Abstract
Objectives Estetrol (E4) is a natural estrogen produced by the human fetal liver. In combination with drospirenone (DRSP) or levonorgestrel (LNG), E4 blocks ovulation and has less effect on haemostatic biomarkers in comparison with ethinylestradiol (EE) combined with DRSP. This study evaluates the impact of several doses of E4/DRSP and E4/LNG on safety parameters such as liver function, lipid metabolism, bone markers and growth endocrine parameters.
Methods This was a dose-finding, single-centre, controlled study performed in healthy women aged 18 to 35 years with a documented pretreatment ovulatory cycle. Participants received 5 mg or 10 mg E4/3 mg DRSP; 5 mg, 10 mg or 20 mg E4/150 μg LNG; or 20 μg EE/3 mg DRSP as a comparator for three consecutive cycles in a 24/4-day regimen. Changes from baseline to end of treatment in liver parameters, lipid metabolism, bone markers and growth endocrinology were evaluated.
Results A total of 109 women were included in the study. Carrier proteins were minimally affected in the E4/DRSP and E4/LNG groups, in comparison with the EE/DRSP group, where a significant increase in sex hormone-binding globulin was observed. Similarly, minor effects on lipoproteins were observed in the E4 groups, and the effects on triglycerides elicited by the E4 groups were significantly lower than those in the EE/DRSP group. No imbalances in bone markers were observed in any groups. No alterations in insulin-like growth factor were observed in the E4 groups.
Conclusions E4-containing combinations have a limited effect on liver function, lipid metabolism, and bone and growth endocrine parameters.
Chinese Abstract
摘要
目的 雌四醇(E4)是来源于人胎儿肝脏的天然雌激素。雌四醇与屈螺酮(DRSP)或左炔诺孕酮(LNG)配伍的复方口服避孕药制剂,能够抑制排卵,同时相较于炔雌醇(EE)与屈螺酮配伍制剂,它对凝血功能的各项指标影响较小。本研究的目的是为了评估不同剂量的雌四醇与屈螺酮以及左炔诺孕酮的配伍制剂对肝功能、脂代谢、骨骼转换标志物、以及生长激素分泌等安全性指标的影响。
方法 本研究为开放的、单中心、剂量定义的对照研究,研究对象为18-35岁、具有良好的排卵周期的健康妇女,受试者分别接受5mg或10mg雌四醇/屈螺酮3mg(E4/DRSP)制剂,5mg、10mg、20mg雌四醇/左炔诺孕酮150ug(E4/LNG)制剂,以及20ug炔雌醇/屈螺酮(EE/DRSP)3mg制剂作为对照,连续三个24/4天周期。自基线到研究结束,测定肝功能、脂代谢、骨转换以及生长激素分泌的各项指标,对其改变进行评估。
结果 109名妇女入选该研究。与EE/DRSP组比较,E4/DRSP以及E4/LNG组肝脏产生的运载蛋白受影响很小,但是EE/DRSP组性激素结合蛋白的含量明显增加。同样,E4组的脂蛋白受影响很小,而且对甘油三酯的影响显著低于EE/DRSP组。在所有研究组中,都没有观察到骨转换的失衡。在E4组,没有观察到胰岛素样生长因子的改变。
结论 含有E4的复方制剂对肝功能、脂代谢、骨骼转换以及生长内分泌的影响有限。
关键词 骨标记物、屈螺酮、内分泌、雌四醇、左炔诺孕酮、脂代谢、肝功能
KEYWORDS: Bone markers, Drospirenone, Endocrinology, Estetrol, Levonorgestrel, Lipid metabolism, Liver function
INTRODUCTION
Combined oral contraceptives (COCs) are a well-known and reliable method of reversible contraception used by women worldwide1. An optimal COC would completely block ovulation and suppress endogenous ovarian activity, while causing minimal changes in haemostasis, lipid metabolism, liver function and growth hormone (GH) endocrinology. In addition, it would provide an adequate spotting and bleeding pattern, with no deterioration in quality of life. The majority of the currently available COCs contain ethinylestradiol (EE), as the estrogen component, and a variety of progestogens including drospirenone (DRSP) and levonorgestrel (LNG).
The estrogens estradiol (E2) and E2 valerate have recently been introduced; however, EE remains the most widely used estrogen in COCs. Although EE has been shown to be safe, it causes subjective side effects (breast tension and tenderness, weight gain, oedema, nausea, vomiting, bloating, headache and mood changes)2. In addition, EE increases the synthesis of various liver proteins, such as lipoproteins, angiotensinogen, sex hormone-binding globulin (SHBG), corticosteroid-binding globulin (CBG) and ceruloplasmin3. Furthermore, the use of EE and its enterohepatic recirculation is related to a doubling of all types of gallbladder diseases4. The most serious adverse effects of EE and other exogenous estrogens are cardiovascular complications, both arterial (hypertension, myocardial and cerebral infarction) and venous (deep vein thrombosis and pulmonary embolism). These cardiovascular complications are rare but serious, especially in young, healthy women5,6. Strategies to reduce the risk of deep vein thrombosis are: (i) lowering the dose of EE; and (ii) substituting EE with E2 or another estrogen7,8.
The development of new estrogens such as estetrol (E4) holds promise for the safety and tolerability of future COCs3. E4 is only synthesised by human fetal liver and is therefore present only during human pregnancy9. Late pregnancy maternal plasma levels are around 1 ng/ml, while fetal plasma levels are 12 to 19 times higher at term9,10. E4 does not bind to SHBG, has a low impact on SHBG production by human hepatocytes, and is mainly excreted in the urine rather than through the biliary route9,11. In postmenopausal women, E4 was found to cause dose-dependent decreases in both the marker of bone resorption (C-telopeptide) and the marker of bone formation (osteocalcin)12. The inhibitory effect was found to be more prominent on bone resorption, suggesting the possibility of positive bone formation12.
Recent studies of uterine and vascular actions of E4 delineate a distinctive profile of estrogen receptor alpha (ERα) modulation. E4 activates the nuclear ERα, but antagonises the membrane-bound ERα. Transgenic mice lacking this membrane receptor fail to ovulate and are infertile. E4 could therefore be particularly suitable for a contraceptive indication13,14.
Preclinical and phase I clinical research has suggested that E4 may be a suitable replacement for EE in COCs12,15. There is preclinical proof that E4 effectively inhibits ovulation in a dose-dependent manner similar to the action of EE16. In a recent open-label phase II dose-finding study (NTR2102/EudraCT 2009-011858-17), two E4/DRSP and three E4/LNG combinations vs. 20 μg EE/3 mg DRSP were investigated for their effects on ovulation inhibition and haemostatic biomarkers (I.J.M. Duijkers, unpublished data; C. Kluft, unpublished data). All doses of 5 mg, 10 mg or 20 mg E4, in combination with DRSP or LNG, blocked ovulation and dose-dependently decreased ovarian activity. In addition, minor effects were observed on haemostatic biomarkers.
In order to more precisely characterise the safety of E4-containing COCs, we evaluated in the present study the pharmacodynamic effects of E4/DRSP and E4/LNG vs. EE/DRSP on liver function, lipid metabolism, bone markers and growth endocrine parameters. This study provides clinically relevant information on E4 pharmacokinetics.
