Abstract
Importance
Postnatally acquired cytomegalovirus (CMV) is typically benign in term infants but, in very low birth weight (VLBW) infants, can cause pneumonitis and sepsis-like illness. Whether postnatal CMV infection results in long-term pulmonary sequelae in these infants is unknown.
Objective
To investigate the relationship between postnatal CMV infection and bronchopulmonary dysplasia (BPD) and mortality in a large, multicenter cohort of VLBW infants.
Design
Propensity-matched retrospective cohort study.
Setting
348 neonatal intensive care units in the United States from 1997–2012.
Participants
Hospitalized VLBW (<1500 g) infants.
Exposures
Postnatal CMV infection was defined as a diagnosis of CMV or detection of CMV from blood, urine, cerebrospinal fluid, or respiratory secretions on or after day of life 21. Infants with a CMV diagnosis or virologic detection of CMV prior to day of life 21 were not considered to have postnatal infection.
Main Outcomes and Measures
We matched infants with postnatal CMV infection 1:1 to comparison infants using propensity scores, and used Poisson regression to examine the effect of postnatal CMV on the combined risk of death or BPD at 36 weeks postmenstrual age. To describe features of postnatal CMV infection, we extracted clinical and laboratory data from 7 days before until 7 days after infants met criteria for postnatal CMV.
Results
Of 101,111 infants, 328 (0.3%) had postnatal CMV infection. We matched a comparison infant to 303 (92%) CMV-infected infants for a final cohort of 606 infants. The median gestational age and birth weight of this cohort were 25 weeks and 730 g, respectively. Postnatal CMV infection was associated with an increased risk of death or BPD at 36 weeks postmenstrual age (risk ratio [RR]: 1.21, 95% confidence interval [CI]: 1.10–1.32) and BPD (RR: 1.33, 95% CI: 1.19–1.50). Changes in cardiorespiratory status associated with postnatal CMV infection included a new requirement for vasopressor medications (9%), intubation for mechanical ventilation (15%), a new oxygen requirement (28%), and death (1.2%).
Conclusions and Relevance
In VLBW infants, postnatal CMV infection was associated with increased risk of BPD. Further studies are needed to determine the role of preventative measures against CMV in this population.
Keywords: cytomegalovirus, bronchopulmonary dysplasia, postnatal CMV infection
Cytomegalovirus (CMV) is the most common perinatal viral infection worldwide.1 CMV may be acquired in utero (congenital CMV infection), at delivery from exposure to maternal genital secretions, or after birth (postnatal CMV infection).2-4 Postnatal infection among infants most frequently results from exposure to virus shed in breast milk and – prior to widespread use of CMV-negative and leukoreduced blood products in premature infants – through blood transfusion.3-6 Postnatal infection is not typically associated with clinical signs in term infants, likely as a result of the relative maturity of the term infant immune system and maternal antibody acquired during the third trimester.7,8 In contrast, very low birth weight (VLBW; birth weight <1500 g) infants with postnatal CMV infection can manifest sepsis-like illness, pneumonitis, hepatitis, or hematological abnormalities.2,6,9 Although many VLBW infants with clinically apparent postnatal CMV experience a deterioration in respiratory status coinciding with infection, it is unclear if this results in long-term pulmonary sequelae such as bronchopulmonary dysplasia (BPD).
In this study, we investigate the relationship between postnatal CMV infection and BPD and mortality in a large, multicenter cohort of VLBW infants. As a secondary objective, we describe the clinical and laboratory characteristics associated with postnatal CMV infection in this cohort to inform diagnostic testing in VLBW infants.
METHODS
STUDY SETTING
We identified VLBW infants hospitalized on day of life (DOL) 21 in neonatal intensive care units (NICU) managed by the Pediatrix Medical Group from 1997–2012. Data were obtained from an electronic medical record that prospectively captures information from daily progress notes generated by clinicians using a computer-assisted tool. The method of data extraction was previously described in detail.10 During the study period, the Pediatrix Medical Group included 348 NICUs in the United States. Data on multiple aspects of patient care are available, including demographics, medications, laboratory and culture results, and diagnoses. This study was approved by the Duke University Institutional Review Board.
POSTNATAL CMV INFECTION
We defined postnatal CMV infection as a diagnosis of “CMV infection,” “acquired CMV infection,” or “congenital CMV infection,” or detection of CMV by culture or polymerase chain reaction (PCR) of blood, urine, cerebrospinal fluid, or respiratory secretions on or after DOL 21. To limit identification of infants who were infected congenitally, infants were not considered to have postnatal CMV if they had a diagnosis of “intracranial calcifications” or any of the following prior to DOL 21: 1) detection of CMV by culture or PCR from any source; 2) a diagnosis of “CMV infection,” “acquired CMV infection,” or “congenital CMV infection”; 3) a diagnosis of “microcephaly”; 4) treatment with ganciclovir, valganciclovir, cidofovir, or foscarnet; or 5) a transaminitis, defined as aspartate transaminase (AST) >150 U/L and alanine aminotransferase (ALT) >90 U/L. For the purposes of this analysis, only postnatal CMV infections occurring at <36 weeks postmenstrual age were included.
