Abstract
Purpose
Mice are commonly used to study conventional outflow physiology. This study examined how physical factors (hydration, temperature, and anterior chamber [AC] deepening) influence ocular perfusion measurements in mice.
Methods
Outflow facility (C) and pressure-independent outflow (Fu) were assessed by multilevel constant pressure perfusion of enucleated eyes from C57BL/6 mice. To examine the effect of hydration, seven eyes were perfused at room temperature, either immersed to the limbus in saline and covered with wet tissue paper or exposed to room air. Temperature effects were examined in 12 eyes immersed in saline at 20°C or 35°C. Anterior chamber deepening was examined in 10 eyes with the cannula tip placed in the anterior versus posterior chamber (PC). Posterior bowing of the iris (AC deepening) was visualized by three-dimensional histology in perfusion-fixed C57BL/6 eyes and by spectral-domain optical coherence tomography in living CD1 mice.
Results
Exposure to room air did not significantly affect C, but led to a nonzero Fu that was significantly reduced upon immersion in saline. Increasing temperature from 20°C to 35°C increased C by 2.5-fold, more than could be explained by viscosity changes alone (1.4-fold). Perfusion via the AC, but not the PC, led to posterior iris bowing and increased outflow.
Conclusions
Insufficient hydration contributes to the appearance of pressure-independent outflow in enucleated mouse eyes. Despite the large lens, AC deepening may artifactually increase outflow in mice. Temperature-dependent metabolic processes appear to influence conventional outflow regulation. Physical factors should be carefully controlled in any outflow studies involving mice.
Keywords: trabecular meshwork, glaucoma, mouse models, intraocular pressure, Schlemm's canal
Mice are a common animal model for studies of aqueous humor dynamics and outflow physiology. The anatomy of the conventional outflow pathway in mice is similar to that of humans with a continuous Schlemm's canal and lamellated trabecular meshwork.1 Like primates, mice possess a ciliary muscle that forms tendinous connections to the elastic fiber net of the trabecular meshwork and the inner wall endothelium of Schlemm's canal.2 Compounds that affect outflow facility in humans, including pilocarpine,2,3 TGF-β2,4,5 latanoprost6–8 prostaglandin EP4 receptor agonist,9–11 and sphingosine 1-phosphate,11,12 similarly affect outflow facility in mice. Recently, mice have been used to validate novel compounds that increase outflow facility based on hits from screening assays of cellular contractility.13
Numerous investigators have measured outflow facility in mice.2,5,6,8,11,14–26 However, on account of the small dimensions of the mouse eye and the low flow rates involved, there is greater potential for physical factors to influence ocular perfusion measurements in mice compared with larger species. For example, evaporation from the surface of the eye, which is more pronounced in smaller eyes that have a larger surface to volume ratio, may lead to dehydration of the corneoscleral shell and artifactually increase the apparent outflow rate. Importantly, this effect would manifest as a pressure-independent outflow during perfusion. Posterior bowing of the iris, known as anterior chamber (AC) deepening, artificially increases outflow facility by applying traction to the trabecular meshwork.27,28 Anterior chamber deepening typically occurs during ocular perfusion via the AC, when the pressure in the AC exceeds that in the posterior chamber (PC). The pressure difference causes the iris–lens channel to collapse like a 1-way valve, preventing pressure equilibration across the iris. Anterior chamber deepening can be prevented by perfusion via the PC, by creating a fluidic shunt across the iris,29 or by iridectomy.30 Note that AC deepening is not synonymous with ‘AC depth,' which represents the distance between the posterior cornea and anterior lens. Because mice have a relatively large crystalline lens, it has been proposed that AC deepening may be negligible in mice,18 but this has not been specifically examined. To address these gaps in knowledge, this study examined the influence of hydration and AC deepening on pressure-dependent and pressure-independent outflow in enucleated mouse eyes. We also examined the effect of temperature that could account for up to 40% variation in apparent outflow facility due to changes in water viscosity between room and physiological temperature.
Methods
Experimental Design
Separate perfusion experiments were designed to assess the effects of hydration, temperature, and AC deepening on pressure-dependent and pressure-independent outflow in enucleated C57BL/6 mouse eyes. To investigate the effect of hydration, eyes were perfused either covered with moist tissue paper and immersed to the limbus in isotonic saline at room temperature or covered with moist tissue paper but otherwise exposed to room air without immersion. To evaluate the effect of temperature, eyes were perfused either at room temperature or at 35°C while covered with moist tissue paper and immersed to the limbus in isotonic saline. To assess the effect of AC deepening, eyes were perfused with the tip of the cannulation needle placed in the AC or PC while covered with moist tissue paper and immersed to the limbus in isotonic saline at 35°C.
To visualize whether AC deepening (posterior iris bowing) occurred during ex vivo perfusion, three-dimensional (3D) reconstructions of the anterior segment were generated from enucleated eyes that were perfusion-fixed with the needle tip in the AC or PC in C57BL/6 mice. Three-dimensional reconstructions were based on several hundred serial sections acquired using a computer-controlled microtome and automated block-face imaging system. To visualize whether AC deepening occurs in vivo, spectral-domain optical coherence tomography (SD-OCT) was used to image the iridocorneal angle in living CD1 mice under anesthesia during AC pressurization. One eye of each CD1 mouse was surgically iridectomized to allow pressure equilibration across the iris, while the iris of the other eye was left untreated.
Animal Husbandry
C57BL/6 mice (Charles River UK Ltd., Margate, UK) between 8 and 14 weeks of age of either sex were used for ex vivo perfusions and for 3D reconstructions in perfusion-fixed eyes. CD1 mice (Jackson Laboratory, Bar Harbor, ME, USA) between 4 and 8 weeks of age of either sex were used for SD-OCT imaging. All mice were fed ad libitum, housed in clear cages at 21°C with a 12 hour light-dark cycle. Experiments were typically performed between 9 AM and 3 PM (lights on at 6 AM). All experiments were performed in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, under the authority of a UK Home Office project license for experiments done at Imperial College London and the Institutional Animal Care and Use Committee for experiments done at Duke University Medical School.
Ex Vivo Ocular Perfusion
Eyes were enucleated within 15 minutes after cervical dislocation and stored in PBS at 4°C to await perfusion, typically within 3 hours. For perfusion, eyes were affixed to a support platform using cyanoacrylate glue (Loctite; Henkel LTD, Hemel Hempstead, UK) applied to the posterior sclera. The support platform was either submerged in isotonic PBS or exposed to room air, depending on the experimental design (see above). All eyes were covered with tissue paper that was kept moist either by wicking from the saline bath or, for experiments where the eye was exposed to room air, by regular drops of saline delivered approximately once every 15 minutes; this latter procedure mimics that used in some of our earlier mouse eye perfusion studies.11,18,19 For temperature studies, the temperature was controlled using a custom-built heater and controller with a thermocouple placed near to the eye so as to maintain eye temperature at approximately 35°C. For room temperature perfusions, the controller was deactivated and the bath was allowed to equilibrate with room air that was typically 20°C.
