Abstract
OBJECTIVE
Early diagnosis of HIV infection in infants with perinatal HIV exposure is critical for clinical management decisions. Positive HIV nucleic acid detection represents the gold standard for the diagnosis of HIV infection status in infants prior to age 18 months, but this test is not universally available, especially in resource poor regions of the world. In this study we tested the hypothesis that the CD4/CD8 T cell ratio can predict HIV infection status in HIV-exposed infants.
METHODS
CD4/CD8 T cell ratios were determined from data of live born, singleton infants who had been prospectively enrolled in the Women and Infants Transmission Study (WITS). Data from 2208 infants with known HIV infection status (179 HIV infected, 2029 uninfected) were analyzed.
RESULTS
Receiver Operating Characteristic (ROC) curves indicated that CD4/CD8 ratio performed better than CD4% for diagnosis of HIV infection as early as age 2 months (p=0.018), and this relationship was unaffected by adjusting for maternal race/ethnicity, infant birth weight, gestational age and gender. At age 4 mos., 90% specificity for HIV diagnosis was associated with 60% sensitivity. For ease of utilization, longitudinal LMS profile-based percentile curves were developed for sensitivity/specificity of CD4/CD8 ratios in HIV-infected and -uninfected infants until age 12 months. At age 6 months, a simplified equation that incorporated sequential CD4/CD8 ratios and hematocrit values resulted in improved ROCs with 94% positive predictive value (PPV) and 98% negative predictive value (NPV). The PPV and NPV remained above 90% in simulated infant populations over a wide range of prevalence of HIV infection.
CONCLUSIONS
In the absence of virologic diagnosis, a presumptive diagnosis of HIV- infection status may be made on the basis of CD4/CD8 ratios in HIV-1-exposed infants after age 2 months; sensitivity and specificity can be further improved at 6 months by using a discriminant analysis equation.
Keywords: HIV diagnosis, perinatal HIV transmission, HIV exposed infants, CD4/CD8 ratio
INTRODUCTION
Mother-to-child transmission (MTCT) of the virus is the main cause of pediatric HIV infection and occurs in about 25% of infants born to HIV infected women who do not receive interventions for the prevention of MTCT (PMTCT).1 In many parts of the developing world where breastfeeding is the norm, postnatal transmission of HIV contributes to alarmingly high rates of MTCT.2 Many HIV-1-infected pregnant women still do not have access to PMTCT programs and their infants remain at high risk for being infected.3 Regardless of the availability of PMTCT, the determination of the HIV infection status of infants born to HIV infected women is critically important for proper management of both HIV-infected and -uninfected infants.4-6
HIV nucleic acid amplification assays, including DNA polymerase chain reaction (PCR), represent the gold standard for diagnosis of HIV infection for HIV-exposed infants. For example, HIV DNA PCR assays have >99% sensitivity and specificity at 6 weeks, even in resource-poor settings.7 However, HIV nucleic acid testing is not available in many resource-poor areas of the world where there is a high burden of HIV infection. In these settings serologic diagnosis may be the only available test, resulting in a delay in diagnosis of HIV exposed infants. This is because the infection status cannot be established until either seroreversion occurs following decay of passively-transferred maternal antibody in uninfected children, or in the case of HIV infected children, until the development of HIV associated clinical symptoms or documented persistence of HIV antibody beyond 18 months, the cut-off for decay of maternal antibody.6 Until appropriate HIV virologic assays for diagnosis of HIV infection become universally available, it is critical to develop alternative diagnostic algorithms for perinatally HIV-exposed infants as quickly after birth as possible. Several approaches are currently under investigation.6,8,9 The present study addresses this problem from an immunologic point-of-view, and evaluates whether CD4/CD8 ratio can be a useful diagnostic tool for HIV-exposed infants.
Several clinical sites in many parts of the world, while lacking HIV nucleic acid testing facilities, have access to T cell subset analysis. Typically, CD4 T cells are used for monitoring the degree of immune suppression in HIV-infected infants, children and adults.10 Concurrent with loss of CD4 T cells, a predominant effect of HIV infection is expansion of CD8 T cells 11,12 which occurs as a component of the immune activation that is a pathogenic feature in HIV infection. We hypothesized that an inverse CD4/CD8 T cell ratio is likely to be a more sensitive discriminator of HIV infection status than depletion of CD4 T lymphocytes alone.
In the present study, we have analyzed CD4/CD8 ratios from a prospective cohort study of infants with perinatal HIV exposure in whom the infection status of the infants had been established by HIV-1 DNA PCR. The objectives of our study were: 1) to determine whether the CD4/CD8 ratio in HIV-exposed infants could discriminate between infected and uninfected infants; 2) to determine the earliest age at which such discrimination could be accomplished; and 3) to determine if the discriminating power could be enhanced by utilizing additional simple laboratory measures.
