefore, interactions were interpreted via an appraisal of consistent patterns of effects [11].
Conclusion
Using innovative, efficient strategies to investigate approaches for treating smokers recruited in primary care, this research identified three intervention components that demonstrated promising effects on abstinence: Preparation NRT, Preparation Counseling, and Intensive Cessation In-Person Counseling. Intensive Cessation Phone Counseling and Extended Medication (16 vs. 8 weeks of combination NRT) demonstrated less evidence of effectiveness. The multiple statistical interactions amongst the different intervention components support the use of factorial experiments to screen intervention components for their main and interactive effects, prior to assembling multi-component treatments. The promising intervention components identified in this research should undergo further evaluation, including an RCT that would determine their effects when they are used together as an integrated treatment.
Supplementary Material
Acknowledgments
We would like to acknowledge the staff at Aurora Health Care, Deancare, and Epic Systems Corporation for their collaboration in this research. We are very grateful to the staff and students at the Center for Tobacco Research and Intervention in the University of Wisconsin School of Medicine and Public Health for their assistance with this research.
Footnotes
Based upon reviewer recommendations the designation of outcomes was altered from what was listed in trial registration materials.
Declaration of Interest: This research was supported by grants 9P50CA143188 and 1K05CA139871 from the National Cancer Institute to the University of Wisconsin Center for Tobacco Research and Intervention and by the Wisconsin Partnership Program. Dr. Collins is also supported by NIH grants P50DA10075 and R01DK097364. This work was carried out in part while Dr. Schlam was a Primary Care Research Fellow supported by a National Research Service Award (T32HP10010) from the Health Resources and Services Administration to the University of Wisconsin Department of Family Medicine. Dr. Cook is also supported by Merit Review Award 101CX00056 from the US Department of Veterans Affairs. Dr. Loh is also supported by NSF grant DMS-1305725.
The authors have received no direct or indirect funding from, nor do they have a connection with, the tobacco, alcohol, pharmaceutical or gaming industries or anybody substantially funded by one of these organizations. Dr. Loh is partially supported by a grant from Eli Lilly and Company for research that is unrelated to smoking or tobacco dependence treatment.
Clinical Trial Registration: NCT01116986
References
- 1.U.S. Department of Health and Human Services. The health consequences of smoking - 50 years of progress: A report of the Surgeon General. Atlanta, GA: 2014. [Google Scholar]
- 2.Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz N, Curry SJ, et al. Treating tobacco use and dependence: 2008 update. Rockville, MD: U.S. Department of Health and Human Services, U.S. Public Health Service; 2008. [Google Scholar]
- 3.Fiore MC, Bailey WC, Cohen SJ. Smoking cessation: Clinical practice guideline No. 18. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research; 1996. [Google Scholar]
- 4.Niaura R, Abrams DB. Smoking cessation: progress, priorities, and prospectus. J Consult Clin Psychol. 2002;70:494–509. doi: 10.1037//0022-006x.70.3.494. [DOI] [PubMed] [Google Scholar]
- 5.Piasecki TM, Baker TB. Any further progress in smoking cessation treatment? Nicotine Tob Res. 2001;3:311–23. doi: 10.1080/14622200110050484. [DOI] [PubMed] [Google Scholar]
