Abstract
Development of methods for scalable biofabrication of uniformly sized tissue spheroids is essential for tissue spheroid-based bioprinting of large size tissue and organ constructs. The most recent scalable technique for tissue spheroid fabrication employs a micromolded recessed template prepared in a non-adhesive hydrogel, wherein the cells loaded into the template self-assemble into tissue spheroids due to gravitational force. In this study, we present an improved version of this technique. A new mold was designed to enable generation of 61 microrecessions in each well of a 96-well plate. The microrecessions were seeded with cells using an EpMotion 5070 automated pipetting machine. After 48 h of incubation, tissue spheroids formed at the bottom of each microrecession. To assess the quality of constructs generated using this technology, 600 tissue spheroids made by this method were compared with 600 spheroids generated by the conventional hanging drop method. These analyses showed that tissue spheroids fabricated by the micromolded method are more uniform in diameter. Thus, use of micromolded recessions in a non-adhesive hydrogel, combined with automated cell seeding, is a reliable method for scalable robotic fabrication of uniform-sized tissue spheroids.
1. Introduction
Organ printing is the computer aided, layer-by-layer additive biofabrication of human tissue and organ constructs [1]. The concept of tissue spheroid-based organ printing is based on using spherical microtissues (tissue spheroids) as building blocks [2–5]. Tissue spheroids are produced through the spontaneous self-assembly process of single-cell suspensions in the absence of an extracellular matrix [6]. The successful use of tissue spheroids as building blocks requires scalability of their biofabrication process, which means that the tissue spheroids must be produced in large quantities, with uniform size, in a time-efficient manner. Many methods have been suggested for tissue spheroid biofabrication, namely: (i) cultivation of a cell suspension in a spinner flask, gyratory shaker, roller bottle [7–10] or on a non-adhesive surface [11, 12], (ii) centrifugation-based compression [13, 14], and (iii) gravity enforced assembly in hanging drops [6, 15]. However, none of these methods fits the scalability criterion, either because spheroid shape and size cannot be controlled during the fabrication process or because the platform does not permit fabrication of tissue spheroids in large number in a time efficient manner [16]. The use of micromolded wells on non-adhesive hydrogels for guided self-assembly is a promising method for scalable fabrication of tissue spheroids [17–20]. Suspended cells are loaded into the fabricated microwells, where they will assemble and conform to the well geometry driven by gravity and hydrodynamic forces. In order to ensure that the self-assembling process produces reproducibly shaped tissue spheroids, it is essential that the microwell templates have a rounded bottom [19, 20]. The goal of this study was to improve the scalability of already existing micromolding methods for tissue spheroid fabrication. To accomplish this, we designed a mold that enables creation of a large number of microrecessions with minimal effort. An automated pipetting system for seeding the microrecessions with cell suspension for biofabrication of uniform-sized tissue spheroids in large quantities was also employed. Development of a method for scalable biofabrication of uniformly shaped tissue spheroids is an important milestone in the advancement of organ printing technology.
2. Materials and methods
2.1. Cell culture
Adipose tissue derived stem cells (ADSCs) (Invitrogen) were expanded in MesenPRO RS basal medium (Invitrogen) supplemented with MesenPRO RS growth supplement (Invitrogen), 1% penicillin/streptomycin (Invitrogen), 1% L-glutamine (Invitrogen), 1% amphotericinB (Sigma-Aldrich). Cells were maintained in a humidified incubator at 37 °C in 5% CO2–95% air atmosphere, with medium change every second day. Cells from fourth passage cultures were used for tissue spheroid fabrication.
2.2. Mold design and fabrication
Solidworks CAD software (Dassalt Systemes) was used to design a mold with a maximum density of micropillars. The mold consists of a rectangular platform, with 96 pillars that spatially correspond to the individual wells of a 96-well culture plate. Each pillar has 61 round-ended micropillars with 0.8 mm height and 0.4 mm diameter. When immersed in agarose, these micropillars cast microrecessions with a rounded bottom for tissue spheroid formation. The computer-aided design (CAD) of the mold was exported into an STL file and printed with a Connex350™ machine using the Polyjet Matrix™, a proprietary multimaterial 3D printing process of the Objet Geometries (Israel). In this process a very thin layer (16 microns) of photo curable polymeric material is jetted onto a build tray where the model is constructed. During this layer-by-layer deposition process, the photopolymer is cured by exposure to UV light immediately after deposition. A gel-like material is used for supporting hanging parts of the model that can be water jet removed.