METHODS
This was an open-label, dose-finding phase II study conducted at a single centre in the Netherlands (Dinox BV, Groningen, the Netherlands) and registered as NTR2102/EudraCT 2009-011858-17. The study was approved by an independent ethics committee and conducted in accordance with the ethical principles established by the Declaration of Helsinki and the International Conference on Harmonization—Good Clinical Practice Guidelines. Written informed consent was obtained from all participants before enrolment in the study.
Participants
Healthy women, aged 18 to 35 years with a BMI between 18 and 30 kg/m2, were eligible for inclusion in the study. The exclusion criteria were in line with the World Health Organization's medical eligibility criteria for COC use17. Women unwilling to use a non-hormonal method of contraception during the study were also excluded. At screening, all women underwent thorough medical and gynaecological examinations, and blood and urine were sampled for routine laboratory analyses. Eligible women had to use a barrier method of contraception during a washout cycle, the pretreatment cycle and the subsequent cycles in the study up to 7 days after the follow-up visit. Moreover, all women had to have at least two spontaneous cycles before starting the study medication.
Treatment and study design
The study consisted of a washout cycle (when using COCs), followed by one pretreatment observational cycle to verify ovulation, three 28-day treatment cycles (cycles 1–3) and one post-treatment cycle. The pretreatment study visit was held on day 3 (± 1) after the start of spontaneous menses. Eligible women were assigned to one of six treatment groups: 5 mg or 10 mg E4 combined with 3 mg DRSP; 5 mg, 10 mg or 20 mg E4 combined with 150 μg LNG; or the comparator 20 μg EE combined with 3 mg DRSP (Yaz; Bayer HealthCare Pharmaceuticals, Berlin, Germany). All subjects were stratified according to the day of ovulation in the pretreatment cycle, except those in the 20 mg E4/LNG group, as this group was added later during the course of the study. The sample size was primarily determined to allow conclusions on ovulation inhibition (I.J.M. Duijkers, unpublished data), which was expected to vary between 1% and 14% in a worst case scenario for three consecutive cycles. As a result, approximately 18 participants had to be included in each treatment group.
The first study treatment was taken on the first day of the next menses and continued over three cycles of 24 days, each followed by 4 days of no treatment (E4 groups) or placebo (comparator) intake. Compliance was assessed by recording study treatment (pill intake) on diary cards. Blood samples for laboratory measurements (liver function, bone biomarkers, growth endocrine parameters) were taken on day 3 (± 1) of the pretreatment cycle, on days 3 (± 1) and 24 (± 1) of cycles 1 and 3, and at the follow-up visit on day 3 (± 1) of the cycle following the post-treatment cycle. Blood samples for pharmacokinetic assessments in subjects allocated to one of the E4/DRSP or E4/LNG groups were taken on day 24 (± 1) of cycles 1 and 3 before the pill intake scheduled for that day. Excretion of E4 and E4 conjugates was investigated by collection of 24 h urine twice between day 21 and 24 of cycle 3 in the 10 mg E4/LNG group only.
Laboratory measurements
Laboratory analyses were performed under the responsibility of the Clinical-Chemical Laboratory, Canisius Wilhelmina Hospital, Nijmegen, the Netherlands. Pharmacokinetic analyses were performed by Xendo Drug Development BV, Groningen, the Netherlands.
Liver function and lipid metabolism
The carrier proteins SHBG, CBG and ceruloplasmin were measured by the respective immunoassays: COBAS ECLIA (Roche Diagnostics, Mannheim, Germany), CBG RIA (BioSource, San Diego, California, USA) and COBAS INTEGRA (Roche Diagnostics). Serum levels of lipids and lipoproteins (HDL-, LDL- and total cholesterol, and triglycerides) were measured by enzymatic (colorimetric) tests (Roche Diagnostics). The liver enzymes aspartate aminotransferase/glutamic-oxalocetic transaminase (ASAT/SGOT), alkaline phosphatase and γ-glutamyl transferase (γGT) were measured by enzymatic (colorimetric) tests (Roche Diagnostics).
Bone parameters
The biomarkers of bone turnover C-telopeptide and osteocalcin were measured by the immunoassays β-CrossLaps/serum and N-MID Osteocalcin (Roche Diagnostics), respectively.
Growth endocrinology
Insulin-like growth factor (IGF)-I and GH were measured using immunometric techniques (Siemens Medical Solutions Diagnostics, Los Angeles, USA), IGF-II by radioimmunoassay (RIA) in Sep-Pak C18 extracts of plasma18, and IGF-binding protein (IGFBP)-1 and -3 by specific RIAs.19,20
Pharmacokinetics
Plasma E4 trough levels were investigated by collection of steady-state samples in the E4 treatment groups 24 h after study medication intake.
The excretion of E4 and E4 conjugates was investigated by collection of 24 h urine at steady state from 10 subjects in the 10 mg E4 treatment groups between day 21 and day 24 of cycle 3. Urinary recovery was expressed as total excretion of E4 in urine compared with daily intake of 10 mg E4.
E4 plasma and urine levels were measured by liquid chromatography, followed by tandem mass spectrometry detection (LC-MS/MS; lower limit of quantification 25 pg/ml). For the E4 conjugates in urine samples, hydrolysis was performed by β-glucuronidase and sulfatase before the measurement of the released E4 by LC-MS/MS. The E4 glucuronide and E4 sulfate levels were then calculated semi-quantitatively, based on the assumption that hydrolysis is complete. Total E4 excretion was calculated by summing the excreted quantities of unconjugated E4, E4 glucuronide and E4 sulfate after the E4 conjugate quantities were corrected using the E4/E4 glucuronide and E4/E4 sulfate molecular weight ratio.
Statistical analysis
This was an exploratory study. The intention-to-treat (ITT) population (subjects who received at least one dose of study treatment and had at least one post-baseline assessment) served as a basis for the statistical analysis. The ITT population was identical to the all-subjects-treated (AST) population.
Primary analyses included the investigation of pharmacodynamic effects on liver function, lipid metabolism and bone biomarkers. Quantitative summary statistics and a summary of change from baseline to treatment cycle 3 day 24 were performed on these parameters, as well as for SHBG at cycle 1 day 24. Box whisker plots for relative changes from baseline to treatment cycle 3 day 24 were prepared for lipid and lipoprotein parameters. Values assessed on pretreatment cycle day 3 served as baseline.
Secondary analyses included the investigation of pharmacodynamic effects on growth endocrine parameters.
In addition, the differences in the changes from baseline to the end of treatment in carrier proteins, lipids parameters, bone biomarkers and growth endocrine parameters were compared using ANOVA between the different groups after having pooled the two E4/DRSP groups and the three E4/LNG groups. In the statistical analyses, p < 0.01 was used as a criterion for statistical significance.
Finally, quantitative summary statistics were performed for pharmacokinetic analyses of E4 steady-state levels and excretion data.