Eyes were cannulated with a 33-G needle connected to a computer-controlled perfusion system optimized for mouse eyes, similar to that described previously.11,18,20 For needle tips placed in the PC, the needle tip was carefully threaded through the pupil and rotated such that the beveled end faced away from the iris so as to avoid potential blockage. Care was taken to avoid puncturing the iris, so as to avoid release of pigment that could obstruct outflow. All cannulations were performed using a 3-axis micromanipulator under a dissection microscope to visualize the position of the needle tip. The perfusion system included a computer controlled syringe pump (PhD Ultra; Harvard Apparatus, Holliston, MA, USA) holding a 25-μL syringe (GasTight; Hamilton, Reno, NV, USA) and a pressure transducer (142PC01G; Honeywell, Morristown, NJ, USA) to measure the perfusion pressure (PP) of the eye. A custom-written algorithm31 programmed in LabVIEW (National Instruments, Austin, TX, USA) was used to automatically adjust the syringe pump flow rate so as to set a user-defined PP . Eyes were perfused at sequential pressure steps, with at least 20 minutes at each step. For hydration and temperature studies, the sequential pressure steps were 4, 8, 15, and 25 mm Hg. For AC deepening studies, 11 pressure steps were used (8, 12, 15, 18, 21, 24, 27, 31, 34, 37, 40 mm Hg). The steady flow rate over the last 10 minutes of each step was averaged and plotted versus PP to calculate outflow facility (C) according to the modified Goldmann's equation
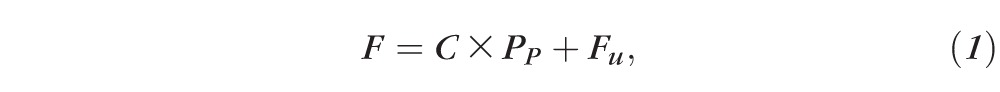 |
where F is the steady perfusion flow rate at each pressure step and Fu is the pressure-independent outflow rate (typically assumed to represent the unconventional outflow). Assuming that C is independent of PP , C may be estimated from Equation 1 based on the slope of the F-PP relationship. Fu is estimated as the extrapolated value of F at PP = 0. Equation 1 assumes that episcleral venous pressure and aqueous humor production are both zero, as appropriate for enucleated eyes, and that perfusion reaches steady state at each perfusion pressure. The perfusion fluid was Dulbecco's PBS containing calcium and magnesium and 5.5 mM glucose, and was filtered (0.22 μm) prior to use (collectively referred to as “DBG”). Hydration experiments used seven unpaired eyes, temperature experiments used 12 unpaired eyes, and AC deepening experiments used 10 unpaired eyes. Statistical analysis was performed using an unpaired, 2-tailed Student's t-test.
An additional five pairs of eyes were used to examine the effect of postmortem time on outflow facility, where one eye was perfused immediately after death and the fellow eye was perfused after enucleation and 3 hours of storage in PBS at 4°C, mimicking the storage conditions used in this study. The eyes were covered with moist tissue paper but otherwise exposed to room temperature air. The flow-pressure relationships and facility were very similar for the two cases (Supplementary Fig. S1), suggesting negligible postmortem changes in outflow facility over 3 hours.
Three-Dimensional Reconstructions of the Anterior Segment Ex Vivo
Three-dimensional reconstructions of the anterior segment were generated to visualize the shape of the iris and to determine whether AC deepening occurs during ex vivo perfusion of enucleated mouse eyes. Three-dimensional reconstructions were created based on serial sagittal sections through the eye acquired using a computer-controlled microtome and automated block-face imaging system that was developed by one coauthor (JCD) and was similar to previous designs.32,33 Images were acquired using episcopic fluorescent image capture where the excitation and emission wavelengths were selected to visualize the intrinsic tissue autofluorescence. Eyes were embedded in paraffin containing an opacifier dye to quench out-of-focus light and thereby obtain episcopic images of only that tissue lying in close proximity to the block face. This system allows visualization of the anterior segment anatomy with resolution comparable to conventional histology (Supplementary Fig. S2).
Enucleated eyes used for 3D reconstruction were perfusion-fixed with 4% paraformaldehyde (PFA) at 40 mm Hg with the needle tip placed either in the AC or PC (N = 1 or 2). As these eyes were not used for facility measurements, a small puncture or iridectomy was intentionally made in the iris for eyes with PC cannulations. Placing the needle tip in the PC or iridectomy eliminates the pressure differential across the iris that would otherwise drive AC deepening if the needle tip were placed in the AC.27,28 So that the eye was fixed at the desired pressure, the perfusion needle was backfilled with a bolus of 5 μL DBG; this allowed the eye to be pressurized and perfused with DBG prior to fixative entering the eye. The flow rate was recorded throughout the perfusion, revealing a decrease in flow rate as fixative entered the eye (Supplementary Fig. S3). The eye was considered adequately fixed when the flow rate reduced to approximately 60% of the initial stable value, consistent with the facility reduction observed following fixation in human eyes.29,34
Perfusion-fixed eyes were removed from the perfusion system and immersed immediately in 4% PFA for an additional 3 to 4 hours. Eyes were then washed in PBS and mounted in 1.8% agarose. The agarose block was then trimmed into a pyramidal shape such that the largest face was parallel to the sagittal plane through the eye; this was used to indicate the orientation of the eye for sagittal sectioning. Agarose-embedded eyes were dehydrated in graded solutions of ethanol (50%, 70%, 90%, and 100%). Eyes were cleared in xylene, then in xylene with 0.5% Sudan II, infiltrated with paraffin, and mounted in a specimen holder. Paraffin contained an opacifier (4% Sudan II dye) that blocked fluorescent emissions from tissue deep within the block so as to limit imaging to tissue at the block face.
Automated serial sections were cut and imaged at 1-μm steps using a Leica RM2165 microtome (Milton Keynes, UK), Nikon AZ100 microscope with a ×4 plan apo objective (NA 0.4; Melville, NY, USA) and ALTA U16M monochrome CCD camera (Apogee Instruments, Logan, UT, USA). Fluorescent excitation/emission filters (540–580 nm/600–660 nm) were chosen to match the autofluorescence spectra of the tissue as first characterized by a wavelength-scan on a confocal microscope (TCS SP5; Leica). The image field was 1.7 mm × 1.7 mm (4096 × 4096 pixels) centered on the AC, and images were downsampled 4-fold to yield a final voxel size of 1.7 μm × 1.7 μm × 1 μm. Semiautomated segmentation was used to define the contour of the iris and cornea, with features drawn manually in every tenth image using an electronic stylus, with automated interpolation in between. Three-dimensional reconstructions of the AC and iris were generated using Amira 3D software (FEI, Hillsboro, OR, USA). Typical reconstructions incorporated 1000 to 1500 serial sections spanning 1.0 to 1.5 mm.
