Abstract
Background
Lung cancer risk is the leading cause of cancer-related deaths worldwide. We conducted a meta-analysis to evaluate the relationship between dairy consumption and lung cancer risk.
Methods
The databases included EMBASE, Medline (PubMed), and Web of Science. The relationship between dairy consumption and lung cancer risk was analyzed by relative risk or odds ratio estimates with 95% confidence intervals (CIs). We identified eight prospective cohort studies, which amounted to 10,344 cases and 61,901 participants.
Results
For milk intake, relative risk was 0.95 (95% CI: 0.76–1.15); heterogeneity was 70.2% (P=0.003). For total dairy product intake, relative risk was 0.96 (95% CI: 0.89–1.03), heterogeneity was 68.4% (P=0.004).
Conclusion
There was no significant association between dairy consumption and lung cancer risk.
Keywords: lung cancer, meta-analysis, milk, dairy products
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide. According to recent estimates, lung cancer causes 1.1 million deaths each year.1,2 In spite of scientific and medical advances in this area, the survival rate of patients with lung cancer has not significantly improved. Therefore, it is crucial to identify lung cancer risk factors to develop and establish preventive strategies.
Researchers have also focused on the effects of diet and on cancer development.3 Studies have shown that fruits, vegetables,4–8 and green tea9 have anticancer effects. However, other studies have not found any associations.10 The consumption of dairy products may play a significant role in tumorigenesis. Several meta-analyses have reported an association between dairy consumption and cancer risk, especially of the prostate and breast.11–13 Epidemiological studies6,14–20 examining the relationship between dairy consumption and lung cancer risk have produced conflicting results; four studies have reported positive associations with milk14–16,20 and another four with total dairy products,15–17,19 while two have reported negative associations with milk18,20 and another three with total dairy products.6,17,18 Given that the association between dairy consumption and lung cancer risk remains unclear, we conducted a meta-analysis21 to assess the relationship between dairy consumption and lung cancer risk. However, the association between dairy consumption and lung cancer risk is unknown and needs to be defined.
Methods
Study search strategy
We designed and performed a meta-analysis based on the Meta-Analysis Guidelines for epidemiological observational studies.22 The databases included EMBASE, Medline (PubMed), and Web of Science. The studies reviewed included case–control and cohort studies that evaluated the relationship between dairy consumption and lung cancer risk. The search terms were lung cancer, lung carcinoma, milk, dairy products, and total dairy foods.
Inclusion criteria
The inclusion criteria were: 1) human clinical trials published in English; 2) studies where dairy product consumption was the main exposure; 3) studies that presented the odds ratio (OR) or relative risk (RR) with 95% confidence interval (CI); and 4) prospective cohort studies.
Data extraction and quality assessment
The data extracted from the studies included the first author, year of publication, average follow-up time, study region, exposure time, number of cases, population characteristics, adjusted ORs, RRs, or hazard ratio (HR) with their 95% CIs and adjustments. Four publications6,15,16,19 reported separate RRs for different dairy products; therefore, the RR estimates with the maximum number of cases within each study were selected.
To assess the quality of the study designs, we evaluated key components of the studies.22 We used Q and I2 statistics to estimate heterogeneity among the studies; any disagreements were resolved by discussion.
Data analyses
The association between total dairy product or milk consumption and lung cancer risk was assessed by RRs. Milk included whole/high-fat milk and skim/low-fat milk; total dairy products included cheese, ice cream, artificial butter, butter, margarine, yoghurt, and other dairy products. Where crude and adjusted RRs were provided, we used the adjusted RRs.
The incidence of lung cancer in humans is low; therefore, the ORs were deemed to be equal to HRs and RRs. RRs and 95% CIs were estimated based on adjusted RRs or ORs for the highest versus the lowest dairy product intake.
We used Q (P≤0.10) and I2 statistics to examine the homogeneity across studies23 and the fixed effects model when substantial heterogeneity was detected.24 Subgroup analyses were conducted on cases ≥300; subgroup analysis was performed when adjusting for smoking status and age. Additionally, we investigated the effect of a single study on the overall risk estimate.