RESULTS
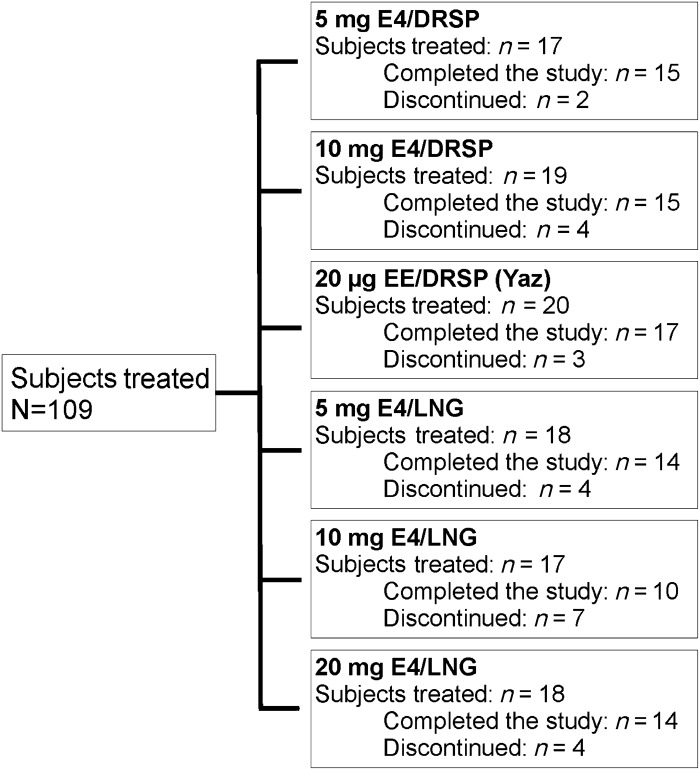
The study was performed from November 2009 until November 2010. In total, 111 subjects were included and assigned to one of the six treatment groups. Two women dropped out before starting study medication, one withdrew her consent and the other became pregnant in the pretreatment cycle. Of the 109 subjects treated, the majority (85/109; 78.0%) completed the study. The proportion of subjects completing the study was similar across the treatment groups (77.8–88.2%), with the exception of the 10 mg E4/LNG group, in which 58.8% (10/17) completed the study (Figure 1). Fifteen subjects discontinued the study during the treatment phase and nine did not complete the post-treatment cycle. Reasons for discontinuation in these cases were adverse events (five subjects: emotional lability and acute bronchitis, tiredness, increased frequency of headache, mood swings, decreased libido and headache, respectively), intracyclic bleeding (one subject), personal reasons (one subject), use of prohibited concomitant medication to treat acute bronchitis (one subject), incorrect study medication intake (one subject), withdrawal of consent (one subject), pregnancy during the post-treatment cycle (one subject) and inability to adhere to the visit schedule (13 subjects).
Figure 1. Allocation of subjects by treatment group (ITT and AST populations).
Overall demographic and baseline characteristics of the 109 subjects treated were generally similar across the treatment groups (Table 1). The mean age was 22.9 years (range 18–33 years). Mean BMI was slightly different between groups and ranged from 21.5 kg/m2 in the 5 mg E4/LNG group to 24.3 kg/m2 in the 20 mg E4/LNG group. The distribution of races was similar among groups and was predominantly white (82.4–100%). The safety and tolerability profile associated with the different combinations is reported in the publication on ovulation inhibition in the same subjects (I.J.M. Duijkers, unpublished data).
Table 1. Main demographics and baseline characteristics (ITT and AST populations).
| Characteristic |
5 mg E4/DRSP n = 17 |
10 mg E4/DRSP n = 19 |
20 μg EE/DRSP n = 20 |
5 mg E4/LNG n = 18 |
10 mg E4/LNG n = 17 |
20 mg E4/LNG n = 18 |
|---|---|---|---|---|---|---|
| Age, years Mean (SD) Range |
24.5 (3.2) 20–33 |
23.7 (3.7) 20–32 |
23.4 (3.9) 18–33 |
22.3 (2.6) 18–28 |
22.4 (2.4) 19–27 |
21.1 (2.3) 18–26 |
| BMI, kg/m2 Mean (SD) Range |
22.7 (2.4) 18.3–26.1 |
23.2 (3.2) 18.8–30.0 |
23.0 (2.9) 19.2–28.3 |
21.5 (1.7) 18.2–24.5 |
21.8 (2.5) 18.7–27.4 |
24.3 (3.4) 19.1–29.8 |
| Race, n (%) White Black Asian Other |
14 (82.4) 1 (5.9) 2 (11.8) 0 |
18 (94.7) 0 0 1 (5.3) |
19 (95.0) 0 1 (5.0) 0 |
16 (88.9) 0 0 2 (11.1) |
17 (100) 0 0 0 |
16 (88.9) 2 (11.1) 0 0 |
LIVER FUNCTION AND LIPID METABOLISM
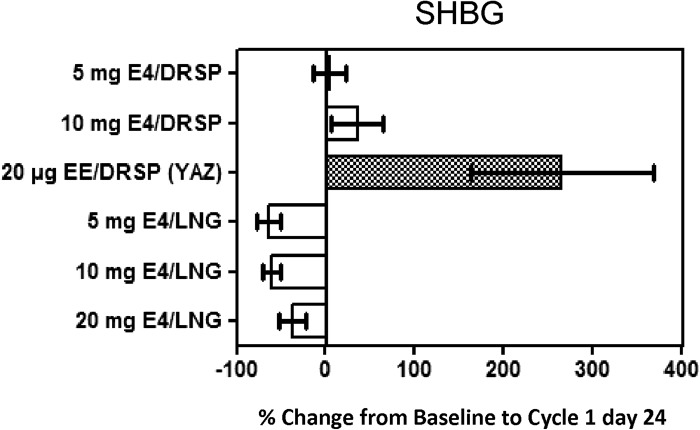
A dose-dependent response was observed in the E4/DRSP and E4/LNG groups for the carrier protein parameters (Table 2; C. Kluft, unpublished data). A decrease in SHBG was observed in the E4/LNG groups (mean change up to − 69.0%). By contrast, increases in SHBG were observed in the 5 mg (7.9%) and 10 mg (44.5%) E4/DRSP groups, but these were still considerably less than the 306.3% observed in the EE/DRSP group. All effects on SHBG were already apparent at treatment cycle 1 (Figure 2).
