Simultaneous activation created larger, more confluent, and more spherical ablation zones with greater temperatures than sequential activation.
Abstract
Purpose
To compare microwave ablation zones created by using sequential or simultaneous power delivery in ex vivo and in vivo liver tissue.
Materials and Methods
All procedures were approved by the institutional animal care and use committee. Microwave ablations were performed in both ex vivo and in vivo liver models with a 2.45-GHz system capable of powering up to three antennas simultaneously. Two- and three-antenna arrays were evaluated in each model. Sequential and simultaneous ablations were created by delivering power (50 W ex vivo, 65 W in vivo) for 5 minutes per antenna (10 and 15 minutes total ablation time for sequential ablations, 5 minutes for simultaneous ablations). Thirty-two ablations were performed in ex vivo bovine livers (eight per group) and 28 in the livers of eight swine in vivo (seven per group). Ablation zone size and circularity metrics were determined from ablations excised postmortem. Mixed effects modeling was used to evaluate the influence of power delivery, number of antennas, and tissue type.
Results
On average, ablations created by using the simultaneous power delivery technique were larger than those with the sequential technique (P < .05). Simultaneous ablations were also more circular than sequential ablations (P = .0001). Larger and more circular ablations were achieved with three antennas compared with two antennas (P < .05). Ablations were generally smaller in vivo compared with ex vivo.
Conclusion
The use of multiple antennas and simultaneous power delivery creates larger, more confluent ablations with greater temperatures than those created with sequential power delivery.
© RSNA, 2015
Introduction
Imaging-guided thermal ablation is an increasingly accepted treatment option for tumors of the liver, kidney, lung, and bone. Improved ablation devices and techniques have resulted in increasing effectiveness, a low rate of serious complications, and a rapid return to the activities of normal daily life (1). However, a high rate of local tumor progression remains the most important factor preventing more widespread adoption of ablation, particularly compared with hepatic resection (2). The primary cause of local tumor progression is an inadequate ablation zone that fails to cover the targeted tumor and a circumferential ablative safety margin (5–10 mm) (3,4). Despite recent improvements, current ablation devices and techniques are unable to consistently treat tumors larger than 3 cm with a single application of energy (5), often necessitating the use of multiple overlapping ablations.
Two methods for generating overlapping ablations have been used: (a) multiple insertions of a single applicator to overlap ablations in a sequential fashion and (b) multiple applicators acting in concert to simultaneously generate a confluent ablation. Although both methods have been used in clinical studies of radiofrequency (RF) and microwave ablation, an optimal delivery method has not yet been determined (6–9). Comparison studies of sequential and simultaneous RF ablation techniques have concluded that simultaneous multiple-electrode delivery creates larger and more confluent ablation zones (10,11). However, RF electrical current cannot be applied continuously during thermal ablation as simultaneous activation of multiple electrodes can lead to ineffective heating in the center of the array (12). Thus, multiple-electrode RF ablations are created by using a switching algorithm (13).
Conversely, electromagnetic waves in the microwave frequency range will transmit through desiccated tissue and can be applied to multiple antennas simultaneously to create a more uniform heating zone (14–16). The relative phases of the applied waves can be controlled to produce constructive interference and enhance tissue heating in and around the antenna array (17,18). Therefore, we hypothesized that simultaneous energy delivery coupled with constructive electromagnetic interference between the antennas can produce larger and more confluent ablations in less time than sequential energy delivery. The purpose of our study was to compare the sequential and simultaneous methods of microwave energy delivery by comparing ablation zones created with each technique in ex vivo and in vivo liver tissue.
Materials and Methods
Studies were performed in both ex vivo and in vivo tissue models (Fig 1). The ex vivo model provided a more controlled test medium owing to the absence of blood flow in large vessels and tissue and the lack of breathing motion. An in vivo model was then used to evaluate the effect of physiologic blood flow. Some authors have financial interests in NeuWave Medical (Madison, Wis), such as stock holdings (C.L.B., F.T.L., J.L.H.), patents issued or pending (C.L.B., F.T.L.), royalties received (F.T.L.), board membership (F.T.L.), consultancy (C.L.B.), or grant support (M.G.L.). NeuWave did not provide financial or material support for this study. All data were collected and controlled by authors without relevant interests in NeuWave.
Figure 1:
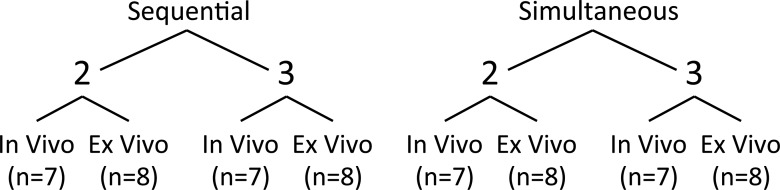
Experimental design. Ex vivo and in vivo tissue models and two- or three-antenna arrays were used to assess differences in ablations created with sequential ablation or simultaneous ablation with multiple antennas.