- 6.Stead LF, Lancaster T. Behavioural interventions as adjuncts to pharmacotherapy for smoking cessation. Cochrane Database Syst Rev. 2012;(12):Art. No.: CD009670. doi: 10.1002/14651858.CD009670.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Collins LM, Murphy SA, Strecher V. The multiphase optimization strategy (MOST) and the sequential multiple assignment randomized trial (SMART): new methods for more potent eHealth interventions. Am J Prev Med. 2007;32(5 Suppl):S112–8. doi: 10.1016/j.amepre.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins LM, Baker TB, Mermelstein RJ, Piper ME, Jorenby DE, Smith SS, et al. The Multiphase Optimization Strategy for engineering effective tobacco use interventions. Ann Behav Med. 2011;41:208–26. doi: 10.1007/s12160-010-9253-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins LM, Murphy SA, Nair VN, Strecher VJ. A strategy for optimizing and evaluating behavioral interventions. Ann Behav Med. 2005;30:65–73. doi: 10.1207/s15324796abm3001_8. [DOI] [PubMed] [Google Scholar]
- 10.Collins LM, Dziak JJ, Kugler KC, Trail JB. Factorial experiments: efficient tools for evaluation of intervention components. Am J Prev Med. 2014;47:498–504. doi: 10.1016/j.amepre.2014.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baker TB, Collins LM, Mermelstein R, Piper ME, Schlam TR, Cook JW, et al. Enhancing the effectiveness of smoking treatment research: conceptual bases and progress. Addiction. doi: 10.1111/add.13154. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cook JW, Baker TB, Fiore MC, Smith SS, Fraser D, Bolt DM, et al. Comparative effectiveness of motivation phase intervention components for use with smokers unwilling to quit: a factorial screening experiment. Addiction. doi: 10.1111/add.13161. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schlam TR, Fiore MC, Smith SS, Fraser D, Bolt DM, Collins LM, et al. Comparative effectiveness of intervention components for producing long-term abstinence from smoking: a factorial screening experiment. Addiction. doi: 10.1111/add.13153. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baker TB, Mermelstein R, Collins LM, Piper ME, Jorenby DE, Smith SS, et al. New methods for tobacco dependence treatment research. Ann Behav Med. 2011;41:192–207. doi: 10.1007/s12160-010-9252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schlam TR, Baker TB. Interventions for tobacco smoking. Annu Rev Clin Psychol. 2013;9:675–702. doi: 10.1146/annurev-clinpsy-050212-185602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kenford SL, Fiore MC, Jorenby DE, Smith SS, Wetter D, Baker TB. Predicting smoking cessation. Who will quit with and without the nicotine patch. JAMA. 1994;271:589–94. doi: 10.1001/jama.271.8.589. [DOI] [PubMed] [Google Scholar]
- 17.Piasecki TM, Fiore MC, Baker TB. Profiles in discouragement: two studies of variability in the time course of smoking withdrawal symptoms. J Abnorm Psychol. 1998;107:238–51. doi: 10.1037//0021-843x.107.2.238. [DOI] [PubMed] [Google Scholar]
- 18.Hughes JR. Effects of abstinence from tobacco: etiology, animal models, epidemiology, and significance: a subjective review. Nicotine Tob Res. 2007;9:329–39. doi: 10.1080/14622200701188927. [DOI] [PubMed] [Google Scholar]
- 19.Jorenby DE, Hatsukami DK, Smith SS, Fiore MC, Allen S, Jensen J, et al. Characterization of tobacco withdrawal symptoms: transdermal nicotine reduces hunger and weight gain. Psychopharmacology (Berl) 1996;128:130–8. doi: 10.1007/s002130050118. [DOI] [PubMed] [Google Scholar]
- 20.Tonstad S, Tonnesen P, Hajek P, Williams KE, Billing CB, Reeves KR. Effect of maintenance therapy with varenicline on smoking cessation: a randomized controlled trial. JAMA. 2006;296:64–71. doi: 10.1001/jama.296.1.64. [DOI] [PubMed] [Google Scholar]
- 21.Stapleton JA, Russell MA, Feyerabend C, Wiseman SM, Gustavsson G, Sawe U, et al. Dose effects and predictors of outcome in a randomized trial of transdermal nicotine patches in general practice. Addiction. 1995;90:31–42. doi: 10.1046/j.1360-0443.1995.901316.x. [DOI] [PubMed] [Google Scholar]