MATERIALS AND METHODS
Women and Infants Transmission Study
The Women and Infants Transmission Study (WITS) was a prospective cohort study of HIV infected women and their children conducted at multiple sites in the U.S. (Boston and Worcester, MA; Houston, TX; Chicago, IL; New York City, NY) and San Juan, Puerto Rico. The design and objectives of this study have been previously described.13 Briefly, beginning in 1989, HIV infected pregnant women were screened and recruited into the study, which followed a standard IRB-approved protocol. Each enrolled woman provided written informed consent for her participation and that of her child. Diagnostic testing of each infant was performed according to a fixed schedule (at birth and at 1, 2, 4, 6, 9, and 12 months of age). The diagnosis of HIV infection in children required two positive HIV-1 DNA polymerase chain reaction (PCR) assays. Early in the WITS, three negative assays were required to declare a child HIV uninfected, with at least one test being performed after the age of 30 days. This rule was later changed to two negative assay results, with one occurring after 30 days of age and the other occurring after four months of age. Flow cytometry was performed using standardized monoclonal antibody panels (Becton Dickinson, San Jose, California) at National Institutes of Health-certified study laboratories.14 The natural history of CD4+ and CD8+ T cell subsets of the WITS cohort has previously been published.15
Study Population for This Analysis
The study population for this analysis was restricted to live born infants with known HIV1 infection status (infected or uninfected), who were singletons or the first-born of a multiple gestation pregnancy, and who were born during the first enrollment in the WITS for their mothers.
Statistical Methods
A power analysis was performed to assess the possibility of detecting statistically significant results for the proposed study. Considering one set of results for each visit, we assumed that data for 150 HIV infected infants and 2500 HIV uninfected infants would be part of the analysis. Under this assumption, it would be possible to detect a change from 0.7 to 0.8 in two ROC curves for CD4% and CD4/CD8 ratio with 80% power when testing at the 0.05 alpha level.
Means, standard deviations, and percentages, where presented, were calculated using SAS procedures [SAS Institute Inc., Version 8, Cary, NC]. Receiver Operating Characteristic (ROC) curves, using the WITS definition of HIV infection as the gold standard, were generated for each visit using the CD4 percentage (CD4%), the ratio of the CD4% and the CD8% (CD4/CD8 ratio), and HIV-1 DNA PCR measures collected at that visit. In order to account for the correlated nature of the paired data when comparing two or more adjusted ROC curves (constructed using data from the same individuals), a non-parametric approach was utilized to correct for the correlation based on generalized U-Statistics methods.16 The hypothesis that the two empirical ROC curves are equal was tested.16,17 Multivariate logistic regression models were fitted (WITS-defined HIV infection status serving as the dependent variable, CD4% and CD4/CD8 ratio as the independent variables). The adjusted analyses also incorporated other covariates. Longitudinal profiles of CD4/CD8 ratio percentiles for HIV infected and HIV uninfected infants were generated using the LMS method.18
A linear discriminant analysis was performed to determine if CD4/CD8 ratios and hematocrit values at different times during infancy could improve on the univariate ROC results for the CD4/CD8 ratio data at age 6 months. Specifically, the CD4/CD8 ratios at 1 month, 2 months, 4 months and 6 months of age and hematocrits at 6 months of age were used to develop a simplified discriminant equation:
By choosing two “priors” for the equation, we were able to establish two regions that allowed the classification of a sizeable number of infants as infected or uninfected. To develop the 0discriminant function into a diagnostic tool, the following cut-points were determined: <9, HIV infected; 9-12, Indeterminate; >12, HIV uninfected. The equation was evaluated in the same way as the CD4/CD8 ratio and the values for the ROC were determined.
RESULTS
Size and Characteristics of the Study Population
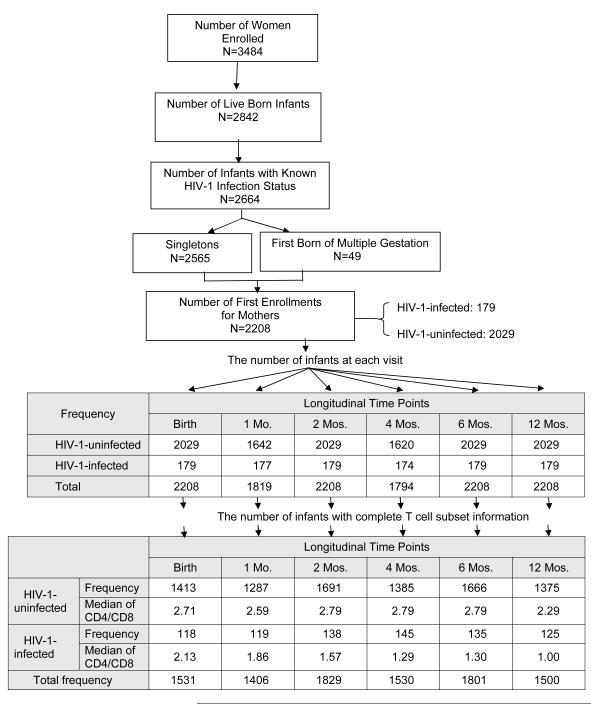
Between 1989 and 2005, 3484 women were enrolled in WITS and 2842 live births occurred. The HIV infection status was determined for 2664 of these 2842 live born infants. Of these 2664 infants with known infection status, 2565 were singletons and 49 were the first born of a multiple gestation pregnancy, and 2208 represented infants born to women during their first enrollment into WITS. Thus, the study population consisted of 2208 infants, of whom 179 were HIV-1-infected and 2029 were uninfected. Figure 1 depicts the derivation of the study population and the number of infants (overall and according to HIV infection status) who completed study visits during the first 12 months on study. Eleven percent of the population was white, 48% black, 35% Hispanic, and 6% other. Seventeen percent of infants were low birth weight. The median gestational age was 38 weeks (range: 25-43 weeks). The overall rate of mother to child transmission of HIV-1 was 8.11%, with this rate decreasing over time: on or before February 28, 1994: 20.54%, from March 1, 1994 to July 31, 1996: 7.19%, and on or after August 1, 1996: 2.27%.