Table 2. Percentage change from baseline at treatment cycle 3 day 24 for carrier proteins, lipoproteins, bone biomarkers, and growth and steroid endocrine parameters (ITT population).
| Parameter |
5 mg E4/DRSP n = 17 |
10 mg E4/DRSP n = 19 |
20 μg EE/DRSP n = 20 |
5 mg E4/LNG n = 18 |
10 mg E4/LNG n = 17 |
20 mg E4/LNG n = 18 |
|---|---|---|---|---|---|---|
| SHBG | 7.9 (26.2) | 44.5 (34.1) | 306.3 (117.7) | − 69.0 (11.8) | − 64.8 (11.9) | − 44.2 (18.0) |
| CBG | 17.1 (16.6) | 28.1 (19.6) | 170.3 (75.6) | − 6.9 (17.2) | 5.9 (13.3) | 25.2 (25.0) |
| Ceruloplasmin | 8.2 (12.2) | 16.1 (11.1) | 69.0 (22.9) | − 5.4 (14.6) | 0.7 (9.9) | 16.2 (6.1) |
| HDL-cholesterol | 8.1 (14.0) | 5.6 (11.5) | 15.2 (11.3) | − 16.9 (20.7) | − 11.9 (14.1) | − 19.0 (10.9) |
| LDL-cholesterol | 6.7 (20.7) | 6.3 (18.3) | − 9.2 (22.1) | − 5.9 (16.1) | − 13.8 (20.2) | 8.9 (17.9) |
| Total cholesterol | 5.2 (9.8) | 5.0 (9.6) | 4.9 (10.3) | − 12.8 (9.1) | − 15.5 (14.4) | − 7.6 (9.1) |
| Triglycerides | 6.4 (36.7) | 10.0 (48.5) | 61.2 (51.2) | − 24.6 (33.7) | − 29.7 (26.5) | − 27.4 (16.5) |
| ASAT/SGOT | − 4.0 (11.9) | 2.0 (22.1) | − 9.6 (25.6) | − 11.6 (24.9) | − 12.4 (21.9) | − 13.3 (18.6) |
| Alkaline phosphatase | − 11.3 (6.6) | − 17.6 (8.6) | − 20.6 (11.8) | − 7.5 (12.5) | − 5.8 (14.9) | − 4.7 (12.5) |
| γGT | − 4.8 (18.5) | − 8.2 (14.6) | − 11.0 (20.9) | − 0.6 (19.8) | 3.6 (16.0) | 2.7 (17.7) |
| C-telopeptide | − 8.6 (16.8) | − 13.4 (20.2) | − 34.9 (17.8) | − 6.4 (22.5) | − 12.4 (23.0) | − 22.4 (18.8) |
| Osteocalcin | − 10.4 (11.1) | − 16.3 (11.9) | − 22.3 (11.7) | − 4.1 (16.6) | 0.8 (19.7) | − 13.0 (16.1) |
| IGF-I | − 5.9 (10.8) | − 11.5 (17.7) | − 41.9 (14.0) | 1.4 (7.4) | 3.4 (20.0) | − 8.8 (12.0) |
| IGF-II | − 0.7 (8.4) | − 2.3 (13.1) | 4.7 (7.7) | − 0.7 (9.4) | 4.8 (18.6) | − 2.2 (6.9) |
| IGFBP-1 | 21.1 (52.3) | 0.0 (30.6) | 190.9 (245.0) | − 7.5 (46.7) | 56.5 (251.5) | 41.9 (83.8) |
| IGFBP-3 | 7.4 (8.0) | 1.4 (11.0) | 3.9 (12.6) | 1.3 (12.4) | 2.0 (12.9) | 16.3 (11.0) |
| GH | 100.0 (191.6) | 314.1 (722.4) | 238.4 (508.4) | 173.9 (755.1) | 357.5 (750.3) | 467.7 (1191.3) |
Values are mean (SD) percentage change.
Figure 2. Mean (SD) percentage change from baseline to cycle 1 day 24 in SHBG (ITT population).
In the E4/DRSP and E4/LNG groups, CBG was marginally affected (mean changes between − 6.9% and 28.1%), whereas a substantial increase was observed in the EE/DRSP group (mean change 170.3%). Ceruloplasmin, the major copper-carrying protein in the blood, is synthesised by hepatocytes under the influence of estrogens21. By increasing copper availability, increased ceruloplasmin levels contribute to the enhanced oxidative stress observed in COC users22. The effect on ceruloplasmin was minimal in the E4/DRSP (8.2% and 16.1%) and E4/LNG groups (between − 5.4% and 16.2%), whereas for the EE/DRSP group a more pronounced increase of 69.0% was noted (Table 2). After pooling the E4/DRSP and E4/LNG groups, SHBG, CBG and ceruloplasmin levels were significantly lower in the E4 groups compared with the EE/DRSP group. The differences in SHBG level between the E4/DRSP and the E4/LNG groups also reached statistical significance but the changes in CBG and ceruloplasmin did not.
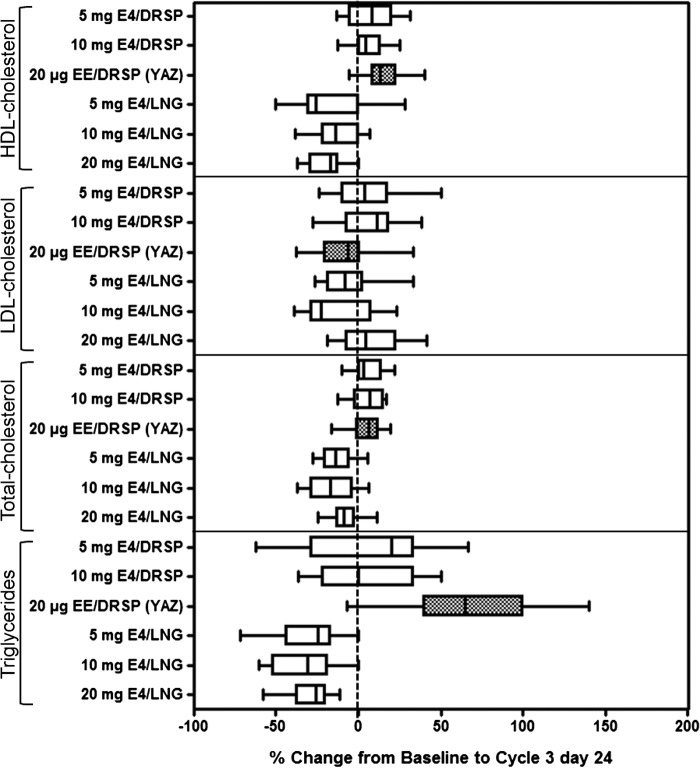
In the DRSP-treated groups, HDL-cholesterol increased in the E4/DRSP and EE/DRSP groups (up to 8.1% and 15.2%, respectively), but decreased in the E4/LNG groups (up to − 19.0%). LDL- cholesterol slightly increased in the E4/DRSP and 20 mg E4/LNG groups (up to 6.7% and 8.9%, respectively), but decreased in the other E4/LNG groups (up to − 13.2%), as well as in the EE/DRSP group (− 9.2%). Consequently, total cholesterol increased slightly in the DRSP-treated groups (from 4.9% to 5.2%), but decreased in the E4/LNG groups (up to − 15.5%). Triglyceride levels decreased by up to − 29.7% in the E4/LNG treatment groups, but increased in the E4/DRSP and EE/DRSP groups (mean change up to 10.0% and 61.2%, respectively) (Table 2, Figure 3).
Figure 3. Box whisker plots for E4/DRSP, E4/LNG (all treatment groups) and EE/DRSP of relative changes from baseline to cycle 3 day 24 for lipid and lipoprotein parameters (ITT population). The edges of the boxes represent the 25th and 75th sample percentiles (quartiles); the vertical line in the boxes shows the median; and whiskers are drawn up to the smallest and largest value within 1.5 times the interquartile range.
When comparing the pooled results of the E4/LNG groups with those of the E4/DRSP groups, the changes in HDL-cholesterol, total cholesterol and triglycerides were all statistically different, while the changes in LDL-cholesterol did not reach statistical significance.
