Abstract
Purpose
The purpose of this study was to compare the histologic features and cytokine profiles of experimental autoimmune uveitis (EAU) and a primed mycobacterial uveitis (PMU) model in rats.
Methods
In Lewis rats, EAU was induced by immunization with interphotoreceptor binding protein peptide, and PMU was induced by immunization with a killed mycobacterial extract followed by intravitreal injection of the same extract. Clinical course, histology, and the cytokine profiles of the aqueous and vitreous were compared using multiplex bead fluorescence immunoassays.
Results
Primed mycobacterial uveitis generates inflammation 2 days after intravitreal injection and resolves spontaneously 14 days later. CD68+ lymphocytes are the predominant infiltrating cells and are found in the anterior chamber, surrounding the ciliary body and in the vitreous. In contrast to EAU, no choroidal infiltration or retinal destruction is noted. At the day of peak inflammation, C-X-C motif ligand 10 (CXCL10), IL-1β, IL-18, and leptin were induced in the aqueous of both models. Interleukin-6 was induced 2-fold in the aqueous of PMU but not EAU. Cytokines elevated in the aqueous of EAU exclusively include regulated on activation, normal T cell expressed and secreted (RANTES), lipopolysaccharide-induced CXC chemokine (LIX), growth-related oncogene/keratinocyte chemokine (GRO/KC), VEGF, monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1α (MIP-1α), and IL-17A. In the vitreous, CXCL10, GRO/KC, RANTES, and MIP-1α were elevated in both models. Interleukin-17A and IL-18 were elevated exclusively in EAU.
Conclusions
Primed mycobacterial uveitis generates an acute anterior and intermediate uveitis without retinal involvement. Primed mycobacterial uveitis has a distinct proinflammatory cytokine profile compared with EAU, suggesting PMU is a good complementary model for study of immune-mediated uveitis. CXCL10, a proinflammatory cytokine, was increased in the aqueous and vitreous of both models and may be a viable therapeutic target.
Keywords: cytokine, uveitis, experimental autoimmune uveoretinitis, lewis rat, CXCl10
Ocular inflammatory diseases (collectively considered uveitis) carry a high risk of vision loss and are estimated to cause 10%–15% of blindness in the United States.1–3 In order to understand the pathogenesis of uveitic disease, a number of animal models, including experimental autoimmune uveitis (EAU), endotoxin-induced uveitis (EIU), experimental autoimmune anterior uveitis, and many others, have been developed over the last 50 years.4 These models each recapitulate a subset of uveitic conditions; however, no single model recapitulates all forms of human uveitis effectively. Experimental autoimmune uveitis has been the most extensively studied and is the primary model system from which the known mechanisms of ocular inflammation have been elucidated.5 However, efforts to develop therapeutics based on predicted results from EAU models in rodents have not consistently translated to success in humans.6 Thus, it may be advantageous to screen potential therapeutic agents through multiple animal models, representing diverse mechanisms of ocular inflammatory disease.
Currently, the only Food and Drug Administration–approved agents specifically approved for treatment of uveitis are intraocular corticosteroids. Two such local treatments are the implantable fluocinolone acetonide device (Retisert; Bausch + Lomb, Rochester, NY, USA) and the sustained release dexamethasone implant (Ozurdex; Allergan, Irvine, CA, USA). Both devices were initially tested in animals using an experimental mycobacterial model of uveitis in rabbits. Efficacy in this model was associated with ultimate efficacy in treatment of human disease.7–12 In this model, rabbits are immunized with a subcutaneous killed mycobacterial extract followed by injection of small amounts of this extract into the vitreous cavity. This produces a robust, acute inflammatory response that can recapitulate chronic inflammation with repeated intraocular injections.9,13 Although this model was used to demonstrate efficacy of time release intravitreal corticosteroids in rabbits, its mechanisms have not been well characterized. Additionally, the model, to date, has been utilized only in rabbits. Ideally, this model would be portable to other species such as rat or mouse, where study of its mechanisms would be aided by superior tools (including genetic mutants and a wider range of reagents) available in these species.
We adapted this model to the rat and renamed it primed mycobacterial uveitis (PMU). Unlike EAU, which is a canonical autoimmune-mediated uveitis model relying on the adaptive immune system, the mechanism of PMU is expected to rely on both innate and adaptive immune responses to mycobacterial extract. Here we outline the experimental protocol, describe clinical and histopathologic findings of PMU in the rat, and provide a cytokine comparison with the established EAU rat model. The results suggest that PMU and EAU in rats produce distinct models of uveitis with overlapping but unique cytokine responses. Several cytokines are elevated in both models, suggesting they may be good targets for diverse forms of uveitis.
Methods
Animals and Induction of Uveitis
The animal study protocol was approved by the Animal Care and Use Committee of the University of Washington (animal study protocol 4184-04) and was compliant with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. All efforts were made to minimize pain and distress in the experimental animals.
Female Lewis rats were purchased from Charles River (Hollister, CA, USA) or Taconic Farms (Oxnard, CA, USA). Induction of PMU in Lewis rats was initiated with a systemic priming consisting of a subcutaneous injection in the right hind flank of 100 μg mycobacterium tuberculosis H37Ra antigen (Difco Laboratories, Detroit, MI, USA) in 0.1 mL 50% peanut oil and 50% PBS suspension. Seven days later, both eyes were injected with 3.75 μg mycobacterium tuberculosis H37Ra antigen suspended in PBS by sonication in a 5-μL volume. For cytokine analysis, animals termed naïve received the PMU subcutaneous prime but not the intravitreal injection. Animals termed EAU naïve were untreated at the time of vitreous collection.
Induction of EAU in Lewis rats was initiated with a subcutaneously injection of 30 μg synthetic bovine interphotoreceptor retinoid-binding protein (IRBP) peptide (residue sequence ADGSSWEGVGVVPDV) in complete Freund's adjuvant (2.5 mg/mL mycobacterium) in the right hind flank.
Clinical Evaluation, Histology, and Immunohistochemistry
For all clinical evaluations (external examinations and color photography), animals were anesthetized with inhaled 5% isoflurane and photographed using a macrophotography system with collinear light source.
For histology, four eyes from two animals were harvested at each time point, placed in 4% paraformaldehyde for 48 hours, and then embedded in paraffin blocks. Four- to 6-μm sections were stained with hematoxylin and eosin. For immunohistochemistry, serial sections were stained with 1:100 mouse anti-rat CD68 (AbD Serotec, Kidlington, UK), 1:400 rabbit anti-human CD3 (DAKO, Carpinteria, CA, USA), and 1:200 mouse anti-human CD20 (AbD Serotec). Sections were deparafinized with three 5-minute washes of xylene and rehydrated with ethanol diluted in water (100%–75%) and then washed in 1× PBS for 5 minutes. Antigen retrieval was performed using citrate buffer pH6 (buffer HEIR L; Thermo Fisher Scientific, Waltham, MA, USA) for 30 minutes in a pressure cooker. Samples were blocked with PBS plus 2.5% horse serum for 30 minutes at room temperature and then incubated with primary antibodies overnight at 4°C. Primary antibodies were removed, and samples were washed with 1× PBS three times for 10 minutes each. Secondary antibody conjugated to horseradish peroxidase (ImmPress; Vector Labs, Burlingame, CA, USA) was added and incubated for 60 minutes at room temperature. Samples were washed with 1× PBS for 10 minutes twice, and 3,3′-diaminobenzidine (DAB) solution was added until color reaction was detected by light microscopy (1–3 minutes). Slides were washed with water and then counterstained with hematoxylin.