Ex Vivo Model
Bovine livers were chosen for this portion of the experiment because of their large size, which minimizes the effect of the insulating liver-air boundary on ablation growth. Livers were harvested at a local slaughterhouse and brought to room temperature (approximately 20°C). Ablations were performed by using a 2.45-GHz clinical microwave ablation system that provides coherent power to all antennas (Certus 140 with PR 15 antennas; NeuWave Medical). Four protocols were evaluated: simultaneous activation of two or three antennas and sequential activation of two or three antennas (eight per group; 32 ablations overall). Antenna spacing was 1.6 cm for two antennas and 2.0 cm for three antennas in an equilateral triangle configuration to ensure adequate overlap of the growing ablations (Fig 2) (14,19,20). An acrylic template was used to ensure consistent placement. Sequential activation was provided by applying 50 W to one antenna for 5 minutes, waiting 5 minutes to simulate antenna repositioning, and repeating the procedure for each additional antenna. Simultaneous activation was provided by applying 50 W to each antenna for a total of 5 minutes for both two- and three-antenna experiments. Thus, the sequential technique took a total of either 15 minutes (two antennas) or 25 minutes (three antennas), whereas the simultaneous technique took 5 minutes in all cases.
Figure 2:
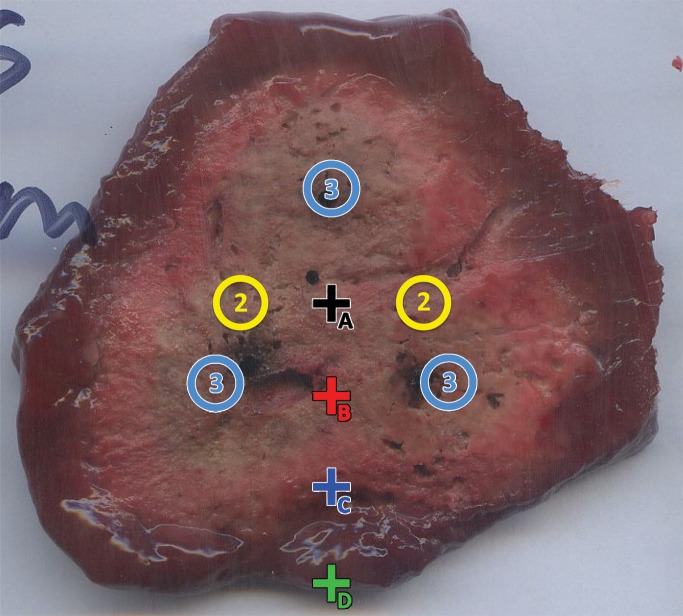
Placement of temperature sensors (+) for two- (2) or three (3)–antenna arrays (◯). Temperature sensors were placed in ex vivo tissue such that sensor A was at center of array, whereas sensors B–D were separated by 1 cm along a radial line.
Temperatures were measured throughout each ablation by using fiber optic sensors as in previous studies (Neoptix, Quebec, Canada) (10). Four temperature probes were placed in a line bisecting an axis of symmetry beginning at the array center, with 1 cm spacing between probes (Fig 2). Temperatures were recorded every second for the entirety of each ablation, including the simulated repositioning times.
In Vivo Model
Animal procedures were approved by our institutional research animal care and use committee and were compliant with regulatory guidelines (21).
Ablations were performed in the livers of eight female domestic swine (weight, 60–70 kg), which were selected on the basis of their size similarity to humans. Animals were initially sedated by using 7 mg/kg intramuscular tiletamine hydrochloride–zolazepam hydrochloride and 2.2 mg/kg intramuscular xylazine hydrochloride. Endotracheal intubation was facilitated with 0.05 mg/kg intramuscular atropine. Isoflurane was used for inhalational anesthesia throughout the duration of the procedure. The liver was exposed by means of a ventral midline incision.
Microwave antennas (PR 15, NeuWave Medical) were placed into the surgically exposed liver via an acrylic spacer (M.B., M.M., C.M.H., with 1–8 years of experience) to ensure accurate and consistent antenna spacing similar to that of ex vivo studies (1.6 cm for two antennas, 2.0 cm for three antennas). Four protocols were again evaluated: simultaneous activation of two or three antennas and sequential activation of two or three antennas (seven per group, 28 ablations overall). The anatomic location of each ablation and the order in which ablations were performed (ie, sequential before simultaneous or vice versa) were randomized to minimize potential bias from the liver anatomy. The antenna maximum power of 65 W was used in vivo to offset the cooling effects of tissue perfusion, with the goal of creating ablations similar to those produced in the ex vivo model. At the end of the procedure, animals were euthanized by using 0.2 mL/kg intravenous barbiturate overdose (390 mg/mL pentobarbital sodium and 50 mg/mL phenytoin sodium). The liver was then removed en bloc for sectioning.
