Abstract
Furfuryl alcohol is considered by the U.S. Environmental Protection Agency to be a high volume production chemical, with over 1 million pounds produced annually. Due to its high production volume and its numerous industrial and consumer uses, there is considerable potential for work-related exposure, as well as exposure to the general population, through pulmonary, oral, and dermal routes of exposure. Human exposure data report a high incidence of asthma in foundry mold workers exposed to furan resins, suggesting potential immunologic effects. Although furfuryl alcohol was nominated and evaluated for its carcinogenic potential by the National Toxicology Program, studies evaluating its immunotoxicity are lacking. The studies presented here evaluated the immunotoxic potential of furfuryl alcohol following exposure by the dermal and pulmonary routes using a murine model. When tested in a combined irritancy local lymph node assay, furfuryl alcohol was identified to be an irritant and mild sensitizer (EC3 = 25.6%). Pulmonary exposure to 2% furfuryl alcohol resulted in enhanced airway hyperreactivity, eosinophilic infiltration into the lungs, and enhanced cytokine production (IL-4, IL-5, and interferon-γ) by ex vivo stimulated lung-associated draining lymphoid cells. Airway hyperreactivity and eosinophilic lung infiltration were augmented by prior dermal exposure to furfuryl alcohol. These results suggest that furfuryl alcohol may play a role in the development of allergic airway disease and encourage the need for additional investigation.
Keywords: furfuryl alcohol, occupational asthma, immunotoxicity
Furfuryl alcohol, also known as furanmethanol, 2-furancar-binol, 2-hydroxymethylfuran, and furfural alcohol, is most commonly used in foundry operations as a binding agent and as a corrosion inhibitor. Properties such as low viscosity, high reactivity, and excellent solvent characteristics make it valuable to a variety of industries. Furfuryl alcohol is also used as an intermediate in the manufacture of resins and wetting agents, a reactive plasticizer for phenolic resins in the manufacture of cold-molded grinding wheels, an intermediate in the synthesis of numerous organic chemicals, and a solvent in cleaning and paint removal operations, as well as in varnishes and dyes. Furfuryl alcohol can initiate polymerization when mixed with foundry sand and exposed to heat or acid catalysts, resulting in vaporization leading to the potential for pulmonary exposure (Virtamo and Tossavainen, 1976). Furfuryl alcohol is also used as a flavoring agent in a variety of consumer food products including coffee, tea, cocoa, milk products, nuts, breads, popcorn, and vegetables (Maga, 1979).
As a result of the ubiquitous presence of furfuryl alcohol, concerns have been raised over potential adverse health effects associated with exposure. Studies have reported furfuryl alcohol to be highly toxic in laboratory animals (Gajewski and Alsdorf, 1949). Exposure of rats to furfuryl alcohol vapor (100 ppm) resulted in decreased weight gain and biochemical changes in the brain (Savolainen and Pfaffli, 1983). Inhalation exposure studies performed by the National Toxicology Program (NTP) reported evidence of carcinogenic activity in both male (nasal) and female (nasal and renal) rats (NTP, 1999). In these studies, signs of severe irritation were observed following inhalation (250 ppm for 16 days) including labored breathing, decreased activity, and nose and eye discharge. Increases in lung eosinophils were observed in both male and female rats, suggesting that furfuryl alcohol exposure may have an impact on immunologic responses.
Consistent with animal studies, human exposure data have reported a high incidence of respiratory disease, including asthma, in foundry workers exposed to furan-based resins (Ahman et al., 1991; Cockcroft et al., 1980; Gomes et al., 2001; Kuo et al., 1999; Low and Mitchell, 1985). A mold maker was found to develop severe asthma after working with a mixture containing furfuryl alcohol, paraformaldehyde, xylene and a catalyst containing sulfuric acid or butyl alcohol. Exposure to the furan resin mixed with a catalyst, as well as pure furfuryl alcohol mixed with sulfuric acid or butyl alcohol, provoked a late asthmatic response and heightened nonallergic bronchial responsiveness to inhaled histamine in this individual (Cockcroft et al., 1980).
Although the majority of studies have focused on the health effects associated with pulmonary exposure to furfuryl alcohol, the skin has also been identified as a relevant route of exposure in humans. Immersion of a human hand in liquid furfuryl for 15 min has been shown to result in the percutaneous absorption of furfuryl alcohol concentrations that equal those absorbed during an 8-h inhalation exposure to 10 mg/m3 furfuryl alcohol, based on urinary metabolites (Sedivec and Flek, 1978). In a separate report, study volunteers exposed dermally to an atmosphere containing 30 mg/m3 furfural vapors, while breathing fresh air through a mask, absorbed 20–30% of the furfuryl alcohol absorbed by other volunteers exposed to furfuryl alcohol through both the dermal and pulmonary routes under the same conditions (Sedivec and Flek, 1978). Based on the previously described occupational uses for furfuryl alcohol, there is the potential for dermal exposure to the neat chemical, which could deliver high concentrations to individuals working with this chemical. As a result of the potential for dermal exposure, a National Institute for Occupational Safety and Health (NIOSH) skin notation has recently been added for furfuryl alcohol to alert employees that excessive exposure to furfuryl alcohol can also occur through this route of exposure.
In addition to the skin notation, other regulations have been created for furfuryl exposure based on results from human exposure data (Apol, 1973; Burton and Rivera, 1972). The current permissible exposure limit for furfuryl alcohol designated by the Occupational Safety and Health Administration is 50 ppm (200 mg/m3), determined as a time-weighted average for up to an 8-h work shift. The NIOSH recommended exposure limit is 10 ppm (40 mg/m3) with a short-term exposure limit of 15 ppm (60 mg/m3). An immediately dangerous to life and health value of 75 ppm was also set by NIOSH based on acute inhalation toxicity data in animals (NIOSH, 2010).
Despite the animal and human reports mentioned above suggesting that furfuryl alcohol exposure may contribute to allergic airway disease, studies investigating the immunotoxic effects of furfuryl alcohol were previously lacking. Therefore, these studies were undertaken to evaluate the immunotoxic effects associated with both dermal and pulmonary exposure to furfuryl alcohol using a murine model.
MATERIALS AND METHODS
Animals
Female BALB/c mice were used in this study. The mice were purchased from Taconic Farms (Germantown, NY) at 6–8 weeks of age. Upon arrival, the animals were allowed to acclimate for a minimum of 5 days. Animals were randomly assigned to treatment groups, weighed, and individually identified via tail markings using a permanent marker or tattoo. A preliminary ANOVA on body weights was performed to insure a homogeneous distribution of animals across treatment groups. The animals were housed at a maximum of five per cage in ventilated plastic shoebox cages with hardwood chip bedding, NIH-31 modified 6% irradiated rodent diet (Harlan Teklad, Indianapolis, IN), and tap water was provided from water bottles, ad libitum. The temperature in the animal facility was maintained between 68°F and 72°F and the relative humidity between 36 and 57%. The light/dark cycle was maintained on 12-h intervals. All animal experiments were performed in the AAALAC accredited NIOSH animal facility in accordance with an animal protocol approved by the Institutional Animal Care and Use Committee.
Chemicals
Furfuryl alcohol (Cas # 98-00-0), methacholine chloride (MCH; Cas # 101-86-0), α-hexylcinnamaldehyde (HCA; Cas # 101-86-0), and toluene 2,4-diisocyanate (TDI; Cas # 584-84-9) were all purchased from Sigma Aldrich (St Louis, MO).
Toxicity and range finding studies
Initial studies were conducted to evaluate the toxicity of furfuryl alcohol following dermal and pulmonary exposure. To select doses for dermal studies, mice were exposed to 25 µl/ear of acetone vehicle or increasing concentrations of furfuryl alcohol (10–75%) once daily for three to four consecutive days. For pulmonary exposure, mice were lightly anesthetized with isoflurane (Abbott Laboratories, 99.9%, Saint-Laurent, Québec, Canada) and exposed to increasing concentrations of furfuryl alcohol (0.5–10%) or vehicle (PBS) by pharyngeal aspiration, using the method described by Rao et al. (2003) once every fourth day for a total of four doses. Systemic toxicity was evaluated by clinical observation (morbidity or extensive irritation) and changes in body weight from preexposure to the time of sacrifice.