The changes in lipid parameters elicited by the comparator (EE/DRSP) were statistically significantly different from those in the pooled E4/LNG groups, except the decrease in LDL-cholesterol. The E4/DRSP combinations did not elicit significant differences in lipid parameters in comparison with EE/DRSP.
Small decreases in the liver enzymes ASAT/ SGOT and alkaline phosphatase (mean changes up to − 13.3% and − 7.5%, respectively) were observed in the E4/LNG groups and in the 5 mg E4/DRSP (− 4.0% and − 11.3%, respectively) and EE/DRSP groups (− 9.6% and − 20.6%, respectively). A small increase in ASAT/SGOT was observed in the 10 mg E4/DRSP group (2.0 %) and a decrease in alkaline phosphatase (− 17.6%). γGT remained stable in all E4/LNG groups, whereas in both E4/DRSP groups and in the EE/DRSP group small decreases were observed (− 4.8%, − 8.2% and − 11.0%, respectively). Due to the large interindividual variations, no statistically significant differences were found between the pooled E4/DRSP and E4/LNG groups or between the E4 groups and the EE/DRSP group in the liver enzymology parameters, except for the alkaline phosphatase level, which was significantly lower in the EE/DRSP group compared with the E4/LNG group.
Bone parameters
In the E4 groups, a dose-related decrease was observed for the biomarkers of bone resorption (C-teloppetide) and bone formation (osteocalcin) (mean changes up to − 22.4% and − 16.3%, respectively). A non-significant greater suppression of bone turnover (− 34.9% and − 22.3%, respectively) was observed in the EE/DRSP groups (Table 2).
The pooled E4/DRSP combinations had significantly less impact on C-telopeptide levels than either of the other combinations. Osteocalcin levels were significantly lower in the EE/DRSP group than in the pooled E4/LNG groups, while no statistically significant differences were seen between the E4-containing combinations and the DRSP-containing combinations.
Growth endocrinology
No clear effects on the growth endocrine parameters IGF-I, IGF-II and IGFBP-3 were observed in any of the E4/DRSP and E4/LNG groups. In the EE/DRSP group, IGF-I was statistically significantly decreased (mean change − 41.9%) in comparison with the pooled E4/DRSP and E4/LNG groups, while no effect was observed on IGF-II and IGFBP-3 (Table 2). An effect on IGFBP-1 was observed in the E4/LNG groups (mean change up to 56.5%), and a limited effect (21.1% and 0%, respectively) in the 5 mg and 10 mg E4/DRSP groups. By contrast, a large increase was observed in the EE/DRSP group (190.9%) (Table 2). This was statistically significantly different from the pooled E4/DRSP and E4/LNG groups. Although there were large inter-individual differences, GH levels increased in all groups. For the E4/DRSP, E4/LNG and EE/DRSP groups, the mean changes were up to 314.1%, 467.7% and 238.4%, respectively, with no statistical differences (Table 2). In the follow-up cycle, a return to baseline levels was observed for all growth endocrine parameters in all groups, except for GH, which remained increased in the E4/DRSP (up to 174.1%), E4/LNG (up to 216.9%) and EE/DRSP (129.9) groups (data not shown).
Pharmacokinetics
A dose-dependent increase was observed in E4 plasma levels. Administration of 20 mg E4/LNG resulted in mean E4 trough levels of 2268 pg/ml and 2005 pg/ml in treatment cycles 1 and 3, respectively (Table 3).
Table 3. E4 plasma trough levels (pg/ml).
| Treatment cycle |
5 mg E4/DRSP n = 17 |
10 mg E4/DRSP n = 19 |
5 mg E4/LNG n = 18 |
10 mg E4/LNG n = 17 |
20 mg E4/LNG n = 18 |
|---|---|---|---|---|---|
| Cycle 1 | 638 (445) | 1361 (455) | 510 (216) | 1006 (395) | 2268 (851) |
| Cycle 3 | 568 (331) | 1366 (387) | 527 (239) | 880 (354) | 2005 (793) |
Values are mean (SD).
In 10 subjects from the 10 mg groups (E4/DRSP or E4/LNG), E4 was primarily excreted in the urine as E4 glucuronide (median 60.7%, range 47.6–77.2%) and, to a lesser extent, as E4 sulfate (median 17.6%, range 13.2–22.1%). Urinary excretion of unconjugated E4 was negligible (range 0.2–0.7%). The median total E4 excretion in urine was 79.7% (range 61.1–99.0%).
DISCUSSION
Findings and interpretation
A clinical programme has been initiated to develop fetal E4 as a replacement for EE in COCs. Ovulation inhibition by E4 has been assessed preclinically,16 and in young healthy women (I.J.M. Duijkers, unpublished data). E4, in combination with DRSP or LNG, has been shown to be effective in suppressing ovulation (I.J.M. Duijkers, unpublished data). In addition, it was observed that E4 had a minor effect on haemostatic biomarkers, both on coagulation and on fibrinolysis (C. Kluft, unpublished data).
Pharmacokinetic studies in early postmenopausal women revealed an elimination half-life (t ½) of E4 of approximately 28 h, allowing once-daily oral administration15. This was recently confirmed in a study in healthy women, aged 18 to 45 years and receiving combined 20 mg E4/LNG tablets, in which a t ½ of 26.9 h was observed (data not published). For this formulation the maximum plasma concentration of E4 was 3490 pg/ml, reached at a t max of 1.3 h. The present observations reveal an E4 trough level of 2005 pg/ml in the third cycle for the same combination.
The present study compared the effects of 5 mg and 10 mg E4 plus DRSP, and 5 mg, 10 mg and 20 mg E4 plus 150 μg LNG, vs. 20 μg EE plus 3 mg DRSP, administered in a 24-day regimen during three cycles, on a series of liver function and endocrine parameters.
Compared with EE/DRSP, the E4/DRSP and E4/LNG combinations were associated with a significantly lower effect on SHBG. EE/DRSP raised SHBG levels over 300% compared with baseline. Similarly, E4/DRSP and E4/LNG had a limited effect on the other carrier proteins CBG and ceruloplasmin, in contrast to increases observed with EE/DRSP. This limited effect on SHBG in healthy young women is a confirmation of previous findings11. These findings suggest that E4 practically does not stimulate the production of SHBG in human hepatocytes, and in vivo E4 has limited influence on the SHBG plasma concentration or E4 availability to target tissues. Moreover, it is considered to be of relevance, since a change in SHBG with a COC could be interpreted as a measure of total estrogenicity and used as a predictor of the risk of venous thromboembolism23–25. Although there is some debate regarding SHBG as a thrombotic marker26,27, the European Medicines Agency recommends SHBG measurements for the estimation of thrombotic safety of a COC28. It was previously demonstrated that EE/DRSP use increases lipid peroxidation. The elevated levels of oxidised LDLs and lipid peroxides were correlated with the increase in plasma levels of copper induced by EE. The increase in plasma copper levels related to COC use is well known and has been attributed to the induction by estrogen of hepatic synthesis of the acute-phase protein ceruloplasmin, the main copper carrier protein29–31. It may, therefore, be hypothesised that the demonstrated low impact of E4 on ceruloplasmin levels may also result in a lower impact of an E4-containing COC on oxidative stress.
