Abstract
We report a Rh-catalyzed, enantioselective silylation of arene C–H bonds directed by a (hydrido)silyl group. (Hydrido)silyl ethers that are formed in situ by hydrosilylation of benzophenone or its derivatives undergo asymmetric C–H silylation in high yield with excellent enantioselectivity in the presence of [Rh(cod)Cl]2 and a chiral bisphosphine ligand. The stereoselectivity of this process also allows enantioenriched diarylmethanols to react with site selectivity at one aryl group over the other. Enantioenriched benzoxasiloles from the silylation process undergo a range of transformations to form C–C, C–O, C–I, or C–Br bonds.
Functionalization of C–H bonds with main-group reagents such as boranes and silanes has become a common synthetic method due to the high regioselectivity of the reaction and diverse applications of the products.1,2 Although some of these reactions generate chiral products, setting stereogenic carbon atoms by the enantioselective functionalization of a C–H bond with such main-group reagents has not been achieved. Takai reported an asymmetric Rh-catalyzed C–H silylation reaction to set a stereogenic silicon center of a spirocyclic silane with up to 81% enantiomeric excess (ee),3 and Shibata and He both reported the silylation of C–H bonds in ferrocenes to generate planar chiral compounds with moderate to high ee’s.4 However, application of the silylation to enantioselective formation of molecules related to products more commonly used in medicinal chemistry has not been reported.5,6
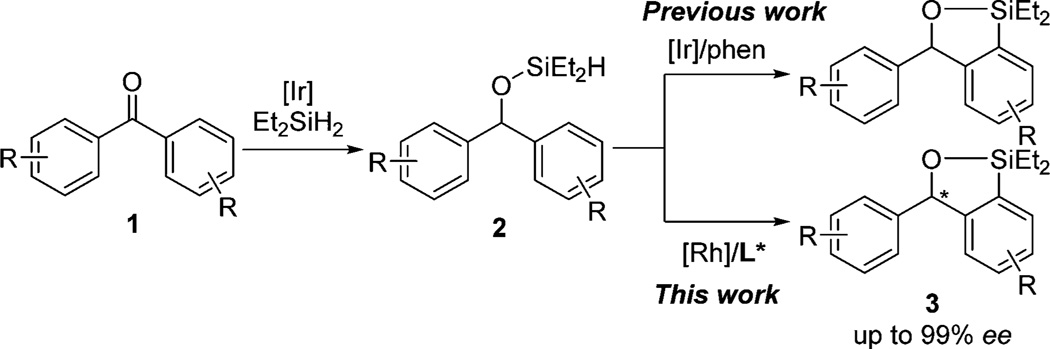
We recently reported the Ir-catalyzed silylation of aromatic C–H bonds to form benzoxasiloles.7 In this reaction, a (hydrido)silyl group is generated in situ from dehydrogenative silylation of an alcohol or hydrosilylation of a ketone and serves as the directing element for C–H activation of the arene (Scheme 1). Benzophenone, a substrate containing enantiotopic phenyl rings, underwent the C–H silylation, creating the potential to develop an enantioselective version of this process. With the appropriate combination of a metal precursor and chiral ligand, an enantioselective C–H silylation could set the stereogenic carbon atom of a diarylmethanol.
Scheme 1.
(Hydrido)silyl-Directed Arene C–H Bond Activation
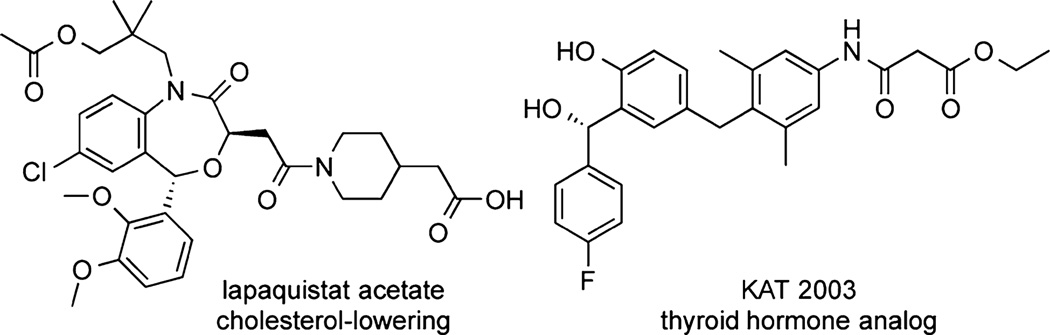
Chiral diarylmethanols are important pharmacophores and can be found in biologically active compounds such as lapaquistat acetate8 and KAT 20039 (Figure 1). Enantioenriched diarylmethanols can be synthesized by asymmetric reduction of benzophenone derivatives or by asymmetric addition of aryl organometallic reagents to aldehydes.10,11 Enantioselecitve C–H bond functionalization of diarylmethanols with silane reagents would be an alternative, mild, catalytic route to this class of compound.