Ablation Zone Measurements
In both ex vivo and in vivo tissues, the proximal and distal aspects of each ablation zone were located by dissection and ablation zone length was measured to the nearest 0.1 mm with a digital caliper. The tissue was then sliced into approximately 5-mm-thick sections in a plane orthogonal to the antenna insertion tract. Section thicknesses were measured to the nearest 0.1 mm. The slices were scanned optically and stored as digital images. Similar to previous studies of multiple-applicator ablation, ablation zone minimum and maximum diameter, area, circularity, and maximum inscribed diameter were measured from the digital images by using ImageJ (v1.47, U. S. National Institutes of Health, Bethesda, Md). Measurements were performed only on the central zone of coagulative necrosis (22,23). Circularity was defined from the isoperimetric quotient, as follows: (4 × π × area)/perimeter2. Maximum inscribed diameter was defined as the maximum circle diameter that fits inside the ablation zone, which is more indicative of the area that could be treated with the ablation than minimum or maximum diameter (10). Ablation volume was estimated by summing the product of ablation area and slice thickness over all slices.
Statistical Analysis
Data were analyzed by using linear mixed effects models. Response variables included the ablation zone maximum diameter, minimum diameter, inscribed diameter, volume, and circularity. Fixed effects included power delivery technique (sequential or simultaneous), number of antennas (two or three), and tissue model (ex vivo or in vivo), whereas animals and ablation location (medial or lateral lobes) were modeled as random effects. Diagnostic plots of the response variables suggested that perfusion in vivo may have a greater effect against the growth of sequential ablations, so an interaction between tissue model and power delivery technique was included. Visual inspection of residual plots did not reveal any obvious violations of normality or homoscedasticity. Maximum tissue temperatures from ex vivo ablations were compared by using the Student t test. P < .05 was considered to indicate a significant difference. Analyses were conducted by using software (R Core Team, 2015, R: A language and environment for statistical computing; R Foundation for Statistical Computing, Vienna, Austria).
Results
Simultaneous versus Sequential Power Delivery
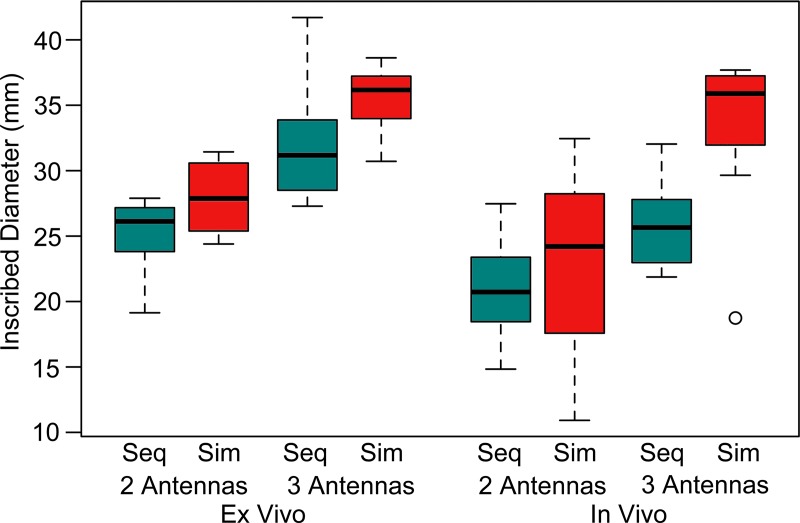
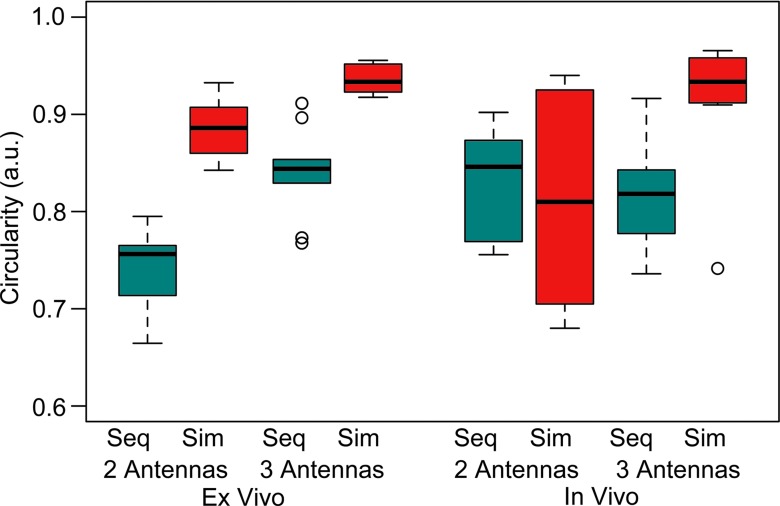
Simultaneous power delivery resulted in larger ablations than did sequential power delivery (Fig 3). Ablation diameters increased by an average of 10% when using simultaneous power delivery, corresponding to an average increase in volume of 15%. A complete tabulation of ablation dimensions and significance testing results can be found in Tables 1 and 2. The most significant differences between sequential and simultaneous power delivery were noted for inscribed diameter (Fig 4). The largest ablations overall were produced with three antennas and simultaneous power delivery. Simultaneous power delivery also created more circular ablations (11% on average) compared with sequential power delivery (P = .0001; Fig 5).