OUTCOMES
The primary outcome was death or BPD at 36 weeks postmenstrual age. Secondary outcomes were BPD and death prior to hospital discharge, considered separately. Infants were classified as having BPD if they received respiratory support (nasal cannula oxygen or continuous positive airway pressure [CPAP], conventional mechanical ventilation, or high-frequency ventilation) continuously from a postmenstrual age of 36 0/7 to 36 6/7 weeks.11 Infants on room air without any respiratory support for at least one day between 36 0/7 and 36 6/7 weeks postmenstrual age were classified as not having BPD. Infants discharged prior to 36 6/7 weeks postmenstrual age who did not receive respiratory support on the day of discharge were classified as not having BPD. The outcome of BPD was considered missing for infants who died before 36 6/7 weeks postmenstrual age or were discharged prior to 36 6/7 weeks postmenstrual age and received respiratory support on the day of discharge.
OTHER DEFINITIONS
We defined small-for-gestational-age as birth weight below the tenth percentile for gestational age (GA), based on Olsen growth curves.12 Sepsis was defined as isolation of one or more bacterial or fungal pathogens from blood. We excluded likely bacterial contaminants, including non-speciated streptococci, Bacillus spp., Corynebacterium spp., and Micrococcus spp. We divided coagulase-negative Staphylococcus infections into three categories: definite, probable, and possible, as previously described.13 Only definite and probable infections were included in this analysis. We considered the following medications to be vasopressors: amrinone, dobutamine, dopamine, epinephrine, milrinone, and norepinephrine. We defined neutropenia as an absolute neutrophil count <1500 cells/μL, thrombocytopenia as a platelet count <100,000/µL, and direct hyperbilirubinemia as a serum direct bilirubin >1.0 mg/dL.
STATISTICAL ANALYSES
We compared outcomes among VLBW infants with postnatal CMV infection to infants without postnatal CMV. Infants without postnatal CMV infection were selected from all VLBW infants hospitalized on DOL 21 who did not meet criteria for postnatal CMV, including infants who were not tested for CMV and infants who tested negative for CMV. Because postnatal CMV infection is more likely to be associated with clinical signs among the subset of VLBW infants with extreme prematurity and significant comorbidities,2 we used propensity score matching to obtain similar populations for comparison. Comorbid conditions and surrogates for severity of illness were assessed in relation to DOL 21, the day on which infants were first at risk for postnatal CMV infection. We included the following variables, including previously reported risk factors for BPD, in a logistic regression model to generate propensity scores: GA; birth weight; small-for-gestational-age status; sex; race; discharge year; NICU site; days of breast milk exposure between DOL 15 and 21; number of days on which surfactant was received; necrotizing enterocolitis, grade III or IV intraventricular hemorrhage, patent ductus arteriosus, and sepsis episode occurring on DOL ≤21; and number of vasopressor medications, type of respiratory support, and fraction of inspired oxygen (FiO2) assessed on DOL 21.14 We included discharge year as a categorical variable in the model to adjust for changes in clinical practice and outcomes over time. We matched 1:1 on the propensity score using nearest-neighbor matching, provided that the difference in the propensity scores of infants with postnatal CMV and their matched comparison infants was <0.01.15 We examined the distributions of propensity scores across groups using histograms and kernel density plots. We assessed covariate balance across groups within both the unmatched and propensity score-matched cohorts using chi-square tests.
We used Poisson regression with a sandwich variance estimator conditioning on the matched pair to determine the effect of postnatal CMV infection on outcomes in the propensity score-matched cohort.16 This modeling approach, as opposed to logistic regression, was appropriate because the odds ratio obtained using logistic regression is an upwardly biased estimate of the risk ratio (RR) when the outcome is not rare (>10%), as was the case for our primary outcome and secondary outcome of BPD.17
We conducted sensitivity analyses to assess whether our findings would have differed had alternative assumptions been made. First, we examined the effect of postnatal CMV infection on outcomes when infants meeting criteria for postnatal CMV based only on diagnostic coding (and not virologic data) were excluded (n=106). In originally defining postnatal CMV infection, we included infants with a diagnosis of “congenital CMV infection” on or after DOL 21 because we felt that the distinction between congenital and postnatal CMV infection may not be accurately assessed. Our second sensitivity analysis thus excluded infants with a diagnosis of “congenital CMV infection” on or after DOL 21 (n=135). Finally, to assess whether the observed association of postnatal CMV with BPD might be related to a transient respiratory deterioration associated with acute CMV infection, we repeated analyses excluding postnatal CMV infections occurring at ≥34 weeks postmenstrual age (n=94). In conducting each of these sensitivity analyses, we first excluded children who met the specified condition, and then repeated the procedures for generating propensity scores and matching comparison infants to infants with postnatal CMV by propensity scores.
To describe the features of postnatal CMV infection, we extracted clinical and laboratory data from 7 days before until 7 days after infants met criteria for postnatal CMV. For each variable, we calculated the maximum (or minimum) value recorded during the time period. We also determined the proportion of infants who died during this time period or required initiation of vasopressor medications, increased level of respiratory support or intubation, and increased FiO2 or initiation of supplemental oxygen on one or more days. All statistical analyses were conducted using STATA version 13.1 (College Station, TX).