Figure 1.
Two enantiopure biologically active compounds containing chiral diarylmethanol motifs.
We report catalysts and conditions for such enantioselective silylation. Combining hydrosilylation of benzophenone or its derivatives and intramolecular silylation of the resulting silyl ether creates chiral, nonracemic diarylmethanols in high enantiopurity. This stereoselective process also enables the site-selective functionalization of enantioenriched chiral diaryl methanols. The products of these functionalization reactions are suitable for a range of transformations at the C–Si bond.
Based on our previous Ir-catalyzed intramolecular silylation of C–H bonds, we focused initially on potential Ir catalysts containing chiral nitrogen, phosphine-nitrogen, and bisphosphine ligands for enantioselective C–H silylation. We found that a catalyst generated from [Ir(cod)Cl]2 and DTBM-Segphos (L32) provided 3a in 90% ee and 80% yield. However, this system required temperatures higher than 80 °C with 4 mol % catalyst loading to form the desired product in good yields. In addition, the reaction under these conditions gave high yields and ee’s only with unsubstituted benzophenone (see the SI for details).
To address these limitations, we tested Rh catalysts, instead of Ir catalysts, based on prior intramolecular silylations of arenes and alkanes, albeit at high temperatures.12 Indeed, we found that the combination of [Rh(cod)Cl]2 and L32 catalyzed the silylation of 2a at 50 °C to give 3a in 90% ee with complete conversion. This preliminary result led to the recent finding in one of our laboratories that Rh-bisphosphine complexes catalyze intermolecular silylations of aromatic C–H bonds at only 45 °C with 2 mol % catalyst.13 However, the scope of the intramolecular, asymmetric silylation catalyzed by [Rh(cod)Cl]2 and L32 was similarly narrow, even though the reaction occurred at lower temperature.
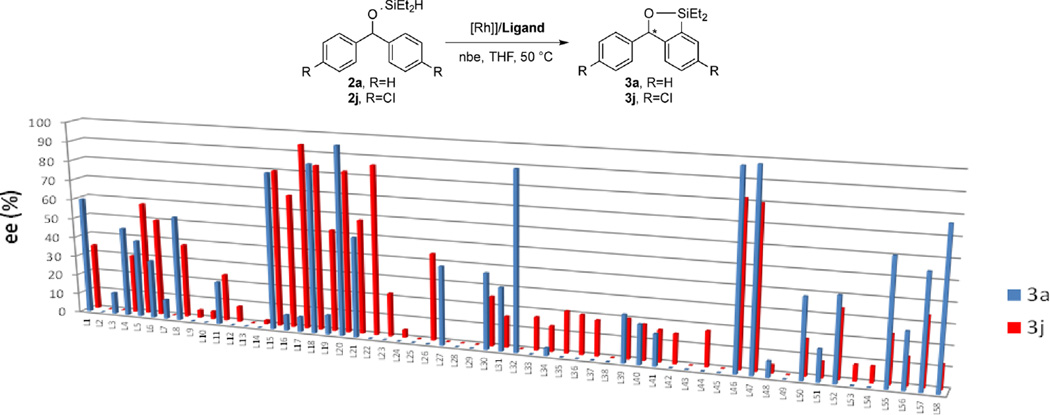
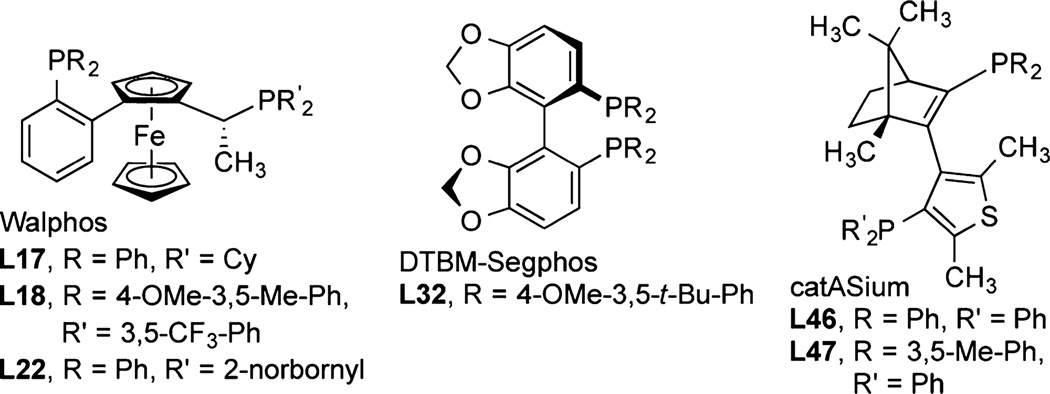