Figure 3:
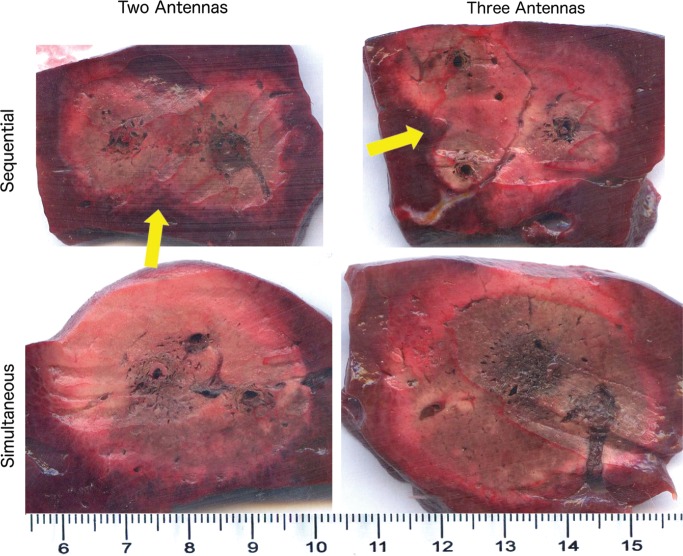
Representative images of two- and three-antenna ablation zones illustrate differences between energy delivery and number of antennas. Sequential ablation zones are markedly less confluent, with clefts noted between each ablation (arrows). Scale is in centimeters.
Table 1.
Ablation Zone Size, Shape, and Temperature Metrics
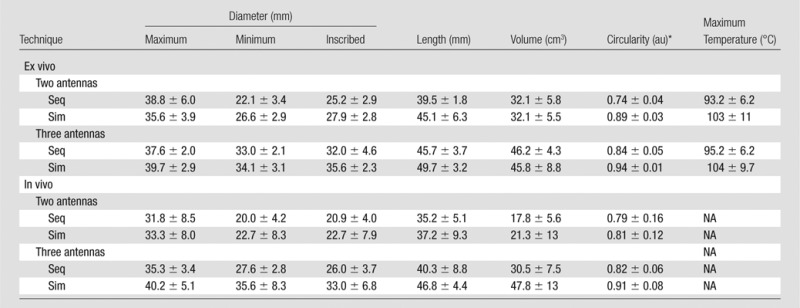
Note.—Data are means ± standard deviations. NA = not applicable, Seq = sequential delivery, Sim = simultaneous delivery.
*au = arbitrary units.
Table 2.
Significance Testing Results with the Linear Mixed Effects Model
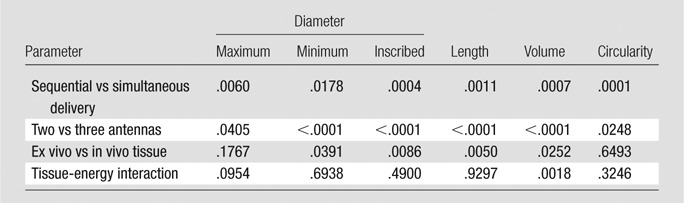
Note.—Data are P values.
Figure 4:
Box-and-whisker plot of maximum inscribed circle diameter. Simultaneous power delivery and the use of three antennas produced larger ablation zones. Ablations were slightly larger in ex vivo model. Seq = sequential, Sim = simultaneous. ◯ = outlier.
Figure 5:
Box-and-whisker plot of circularity. Simultaneous power delivery and use of three antennas produced significantly greater ablation circularity. Unlike ablation size metrics, circularity was not influenced significantly by tissue model. Seq = sequential, Sim = simultaneous. ◯ = outliers.