Combined local lymph node and irritancy assay
To determine the irritancy and sensitization potential of furfuryl alcohol, a combined local lymph node assay (LLNA) was conducted. Furfuryl alcohol dosing concentrations (10–75%) and vehicle (acetone) were selected based on initial range finding toxicity studies. The LLNA was performed according to the method described in the Interagency Coordination Committee on the Validation of Alternative Methods Peer Review Panel report (National Institute of Environmental Health Sciences [NIEHS], 1999) with minor modifications. Briefly, mice (five per group) were exposed topically to acetone vehicle, increasing concentrations of furfuryl alcohol, or positive control (30% HCA) on the dorsal surface of each ear (25 µl/ear) for three consecutive days. HCA is an accepted and well-characterized positive control for the LLNA (NIEHS, 1999). TDI (2.5%) was used as a positive control for the irritancy portion of the experiment. Irritancy measurements were performed as previously described (Woolhiser et at, 1999). The thickness of the right and left ear pinnae of each mouse was measured using a modified engineer’s micrometer (Mitutoyo Co., Japan) before the first chemical administration and 24 h following the final exposure. The mean percentage of ear swelling was calculated based on the following equation: ([mean postchallenge ear thickness — mean prechallenge ear thickness] / mean prechallenge thickness) × 100. Animals were allowed to rest for 2 days following the last exposure. On day 6, mice were injected intravenously via the lateral tail vein with 20 µCi 3H-thymidine (specific activity 2 Ci/mmol; Dupont NFN, Boston, MA). Five hours after 3H-thymidine injection, animals were euthanized via CO2 inhalation, and the left and right cervical draining lymph nodes (DLNs) located at the bifurcation of the jugular vein were excised and pooled for each animal. Single cell suspensions were made and incubated overnight in 5% trichloroacetic acid, and samples were counted using a Packard Tri-Carb 2500TR liquid scintillation analyzer (PerkinElmer, Inc., Waltham, MA). Stimulation indices (SI) were calculated by dividing the mean disintegrations per minute (DPM) per test group by the mean DPM for the vehicle control group. EC3 values (concentration of chemical required to induce a threefold increase over the vehicle control) were calculated based on the equation from Basketter et al. (1999).
Phenotypic analysis of DLN cells following dermal furfuryl alcohol exposure
To determine if furfuryl alcohol induced an IgE-mediated type I response, the absolute number and percentage of IgE+B220+ cells in the DLNs were quantitated after dermal exposure to furfuryl alcohol. For the phenotypic analysis, furfuryl alcohol was tested at concentrations up to 75%. Lymph node cell phenotypes were analyzed using flow cytometry, as described by Manetz and Meade (1999). Mice were exposed to acetone, increasing concentrations of furfuryl alcohol, or TDI (2.5%) positive control topically on the dorsal surface of each ear (25 µl/ear) for four consecutive days. TDI is commonly used by this laboratory as a Th2 positive control when evaluating low-molecular-weight chemicals. Animals were allowed to rest for 6 days after the final exposure and then euthanized on day 10 by CO2 inhalation. DLNs were collected (two nodes/animal/tube) in 2 ml PBS and were dissociated using the frosted ends of two microscope slides. Cell counts were performed using a Coulter Counter (Z2 model; Beckman Coulter, Fullerton, CA), and 1 × 106 cells per sample were added to the wells of a 96-well plate. Cells were washed using flow staining buffer (1% bovine serum albumin/0.1% sodium azide in PBS) and then incubated with Fc block (clone 2.4G2). The cells were then incubated with anti-CD45RA/B220 (PE, clone RA3–6B2) and anti-IgE antibodies (FITC, clone R-35–72) or the appropriate isotype controls, diluted in staining buffer, washed, and incubated with propidium iodine (PI). All antibodies and isotype controls were purchased from BD Bioscience, Pharmingen (San Jose, CA). After a final wash, cells were resuspended in staining buffer and analyzed with a BD FACSCaliber Flow Cytometer using a PI viability gate.
Pulmonary exposure to furfuryl alcohol
For pulmonary exposure studies, mice were lightly anesthetized using isoflurane and exposed to increasing concentrations of furfuryl alcohol (50 µl of 0.5, 1, or 2% solution in PBS) or PBS (vehicle control) by pharyngeal aspiration using the method previous described by Rao et al. For the initial studies investigating the effects of pulmonary exposure alone, mice (five per group) were exposed to furfuryl alcohol every fourth day for 3 weeks for a total of eight doses. For the studies investigating whether prior dermal exposure to furfuryl alcohol is capable of enhancing pulmonary responses, mice (seven per group) were sensitized on the dorsal surface of both ears with 25 µl/ear of acetone vehicle control or increasing concentrations of furfuryl alcohol (25, 50, or 75%) on days 1–4 of the experiment. Mice were then challenged with 2% furfuryl alcohol via pharyngeal aspiration on days 5, 9, 13, and 17 for a total of four aspirations.
Airway hyperreactivity
Twenty-four hours after the final pulmonary exposure, airway responsiveness was assessed as changes in airway function following challenge with aerosolized MCH using the Buxco unrestrained whole-body plethysmography system. An initial 5-min baseline PenH reading was obtained prior to challenge with increasing concentrations of aerosolized MCH (10, 25, and 50 mg/ml in PBS). For each concentration of MCH, average PenH values were collected every 30 s for 5 min. MCH exposure occurred only for the first 3 min of this 5-min period. During the final 2 min, mice were exposed to fresh air alone. Average PenH values for each 5-min period were plotted versus the MCH concentration and used as a measure of airway responsiveness.
Bronchial alveolar lavage-cellular infiltrate phenotyping
Bronchioalveolar lavage (BAL) samples were collected 24 h after final airway challenge. Following euthanasia, lungs were perfused, via the right ventricle of the heart, with 10 ml PBS to remove blood cells present within the lung vasculature. BAL samples were then collected by cannulating the trachea of each mouse and lavaging three times, each time with 1 ml of sterile PBS. Approximately 2 ml of bronchial lavage fluid was recovered from each mouse. Suspended cells were pelleted by centrifugation (5 min at 486 × g) and resuspended in 200 µl of flow staining buffer containing the following combinations of fluorochrome conjugated antibodies to identify infiltrating eosinophil, neutrophil, and alveolar macrophage (CD45-APC, Siglec-F-PE, CD11c–biotin, and LY6G–FITC) or B- and T-cell (CD45-APC, CD45R/B220-PE, and CD3-FITC) populations. Cell suspensions were incubated with labeled antibodies on ice in the dark for 30 min and washed. Samples stained with biotin-labeled anti-CD11c (1:100 dilutions in flow staining buffer) were resuspended in flow staining buffer containing streptavidin PercP, incubated for an additional 30 min on ice in the dark, and washed. All samples were then fixed by resuspending in 100 µl BD cytofixation buffer (BD Bioscience, San Diego, CA) and incubating for 15 min. Cells were then washed, resuspended in flow staining buffer, and enumerated using a FACSCaliber Flow Cytometer (BD Bioscience, San Jose, CA) within 48 h. Lung cellular infiltrates were identified phenotypically based upon the distinct expression of the following cell surface markers as previously reported by Stevens et al. (2007): eosinophils (CD45hi, LY6Glow, Siglec Fhi, CD11clow), neutrophils (CD45hi, LY6Ghi, CD11clow), and alveolar macrophages (CD45hi, LY6Glow, Siglec Fhi, CD11chi). The absolute number of gated cells corresponding to each cellular phenotype was determined using AccuCheck Counting Beads (Invitrogen, Camarillo, CA) according to the manufacturer’s instructions.
Ex vivo analysis of cytokine production from lung-associated DLN cells
Lung-associated lymph nodes (LALN) were collected in 3 ml PBS from mice 24 h after final pulmonary exposure. Single cell suspensions of lymph node cells were made by grinding the tissue between the frosted ends of two microscope slides. Cells were counted using a Coulter Counter (Z1 model; Beckman Coulter, Brea, CA), adjusted to 1 × 106 cells/ml in sterile RPMI media containing 10% fetal calf serum (FCS), and seeded in 48-well plates (500,000 cells per well). Cells were then stimulated with α-CD3 + α-CD28 antibodies (2 µg/ml each; BD Bioscience, Pharmingen) for 24 h at 37°C and 5% CO2. Supernatants were analyzed for IL-4, IL-5, and interferon (IFN)-γ levels using OptEIA ELISA kits purchased from BD Biosciences according to the manufacturer’s instructions.