Figure 1.
Derivation of the Study Population and Median CD4/CD8 Ratios at Each Study Visit
CD4/CD8 Ratio and HIV Infection Status in Infancy
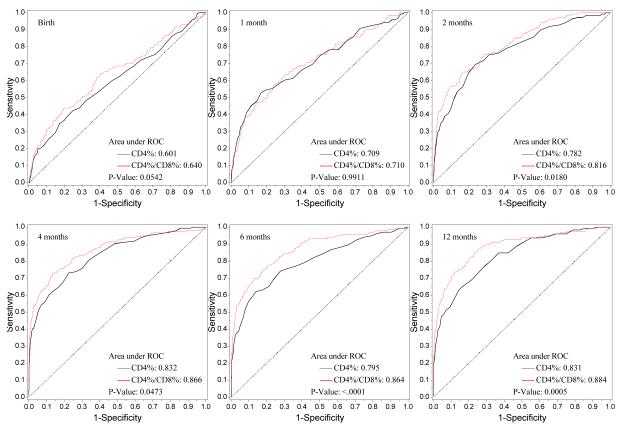
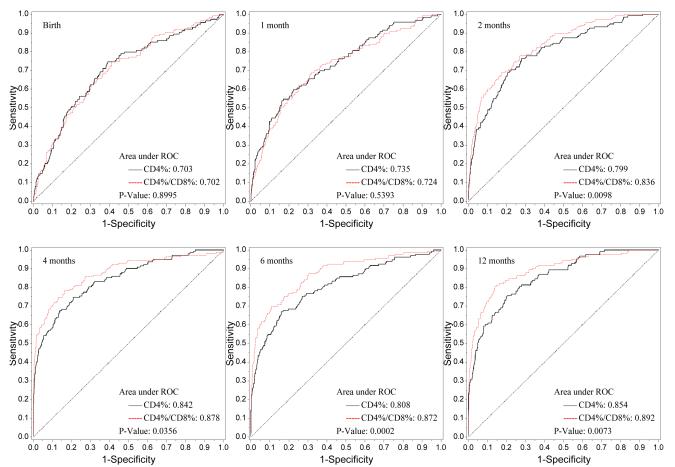
The number of infants at each study visit with complete flow cytometry data, as well as the median CD4/CD8 ratio for HIV-1-infected and uninfected infants, are shown in Figure 1. Nonparametric comparisons were carried out to test the hypothesis that the areas under the ROC curves for CD4% and the CD4/CD8 ratio were equal. In unadjusted and adjusted analyses, infant HIV-1 infection status served as the response variable and CD4% or CD4/CD8 ratio as the independent variable. Figure 2 shows the receiver operating characteristic (ROC) curves by visit (birth and 1, 2, 4, 6, 12 months) for the CD4/CD8 ratio at that visit for determination of HIV-1 infection status. The more pronounced the convexity to the upper left indicates greater diagnostic capability. This is measured in terms of the area under the curve (AUC), computed at each visit based on the logistic regression modeling, which is annotated at the lower right of each curve. Early ROC curves show AUCs close to 0.7. Taking the one month ROC curve as an example, the AUC is 0.71. In order for the CD4/CD8 ratio to have 95% sensitivity, the specificity of the CD4/CD8 ratio is 15%. By the time of the four-month visit, the AUC has improved to 0.866. The curve shows that in order for the four month CD4/CD8 ratio to have a sensitivity of 90%, the specificity of the ratio is 60%. Adjusting these analyses for maternal race/ethnicity and infant gender, birth weight and gestational age did not change the overall relationship between CD4/CD8 ratio and HIV infection status of the infants (Figure 3).
Figure 2.
ROC Plot of CD4% vs. CD4/CD8 (Univariate Analyses)
Figure 3.
ROC Plot of CD4% vs. CD4/CD8 (Adjusted for Other Covariates)
The p-values for the generalized U-statistics comparing the correlated ROC curves (CD4% and CD4/CD8 ratio) are at the bottom right of each plot. There was significant evidence that the CD4/CD8 ratio had better diagnostic accuracy than CD4% in discriminating between HIV infected and uninfected infants at or after 2 months of age in both univariate and multivariate analyses respectively [2 months (p=0.018, 0.001), 4 months (p=0.047, 0.036), 6 months (p< 0.0001, 0.0002) and 12 months (p=0.0005, p=0.007)]. There is a marginal difference between the two diagnostic markers at birth (p=0.054).