To identify a catalyst that would react with a broader range of diarylmethanols, we evaluated the reaction of an unsubstituted and a substituted diarylmethyl silyl ether, 2a and 2j, respectively, catalyzed by 5 mol % [Rh(cod)Cl]2 and 58 commercially available bisphosphines, phosphine-nitrogen ligands, and dinitrogen ligands (Figure 2; see the SI for a complete list of ligands). The reactions were conducted with 2 equiv of norbornene (nbe) as an H2 acceptor at 50 °C in THF. In contrast to the catalyst generated from L32, which was active and selective for reaction of only substrate 2a, those derived from members of the Walphos (L15–L22) and catASium (L46, L47) classes of ligands (Figure 3) generated catalysts that formed the desired silylation products with high ee’s for both of the test substrates and a range of additional diarylmethyl silyl ethers (vide inf ra). The highest enantioselectivity (99% ee) for reaction of diarylmethyl silyl ether 2a was obtained with catalysts containing L46 or L47, but the highest enantioselectivity for reactions of silyl ether 2j was obtained with catalysts containing Walphos ligands L17, L18, and L22. In general, we observed that the catalysts generating large amounts of byproducts also reacted with low ee’s.14 The reaction occurred with equivalent enantioselectivities and yields when it was conducted with a lower loading of catalyst (1 mol %) and fewer equivalents of norbornene (1.2 equiv) as when it was conducted with 5 mol % of catalyst and 2 equiv of norbornene.
Figure 2.
Results from evaluation of 58 ligands for the asymmetric silylation of diarylmethyl silyl ethers 2a and 2j (% ee). Conditions: [Rh(cod)Cl]2 (2.5 mol %), ligand (5 mol %), nbe (2 equiv), THF, 50 °C. Selected ligand categories: Josiphos class (L1–L12), Walphos class (L15–L22), Segphos class (L30–L33), and catASium class (L46, L47).
Figure 3.
Ligands in the Rh complexes that catalyze asymmetric silylation with high ee.
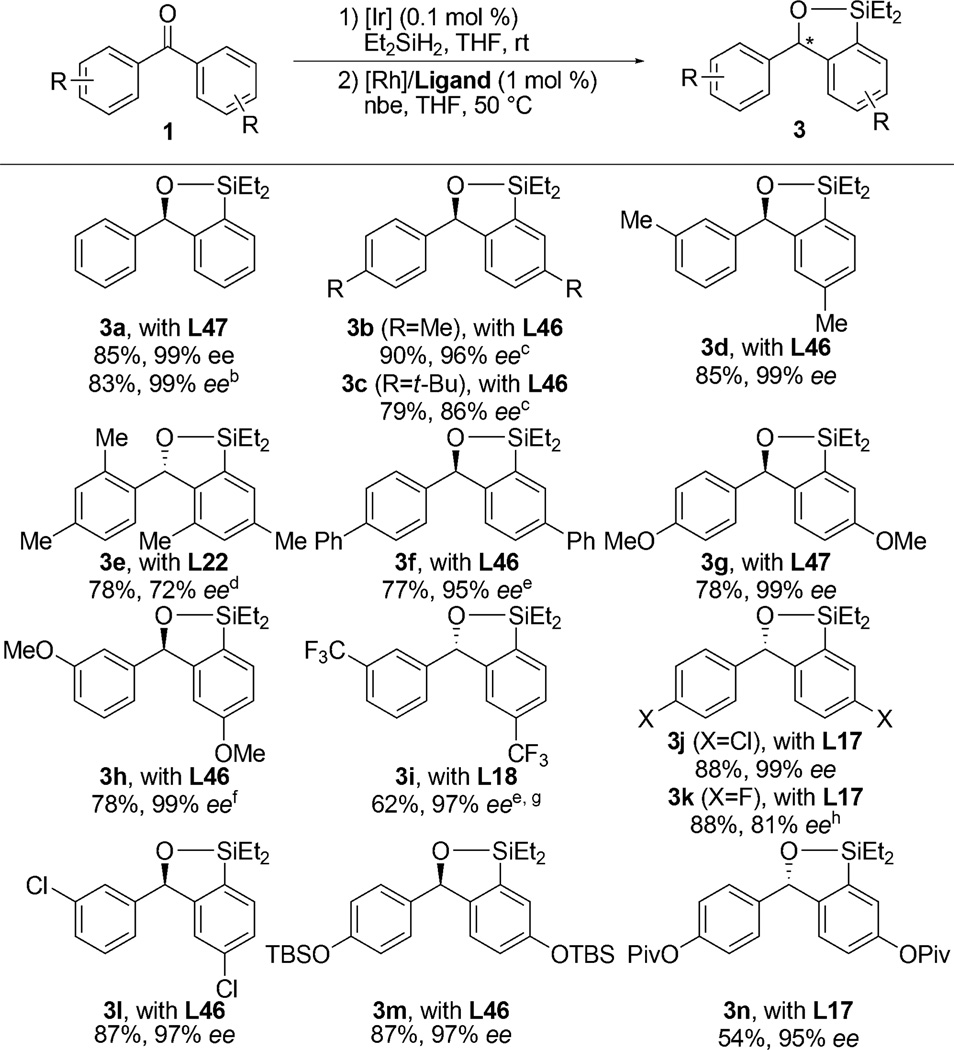