RESULTS
PATIENT CHARACTERISTICS
We identified 101,111 VLBW infants, and 328 (0.3%) infants met study criteria for postnatal CMV infection (Figure 1). Of these infants, 144 (44%) met criteria for postnatal CMV based on diagnosis and virologic testing, 106 (32%) based on diagnosis only, and 78 (24%) based on virologic testing only. The sources of specimens for the 222 infants with virologic testing were urine (72%), trachea (12%), blood (8%), and nasopharynx (8%). We matched a comparison infant to 303 of 328 (92%) infants with postnatal CMV infection. For the remaining 25 infants with postnatal CMV, data were missing for one or more variables included in the model to generate propensity scores. The 606 infants in the final propensity score-matched cohort were from 70 NICU sites. Propensity score matching resulted in a population that was closely matched on baseline characteristics, with no statistically significant differences in covariates observed across the two groups (Table 1). Median (25th percentile, 75th percentile) GA and birth weight of infants in the propensity score-matched cohort were 25 weeks (24–27) and 730 g (611–915), respectively.
Figure 1.
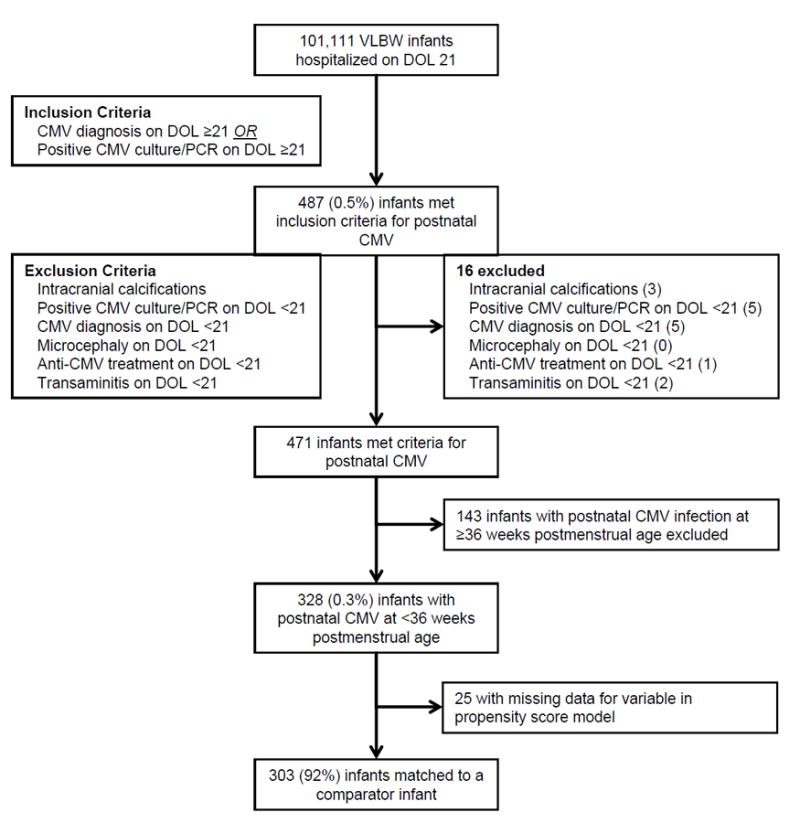
Participant Flow Diagram
Table 1.
Characteristics of the Study Population
| Unmatched Cohort (n=101,111) |
Propensity Score-Matched Cohort (n=606) |
|||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Postnatal CMV (n=328) |
No Postnatal CMV (n=100,783) |
Standardized Mean Difference |
P | Postnatal CMV (n=303) |
No Postnatal CMV (n=303) |
Standardized Mean Difference |
P | |
| Demographics | ||||||||
| Gestational age at birth, weeks | <0.001 | 0.90 | ||||||
| ≤24 | 34% | 7% | 0.73 | 35% | 34% | 0.02 | ||
| 25–28 | 58% | 41% | 0.35 | 58% | 60% | -0.03 | ||
| 29–31 | 7% | 39% | -0.81 | 7% | 7% | 0.03 | ||
| ≥32 | <1% | 13% | -0.54 | 0% | 0% | -a | ||
| Birth weight category, g | <0.001 | 0.82 | ||||||
| <500 | 9% | 1% | 0.36 | 9% | 8% | 0.04 | ||
| 500–749 | 45% | 13% | 0.77 | 46% | 48% | -0.04 | ||
| 750–999 | 28% | 22% | 0.14 | 29% | 26% | 0.06 | ||
| 1001–1499 | 18% | 64% | -1.07 | 17% | 19% | -0.04 | ||
| Small for gestational age | 21% | 23% | -0.05 | 0.37 | 20% | 17% | 0.08 | 0.35 |
| Male sex | 54% | 50% | 0.09 | 0.10 | 55% | 52% | 0.06 | 0.46 |
| Race/ethnicity | 0.02 | 0.39 | ||||||
| White | 42% | 49% | -0.14 | 41% | 39% | 0.06 | ||
| African-American | 26% | 27% | -0.01 | 27% | 23% | 0.08 | ||
| Hispanic | 25% | 19% | 0.13 | 24% | 30% | -0.13 | ||
| Other | 7% | 5% | 0.08 | 8% | 8% | -0.01 | ||
| Discharge year | <0.001 | 0.82 | ||||||
| 1997–2000 | 3% | 10% | -0.29 | 3% | 3% | -0.02 | ||
| 2001–2004 | 20% | 21% | -0.03 | 19% | 17% | 0.08 | ||
| 2005–2008 | 35% | 33% | 0.04 | 35% | 36% | -0.02 | ||
| 2009–2012 | 42% | 36% | 0.13 | 43% | 44% | -0.03 | ||
| Breast milk exposure on DOL 14–21 | 0.003 | 0.44 | ||||||
| 0 days | 37% | 38% | -0.04 | 37% | 32% | 0.10 | ||
| 1–6 days | 36% | 28% | 0.17 | 36% | 39% | -0.06 | ||
| 7 days | 27% | 33% | -0.14 | 27% | 29% | -0.04 | ||
| Co-morbidities on DOL ≤21 | ||||||||
| Necrotizing enterocolitis | 4% | 3% | 0.04 | 0.42 | 3% | 2% | 0.06 | 0.46 |
| Patent ductus arteriosus | 73% | 42% | 0.65 | <0.001 | 74% | 74% | <0.001 | >0.99 |
| Grade 3 or 4 intraventricular hemorrhage | 11% | 6% | 0.20 | <0.001 | 11% | 10% | 0.04 | 0.60 |
| Sepsis | 18% | 8% | 0.32 | <0.001 | 18% | 16% | 0.07 | 0.39 |
| Treatments | ||||||||
| One or more vasopressor medications on DOL 21 | 7% | 2% | 0.22 | <0.001 | 6% | 6% | 0.03 | 0.73 |