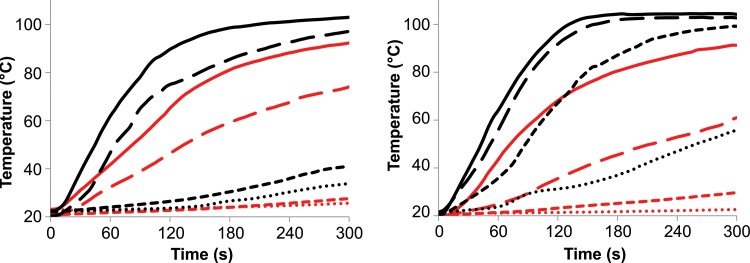
Although the differences between simultaneous and sequential delivery were less appreciable in the ex vivo tissue model, temperatures measured ex vivo increased at a faster rate and to a higher maximum when using simultaneous power delivery, which suggests that simultaneous power delivery may be of benefit in a perfused model (Fig 6).
Figure 6:
Graphs show mean temperatures recorded during simultaneous (black) and sequential (red) power delivery at points A (solid), B (long dashes), C (short dashes), and D (dots) in Figure 2. Two-antenna (left) and three-antenna (right) ablations are shown. Heating was faster when using simultaneous power delivery compared with sequential energy delivery (16°C/sec vs 10°C/sec for two antennas; 14°C/sec and 8.7°C/sec for three antennas; P < .001).
Two versus Three Antennas
The number of antennas had a profound influence on ablation geometry. Ablation zone diameters and volume were larger with three antennas than with two antennas for either tissue model or power delivery technique (P < .05). Of note, the number of antennas was the most significant factor on minimum diameter and inscribed diameter but not on maximum diameter. Ablation zones created with three antennas were also more circular than those created with two antennas (P = .025; Fig 3).
Ex Vivo versus in Vivo Tissue Model
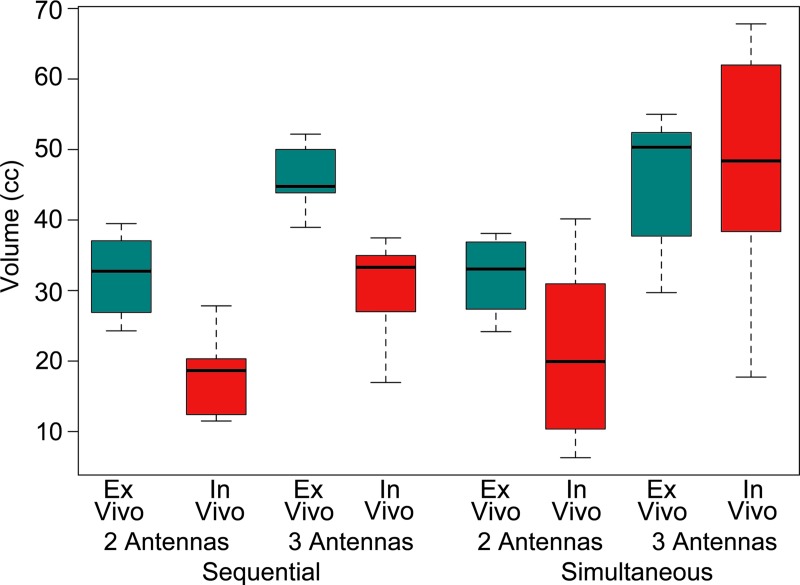
As expected, ablations created in the in vivo liver model were generally smaller than those created ex vivo. The effect of tissue type was significant for all size metrics except maximum diameter (P < .05) and appeared more pronounced when using sequential energy delivery; however, the interaction between delivery technique and tissue model was not significant (P > .10). Unlike the number of antennas or energy delivery technique, the tissue model did not have an effect on the circularity of ablations (P = .65; Fig 7).
Figure 7:
Box-and-whisker plot of volume with side-by-side comparison of tissue type. Ablations were slightly larger in ex vivo model, but the effect of tissue model was reduced by using simultaneous energy delivery or three antennas.
Other Results
There was an observable trend toward larger ablation zones in the medial lobes of the animal, which appeared to be due to a bias in the number of three-antenna ablations performed in the medial lobes (five two-antenna and nine three-antenna ablations). Ablation location was not found to be a significant factor in mixed model analysis (P > .13, all metrics). The animal was a significant factor in the maximum diameter and volume models (P < .05) but not in any other metric (P > .19).
Discussion
The results of our study suggest that microwave ablations created by using simultaneous activation of multiple microwave antennas are larger, are more confluent, and achieve greater peripheral temperatures than ablations created by using sequential activation. These differences were noted despite the fact that simultaneous activation required only one-third to one-half of the ablation time required for sequential activation. The effect of simultaneous activation was most marked in vivo and with three antennas.