Cytokine Analysis
Samples were tested using the MilliplexMAP rat cytokine/chemokine premixed 27 plex immunology multiplex assay (Millipore, Billerica, MA, USA). The aqueous and vitreous levels of 27 cytokines were measured. The cytokines measured were granulocyte-colony stimulating factor (G-CSF), eotaxin, granulocyte monocyte-colony stimulating factor (GM-CSF), IL-1α, macrophage inflammatory protein-1α (MIP-1α), IL-2, epidermal growth factor (EGF), IL-13, IL-12p70, IL-5, monocyte chemoattractant protein-1 (MCP-1), IFN-γ–induced protein 10 (IP-10), fractalkine, lipopolysaccharide-induced CXC chemokine (LIX), MIP-2, leptin, IL-4, IL-6, IL-10, IFN-γ, IL-17A, Il-18, growth-related oncogene/keratinocyte chemokine (GRO/KC), VEGF, TNF-α, and regulated on activation, normal T cell expressed and secreted (RANTES). From individual eyes, aqueous was aspirated and protease inhibitor added. The vitreous was separated from the retina, diluted in 150 μL PBS and 1× protease inhibitor (Sigma-Aldrich, St. Louis, MO, USA), and homogenized. The sample was spun down for 1 minute at 10,000g, and supernatant was collected. All samples were stored at −80°C prior to testing. Prior to analysis, aqueous and vitreous samples were diluted (1:1) in RIPA buffer (RIPA, PMSF, PIC) according to the manufacturer's protocol. Samples were analyzed using the MAGPIC system (Luminex, Austin, TX, USA) with xPonent software version 4.2 (Millipore). Samples with calculated concentrations below the lowest limit of detection were given a value of the lowest limit of detection for statistical analysis. Data analysis was performed using Milliplex Analyst Standard Version 5.1 software (Millipore). All cytokine data are presented as median fluorescence intensity (MFI) without conversion to concentration in order to decrease induced error.14
Statistical Analysis
Change from naïve mean MFI was analyzed using a 1-way ANOVA model with a 1-step Sidak correction for multiple comparisons. Prior to ANOVA analysis, outliers with a z score >2 or ≤2 were removed from the calculation of the mean.15 Between-group comparisons were performed in a pairwise fashion using either a Bonferroni or Games-Howell test. Post hoc analysis was performed only for groups with a significant variance detected by ANOVA. P < 0.05 was considered statistically significant.
Results
Clinical and Histologic Disease Activity in the PMU Rat Model
Primed mycobacterial uveitis in the rat is initiated with a subcutaneous injection of a 100-μg suspension of mycobacterium tuberculosis H37Ra antigen in oil, followed 7 days later by an intravitreal injection of 3.75 μg mycobacterium tuberculosis H37Ra antigen in PBS (Fig. 1). A robust anterior inflammatory response is seen starting 2 days after intraocular injection. Clinically, the inflammation can be detected by the formation of a pupillary membrane and engorgement of the corneal vasculature (Figs. 2B, 2C).
Figure 1.
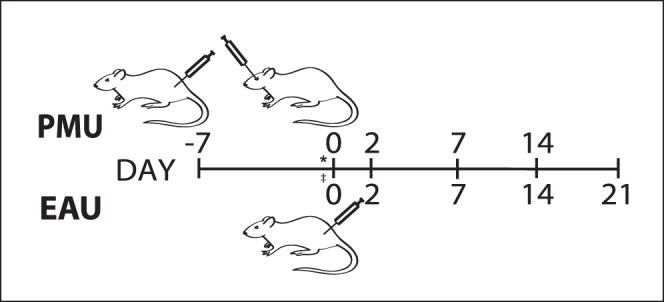
Induction of PMU and EAU in rats. For PMU, a subcutaneous “systemic prime” of killed mycobacterium tuberculosis H37Ra is given on day −7. On day 0, uveitis is induced with an intravitreal injection of the killed mycobacterium extract in PBS. Ocular inflammation peaks on day 2 and resolves spontaneously by day 14. Experimental autoimmune uveitis is induced by immunization with IRBP peptide (ADGSSWEGVGVVPDV) in complete Freund's adjuvant on day 0. Ocular inflammation peaks on day 14 and spontaneously resolves on day 21. Ocular inflammation was assessed on days 2, 7, and 14 for PMU and days 2, 7, 14, and 21 for EAU. Naïve samples were collected from animals at day 0 in both models (*PMU Naïve; ‡EAU naïve).
Figure 2.
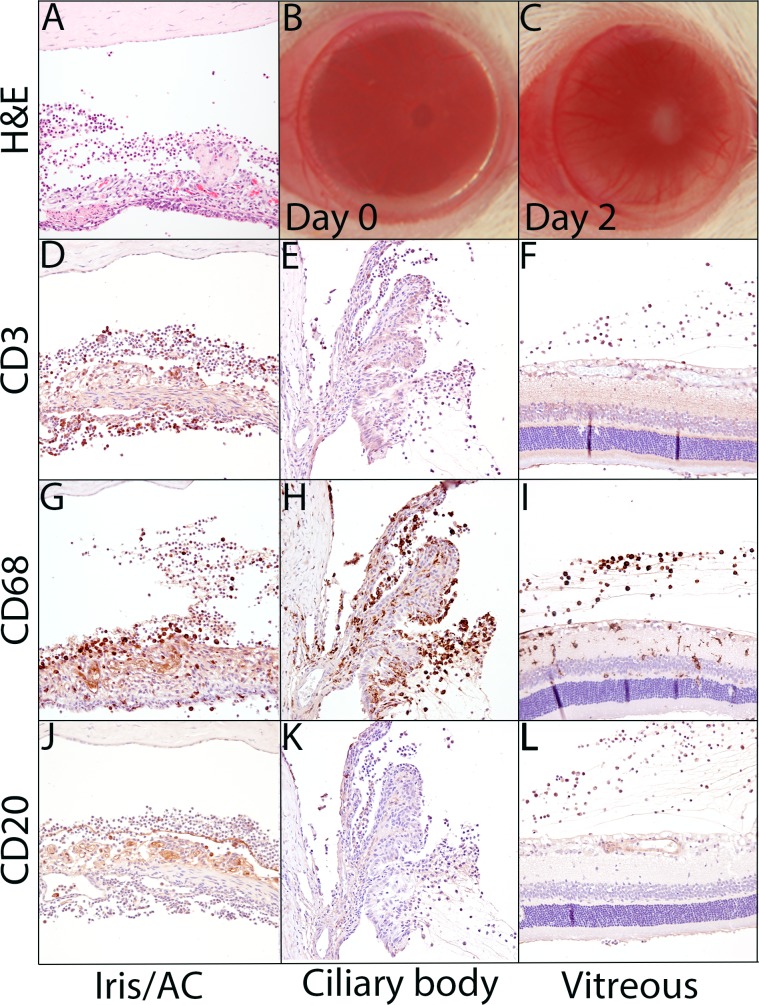
Primed mycobacterial uveitis generates a CD68-predominant inflammatory response in the anterior chamber, vitreous, and surrounding the ciliary body without retinal degeneration. (A) Hematoxylin and eosin–stained section of iris and anterior chamber demonstrating inflammatory cells in the aqueous, iris vasculature dilation, and epithelioid macrophages forming a multinucleated giant cell on the anterior iris surface. (B) Uninflamed eye at PMU day 0, prior to intravitreal injection. (C) The PMU eye during peak inflammation (day 2) demonstrating dilated corneal vasculature, hypopyon, and pupillary membrane. Immunohistochemistry with anti-CD3 (D–F), anti-CD68 (G–I), and anti-CD20 (J–L) antibodies identifies a predominance of CD68- and CD3-positive inflammatory cells with few CD20-positive cells. The inflammation is predominantly noted in the anterior chamber (D, G, J), surrounding the ciliary processes (E, H, K), and in the vitreous (F, I, J). CD68-positive cells are seen within the retina (J), but retinal damage was not noted.
Using hematoxylin and eosin staining, inflammatory cells are seen to infiltrate the anterior chamber, vitreous, and surround the ciliary processes (Fig. 2). Granulomatous inflammation was identified on the iris (Fig. 2A) and subconjunctivally at the site of intravitreal injection (data not shown). Inflammation resolves (as assessed by histology) by day 14 without apparent permanent sequelae.
Immunohistochemistry was performed to identify the inflammatory cell types (Figs. 2D–L). CD68+ macrophages are the predominant inflammatory cells present at peak inflammation, along with substantial numbers of CD3+ T lymphocytes, whereas CD20+ B cells are rarely seen. In contrast to EAU, neither choroidal infiltration nor retinal inflammation with loss of photoreceptors was observed.