Data analyses were performed with STATA version 12.0 (StataCorp LP, College Station, TX, USA). P<0.05 was considered to be statistically significant. We used Egger’s linear regression and Begg’s rank correlation methods to evaluate potential publication bias.25,26
Results
Studies
Figure 1 illustrates detailed steps of study selection. A total of eight studies were included in our meta-analysis.
Figure 1.

Search strategy and selection of studies for inclusion in the meta-analysis.
Abbreviation: TDF, total dairy food.
Study characteristics
The characteristics of the eight studies are presented in Table 1. The studies were published in 1996–2014. Six studies were performed in Europe, one was conducted in the USA, and one was performed in Iran. The number of participants varied from 479 to 53,570, with a total of 58,997 subjects, and the lung cancer cases ranged from 124 to 4,287, with a total of 8,857 cases. Among the eight studies, seven reported data on total dairy product intake and seven reported data on milk intake. The follow-up ranged from 3 to 65 years, with a median follow-up of 4.5 years. Most studies were matched or adjusted for family history, body mass index, total energy intake, and age.
Table 1.
Characteristics of the eight prospective cohort studies included in the meta-analysis
| Reference | Population | Duration (years) | No of cases | Assessment | Exposure | Consumption levels | OR or RR (95% CI) | Adjustment for potential confounders |
|---|---|---|---|---|---|---|---|---|
| Axelsson et al14 | 1,286; age: ≥75 years; Scandinavian | 4 | 308 | Q | Milk | High vs low | 1.73 (1–3.01) | Age, number of cigarettes/day, number of years smoking cigarettes (continuous variables), marital status (four classes) and socioeconomic job classification (seven classes), smoking status, fruit and vegetable consumption |
| Nyberg et al15 | 479; age: ≥30 years never-smokers; Stockholm | 6 | 124 124 |
Q&FFQ | Milk TDF |
≥2 vs 0 glasses/day High vs low |
1.24 (0.71–2.17) 1.21 (0.61–2.39) |
Sex, age, smoking status, residence (urban vs rural), occupational exposure, fruit consumption |
| Hosseini et al16 | 754; age: ≥75 years; Iran | 3 | 482 482 |
Q | Milk TDF |
Upper third vs lower third Upper third vs lower third |
2.64 (1.54–4.51) 2.94 (1.79–4.82) |
Family history of lung cancer, total energy intake, smoking status, BMI |
| Park et al17 | 53,570 AARP members; age: 50–71 years | 7 | 4,287 2,457 |
Q Q |
TDF TDF |
Q5 vs Q1 Q5 vs Q1 |
1.05 (0.95–1.16) (men) 0.92 (0.81–1.04) (women) |
Race/ethnicity, education, marital status, BMI, family history of lung cancer, physical activity, alcohol consumption, red meat intake, total energy intake Race/ethnicity, BMI, maternal age at first delivery, number of children, age at menopause, education, marital status, family history of lung cancer, physical activity, MHT use, smoking status, and intakes of red meat, alcohol, fat, and total energy |
| Rachtan19 | 594; median age: 61 years; Cracow | 6 | 242 | Q | TDF | High vs low | 1.62 (0.94–2.81) | Age, smoking status, alcohol consumption (beer and vodka), siblings with cancer, tuberculosis, place of residence, occupational exposure |
| Axelsson et al20 | 1,452; lung cancer patients; age: ≥75 years; Scandinavian | 5 3 |
177 359 |
FFQ | Milk Milk |
High vs low High vs low |
1.9 (1.1–3.3) (women) 1.9 (1.3–2.9) (men) |
Age (continuous variable), number of cigarettes smoked per day (four classes), number of years smoking cigarettes (five classes), marital status (four classes), consumption of vegetables, fruits, and milk |
| Brennan et al6 | 1,551; age: 0–75 years | 3 | 496 500 |
FFQ | TDF Milk |
High vs low High vs low |
0.7 (0.5–1.0) 0.8 (0.6–1.2) |
Age and sex |
| Van der Pols et al18 | 2,215; average age: 8 years; United Kingdom | 65 | 153 153 |
Q | TDF Milk |
≥1.2 vs <0.5 cups/day >1.2 cups vs <0.5 cup |
0.66 (0.39–1.10) 0.65 (0.40–1.08) |
Age, weight, height, region, season, and intake of total energy, fruits, and vegetables |
Abbreviations: BMI, body mass index; CI, confidence interval; FFQ, Food Frequency Questionnaire; Q, quintile; OR, odds ratio; RR, relative risk; TDF, total dairy food; No, number; AARP, American Association of Retired Persons; MHT, menopausal hormone therapy.