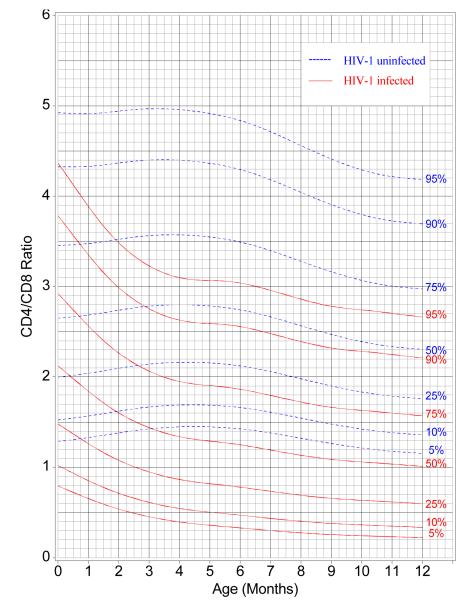
Figure 4 uses LMS curves to present centiles for CD4/CD8 ratios as a function of infants’ ages. The curves were generated at percentiles of 5, 10, 25, 50, 75, 90, and 95 by HIV-1 infection status (infected and uninfected) using the LMS method.17 For any value of y, one can obtain the specificity (100 minus the centile for the uninfected infants) and the sensitivity (the centile for the infected infants) for any age. From birth to four months of age, the CD4/CD8 ratio decreased for HIV infected infants, but increased slightly for uninfected infants. After four months of age, the discriminatory power (separation of the two sets of centile measurements) of the ratio stabilized, with the LMS percentile curves showing a slight decrease in the values of the ratio from four months of age to 12 months of age for both HIV-1-infected and uninfected infants.
Figure 4.
Reference Centile Curves for CD4/CD8 by HIV-1 Infection Status: 5th, 10th, 25th, Median, 75th, 90th and 95th Percentiles
To assess whether maternal receipt of antiretroviral (ARV) drugs was associated with outcome measures, we determined the values for CD4/CD8 ratios in infected and uninfected infants according to in utero exposure to maternal ARV drugs. Figure 5a shows the mean of CD4/CD8 ratios from birth to 6 months by HIV infection status and maternal ARV usage. Similarly, the association of maternal ARV drugs and infant hematocrit was evaluated (Figure 5b), as in utero exposure to ARV drugs has been reported to influence hematological indices, albeit transiently.19, 29 Maternal ARV drugs did not appear to affect the CD4/CD8 ratio or hematocrit among the HIV infected or uninfected infants.
Figure 5.
a. Mean of CD4/CD8 ratio and b. Mean of hematocrit by age in HIV-1-infected and HIV-1-uninfected infants whose mothers did or did not receive antepartum or intrapartum antiretroviral drugs (ARVs)
Discriminant Analysis incorporating CD4/CD8 Ratio and Hematocrit
To determine if we could improve the diagnostic potential of using CD4/CD8 ratio, we developed a discriminant analysis algorithm which could be used to make a presumptive diagnosis of HIV-1 infection based on CD4/CD8 ratio and hematocrit data at time points up to six months of age. There were 88 HIV infected and 955 uninfected infants with complete data for this analysis. The CD4/CD8 ratios of these subjects at 1 month, 2 months, 4 months and 6 months of age were significantly related to HIV infection status (p-values < 0.0001). The hematocrits at 4 and 6 months of age also were significantly related to HIV infection status (p < 0.0001). The discriminant function was developed as described above, and, evaluating the resulting discriminant equation in the same way as the CD4/CD8 ratio, the values for the ROC increases at the six-month evaluation to 0.895 and 0.899 for the unadjusted and adjusted analyses respectively. These ROC values are superior to the ROC values obtained with CD4/CD8 ratios alone.
Applying this discriminant diagnosis rule to the WITS data, the presumptive diagnosis and actual infection status in the study cohort are shown in Table 1. Sixteen, 54 and 18 HIV infected infants were respectively classified as HIV infected, indeterminate, and HIV uninfected. Similarly, 1, 144 and 810 HIV uninfected infants were respectively classified as HIV infected, indeterminate, and HIV uninfected. The positive and negative predictive values for the discriminant function when applied to the WITS data were 94% and 98% respectively.
Table 1.