Total serum IgE
Following euthanasia of animals used in the phenotypic analysis assays (dermal exposure) and airway hyperreactivity (AHR) (pulmonary exposure), blood samples were collected via cardiac puncture or transection of the abdominal aorta. Sera were separated by centrifugation and frozen at −20°C for subsequent analysis of IgE by ELISA. A standard colorimetric sandwich ELISA was performed as previously described (Butler, 2000). All antibodies and isotype controls were purchased from BD Bioscience, Pharmingen. In brief, 96-well flat bottom plates (Dynatech Immulon-2) were coated with (2 µg/ml in PBS) purified monoclonal rat anti-mouse IgE antibody (clone R35–72; 2 µg/ml, diluted in 0.05M carbonate-bicarbonate buffer, pH 9.6), sealed with plate sealers, and incubated overnight at 4°C. The plates were washed three times with PBS/Tween 20 and then blocked for 1 h with diluent (2% FCS and 0.05% sodium azide) at room temperature. Initial serum samples were diluted 1:10 in diluent, and the IgE control standard (mouse IgE α-TNP; clone C38-2) was prepared at 500 ng/ml. Serum samples and IgE control standard were serially diluted (1:2) through eight wells, added to the coated plates in a 100 µl volume, and incubated at room temperature for 1 h. The plates were then washed three times with PBS/Tween 20. Biotin-conjugated rat anti-mouse IgE (clone R35–92; 2 µg/ml) was added in a 100 µl volume, and plates were incubated at room temperature for 1 h. The plates were again washed three times with PBS/Tween 20. Streptavidin-alkaline phosphatase (BD Bioscience, Pharmingen Cat# 554065; 100 µl of a 1:400 dilution) was added, and plates were incubated for 1 h at room temperature. P-Nitrophenyl phosphate (Sigma Cat# N-9389) was used as the alkaline phosphatase substrate and added to the plates in a 100 µl volume. The plates were allowed to develop for up to 30 min at room temperature or until the optical density reading of the highest standard reached 3.0. Absorbance was determined using a SpectraMax Vmax Plate Reader (Molecular Devices, Sunnyvale, CA) at 405–605 nm. Data analysis was performed using the IBM SoftMax Pro 3.1 (Molecular Devices), and the IgE concentrations for each sample were interpolated from a standard curve using multipoint analysis.
Histopathology
Lungs from mice dermally exposed to furfuryl alcohol or PBS and then subsequently challenged by pulmonary aspiration as described above were insulfated with 10% formalin and collected for histopathology. Lung tissue for histopathology was embedded in paraffin, sectioned at five microns, and stained with hematoxylin and eosin, Alcian Blue/PAS, Masons trichrome, and Sirius Red. Lungs from two mice per sensitization and challenge combination were evaluated for changes by a veterinary pathologist. Semiquantitative pathology scores were the sum of the severity and distribution of the histopathologic changes as previously described (Hubbs et al., 2008).
Statistical analysis
For analysis of animal studies, the data were first tested for homogeneity using the Bartlett’s chi-square test. If homogeneous, a one-way ANOVA was conducted. If the ANOVA showed significance at p < 0.05 or less, the Dunnett’s multiple range t-test was used to compare treatment groups with the control group. LLNA data were further evaluated by calculating the DPM values of the 3H incorporation from the DLNs of the animals from each group. The data were compared between groups by using SI values calculated from the DPM values of each group. Linear trend analysis was performed to determine if furfuryl alcohol had exposure concentration-related effects for specific endpoints. Differences were considered to be significant if p < 0.01 as compared with vehicle controls. SAS/STAT software (Version 9.2 of the SAS system for Windows; SAS Institute, Cary, NC) was used to analyze PenH. A PROC MIXED was utilized to run a two-way factorial ANOVA with concentration of MCH treated as a repeated measure to account for multiple measures in individual animals. Treatment comparisons between treatment groups were then calculated at each level of MCH utilizing the “slice” option. All differences were considered significant at p < 0.05.
RESULTS
Dermal Exposure to Furfuryl Alcohol
Dermal irritation and sensitization
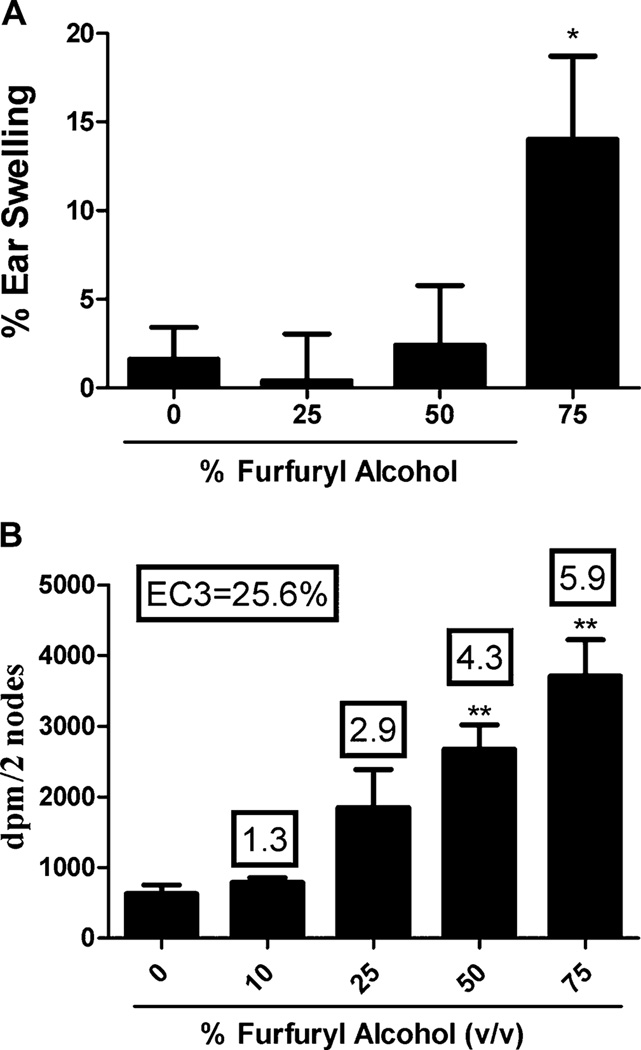
Initial concentration range finding studies found no overt dermal toxicity at exposure to 75% furfuryl alcohol; therefore, this was the maximum concentration used for the dermal studies. The irritancy and sensitization potential of furfuryl alcohol were evaluated using a combined irritancy/LLNA. A significant increase (p < 0.05) in ear swelling (14%) was observed in mice following dermal exposure to 75% furfuryl alcohol (Fig. 1A). The positive control (2.5% TDI) resulted in a statistically significant increase (66%) in ear swelling 24 h after final exposure. In the LLNA, a dose responsive increase (Linear Trend Test; p < 0.01) in proliferation was observed in animals exposed to increasing concentrations of furfuryl alcohol reaching statistical significance (p < 0.05) in the 50 and 75% exposure groups (Fig. 1B). The calculated EC3 value for furfuryl alcohol was 25.6%. HCA (30%) was used for the positive control for the LLNA and resulted in an average SI value of 7.2.
FIG. 1.
Irritancy and sensitization following dermal furfuryl alcohol exposure. Analysis of irritation (A) and sensitization (B) following a 4-day dermal exposure to furfuryl alcohol. Bars represent the mean ± SE of five mice per group. Numbers above bars represent SI. Levels of statistical significance are designated as *p ≤ 0.05 and **p ≤ 0.01 as compared with acetone control.
Elevations in local and total IgE
Although the LLNA is effective at identifying potential sensitizers based upon their ability to induce lymphoid cell proliferation, the LLNA does not differentiate between IgE-mediated (Th2) and T cell– mediated (Th1) hypersensitivity responses. Therefore, to further characterize the effects of furfuryl alcohol-mediated sensitization, phenotypic analysis of B220+ and IgE+B220+ cell populations within the DLN of exposed mice was performed. Consistent with LLNA data, phenotypic analysis of DLN isolated from mice exposed to furfuryl alcohol showed a dose responsive increase (Linear Trend Test; p < 0.01) in both the percent and absolute number of B220+ and IgE+B220+ cells in the DLN as compared with vehicle control (Table 1). Exposure to furfuryl alcohol significantly increased the percentage of IgE+B220+ (24.2 ± 5.3 at 75%) and B220+ cell populations (35.1 ± 1.9 at 75%) in the DLN at all concentrations tested (25–75%). The absolute number of B220+ cells in furfuryl alcohol sensitized mice was significantly elevated at all concentrations, whereas the absolute number of IgE+B220+ cell was significantly elevated following exposure to 75% furfuryl alcohol. Mice exposed to 75% furfuryl alcohol also exhibited a mild but statistically significant increase in serum IgE antibody production (493 ± 98 ng/ml; p < 0.05) in comparison with those exposed only to the acetone vehicle control (180 ± 65 ng/ml; Table 1). No changes in organ or body weights were observed in these animals (data not shown). TDI (2.5%) was used as an IgE positive control for these studies and resulted in significant mean elevations of total IgE (1356 ± 37 ng/ml), IgE+B220+ (38.7 ± 4.6%), and B220+ (41.5 ± 4.5%).