SD-OCT Imaging of Anterior Segment in Vivo
Spectral-domain OCT imaging of the anterior segment was used to visualize the shape of the iris and to determine whether AC deepening occurs during ocular perfusion of living mice. Spectral-domain OCT was performed in six CD1 mice before and after AC cannulation and pressurization to 15, 25, or 45 mm Hg. One to 2 weeks prior to SD-OCT imaging, the left eye of six mice were surgically iridectomized to create an opening through the iris so as to theoretically eliminate any pressure gradient between the AC and PC, following established techniques.30 The right eye was not iridectomized and was used as an untreated control. For iridectomy, mice were anesthetized by intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg), and a drop of topical proparacaine. A straight approximately 2-mm clear-corneal wound following the limbus was made with a Beaver 75 blade (Beaver-Visitec International, Inc., Waltham, MA, USA) under an operating microscope, and the iris was grasped using 0.12 forceps. A small hole (∼0.5 mm) was cut through the iris using Vannas scissors (Bausch & Lomb Surgical, Inc., Rancho Cucamonga, CA, USA) creating an iridectomy that persisted until the time of perfusion (Supplementary Fig. S4). Erythromycin was applied to the cornea, and the animals were maintained on a warm water-circulating blanket to await recovery from anesthesia.
For SD-OCT imaging, mice were anesthetized as described above and placed on a custom-made OCT platform designed specifically for mice.23,30 Both the iridectomized and noniridectomized eyes were imaged sequentially, alternating the order between experiments. A pulled glass micropipette filled with PBS was inserted through the cornea into the AC using a micromanipulator. The micropipette was connected to a manometric column and pressure transducer (142PC05D; Honeywell) to adjust and monitor IOP. Anterior chamber deepening was visualized using a commercial SD-OCT system (Bioptigen Envisu R2200, Morrisville, NC, USA) with a 180 nm Superlum Broadlighter (Carrigtwohill, Ireland) source providing 2-μm axial resolution, and a 12-mm telecentric lens bore for anterior segment OCT imaging. The OCT probe was aimed at the inferior lateral limbus to visualize the corneoscleral angle, while IOP was set by the height of the manometric column. At each IOP step (15, 25, or 45 mm Hg), a sequence of repeated B-scans (each containing 1000 A-scans spanning 1 mm in lateral length) were captured, registered, and averaged to create a high signal-to-noise-ratio image of the anterior segment. Image registration was performed utilizing StackReg registration plug-in35 for ImageJ software (http://imagej.nih.gov/ij/; provided in the public domain by the National Institutes of Health, Bethesda, MD, USA).
Results
The Influence of Hydration
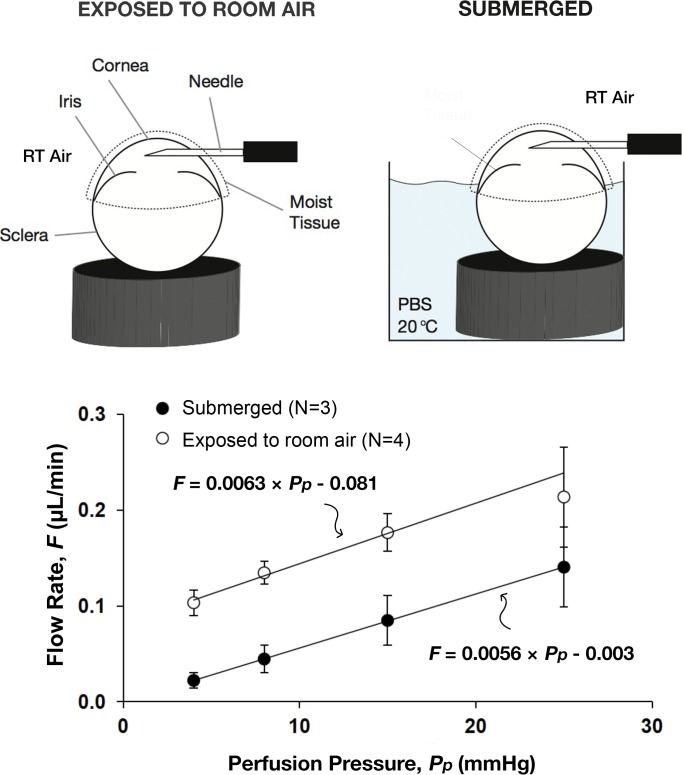
At each perfusion pressure, the flow rate was larger in the air-exposed eye compared with the immersed eye (P < 0.01 for 4, 8, 15 mm Hg; P = 0.13 for 25 mm Hg; Fig. 1). Likewise, the estimated flow rate at zero pressure (Fu) was larger in the air-exposed relative to the immersed eye (0.081 ± 0.030 vs. −0.0003 ± 0.004 μL/min; mean ± SD; N = 3 vs. 4; P = 0.011), and Fu was indistinguishable from zero in the immersed eye. Despite the offset in the flow rate, the slope of the flow-pressure relationship (C) was similar between immersed and air-exposed eyes (0.0056 ± 0.0016 vs. 0.0063 ± 0.0036 μL/min/mm Hg; P = 0.75). These data imply that insufficient hydration artifactually increases pressure-independent outflow and therefore estimates of Fu based on Equation 1, although no significant effect on C was observed.
Figure 1.
The flow-pressure relationship of mouse eyes perfused while exposed to room air or while immersed to the limbus in a bath of isotonic saline. In both cases, eyes were covered with moist tissue paper and perfused at room temperature (RT) via the AC. The zero-pressure intercept (representing the pressure-independent outflow rate, Fu) was nonzero in eyes exposed to room air, but was indistinguishable from zero in immersed eyes. The slope of the flow-pressure relationship (representing the pressure-dependent outflow facility, C) was similar in both cases. Error bars, SD.
The Influence of Temperature
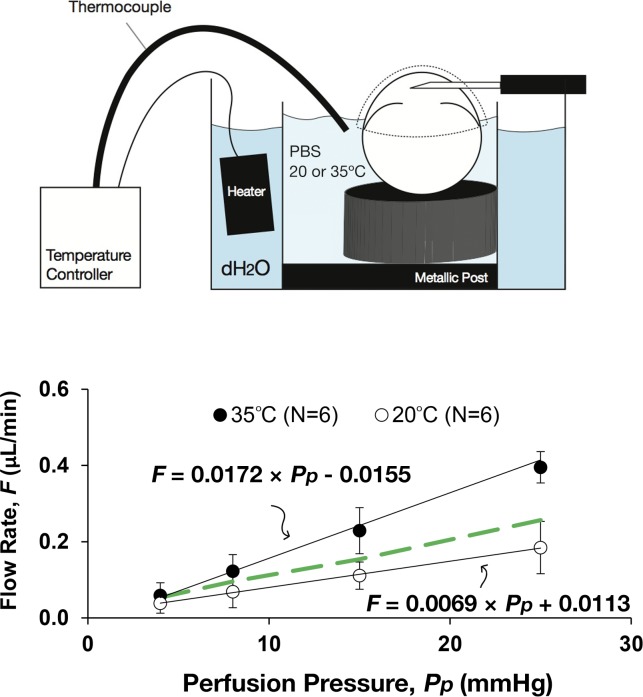
C was 2.5-fold larger in perfusions conducted at 35°C (0.0172 ± 0.0028 μL/min/mm Hg, N = 6) relative to room temperature at approximately 20°C (0.0069 ± 0.0021 μL/min/mm Hg, N = 6; P = 4 × 10−5; Fig. 2). The temperature-dependent increase in C exceeded the 1.4-fold increase predicted based on temperature-dependent changes in viscosity36 (P = 0.004), suggesting that temperature-dependent metabolic processes partly contribute to outflow regulation in mice. In contrast, Fu was unaffected by temperature in immersed eyes and was not statistically different from zero at either 35°C (−0.0155 ± 0.0385 μL/min, N = 6) or approximately 20°C (0.0113 ± 0.0248 μL/min, N = 6), consistent with the hydration results presented above. These data suggest that temperature significantly influences estimates of C but has little effect on estimates of Fu.