Main results
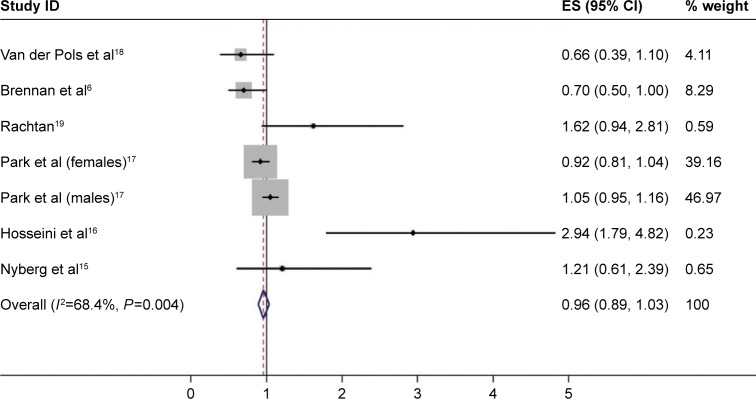
Total dairy intake was inconsistent among seven studies. For the final RR, the highest versus the lowest total dairy consumption was 0.96 (95% CI: 0.89–1.03); heterogeneity was 68.4% (P=0.004). Total milk intake was similarly inconsistent among the seven studies. The final RR was 0.95 (95% CI: 0.76–1.15); heterogeneity was 70.2% (P=0.003). The pooled RRs for the highest versus the lowest total milk intake and total dairy food consumption and lung cancer risk are shown in Figures 2 and 3. Based on the results, there was no significant association between dairy consumption and lung cancer risk.
Figure 2.
Forest plot of studies examining the association between milk intake and lung cancer risk.
Abbreviations: CI, confidence interval; ES, effect size; ID, identification.
Figure 3.
Forest plot of studies examining the association between total dairy product intake and lung cancer risk.
Abbreviations: CI, confidence interval; ES, effect size; ID, identification.
Subgroup analyses
Table 2 shows the results of the subgroup analyses. We conducted a subgroup analysis for dairy product consumption after adjusting for smoking status and age. For total dairy product and milk consumption, there was no significant association with lung cancer risk. A sensitivity analysis revealed that RR ranged from 0.66 (95% CI: 0.39–1.10) to 2.94 (95% CI: 1.79–4.82) for total dairy food consumption and from 0.65 (95% CI: 0.40, 1.08) to 2.64 (95% CI: 1.54–4.51) for milk consumption.
Table 2.
RRs of lung cancer in relation to milk and total dairy food consumption
| Group | Total dairy food
|
Milk
|
||||||
|---|---|---|---|---|---|---|---|---|
| No of studies | RR (95% CI) | Pheterogeneity | I2 (%) | No of studies | RR (95% CI) | Pheterogeneity | I2 (%) | |
| Total cases | 7 | 0.96 (0.89–1.03) | 0.004 | 68.4 | 7 | 0.95 (0.76–1.15) | 0.003 | 70.2 |
| >300 | 4 | 0.97 (0.90–1.04) | 0.003 | 78.5 | 4 | 1.05 (0.78–1.31) | 0.004 | 77.2 |
| ≤300 | 3 | 0.83 (0.52–1.14) | 0.115 | 53.7 | 3 | 0.84 (0.54–1.14) | 0.052 | 66.2 |
| Adjustments in models | ||||||||
| Smoking | 4 | 0.95 (0.83–1.06) | 0.027 | 67.3 | 5 | 1.71 (1.29–2.13) | 0.488 | <0.01 |
| Age | 4 | 0.75 (0.56–0.95) | 0.191 | 36.9 | 6 | 0.92 (0.72–1.12) | 0.010 | 66.9 |
Abbreviations: CI, confidence interval; RR, relative risk; No, number.