TABLE 1.
Phenotypic and Total Serum IgE Analysis
| Dose group (furfuryl alcohol) (%) | IgE+B220+ (%) | Total IgE+B220+ (× 107) | B220+ (%) | Total B220+ (× 107) | Total IgE (ng/ml) |
|---|---|---|---|---|---|
| 0a | 4.0 ± 1.1 | 2.8 ± 1.1 | 16.3 ± 2.1 | 10.1 ± 2.2 | 180 ± 65 |
| 25 | 5.7 ± 2.2* | 5.7 ± 2.2 | 29.5 ± 2.8** | 24.5 ± 4.2* | 491 ± 58 |
| 50 | 16.0 ± 2.8** | 13.9 ± 3.0 | 26.8 ± 2.8* | 23.4 ± 3.4* | 366 ± 47 |
| 75 | 24.2 ± 5.3** | 24.6 ± 7.3** | 35.1 ± 1.9** | 33.8 ± 5.0** | 493 ± 98* |
Note. Values represent the mean ± SE from five animals per group.
Represents acetone vehicle control.
Statistical significance is designated as *p ≤ 0.05 and **p ≤ 0.01.
Pulmonary Exposure to Furfuryl Alcohol
Enhanced AHR, IgE, and eosinophils
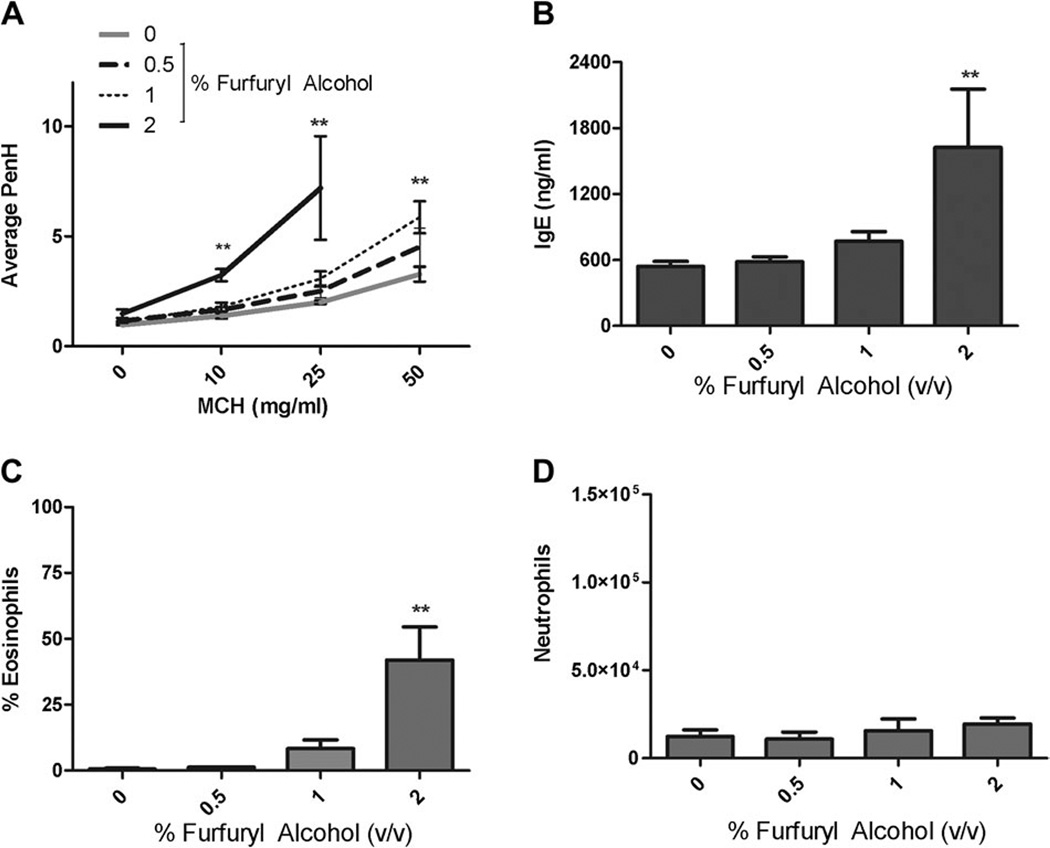
Results from the concentration range finding study identified the maximum pulmonary dose that was tolerated by the animals to be 2%. Animals exposed to concentrations greater than 2% were found to be lethargic, exhibited weight loss, and had ruffled fur. As expected, exposure to increasing concentrations of MCH resulted in increasing AHR in the vehicle control group. Repeated pulmonary exposure (eight doses) to low concentrations of furfuryl alcohol (0.5%) had little effect on airway responsiveness however; repeated pulmonary exposure (eight doses) to both 1 and 2% furfuryl alcohol significantly enhanced airway responsiveness to aerosolized MCH (Fig. 2A; Table 2). Following challenge with 10 and 25 mg/ml MCH, the average PenH values of mice exposed to 2% furfuryl alcohol were 3.28 ± 0.28 and 7.21 ± 2.36 compared with the vehicle control values of 1.39 ± 0.13 and 2.01 ± 0.08, respectively. Due to the strong airway responses induced by challenge with 25 mg/ml MCH in mice exposed to 2% furfuryl alcohol, the 50 mg/ml MCH challenge was not conducted. Enhanced airway responses following exposure to 1% furfuryl alcohol reached statistical significance only after challenge with 50 mg/ml MCH, resulting in an average PenH value of 5.9 ± 0.72 compared with a vehicle control value of 3.23 ± 0.42. The absolute number of eosinophils in the BAL fluid of mice exposed to 2% furfuryl alcohol was significantly enhanced (153,075 ± 86,269) in comparison with vehicle controls (1448 ± 773; Fig. 2C). Although a modest increase in the number of eosinophils isolated from the BAL fluid of the 1% exposure group was identified, the levels did not reach statistical significance. Consistent with these findings, serum IgE levels were increased approximately threefold, from 541 ± 46 pg/ml in vehicle control mice to 1625 ± 530 pg/ml in mice exposed to 2% furfuryl alcohol (Fig. 2B). Pulmonary exposure to furfuryl alcohol had no effect on the total number of neutrophils present in BAL fluid (Fig. 2D). No changes in organ or body weights were observed in these animals (data not shown).
FIG. 2.
Effect of pulmonary furfuryl alcohol exposure. Airway hyperreactivity (A), total IgE (B), eosinophil infiltrates (C), and neutrophil infiltrates (D) were evaluated in mice following pulmonary exposure to furfuryl alcohol. Mice were exposed to furfuryl alcohol every fourth day for 3 weeks for a total of eight doses. Bars represent mean values ± SE of five mice per group with the exception of the 2% exposure group, which had only four mice. Levels of statistical significance are designated as **p ≤ 0.01 as compared with the vehicle control.
TABLE 2.
Assessment of Airway Hyperreactivity following Furfuryl Alcohol Exposure
| Pulmonarya |
Dermal + pulmonaryb |
|||||||
|---|---|---|---|---|---|---|---|---|
| Furfuryl alcohol | 0 mg/ml | 10 mg/ml | 25 mg/ml | 50 mg/ml | Furfuryl alcohol | 0 mg/ml | 10 mg/ml | 25 mg/ml |
| 0% | 0.9 ± 0.02 | 1.4 ± 0.13 | 2.0 ± 0.08 | 3.3 ± 0.33 | 0% | 1.1 ± 0.05 | 1.6 ± 0.04 | 3.0 ± 0.49 |
| 0.5% | 1.1 ± 0.04 | 1.7 ± 0.11 | 2.5 ± 0.29 | 4.5 ± 0.86 | 2% + 0% | 1.3 ± 0.09 | 2.5 ± 0.39 | 5.5 ± 1.7 |
| 1% | 1.1 ± 0.03 | 1.8 ± 0.20 | 3.1 ± 0.34 | 5.9 ± 0.72* | 2% + 25% | 1.5 ± 0.09 | 3.6 ± 1.0 | 10.8 ± 2.8 |
| 2% | 1.5 ± 0.19 | 3.2 ± 0.28* | 7.2 ± 2.36* | 2% + 50% | 1.2 ± 0.04 | 5.1 ± 0.97 | 12.6± 3.1*,† | |
| 2% + 75% | 2.3 ± 0.38 | 6.8 ± 2.0*,† | 13.6 ± 2.6*,† | |||||
Mice (five per group) were exposed to furfuryl alcohol every fourth day for 3 weeks for a total of eight doses (Fig. 2A).