Both the E4/DRSP and E4/LNG combinations showed minor effects on lipid levels (HDL- and LDL-cholesterol). In comparison with EE/DRSP, the pooled E4/DRSP group was associated with a non-significant increase in HDL- and LDL-cholesterol levels and, consequently, in total cholesterol. In accordance with data from the literature, the EE/DRSP combination increased the level of HDL-cholesterol and decreased the level of LDL-cholesterol, leading to an increased level of total cholesterol32–37. All E4/LNG regimens reduced plasma triglyceride levels by approximately 30% (statistically significantly different from EE/DRSP), whereas the E4/DRSP combinations non-significantly raised triglyceride levels by 10%. The impact of EE/DRSP on the rise in triglyceride levels was more pronounced (approximately 60%). Increased triglyceride levels are considered a marker for cardiometabolic diseases, and it has been suggested that long-term use of COCs might increase the risk of acute metabolic syndrome38. Although a retrospective cohort study failed to confirm this association, the lack of increase in triglyceride levels in women exposed to E4-containing COCs might be beneficial39.
A balance between bone resorption and bone formation maintains the regulation of bone mineral density. E4 acts like a weak estrogen on several body systems, including liver function, but displays a comparable potency to that of EE on others such as bone turnover. This finding has been demonstrated in an osteoporosis rat model12,40. The present study did not detect any imbalances after treatment with E4/DRSP, E4/LNG or the comparator EE/DRSP in serum osteocalcin and C-telopeptide. Serum osteocalcin is produced almost exclusively by osteoblasts and is a sensitive marker of bone formation that correlates with histomorphometric measurements of bone formation in bone biopsy specimens41,42. C-telopeptide is a sensitive biomarker of bone degradation and turnover43. Previous short-term studies confirm the usefulness of these markers to document the effect of COC on bone metabolism. For example, serum osteocalcin levels were somewhat, but not significantly, lower during short-term (3 months) E2 valerate/dienogest COC pill use in comparison with basal values44. Serum osteocalcin was unchanged in women receiving a contraceptive vaginal ring or dermal patch for 6 or 12 months45. The decreased bone turnover observed in the present study with the E4/DRSP, E4/LNG and EE/DRSP COCs is indicative of a similar positive influence on bone turnover in young post-adolescent women.
No clear effects on IGF growth parameters were observed in any of the E4/DRSP or E4/LNG groups, but a decrease in IGF-I was noted in the women who received EE/DRSP, similar to that reported for other COCs containing EE/dienogest or EE/LNG46. No relevant differences were observed in plasma concentrations of IGFBP-1 and IGFBP-3 following E4/DRSP or E4/LNG. IGF-I is produced by the liver and excreted in the circulation, where it binds to IGFBP-1 and IGFBP-3. Because circulating IGF-I is mainly of hepatic derivation, its suppression by estrogen is probably a hepatocellular effect of the estrogen, whereas GH increase seems to be a consequence of IGF-I reduction47. As a result, hormonal contraceptives can modulate the GH/IGF-I axis during the reproductive years. The currently observed changes in levels of IGF-I and IGF-II, as well as in GH and IGFBP-1 and IGFBP-3 levels, with the EE/DRSP combination, are probably the consequence of the potent estrogenic action of EE on the hepatocytes. Hence, the negligible impact of E4 on liver function does not result in significant changes in IGF-I plasma concentrations.
Strengths and weaknesses of the study
This study is part of an exploratory open-label, dose-finding phase II study (NTR2102/EudraCT 2009-011858-17) investigating the efficacy and safety of COCs containing the new estrogen E4. In this initial report, the effects of two E4/DRSP and three E4/LNG combinations vs. 20 μg EE/3 mg DRSP were evaluated on ovulation inhibition, biomarkers of haemostasis and on liver function (present paper). The information gained from this initial programme is that E4, in combination with DRSP or LNG, at all doses tested, blocked ovulation and dose-dependently reduced ovarian activity (I.J.M. Duijkers, unpublished data). In addition, E4 had minor effects on haemostatic biomarkers (C. Kluft, unpublished data). Together with the findings in the present study (showing a limited effect on hepatic, lipid, bone and growth endocrine parameters), relevant original information has been obtained on contraceptive efficacy and safety of COCs containing E4 as the estrogen component. Finally, the pharmacokinetic data demonstrate that most of the E4 is bioavailable and is excreted in the urine as sulfate and glucuronide conjugates, in contrast to other estrogens, which are mainly excreted through the bile. This urinary excretion pattern may convey a significant advantage, since COCs containing EE significantly increase the incidence of gallbladder diseases48.
These preliminary safety data require confirmation and further evaluation in a larger population of healthy women exposed for longer periods of time to various combinations of E4/DRSP or E4/LNG. Longer term and larger studies are necessary to select a definitive regimen that not only efficiently blocks ovulation and adequately inhibits ovarian function but also delivers excellent quality of life and a satisfactory vaginal spotting and bleeding pattern. More detailed and focused studies should then confirm the minimal impact of the selected regimen on carbohydrate, lipid and lipoprotein metabolism, on oxidative stress markers, coagulation and fibrinolysis markers, and on bone metabolism.
Differences in results and conclusions in relation to other studies
The present study confirms earlier findings of E4 on liver cell metabolism and bone-sparing effects12,15. The effects of the EE/DRSP combination on bone turnover and bone mineral density have recently been investigated49,50. The positive influence of short-term EE/DRSP on bone turnover in young fertile women49 was in line with the findings in the present study, which did not detect any imbalances. However, a decrease in bone formation was observed after EE/DRSP administration during six consecutive cycles50.
Relevance of the findings: Implications for clinicians
According to preclinical and phase I clinical research, E4 seems suitable to replace EE in COCs12,15. This has been confirmed in healthy young women, where suppression of ovarian activity and inhibition of ovulation have been demonstrated (I.J.M. Duijkers, unpublished data). Also, based on the results of the present study, E4-containing COCs appear to interfere less with lipid metabolism and coagulation parameters while maintaining adequate bone protection. The available data will assist in selecting the optimal doses of E4 and progestogen for further evaluation.
Unanswered questions and future research
So far, there is substantial information on the pharmacological, pharmacokinetic and ovulation inhibition activities of E4 12. However, current knowledge on the bleeding pattern is minimal due to the limited number of subjects treated. Future trials are needed to provide information on the bleeding pattern of the E4 COC to be selected.
In a recent review of the pharmacological profile of estrogens in COCs, it was stated that new estrogens such as E4 differ from EE with regard to their pharmacological properties and target organs3. These differences hold promise for the safety and tolerability of future COCs.
CONCLUSION
The present study shows that, compared with the EE/DRSP combination, both E4/DRSP and E4/LNG have a limited effect on liver function, lipid metabolism, and bone and growth endocrine parameters.
ACKNOWLEDGEMENTS
The authors wish to thank Jan Egberts and Merel Hazewindus (CHC Europe) for providing support in manuscript preparation.