| Maximum level of respiratory support on DOL 21 | <0.001 | 0.72 | ||||||
| Room air | 5% | 41% | -0.96 | 5% | 4% | 0.07 | ||
| Nasal cannula oxygen or CPAP | 32% | 37% | -0.12 | 33% | 36% | -0.08 | ||
| Conventional mechanical ventilation | 43% | 16% | 0.60 | 41% | 39% | 0.03 | ||
| High-frequency ventilation | 21% | 5% | 0.49 | 21% | 21% | 0.02 | ||
| Maximum FiO2 on DOL 21 | <0.001 | 0.89 | ||||||
| 21% | 18% | 59% | -0.93 | 18% | 18% | -0.02 | ||
| 22–50% | 67% | 33% | 0.72 | 67% | 68% | -0.01 | ||
| 51–100% | 15% | 8% | 0.24 | 15% | 14% | 0.04 | ||
| Receipt of surfactant on DOL ≤21 | <0.001 | 0.49 | ||||||
| 0 days | 14% | 42% | -0.64 | 15% | 12% | 0.09 | ||
| 1 day | 61% | 42% | 0.39 | 61% | 61% | -0.01 | ||
| ≥2 days | 24% | 16% | 0.20 | 24% | 27% | -0.06 | ||
Abbreviations: CMV, cytomegalovirus; DOL, day of life; CPAP, continuous positive airway pressure; FiO2, fraction of inspired oxygen.
Standardized mean difference was not calculated because no infants had gestational age at birth 32 weeks.
OUTCOMES
In the propensity score-matched cohort, infants with postnatal CMV infection were discharged (or died) at a median of 40.6 weeks postmenstrual age. Infants without postnatal CMV infection were discharged (or died) at a median of 38.6 weeks postmenstrual age. Primary and secondary outcomes by postnatal CMV infection status in this cohort are shown in Table 2. Overall, 69% of infants died or met criteria for BPD at 36 weeks postmenstrual age, including 76% of infants with postnatal CMV infection and 63% of infants without postnatal CMV. Postnatal CMV infection was associated with an increased risk of death or BPD at 36 weeks postmenstrual age (RR: 1.21, 95% confidence interval [CI]: 1.10–1.32) and BPD (RR: 1.33, 95% CI: 1.19–1.50). There was no significant association between postnatal CMV and death prior to hospital discharge (RR: 0.71, 95% CI: 0.43–1.15). The effect of postnatal CMV infection on primary and secondary outcomes was substantively unchanged in sensitivity analyses (eTable 1).
Table 2.
Outcomes of VLBW Infants According to Postnatal CMV Infection, Propensity Score-Matched Cohort (n=606)
| Postnatal CMV (n=303) | No Postnatal CMV (n=303) | Adjusted RR (95% CI) | P | |
|---|---|---|---|---|
| Primary outcome | ||||
| Death or BPD at 36 weeks postmenstrual age | 76% | 63% | 1.21 (1.10, 1.32) | <0.001 |
| Secondary outcomes | ||||
| BPD | 71% | 53% | 1.33 (1.19, 1.50) | <0.001 |
| Death prior to hospital discharge | 8% | 11% | 0.71 (0.43, 1.16) | 0.17 |
Abbreviations: VLBW, very low birth weight; CMV, cytomegalovirus; RR, risk ratio; CI, confidence interval; BPD, bronchopulmonary dysplasia.
CLINICAL AND LABORATORY CHARACTERISTICS OF POSTNATAL CMV INFECTION
Median (25th percentile, 75th percentile) GA and birth weight of the 328 infants with postnatal CMV infection were 25 (24–27) weeks and 730 (618–903) g. Median (25th percentile, 75th percentile) postnatal age at CMV diagnosis was 49 (38–60) days (Table 3). We observed one or more changes in cardiorespiratory status or laboratory abnormalities in 293 (89%) infants within 7 days before or after CMV diagnosis. Changes in cardiorespiratory status included a new requirement for vasopressor medications (9%), intubation for mechanical ventilation (15%), a new oxygen requirement (28%), and death (1.2%). Among infants with available data, thrombocytopenia (66%), direct hyperbilirubinemia (66%), and neutropenia (34%) were the most frequent laboratory abnormalities.