Mice (seven per group) were sensitized with furfuryl alcohol (25, 50, or 75%) on days 1–4 of the experiment and then challenged with 2% furfuryl alcohol via pharyngeal aspiration on days 5, 9, 13, and 17 (Fig. 4A).
Values represent the mean PenH ± SE following MCH challenge (0, 10, 25, and 50 mg/ml); statistical significance is designated as *p ≤ 0.01 as compared with the vehicle control or †p ≤ 0.01 as compared with the 2% furfuryl alcohol pulmonary exposure control (2% + 0%).
Enhanced cytokine production by LALN
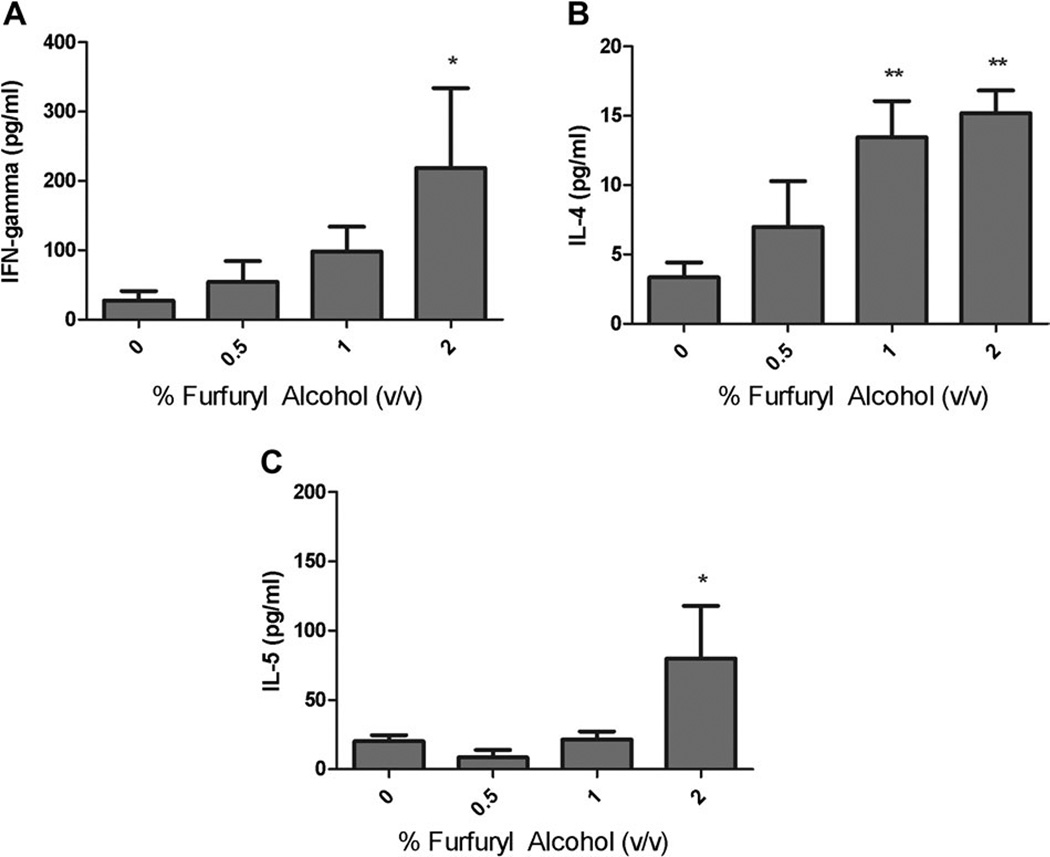
Pulmonary exposure to furfuryl alcohol resulted in a mixed Th1/Th2 immune response with dose responsive increases (Linear Trend Test; p < 0.01) in IL-4 and IFN-γ. Statistically significant increases in IFN-γ (219 ± 115 pg/ml), IL-4 (15 ± 2 pg/ml), and IL-5 (80 ± 38pg/ml) production by LALN cells (Fig. 3) were observed following pulmonary exposure to 2% furfuryl alcohol compared with the vehicle control mice.
FIG. 3.
Cytokine production by lung-associated draining lymphoid cells following pulmonary exposure to furfuryl alcohol. Analysis of (A) IFN-γ, (B) IL-4, and (C) IL-5 production by LALN following pulmonary exposure to furfuryl alcohol. Mice were exposed to furfuryl alcohol every fourth day for 3 weeks for a total of eight doses. Bars represent mean ± SE for each group of five mice, with the exception of the 2% exposure group, which had only four. Levels of statistical significance are denoted as *p ≤ 0.05 and **p ≤ 0.01 as compared with vehicle controls.
Prior dermal exposure to furfuryl alcohol enhances allergic airway responses
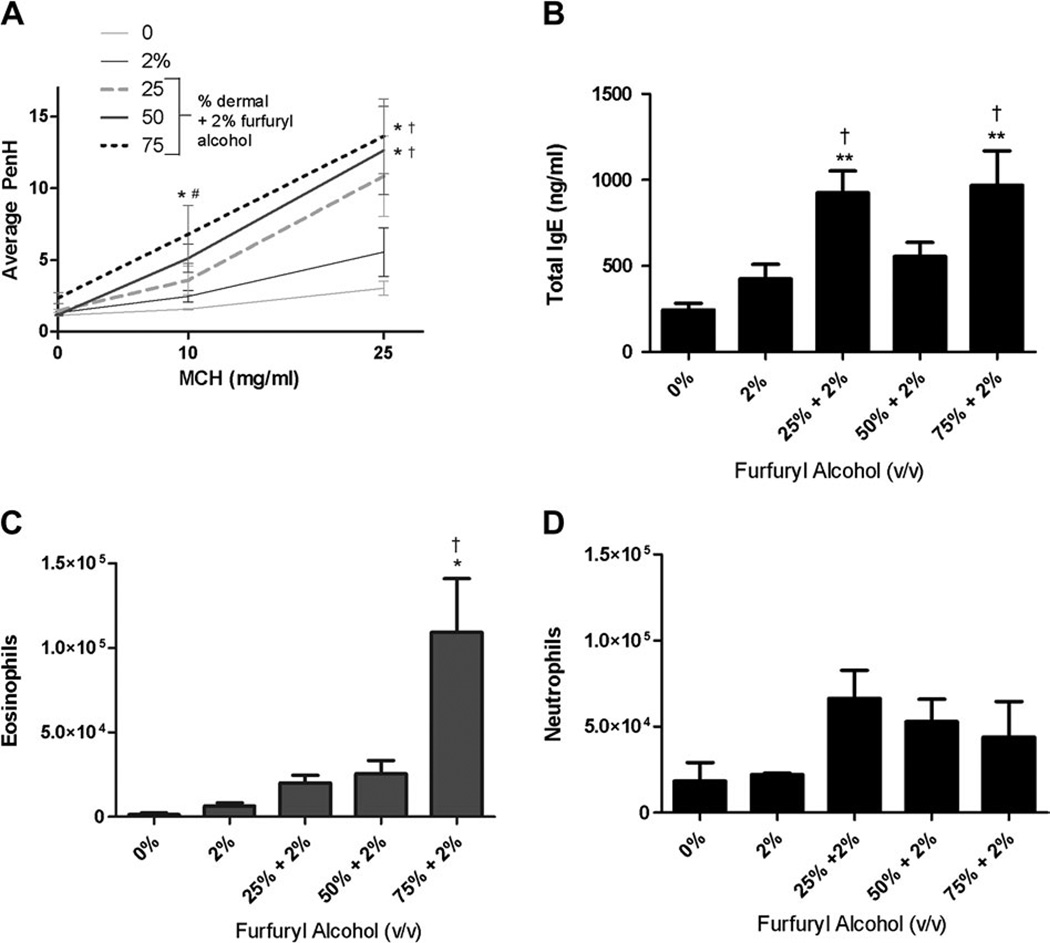
Although airway responses induced by repeat pulmonary exposure (four doses) with 2% furfuryl alcohol were enhanced (Fig. 4A; Table 2), the increase in average PenH values did not reach statistical significance when compared with vehicle controls. However, when mice were first treated topically (50–75%) and then subsequently challenged (2%; four doses) with furfuryl alcohol statistically significant enhancements of AHR were detected in comparison with the vehicle control mice as well as in comparison with mice that received only pulmonary exposure to 2% furfuryl alcohol (Fig. 4A; Table 2). The average PenH values following MCH challenge (25 mg/ml) were 13.1 ± 3.2 (50% dermal + 2% pulmonary challenge) and 14.3 ± 3.7 (75% dermal + 2% pulmonary challenge). These values were more than double the average PenH value that was measured in mice receiving only pulmonary exposure (5.5 ± 1.7). Due to the strong airway responses induced by challenge with 25 mg/ml MCH in mice exposed to furfuryl alcohol, the 50 mg/ml MCH challenge was not conducted for any of the groups. Mice that were exposed to furfuryl alcohol (75%) by dermal route only showed no alterations in AHR and had PenH values similar to those measured in vehicle controls (data not shown). In addition to heightened airway reactivity, eosinophilic infiltration in BAL fluid was also increased in mice that received furfuryl alcohol via dermal and pulmonary routes compared with the vehicle control or those that received only pulmonary exposures (Fig. 4B). The largest increase in eosinophilic infiltration (109,309 ± 31,884) was observed in mice topically exposed to 75% furfuryl alcohol and then challenged with 2% compared with the BAL collected from mice only exposed to 2% furfuryl alcohol (6291 ± 1873). Consistent with these findings, serum IgE levels were statistically elevated in the mice exposed via dermal and pulmonary routes (Fig. 4B) compared with vehicle control and 2% furfuryl alcohol exposure groups. Mice that were exposed to furfuryl alcohol by dermal (75%) route only also had a statistically significant increase (530 ± 81 pg/ml) in total IgE compared with the vehicle control (246 ± 37 pg/ml). Although not statistically significant, prior dermal exposure to furfuryl alcohol increased the number of neutrophils in the BAL fluid as compared with both vehicle control mice and mice exposed to 2% furfuryl alcohol via the airways alone (Fig. 4D). Mice that were exposed to furfuryl alcohol by dermal (75%) route alone showed no alterations in total eosinophils or neutrophils (data not shown). No changes in organ or body weights were observed in these animals (data not shown).