Figure 2.
The flow-pressure relationship of paired mouse eyes perfused at room temperature (∼20°C) or physiological temperature (35°C). In both cases, eyes were immersed to the limbus in isotonic saline, covered with moist tissue paper and perfused through the AC. The slope of the flow-pressure relationship (representing the pressure-dependent outflow facility, C) increased from room to physiological temperature, but the increase was more than could be attributed to viscosity changes alone (indicated by the green dashed line). The zero-pressure intercept (representing the pressure-independent outflow rate, Fu) was similar in both cases and not statistically different from zero in either case. Error bars, SD.
The Influence of Anterior Chamber Deepening
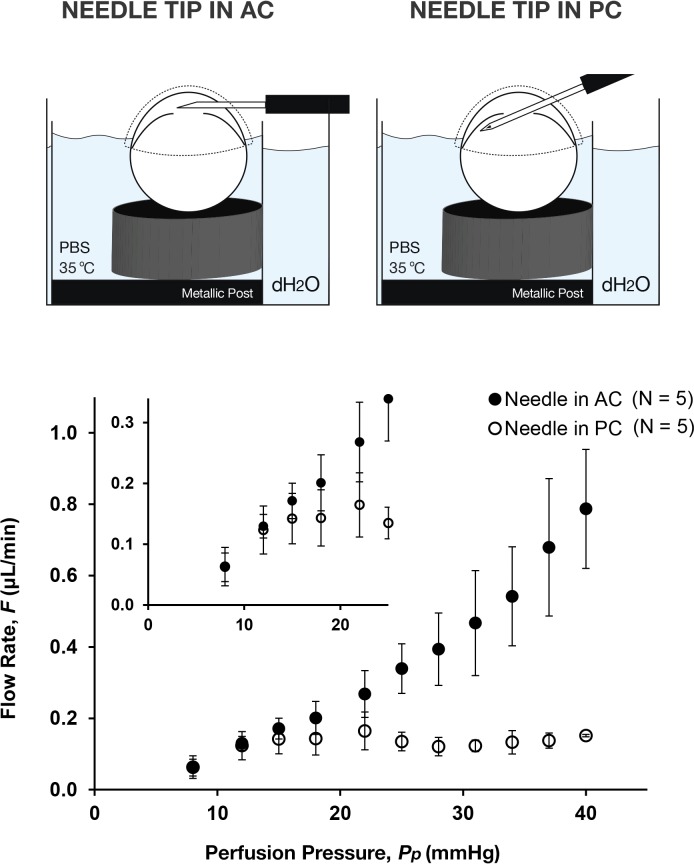
With the needle tip positioned in the AC, the perfusion flow rate increased incrementally with increasing pressure up to 40 mm Hg (Fig. 3). The form of the flow-pressure relationship was nonlinear, trending upward with increasing pressure, and this nonlinearity was most pronounced above 22 mm Hg. When the needle tip was positioned in the PC, however, the perfusion flow rate reached a plateau at approximately 15 mm Hg without any further apparent increase in flow rate for perfusion pressures up to 40 mm Hg. The difference in flow rate between AC and PC cannulations was statistically significant for all pressures above 25 mm Hg (P < 0.021). Likewise, the range of measured flow rates (as indicated by the size of the error bars) increased with perfusion pressure for the AC cannulations, but remained relatively constant for the PC cannulations, suggesting that there is larger variability in the flow rate between eyes with AC cannulation. These data demonstrate that the position of the needle tip in the AC versus PC has a strong influence on perfusion measurements in mice and would most likely influence estimates of outflow facility, particularly for perfusion pressures greater than 15 mm Hg.
Figure 3.
The flow-pressure relationship of mouse eyes perfused with the needle tip in the AC or PC. In both cases, eyes were immersed to the limbus in isotonic saline, covered with moist tissue paper and perfused at physiological temperature (35°C). The flow-pressure relationship was nonlinear in both cases, trending upward above approximately 22 mm Hg with an AC cannulation but reaching a plateau above approximately 15 mm Hg with a PC cannulation. Inset shows a magnified version of the same flow-pressure data near the point where AC and PC cannulations diverge. Error bars, SD.
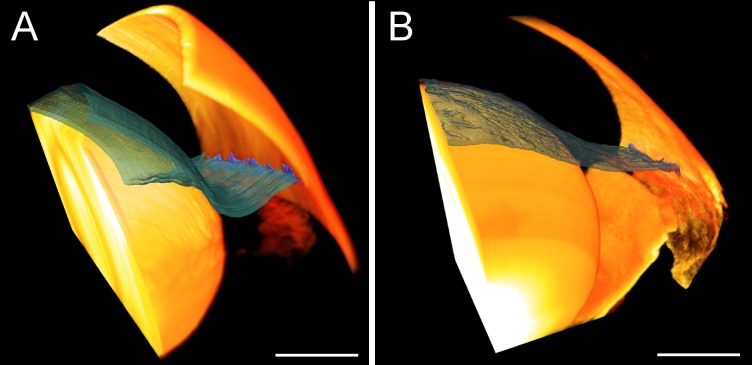
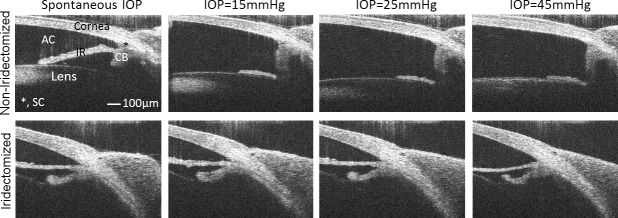
To visualize AC deepening, the position of the iris was imaged in perfusion-fixed and living mouse eyes with and without PC cannulation and/or iridectomy. Histologic examination of perfusion-fixed eyes revealed posterior bowing of the iris when the perfusion was performed with the needle tip positioned in the AC without iridectomy (Fig. 4A, Supplementary Fig. S5A). When the cannula tip was positioned in the PC with iridectomy, however, AC deepening was eliminated and there was no posterior bowing of the iris (Fig. 4B, Supplementary Fig. S5B). Similar observations were found in living mice by SD-OCT where posterior displacement of the iris was observed in noniridectomized eyes for perfusion pressures of 15 mm Hg or more (Fig. 5). In contrast, no posterior iris displacement was observed in the fellow iridectomized eyes, where pressure presumably equilibrated between the AC and PC. These data reveal that AC deepening occurs during perfusion of mouse eyes, both in vivo and ex vivo, unless precautions are taken to eliminate the pressure differential across the iris, and AC deepening may significantly influence the measured value of outflow facility.