HIV-1 infection status based on discriminant analysis and actual HIV-1 infection status
| Discriminant value | Presumptive Classification |
Classification Results based on Discriminant Analysis | |
|---|---|---|---|
|
in HIV-1-Infected
No. (%) |
in HIV-1-Uninfected No. (%) |
||
| <9 | HIV-1-infected | 16 (18) | 1 (.001) |
| 12-9 | Indeterminate | 54 (61) | 144 (15) |
| >12 | HIV-1-uninfected | 18 (20) | 810 (85) |
Effects of prevalence of HIV infection on diagnosis
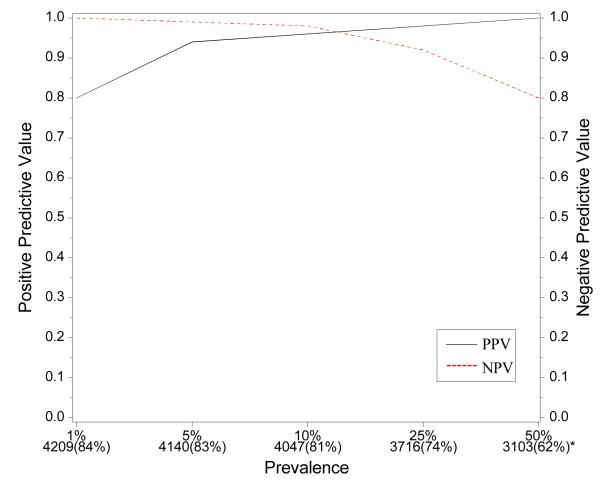
Developing and testing the discriminant on the same population can lead to bias in terms of the degree to which the classification agrees with the final HIV infection status of the infant. Further, the agreement table was developed under a fixed prevalence of HIV infection (as was observed in the WITS cohort). To address this potential bias and to investigate how different prevalence values could impact the classification probabilities of the discriminant, we simulated infant populations with different prevalences, as shown in Figure 6, in the following way. A simulation population of 5000 infants was constructed by stratifying the WITS population into HIV infected and uninfected infants. Then we randomly selected pre-specified numbers of HIV infected and uninfected infants with replacement to obtain the simulated population of 5000 infants. For instance, to obtain a simulated population with a 10% prevalence of HIV infection, we selected with replacement 500 “infants” from the HIV infected group in the WITS population and 4500 “infants” from the HIV uninfected group in WITS. The bottom of the figure shows the number and proportion of infants who were classified as HIV-1-infected or HIV uninfected [ranging from 62% (when the prevalence of HIV infection is 50%) to 84%]. The positive and negative predictive values remain above 90% over a wide range of prevalence of HIV infection.
Figure 6.
PPV and NPV based on discriminant function for simulated data with various prevalences of HIV-1 infection among Infants. * Indicates number and proportion of children who could be classified using CD4/CD8 ratio and hematocrit measurements
DISCUSSION
In this study, we evaluated a surrogate measure, the CD4/CD8 ratio, for its ability to discriminate between HIV infected and uninfected infants born to HIV infected women. Our analyses suggest that in situations where virologic diagnosis is not feasible and facilities exist for CD4 and CD8 T cell determinations, the CD4/CD8 ratio can play a valuable role in providing a presumptive diagnosis of HIV infection and can aid in the management of HIV-exposed infants born to HIV infected women.
The rationale for selecting CD4/CD8 ratio as a potential diagnostic test was made on the basis of several known facts about HIV pathogenesis. It is well established that CD4% are normally extremely high in infants relative to CD8% at birth and during the first several months of life 21,22 and that they gradually decline with age.21,23 Although CD4 T cell counts of HIV infected infants decline faster than those of uninfected infants, 15-18, 24 depletion of CD4 T cells in HIV infected infants does not become apparent early in life except in rare instances where immunosuppression is evident at birth.25, 26 In contrast to CD4 T cells, CD8 T cells show a greater turnover and are greatly influenced by the immune activation that accompanies HIV infection.27, 28 The ensuing CD8 T cell expansion begins early in HIV-1 infection, contributing to the altered CD4/CD8 ratio which becomes evident even in acute HIV infection in adults 29,30 and precedes detection of loss of CD4 T cells. The expansion of CD8 T cells and known relationship of CD4/CD8 ratio with HIV disease progression in HIV infected adults 31 led us to hypothesize that a similar CD8 T cell expansion occurs in HIV infected infants, making CD4/CD8 ratio a more attractive measure than CD4 T cell counts for diagnosis. Such a presumption assumes that the infant is otherwise healthy, without evidence of concurrent co-infection (fungal, parasitic, viral) which could effect CD8 T cell expansion. An additional advantage of the CD4/CD8 ratio is that it does not matter whether the ratio is derived from percentages or absolute numbers of CD4 and CD8 T cells.
To assess the value of the CD4/CD8 ratio as a diagnostic test, we made use of data from a cohort of North American infants born to HIV infected mothers with known HIV infection status based on virological testing. First, we compared CD4% and CD4/CD8 ratios in known HIV infected and uninfected infants. At birth, the difference between the two measures was marginal, but starting at two months of age, there was significant evidence that the CD4/CD8 ratio had better accuracy than CD4% in discriminating between HIV infected and uninfected infants in both univariate analysis and multivariate analyses. The discriminatory power at or after four months of age resulted in a trade off of approximately 60% specificity for 90% sensitivity or 65% sensitivity for a specificity of 90%, still far less than the values obtained when using PCR results (sensitivity 99%, specificity 98%).
In order to simplify the utilization of a given CD4/CD8 value at a particular age by a health care provider, percentile curves for CD4/CD8 ratios were generated by HIV infection status (infected and uninfected) as a function of age in months when the CD4% and CD8% determinations were made. As expected the CD4/CD8 ratio was high in both HIV infected and uninfected infants at birth. While there was considerable overlap at intermediate values, those at the upper or lower ends of the range of CD4/CD8 ratios were clearly able to discriminate between HIV-1 infected and uninfected infants. Importantly, the curves provide information about the sensitivity and specificity of a given ratio for diagnosis of HIV infection at all ages until age 12 months. With the caveat that data should ideally be developed for the local population, the percentile curves presented herein can be cautiously used by health care providers to determine the potential risk of the infant being infected or not, and combine it with other parameters including clinical judgment to facilitate management decisions.