FIG. 4.
Effect of prior dermal exposure on airway responses induced by pulmonary exposure to furfuryl alcohol. Airway hyperreactivity (A), IgE (B), eosinophils (C), and neutrophils (D) were evaluated in mice dermally sensitized to furfuryl alcohol (25–75%) and challenged with furfuryl alcohol (2%; four doses) via the airways. Mice were sensitized with furfuryl alcohol (25, 50, or 75%) on days 1–4 of the experiment and then challenged with 2% furfuryl alcohol via pharyngeal aspiration on days 5, 9, 13, and 17. Error bars represent mean ± SE of seven mice per group. Statistical significance is designated as †p ≤ 0.01 as compared with the 2% furfuryl alcohol pulmonary exposure control (four doses) or *p ≤ 0.01 as compared with the vehicle control.
Histopathology
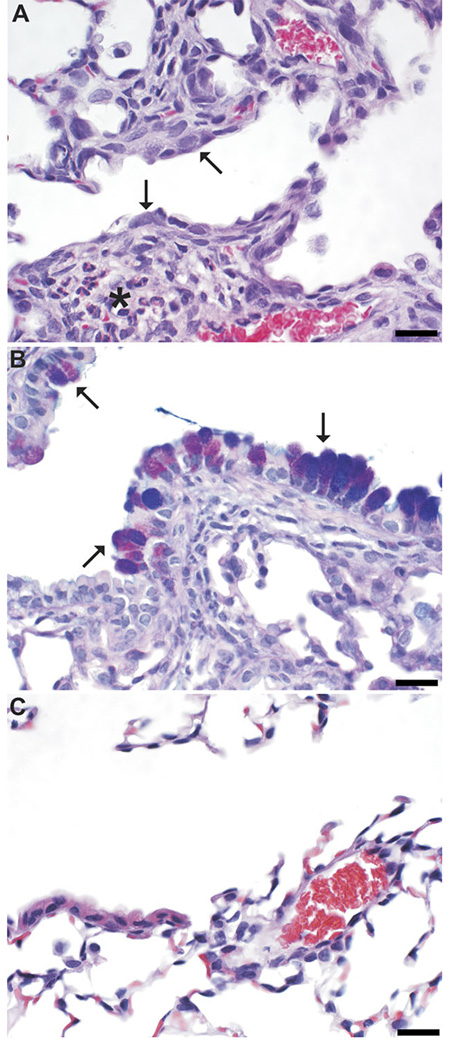
Aspiration with furfuryl alcohol (2%; four doses) produced consistent changes within the lung that were centered at the bronchioloalveolar junction (Table 3). These changes include multifocal histiocytic to histiocytic and eosinophilic to eosinophilic and neutrophilic bronchointerstitial pneumonia with bronchiolar and alveolar epithelial hypertrophy and hyperplasia (Fig. 5A). In addition, bronchiolar epithelial disorganization and mucous metaplasia were consistently associated with furfuryl alcohol aspiration (Fig. 5B). Mice aspirating PBS did not have bronchointerstitial pneumonia or airway epithelial changes (Fig. 5C). Dermal sensitization was necessary for the development of furfuryl alcohol–induced eosinophilic pneumonia except in one mouse exposed to furfuryl without dermal sensitization, which developed a multifocal, mild, histiocytic bronchointerstitial pneumonia without a major eosinophilic component. Furfuryl alcohol–induced airway epithelial changes (airway mucous metaplasia) occurred irrespective of dermal sensitization (Table 3).
TABLE 3.
Pulmonary Histopathology Findings in Mice Aspirating Furfuryl Alcohol with and without Prior Sensitization
| Pulmonary challengea |
|||
|---|---|---|---|
| PBS | 2% furfuryl alcohol | ||
| Dermal sensitization | Acetone | Eosinophilic pneumonia 0/2 (0)b | Eosinophilic pneumonia 0/2 (3)b,c |
| Airway mucous metaplasia 0/2 (0) | Airway mucous metaplasia 2/2 (5) | ||
| 25% furfuryl alcohol | Not done | Eosinophilic pneumonia 2/2 (6.5)b | |
| Airway mucous metaplasia 2/2 (6) | |||
| 50% furfuryl alcohol | Not done | Eosinophilic pneumonia 2/2 (6)b | |
| Airway mucous metaplasia 2/2 (6) | |||
| 75% furfuryl alcohol | Eosinophilic pneumonia 0/2 (0)b | Eosinophilic pneumonia 2/2 (6.5)b | |
| Airway mucous metaplasia 0/2 (0) | Airway mucous metaplasia 2/2 (6) | ||
Data are presented as the number affected/number evaluated followed by the mean pathology score of all animals in that exposure.
Eosinophilic pneumonia includes pneumonias classified as eosinophilic and neutrophilic, pneumonias classified as histiocytic and eosinophilic, and pneumonias classified as eosinophilic and histiocytic. All pneumonia involve both bronchioles and the nearby interstitium (bronchointerstitial).
One mouse in this group developed multifocal mild histiocytic bronchointerstitial pneumonia with a small number of neutrophils, lymphocytes, and eosinophils as lesser contributors to the inflammation.
FIG. 5.
Histopathologic changes after aspiration of furfuryl alcohol in mice sensitized by prior dermal sensitization. In hematoxylin and eosin (H&E)-stained sections of furfuryl alcohol–exposed (pulmonary and dermal) mice (A), the bronchiolar epithelium is hypertrophied, hyperplastic, and mildly disorganized (arrows), and the peribronchiolar interstitium is infiltrated by histiocytes and eosinophils (*). In AB/PAS-stained sections from furfuryl alcohol–exposed mice (B), mucous metaplasia (arrows) is demonstrated by magenta to purple staining of hypertrophied bronchiolar epithelial cells. Vehicle control mice were within normal histologic limits (C; H&E stained section). Bar is 20 µm.
DISCUSSION
During the past several decades, a remarkable increase in the prevalence of asthma has been noted in the United States and other industrialized nations (Moorman et al., 2007). It is now estimated that one in eight individuals suffer from the disease (Busse and Lemanske, 2001). In addition to improved hygiene and reduced exposure to pathogens, individuals living in industrial societies are also exposed to an increasing number and amounts of chemicals (Busse and Lemanske, 2001; Umetsu et al., 2002). Exposure to chemicals has been shown to enhance the development of respiratory illness, especially in the occupational setting where exposure can be high. Work-related asthma is currently the most frequently diagnosed occupational respiratory illness, costing an estimated $400 million dollars per year, and is responsible for approximately 10–25% of asthma occurring in adults (Petsonk, 2002). Of the 250 substances suspected of causing occupationally induced asthma, approximately 90 are low-molecular-weight chemical compounds.