Figure 4.
Anterior chamber deepening in enucleated mouse eyes as visualized by 3D reconstructions of the anterior segment. Eyes perfused via the AC (A) show posterior iris displacement and an enlarged iridocorneal angle relative to eyes perfused via PC (B). Both eyes were perfusion-fixed at 40 mm Hg. The iris is shown in blue-green and the cornea and lens in yellow. Scale bars: 0.5 mm. Individual sections used to generate the 3D reconstructions are shown in Supplementary Fig. S5.
Figure 5.
Anterior chamber deepening in living CD1 mice as visualized by SD-OCT. Some eyes were surgically iridectomized 1 to 2 weeks prior to imaging to eliminate the pressure differential across the iris. Posterior iris displacement was observed in noniridectomized eyes for all pressures greater than spontaneous IOP (top row), while posterior iris displacement was eliminated in the iridectomized eyes over the same pressure range (bottom row). The asterisk shows the location of Schlemm's canal. All panels are presented at the same magnification. CB, ciliary body; IR, iris.
Discussion
This study showed that hydration, temperature, and AC deepening strongly influence the measured flow-pressure relationship in enucleated mouse eyes. Exposing the eye to room air during perfusion increased the pressure-independent outflow, an effect that was eliminated by immersing the eye in isotonic saline. Increasing eye temperature from room to physiological temperature led to an increase in outflow facility that exceeded the increase attributable to reduced perfusate viscosity. Anterior chamber deepening appeared to alter the shape of the flow-pressure relationship, and AC deepening occurred regardless of whether the perfusion was performed in vivo or ex vivo, unless an open communication was made across the iris. These results reveal that ocular perfusion in mice is sensitive to physical factors that must be carefully controlled in order to accurately assess murine outflow physiology.
Although mice are now commonly used in studies of outflow physiology, there is a key unresolved question regarding the magnitude of unconventional (i.e., nontrabecular) outflow in mice. This question is important because unconventional outflow in humans typically accounts for less than one-half of total outflow,37–39 and human IOP depends more strongly on conventional outflow. If mice are to be considered a reasonable model for aqueous humor dynamics in humans, then the majority of outflow in mice should also pass through the conventional (i.e., trabecular) outflow pathway. However, several studies,6,15,16,40 including early studies from our own group,18,19 have suggested that unconventional outflow in mice comprises the majority of total outflow, up to 80% or more according to some reports.6,15,16 Estimates of unconventional outflow have been reported to be larger in younger mice,26 consistent with the age-related decline of unconventional outflow reported in primates,41,42 although there may be variability between mouse strains.26 Nevertheless, most higher estimates of unconventional outflow in mice6,15,16,18,19,40 were based on indirect perfusion-based estimates of Fu, defined as the apparent pressure-independent outflow that was determined by fitting Goldmann's equation or Equation 1 to the measured flow-pressure perfusion data.
Insufficient hydration may lead to a significant artificial elevation in pressure-independent outflow, as demonstrated in the current study, wherein immersing the eye in isotonic saline drastically reduced Fu, presumably by eliminating evaporation from the ocular surface. It remains an open question whether hydration influences in vivo perfusions, particularly if the eye is exposed to room air or fixed open without hydrating drops, although the conjunctiva and adnexal tissues might limit changes in hydration state in vivo. Furthermore, indirect estimation of Fu typically assumes that outflow facility is independent of pressure, which would be consistent with a linear flow-pressure relationship. Nonlinearity associated with AC deepening or Schlemm's canal collapse (see below) would therefore introduce errors in the estimation of Fu. We therefore conclude that indirect assessments of pressure-independent outflow, at least in enucleated eyes, are sensitive to physical factors associated with the perfusion, and future work should consider how these factors affect in vivo estimates of unconventional outflow.
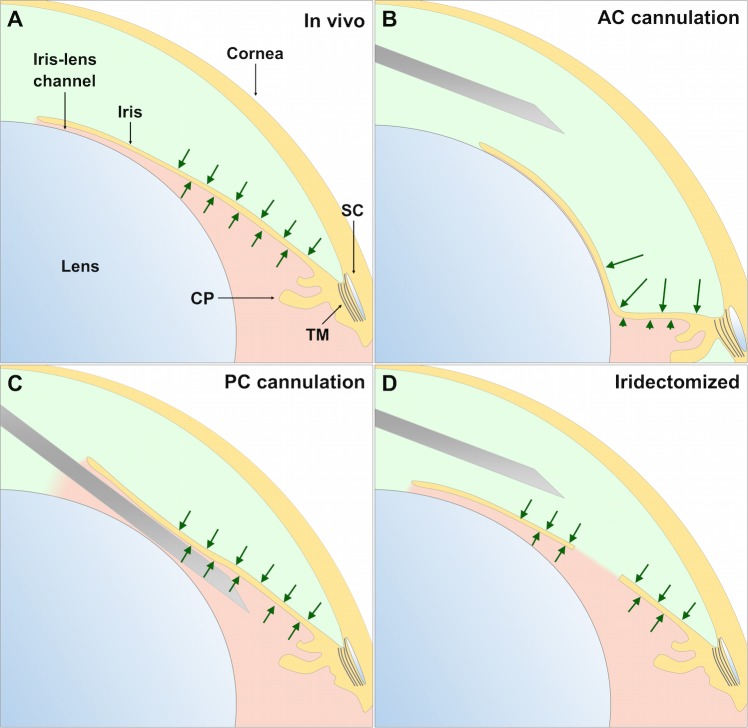
Figure 6 illustrates schematically the mechanism by which iridectomy or PC cannulation prevents AC deepening by neutralizing the pressure gradient across the iris. In vivo, aqueous humor flows from the PC to the AC maintaining patency of the iris-lens channel, resulting in equivalent pressures on either side of the iris (Fig. 6A). When enucleated eyes are perfused through the AC, there is no inflow and the iris-lens channel collapses, creating an effective 1-way valve. As fluid is perfused into the AC, the AC pressure exceeds that in the PC, and the resulting pressure difference across the iris drives AC deepening (Fig. 6B). For in vivo cannulations, there is both inflow from the PC and perfusion via the AC. This creates a complex mechanical environment where the pressure gradient across the iris depends on the balance between the flow rates, and based on our present data it appears that AC deepening may indeed occur during AC cannulation in vivo under ketamine/xylazine anesthesia. Perfusion through the PC (Fig. 6C) or iridectomy (Fig. 6D) eliminates AC deepening by maintaining an open channel across the iris that neutralizes the pressure gradient between the AC and PC. In this study, AC deepening was observed in mice of different ages, strains, and sex, both ex vivo and in vivo, despite the large crystalline lens, and AC deepening has been observed in other species including humans.27–29 It thus seems most probable that the mechanisms responsible for AC deepening are associated with the mechanics of AC cannulation, as opposed to subtle anatomical differences between mouse strains, ages, or sexes.