Peak Cytokine and Chemokine Analysis in PMU and EAU
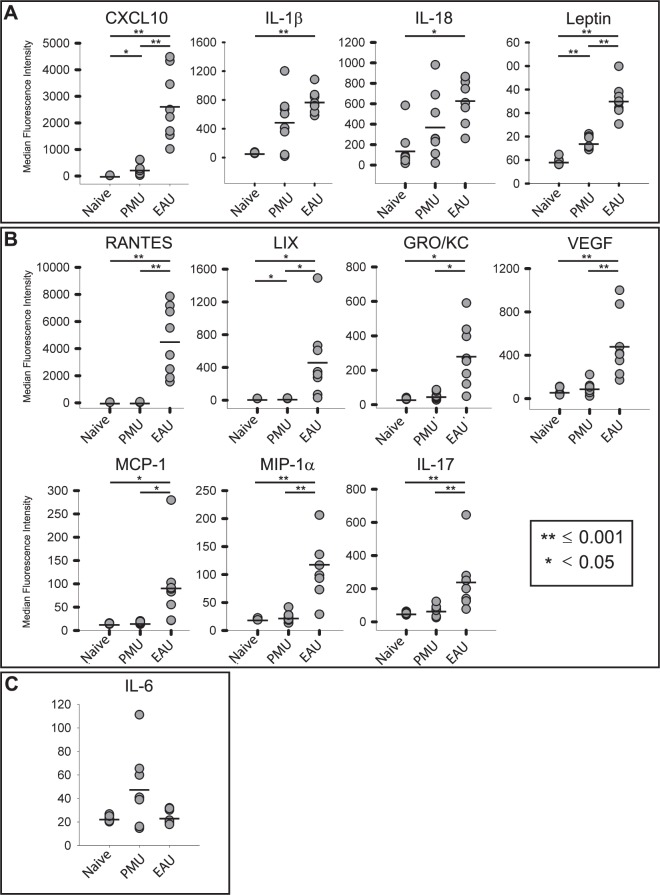
Aqueous and vitreous were collected on the day of peak inflammation from PMU (day 2) and EAU (day 14) for analysis of 27 cytokines using the Luminex multiplex analysis. In the aqueous of PMU eyes, five cytokines were induced ≥1.5-fold over baseline levels (Table 1; Fig. 3). Two of these cytokines, CXCL10 and leptin, were significantly induced compared with naïve eyes. CXCL10 and IL-1β were the most highly-induced cytokines in PMU aqueous, with 9.7-fold (P = 0.002) and 7.3-fold (P = 0.07) induction over baseline, respectively. Other induced proinflammatory cytokines in PMU aqueous were IL-18 (2.6-fold, P = 0.15), IL-6 (2.1-fold, P = 0.13), leptin (1.8-fold, P < 0.001), GRO/KC (1.5-fold, P = 0.08), and VEGF (1.5-fold, P = 0.75).
Table 1.
Aqueous Cytokine Induction in PMU and EAU
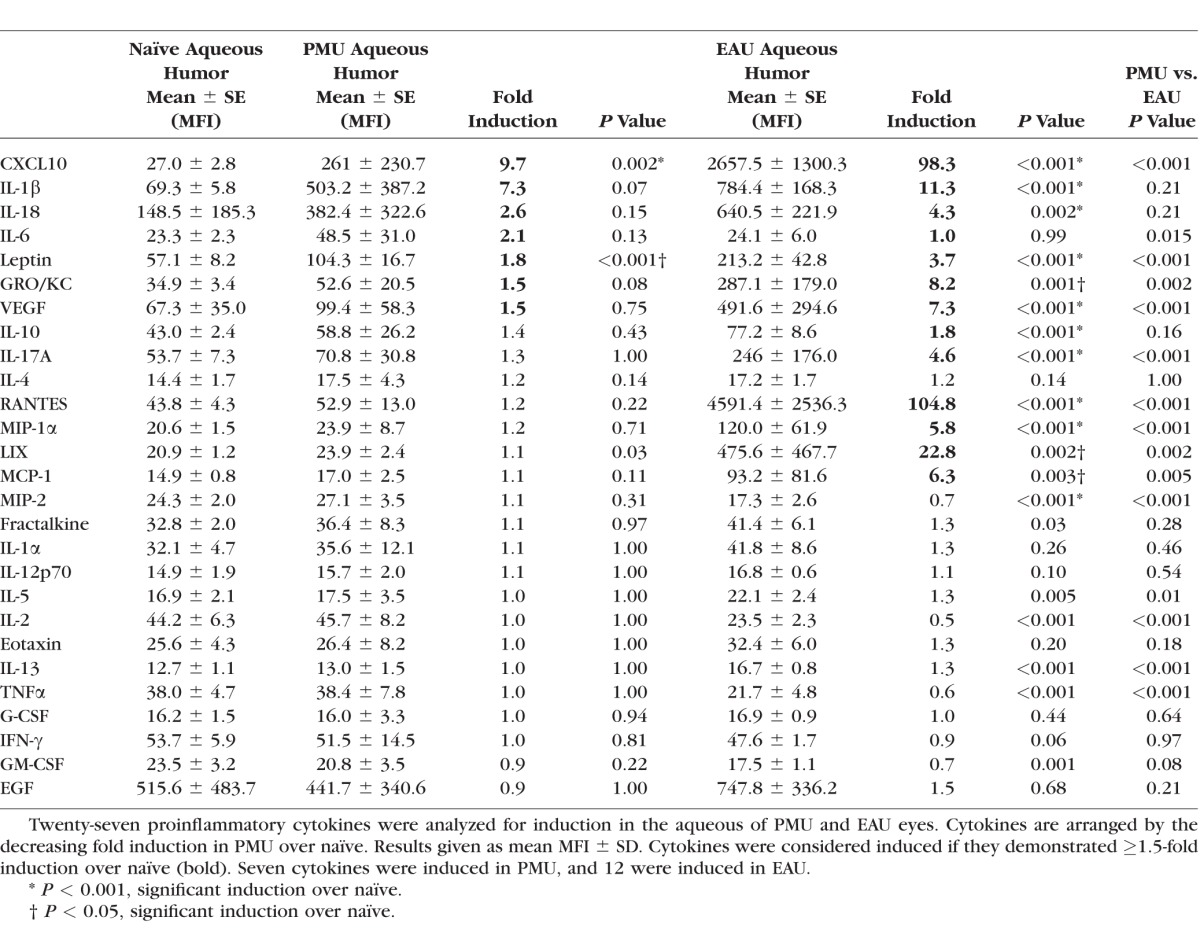
Figure 3.
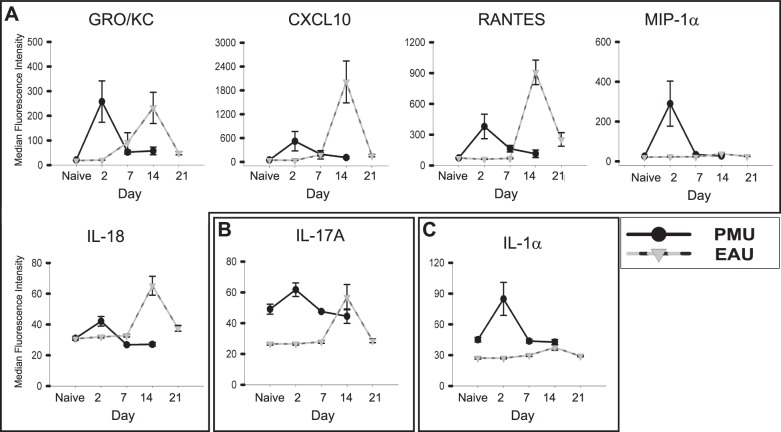
Comparison of highly-induced proinflammatory cytokines in the aqueous. Aqueous was collected from PMU naïve, PMU, and EAU eyes. Cytokine levels are expressed in MFI. The MFI from each sample is represented as a gray circle. The bar represents the mean MFI. (A) Cytokines with >1.5-fold induction in both PMU and EAU. (B) Cytokines with >1.5-fold induction in EAU only. The LIX levels in PMU were significantly elevated (P = 0.03), but with only 1.1-fold induction. (C) Interleukin-6 is only induced in PMU (2.1-fold, P = 0.13). Significant differences are indicated by *P < 0.05 and **P ≤ 0.001. Nonsignificant differences are not indicated. Cytokine levels are expressed in MFI.