These studies evaluated the allergic potential of the low-molecular-weight chemical, furfuryl alcohol, following dermal and pulmonary exposure. It is important to note that these acute exposure studies were conducted for the purpose of hazard identification. The exposure regimes employed in these investigations delivered relatively high doses of furfuryl alcohol to the skin (25–75%) and lungs (0.5–2%) of a mouse. However, although the general public would not likely encounter concentrations, this high occupational exposure to furfuryl alcohol can often be to the neat chemical. Furfuryl alcohol was identified as an irritant and sensitizing chemical following dermal exposure, due to its capacity to induce ear swelling and significant DLN cell proliferation. The calculated EC3 value for furfuryl alcohol, based on LLNA results, was 25.6% classifying it as a mild sensitizer (Loveless et al., 2010). Supporting a role for respiratory sensitization, significant increases in both local (IgE+B220+ cells) and total serum IgE were observed. Manetz and Meade (1999) have previously shown that select chemicals capable of inducing IgE-mediated allergic responses have comparable peak increases in the percent IgE+B220+ and B220+ populations, which tend to become significantly elevated at equivalent chemical concentrations. A similar trend was observed after treatment with 75% furfuryl alcohol (IgE+B220+ population increased to 24.2 ± 5.3% and B220+ population increased to 35.1 ± 1.9% of total lymphocytes). Since the elevation in IgE+B220+ cell populations relates to the local binding of soluble IgE to the CD23 receptor on B cells in the DLNs, it would be expected to occur before IgE elevations in the serum and possibly be detected following exposure to lower concentrations (Cheng et al., 2010). This is consistent with the observed results. The furfuryl alcohol concentration that induced significant increases in the IgE+B220+ cell population (25%) was lower than the concentration significantly elevating total serum IgE (75%). In addition, while significant increases in total IgE (493 ± 98 mg/ml) were detected, the values were low. It is possible that due to the mild sensitization potential of furfuryl alcohol, a longer exposure protocol (> 4 days) may be required to induce higher levels of total IgE in the serum (Fukuyama et al., 2009). Consistent with this hypothesis, repeated pulmonary exposure (eight doses) to 2% furfuryl alcohol over a 3-week period, resulted in higher total serum IgE levels (1625 ± 530 pg/ml; Fig. 2B) than those documented for the mice exposed to just four pulmonary exposures (425 ± 84 pg/ml; Fig. 4B).
Since case report studies and the results from dermal exposure studies suggested a potential link between furfuryl alcohol and IgE-mediated sensitization, additional studies were conducted to determine if pulmonary exposure to furfuryl alcohol could enhance airway hyperresponsiveness and the recruitment of immune cells to the lungs. Consistent with the onset of IgE-mediated allergic asthma, repeat pulmonary exposure (eight doses) to furfuryl alcohol resulted in increases in AHR, eosinophils, type IV hypersensitivity-mediating cytokines, and IgE. Cytokines induced by CD4+ T cells have been shown to be essential mediators of IgE-mediated allergic diseases, such as asthma. Upon activation, T-helper (Th) cells undergo differentiation into functionally distinct effector subsets. Although Th1 cells typically produce IFN-γ and IL-12 and regulate cellular immunity, Th2 cells are associated with orchestrating the inflammatory response associated with IgE-mediated allergy through the production of IL-4, IL-5, and IL-13. Although expression levels of both IL-4 and IL-5 were enhanced following repeated pulmonary exposure to furfuryl alcohol, so was the production of the Th1 cytokine IFN-γ. Although similar mixed Th2-Th1 cytokine profiles have been observed following exposure to respiratory sensitizers, such as TDI (Johnson et al., 2007), the specific role that IFN-γ plays in respiratory sensitizer-induced airway hyperreactivity remains to be defined.
Histopathologic analysis of lungs isolated from select mice in this study provided further support for respiratory sensitization. Airway mucous (goblet) cell metaplasia was identified in all mice following respiratory tract exposure to 2% furfuryl alcohol, irrespective of prior dermal sensitization (Fig. 5). These findings are consistent with a previous 14-week furfuryl alcohol rat inhalation study demonstrating increased numbers of mucous cells in the respiratory epithelium of the nose (NTP, 1999). In humans, hyperplasia of airway mucous cells located specifically in the bronchiolar respiratory epithelium is a hallmark of asthma pathogenesis. Abnormalities in the number of mucous cells within this region, as well as changes in the amount of both their stored and secreted mucin, is believed to contribute to the clinical manifestations associated with the disease, such as sputum production, airway narrowing, and accelerated loss of lung function (Lai and Rogers; Warner and Knight, 2008). Computational fluid dynamic and physiologically based pharmacokinetic modeling would help determine how much furfuryl alcohol would reach the bronchiolar epithelium of man, adding to the risk assessment for this chemical (Frederick et al., 2002).
It is not known if excessive dermal exposure to furfuryl alcohol may enhance respiratory responses contributing to occupational asthma by modulating responses in the respiratory tract. Therefore, the effects of prior dermal furfuryl alcohol exposure on furfuryl alcohol–induced airway responses were evaluated. Dermal exposure to sensitizing chemicals, such as TDI and trimellitic anhydride, has previously been shown to induce sensitization of the respiratory tract and enhance respiratory responses upon pulmonary challenge (Howell et al., 2002; Zhang et al., 2004). Consistent with these reports, the studies described here found that prior dermal exposure to furfuryl alcohol could further enhance airway hyperresponsiveness, IgE production, and immune cell infiltrates in the lung compared with those mice that had only pulmonary exposures. In addition, although the number of mice examined for each exposure group was low, eosinophilic pneumonia was demonstrated by histopathology if, and only if, the mice received a pulmonary challenge with furfuryl alcohol after dermal sensitization, irrespective of the dose used for dermal sensitization.
Although the data described here support a role for furfuryl alcohol in allergic airway disease, it cannot definitively be stated that the pulmonary responses identified were not due to an irritant effect because the effect of a single pulmonary exposure to furfuryl alcohol was not investigated. Pulmonary responses caused by irritation typically cause increases in the number of neutrophils, as they play a significant role in injury-induced inflammation and repair. However, this was not the case for the pulmonary exposures conducted in these studies. It is also important to note that the enhanced pause (PenH) value generated from the pulmonary airway hyperreactivity studies is an indirect measurement of flows and volumes based on software algorithms, which calculate a physiological value from a measured value. Although it is important to mention this limitation associated with PenH, whole-body plethysmography provides the benefits of end-point analysis on unrestrained conscious animals.
In summary, furfuryl alcohol was identified as a low-molecular-weight chemical sensitizer, potentially capable of inducing IgE-mediated allergic responses and thus contributing to the rising incidence of occupational respiratory disease. Exposure to furfuryl alcohol–induced sensitization following both dermal and pulmonary exposures when tested in a murine model. Consistent with case report studies describing a high incidence of respiratory disease in workers exposed to furfuryl alcohol, repeated respiratory exposure to furfuryl alcohol enhanced airway hyperreactivity, IgE, lung eosinophilic infiltration, and enhanced cytokine production. Prior dermal exposure to furfuryl alcohol was shown to further enhance these responses. Together, these results suggest that furfuryl alcohol may play a role in allergic airway disease and encourage additional investigation into immunotoxic effects associated with furfuryl alcohol exposure.
ACKNOWLEDGMENTS
The authors would like to thank Patsy Willard, HT, ASCP for preparing the histopathology slides.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention.
