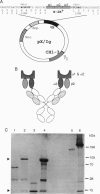
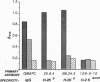
Abstract
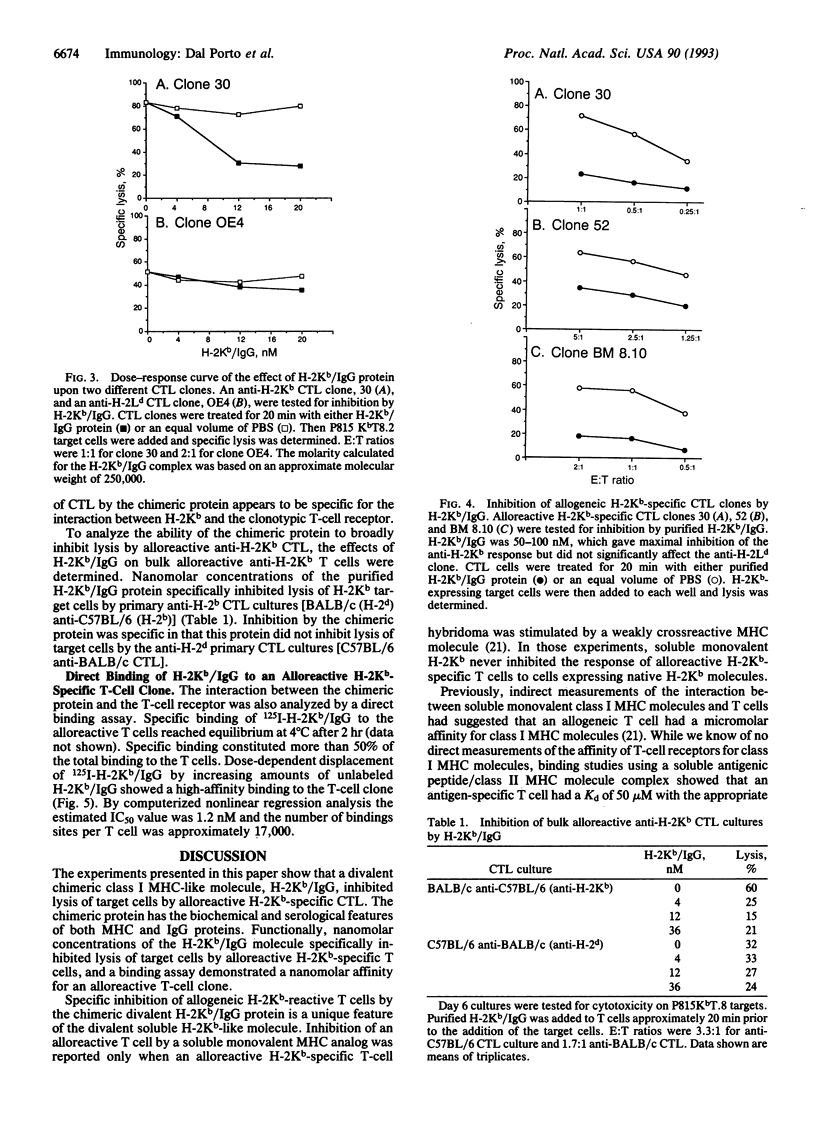
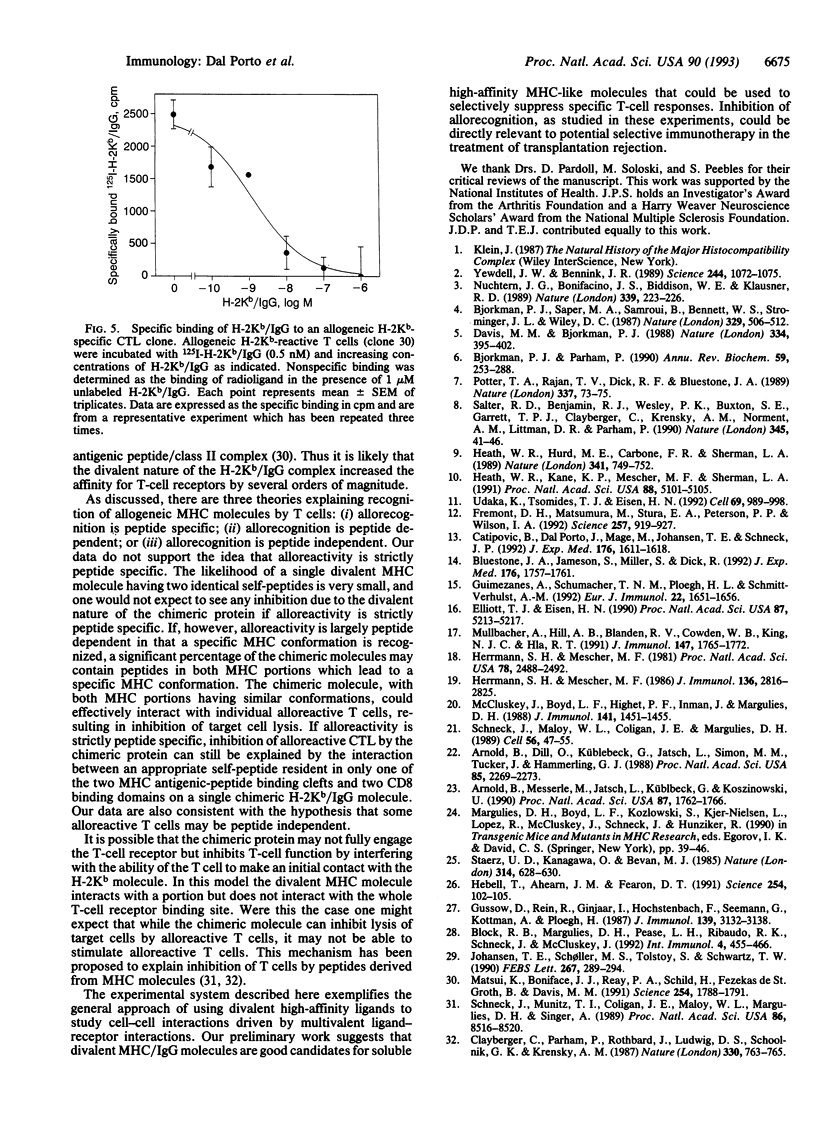
Genetically engineered or chemically purified soluble monovalent major histocompatibility complex (MHC) molecules, which have previously been used to study T cells, have not blocked cytotoxic T-cell responses. Here we describe a genetically engineered divalent class I MHC molecule which inhibits lysis of target cells by alloreactive cytotoxic T cells. This protein, H-2Kb/IgG, was generated as a fusion protein between the extracellular domains of a murine class I polypeptide, H-2Kb, and an immunoglobulin heavy chain polypeptide. The chimeric protein has serological and biochemical characteristics of both the MHC and IgG polypeptides. Nanomolar concentrations of H-2Kb/IgG inhibited lysis of H-2Kb-expressing target cells not only by alloreactive H-2Kb-specific T-cell clones but also by alloreactive H-2Kb-specific primary T-cell cultures. A direct binding assay showed high-affinity binding between the H-2Kb/IgG molecule and an H-2Kb-specific alloreactive T-cell clone. Unlabeled H-2Kb/IgG displaced 125I-labeled H-2Kb/IgG from T cells with an IC50 of 1.2 nM.
Full text
PDF
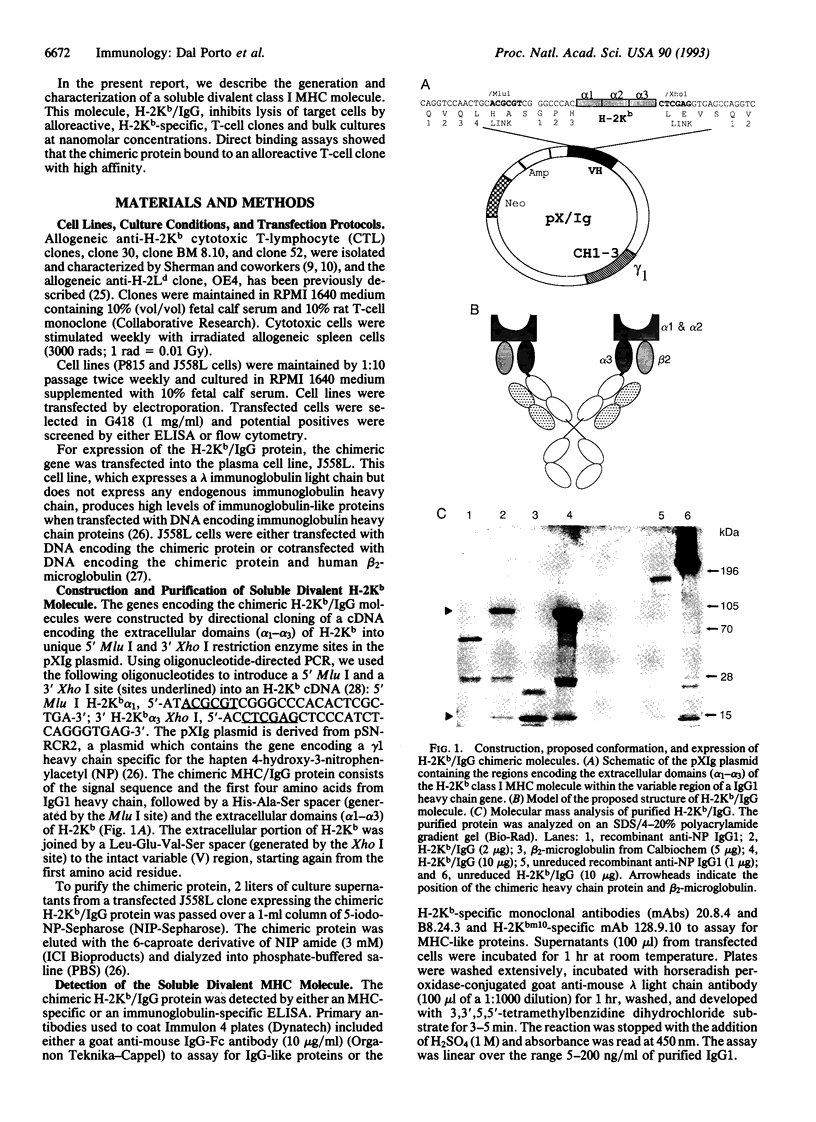
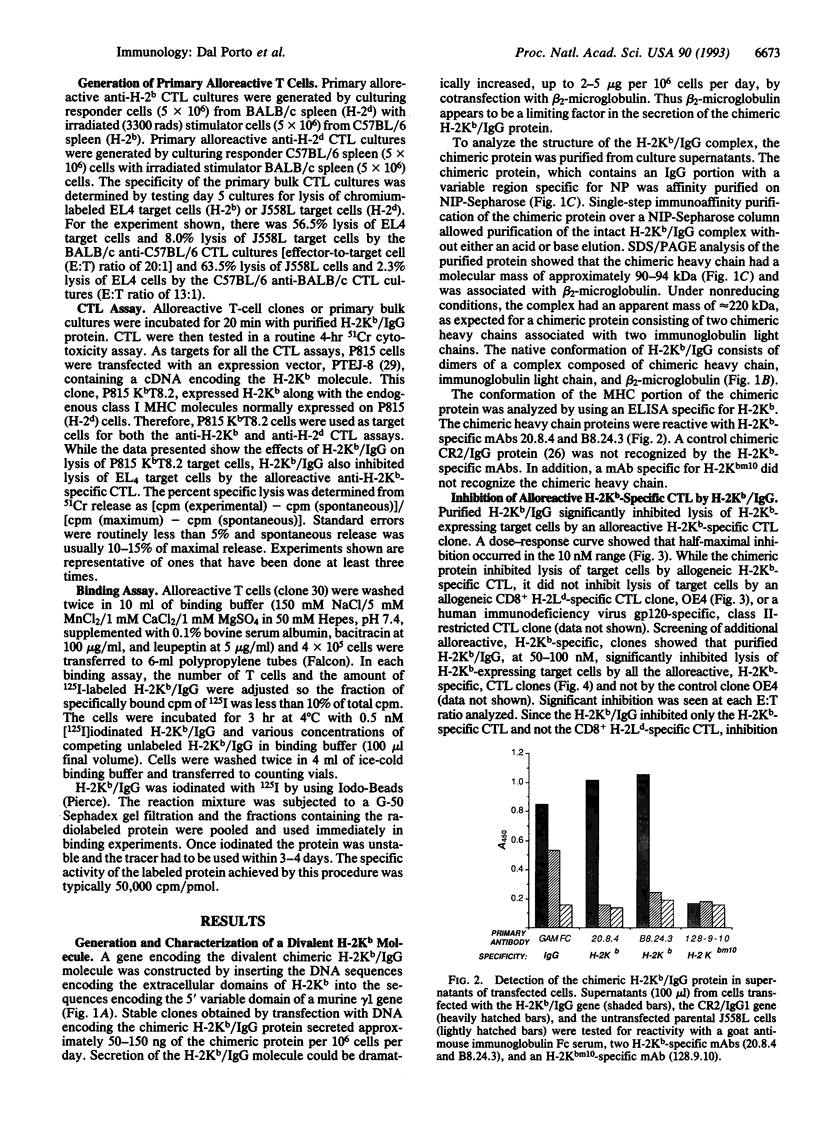
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold B., Dill O., Küblbeck G., Jatsch L., Simon M. M., Tucker J., Hämmerling G. J. Alloreactive immune responses of transgenic mice expressing a foreign transplantation antigen in a soluble form. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2269–2273. doi: 10.1073/pnas.85.7.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold B., Messerle M., Jatsch L., Küblbeck G., Koszinowski U. Transgenic mice expressing a soluble foreign H-2 class I antigen are tolerant to allogeneic fragments presented by self class I but not to the whole membrane-bound alloantigen. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1762–1766. doi: 10.1073/pnas.87.5.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkman P. J., Parham P. Structure, function, and diversity of class I major histocompatibility complex molecules. Annu Rev Biochem. 1990;59:253–288. doi: 10.1146/annurev.bi.59.070190.001345. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987 Oct 8;329(6139):506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- Blok R., Margulies D. H., Pease L., Ribaudo R. K., Schneck J., McCluskey J. CD8 expression alters the fine specificity of an alloreactive MHC class I-specific T hybridoma. Int Immunol. 1992 Apr;4(4):455–466. doi: 10.1093/intimm/4.4.455. [DOI] [PubMed] [Google Scholar]
- Bluestone J. A., Jameson S., Miller S., Dick R., 2nd Peptide-induced conformational changes in class I heavy chains alter major histocompatibility complex recognition. J Exp Med. 1992 Dec 1;176(6):1757–1761. doi: 10.1084/jem.176.6.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catipović B., Dal Porto J., Mage M., Johansen T. E., Schneck J. P. Major histocompatibility complex conformational epitopes are peptide specific. J Exp Med. 1992 Dec 1;176(6):1611–1618. doi: 10.1084/jem.176.6.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayberger C., Parham P., Rothbard J., Ludwig D. S., Schoolnik G. K., Krensky A. M. HLA-A2 peptides can regulate cytolysis by human allogeneic T lymphocytes. Nature. 1987 Dec 24;330(6150):763–765. doi: 10.1038/330763a0. [DOI] [PubMed] [Google Scholar]