In the aqueous from EAU, 12 cytokines were induced, and all were significantly elevated above naïve (Table 1; Fig. 3). Similar to PMU, CXCL10 was highly induced (98.3-fold, P < 0.001). Additional similarities with PMU included IL-1β (11.3-fold, P < 0.001), IL-18 (4.3-fold, P = 0.002), and leptin (3.7-fold, P < 0.001). Proinflammatory cytokines that were significantly induced in EAU but not in PMU included RANTES (104-fold, P < 0.001), LIX (22.8-fold, P = 0.002), GRO/KC (8.2-fold, P < 0.001), VEGF (7.3-fold, P < 0.001), MCP-1 (6.3-fold, P = 0.003), MIP-1α (5.8-fold, P < 0.001), IL-17A (4.6-fold, P < 0.001), IL-18 (4.3-fold, P = 0.002), and IL-10 (1.8-fold, P < 0.001).
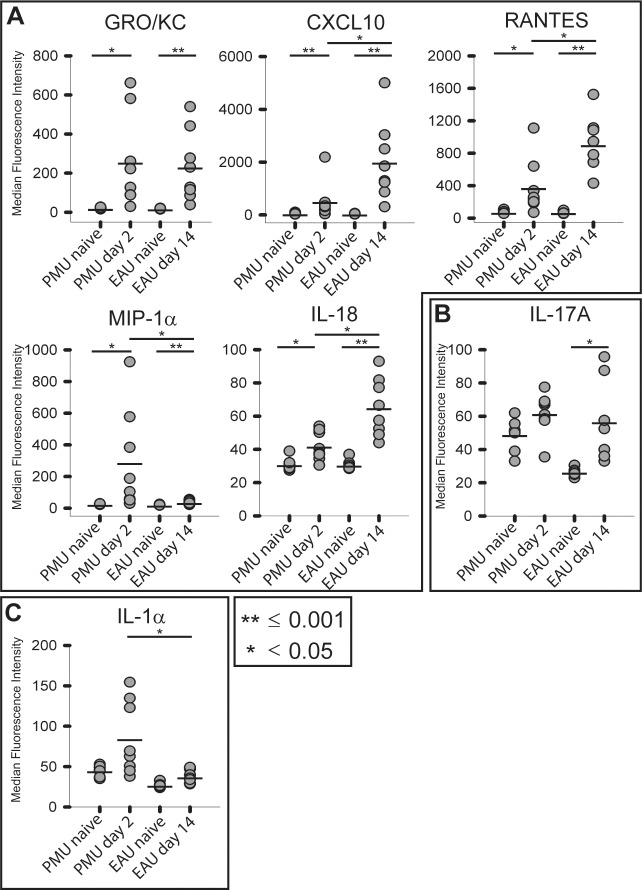
In the vitreous of both models, GRO/KC, CXCL10, RANTES, MIP-1α, and IL-18 were all significantly induced (Tables 2, 3; Fig. 4). CXCL10 was the most highly induced cytokine in EAU (43.7-fold, P < 0.001) and the most significantly induced cytokine in PMU (10.8-fold, P < 0.001). GRO/KC had the highest fold induction in PMU (12.4-fold, P = 0.003) and was similarly induced in EAU (12.5-fold, P = 0.001). Overall, induced cytokine levels were higher in EAU than in PMU, except for MIP-1α (PMU, 11.3-fold versus EAU, 2.1-fold, P = 0.05) and IL-1α (PMU, 1.9-fold versus EAU, 1.4-fold, P = 0.03). Interleukin-17A was only significantly induced in EAU (2.1-fold, P = 0.01).
Table 2.
Vitreous Cytokine Induction in PMU
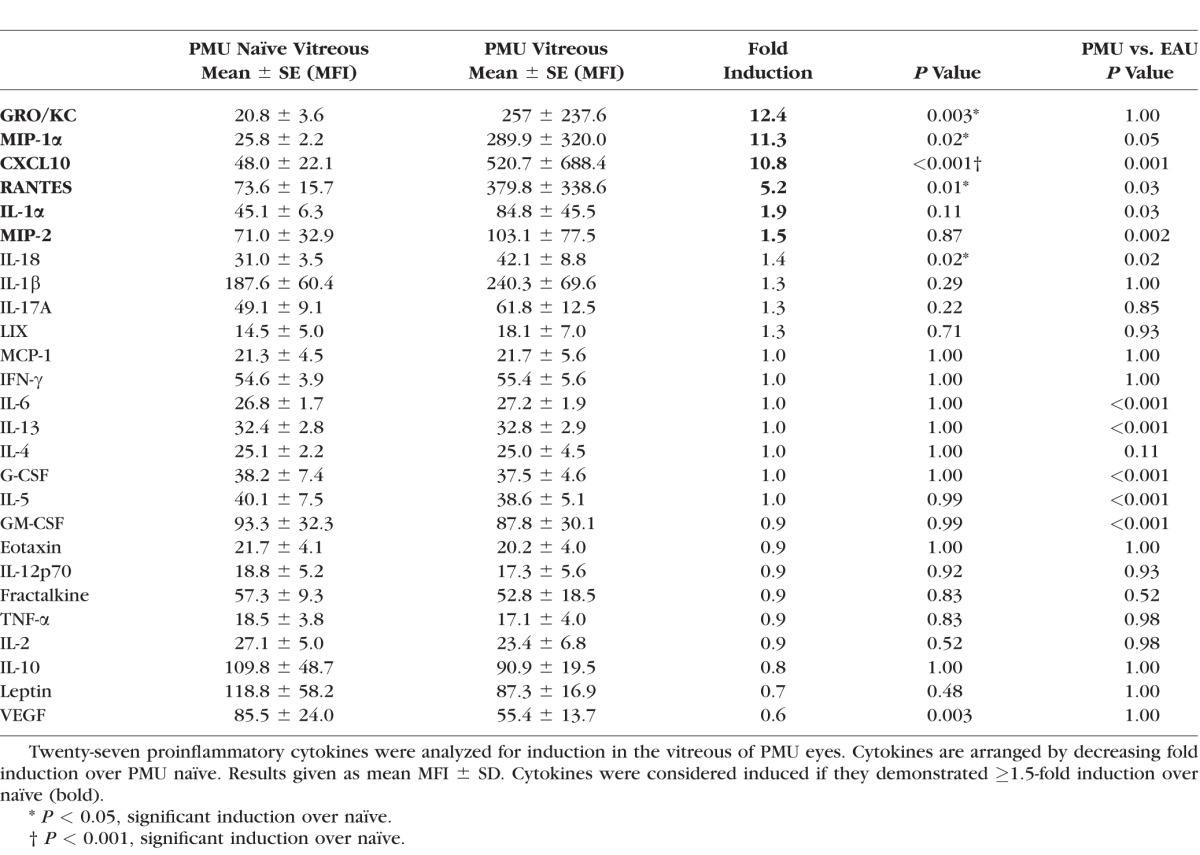
Table 3.