The predictive value of CD4/CD8 ratio is further improved by incorporating hematocrit, a simple laboratory marker that is relatively easy to access in low resource areas. Markers such as total lymphocyte count, hemoglobin and serum albumin have previously been shown to be a useful surrogate for disease progression.32 We developed a simple discriminant analysis algorithm using the CD4/CD8 ratios at different time points and the hematocrit values at age 4 6 months. By varying the Bayes prior to the analysis, it was possible to develop a testing strategy that had a 94% positive predictive value and a 98% negative predictive value. The ability to rule out HIV infection was particularly strong using this analysis. While such information would not completely eliminate the need to continue monitoring for HIV infection, it could greatly minimize monitoring requirements and alleviate anxiety of the caregiver. In situations where safe alternatives to breast feeding are available, the probable diagnosis of not being infected by perinatal MTCT would allow measures to be taken to prevent infection after birth. The enhanced ability to make a presumptive diagnosis of “HIV infected” would be of paramount importance for setting up the plan for treatment and monitoring as per local guidelines. A limited number of infants are expected to fall in the indeterminate category classification by the discriminant value. Subjects in this category should ideally be subjected to virologic testing for a definitive diagnosis, thereby limiting nucleic acid testing to a few cases, instead of applying it for all HIV-exposed infants. Without virologic testing, the status of indeterminate cases would depend upon clinical staging or serologic testing during follow-up
It has been reported that antenatal antiretroviral prophylaxis can transiently influence hematological indices of HIV-exposed infants.19 However, we did not find any differences in either CD4/CD8 ratio or hematocrit between HIV infected infants whose mothers did or did not receive ARV drugs. Also, no differences were observed for the HIV uninfected infants between those whose mothers did or did not receive ARV drugs. Thus, this type of discriminant analysis is not affected by antiretroviral drug intervention in the mothers.
The predictive value of diagnostic tests is influenced by the prevalence of that particular condition. The WITS cohort was established prior to routine implementation of ARV prophylaxis for prevention of MTCT transmission of HIV but continued enrollment into the current era when both ARV prophylaxis and cesarean section before labor and before ruptured membranes are utilized for prevention of such transmission. In a simulated population, with the prevalence of HIV infection among infants ranging from 1% to 50%, using both the CD4/CD8 ratio and the hematocrit, the diagnosis of HIV infection could be made for 61-84% of infants by six months of age. CD4/CD8 ratio performed better with increasing age, and it might also be worthwhile evaluating it as a tool for monitoring disease progression or response to treatment.
We conclude that the CD4/CD8 ratio performs well as a diagnostic assay for HIV infection in infants, and its diagnostic potential may be enhanced when used in conjunction with other clinical or laboratory assessments, e.g. hematocrit. Several new cost effective methods are being investigated for obtaining CD4 and CD8 T cell counts 33, potentially leading to wider access to CD4/CD8 ratio. The diagnostic utility of the CD4/CD8 ratio in HIV exposed infants has received limited attention to date. This is the first study utilizing a large database of HIV exposed infants in whom the virologic diagnosis was established by DNA PCR and CD4/CD8 ratios were performed. Only one previous study utilizing the CD4/CD8 ratio has been reported in HIV exposed infants 34 in which there was a strong correlation between lower CD4/CD8 ratios and positive HIV-1 DNA PCR results. However, this was a small study. Our results suggest that a study is warranted in those resource-poor situations where CD4/CD8 ratio is feasible, and virologic diagnosis can be performed in order to validate the findings of our study. With higher rates of MTCT of HIV, the sensitivity and specificity are expected to be higher. In resource-poor settings without routine availability of virologic assays for infant diagnosis, establishing a presumptive diagnosis of HIV infection by surrogate assays such as the CD4/CD8 ratio early in life may allow timely intervention for HIV exposed infants to improve outcomes.
Acknowledgments
Funding: The WITS study was supported by the following grants from the NIH: U01 AI 034858, U01 DA 015054, U01 DA 015053, U01 AI 034841, U01 HD 036117, U01 HD 041983, N01 AI 085339, U01 AI 050274-01; NIH GCRC RR000188; NIH GCRC RR000645
APPENDIX. Principal Investigators, study coordinators and program officers for The Women and Infants Transmission Study
Clemente Diaz, Edna Pacheco-Acosta (University of Puerto Rico, San Juan, PR); Ruth Tuomala, Ellen Cooper, Donna Mesthene (Boston/Worcester Site, Boston, MA); Phil LaRussa, Alice Higgins (Columbia Presbyterian Hospital, New York, NY); Sheldon Landesman, Herman Mendez, Ava Dennie (State University of New York, Brooklyn, NY); Kenneth Rich, Delmyra Turpin (University of Illinois at Chicago, Chicago, IL); William Shearer, Norma Cooper (Baylor College of Medicine, Houston, TX); Joana Rosario (National Institute of Allergy and Infectious Diseases, Bethesda, MD); Kevin Ryan, (National Institute of Child Health and Human Development, Bethesda, MD); Vincent Smeriglio, Katherine Davenny (National Institute on Drug Abuse, Bethesda, MD); and Bruce Thompson (Clinical Trials & Surveys Corp., Baltimore, MD). Scientific Leadership Core: Kenneth Rich (PI), Delmyra Turpin (Study Coordinator).