- Davis M. M., Bjorkman P. J. T-cell antigen receptor genes and T-cell recognition. Nature. 1988 Aug 4;334(6181):395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- Elliott T. J., Eisen H. N. Cytotoxic T lymphocytes recognize a reconstituted class I histocompatibility antigen (HLA-A2) as an allogeneic target molecule. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5213–5217. doi: 10.1073/pnas.87.13.5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremont D. H., Matsumura M., Stura E. A., Peterson P. A., Wilson I. A. Crystal structures of two viral peptides in complex with murine MHC class I H-2Kb. Science. 1992 Aug 14;257(5072):919–927. doi: 10.1126/science.1323877. [DOI] [PubMed] [Google Scholar]
- Guimezanes A., Schumacher T. N., Ploegh H. L., Schmitt-Verhulst A. M. A viral peptide can mimic an endogenous peptide for allorecognition of a major histocompatibility complex class I product. Eur J Immunol. 1992 Jun;22(6):1651–1654. doi: 10.1002/eji.1830220647. [DOI] [PubMed] [Google Scholar]
- Güssow D., Rein R., Ginjaar I., Hochstenbach F., Seemann G., Kottman A., Ploegh H. L. The human beta 2-microglobulin gene. Primary structure and definition of the transcriptional unit. J Immunol. 1987 Nov 1;139(9):3132–3138. [PubMed] [Google Scholar]
- Heath W. R., Hurd M. E., Carbone F. R., Sherman L. A. Peptide-dependent recognition of H-2Kb by alloreactive cytotoxic T lymphocytes. Nature. 1989 Oct 26;341(6244):749–752. doi: 10.1038/341749a0. [DOI] [PubMed] [Google Scholar]
- Heath W. R., Kane K. P., Mescher M. F., Sherman L. A. Alloreactive T cells discriminate among a diverse set of endogenous peptides. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5101–5105. doi: 10.1073/pnas.88.12.5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebell T., Ahearn J. M., Fearon D. T. Suppression of the immune response by a soluble complement receptor of B lymphocytes. Science. 1991 Oct 4;254(5028):102–105. doi: 10.1126/science.1718035. [DOI] [PubMed] [Google Scholar]