The scope of the Rh-catalyzed asymmetric silylation is shown in Table 1. Hydrosilylation of benzophenones 1a–1n with diethylsilane catalyzed by 0.05 mol % [Ir(cod)OMe]2 formed (hydrido)silyl ethers 2a–2n. In the presence of 1.0 mol%of both [Rh(cod)Cl]2 and ligand with 1.2 equiv of norbornene as an H2 acceptor, a wide range of the silyl ethers were converted to benzoxasiloles 3a–3n in good to excellent yields with excellent enantioselectivities (Table 1).15 For the reaction of each substrate, ligands L17, L18, L22, L46, and L47 were examined to determine which one provided the highest yield and ee. Unsubstituted benzophenone (1a) and alkyl- or aryl-substituted benzophenones containing meta and para substituents (1b–1d, 1f) yielded the desired products in high yields (77–90%) and good to excellent enantioselectivities (86–99% ee) with either L46 or L47. Varying the electronic properties of the arene had little influence on the yield and ee (1g–1i). Although silyl ethers containing aryl bromides formed substantial amounts of byproducts resulting from proto-debromination, those substituted with a chloro group (1j, 1l) or a fluoro group (1k) reacted in good yield and good to excellent ee. In addition, functional groups containing oxygen, such as silyl ethers (1m) and esters (1n), were tolerated under the reaction conditions. However, reactions with a substrate bearing substituents at the ortho positions (1e) required a higher temperature to reach full conversion, and a lower ee of 72% was observed. The asymmetric silylation reaction occurred with 5 mmol of substrate to provide more than 1 g of the enantioenriched benzoxasilole (3a) in a yield and ee similar to those of the reactions conducted on a 1 mmol scale.
Table 1.
Scope of Enantioselective C–H Silylationa
Isolated yields for reactions conducted on a 1.0 mmol scale following purification by silica gel chromatography. The ee values were determined by chiral HPLC analysis. The absolute configuration was assigned by analogy (see SI for details).
5.0 mmol scale.
The ee values were determined after iodination.
The reaction was conducted at 80 °C.
0.1 mol % [Ir(cod)OMe]2 was used for the first step.
The corresponding diarylmethanol was used as a substrate.
The ee value was determined after Tamao–Fleming oxidation.
The reaction was conducted at room temperature.