Discussion
To the best of our knowledge, this is the first meta-analysis to investigate dairy consumption and lung cancer risk. We found that for milk intake, the RR was 0.95 (95% CI: 0.76–1.15) and the heterogeneity was 70.2% (P=0.003). For total dairy product intake, the RR was 0.96 (95% CI: 0.89–1.03) and the heterogeneity was 68.4% (P=0.004). Subgroup analysis for dairy product consumption suggested that there was no significant association with lung cancer risk.
Dairy consumption may play a role in cancer development.27 However, the evidence from observational studies is inconclusive. The mechanism of any relationship between the consumption of dairy products and the risk of lung cancer remains unclear. There are reports of associations between lung cancer and insulin-like growth factor I,28,29 poor vitamin D status,30,31 and polychlorinated biphenyls,32–34 suggesting possible roles for these factors in tumorigenesis. Previous analyses have led to conflicting findings regarding the association of dairy consumption and lung cancer risk. Two analyses have provided evidence that excess of 25-hydroxy vitamin D may be associated with a reduced risk of lung cancer, especially in subjects with vitamin D deficiency.35,36
Our meta-analysis had several strengths. To improve the statistical power, we included several studies, all of which had a prospective cohort design. This design minimizes selection bias and recall, which is a limitation of retrospective studies. However, there were several limitations in our study. The number of studies included was relatively small, and only studies written in English were considered. Additionally, it is possible that there were unexamined or uncontrolled confounding factors in the included studies, for example, smoking, which is known to be an important risk factor for lung cancer.37–39
There was heterogeneity across the studies in terms of total dairy product and milk consumption, which is not surprising considering the differences in the study designs and in the study populations. Heterogeneity may also have been a consequence of different durations of exposure to dietary products and different dietary habits.
In conclusion, our meta-analysis, which involved eight studies, revealed no significant association between dairy consumption and lung cancer risk. Large cohort studies were adjusted to take account of potential confounding factors, including total energy intake, body mass index, age, and other dietary factors, which are highly correlated with milk and/or total dairy consumption. Further well-designed studies are required.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (NSFC-2013-81372022), and Guangdong Natural Science Foundation (2012B061700074).
Footnotes
Disclosure
The authors confirm that they were not involved in studies included in the meta-analysis. The authors report no conflicts of interest in this work.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis C, Siegel R, Bandi P, Jemal A. Cancer statistics 2011. CA Cancer J Clin. 2011;61(6):409–418. doi: 10.3322/caac.20134. [DOI] [PubMed] [Google Scholar]
- 3.Wiseman M. The second World Cancer Research Fund/American Institute for Cancer Research expert report: food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc. 2008;67(3):253–256. doi: 10.1017/S002966510800712X. [DOI] [PubMed] [Google Scholar]
- 4.Ziegler RG, Mayne ST, Swanson CA. Nutrition and lung cancer. Cancer Causes Control. 1996;7(1):157–177. doi: 10.1007/BF00115646. [DOI] [PubMed] [Google Scholar]