- 22.Gwaltney CJ, Metrik J, Kahler CW, Shiffman S. Self-efficacy and smoking cessation: a meta-analysis. Psychol Addict Behav. 2009;23:56–66. doi: 10.1037/a0013529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirchner TR, Shiffman S, Wileyto EP. Relapse dynamics during smoking cessation: recurrent abstinence violation effects and lapse-relapse progression. J Abnorm Psychol. 2012;121:187–97. doi: 10.1037/a0024451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piasecki TM, Jorenby DE, Smith SS, Fiore MC, Baker TB. Smoking withdrawal dynamics: I. Abstinence distress in lapsers and abstainers. J Abnorm Psychol. 2003;112:3–13. [PubMed] [Google Scholar]
- 25.Glasgow RE, Orleans CT, Wagner EH. Does the chronic care model serve also as a template for improving prevention? Milbank Q. 2001;79:579–612. iv–v. doi: 10.1111/1468-0009.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLellan A, Lewis DC, O’Brein CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284:1689–95. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- 27.Riley WT, Glasgow RE, Etheredge L, Abernethy AP. Rapid, responsive, relevant (R3) research: a call for a rapid learning health research enterprise. Clin Transl Med. 2013;2:10. doi: 10.1186/2001-1326-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rose JE, Herskovic JE, Behm FM, Westman EC. Precessation treatment with nicotine patch significantly increases abstinence rates relative to conventional treatment. Nicotine Tob Res. 2009;11:1067–75. doi: 10.1093/ntr/ntp103. [DOI] [PubMed] [Google Scholar]
- 29.Rose JE, Behm FM, Westman EC, Kukovich P. Precessation treatment with nicotine skin patch facilitates smoking cessation. Nicotine Tob Res. 2006;8:89–101. doi: 10.1080/14622200500431866. [DOI] [PubMed] [Google Scholar]
- 30.Lindson N, Aveyard P. An updated meta-analysis of nicotine preloading for smoking cessation: investigating mediators of the effect. Psychopharmacology (Berl) 2011;214:579–92. doi: 10.1007/s00213-010-2069-3. [DOI] [PubMed] [Google Scholar]
- 31.Fiore MC, Bailey WC, Cohen SJ. Treating tobacco use and dependence: Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services, U.S. Public Health Service; 2000. [Google Scholar]
- 32.Shiffman S. Coping with temptations to smoke. J Consult Clin Psychol. 1984;52:261–7. doi: 10.1037//0022-006x.52.2.261. [DOI] [PubMed] [Google Scholar]
- 33.Williams GC, McGregor HA, Sharp D, Levesque C, Kouides RW, Ryan RM, et al. Testing a self-determination theory intervention for motivating tobacco cessation: supporting autonomy and competence in a clinical trial. Health Psychol. 2006;25:91–101. doi: 10.1037/0278-6133.25.1.91. [DOI] [PubMed] [Google Scholar]
- 34.Williams G, Minicucci D, Kouides R, Levesque C, Chirkov V, Ryan R, et al. Self-determination, smoking, diet and health. Health Educ Res. 2002;17:512–21. doi: 10.1093/her/17.5.512. [DOI] [PubMed] [Google Scholar]
- 35.Abrams DB, Monti PM, Carey KB, Pinto RP, Jacobus SI. Reactivity to smoking cues and relapse: Two studies of discriminant validity. Behav Res Ther. 1988;26:225–33. doi: 10.1016/0005-7967(88)90003-4. [DOI] [PubMed] [Google Scholar]
- 36.Droungas A, Ehrman RN, Childress AR, O’Brien CP. Effect of smoking cues and cigarette availability on craving and smoking behavior. Addict Behav. 1995;20:657–73. doi: 10.1016/0306-4603(95)00029-c. [DOI] [PubMed] [Google Scholar]
- 37.Juliano LM, Brandon TH. Reactivity to instructed smoking availability and environmental cues: evidence with urge and reaction time. Exp Clin Psychopharmacol. 1998;6:45–53. doi: 10.1037//1064-1297.6.1.45. [DOI] [PubMed] [Google Scholar]
- 38.Juliano LM, Brandon TH. Effects of nicotine dose, instructional set, and outcome expectancies on the subjective effects of smoking in the presence of a stressor. J Abnorm Psychol. 2002;111:88–97. doi: 10.1037//0021-843x.111.1.88. [DOI] [PubMed] [Google Scholar]