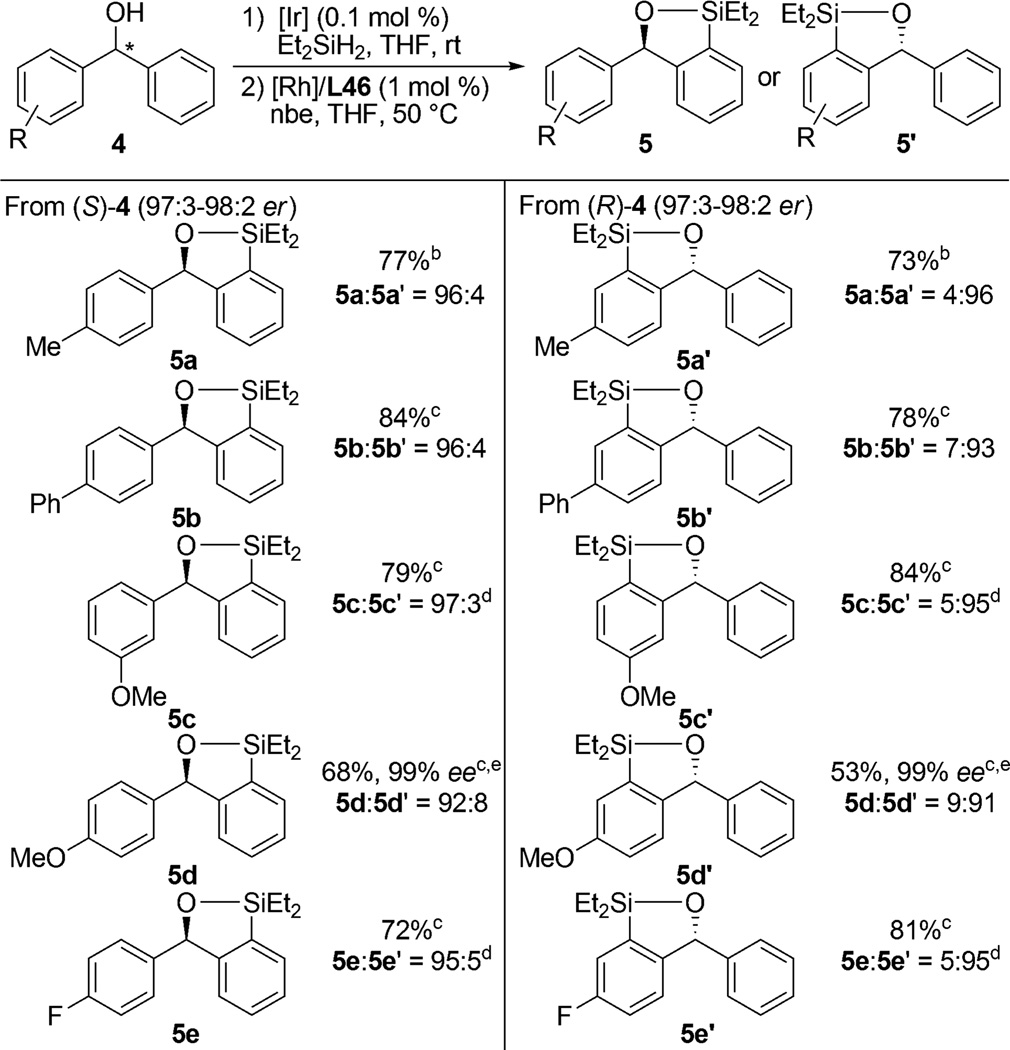
Nonracemic chiral catalysts can react with two enantiomers of a substrate to form two constitutional isomers of the products selectively.16 We exploited the high stereoselectivity of our C–H silylation reactions for site-selective silylations of enantioenriched diarylmethanols (Table 2). Selective C–H bond functionalization at one aryl group over the other of a chiral diarylmethanol in the absence of distinct steric properties is challenging. However, reaction of (hydrido)silyl ethers, prepared by dehydrogenative silylation of chiral diarylmethanol enantiomers 4, in the presence of a highly enantioselective catalyst should convert the two enantiomers into the two different constitutional isomers, 5 and 5′ with high site selectivity. As shown in Table 2, a variety of enantioenriched diarylmethanols were shown to react with high site selectivity controlled by the configuration of the catalyst. The (S)-enantiomer of 4 was transformed selectively into 5, while the (R)-enantiomer of 4 was transformed selectively into 5′.
Table 2.
Scope of Site-Selective C–H Silylationa
Isolated yields for reactions conducted on a 0.25 mmol scale following purification by silica gel chromatography. Combined yields of both constitutional isomers are reported. Regioisomeric ratio was determined by GC analysis of the crude reaction mixture unless otherwise noted.
The enantiomer ratio of the starting material was 98:2.
The enantiomer ratio of the starting material was 97:3.
Regioisomeric ratio was determined by 1H NMR spectroscopy of the crude reaction mixture.
Isolated yield of the single isomer.