Table 3.
Clinical and Laboratory Characteristics of VLBW Infants with Postnatal CMV Infection (n=328)
| Postnatal CMVa (n=328) | |
|---|---|
| Postnatal age at CMV diagnosis, days | |
| 21–34 | 20% |
| 35–60 | 54% |
| ≥61 | 27% |
| Postmenstrual age at CMV diagnosis, weeks | |
| ≤30 | 25% |
| 31–33 | 46% |
| ≥34 | 29% |
| Cardiorespiratory status | |
| Received ≥1 vasopressor medications | 12% |
| New requirement for vasopressor medications | 9% |
| Maximum level of respiratory support | |
| Room air | 6% |
| Nasal cannula oxygen or CPAP | 32% |
| Conventional mechanical ventilation | 45% |
| High-frequency ventilation | 18% |
| Required increased level of respiratory support | 25% |
| Required intubation for mechanical ventilation | 15% |
| Maximum FiO2 | |
| 21% | 8% |
| 22–50% | 51% |
| 51–100% | 41% |
| Required increased FiO2 | 58% |
| New oxygen requirement | 28% |
| Death | 1.2% |
| Laboratory abnormalities | |
| Absolute neutrophil count <1500 cells/μL | 34% |
| Absolute lymphocyte count <2500 cells/μL | 26% |
| Platelet count <100,000/μL | 66% |
| Transaminitisb | 16% |
| Direct bilirubin >1.0 mg/dL | 66% |
| Change in cardiorespiratory status or laboratory abnormality | 89% |
Abbreviations: VLBW, very low birth weight; CMV, cytomegalovirus; CPAP, continuous positive airway pressure; FiO2, fraction of inspired oxygen; μL, microliter; mg, milligram; dL, deciliter.
Percentages may not sum to total because of rounding.
Defined as aspartate transaminase (AST) >150 U/L and alanine aminotransferase (ALT) >90 U/L.
DISCUSSION
We present findings from the largest reported cohort of VLBW infants with postnatal CMV infection to date. Our results indicate that postnatal CMV infection at <36 weeks postmenstrual age is associated with an increased risk of BPD. Postnatal CMV was not associated with death before hospital discharge.
There are several plausible mechanisms by which postnatal CMV infection might increase the risk of BPD. Damage to lung tissue may occur as a direct result of viral infection or indirectly through the immune response to the virus.18 Infection of the lung by CMV is characterized by a mononuclear inflammatory process, deposition of fibrin, hemorrhage, and sloughing of lung epithelial cells.19 CMV might also increase BPD risk by causing a deterioration in respiratory status that leads to increased exposure to other known risk factors, such as prolonged mechanical ventilation.
A potential association between postnatal CMV and BPD was first observed in the 1970’s. Whitley et al. described two infants who developed a protracted pneumonitis with histopathology suggesting a causative role for CMV.20 Roughly 10 years later, investigators reported radiographic findings consistent with BPD in 24 of 32 (75%) infants diagnosed with postnatal CMV infection compared with 12 of 32 (38%) controls in a single center study.21 Other case reports have since attributed multicystic lung disease, interstitial fibrosis, and pulmonary hypertension to postnatal CMV infection.22,23
However, postnatal CMV infection was not associated with BPD in several recent prospective studies. In contrast to earlier studies, these cohorts identified incident CMV infections through serial virologic monitoring, and the majority of the identified infections were not clinically apparent. Neuberger et al. monitored the premature infants of CMV-seropositive mothers with biweekly CMV culture and PCR of urine, comparing the clinical course of 40 infants with postnatal CMV infection to matched controls. They found no association between postnatal CMV and BPD, although the overall incidence of BPD was low (16%).24,25 Nijman et al. prospectively screened 315 infants born at <32 weeks GA, identifying 39 infants with postnatal CMV infection. BPD, defined in this study as the requirement for FiO2 ≥30% or positive pressure ventilation at 36 weeks postmenstrual age, was diagnosed in 0% of infants with postnatal CMV infection and 2% of infants without postnatal CMV.26 Eighty-five percent of infants in this cohort did not have clinical signs or laboratory abnormalities that could be attributed to CMV infection.26 Finally, Prosch et al. collected urine samples and tracheal or pharyngeal aspirates from 66 VLBW infants during the first month of life. BPD developed in 12% of non-CMV-infected infants and 29% of infants with CMV infection, including 3 of 4 (75%) infants with postnatal infection.27 There were no significant differences in the prevalence of BPD by CMV infection status, although the small number of postnatally infected infants precluded specific comparisons with this group.27
The incidence of postnatal CMV infection in our cohort was lower than was reported in most prior studies. In a recent meta-analysis of 17 studies, the risk of postnatal CMV infection among VLBW infants in the United States was estimated to be 6.5%, with 1.4% of infants developing a sepsis-like syndrome.28 Only 0.3% of VLBW infants in our cohort met criteria for postnatal CMV infection at <36 weeks postmenstrual age. This comparatively low incidence likely reflects non-recognition of postnatal CMV infections associated with no or few clinical signs. Specifically, we identified infants with postnatal CMV infection based on physician diagnoses or virologic testing in a non-research setting. Although CMV screening practices at our NICU sites were unavailable, few NICUs in the United States were routinely screening for postnatal CMV infection during the study period. Thus, the majority of CMV-infected infants identified in our cohort had clinical signs or laboratory abnormalities consistent with postnatal infection. Moreover, establishing a diagnosis of postnatal CMV in practice is challenging, even in the presence of clinical signs, given substantial overlap with the presentation of bacterial or fungal sepsis. Hence, many of the postnatal CMV infections in our cohort may further represent the small minority of infections that result in severe or protracted symptoms. While our identification of infants with postnatal CMV reflects standard practice in most NICUs during the study period, our findings may not be generalizable to settings that routinely screen VLBW infants for CMV. Large prospective studies are needed to determine whether CMV acquisition without clinical signs is associated with BPD.