Figure 6.
An illustration of the mechanism of AC deepening and strategies to eliminate AC deepening in mice. Light green indicates AC and light pink indicates the PC. (A) In vivo there is no pressure difference across the iris (indicated by equal sized arrows), as aqueous humour secreted from the ciliary processes (CP) passes from the PC to the AC through the iris–lens channel. (B) Perfusion via the AC elevates the pressure acting on the anterior surface of the iris relative to that in the PC (arrow length implies magnitude), inducing posterior bowing of the iris and collapse of the iris–lens channel. Deformation of the iris applies traction to the trabecular meshwork, artifactually increasing outflow facility. (C) Perfusion via the PC emulates aqueous humour secretion and allows the iris–lens channel to remain open throughout its entire circumference. (D) Surgical iridectomy produces an artificial shunt between the AC and PC, equalizing the pressures, and thereby negating AC deepening. Needle scaled by approximately 1:3 for clarity.
By applying traction to the TM, AC deepening increases outflow facility. In human eyes, the effect of AC deepening on outflow facility can be eliminated by cyclodialysis,29 suggesting that the ciliary muscle mediates the effect on outflow by transmitting tension from the iris to the trabecular meshwork. In mice, the ciliary muscle contains only a few longitudinal fibers2,43 without circular or radial fibers as observed in primates,44 and mice, like primates,45 exhibit tendinous and elastic fiber connections tethering the longitudinal ciliary muscle to the trabecular meshwork and ciliary body/iris root.2 In mice, as in primates,3 contraction of the ciliary muscle by pilocarpine applies traction to the trabecular meshwork, increases outflow facility,2,30 widens Schlemm's canal lumen,30 and decreases IOP.30,46,47 Presumably, these same tendons and elastic fiber connections mediate iridial tension generated during AC deepening in mice so as to increase trabecular outflow.
A nonlinear increasing flow-pressure relationship was observed in enucleated mouse eyes perfused via the AC, consistent with the facility increase attributed to AC deepening in enucleated human eyes.27,28 When perfused through the PC, however, the flow increased for pressures up to approximately 15 mm Hg in enucleated mouse eyes, but then exhibited a plateau with a negligible further increase in flow for further increases in pressure. This behavior is consistent with the pressure-dependent decrease in outflow facility observed in human eyes after eliminating AC deepening,28,48 which has been attributed to Schlemm's canal collapse.48 Although we were unable to resolve Schlemm's canal dimensions, a recent study by Li et al.,30 using customized image processing techniques for SD-OCT in living mice, reported almost complete collapse at 20 mm Hg. Furthermore, an early computational study predicted a flow-pressure relationship that reaches a plateau once Schlemm's canal becomes fully collapsed.49 Future studies should more closely examine the relationships between Schlemm's canal collapse and outflow facility in mice, as well as how the resulting nonlinearity in the flow-pressure relationship influences indirect estimates of pressure-independent outflow.
Regardless of whether perfusions are performed in vivo or ex vivo, AC deepening may confound ocular perfusion measurements in mice unless precautions are taken to eliminate the pressure difference across the iris. Possible approaches include placing the needle tip in the PC or performing an iridectomy or iridotomy. Alternatively, our data suggest that the influence of AC deepening on outflow may be reduced if perfusion pressures are kept below the pressure at which the flow-pressure relationships between AC and PC cannulations appear to diverge. Based on our SD-OCT data, AC deepening may indeed occur in living mice, but as we did not perform in vivo perfusion measurements, the influence of AC deepening on outflow measurements in vivo requires further investigation.
In the temperature-controlled experiments, outflow facility was 2.5-fold greater at physiological temperature compared with room temperature. Importantly, this facility increase was more than could be attributed to temperature-dependent changes in perfusate viscosity (that would predict only a 1.4-fold increase36). This suggests a possible metabolic component to trabecular outflow in mice that is suppressed at room temperature. Alternatively, the biomechanical stiffness of cells comprising the trabecular outflow pathway may change in response to temperature so as to influence outflow resistance.50 Regardless of the mechanism, these data conflict with prior studies in human eyes concluding that any temperature-dependent change in outflow facility can be attributed almost entirely to changes in perfusate viscosity51 and that inhibitors of cellular metabolism have little effect on outflow facility.52,53 While we cannot explain why mice exhibit a temperature-dependent facility, the discrepancy with prior studies merits further investigation and may be attributable to species differences or to differences in the postmortem time (prior studies used human eyes within 48 hours of death51 compared the current study that used mouse eyes within 3 hours of death).
In vivo perfusions undoubtedly provide a better model, relative to ex vivo, of the physiological ocular environment including all factors involved in aqueous humor dynamics. However, the increased complexity of the in vivo environment complicates measurements of individual parameters. An advantage of ex vivo perfusions is that episcleral venous pressure and aqueous humor inflow are reduced to zero upon enucleation. The Goldmann's equation thereby reduces to Equation 1 with only two unknowns (C and Fu), rather than four unknowns as for the in vivo case (C, Fu, Pe, and Fin). While various methods have been proposed to estimate all parameters of aqueous humor dynamics within individual living mice,8,15 such measurements are difficult and highly dependent on the validity of several assumptions. Additional factors such as innervation and blood supply, which may affect ciliary muscle tone and choroidal blood volume, would further complicate in vivo measurements. Ex vivo perfusions, however, require that eyes be removed from the orbit and affixed to a support platform, and thus small changes in ocular geometry or the presence of an adhesive could influence ex vivo measurements of facility. However, as a similar mounting procedure was applied to all eyes, its effect would be unlikely to diminish the ability to resolve differences between eyes. Extended postmortem times could introduce hypoxia or tissue degradation, although these effects appear to be negligible in freshly extracted tissue (i.e., perfusions commencing within 3 hours of enucleation; Supplementary Fig. S1).
In conclusion, ocular perfusion measurements in mice are sensitive to a number of physical factors that may significantly influence assessment of pressure-dependent and pressure-independent outflow. These studies clarify valuable guidelines for future perfusion studies. Ocular perfusions should be conducted at physiological temperature with the eyes kept hydrated by immersion in isotonic saline. For in vivo perfusions where immersion is impractical, investigators must be aware that evaporative losses or dehydration of the eye may contribute to the appearance of pressure-independent outflow, particularly if the eyelids are fixed open or if the eye is proptosed during perfusion. Anterior chamber deepening may be avoided by performing a surgical iridectomy, iridotomy, or by perfusing through the PC. Alternatively, it might be possible to reduce the effects of AC deepening by maintaining low perfusion pressures, although further studies are necessary to investigate AC deepening and its effect on outflow both in vivo and ex vivo.
Supplementary Material
Acknowledgments
The authors thank Juan Reynaud for technical support and expertise for the 3D automated histology system. The authors thank Larry Kagemann, PhD, for his helpful advice regarding OCT in mice. The authors are grateful for the assistance of Michel Boussommier-Calleja for the drawings shown in Figures 1 through 3.