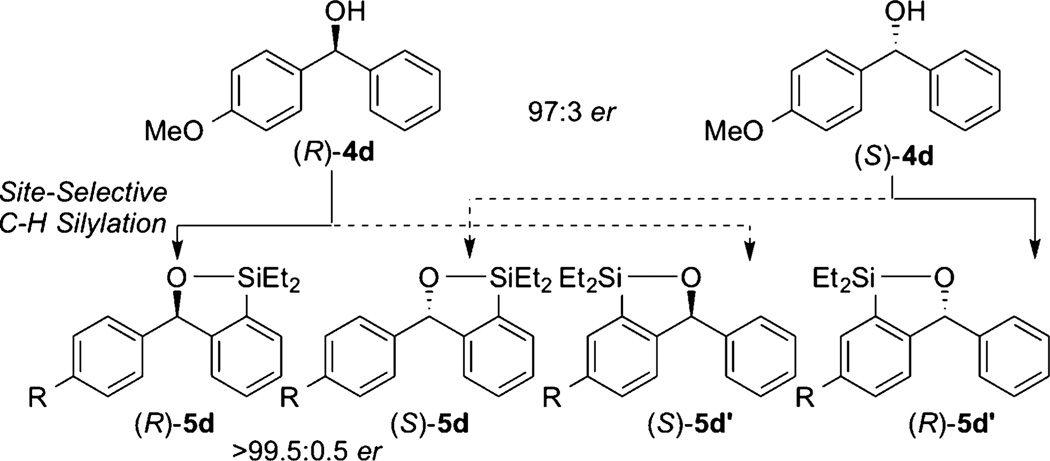
According to the Horeau principle, the ee amplifies as two enantioselective operations occur on the same substrate successively.17 In our case, the synthesis of enantioenriched substrate 4 can be considered the first enantioselective operation of this scenario, and our silylation reaction considered the second enantioselective operation that improves the ee of the diarylmethanol 4 (Figure 4). Consistent with this reasoning, the ee’s of the constitutional isomers of products 5d and 5d′ (>99% ee) were higher than those of the starting materials (94% ee). Thus, this method can be used to upgrade the ee of enantioenriched diarylmethanols.
Figure 4.
Amplification of enantiomeric excess of enantioenriched diarylmethanols by site-selective C–H silylation.
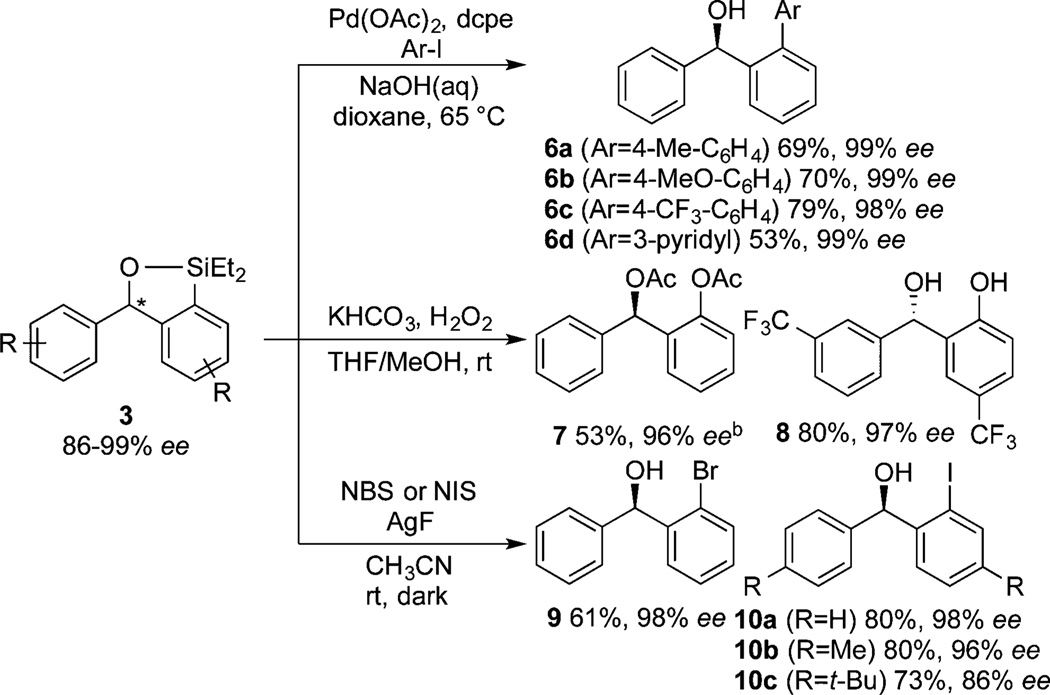
The enantioenriched benzoxasiloles formed in these reactions are useful synthetic intermediates because the Ar–Si linkage can be converted into carbon–carbon or carbon–heteroatom bonds without any erosion of ee (Scheme 2). Under conditions we developed for coupling of silyl ethers,7,18 Pd-catalyzed Hiyama cross-coupling of the enantioenriched products with aryl iodides formed benzoxasilole 3a in good yields without any erosion of enantiomeric purity (98–99% ee). Electron-neutral (6a), electron-rich (6b), and electron-poor (6c) aryl iodides and a heteroaryl iodide (6d) were suitable coupling partners. In addition, the enantioenriched benzoxasiloles underwent Tamao–Fleming oxidation to form carbon–oxygen bonds. Treatment of benzoxasiloles 3 with aqueous H2O2 and KHCO3 gave diol products; 3a and 3i were converted to diacetate 7 and diol 8, respectively, without any significant change in ee. Finally, halogenation of 3 in the presence of silver fluoride and N-halosuccinimide formed the brominated and iodinated diarylmethanols 9 and 10 in good yields while maintaining high ee’s.