Vitreous Cytokine Induction in EAU
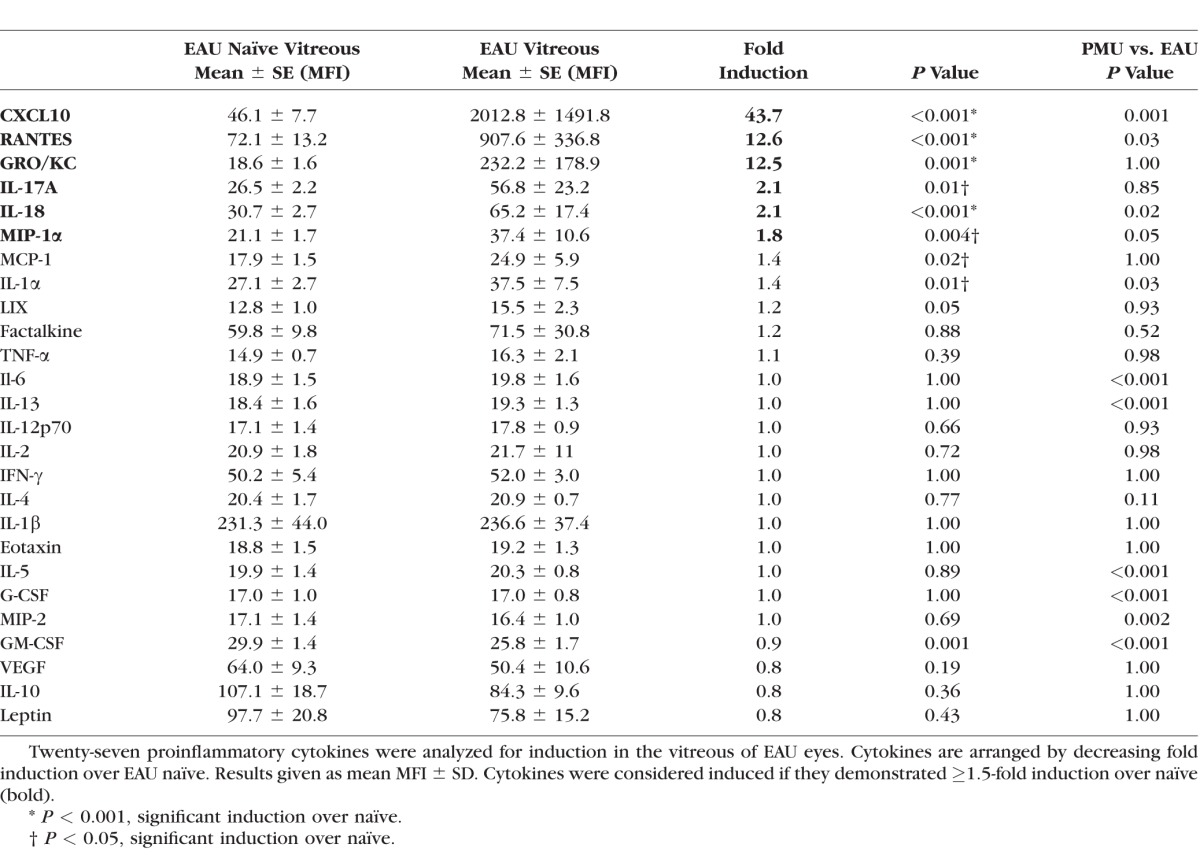
Figure 4.
Comparison of highly-induced proinflammatory cytokines in the vitreous. Vitreous was collected from PMU naïve, PMU, and EAU eyes. Cytokine levels are expressed in MFI. The MFI from eight animals per condition is represented as a gray circle. The bar represents the mean MFI. (A) Cytokines with >1.5-fold induction in both PMU and EAU. Interleukin-18 in PMU was only elevated 1.4-fold, but this was significant (P = 0.02) (B) Interleukin-17 was induced 2.1-fold in EAU only (P = 0.01). (C) Interleukin-61α was induced in PMU (1.9-fold, P = 0.11). Significant differences are indicated by *P < 0.05 and **P ≤ 0.001. Nonsignificant differences are not indicated.
Cytokine Time Course in PMU and EAU
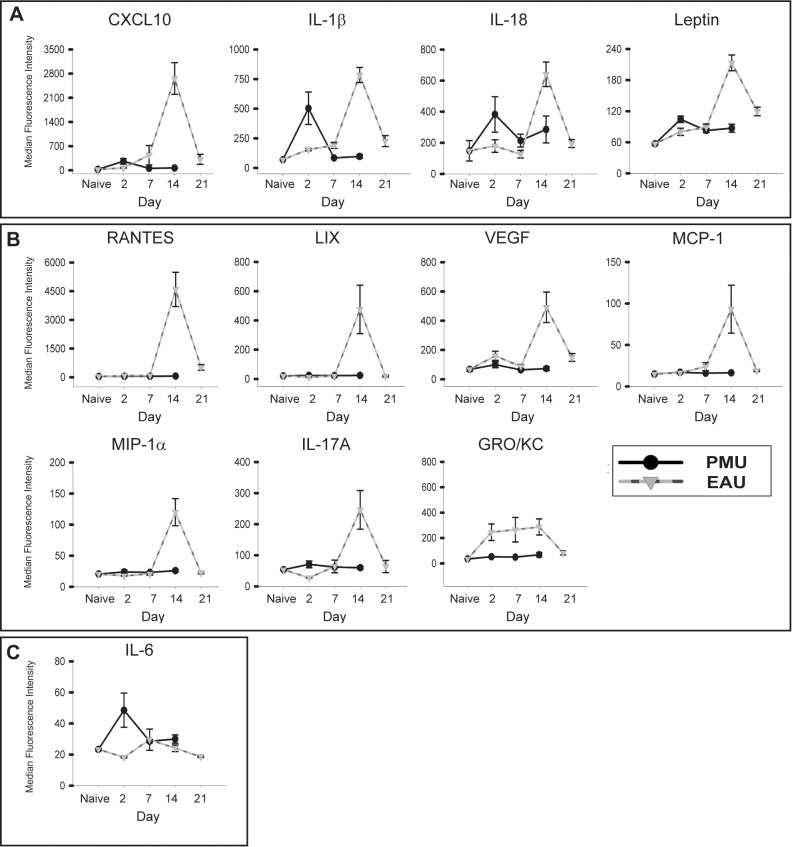
Cytokines that function as key mediators of the clinical manifestations inflammation would be expected to demonstrate peak levels on day 2 in PMU and day 14 in EAU. To determine which cytokines display this pattern of expression, cytokine levels were assessed from aqueous and vitreous samples taken from eyes on days 2, 7, and 14 for PMU and 2, 7, 14, and 21 for EAU. In the aqueous (Fig. 5) and the vitreous (Fig. 6), cytokines that were induced during inflammation exhibited the expected monophasic increase in concentration concordant with the day of peak clinical inflammation. Aqueous GRO/KC in EAU was the only exception demonstrating an early elevation at day 2 that was sustained through day 14 before declining to baseline levels by day 21. In the vitreous, GRO/KC, CXCL10, RANTES, MIP-1α, and IL-18 demonstrate a concordant peak with inflammation in both PMU and EAU (Fig. 6A); MIP-1α had a monophasic peak in both PMU and EAU, but the magnitude of induction was significantly higher in PMU than EAU (P = 0.05) leading to a flattened appearance of the EAU peak. The pattern of IL-17A expression suggests an association with peak inflammation in both models; however, although the association with EAU was significant, in PMU at day 2, there is only a small and nonsignificant increase in PMU IL-17A levels (1.3-fold, P = 0.22). The IL-1α level also demonstrated a statistically nonsignificant peak in PMU at day 2 (1.9-fold, P = 0.11) that was elevated above the EAU peak (1.4-fold above EAU naïve, P = 0.01). However, PMU naïve levels were elevated above EAU naïve levels, which may have contributed to this difference.
Figure 5.
Patterns of proinflammatory cytokine induction in the PMU and EAU aqueous. Cytokine levels from the aqueous in PMU (solid line) and EAU (dotted line) were measured on days 2, 7, 14, and 21 (EAU only). Each point represents the average from eight animals (16 eyes) analyzed per day. Bars indicate SE. (A) CXCL10, IL-1β, IL-18, and leptin demonstrate a pattern of a monophasic induction corresponding with the peak of clinical inflammation in both models (day 2 in PMU and day 14 in EAU). (B) RANTES, LIX, VEGF, MCP-1, MIP-1α, IL-17A, and GRO/KC are not induced in PMU but are induced in EAU. (C) Interleukin-6 demonstrates a concordant peak in PMU but not EAU.
Figure 6.
Vitreous patterns of proinflammatory cytokine concentrations in PMU and EAU. Cytokine levels from the vitreous in PMU (solid line) and EAU (dotted line) were measured on days 2, 7, 14, and 21 (EAU only). Each point represents the average from eight animals (16 eyes) analyzed per day. Bars indicate SE. (A) GRO/KC, CXCL10, RANTES, MIP-1α, and IL-18 have similar baseline naïve levels in both models that demonstrate a monophasic peak with inflammation. (B) Interleukin-17A levels are elevated in PMU naïve eyes compared with EAU naïve and are only significantly induced during inflammation in EAU. (C) Interleukin-1α levels demonstrate a monophasic peak with inflammation in PMU.
Discussion
We describe here the successful application of a well-established rabbit model of uveitis to the rat. This model, which we have descriptively named PMU, generates an acute anterior and intermediate uveitis that differs from the well-established EAU model in its time course and intensity of ocular inflammation, in its absence of retinal or choroidal involvement, and in its distinct cytokine profile in the inflamed aqueous and vitreous. However, despite these differences, a core group of cytokines that were elevated in both models were identified, suggesting that these might be important mediators of inflammation in multiple forms of uveitis.