REFERENCES
- Ahman M, Alexandersson R, Ekholm U, Bergstro¨m B, Dahlqvist M, Ulfvarson U. Impeded lung function in moulders and coremakers handling furan resin sand. Int. Arch. Occup. Environ. Health. 1991;63:175–180. doi: 10.1007/BF00381565. [DOI] [PubMed] [Google Scholar]
- Apol A. Health Hazard Evaluation Toxicity Determination Report No. 73-116-85. Tigard, OR: Western Foundry; 1973. [Google Scholar]
- Basketter DA, Lea LJ, Dickens A, Briggs D, Pate I, Dearman RJ, Kimber I. A comparison of statistical approaches to the derivation of EC3 values from local lymph node assay dose responses. J. Appl. Toxicol. 1999;19:261–266. doi: 10.1002/(sici)1099-1263(199907/08)19:4<261::aid-jat572>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Burton DJ, Rivera RO. Health Hazard Evaluation/Toxicity Determination Report 72-10-15. Salt Lake City, UT: May Foundry; 1972. p. 16. [Google Scholar]
- Busse WW, Lemanske RF., Jr Asthma. N. Engl. J. Med. 2001;344:350–362. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- Butler JE. Enzyme-linked immunosorbent assay. J. Immunoassay. 2000;21:165–209. doi: 10.1080/01971520009349533. [DOI] [PubMed] [Google Scholar]
- Cheng LE, Wang ZE, Locksley RM. Murine B cells regulate serum IgE levels in a CD23-dependent manner. J. Immunol. 2010;185:5040–5047. doi: 10.4049/jimmunol.1001900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft DW, Cartier A, Jones G, Tarlo SM, Dolovich J, Hargreave FE. Asthma caused by occupational exposure to a furan-based binder system. J. Allergy Clin. Immunol. 1980;66:458–463. doi: 10.1016/0091-6749(80)90006-8. [DOI] [PubMed] [Google Scholar]
- Frederick CB, Lomax LG, Black KA, Finch L, Scribner HE, Kimbell JS, Morgan KT, Subramaniam RP, Morris JB. Use of a hybrid computational fluid dynamics and physiologically based inhalation model for interspecies dosimetry comparisons of ester vapors. Toxicol. Appl. Pharmacol. 2002;183:23–40. doi: 10.1006/taap.2002.9451. [DOI] [PubMed] [Google Scholar]
- Fukuyama T, Tajima Y, Ueda H, Hayashi K, Shutoh Y, Saito TR, Harada T, Kosaka T. Investigation of the chemical-induced selective type II (T(H)2) allergic response in mice: Effect of the length of the sensitizing phase. J. Immunotoxicol. 2009;6:75–83. doi: 10.1080/15476910902891319. [DOI] [PubMed] [Google Scholar]
- Gajewski JE, Alsdorf WR. Studies on furan compounds: Toxicity and pharmacological action of furfuryl alcohol. Fed. Proc. 1949;8:294. [Google Scholar]
- Gomes J, Lloyd OL, Norman NJ, Pahwa P. Dust exposure and impairment of lung function at a small iron foundry in a rapidly developing country. Occup. Environ. Med. 2001;58:656–662. doi: 10.1136/oem.58.10.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell MD, Weissman DN, Jean Meade B. Latex sensitization by dermal exposure can lead to airway hyperreactivity. Int. Arch. Allergy Immunol. 2002;128:204–211. doi: 10.1159/000064253. [DOI] [PubMed] [Google Scholar]
- Hubbs AF, Goldsmith WT, Kashon ML, Frazer D, Mercer RR, Battelli LA, Kullman GJ, Schwegler-Berry D, Friend S, Castranova V. Respiratory toxicologic pathology of inhaled diacetyl in Sprague-Dawley rats. Toxicol. Pathol. 2008;36:330–344. doi: 10.1177/0192623307312694. [DOI] [PubMed] [Google Scholar]
- Johnson VJ, Yucesoy B, Reynolds JS, Fluharty K, Wang W, Richardson D, Luster MI. Inhalation of toluene diisocyanate vapor induces allergic rhinitis in mice. J. Immunol. 2007;179:1864–1871. doi: 10.4049/jimmunol.179.3.1864. [DOI] [PubMed] [Google Scholar]
- Kuo HW, Chang CL, Liang WM, Chung BC. Respiratory abnormalities among male foundry workers in central Taiwan. Occup. Med. (Lond.) 1999;49:499–505. doi: 10.1093/occmed/49.8.499. [DOI] [PubMed] [Google Scholar]
- Lai HY, Rogers DF. Mucus hypersecretion in asthma: Intracellular signalling pathways as targets for pharmacotherapy. Curr. Opin. Allergy Clin. Immunol. 2010;10:67–76. doi: 10.1097/ACI.0b013e328334643a. [DOI] [PubMed] [Google Scholar]
- Loveless SE, Api AM, Crevel RW, Debruyne E, Gamer A, Jowsey IR, Kern P, Kimber I, Lea L, Lloyd P, et al. Potency values from the local lymph node assay: Application to classification, labelling and risk assessment. Regul. Toxicol. Pharmacol. 2010;56:54–66. doi: 10.1016/j.yrtph.2009.08.016. [DOI] [PubMed] [Google Scholar]
- Low I, Mitchell C. Respiratory disease in foundry workers. Br. J. Ind. Med. 1985;42:101–105. doi: 10.1136/oem.42.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maga JA. Furans in foods. CRC Crit. Rev. Food Sci. Nutr. 1979;11:355–400. doi: 10.1080/10408397909527268. [DOI] [PubMed] [Google Scholar]
- Manetz TS, Meade BJ. Development of a flow cytometry assay for the identification and differentiation of chemicals with the potential to elicit irritation, IgE-mediated, or T cell-mediated hypersensitivity responses. Toxicol. Sci. 1999;48:206–217. doi: 10.1093/toxsci/48.2.206. [DOI] [PubMed] [Google Scholar]
- Moorman JE, Rudd RA, Johnson CA, King M, Minor P, Bailey C, Scalia MR, Akinbami LJ. National surveillance for asthma—United States, 1980–2004. MMWR Surveill. Summ. 2007;56:1–54. [PubMed] [Google Scholar]
- National Institute of Environmental Health Sciences (NIEHS) National Institute of Environmental Health Sciences; the murine local lymph node assay: A test method for assessing the allergic contact dermatitis potential of chemicals/compounds. Fed. Regist. 1999;64:14006–14007. [PubMed] [Google Scholar]
- National Institute for Occupational Safety and Health (NIOSH) NIOSH Pocket Guide to Chemical Hazards; Furfuryl Alcohol. National Institute for Occupational Safety and Health; 2010. [Accessed June 28, 2011]. Available at: http://www.cdc.gov/niosh/npg/npgd0298.html. [Google Scholar]
- National Toxicology Program (NTP) Toxicology and Carcinogenesis Studies of Furfural (CAS No. 98-01-1) in F344/N Rats and B6C3F Mice (Inhalation studies). Technical report. Series No. 382. NIH Publication No. 99-3972. U.S. Department of Health and Human Services. Public Health Service. Research Triangle Park, NC: National Institutes of Health; 1999. [Google Scholar]
- Petsonk EL. Work-related asthma and implications for the general public. Environ. Health Perspect. 2002;110(Suppl. 4):569–572. doi: 10.1289/ehp.02110s4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao GV, Tinkle S, Weissman DN, Antonini JM, Kashon ML, Salmen R, Battelli LA, Willard PA, Hoover MD, Hubbs AF. Efficacy of a technique for exposing the mouse lung to particles aspirated from the pharynx. J. Toxicol. Environ. Health A. 2003;66:1441–1452. doi: 10.1080/15287390306417. [DOI] [PubMed] [Google Scholar]
- Savolainen H, Pfaffli P. Neurotoxicity of furfuryl alcohol vapor in prolonged inhalation exposure. Environ. Res. 1983;31:420–427. doi: 10.1016/0013-9351(83)90020-8. [DOI] [PubMed] [Google Scholar]
- Sedivec V, Flek J. Biologic monitoring of persons exposed to furfural vapors. Int. Arch. Occup. Environ. Health. 1978;42:41–49. doi: 10.1007/BF00385710. [DOI] [PubMed] [Google Scholar]
- Stevens WW, Kim TS, Pujanauski LM, Hao X, Braciale TJ. Detection and quantitation of eosinophils in the murine respiratory tract by flow cytometry. J. Immunol. Methods. 2007;327:63–74. doi: 10.1016/j.jim.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umetsu DT, McIntire JJ, Akbari O, Macaubas C, DeKruyff RH. Asthma: An epidemic of dysregulated immunity. Nat. Immunol. 2002;3:715–720. doi: 10.1038/ni0802-715. [DOI] [PubMed] [Google Scholar]
- Virtamo M, Tossavainen A. Gases formed from furan binding agents.. Scand. J. Work Environ. Health. 1976;2(Suppl. 1):50–53. doi: 10.5271/sjweh.2832. [DOI] [PubMed] [Google Scholar]
- Warner SM, Knight DA. Airway modeling and remodeling in the pathogenesis of asthma. Curr. Opin. Allergy Clin. Immunol. 2008;8:44–48. doi: 10.1097/ACI.0b013e3282f3b5cb. [DOI] [PubMed] [Google Scholar]
- Woolhiser MR, Munson AE, Meade BJ. Role of sensitization routes in the development of type I hypersensitivity to natural rubber latex in mice. Am. J. Ind. Med. 1999;36(Suppl.1):139–141. doi: 10.1002/(sici)1097-0274(199909)36:1+<139::aid-ajim49>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Zhang XD, Fedan JS, Lewis DM, Siegel PD. Asthmalike biphasic airway responses in Brown Norway rats sensitized by dermal exposure to dry trimellitic anhydride powder. J. Allergy Clin. Immunol. 2004;113:320–326. doi: 10.1016/j.jaci.2003.11.047. [DOI] [PubMed] [Google Scholar]