- 39.Hughes JR, Carpenter MJ. Does smoking reduction increase future cessation and decrease disease risk? A qualitative review. Nicotine Tob Res. 2006;8:739–49. doi: 10.1080/14622200600789726. [DOI] [PubMed] [Google Scholar]
- 40.Cinciripini PM, Lapitsky L, Seay S, Wallfisch A, Kitchens K, Van Vunakis H. The effects of smoking schedules on cessation outcome: can we improve on common methods of gradual and abrupt nicotine withdrawal? J Consult Clin Psychol. 1995;63:388–99. doi: 10.1037//0022-006x.63.3.388. [DOI] [PubMed] [Google Scholar]
- 41.Cinciripini PM, Wetter DW, McClure JB. Scheduled reduced smoking: effects on smoking abstinence and potential mechanisms of action. Addict Behav. 1997;22:759–67. doi: 10.1016/s0306-4603(97)00061-0. [DOI] [PubMed] [Google Scholar]
- 42.Farkas AJ. When does cigarette fading increase the likelihood of future cessation? Ann Behav Med. 1999;21:71–6. doi: 10.1007/BF02895036. [DOI] [PubMed] [Google Scholar]
- 43.Baker TB, Brandon TH, Chassin L. Motivational influences on cigarette smoking. Annu Rev Psychol. 2004;55:463–91. doi: 10.1146/annurev.psych.55.090902.142054. [DOI] [PubMed] [Google Scholar]
- 44.McCarthy DE, Piasecki TM, Fiore MC, Baker TB. Life before and after quitting smoking: an electronic diary study. J Abnorm Psychol. 2006;115:454–66. doi: 10.1037/0021-843X.115.3.454. [DOI] [PubMed] [Google Scholar]
- 45.Brandon TH, Tiffany ST, Obremski KM, Baker TB. Postcessation cigarette use: The process of relapse. Addict Behav. 1990;15:105–14. doi: 10.1016/0306-4603(90)90013-n. [DOI] [PubMed] [Google Scholar]
- 46.Garvey AJ, Bliss RE, Hitchcock JL, Heinold JW, Rosner B. Predictors of smoking relapse among self-quitters: A report from the Normative Aging Study. Addict Behav. 1992;17:367–77. doi: 10.1016/0306-4603(92)90042-t. [DOI] [PubMed] [Google Scholar]
- 47.Shiffman S, Balabanis M. Do drinking and smoking go together? Alcohol Health Res World. 1996;20:107–10. [PMC free article] [PubMed] [Google Scholar]
- 48.Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology (Berl) 2006;184:353–66. doi: 10.1007/s00213-005-0178-1. [DOI] [PubMed] [Google Scholar]
- 49.Kenny PJ, Markou A. Conditioned nicotine withdrawal profoundly decreases the activity of brain reward systems. J Neurosci. 2005;25:6208–12. doi: 10.1523/JNEUROSCI.4785-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shiffman S, Scharf DM, Shadel WG, Gwaltney CJ, Dang Q, Paton SM, et al. Analyzing milestones in smoking cessation: illustration in a nicotine patch trial in adult smokers. J Consult Clin Psychol. 2006;74:276–85. doi: 10.1037/0022-006X.74.2.276. [DOI] [PubMed] [Google Scholar]
- 51.Ferguson SG, Gitchell JG, Shiffman S. Continuing to wear nicotine patches after smoking lapses promotes recovery of abstinence. Addiction. 2012;107:1349–53. doi: 10.1111/j.1360-0443.2012.03801.x. [DOI] [PubMed] [Google Scholar]
- 52.Japuntich SJ, Piper ME, Leventhal AM, Bolt DM, Baker TB. The effect of five smoking cessation pharmacotherapies on smoking cessation milestones. J Consult Clin Psychol. 2011;79:34–42. doi: 10.1037/a0022154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cook JW, Piper ME, Leventhal AM, Schlam TR, Fiore MC, Baker TB. Anhedonia as a component of the tobacco withdrawal syndrome. J Abnorm Psychol. 2015;124:215–25. doi: 10.1037/abn0000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013;5:CD009329. doi: 10.1002/14651858.CD009329.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bolt DM, Piper ME, Theobald WE, Baker TB. Why two smoking cessation agents work better than one: role of craving suppression. J Consult Clin Psychol. 2012;80:54–65. doi: 10.1037/a0026366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bohadana A, Nilsson F, Rasmussen T, Martinet Y. Nicotine inhaler and nicotine patch as a combination therapy for smoking cessation: a randomized, double-blind, placebo-controlled trial. Arch Intern Med. 2000;160:3128–34. doi: 10.1001/archinte.160.20.3128. [DOI] [PubMed] [Google Scholar]