- 5.Hu J, Johnson K, Mao Y, et al. A case-control study of diet and lung cancer in Northeast China. Int J Cancer. 1997;71(6):924–931. doi: 10.1002/(sici)1097-0215(19970611)71:6<924::aid-ijc2>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 6.Brennan P, Fortes C, Butler J, et al. A multicenter case-control study of diet and lung cancer among nonsmokers. Cancer Causes Control. 2000;11(1):49–58. doi: 10.1023/a:1008909519435. [DOI] [PubMed] [Google Scholar]
- 7.Speizer FE, Colditz GA, Hunter DJ, Rosner B, Hennekens C. Prospective study of smoking, antioxidant intake, and lung cancer in middle-aged women (USA) Cancer Causes Control. 1999;10(5):475–482. doi: 10.1023/a:1008931526525. [DOI] [PubMed] [Google Scholar]
- 8.Voorips LE, Goldbohm RA, Verhoeven DT, et al. Vegetable and fruit consumption and lung cancer risk in the Netherlands cohort study on diet and cancer. Cancer Causes Control. 2000;11(2):101–105. doi: 10.1023/a:1008906706084. [DOI] [PubMed] [Google Scholar]
- 9.Tang N, Wu Y, Zhou B, Wang B, Yu R. Green tea, black tea consumption and risk of lung cancer: a meta-analysis. Lung Cancer. 2009;65(3):274–283. doi: 10.1016/j.lungcan.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Breslow RA, Graubard BI, Sinha R, Subar AF. Diet and lung cancer mortality: a 1987 National Health Interview Survey Cohort study. Cancer Causes Control. 2000;11(5):419–431. doi: 10.1023/a:1008996208313. [DOI] [PubMed] [Google Scholar]
- 11.Qin LQ, Xu JY, Wang PY, Kaneko T, Hoshi K, Sato A. Milk consumption is a risk factor for prostate cancer: meta-analysis of case-control studies. Nutr Cancer. 2009;48(1):22–27. doi: 10.1207/s15327914nc4801_4. [DOI] [PubMed] [Google Scholar]
- 12.Dong JY, Zhang L, He K, Qin LQ. Dairy consumption and risk of breast cancer: a meta-analysis of prospective cohort studies. Breast Cancer Res Treat. 2011;127(1):23–31. doi: 10.1007/s10549-011-1467-5. [DOI] [PubMed] [Google Scholar]
- 13.Dong JY, Qin LQ. Soy isoflavones consumption and risk of breast cancer incidence or recurrence: a meta-analysis of prospective studies. Breast Cancer Res Treat. 2011;125(2):315–323. doi: 10.1007/s10549-010-1270-8. [DOI] [PubMed] [Google Scholar]
- 14.Axelsson G, Liljeqvist T, Andersson L, Bergman B, Rylander R. Dietary factors and lung cancer among men in West Sweden. Int J Epidemiol. 1996;25(1):32–39. doi: 10.1093/ije/25.1.32. [DOI] [PubMed] [Google Scholar]
- 15.Nyberg F, Agrenius V, Svartengren K, Svensson C, Pershagen G. Dietary factors and risk of lung cancer in never-smokers. Fredrik Int J Cancer. 1998;78(4):430–436. doi: 10.1002/(sici)1097-0215(19981109)78:4<430::aid-ijc7>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 16.Hosseini M, Naghan PA, Jafari AM, et al. Nutrition and lung cancer: a case control study in Iran. BMC Cancer. 2014;14(1):860. doi: 10.1186/1471-2407-14-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park Y, Leitzmann MF, Subar AF, Hollenbeck A, Schatzkin A. Dairy food calcium, and risk of cancer in the NIH-AARP diet and health study. Arch Intern Med. 2009;169(4):391–401. doi: 10.1001/archinternmed.2008.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van der Pols JC, Bain C, Gunnell D, Smith GD, Frobisher C, Martin RM. Childhood dairy intake and adult cancer risk: 65-y follow-up of the Boyd Orr cohort. Am J Clin Nutr. 2007;86(6):1722–1729. doi: 10.1093/ajcn/86.5.1722. [DOI] [PubMed] [Google Scholar]
- 19.Rachtan J. Dietary habits and lung cancer risk among Polish women. Acta Oncol. 2002;41(4):389–394. doi: 10.1080/028418602760169451. [DOI] [PubMed] [Google Scholar]
- 20.Axelsson G, Rylander R. Diet as risk for lung cancer: a Swedish case-control study. Nutr Cancer. 2002;44(2):145–151. doi: 10.1207/S15327914NC4402_04. [DOI] [PubMed] [Google Scholar]