Animal models of disease are required for the development of new medical therapies, and the use of multiple animal models in preclinical drug development can help improve the likelihood of benefit in human trials. The rabbit version of PMU model has already been used in preclinical testing for therapies that subsequently showed efficacy in human uveitis.7,9 However, PMU has not been mechanistically characterized beyond the initial reports in rabbits.8,9,13 We show in the current studies that, in rats, the inflammatory response primarily affects the anterior segment and vitreous with sparing of the retina and choroid. Primed mycobacterial uveitis features substantial macrophage involvement as manifest by the dominance of CD68-positive cells. This finding, as well as the observation of noncaseating granulomas, suggests that PMU may be a model of human conditions such as sarcoid-associated uveitis. Consistent with this, the cytokine profiles observed in rats with PMU were similar to those seen in human sarcoidosis.16 This suggests that PMU is a good model for granulomatous anterior and intermediate uveitis and may be complementary to the spectrum of posterior segment diseases (such as sympathetic ophthalmia) modeled by EAU.
In this study, we identified cytokines and chemokines that increased in concentration at the peak of inflammation in two uveitis models. In general, cytokine concentrations were higher in EAU than in PMU, reflecting an overall difference in disease severity between the models; at the doses of intravitreal mycobacterium used for PMU in this study, EAU generated a more severe overall inflammatory response than PMU. We expected to find that PMU and EAU would have distinctly different cytokine profiles due to the different mechanisms of uveitis induction in each model (i.e., primarily innate immune response in PMU versus a sustained adaptive response in EAU). Instead, we found many cytokines with increased concentration during peak inflammation in both models, including (in the aqueous) CXCL10, IL-1β, IL-18, and leptin, and (in the vitreous) CXCL10, GRO/KC, and RANTES. Induced cytokines also demonstrated a peak in concentration that coincided with the day of maximum clinical inflammation in both models, supporting the hypothesis that they play a key role in active ocular inflammation (i.e., a cytokine that peaks in expression after peak inflammation is more likely to be involved in resolution of inflammation).
CXCL10 stands out as the chemokine that was consistently and significantly elevated in both the anterior and posterior segments for both uveitis models. In addition, CXCL10 (also known as IP-10) has been identified at high concentrations in the aqueous and vitreous of patients with multiple forms of uveitis, suggesting this may be a common mediator of inflammation in ocular inflammation.16–20 CXCL10 is a member of the C-X-C chemokines and a ligand for CXCR3. It is produced from leukocytes, neutrophils, eosinophils, monocytes, and endothelial cells in response to IFN-γ and recruits leukocytes to sites of inflammation.21 The CXCL10/CXCR3 combination plays an important role in the recruitment of autoreactive T cells into sites of Type 1 helper T cell (TH1)-driven inflammation in diseases such as type 1 diabetes, Graves disease, and rheumatoid arthritis.22 Targeting chemokines is a relatively new area for the treatment of inflammatory diseases, but an antibody directed against CXCL10 (MDX-1100; Medarex, Princeton, NJ, USA) has recently been shown to have efficacy in combination with methotrexate in patients with rheumatoid arthritis.23,24 Our data suggest that CXCL10 may also be a good therapeutic target for ocular inflammation.
Leptin was also significantly elevated in the aqueous of both EAU and PMU. Although leptin is best known for its hormonal role in signaling satiety, there is a growing body of evidence linking leptin to autoimmune diseases by inducing TH1 and TH17 T-cell polarization, inhibiting regulatory T-cell proliferation, and influencing dendritic cell maturation.25–29 Leptin levels are also increased in the serum of patients with Vogt-Koyanagi-Harada syndrome and Beçhet's disease and in the inflamed aqueous of rats with EIU.30–32 The finding of elevated leptin levels in both EAU and PMU suggests that further research into leptin function in ocular inflammation is warranted.
Surprisingly, the cytokine profile in the vitreous differed significantly from that seen in aqueous (despite fluid being freely diffusible between the two chambers). In the vitreous, chemokines such as CXCL10 (IP-10), GRO/KC (CXCL1), and RANTES (CCL5) dominated the inflammatory response. This supports previous studies in EAU that identified marked increases in mRNA expression of CXCL10 and CCL5 in the posterior segment of EAU mouse eyes.33 CXCL1 is a member of the C-X-C chemokines and is produced by macrophages and neutrophils. In contrast with CXCL10, it is primarily a chemoattractant for neutrophils rather than activated T cells. RANTES or CCL5 is a member of the C-C family of chemokines and is a ligand for CCR5 found on TH1 lymphocytes.34 CCL5 is expressed in EAU retinas at high levels during inflammation, and the CCR5 receptor is important for T-cell trafficking in EAU.35,36 Each of these chemokines has also been identified to play a role in ocular inflammation in humans. CXCL1 in the cornea is required for the neutrophilic infiltrate in recurrent herpes simplex virus (HSV) keratitis37 and is elevated in the serum of patients with active Beçhet's disease.38 Both CXCL1 and CXCL10 are elevated in the aqueous of patients with presumed tuberculous uveitis19 and in multiple forms of noninfectious uveitis.18 CCL5 levels have shown elevation in patients with intermediate uveitis,39 but in patients with multiple forms of uveitis, this result was not repeated.40 Overall, these human studies are consistent with our data and suggests that local levels of CXCL1, CXCL10, and CCL5 are important in ocular inflammation.
Interleukin-17 showed differential expression between PMU and EAU. Interleukin-17 was significantly induced in EAU in both the aqueous and vitreous, but not in PMU. Interleukin-17 is an important mediator of EAU, as treatment with an anti–IL-17 antibody inhibits EAU.41 Further work will be needed to determine whether IL-17 is necessary for PMU inflammation. However, the absence of significant induction of IL-17 in PMU suggests it has at best a limited role in this form of uveitis. This difference will be important for the preclinical trials of targeted immunotherapy and may help avoid the risk of targeting a model specific mechanism that is not central to the heterogeneous collection of human uveitic diseases.6
We were surprised that intraocular TNF-α levels were not elevated in either model.42 A previous study that evaluated the cytokine levels in whole homogenized eyes of rats with EAU identified elevated TNF-α and IFN-γ levels.43 In addition, TNF-α production has been demonstrated in retinal microglia during the induction of EAU,44 and increased mRNA expression of TNF-α has also been identified in the iris and ciliary body at the peak of EAU inflammation.45 In the experiments reported here, the retinal structures were separated from the aqueous and vitreous prior to cytokine analysis. Thus, the intraocular fluids may have not have the same distribution of these cytokines as the ocular structural components and may explain our results. In support of this idea, low aqueous levels of TNF-α in patients with active intermediate uveitis and idiopathic anterior uveitis have also been reported.39,46 However, high levels of aqueous TNF-α have also been reported.40
Further refinements of the PMU model would be desirable. Previous work in rabbits has demonstrated that the severity of the inflammatory response can be modulated by varying the concentration and subfraction of H37Ra antigen used in the intravitreal injection. It is likely that optimization of antigen load would have similar effect in small rodents and possible that modulation of antigen load could be used to create models of variable severity. In the rabbit, repeated intraocular injection can simulate recurrent inflammation,13 whereas repeated injection in rats or mice would be challenging, it is possible, and may allow for a model of relapsing/remitting uveitis. The utility of the PMU model would be enhanced by development and validation of a histologic, imaging, and clinical scoring system, as has been done for EAU.47,48
The PMU model shares with the EIU model the generation of innate immune responses in the eye by a pathogen association molecular pattern (PAMP). Endotoxin-induced uveitis is triggered by systemic or local administration of the PAMP lipopolysaccharide (LPS) and is mediated by Toll-like receptor 4 (TLR4).49–51 Although LPS is characteristically associated with Gram-negative bacteria, it is possible that some of the inflammation observed in PMU is attributable to this mechanism, as other ligands have also been shown to activate TLR4.52,53 However, PMU also features T- and B-cell infiltrates characteristic of adaptive immunity; we hypothesize these are secondary to the immunization with mycobacterial extract prior to injection in the eye. This hypothesis is being tested in ongoing experiments comparing inflammation observed in primed versus unprimed animals following intravitreal tubercular extract injection. Similarly, further studies are necessary to determine to what extent the observed immune responses are specific to tubercular antigen compared with other potential stimuli such as other bacterial extracts or viral proteins. The observation that the fellow (uninjected) eye remains free of measurable inflammation in PMU, however, suggests that the systemic priming event has not triggered a persistent EIU condition (as this would likely be bilateral). Primed mycobacterial uveitis thus likely represents a unique mixed-mechanism model incorporating both innate and adaptive arms of immune response in the eye.