Despite numerous technologic advances in the past decade that have increased the ablation zone size created by a single microwave antenna, there is a persistent need for larger ablation zones to account for both the tumor and an ablative margin. Lack of an adequate margin (at least 5 mm for hepatocellular carcinoma and 10 mm for hepatic metastases) is associated with an increase in local tumor progression and a decrease in survival (3,4). Even minimally irregular tumors or deviations in antenna placement of a few millimeters can result in inadequate margins when a single-applicator technique is used. This is primarily due to a precipitous drop in tissue heating away from a single centrally placed applicator, resulting in the coolest temperatures applied to the tumor periphery (24), where high biologic activity and microsatellites are located (25). Hence, virtually all cases of local tumor progression after thermal ablation appear at the periphery of ablation zones.
The use of multiple overlapping ablations provides the dual advantages of creating a larger ablation zone and more aggressively treating the tumor periphery (24). Overlapping microwave ablations can be achieved in one of two ways: (a) insertion of a single antenna with sequential activation to multiple locations or (b) insertion of multiple antennas with simultaneous activation of all antennas. The results of this study demonstrate that these methods do not result in equivalent ablation zones. In clinical practice, the sequential technique is hampered by difficulty in precisely repositioning the antenna after previous ablations owing to obscuration of the tumor by gas bubbles, bleeding, tissue shrinkage, and charring. Despite these limitations, the sequential method appears to be in widespread clinical use (26–30). The primary advantage of the sequential technique appears to be a lower monetary cost for one antenna compared with multiple antennas.
Ablation zone sizes were generally smaller in the normally perfused in vivo liver model compared with the ex vivo model, but the difference was less substantial when using simultaneous power delivery and three antennas. This provides evidence to suggest that a greater number of antennas or greater total power in the array can more effectively overcome the heat-sinking effect of blood perfusion. Other factors, such as tissue permittivity, thermal conductivity, and permeability, may also differ between ex vivo and in vivo tissues. The effect of such factors on ablation size was not the focus of our study but is likely to be lower than the effect of perfusion on the basis of previous analyses (31).
The results of our study corroborate similar studies with RF ablation, where the use of multiple rapidly switched electrodes created larger and more confluent ablation zones than did overlapping sequential ablations (10). Compared with the ablation zone sizes created during experiments with multiple RF electrodes, the microwave ablation zones in our study were larger, even though the ablation time was less than half that of the RF study. Because that particular RF ablation system relied on rapid switching between electrodes to simultaneously produce multiple ablations, only one electrode was active at a given time to avoid counterproductive electrical interference (12). Therefore, multiple-electrode RF ablation can only benefit from thermal synergy without a contribution from overlapping electrical fields. The larger ablation sizes produced with microwaves in our study may indicate more effective heating with microwave energy in general or may also be additional evidence of the enhanced efficiency of in-phase antenna arrays. However, a direct RF versus microwave comparison was beyond the scope of this study.
There were certain limitations in our study. All ablations were performed in normal liver because of the paucity of large-animal tumor models. For this reason, we evaluated two extremes of tissue perfusion. Normally perfused liver represents a “worst possible case” in terms of perfusion-mediated cooling and vascular heat sinks, whereas ex vivo liver lacks any blood perfusion. Neither model is a perfect surrogate for human tumors or pathologic liver; however, together they illustrate the spectrum of blood flow that may be encountered in a clinical environment (32). The swine liver anatomy also differs from that in humans in that it contains four separate lobes, each containing a vascular pedicle and flatter than a human liver of the same volume. Additional clinical study will be needed to determine the impact of the methods evaluated here.
Our results may not be representative of all microwave systems, particularly those without coherent power delivery. Only a single combination of power, time, and antenna spacing was evaluated to minimize the number of live animals required. Our settings were selected on the basis of preliminary studies and guidelines provided by the microwave ablation device manufacturer and were consistent with those used in previous studies and clinical practice (15,16). Our results are concordant with those of previous multiple applicator studies, so we believe that the overall conclusions of the study would not substantially change at powers or times within the bounds of clinical utility.
In summary, the results of our study suggest that microwave ablations created with sequentially overlapping ablations by using a single antenna are not equivalent to those created with multiple antennas powered simultaneously. Simultaneous activation of microwave antennas created larger, more confluent, and rounder ablation zones with greater temperatures than sequential activation. The differences between techniques were most marked when using three antennas or in a perfused tissue model.
Advances in Knowledge
■ Simultaneous delivery of power to multiple antennas can create larger ablation zones than multiple insertions of a single antenna (P < .05).
■ Simultaneous ablations were significantly more circular than sequential ablations independent of the presence of blood perfusion (P = .0001).
■ Blood perfusion decreased the size of all ablations, but the effect was slightly lower with simultaneous energy delivery or three antennas.