Scheme 2.
Functionalization of Enantioenriched Benzoxasilolesa
aIsolated yields for reactions following purification by silica gel chromatography. The ee values were determined by chiral HPLC analysis. The absolute configuration was assigned by analogy (see SI for details). bDiol product was acetylated with Ac2O before isolation.
In summary, we have shown that the silylation of aryl C–H bonds can create a new class of enantioselective C–H bond functionalization. Our enantioselective silylation of aromatic C–H bonds directed by a (hydrido)silyl group occurs in high yield with excellent enantioselectivity when catalyzed by the combination of [Rh(cod)Cl]2 and chiral bisphosphine ligands from the Walphos and catASium families. In addition, we showed that this catalyst system is capable of site-selective silylation of enantioenriched chiral diarylmethanols due to its strong catalyst control. Lastly, we demonstrated that the C–Si bond of the enantioenriched benzoxasiloles can be converted to C–C, C–O, C–I, or C–Br bonds without erosion of enantiomeric excess. Studies to expand the scope of enantioselective silylation of C–H bonds and to gain insight into the factors controlling enantioselectivity are ongoing in our laboratories.
Supplementary Material
ACKNOWLEDGMENTS
T.L. and J.F.H. thank the NSF (GM-55382) for support of this work. T.L. thanks the Samsung Scholarship for a graduate fellowship.
Footnotes
ASSOCIATED CONTENT
Experimental details and characterization data for new compounds. The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.5b03091.
The authors declare no competing financial interest.
REFERENCES
- 1.(a) Mkhalid IAI, Barnard JH, Marder TB, Murphy JM, Hartwig JF. Chem. Rev. 2010;110:890. doi: 10.1021/cr900206p. [DOI] [PubMed] [Google Scholar]; (b) Hartwig JF. Acc. Chem. Res. 2012;45:864. doi: 10.1021/ar200206a. [DOI] [PubMed] [Google Scholar]
- 2.Cheng C, Hartwig JF. Chem. Rev. 2015 doi: 10.1021/cr5006414. [DOI] [PubMed] [Google Scholar]
- 3.Kuninobu Y, Yamauchi K, Tamura N, Seiki T, Takai K. Angew. Chem., Int. Ed. 2013;52:1520. doi: 10.1002/anie.201207723. [DOI] [PubMed] [Google Scholar]
- 4. Shibata T, Shizuno T, Sasaki T. Chem. Commun. 2015;51:7802. doi: 10.1039/c5cc00723b. While this manuscript was under review, He et al. reported an asymmetric silylation of ferrocenes similar to that in ref 4a: Zhang Q-W, An ZA, Liu L-C, Yue Y, He W. Angew. Chem., Int. Ed. 2015
- 5.For reviews on enantioselective C–H bond functionalization, see: Giri R, Shi BF, Engle KM, Maugel N, Yu JQ. Chem. Soc. Rev. 2009;38:3242. doi: 10.1039/b816707a. Doyle MP, Duffy R, Ratnikov M, Zhou L. Chem. Rev. 2010;110:704. doi: 10.1021/cr900239n. Wencel-Delord J, Colobert F. Chem.—Eur. J. 2013;19:14010. doi: 10.1002/chem.201302576. Zheng C, You S-L. RSC Adv. 2014;4:6173.