The last decade has seen a critical reevaluation of the role of innate immunity in the genesis of chronic inflammatory diseases, particularly diseases classified as autoinflammatory.54–56 Sarcoidosis, Beçhet's disease, and HLA-B27–associated uveitis have features consistent with autoinflammatory conditions.57 Sarcoidosis in particular has been increasingly associated with a chronic mixed innate and acquired immune response to mycobacterial proteins, even in the absence of productive infection.58 Thus, with the use of mycobacterial antigens and a potential role of both arms of the immune system to contribute to ocular inflammation, the PMU model may prove to be a useful model to study autoinflammatory forms of uveitis such as sarcoidosis and to compliment the spectrum of autoimmune forms of uveitis modeled by EAU and EIU.
Acknowledgments
Supported by National Institutes of Health (NIH) Award K08EY023998 (KLP), the Burroughs- Wellcome Translational Scientist Award, an unrestricted award from Research to Prevent Blindness, and NIH Grant P30 EY001730 (RVG).
Disclosure: K.L. Pepple, None; L. Rotkis, Alcon Pharmaceuticals (F); J. Van Grol, Alcon, Inc. (E), Novartis Pharmaceuticals (E); L. Wilson, Alcon Pharmaceuticals (F); A. Sandt, Alcon Pharmaceuticals (F); D.L. Lam, None; E. Carlson, Alcon, Inc. (E), Novartis Pharmaceuticals (E); R.N. Van Gelder, Alcon Pharmaceuticals (F)
References
- 1. Rothova A,, Suttorp-van Schulten MS, Frits Treffers W, Kijlstra A. Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol. 1996; 80: 332–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Durrani OM,, Tehrani NN,, Marr JE,, Moradi P,, Stavrou P,, Murray PI. Degree duration, and causes of visual loss in uveitis. Br J Ophthalmol. 2004; 88: 1159–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nussenblatt RB. The natural history of uveitis. Int Ophthalmol. 1990; 14: 303–308. [DOI] [PubMed] [Google Scholar]
- 4. Forrester JV,, Klaska IP,, Yu T,, Kuffova L. Uveitis in mouse and man. Int Rev Immunol. 2013; 32: 76–96. [DOI] [PubMed] [Google Scholar]
- 5. Horai R,, Caspi RR. Cytokines in autoimmune uveitis. J Interferon Cytokine Res. 2011; 31: 733–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dick AD,, Tugal-Tutkun I,, Foster S,, et al. Secukinumab in the treatment of noninfectious uveitis: results of three randomized, controlled clinical trials. Ophthalmology. 2013; 120: 777–787. [DOI] [PubMed] [Google Scholar]
- 7. Mruthyunjaya P,, Khalatbari D,, Yang P,, et al. Efficacy of low-release-rate fluocinolone acetonide intravitreal implants to treat experimental uveitis. Arch Ophthalmol. 2006; 124: 1012–1018. [DOI] [PubMed] [Google Scholar]
- 8. Cousins SW. T cell activation within different intraocular compartments during experimental uveitis. Dev Ophthalmol. 1992; 23: 150–155. [DOI] [PubMed] [Google Scholar]
- 9. Cheng CK,, Berger AS,, Pearson PA,, Ashton P,, Jaffe GJ. Intravitreal sustained-release dexamethasone device in the treatment of experimental uveitis. Invest Ophthalmol Vis Sci. 1995; 36: 442–453. [PubMed] [Google Scholar]
- 10. Lowder C,, Belfort R,, Jr.,, Lightman S,, et al. Dexamethasone intravitreal implant for noninfectious intermediate or posterior uveitis. Arch Ophthalmol. 2011; 129: 545–553. [DOI] [PubMed] [Google Scholar]
- 11. Jaffe GJ,, Martin D,, Callanan D,, Pearson PA,, Levy B,, Comstock T. Fluocinolone acetonide implant (Retisert) for noninfectious posterior uveitis: thirty-four-week results of a multicenter randomized clinical study. Ophthalmology. 2006; 113: 1020–1027. [DOI] [PubMed] [Google Scholar]
- 12. Callanan DG,, Jaffe GJ,, Martin DF,, Pearson PA,, Comstock TL. Treatment of posterior uveitis with a fluocinolone acetonide implant: three-year clinical trial results. Arch Ophthalmol. 2008; 126: 1191–1201. [DOI] [PubMed] [Google Scholar]
- 13. Jaffe GJ,, Yang CS,, Wang XC,, Cousins SW,, Gallemore RP,, Ashton P. Intravitreal sustained-release cyclosporine in the treatment of experimental uveitis. Ophthalmology. 1998; 105: 46–56. [DOI] [PubMed] [Google Scholar]
- 14. Won JH,, Goldberger O,, Shen-Orr SS,, Davis MM,, Olshen RA. Significance analysis of xMap cytokine bead arrays. Proc Natl Acad Sci U S A. 2012; 109: 2848–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Van Seist M,, Jolicoeur P. A solution to the effect of sample size on outlier elimination. Qtly J Exper Psychol. 1994; 47: 631–650. [Google Scholar]
- 16. Nagata K,, Maruyama K,, Uno K,, et al. Simultaneous analysis of multiple cytokines in the vitreous of patients with sarcoid uveitis. Invest Ophthalmol Vis Sci. 2012; 53: 3827–3833. [DOI] [PubMed] [Google Scholar]
- 17. Ang M,, Cheung G,, Vania M,, et al. Aqueous cytokine and chemokine analysis in uveitis associated with tuberculosis. Mol Vis. 2012; 18: 565–573. [PMC free article] [PubMed] [Google Scholar]
- 18. El-Asrar AM,, Al-Obeidan SS,, Kangave D,, et al. CXC chemokine expression profiles in aqueous humor of patients with different clinical entities of endogenous uveitis. Immunobiology. 2011; 216: 1004–1009. [DOI] [PubMed] [Google Scholar]
- 19. Abu El-Asrar AM,, Struyf S,, Kangave D,, et al. Cytokine and CXC chemokine expression patterns in aqueous humor of patients with presumed tuberculous uveitis. Cytokine. 2012; 59: 377–381. [DOI] [PubMed] [Google Scholar]
- 20. van Kooij B,, Rothova A,, Rijkers GT,, de Groot-Mijnes JD. Distinct cytokine and chemokine profiles in the aqueous of patients with uveitis and cystoid macular edema. Am J Ophthalmol. 2006; 142: 192–194. [DOI] [PubMed] [Google Scholar]
- 21. Neville LF,, Mathiak G,, Bagasra O. The immunobiology of interferon-gamma inducible protein 10 kD (IP-10): a novel pleiotropic member of the C-X-C chemokine superfamily. Cytokine Growth Factor Rev. 1997; 8: 207–219. [DOI] [PubMed] [Google Scholar]
- 22. Antonelli A,, Ferrari SM,, Giuggioli D,, Ferrannini E,, Ferri C,, Fallahi P. Chemokine (C-X-C motif) ligand (CXCL)10 in autoimmune diseases. Autoimmun Rev. 2014; 13: 272–280. [DOI] [PubMed] [Google Scholar]
- 23. Yellin M,, Paliienko I,, Balanescu A,, et al. A phase II, randomized, double-blind, placebo-controlled study evaluating the efficacy and safety of MDX-1100, a fully human anti-CXCL10 monoclonal antibody, in combination with methotrexate in patients with rheumatoid arthritis. Arthritis Rheum. 2012; 64: 1730–1739. [DOI] [PubMed] [Google Scholar]
- 24. Proudfoot AE,, Power CA,, Rommel C,, Wells TN. Strategies for chemokine antagonists as therapeutics. Semin Immunol. 2003; 15: 57–65. [DOI] [PubMed] [Google Scholar]
- 25. Cojocaru M,, Cojocaru IM,, Silosi I,, Rogoz S. Role of leptin in autoimmune diseases. Maedica (Buchar). 2013; 8: 68–74. [PMC free article] [PubMed] [Google Scholar]