Declaration of interest: The authors declare no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
MM, CM and J-MF are employees of Estetra SPRL; CK is an employee of the Contract Organisation, Dinox BV, which performed the study; YZ and HCB are employees of Pantarhei Bioscience BV.
REFERENCES
- United Nations World contraceptive use 2011. www.unorg/esa/population/publications/contraceptivE2011/wallchart_frontpdf
- Westhoff CL, Heartwell S, Edwards S, et al. Oral contraceptive discontinuation: Do side effects matter? Am J Obstet Gynecol. 2007;196:412. doi: 10.1016/j.ajog.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitzer J. Pharmacological profile of estrogens in oral contraception. Minerva Ginecol. 2011;63:299–304. [PubMed] [Google Scholar]
- Cirillo DJ, Wallace RB, Rodabough RJ, et al. Effect of estrogen therapy on gallbladder disease. JAMA. 2005;293:330–9. doi: 10.1001/jama.293.3.330. [DOI] [PubMed] [Google Scholar]
- Kiley J, Hammond C. Combined oral contraceptives: A comprehensive review. Clin Obstet Gynecol. 2007;50:868–77. doi: 10.1097/GRF.0b013e318159c06a. [DOI] [PubMed] [Google Scholar]
- Stegeman BH, de Bastos M, Rosendaal FR, et al. Different combined oral contraceptives and the risk of venous thrombosis: Systematic review and network meta-analysis. BMJ. 2013;347:f5298. doi: 10.1136/bmj.f5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg MJ, Meyers A, Roy V. Efficacy, cycle control, and side effects of low- and lower-dose oral contraceptives: A randomized trial of 20 micrograms and 35 micrograms estrogen preparations. Contraception. 1999;60:321–9. doi: 10.1016/s0010-7824(99)00109-2. [DOI] [PubMed] [Google Scholar]
- Hoffmann H, Moore C, Zimmermann H, et al. Approaches to the replacement of ethinylestradiol by natural 17beta-estradiol in combined oral contraceptives. Exp Toxicol Pathol. 1998;50:458–64. doi: 10.1016/s0940-2993(98)80034-1. [DOI] [PubMed] [Google Scholar]
- Holinka CF, Diczfalusy E, Coelingh Bennink HJ. Estetrol: A unique steroid in human pregnancy. J Steroid Biochem Mol Biol. 2008;110:138–43. doi: 10.1016/j.jsbmb.2008.03.027. [DOI] [PubMed] [Google Scholar]
- Coelingh Bennink F, Holinka CF, Visser M, et al. Maternal and fetal estetrol levels during pregnancy. Climacteric. 2008;11((Suppl.1)):69–72. doi: 10.1080/13697130802056321. [DOI] [PubMed] [Google Scholar]
- Hammond GL, Hogeveen KN, Visser M, et al. Estetrol does not bind sex hormone binding globulin or increase its production by human HepG2 cells. Climacteric. 2008;11((Suppl.1)):41–6. doi: 10.1080/13697130701851814. [DOI] [PubMed] [Google Scholar]
- Visser M, Coelingh Bennink HJ. Clinical applications for estetrol. J Steroid Biochem Mol Biol. 2009;114:85–9. doi: 10.1016/j.jsbmb.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Abot A, Fontaine C, Buscato M, et al. The uterine and vascular actions of estetrol delineate a distinctive profile of estrogen receptor alpha modulation, uncoupling nuclear and membrane activation. EMBO Mol Med. 2014;6:1328–46. doi: 10.15252/emmm.201404112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adlanmerini M, Solinhac R, Abot A, et al. Mutation of the palmitoylation site of estrogen receptor alpha in vivo reveals tissue-specific roles for membrane versus nuclear actions. Proc Natl Acad Sci U S A. 2014;111:283–90. doi: 10.1073/pnas.1322057111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelingh Bennink HJ, Holinka CF, Diczfalusy E. Estetrol review: profile and potential clinical applications. Climacteric. 2008;11((Suppl.1)):47–58. doi: 10.1080/13697130802073425. [DOI] [PubMed] [Google Scholar]
- Coelingh Bennink HJ, Skouby S, Bouchard P, et al. Ovulation inhibition by estetrol in an in vivo model. Contraception. 2008;77:186–90. doi: 10.1016/j.contraception.2007.11.014. [DOI] [PubMed] [Google Scholar]
- World Health Organization . Medical eligibility criteria for contraceptive use. 4th edn. Geneva: WHO; 2010. Combined hormonal contraceptives (CHCs) pp. 15–44. [PubMed] [Google Scholar]
- Rikken B, van Doorn J, Ringeling A, et al. Plasma levels of insulin-like growth factor (IGF)-I, IGF-II and IGF-binding protein-3 in the evaluation of childhood growth hormone deficiency. Horm Res. 1998;50:166–76. doi: 10.1159/000023268. [DOI] [PubMed] [Google Scholar]
- de Boer L, Hoogerbrugge CM, van Doorn J, et al. Plasma insulin-like growth factors (IGFs), IGF-binding proteins (IGFBPs), acid-labile subunit (ALS) and IGFBP-3 proteolysis in individuals with clinical characteristics of Sotos syndrome. J Pediatr Endocrinol Metab. 2004;17:615–27. doi: 10.1515/jpem.2004.17.4.615. [DOI] [PubMed] [Google Scholar]
- de Vries BB, Robinson H, Stolte-Dijkstra I, et al. General overgrowth in the fragile X syndrome: Variability in the phenotypic expression of the FMR1 gene mutation. J Med Genet. 1995;32:764–9. doi: 10.1136/jmg.32.10.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyulikhandanova NE, Tsymbalenko NV, Platonova NA, et al. Regulation of ceruloplasmin gene in mammals. Bull Exp Biol Med. 2004;137:485–9. doi: 10.1023/b:bebm.0000038160.60211.6f. [DOI] [PubMed] [Google Scholar]
- De Groote D, Perrier d’Hauterive S, Pintiaux A, et al. Effects of oral contraception with ethinylestradiol and drospirenone on oxidative stress in women 18–35 years old. Contraception. 2009;80:187–93. doi: 10.1016/j.contraception.2009.02.015. [DOI] [PubMed] [Google Scholar]
- Odlind V, Milsom I, Persson I, et al. Can changes in sex hormone binding globulin predict the risk of venous thromboembolism with combined oral contraceptive pills? Acta Obstet Gynecol Scand. 2002;81:482–90. [PubMed] [Google Scholar]
- Raps M, Helmerhorst F, Fleischer K, et al. Sex hormone-binding globulin as a marker for the thrombotic risk of hormonal contraceptives. J Thromb Haemost. 2012;10:992–7. doi: 10.1111/j.1538-7836.2012.04720.x. [DOI] [PubMed] [Google Scholar]
- Stegeman BH, Helmerhorst FM, Vos HL, et al. Sex hormone-binding globulin levels are not causally related to venous thrombosis risk in women not using hormonal contraceptives. J Thromb Haemost. 2012;10:2061–7. doi: 10.1111/j.1538-7836.2012.04878.x. [DOI] [PubMed] [Google Scholar]
- Kluft C, Skouby SO, Jespersen J, et al. Sex hormone-binding globulin as a marker for the thrombotic risk of hormonal contraceptives: A rebuttal. J Thromb Haemost. 2013;11:394–5. doi: 10.1111/jth.12067. [DOI] [PubMed] [Google Scholar]