Footnotes
Presented in part: 14th Conference on Retroviruses and Opportunistic Infections (February 25-28, 2007; Los Angeles, California) (abstract 686)
REFERENCES
- 1.Read JS. Prevention of mother-to-child transmission of HIV. In: Zeichner SL, Read JS, editors. Textbook of Pediatric HIV Care. Cambridge University Press; Cambridge, England: 2005. pp. 111–133. [Google Scholar]
- 2.Coovadia HM, Rollins NC, Bland RM, et al. Mother-to-child transmission of HIV-1 infection during exclusive breastfeeding in the first 6 months of life: an intervention cohort study. Lancet. 2007 Mar 31;369:1107–1116. doi: 10.1016/S0140-6736(07)60283-9. [DOI] [PubMed] [Google Scholar]
- 3.Little K, Newell ML, Luo C, Ngongo N, Borja MC, McDermott P. Estimating the number of vertically HIV-infected children eligible for antiretroviral treatment in resource-limited settings. Int J Epidemiol. 2007;36:679–87. doi: 10.1093/ije/dym019. [DOI] [PubMed] [Google Scholar]
- 4.Paintsil E, Andiman WA. Care and management of the infant of the HIV-1-infected mother. Semin Perinatol. 2007;31:112–123. doi: 10.1053/j.semperi.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Little K, Thorne C, Luo C, et al. Disease progression in children with vertically-acquired HIV infection in sub-Saharan Africa: reviewing the need for HIV treatment. Curr HIV Res. 2007;5:139–153. doi: 10.2174/157016207780077002. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen K, Bryson YJ. Diagnosis of HIV infection in children. Pediatr Clin North Am. 2000;47:39–63. doi: 10.1016/s0031-3955(05)70194-2. [DOI] [PubMed] [Google Scholar]
- 7.Sherman GG, Cooper PA, Coovadia AH, et al. Polymerase chain reaction for diagnosis of human immunodeficiency virus infection in infancy in low resource settings. Pediatr Infect Dis J. 2005;24:993–997. doi: 10.1097/01.inf.0000187036.73539.8d. [DOI] [PubMed] [Google Scholar]
- 8.Fiscus SA, Wiener J, Abrams EJ, Bulterys M, Cachaefeiro A, Respess RA. Ultra-sensitive P24 Antigen Assay for the Diagnosis of Perinatal HIV-1 Infection. J Clin Microbiol. doi: 10.1128/JCM.00813-07. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patton JC, Akkers E, Coovadia AH, Meyers TM, Stevens WS, Sherman GG. Evaluation of dried whole blood spots obtained by heel or finger stick as an alternative to venous blood for diagnosis of human immunodeficiency virus type 1 infection in vertically exposed infants in the routine diagnostic laboratory. Clin Vaccine Immunol. 2007;14:201–203. doi: 10.1128/CVI.00223-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiappini E, Galli L, Tovo PA, et al. Virologic, immunologic, and clinical benefits from early combined antiretroviral therapy in infants with perinatal HIV-1 infection. Aids. 2006;20:207–215. doi: 10.1097/01.aids.0000200529.64113.3e. [DOI] [PubMed] [Google Scholar]
- 11.Chinen J, Easley KA, Mendez H, Shearer WT. Decline of CD3-positive T-cell counts by 6 months of age is associated with rapid disease progression in HIV-1--infected infants. J Allergy Clin Immunol. 2001;108:265–268. doi: 10.1067/mai.2001.116573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shearer WT, Easley KA, Goldfarb J, et al. Evaluation of immune survival factors in pediatric HIV-1 infection. Ann N Y Acad Sci. 2000;918:298–312. doi: 10.1111/j.1749-6632.2000.tb05499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheon A, Fox H, Rich K, et al. for The Women and Infants Transmission Study Group The Women and Infants Transmission Study (WITS) of maternal-infant HIV transmission: study design, methods, and baseline data. J Womens Health. 1996;5:69–78. [Google Scholar]
- 14.Calvelli T, Denny TN, Paxton H, Gelman R, Kagan J. Guideline for flow cytometric immunophenotyping: a report from the National Institute of Allergy and Infectious Diseases, Division of AIDS. Cytometry. 1993;14:702–715. doi: 10.1002/cyto.990140703. [DOI] [PubMed] [Google Scholar]
- 15.Rich KC, et al. Lymphocyte plenotyping in infants: maturation of lymphocyte subpoplations and the effects of HIV infection. Clin Immunol Immunopath. 1997;85:273–281. doi: 10.1006/clin.1997.4439. [DOI] [PubMed] [Google Scholar]
- 16.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 17.Mandrekar JN, Mandrekar SJ. SUGI 30 Proceedings: Statistical methods in diagnostic medicine using SAS software. Vol 12:505–513. [Google Scholar]
- 18.Cole TJ, Green PJ. Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med. 1992;11:1305–1319. doi: 10.1002/sim.4780111005. [DOI] [PubMed] [Google Scholar]