- 26. Matarese G,, Leiter EH,, La Cava A. Leptin in autoimmunity: Many questions some answers. Tissue Antigens. 2007; 70: 87–95. [DOI] [PubMed] [Google Scholar]
- 27. Matarese G,, Moschos S,, Mantzoros CS. Leptin in immunology. J Immunol. 2005; 174: 3137–3142. [DOI] [PubMed] [Google Scholar]
- 28. Matarese G,, Carrieri PB,, La Cava A,, et al. Leptin increase in multiple sclerosis associates with reduced number of CD4(+)CD25+ regulatory T cells. Proc Natl Acad Sci U S A. 2005; 102: 5150–5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pucino V,, De Rosa V,, Procaccini C,, Matarese G. Regulatory T cells leptin and angiogenesis. Chem Immunol Allergy. 2014; 99: 155–169. [DOI] [PubMed] [Google Scholar]
- 30. Liu L,, Yang P,, He H,, et al. Leptin increases in Vogt-Koyanagi-Harada (VKH) disease and promotes cell proliferation and inflammatory cytokine secretion. Br J Ophthalmol. 2008; 92: 557–561. [DOI] [PubMed] [Google Scholar]
- 31. Evereklioglu C,, Inaloz HS,, Kirtak N,, et al. Serum leptin concentration is increased in patients with Behcet's syndrome and is correlated with disease activity. Br J Dermatol. 2002; 147: 331–336. [DOI] [PubMed] [Google Scholar]
- 32. Kalariya NM,, Shoeb M,, Ansari NH,, Srivastava SK,, Ramana KV. Antidiabetic drug metformin suppresses endotoxin-induced uveitis in rats. Invest Ophthalmol Vis Sci. 2012; 53: 3431–3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Keino H,, Takeuchi M,, Kezuka T,, Yamakawa N,, Tsukahara R,, Usui M. Chemokine and chemokine receptor expression during experimental autoimmune uveoretinitis in mice. Graefes Arch Clin Exp Ophthalmol. 2003; 241: 111–115. [DOI] [PubMed] [Google Scholar]
- 34. Bonecchi R,, Bianchi G,, Bordignon PP,, et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998; 187: 129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Crane IJ,, McKillop-Smith S,, Wallace CA,, Lamont GR,, Forrester JV. Expression of the chemokines MIP-1alpha, MCP-1, and RANTES in experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 2001; 42: 1547–1552. [PubMed] [Google Scholar]
- 36. Crane IJ,, Xu H,, Wallace C,, et al. Involvement of CCR5 in the passage of Th1-type cells across the blood-retina barrier in experimental autoimmune uveitis. J Leukoc Biol. 2006; 79: 435–443. [DOI] [PubMed] [Google Scholar]
- 37. West DM,, Del Rosso CR,, Yin XT,, Stuart PM. CXCL1 but not IL-6 is required for recurrent herpetic stromal keratitis. J Immunol. 2014; 192: 1762–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kato Y,, Yamamoto T. Serum levels of GRO-alpha are elevated in association with disease activity in patients with Behcet's disease. Int J Dermatol. 2012; 51: 286–289. [DOI] [PubMed] [Google Scholar]
- 39. Valentincic NV,, de Groot-Mijnes JD,, Kraut A,, Korosec P,, Hawlina M,, Rothova A. Intraocular and serum cytokine profiles in patients with intermediate uveitis. Mol Vis. 2011; 17: 2003–2010. [PMC free article] [PubMed] [Google Scholar]
- 40. Curnow SJ,, Falciani F,, Durrani OM,, et al. Multiplex bead immunoassay analysis of aqueous humor reveals distinct cytokine profiles in uveitis. Invest Ophthalmol Vis Sci. 2005; 46: 4251–4259. [DOI] [PubMed] [Google Scholar]
- 41. Zhang R,, Qian J,, Guo J,, Yuan YF,, Xue K. Suppression of experimental autoimmune uveoretinitis by anti-IL-17 antibody. Curr Eye Res. 2009; 34: 297–303. [DOI] [PubMed] [Google Scholar]
- 42. Dick AD,, Forrester JV,, Liversidge J,, Cope AP. The role of tumour necrosis factor (TNF-alpha) in experimental autoimmune uveoretinitis (EAU). Prog Retin Eye Res. 2004; 23: 617–637. [DOI] [PubMed] [Google Scholar]
- 43. Okada AA,, Sakai J,, Usui M,, Mizuguchi J. Intraocular cytokine quantification of experimental autoimmune uveoretinitis in rats. Ocul Immunol Inflamm. 1998; 6: 111–120. [DOI] [PubMed] [Google Scholar]
- 44. Rao NA,, Kimoto T,, Zamir E,, et al. Pathogenic role of retinal microglia in experimental uveoretinitis. Invest Ophthalmol Vis Sci. 2003; 44: 22–31. [DOI] [PubMed] [Google Scholar]
- 45. de Kozak Y,, Verwaerde C. Cytokines in immunotherapy of experimental uveitis. Int Rev Immunol. 2002; 21: 231–253. [DOI] [PubMed] [Google Scholar]
- 46. Santos Lacomba M,, Marcos Martin C,, Gallardo Galera JM,, et al. Aqueous humor and serum tumor necrosis factor-alpha in clinical uveitis. Ophthalmic Res. 2001; 33: 251–255. [DOI] [PubMed] [Google Scholar]
- 47. Chu CJ,, Herrmann P,, Carvalho LS,, et al. Assessment and in vivo scoring of murine experimental autoimmune uveoretinitis using optical coherence tomography. PLoS One. 2013; 8: e63002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gadjanski I,, Williams SK,, Hein K,, Sattler MB,, Bahr M,, Diem R. Correlation of optical coherence tomography with clinical and histopathological findings in experimental autoimmune uveoretinitis. Exp Eye Res. 2011; 93: 82–90. [DOI] [PubMed] [Google Scholar]
- 49. Kezic J,, Taylor S,, Gupta S,, Planck SR,, Rosenzweig HL,, Rosenbaum JT. Endotoxin-induced uveitis is primarily dependent on radiation-resistant cells and on MyD88 but not TRIF. J Leukoc Biol. 2011; 90: 305–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Poltorak A,, He X,, Smirnova I,, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998; 282: 2085–2088. [DOI] [PubMed] [Google Scholar]
- 51. Li Q,, Peng B,, Whitcup SM,, Jang SU,, Chan CC. Endotoxin induced uveitis in the mouse: susceptibility and genetic control. Exp Eye Res. 1995; 61: 629–632. [DOI] [PubMed] [Google Scholar]
- 52. Schiopu A,, Cotoi OS. S100A8 and S100A9: DAMPs at the crossroads between innate immunity, traditional risk factors, and cardiovascular disease. Mediators Inflamm. 2013; 2013: 828354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jiménez-Dalmaroni MJ,, Gerswhin ME,, Adamopoulos IE. The critical role of toll-like receptors—from microbial recognition to autoimmunity: a comprehensive review. Autoimmun Rev. 2016; 15: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Willermain F,, Rosenbaum JT,, Bodaghi B,, et al. Interplay between innate and adaptive immunity in the development of non-infectious uveitis. Prog Retin Eye Res. 2012; 31: 182–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Henao-Mejia J,, Elinav E,, Jin C,, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012; 482: 179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Doria A,, Zen M,, Bettio S,, et al. Autoinflammation and autoimmunity: bridging the divide. Autoimmun Rev. 2012; 12: 22–30. [DOI] [PubMed] [Google Scholar]
- 57. Rosenbaum JT,, Kim HW. Innate immune signals in autoimmune and autoinflammatory uveitis. Int Rev Immunol. 2013; 32: 68–75. [DOI] [PubMed] [Google Scholar]
- 58. Brownell I,, Ramirez-Valle F,, Sanchez M,, Prystowsky S. Evidence for mycobacteria in sarcoidosis. Am J Respir Cell Mol Biol. 2011; 45: 899–905. [DOI] [PMC free article] [PubMed] [Google Scholar]