Supported by grants from National Glaucoma Research Program of The BrightFocus Foundation (Formerly the American Health Assistance Foundation; Clarksburg, MD, USA), the National Eye Institute (EY022359 to WDS and DRO and EY018152 to JCD; Bethesda, MD, USA), an International Research Scholar Award from Research to Prevent Blindness (DRO; New York, NY, USA), an Entente Cordiale studentship managed by the British Council (ABC; Paris, France) and Medical Engineering Solutions in Osteoarthritis Centre of Excellence funded by the Wellcome Trust and the EPSRC (088844/Z/09/Z; London, UK).
Disclosure: A. Boussommier-Calleja, None; G. Li, None; A. Wilson, None; T. Ziskind, None; O.E. Scinteie, None; N.E. Ashpole, None; J.M. Sherwood, None; S. Farsiu, None; P. Challa, None; P. Gonzalez, None; J.C. Downs, None; C.R. Ethier, None; W.D. Stamer, None; D.R. Overby, None
References
- 1. Smith RS,, Zabaleta A,, Savinova OV,, John SW. The mouse anterior chamber angle and trabecular meshwork develop without cell death. BMC Dev Biol. 2001; 1: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Overby DR,, Bertrand J,, Schicht M,, Paulsen F,, Stamer WD,, Lütjen-Drecoll E. The structure of the trabecular meshwork, its connections to the ciliary muscle, and the effect of pilocarpine on outflow facility in mice. Invest Ophthalmol Vis Sci. 2014; 55: 3727–3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bárány EH. The mode of action of pilocarpine on outflow resistance in the eye of a primate (Cercopithecus ethiops). Invest Ophthalmol. 1962; 1: 712–727. [PubMed] [Google Scholar]
- 4. Gottanka J,, Chan D,, Eichhorn M,, Lütjen-Drecoll E,, Ethier CR. Effects of TGF- β2 in perfused human eyes. Invest Ophthalmol Vis Sci. 2004; 45: 153–158. [DOI] [PubMed] [Google Scholar]
- 5. Shepard AR,, Millar JC,, Pang I-H,, Jacobson N,, Wang WH,, Clark AF. Adenoviral gene transfer of active human transforming growth factor-β2 elevates intraocular pressure and reduces outflow facility in rodent eyes. Invest Ophthalmol Vis Sci. 2010; 51: 2067–2076. [DOI] [PubMed] [Google Scholar]
- 6. Crowston JG,, Aihara M,, Lindsey JD,, Weinreb RN. Effect of latanoprost on outflow facility in the mouse. Invest Ophthalmol Vis Sci. 2004; 45: 2240–2245. [DOI] [PubMed] [Google Scholar]
- 7. Lim KS,, Nau CB,, O'Byrne MM,, et al. Mechanism of action of bimatoprost, latanoprost, and travoprost in healthy subjects. A crossover study. Ophthalmology. 2008; 115: 790–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Millar JC,, Clark AF,, Pang I-H. Assessment of aqueous humor dynamics in the mouse by a novel method of constant-flow infusion. Invest Ophthalmol Vis Sci. 2011; 52: 685–694. [DOI] [PubMed] [Google Scholar]
- 9. Woodward DF,, Nilsson SFE,, Toris CB,, Kharlamb AB,, Nieves AL,, Krauss AH-P. Prostanoid EP4 receptor stimulation produces ocular hypotension by a mechanism that does not appear to involve uveoscleral outflow. Invest Ophthalmol Vis Sci. 2009; 50: 3320–3328. [DOI] [PubMed] [Google Scholar]
- 10. Millard LH,, Woodward DF,, Stamer WD. The role of the prostaglandin EP4 receptor in the regulation of human outflow facility. Invest Ophthalmol Vis Sci. 2011; 52: 3506–3513. [DOI] [PubMed] [Google Scholar]
- 11. Boussommier-Calleja A,, Bertrand J,, Woodward DF,, Ethier CR,, Stamer WD,, Overby DR. Pharmacologic manipulation of conventional outflow facility in ex vivo mouse eyes. Invest Ophthalmol Vis Sci. 2012; 53: 5838–5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sumida GM,, Stamer WD. S1P2 receptor regulation of sphingosine-1-phosphate effects on conventional outflow physiology. Am J Physiol. 2011; 300: C1164–C1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Park CY,, Zhou EH,, Tambe D,, et al. High-throughput screening for modulators of cellular contractile force. Integr Biol. 2015; 7: 1318–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang D,, Vetrivel L,, Verkman AS. Aquaporin deletion in mice reduces intraocular pressure and aqueous fluid production. J Gen Physiol. 2002; 119: 561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aihara M,, Lindsey JD,, Weinreb RN. Aqueous humor dynamics in mice. Invest Ophthalmol Vis Sci. 2003; 44: 5168–5173. [DOI] [PubMed] [Google Scholar]
- 16. Zhang Y,, Davidson BR,, Stamer WD,, Barton JK,, Marmorstein LY,, Marmorstein AD. Enhanced inflow and outflow rates despite lower IOP in bestrophin-2-deficient mice. Invest Ophthalmol Vis Sci. 2009; 50: 765–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Camras LJ,, Sufficool KE,, Camras CB,, Fan S,, Liu H,, Toris CB. Duration of anesthesia affects intraocular pressure, but not outflow facility in nice. Curr Eye Res. 2010; 35: 819–827. [DOI] [PubMed] [Google Scholar]
- 18. Lei Y,, Overby DR,, Boussommier-Calleja A,, Stamer WD,, Ethier CR. Outflow physiology of the mouse eye: pressure dependence and washout. Invest Ophthalmol Vis Sci. 2011; 52: 1865–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stamer WD,, Lei Y,, Boussommier-Calleja A,, Overby DR,, Ethier CR. eNOS a pressure-dependent regulator of intraocular pressure. Invest Ophthalmol Vis Sci. 2011; 52: 9438–9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boussommier-Calleja A,, Overby DR. The influence of genetic background on conventional outflow facility in mice. Invest Ophthalmol Vis Sci. 2013; 54: 8251–8258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kumar S,, Shah S,, Tang HM,, Smith M,, Borrás T,, Danias J. Tissue plasminogen activator in trabecular meshwork attenuates steroid induced outflow resistance in mice. PLoS One. 2013; 8: e72447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kumar S,, Shah S,, Deutsch ER,, Tang HM,, Danias J. Triamcinolone acetonide decreases outflow facility in C57BL/6 mouse eyes. Invest Ophthalmol Vis Sci. 2013; 54: 1280–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li G,, Farsiu S,, Qiu J,, et al. Disease progression in iridocorneal angle tissues of BMP2-induced ocular hypertensive mice with optical coherence tomography. Mol Vis. 2014; 20: 1695–1709. [PMC free article] [PubMed] [Google Scholar]