- Herrmann S. H., Mescher M. F. Secondary cytolytic T lymphocyte stimulation by purified H-2Kk in liposomes. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2488–2492. doi: 10.1073/pnas.78.4.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann S. H., Mescher M. F. The requirements for antigen multivalency in class I antigen recognition and triggering of primed precursor cytolytic T lymphocytes. J Immunol. 1986 Apr 15;136(8):2816–2825. [PubMed] [Google Scholar]
- Johansen T. E., Schøller M. S., Tolstoy S., Schwartz T. W. Biosynthesis of peptide precursors and protease inhibitors using new constitutive and inducible eukaryotic expression vectors. FEBS Lett. 1990 Jul 16;267(2):289–294. doi: 10.1016/0014-5793(90)80947-h. [DOI] [PubMed] [Google Scholar]
- Matsui K., Boniface J. J., Reay P. A., Schild H., Fazekas de St Groth B., Davis M. M. Low affinity interaction of peptide-MHC complexes with T cell receptors. Science. 1991 Dec 20;254(5039):1788–1791. doi: 10.1126/science.1763329. [DOI] [PubMed] [Google Scholar]
- McCluskey J., Boyd L. F., Highet P. F., Inman J., Margulies D. H. T cell activation by purified, soluble, class I MHC molecules. Requirement for polyvalency. J Immunol. 1988 Sep 1;141(5):1451–1455. [PubMed] [Google Scholar]
- Müllbacher A., Hill A. B., Blanden R. V., Cowden W. B., King N. J., Hla R. T. Alloreactive cytotoxic T cells recognize MHC class I antigen without peptide specificity. J Immunol. 1991 Sep 15;147(6):1765–1772. [PubMed] [Google Scholar]
- Nuchtern J. G., Bonifacino J. S., Biddison W. E., Klausner R. D. Brefeldin A implicates egress from endoplasmic reticulum in class I restricted antigen presentation. Nature. 1989 May 18;339(6221):223–226. doi: 10.1038/339223a0. [DOI] [PubMed] [Google Scholar]