- 6.For representative examples of enantioselective C–H bond functionalization with diverse strategies, see: Shi B-F, Maugel N, Zhang Y-H, Yu J-Q. Angew. Chem., Int. Ed. 2008;47:4882. doi: 10.1002/anie.200801030. Shi BF, Zhang YH, Lam JK, Wang DH, Yu JQ. J. Am. Chem.. Soc. 2010;132:460. doi: 10.1021/ja909571z. Wasa M, Engle KM, Lin DW, Yoo EJ, Yu JQ. J. Am. Chem. Soc. 2011;133:19598. doi: 10.1021/ja207607s. Nakanishi M, Katayev D, Besnard C, Kundig EP. Angew. Chem., Int. Ed. 2011;50:7438. doi: 10.1002/anie.201102639. Pan S, Endo K, Shibata T. Org. Lett. 2011;13:4692. doi: 10.1021/ol201907w. Hyster TK, Knorr L, Ward TR, Rovis T. Science. 2012;338:500. doi: 10.1126/science.1226132. Ye BH, Cramer N. Science. 2012;338:504. doi: 10.1126/science.1226938. Saget T, Cramer N. Angew. Chem., Int. Ed. 2012;51:12842. doi: 10.1002/anie.201207959. Cheng XF, Li Y, Su YM, Yin F, Wang JY, Sheng J, Vora HU, Wang XS, Yu JQ. J. Am. Chem. Soc. 2013;135:1236. doi: 10.1021/ja311259x. Chu L, Wang XC, Moore CE, Rheingold AL, Yu JQ. J. Am. Chem. Soc. 2013;135:16344. doi: 10.1021/ja408864c. Chu L, Xiao KJ, Yu JQ. Science. 2014;346:451. doi: 10.1126/science.1258538. Xiao KJ, Lin DW, Miura M, Zhu RY, Gong W, Wasa M, Yu JQ. J. Am. Chem. Soc. 2014;136:8138. doi: 10.1021/ja504196j. Chan KSL, Fu H-Y, Yu JQ. J. Am. Chem. Soc. 2015;137:2042. doi: 10.1021/ja512529e.
- 7.Simmons EM, Hartwig JF. J. Am. Chem. Soc. 2010;132:17092. doi: 10.1021/ja1086547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miki T, Kori M, Mabuchi H, Tozawa R, Nishimoto T, Sugiyama Y, Teshima K, Yukimasa H. J. Med. Chem. 2002;45:4571. doi: 10.1021/jm020234o. [DOI] [PubMed] [Google Scholar]
- 9.Nishimura T, Taji H, Harada N. Chirality. 2004;16:13. doi: 10.1002/chir.10301. [DOI] [PubMed] [Google Scholar]
- 10.For a review on enantioselective synthesis of diarylmethanols, see: Schmidt F, Stemmler RT, Rudolph J, Bolm C. Chem. Soc. Rev. 2006;35:454. doi: 10.1039/b600091f.
- 11.For representative examples of enantioselective synthesis of diarylmethanols, see: Dosa PI, Ruble JC, Fu GC. J. Org. Chem. 1997;62:444. doi: 10.1021/jo962156g. Corey EJ, Helal CJ. Angew. Chem., Int. Ed. 1998;37:1986. doi: 10.1002/(SICI)1521-3773(19980817)37:15<1986::AID-ANIE1986>3.0.CO;2-Z. Ohkuma T, Koizumi M, Ikehira H, Yokozawa T, Noyori R. Org. Lett. 2000;2:659. doi: 10.1021/ol9904139. Bolm C, Rudolph J. J. Am. Chem. Soc. 2002;124:14850. doi: 10.1021/ja028518l. Braga AL, Ludtke DS, Vargas F, Paixao MW. Chem. Commun. 2005:2512. doi: 10.1039/b501485a. Truppo MD, Pollard D, Devine P. Org. Lett. 2007;9:335. doi: 10.1021/ol0627909. Yamamoto Y, Kurihara K, Miyaura N. Angew. Chem., Int. Ed. 2009;48:4414. doi: 10.1002/anie.200901395.
- 12.(a) Ureshino T, Yoshida T, Kuninobu Y, Takai K. J. Am. Chem. Soc. 2010;132:14324. doi: 10.1021/ja107698p. [DOI] [PubMed] [Google Scholar]; (b) Kuninobu Y, Nakahara T, Takeshima H, Takai K. Org. Lett. 2013;15:426. doi: 10.1021/ol303353m. [DOI] [PubMed] [Google Scholar]
- 13.Cheng C, Hartwig JF. Science. 2014;343:853. doi: 10.1126/science.1248042. [DOI] [PubMed] [Google Scholar]
- 14.We observed hydrosilylation of norbornene in some cases.
- 15.We performed a control experiment on 2a that did not contain residual iridium and found that the yield and ee of 3a remained identical.
- 16.(a) Walsh PJ, Kozlowski MC. Fundamentals of asymmetric catalysis. Sausalito, CA: University Science Books; 2009. [Google Scholar]; (b) Miller LC, Sarpong R. Chem. Soc. Rev. 2011;40:4550. doi: 10.1039/c1cs15069c. [DOI] [PubMed] [Google Scholar]
- 17.Rautenstraunch V. Bull. Soc. Chim. Fr. 1994;131:515. [Google Scholar]
- 18.Denmark SE, Regens CS. Acc. Chem. Res. 2008;41:1486. doi: 10.1021/ar800037p. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.