- 24. Overby DR,, Bertrand J,, Tektas O-Y,, et al. Ultrastructural changes associated with dexamethasone-induced ocular hypertension in mice. Invest Ophthalmol Vis Sci. 2014; 55: 4922–4933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ko MK,, Yelenskiy A,, Gonzalez JM,, Tan JCH. Feedback-controlled constant-pressure anterior chamber perfusion in live mice. Mol Vis. 2014; 20: 163–170. [PMC free article] [PubMed] [Google Scholar]
- 26. Millar JC,, Phan TN,, Pang I-H,, Clark AF. Strain and age effects on aqueous humor dynamics in the mouse. Invest Ophthalmol Vis Sci. 2015; 56: 5764–5776. [DOI] [PubMed] [Google Scholar]
- 27. Ellingsen BA,, Grant WM. The relationship of pressure and aqueous outflow in enucleated human eyes. Invest Ophthalmol. 1971; 10: 430–437. [PubMed] [Google Scholar]
- 28. Brubaker RF. The effect of intraocular pressure on conventional outflow resistance in the enucleated human eye. Invest Ophthalmol Vis Sci. 1975; 14: 286–292. [PubMed] [Google Scholar]
- 29. Grant WM. Experimental aqueous perfusion in enucleated human eyes. Arch Ophthalmol. 1963; 69: 783–801. [DOI] [PubMed] [Google Scholar]
- 30. Li G,, Farsiu S,, Chiu SJ,, et al. Pilocarpine-induced dilation of Schlemm's canal and prevention of lumen collapse at elevated intraocular pressures in living mice visualized by OCT. Invest Ophthalmol Vis Sci. 2014; 55: 3737–3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Overby D,, Gong H,, Qiu G,, Freddo TF,, Johnson M. The mechanism of increasing outflow facility during washout in the bovine eye. Invest Ophthalmol Vis Sci. 2002; 43: 3455–3464. [PubMed] [Google Scholar]
- 32. Weninger WJ,, Mohun T. Phenotyping transgenic embryos: a rapid 3-D screening method based on episcopic fluorescence image capturing. Nat Genet. 2002; 30: 59–65. [DOI] [PubMed] [Google Scholar]
- 33. Rosenthal J,, Mangal V,, Walker D,, Bennett M,, Mohun TJ,, Lo CW. Rapid high resolution three dimensional reconstruction of embryos with episcopic fluorescence image capture. Birth Defects Res C Embryo Today. 2004; 72: 213–223. [DOI] [PubMed] [Google Scholar]
- 34. Sit AJ,, Coloma FM,, Ethier CR,, Johnson M. Factors affecting the pores of the inner wall endothelium of Schlemm's canal. Invest Ophthalmol Vis Sci. 1997; 38: 1517–1525. [PubMed] [Google Scholar]
- 35. Thévenaz P,, Ruttimann UE,, Unser M. A pyramid approach to subpixel registration based on intensity. IEEE Trans Image Process. 1998; 7: 27–41. [DOI] [PubMed] [Google Scholar]
- 36. Haynes WM. CRC Handbook of Chemistry and Physics. Boca Raton: CRC Press; 2013. [Google Scholar]
- 37. Bill A,, Phillips CI. Uveoscleral drainage of aqueous humour in human eyes. Exp Eye Res. 1971; 12: 275–281. [DOI] [PubMed] [Google Scholar]
- 38. Toris CB,, Koepsell SA,, Yablonski ME,, Camras CB. Aqueous humor dynamics in ocular hypertensive patients. J Glaucoma. 2002; 11: 253–258. [DOI] [PubMed] [Google Scholar]
- 39. Nau CB,, Malihi M,, McLaren JW,, Hodge DO,, Sit AJ. Circadian variation of aqueous humor dynamics in older healthy adults. Invest Ophthalmol Vis Sci. 2013; 54: 7623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee YS,, Tresguerres M,, Hess K,, et al. Regulation of anterior chamber drainage by bicarbonate-sensitive soluble adenylyl cyclase in the ciliary body. J Biol Chem. 2011; 286: 41353–41358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Toris CB,, Yablonski ME,, Wang YL,, Camras CB. Aqueous humor dynamics in the aging human eye. Am J Ophthalmol. 1999; 127: 407–412. [DOI] [PubMed] [Google Scholar]
- 42. Gabelt BT,, Gottanka J,, Lütjen-Drecoll E,, Kaufman PL. Aqueous humor dynamics and trabecular meshwork and anterior ciliary muscle morphologic changes with age in rhesus monkeys. Invest Ophthalmol Vis Sci. 2003; 44: 2118–2125. [DOI] [PubMed] [Google Scholar]
- 43. Ko MK,, Tan JCH. Contractile markers distinguish structures of the mouse aqueous drainage tract. Mol Vis. 2013; 19: 2561–2570. [PMC free article] [PubMed] [Google Scholar]
- 44. Tamm ER,, Lütjen-Drecoll E. Ciliary body. Microsc Res Tech. 1996; 33: 390–439. [DOI] [PubMed] [Google Scholar]
- 45. Rohen JW,, Futa R,, Lütjen-Drecoll E. The fine structure of the cribriform meshwork in normal and glaucomatous eyes as seen in tangential sections. Invest Ophthalmol Vis Sci. 1981; 21: 574–585. [PubMed] [Google Scholar]
- 46. Avila MY,, Carré DA,, Stone RA,, Civan MM. Reliable measurement of mouse intraocular pressure by a servo-null micropipette system. Invest Ophthalmol Vis Sci. 2001; 42: 1841–1846. [PubMed] [Google Scholar]
- 47. Akaishi T,, Odani-kawabata N,, Ishida N,, Nakamura M. Ocular hypotensive effects of anti-glaucoma agents in mice. J Ocul Pharmacol Ther. 2009; 25: 401–408. [DOI] [PubMed] [Google Scholar]
- 48. Moses RA. The effect of intraocular pressure on resistance to outflow. Surv Ophthalmol. 1977; 22: 88–100. [DOI] [PubMed] [Google Scholar]
- 49. Johnson MC,, Kamm RD. The role of Schlemm's canal in aqueous outflow from the human eye. Invest Ophthalmol Vis Sci. 1983; 24: 320–325. [PubMed] [Google Scholar]
- 50. Overby DR,, Zhou EH,, Vargas-Pinto R,, et al. Altered mechanobiology of Schlemm's canal endothelial cells in glaucoma. Proc Natl Acad of Sci U S A. 2014; 111: 13876–13881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. VanBuskirk EM,, Grant WM. Influence of temperature and the question of involvement of cellular metabolism in aqueous outflow. Am J Ophthalmol. 1974; 77: 565–572. [DOI] [PubMed] [Google Scholar]
- 52. Bárány EH. In vitro studies of the resistance to flow through the angle of the anterior chamber. Acta Soc Med Upsal. 1954; 59: 260–276. [PubMed] [Google Scholar]
- 53. Epstein DL,, Hashimoto JM,, Anderson PJ,, Grant WM. Effect of iodoacetamide perfusion on outflow facility and metabolism of the trabecular meshwork. Invest Ophthalmol Vis Sci. 1981; 20: 625–631. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.