- 21.Nordmann AJ, Kasenda B, Briel M. Meta-analyses: what they can and cannot do. Swiss Med Wkly. 2012;142:W13518. doi: 10.4414/smw.2012.13518. [DOI] [PubMed] [Google Scholar]
- 22.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 25.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 26.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parodi PW. Cows’ milk fat components as potential anticarcinogenic agents. J Nutr. 1997;127(6):1055–1060. doi: 10.1093/jn/127.6.1055. [DOI] [PubMed] [Google Scholar]
- 28.Voskuil DW, Vrieling A, van’t Veer LJ, Kampman E, Rookus MA. The insulin-like growth factor system in cancer prevention: potential of dietary intervention strategies. Cancer Epidemiol Biomarkers Prev. 2005;14(1):195–203. [PubMed] [Google Scholar]
- 29.Hoppe C, Udam TR, Lauritzen L, Molgaard C, Juul A, Michaelsen KF. Animal protein intake, serum insulin-like growth factor I, and growth in healthy 2.5-y-old Danish children. Am J Clin Nutr. 2004;80(2):447–452. doi: 10.1093/ajcn/80.2.447. [DOI] [PubMed] [Google Scholar]
- 30.Tseng M, Breslow RA, Graubard BI, Ziegler RG. Dairy, calcium, and vitamin D intakes and prostate cancer risk in the National Health and Nutrition Examination Epidemiologic Follow-up Study cohort. Am J Clin Nutr. 2005;81(5):1147–1154. doi: 10.1093/ajcn/81.5.1147. [DOI] [PubMed] [Google Scholar]
- 31.Giovannucci E. Dietary influences of 1, 25(OH) 2 vitamin D in relation to prostate cancer: a hypothesis. Cancer Causes Control. 1998;9(6):567–582. doi: 10.1023/a:1008835903714. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Millikan RC, Bell DA, et al. Polychlorinated biphenyls, cyto-chromeP4501A1 (CYP1A1) polymorphisms, and breast cancer risk among African American women and white women in North Carolina: a population-based case-control study. Breast Cancer Res. 2005;7(1):R12–R18. doi: 10.1186/bcr941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laden F, Ishibe N, Hankinson SE, et al. Polychlorinated biphenyls, cytochrome P450 1A1, and breast cancer risk in the Nurses’ Health Study. Cancer Epidemiol Biomarkers Prev. 2002;11(12):1560–1565. [PubMed] [Google Scholar]
- 34.Schaum J, Schuda L, Wu C, Sears R, Ferrario J, Andrews K. A national survey of persistent, bioaccumulative, and toxic (PBT) pollutants in the United States milk supply. J Expo Anal Environ Epidemiol. 2003;13(3):177–186. doi: 10.1038/sj.jea.7500269. [DOI] [PubMed] [Google Scholar]
- 35.Cheng TY, Neuhouser ML. Serum 25-hydroxy vitamin D, vitamin A, and lung cancer mortality in the US population: a potential nutrient-nutrient interaction. Cancer Causes Control. 2012;23(9):1557–1565. doi: 10.1007/s10552-012-0033-8. [DOI] [PubMed] [Google Scholar]
- 36.Wang X, Cui J, Gu J, He H, Li B, Li W. Plasma 25-hydroxy vitamin D deficiency is associated with the risk of non-small cell lung cancer in a Chinese population. Cancer Biomark. 2015;15(5):663–668. doi: 10.3233/CBM-150506. [DOI] [PubMed] [Google Scholar]
- 37.Pesch B, Kendzia B, Gustavsson P, et al. Cigarette smoking and lung cancer – relative risk estimates for the major histological types from a pooled analysis of case-control studies. Int J Cancer. 2012;131(5):1210–1219. doi: 10.1002/ijc.27339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Papadopoulos A, Guida F, Cénée S, et al. Cigarette smoking and lung cancer in women: results of the French ICARE case-control study. Lung Cancer. 2011;74(3):369–377. doi: 10.1016/j.lungcan.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 39.Papadopoulos A, Guida F, Leffondré K, et al. Heavy smoking and lung cancer: are women at higher risk? Result of the ICARE study. Br J Cancer. 2014;110(5):1385–1391. doi: 10.1038/bjc.2013.821. [DOI] [PMC free article] [PubMed] [Google Scholar]