The clinical signs and laboratory abnormalities associated with postnatal CMV infection in our cohort were generally similar to those previously reported in smaller studies.2,6,29 Notably, however, direct hyperbilirubinemia and thrombocytopenia were substantially more common in our cohort than in prior studies. While these results may accurately reflect the true prevalence of these findings in clinically apparent postnatal CMV infections, it is also possible that these laboratory abnormalities prompt clinicians to consider CMV infection in VLBW infants.
Breast milk has established nutritive and immunological benefits and reduces the incidence of late-onset sepsis and necrotizing enterocolitis in premature infants.30,31 However, with the practice of transfusion of CMV-seronegative or leukoreduced blood, breastfeeding is also the primary route of CMV acquisition among infants in the United States.5 Up to half of pregnant mothers are CMV-seropositive, and over 80% of these mothers shed CMV in their breast milk.32-34 Pasteurization of breast milk eliminates infectious virus but diminishes the beneficial properties of the milk, while freezing reduces but does not eliminate CMV transmission.34-36 Our study suggests that alternative strategies are needed to prevent CMV transmission to VLBW infants while preserving the beneficial properties of breast milk.
Our study has a number of limitations, most of which relate to the retrospective observational design. First, criteria for postnatal CMV infection were based on virologic testing and physician diagnoses in a non-research setting. As a result, the vast majority of infants in our cohort had signs of CMV infection, which generally represents only 10-33% of infections among VLBW infants.26,28,29 Second, given that most congenital CMV infections are not associated with clinical signs and that prolonged urinary shedding and viremia are common, some infants in our cohort may have had congenital infections.37 To minimize this possibility, we excluded infants with CMV-related diagnoses, characteristic laboratory abnormalities, or treatment with antivirals with activity against CMV prior to DOL 21. Moreover, pneumonitis is infrequent among infants with congenital infection, so misclassification would tend to bias our results toward the null.18 We did not consider BPD and death in a single model as competing risk events, choosing instead to examine the effect of postnatal CMV on a composite outcome variable. Finally, we cannot exclude the possibility of unmeasured confounding variables. However, infants with CMV were closely matched to comparison infants on other known risk factors for BPD, including all variables that predicted risk of BPD in a recent multicenter study.38
Among VLBW infants, postnatal CMV infection was associated with an increased risk of BPD. Further research is needed to define the long-term sequelae of postnatal CMV on pulmonary and neurological outcomes and develop novel CMV prevention measures to permit safe breast milk feeding by VLBW infants.
Supplementary Material
Acknowledgments
Drs. Benjamin Jr. and Smith receive research support from Cempra Pharmaceuticals (subaward to HHSO100201300009C) and industry for neonatal and pediatric drug development (www.dcri.duke.edu/research/coi.jsp).
Funding/Support: Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) under award number UL1TR001117 and a New Innovator Award from the NIH Office of the Director under award number DP2HD2075699. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Dr. Kelly is supported by a training grant from the NIH (T32-HD060558). Dr. Benjamin Jr. receives support from the NIH (award 2K24HD058735-06), National Center for Advancing Translational Sciences (award UL1TR001117), National Institute of Child Health and Human Development (contract HHSN275201000003I), and National Institute of Allergy and Infectious Diseases (contract HHSN272201500006I). Dr. Laughon receives support from the U.S. government for his work in pediatric and neonatal clinical pharmacology (Government Contract HHSN267200700051C, PI: Benjamin under the Best Pharmaceuticals for Children Act) and from NICHD (K23HD068497). Dr. Smith receives salary support for research from the NIH and the National Center for Advancing Translational Sciences of the NIH (UL1TR001117), the National Institute of Child Health and Human Development (HHSN275201000003I and 1R01-HD081044-01) and the Food and Drug Administration (1R18-FD005292-01). Dr. Permar receives support from the National Institute of Child Health and Human Development (DPHD2075699, R03HD072796) and National Institute of Allergy and Infectious Diseases (R21AI0694, R01AI06380, P30AI064518, P01-AI117915-01).
Role of the Funding Sources: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Authors’ Contributions: Dr. Kelly had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis; he designed and led the project, collected and analyzed data, drafted the manuscript, and approved the final manuscript as submitted. Dr. Benjamin provided statistical analysis, critically reviewed the manuscript, and approved the final manuscript as submitted. Drs. Puopolo, Laughon, and Benjamin Jr. critically reviewed the manuscript and approved the final manuscript as submitted. Dr. Clark assisted with database creation and design, critically reviewed the manuscript, and approved the final manuscript as submitted. Drs. Smith and Permar aided in project design and implementation, provided mentorship to Dr. Kelly throughout the project, critically reviewed the manuscript, and approved the final manuscript as submitted.