- Raps M, Helmerhorst FM, Fleischer K, et al. Sex hormone-binding globulin as a marker for the thrombotic risk of hormonal contraceptives: reply to a rebuttal. J Thromb Haemost. 2013;11:396–7. doi: 10.1111/jth.12080. [DOI] [PubMed] [Google Scholar]
- Committee for Medical Products for Human Use (CPMP) Guideline on clinical investigation of steroid contraceptives in women. London: European Medicines Agency; 2005. [Google Scholar]
- Akhter S, Shamsuzzaman AK, Banarjee M, et al. Serum copper in rural women taking combined oral contraceptive. Mymensingh Med J. 2006;15:25–9. doi: 10.3329/mmj.v15i1.20. [DOI] [PubMed] [Google Scholar]
- Berg G, Kohlmeier L, Brenner H. Effect of oral contraceptive progestins on serum copper concentration. Eur J Clin Nutr. 1998;52:711–5. doi: 10.1038/sj.ejcn.1600631. [DOI] [PubMed] [Google Scholar]
- Benes B, Spevackova V, Smid J, et al. Effects of age, BMI, smoking and contraception on levels of Cu, Se and Zn in the blood of the population in the Czech Republic. Cent Eur J Public Health. 2005;13:202–7. [PubMed] [Google Scholar]
- Agren UM, Anttila M, Maenpaa-Liukko K, et al. Effects of a monophasic combined oral contraceptive containing nomegestrol acetate and 17beta-oestradiol compared with one containing levonorgestrel and ethinylestradiol on haemostasis, lipids and carbohydrate metabolism. Eur J Contracept Reprod Health Care. 2011;16:444–57. doi: 10.3109/13625187.2011.604450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitruk-Ware R. Pharmacology of different progestogens: The special case of drospirenone. Climacteric. 2005;8(Suppl:4–12. doi: 10.1080/13697130500330382. [DOI] [PubMed] [Google Scholar]
- Endrikat J, Klipping C, Cronin M, et al. An open label, comparative study of the effects of a dose-reduced oral contraceptive containing 20 microg ethinyl estradiol and 100 microg levonorgestrel on hemostatic, lipids, and carbohydrate metabolism variables. Contraception. 2002;65:215–21. doi: 10.1016/s0010-7824(01)00316-x. [DOI] [PubMed] [Google Scholar]
- Scharnagl H, Petersen G, Nauck M, et al. Double-blind, randomized study comparing the effects of two monophasic oral contraceptives containing ethinylestradiol (20 microg or 30 microg) and levonorgestrel (100 microg or 150 microg) on lipoprotein metabolism. Contraception. 2004;69:105–13. doi: 10.1016/j.contraception.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Skouby SO, Endrikat J, Dusterberg B, et al. A 1-year randomized study to evaluate the effects of a dose reduction in oral contraceptives on lipids and carbohydrate metabolism: 20 microg ethinyl estradiol combined with 100 microg levonorgestrel. Contraception. 2005;71:111–7. doi: 10.1016/j.contraception.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Tuppurainen M, Klimscheffskij R, Venhola M, et al. The combined contraceptive vaginal ring (NuvaRing) and lipid metabolism: A comparative study. Contraception. 2004;69:389–94. doi: 10.1016/j.contraception.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Josse AR, Garcia-Bailo B, Fischer K, et al. Novel effects of hormonal contraceptive use on the plasma proteome. PLoS One. 2012;7:e45162. doi: 10.1371/journal.pone.0045162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz BE, Henry N, Goldberg RB. Long-term oral contraceptive treatment, metabolic syndrome and measures of cardiovascular risk in pre-menopausal women: National Health and Nutrition Examination Survey 1999–2004. Gynecol Endocrinol. 2009;25:441–9. doi: 10.1080/09513590902770149. [DOI] [PubMed] [Google Scholar]
- Coelingh Bennink HJ, Heegaard AM, Visser M, et al. Oral bioavailability and bone-sparing effects of estetrol in an osteoporosis model. Climacteric. 2008;11((Suppl. 1)):2–14. doi: 10.1080/13697130701798692. [DOI] [PubMed] [Google Scholar]
- Delmas PD. Biochemical markers of bone turnover for the clinical assessment of metabolic bone disease. Endocrinol Metab Clin North Am. 1990;19:1–18. [PubMed] [Google Scholar]
- Robins SP. Biochemical markers of bone metabolism. CPD Bull Clin Biochem. 1999;1:116–21. [Google Scholar]
- Rosen HN, Moses AC, Garber J, et al. Serum CTX: A new marker of bone resorption that shows treatment effect more often than other markers because of low coefficient of variability and large changes with bisphosphonate therapy. Calcif Tissue Int. 2000;66:100–3. doi: 10.1007/pl00005830. [DOI] [PubMed] [Google Scholar]
- Di Carlo C, Gargano V, Sparice S, et al. Short-term effects of an oral contraceptive containing oestradiol valerate and dienogest on bone metabolism and bone mineral density: An observational, preliminary study. Eur J Contracept Reprod Health Care. 2013;18:388–93. doi: 10.3109/13625187.2013.811483. [DOI] [PubMed] [Google Scholar]
- Massaro M, Di Carlo C, Gargano V, et al. Effects of the contraceptive patch and the vaginal ring on bone metabolism and bone mineral density: A prospective, controlled, randomized study. Contraception. 2010;81:209–14. doi: 10.1016/j.contraception.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Balogh A, Kauf E, Vollanth R, et al. Effects of two oral contraceptives on plasma levels of insulin-like growth factor I (IGF-I) and growth hormone (hGH) Contraception. 2000;62:259–69. doi: 10.1016/s0010-7824(00)00176-1. [DOI] [PubMed] [Google Scholar]
- Weissberger AJ, Ho KK, Lazarus L. Contrasting effects of oral and transdermal routes of estrogen replacement therapy on 24 h growth hormone (GH) secretion, insulin-like growth factor I, and GH-binding protein in postmenopausal women. J Clin Endocrinol Metab. 1991;72:374–81. doi: 10.1210/jcem-72-2-374. [DOI] [PubMed] [Google Scholar]
- Thijs C, Knipschild P. Oral contraceptives and the risk of gallbladder disease: A meta-analysis. Am J Public Health. 1993;83:1113–20. doi: 10.2105/ajph.83.8.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargano V, Massaro M, Morra I, et al. Effects of two low-dose combined oral contraceptives containing drospirenone on bone turnover and bone mineral density in young fertile women: A prospective controlled randomized study. Contraception. 2008;78:10–5. doi: 10.1016/j.contraception.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Paoletti AM, Orru M, Lello S, et al. Short-term variations in bone remodeling markers of an oral contraception formulation containing 3 mg of drospirenone plus 30 microg of ethinyl estradiol: Observational study in young postadolescent women. Contraception. 2004;70:293–8. doi: 10.1016/j.contraception.2004.04.004. [DOI] [PubMed] [Google Scholar]