- 57.Sweeney CT, Fant RV, Fagerstrom KO, McGovern JF, Henningfield JE. Combination nicotine replacement therapy for smoking cessation: rationale, efficacy and tolerability. CNS Drugs. 2001;15:453–67. doi: 10.2165/00023210-200115060-00004. [DOI] [PubMed] [Google Scholar]
- 58.Fraser D, Christiansen BA, Adsit R, Baker TB, Fiore MC. Electronic health records as a tool for recruitment of participants’ clinical effectiveness research: lessons learned from tobacco cessation. Transl Behav Med. 2013;3:244–52. doi: 10.1007/s13142-012-0143-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Piper ME, Baker TB, Mermelstein R, Collins LM, Fraser DL, Jorenby DE, et al. Recruiting and engaging smokers in treatment in a primary care setting: developing a chronic care model implemented through a modified electronic health record. Transl Behav Med. 2013;3:253–63. doi: 10.1007/s13142-012-0178-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Collins LM, Dziak JJ, Li R. Design of experiments with multiple independent variables: a resource management perspective on complete and reduced factorial designs. Psychol Methods. 2009;14:202–24. doi: 10.1037/a0015826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hollis JF, McAfee TA, Fellows JL, Zbikowski SM, Stark M, Riedlinger K. The effectiveness and cost effectiveness of telephone counselling and the nicotine patch in a state tobacco quitline. Tob Control. 2007;16(Suppl 1):i53–9. doi: 10.1136/tc.2006.019794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu SH, Stretch V, Balabanis M, Rosbrook B, Sadler G, Pierce JP. Telephone counseling for smoking cessation: Effects of single-session and multiple-session interventions. J Consult Clin Psychol. 1996;64:202–11. doi: 10.1037//0022-006x.64.1.202. [DOI] [PubMed] [Google Scholar]
- 63.Zhu SH, Tedeschi G, Anderson CM, Rosbrook B, Byrd M, Johnson CE, et al. Telephone counseling as adjuvant treatment for nicotine replacement therapy in a “real-world” setting. Prev Med. 2000;31:357–63. doi: 10.1006/pmed.2000.0720. [DOI] [PubMed] [Google Scholar]
- 64.Zhu SH, Anderson CM, Tedeschi GJ, Rosbrook B, Johnson CE, Byrd M, et al. Evidence of real-world effectiveness of a telephone quitline for smokers. New Engl J Med. 2002;347:1087–93. doi: 10.1056/NEJMsa020660. [DOI] [PubMed] [Google Scholar]
- 65.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 66.Robinson SM, Sobell LC, Sobell MB, Leo GI. Reliability of the Timeline Followback for cocaine, cannabis, and cigarette use. Psychol Addict Behav. 2014;28:154–62. doi: 10.1037/a0030992. [DOI] [PubMed] [Google Scholar]
- 67.IBM Corporation. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corporation; 2013. [Google Scholar]
- 68.Heatherton TF, Kozlowski LT, Frecker RC, Rickert W, Robinson J. Measuring the heaviness of smoking: using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. Br J Addict. 1989;84:791–9. doi: 10.1111/j.1360-0443.1989.tb03059.x. [DOI] [PubMed] [Google Scholar]
- 69.Hedeker D, Mermelstein RJ, Demirtas H. Analysis of binary outcomes with missing data: missing = smoking, last observation carried forward, and a little multiple imputation. Addiction. 2007;102:1564–73. doi: 10.1111/j.1360-0443.2007.01946.x. [DOI] [PubMed] [Google Scholar]
- 70.Collins LM, Trail JB, Kugler KC, Baker TB, Piper ME, Mermelstein RJ. Evaluating individual intervention components: making decisions based on the results of a factorial screening experiment. Transl Behav Med. 2014;4:238–51. doi: 10.1007/s13142-013-0239-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schnoll RA, Patterson F, Wileyto EP, Heitjan DF, Shields AE, Asch DA, et al. Effectiveness of extended-duration transdermal nicotine therapy: a randomized trial. Ann Intern Med. 2010;152:144–51. doi: 10.7326/0003-4819-152-3-201002020-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.