Conflict of Interest Disclosures: The other authors have no financial relationships to disclose.
References
- 1.Alford CA, Stagno S, Pass RF, Britt WJ. Congenital and perinatal cytomegalovirus infections. Rev Infect Dis. 1990;12(Suppl 7):S745–S753. doi: 10.1093/clinids/12.supplement_7.s745. [DOI] [PubMed] [Google Scholar]
- 2.Capretti MG, Lanari M, Lazzarotto T, et al. Very low birth weight infants born to cytomegalovirus-seropositive mothers fed with their mother’s milk: a prospective study. J Pediatr. 2009;154(6):842–848. doi: 10.1016/j.jpeds.2008.12.046. [DOI] [PubMed] [Google Scholar]
- 3.Hamprecht K, Maschmann J, Jahn G, Poets CF, Goelz R. Cytomegalovirus transmission to preterm infants during lactation. J Clin Virol. 2008;41(3):198–205. doi: 10.1016/j.jcv.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 4.de Cates CR, Gray J, Roberton NR, Walker J. Acquisition of cytomegalovirus infection by premature neonates. J Infect. 1994;28(1):25–30. doi: 10.1016/s0163-4453(94)94037-1. [DOI] [PubMed] [Google Scholar]
- 5.Josephson CD, Caliendo AM, Easley KA, et al. Blood transfusion and breast milk transmission of cytomegalovirus in very low-birth-weight infants : a prospective cohort study. JAMA Pediatr. 2014;168(11):1054–1062. doi: 10.1001/jamapediatrics.2014.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adler SP, Chandrika T, Lawrence L, Baggett J. Cytomegalovirus infections in neonates acquired by blood transfusions. Pediatr Infect Dis. 1983;2(2):114–118. doi: 10.1097/00006454-198303000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Mussi-Pinhata MM, Pinto PC, Yamamoto AY, et al. Placental transfer of naturally acquired, maternal cytomegalovirus antibodies in term and preterm neonates. J Med Virol. 2003;69(2):232–239. doi: 10.1002/jmv.10271. [DOI] [PubMed] [Google Scholar]
- 8.Nijman J, van Loon AM, Krediet TG, Verboon-Maciolek MA. Maternal and neonatal anti-cytomegalovirus IgG level and risk of postnatal cytomegalovirus transmission in preterm infants. J Med Virol. 2013;85(4):689–695. doi: 10.1002/jmv.23511. [DOI] [PubMed] [Google Scholar]
- 9.Ballard RA, Drew WL, Hufnagle KG, Riedel PA. Acquired cytomegalovirus infection in preterm infants. Am J Dis Child. 1979;133(5):482–485. doi: 10.1001/archpedi.1979.02130050026005. [DOI] [PubMed] [Google Scholar]
- 10.Thorp JA, Jones PG, Peabody JL, Knox E, Clark RE. Effect of antenatal and postnatal corticosteroid therapy on weight gain and head circumference growth in the nursery. Obstet Gynecol. 2002;99(1):109–115. doi: 10.1016/s0029-7844(01)01657-x. [DOI] [PubMed] [Google Scholar]
- 11.Trembath A, Hornik CP, Clark R, Smith PB, Daniels J, Laughon M. Comparative effectiveness of surfactant preparations in premature infants. J Pediatr. 2013;163(4):955–960. doi: 10.1016/j.jpeds.2013.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. New intrauterine growth curves based on United States data. Pediatrics. 2010;125(2):e214–e224. doi: 10.1542/peds.2009-0913. [DOI] [PubMed] [Google Scholar]
- 13.Hornik CP, Benjamin DK, Becker KC, et al. Use of the complete blood cell count in early-onset neonatal sepsis. Pediatr Infect Dis J. 2012;318:799–802. doi: 10.1097/INF.0b013e318256905c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Sturmer T. Variable selection for propensity score models. Am J Epidemiol. 2006;163(12):1149–1156. doi: 10.1093/aje/kwj149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39(1):33–38. [Google Scholar]
- 16.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Kai FY. What’s the relative risk?: a method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280(19):1690–1691. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 18.Coclite E, Di Natale C, Nigro G. Congenital and perinatal cytomegalovirus lung infection. J Matern Fetal Neonatal Med. 2013;26(17):1671–1675. doi: 10.3109/14767058.2013.794207. [DOI] [PubMed] [Google Scholar]
- 19.McGuinness G, Scholes JV, Garay SM, Leitman BS, McCauley DI, Naidich DP. Cytomegalovirus pneumonitis: spectrum of parenchymal CT findings with pathologic correlation in 21 AIDS patients. Radiology. 1994;192(2):451–459. doi: 10.1148/radiology.192.2.8029414. [DOI] [PubMed] [Google Scholar]
- 20.Whitley RJ, Brasfield D, Reynolds DW, Stagno S, Tiller RE, Alford CA. Protracted pneumonitis in young infants associated with perinatally acquired cytomegaloviral infection. J Pediatr. 1976;89(1):16–22. doi: 10.1016/s0022-3476(76)80919-5. [DOI] [PubMed] [Google Scholar]