Implications for Patient Care
■ The larger, more confluent ablations created by means of simultaneous use of multiple antennas may improve ablative margins and decrease the likelihood of tumor recurrence.
■ Simultaneous activation of multiple antennas decreases ablation time and circumvents the need to precisely reposition a single antenna after previous ablations.
Acknowledgments
Acknowledgment
We thank Lisa Sampson, BS, for her insightful support and expert assistance with the animal experiments.
Received September 8, 2014; revision requested November 3; revision received April 9, 2015; accepted April 21; final version accepted May 1.
Funding: This research was supported by the National Institutes of Health (grants R01 CA142737 and R01 CA14937).
Disclosures of Conflicts of Interest: C.M.H. disclosed no relevant relationships. M.M. disclosed no relevant relationships. M.B. disclosed no relevant relationships. F.T.L. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: institution receives money for board membership from NeuWave Medical; institution receives money for patent from NeuWave Medical; receives royalties from Covidien. Other relationships: disclosed no relevant relationships. M.G.L. Activities related to the present article: received a grant from NeuWave Medical. Activities not related to the present article: received a grant from GE Healthcare. Other relationships: disclosed no relevant relationships. J.L.H. Activities related to the present article: is a stockholder and medical advisor for NeuWave Medical. Activities not related to the present article: is a stockholder in Cellectar Biosciences. Other relationships: has a patent issued. T.Z. disclosed no relevant relationships. C.L.B. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a paid consultant for NeuWave Medical; has a patent with NeuWave Medical; owns stock/stock options in NeuWave Medical. Other relationships: disclosed no relevant relationships.
Abbreviation:
- RF
- radiofrequency
References
- 1.Goldberg SN, Ahmed M. Minimally invasive image-guided therapies for hepatocellular carcinoma. J Clin Gastroenterol 2002;35(5,Suppl 2):S115–S129. [DOI] [PubMed] [Google Scholar]
- 2.Mulier S, Ni Y, Jamart J, Ruers T, Marchal G, Michel L. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg 2005;242(2):158–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakazawa T, Kokubu S, Shibuya A, et al. Radiofrequency ablation of hepatocellular carcinoma: correlation between local tumor progression after ablation and ablative margin. AJR Am J Roentgenol 2007;188(2):480–488. [DOI] [PubMed] [Google Scholar]
- 4.Liu CH, Arellano RS, Uppot RN, Samir AE, Gervais DA, Mueller PR. Radiofrequency ablation of hepatic tumours: effect of post-ablation margin on local tumour progression. Eur Radiol 2010;20(4):877–885. [DOI] [PubMed] [Google Scholar]
- 5.Mulier S, Ruers T, Jamart J, Michel L, Marchal G, Ni Y. Radiofrequency ablation versus resection for resectable colorectal liver metastases: time for a randomized trial? An update. Dig Surg 2008;25(6):445–460. [DOI] [PubMed] [Google Scholar]
- 6.Laeseke PF, Frey TM, Brace CL, et al. Multiple-electrode radiofrequency ablation of hepatic malignancies: initial clinical experience. AJR Am J Roentgenol 2007;188(6):1485–1494. [DOI] [PubMed] [Google Scholar]
- 7.Curley SA, Izzo F, Delrio P, et al. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg 1999;230(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vogl TJ, Straub R, Zangos S, Mack MG, Eichler K. MR-guided laser-induced thermotherapy (LITT) of liver tumours: experimental and clinical data. Int J Hyperthermia 2004;20(7):713–724. [DOI] [PubMed] [Google Scholar]
- 9.Shibata T, Niinobu T, Ogata N, Takami M. Microwave coagulation therapy for multiple hepatic metastases from colorectal carcinoma. Cancer 2000;89(2):276–284. [PubMed] [Google Scholar]
- 10.Brace CL, Sampson LA, Hinshaw JL, Sandhu N, Lee FT, Jr. Radiofrequency ablation: simultaneous application of multiple electrodes via switching creates larger, more confluent ablations than sequential application in a large animal model. J Vasc Interv Radiol 2009;20(1):118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JM, Han JK, Kim HC, et al. Multiple-electrode radiofrequency ablation of in vivo porcine liver: comparative studies of consecutive monopolar, switching monopolar versus multipolar modes. Invest Radiol 2007;42(10):676–683. [DOI] [PubMed] [Google Scholar]