- 19.Pacheco SE, McIntosh K, Lu M, et al. Effect of perinatal antiretroviral drug exposure on hematologic values in HIV-uninfected children: An analysis of the women and infants transmission study. J Infect Dis. 2006;194:1089–1097. doi: 10.1086/507645. [DOI] [PubMed] [Google Scholar]
- 20.Mussi-Pinhata M, do Carmo Rego M, Freimanis L, et al. Maternal antiretroviral exposure during pregnancy and infant hematological and liver enzyme abnormalities. Ped Infect Dis J. 2007 [in press] [Google Scholar]
- 21.McKinney RE, Jr., Wilfert CM. Lymphocyte subsets in children younger than 2 years old: normal values in a population at risk for human immunodeficiency virus infection and diagnostic and prognostic application to infected children. Pediatr Infect Dis J. 1992;11:639–644. [PubMed] [Google Scholar]
- 22.Shearer WT, Rosenblatt HM, Gelman RS, et al. Lymphocyte subsets in healthy children from birth through 18 years of age: the Pediatric AIDS Clinical Trials Group P1009 study. J Allergy Clin Immunol. 2003;112:973–980. doi: 10.1016/j.jaci.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Shearer WT, Easley KA, Goldfarb J, et al. Prospective 5-year study of peripheral blood CD4, CD8, and CD19/CD20 lymphocytes and serum Igs in children born to HIV-1 women. The P(2)C(2) HIV Study Group. J Allergy Clin Immunol. 2000;106:559–566. doi: 10.1067/mai.2000.109433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shearer WT, Rosenblatt HM, Schluchter MD, Mofenson LM, Denny TN. Immunologic targets of HIV infection: T cells. NICHD IVIG Clinical Trial Group, and the NHLBI P2C2 Pediatric Pulmonary and Cardiac Complications of HIV Infection Study Group. Ann N Y Acad Sci. 1993;693:35–51. doi: 10.1111/j.1749-6632.1993.tb26255.x. [DOI] [PubMed] [Google Scholar]
- 25.Nahmias AJ, Clark WS, Kourtis AP, et al. Thymic dysfunction and time of infection predict mortality in human immunodeficiency virus-infected infants. CDC Perinatal AIDS Collaborative Transmission Study Group. J Infect Dis. 1998;178:680–685. doi: 10.1086/515368. [DOI] [PubMed] [Google Scholar]
- 26.Kourtis AP, Ibegbu C, Nahmias AJ, et al. Early progression of disease in HIV-infected infants with thymus dysfunction. N Engl J Med. 1996;335:1431–1436. doi: 10.1056/NEJM199611073351904. [DOI] [PubMed] [Google Scholar]
- 27.Hazenberg MD, Stuart JW, Otto SA, et al. T-cell division in human immunodeficiency virus (HIV)-1 infection is mainly due to immune activation: a longitudinal analysis in patients before and during highly active antiretroviral therapy (HAART) Blood. 2000;95:249–255. [PubMed] [Google Scholar]
- 28.Sachsenberg N, Perelson AS, Yerly S, et al. Turnover of CD4+ and CD8+ T lymphocytes in HIV-1 infection as measured by Ki-67 antigen. J Exp Med. 1998;187:1295–1303. doi: 10.1084/jem.187.8.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mattapallil JJ, Letvin NL, Roederer M. T-cell dynamics during acute SIV infection. Aids. 2004;18:13–23. doi: 10.1097/00002030-200401020-00002. [DOI] [PubMed] [Google Scholar]
- 30.Zaunders J, Carr A, McNally L, Penny R, Cooper DA. Effects of primary HIV-1 infection on subsets of CD4+ and CD8+ T lymphocytes. Aids. 1995;9:561–566. doi: 10.1097/00002030-199506000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Phillips AN, Sabin CA, Elford J, Bofill M, Lee CA, Janossy G. CD8 lymphocyte counts and serum immunoglobulin A levels early in HIV infection as predictors of CD4 lymphocyte depletion during 8 years of follow-up. Aids. 1993;7:975–980. doi: 10.1097/00002030-199307000-00011. [DOI] [PubMed] [Google Scholar]
- 32.Mofenson LM, Harris DR, Moye J, et al. Alternatives to HIV-1 RNA concentration and CD4 count to predict mortality in HIV-1-infected children in resource-poor settings. Lancet. 2003;362:1625–1627. doi: 10.1016/s0140-6736(03)14825-8. [DOI] [PubMed] [Google Scholar]
- 33.Gomo E, Ndhlovu P, Vennervald BJ, Nyazema N, Friis H. Enumeration of CD4 and CD8 T-cells in HIV infection in Zimbabwe using a manual immunocytochemical method. Cent Afr J Med. 2001;47:64–70. doi: 10.4314/cajm.v47i3.8596. [DOI] [PubMed] [Google Scholar]
- 34.Zijenah LS, Katzenstein DA, Nathoo KJ, et al. T lymphocytes among HIV-infected and -uninfected infants: CD4/CD8 ratio as a potential tool in diagnosis of infection in infants under the age of 2 years. J Transl Med. 2005;3:6. doi: 10.1186/1479-5876-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]