- 21.Sawyer MH, Edwards DK, Spector SA. Cytomegalovirus infection and bronchopulmonary dysplasia in premature infants. Am J Dis Child. 1987;141(3):303–305. doi: 10.1001/archpedi.1987.04460030081030. [DOI] [PubMed] [Google Scholar]
- 22.Bradshaw JH, Moore PP. Perinatal cytomegalovirus infection associated with lung cysts. J Paediatr Child Health. 2003;39(7):563–566. doi: 10.1046/j.1440-1754.2003.00219.x. [DOI] [PubMed] [Google Scholar]
- 23.Koklu E, Karadag A, Tunc T, Altun D, Sarici SU. Congenital cytomegalovirus infection associated with severe lung involvement in a preterm neonate: a causal relationship? Eur J Pediatr. 2009;168(11):1409–1412. doi: 10.1007/s00431-009-0941-0. [DOI] [PubMed] [Google Scholar]
- 24.Neuberger P, Hamprecht K, Vochem M, et al. Case-control study of symptoms and neonatal outcome of human milk-transmitted cytomegalovirus infection in premature infants. J Pediatr. 2006;148(3):326–331. doi: 10.1016/j.jpeds.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 25.Ehrenkranz RA, Walsh MC, Vohr BR, et al. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116(6):1353–1360. doi: 10.1542/peds.2005-0249. [DOI] [PubMed] [Google Scholar]
- 26.Nijman J, de Vries LS, Koopman-Esseboom C, Uiterwaal CS, van Loon AM, Verboon-Maciolek MA. Postnatally acquired cytomegalovirus infection in preterm infants: a prospective study on risk factors and cranial ultrasound findings. Arch Dis Child Fetal Neonatal Ed. 2012;97(4):F259–F263. doi: 10.1136/archdischild-2011-300405. [DOI] [PubMed] [Google Scholar]
- 27.Prosch S, Lienicke U, Priemer C, et al. Human adenovirus and human cytomegalovirus infections in preterm newborns: no association with bronchopulmonary dysplasia. Pediatr Res. 2002;52(2):219–224. doi: 10.1203/00006450-200208000-00014. [DOI] [PubMed] [Google Scholar]
- 28.Lanzieri TM, Dollard SC, Josephson CD, Schmid DS, Bialek SR. Breast milk-acquired cytomegalovirus infection and disease in VLBW and premature infants. Pediatrics. 2013;131(6):e1937–e1945. doi: 10.1542/peds.2013-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar ML, Nankervis GA, Cooper AR, Gold E. Postnatally acquired cytomegalovirus infections in infants of CMV-excreting mothers. J Pediatr. 1984;104(5):669–673. doi: 10.1016/s0022-3476(84)80941-5. [DOI] [PubMed] [Google Scholar]
- 30.Sullivan S, Schanler RJ, Kim JH, et al. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J Pediatr. 2010;156(4):562–567.e1. doi: 10.1016/j.jpeds.2009.10.040. [DOI] [PubMed] [Google Scholar]
- 31.Schanler RJ, Lau C, Hurst NM, Smith EO. Randomized trial of donor human milk versus preterm formula as substitutes for mothers’ own milk in the feeding of extremely premature infants. Pediatrics. 2005;116(2):400–406. doi: 10.1542/peds.2004-1974. [DOI] [PubMed] [Google Scholar]
- 32.Colugnati FA, Staras SA, Dollard SC, Cannon MJ. Incidence of cytomegalovirus infection among the general population and pregnant women in the United States. BMC Infect Dis. 2007;7:71. doi: 10.1186/1471-2334-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vochem M, Hamprecht K, Jahn G, Speer CP. Transmission of cytomegalovirus to preterm infants through breast milk. Pediatr Infect Dis J. 1998;17(1):53–58. doi: 10.1097/00006454-199801000-00012. [DOI] [PubMed] [Google Scholar]
- 34.Hayashi S, Kimura H, Oshiro M, et al. Transmission of cytomegalovirus via breast milk in extremely premature infants. J Perinatol. 2011;31(6):440–445. doi: 10.1038/jp.2010.150. [DOI] [PubMed] [Google Scholar]
- 35.Lombardi G, Garofoli F, Manzoni P, Stronati M. Breast milk-acquired cytomegalovirus infection in very low birth weight infants. J Matern Fetal Neonatal Med. 2012;25(Suppl 3):57–62. doi: 10.3109/14767058.2012.712345. [DOI] [PubMed] [Google Scholar]
- 36.Jim WT, Shu CH, Chiu NC, et al. Transmission of cytomegalovirus from mothers to preterm infants by breast milk. Pediatr Infect Dis J. 2004;23(9):848–851. doi: 10.1097/01.inf.0000137571.35541.55. [DOI] [PubMed] [Google Scholar]
- 37.Pass RF, Stagno S, Britt WJ, Alford CA. Specific cell-mediated immunity and the natural history of congenital infection with cytomegalovirus. J Infect Dis. 1983;148(6):953–961. doi: 10.1093/infdis/148.6.953. [DOI] [PubMed] [Google Scholar]
- 38.Laughon MM, Langer JC, Bose CL, et al. Prediction of bronchopulmonary dysplasia by postnatal age in extremely premature infants. Am J Respir Crit Care Med. 2011;183(12):1715–1722. doi: 10.1164/rccm.201101-0055OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