- 12.Haemmerich D, Lee FT, Jr, Schutt DJ, et al. Large-volume radiofrequency ablation of ex vivo bovine liver with multiple cooled cluster electrodes. Radiology 2005;234(2):563–568. [DOI] [PubMed] [Google Scholar]
- 13.Haemmerich D, Lee FT, Jr. Multiple applicator approaches for radiofrequency and microwave ablation. Int J Hyperthermia 2005;21(2):93–106. [DOI] [PubMed] [Google Scholar]
- 14.Brace CL, Laeseke PF, Sampson LA, Frey TM, van der Weide DW, Lee FT, Jr. Microwave ablation with multiple simultaneously powered small-gauge triaxial antennas: results from an in vivo swine liver model. Radiology 2007;244(1):151–156. [DOI] [PubMed] [Google Scholar]
- 15.Oshima F, Yamakado K, Nakatsuka A, Takaki H, Makita M, Takeda K. Simultaneous microwave ablation using multiple antennas in explanted bovine livers: relationship between ablative zone and antenna. Radiat Med 2008;26(7):408–414. [DOI] [PubMed] [Google Scholar]
- 16.Wright AS, Lee FT, Jr, Mahvi DM. Hepatic microwave ablation with multiple antennae results in synergistically larger zones of coagulation necrosis. Ann Surg Oncol 2003;10(3):275–283. [DOI] [PubMed] [Google Scholar]
- 17.Brace CL. Microwave ablation technology: what every user should know. Curr Probl Diagn Radiol 2009;38(2):61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turner PF. Interstitial equal-phased arrays for EM hyperthermia. IEEE Trans Microw Theory Tech 1986;34(5):572–578. [Google Scholar]
- 19.Lubner MG, Ziemlewicz TJ, Hinshaw JL, Lee FT, Jr, Sampson LA, Brace CL. Creation of short microwave ablation zones: in vivo characterization of single and paired modified triaxial antennas. J Vasc Interv Radiol 2014;25(10):1633–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laeseke PF, Sampson LA, Frey TM, et al. Multiple-electrode radiofrequency ablation: comparison with a conventional cluster electrode in an in vivo porcine kidney model. J Vasc Interv Radiol 2007;18(8):1005–1010. [DOI] [PubMed] [Google Scholar]
- 21.National Research Council . Guide for the care and use of laboratory animals. 8th ed. Washington, DC: National Academies Press, 2011. [Google Scholar]
- 22.Bhardwaj N, Strickland AD, Ahmad F, Atanesyan L, West K, Lloyd DM. A comparative histological evaluation of the ablations produced by microwave, cryotherapy and radiofrequency in the liver. Pathology 2009;41(2):168–172. [DOI] [PubMed] [Google Scholar]
- 23.Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria—a 10-year update. Radiology 2014;273(1):241–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laeseke PF, Lee FT, Jr, van der Weide DW, Brace CL. Multiple-antenna microwave ablation: spatially distributing power improves thermal profiles and reduces invasiveness. J Interv Oncol 2009;2(2):65–72. [PMC free article] [PubMed] [Google Scholar]
- 25.Okusaka T, Okada S, Ueno H, et al. Satellite lesions in patients with small hepatocellular carcinoma with reference to clinicopathologic features. Cancer 2002;95(9):1931–1937. [DOI] [PubMed] [Google Scholar]
- 26.Huang S, Yu J, Liang P, et al. Percutaneous microwave ablation for hepatocellular carcinoma adjacent to large vessels: a long-term follow-up. Eur J Radiol 2014;83(3):552–558. [DOI] [PubMed] [Google Scholar]
- 27.Yin XY, Xie XY, Lu MD, et al. Percutaneous thermal ablation of medium and large hepatocellular carcinoma: long-term outcome and prognostic factors. Cancer 2009;115(9):1914–1923. [DOI] [PubMed] [Google Scholar]
- 28.Livraghi T, Goldberg SN, Lazzaroni S, et al. Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology 2000;214(3):761–768. [DOI] [PubMed] [Google Scholar]
- 29.Andreano A, Galimberti S, Franza E, et al. Percutaneous microwave ablation of hepatic tumors: prospective evaluation of postablation syndrome and postprocedural pain. J Vasc Interv Radiol 2014;25(1):97–105, e1–e2. [DOI] [PubMed] [Google Scholar]
- 30.Livraghi T, Meloni F, Solbiati L, Zanus G; Collaborative Italian Group using AMICA system. Complications of microwave ablation for liver tumors: results of a multicenter study. Cardiovasc Intervent Radiol 2012;35(4):868–874. [DOI] [PubMed] [Google Scholar]
- 31.Duck FA. Physical properties of tissue: a comprehensive reference book. London, England: Academic Press, 1990. [Google Scholar]
- 32.Goldberg SN, Meloni F, Torosatti N, Nissenbaum I, Appelbaum L, Solbiati L. Parameter characterization for microwave tumor ablation: comparison of experimental and HCC data [abstr]. Presented at the World Conference on Interventional Oncology, New York, NY, 2011; 63. [Google Scholar]