- Potter T. A., Rajan T. V., Dick R. F., 2nd, Bluestone J. A. Substitution at residue 227 of H-2 class I molecules abrogates recognition by CD8-dependent, but not CD8-independent, cytotoxic T lymphocytes. Nature. 1989 Jan 5;337(6202):73–75. doi: 10.1038/337073a0. [DOI] [PubMed] [Google Scholar]
- Salter R. D., Benjamin R. J., Wesley P. K., Buxton S. E., Garrett T. P., Clayberger C., Krensky A. M., Norment A. M., Littman D. R., Parham P. A binding site for the T-cell co-receptor CD8 on the alpha 3 domain of HLA-A2. Nature. 1990 May 3;345(6270):41–46. doi: 10.1038/345041a0. [DOI] [PubMed] [Google Scholar]
- Schneck J., Maloy W. L., Coligan J. E., Margulies D. H. Inhibition of an allospecific T cell hybridoma by soluble class I proteins and peptides: estimation of the affinity of a T cell receptor for MHC. Cell. 1989 Jan 13;56(1):47–55. doi: 10.1016/0092-8674(89)90982-3. [DOI] [PubMed] [Google Scholar]
- Schneck J., Munitz T., Coligan J. E., Maloy W. L., Margulies D. H., Singer A. Inhibition of allorecognition by an H-2Kb-derived peptide is evidence for a T-cell binding region on a major histocompatibility complex molecule. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8516–8520. doi: 10.1073/pnas.86.21.8516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staerz U. D., Kanagawa O., Bevan M. J. Hybrid antibodies can target sites for attack by T cells. Nature. 1985 Apr 18;314(6012):628–631. doi: 10.1038/314628a0. [DOI] [PubMed] [Google Scholar]
- Udaka K., Tsomides T. J., Eisen H. N. A naturally occurring peptide recognized by alloreactive CD8+ cytotoxic T lymphocytes in association with a class I MHC protein. Cell. 1992 Jun 12;69(6):989–998. doi: 10.1016/0092-8674(92)90617-l. [DOI] [PubMed] [Google Scholar]
- Yewdell J. W., Bennink J. R. Brefeldin A specifically inhibits presentation of protein antigens to cytotoxic T lymphocytes. Science. 1989 Jun 2;244(4908):1072–1075. doi: 10.1126/science.2471266. [DOI] [PubMed] [Google Scholar]