2.3. Fabrication of tissue spheroids
2.3.1. Fabrication of tissue spheroids in the micromolded recessions
A suspension of 2% agarose (VWR) in sterile water was prepared and sterilized by autoclaving. Wells of 96-well plates were loaded with 150 μl molten agarose and the sterilized molds were inserted into the plates. After the agarose was cooled, the molds were removed. Each well of the 96-well plates had 61 microrecessions with the same dimensions as the micropillars from the molds. The wells were filled with culture medium (above) and incubated for 24 h at 37 °C for equilibration of the agarose gel, with a medium change after the first 12 h (figures 1(a) and (b)). Cells from confluent ADSC layers were harvested using 0.25% trypsin/EDTA (Invitrogen) for 5 min in a humidified incubator. Cells were counted and their concentration was adjusted to 1.9 × 106 cells ml−1. Next, equilibration medium was removed from the wells of the 96-well plates and replaced with 300 μl of cell suspension using the epMotion 5070 automated pipetting system equipped with the eight-channel dispensing tool and epBlue controlling software (Eppendorf). The seeded 96-well plates were incubated at 37 °C in a humidified incubator for 48 h, during which time tissue spheroids formed at the bottom of the microrecessions (figures 1(c) and (d)). Micrographs of the microrecessions were taken before seeding, 1 h after seeding and at the end of the 48 h of incubation using a Leica DMI 4000B inverted microscope equipped with a Hamamatsu Orca camera. To release the tissue spheroids from the bottom of the microrecession, we created a gentle vortex by repeated pipetting. Spheroids were collected in a Petri dish with a pipette and imaged using a Dage-MTI RC300 camera mounted on a Leica MZ12 stereomicroscope.
Figure 1.
Scheme of the concept: the mold with the micropillars is inserted on the wells of 96-well plates previously filled with molten agarose (a). When the mold is removed, microrecessions are left in the agarose (b). The microrecessions are filled with cell suspension (c) that over 48 h will self-assemble into tissue spheroids at the bottom of the microrecessions (d).
2.3.2. Fabrication of tissue spheroids by the hanging drop method for control
Cells from confluent ADSC layers were harvested using 0.25% trypsin/EDTA for 5 min in a humidified incubator. Cells were counted and the concentration of cell suspension adjusted to 106 cell ml−1 (2.5 × 104 cells/25 μl). Using this suspension, 25 μl droplets were placed on the lid of Petri dishes that were inverted and incubated at 37 °C in the humidified incubator for 48 h. During this incubation time, the cells self-assembled into tissue spheroids at the bottom of the droplets due to gravitational force. For collection, lids were inverted and the tissue spheroids were collected in a Petri dish with a pipette and imaged using a Dage-MTI RC300 camera mounted on a Leica MZ12 stereomicroscope.
2.4. Measurements and statistics
Using Image J software, the images were thresholded to eliminate the background signal and highlight the tissue spheroids. The area of tissue spheroids was measured and diameter was calculated from an assumed circular geometry. Descriptive statistical analysis regarding the uniformity of the diameters of the tissue spheroids was performed using StatPlus: mac LE software.
3. Results
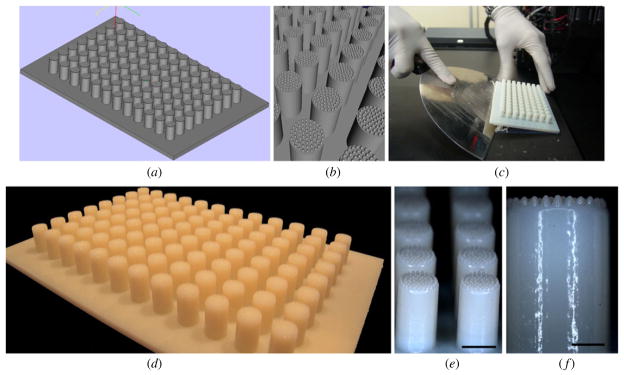
Using CAD we designed a mold that enables casting of 61 microrecessions in each well of a 96-well plate that contains molten sterile agarose. Based on the CAD image (figures 2(a) and (b)), the mold was fabricated in a Connex350™ machine using the Polyjet Matrix™ 3D printing process (figure 2(c)). This process can produce precise models with very fine details and excellent surface finishing without the need of any post-curing processing. The larger pillars of the printed mold fit perfectly into the wells from the 96-well plates. Small variations between the dimensions of the micropillars were observed, but they were not significant enough to interfere with use of the mold for spheroid fabrication (figures 2(d)–(f)).
Figure 2.
Design and fabrication of the mold: (a) CAD image of the mold; (b) higher magnification of the CAD image of individual pillars with 61 micropillars; (c) image of the fabricated mold as it is removed from the printing machine; (d) image of the actual mold; ((e), (f)) closer views on the individual pillars. Scale bar: 5 mm (e), 2 mm (f).
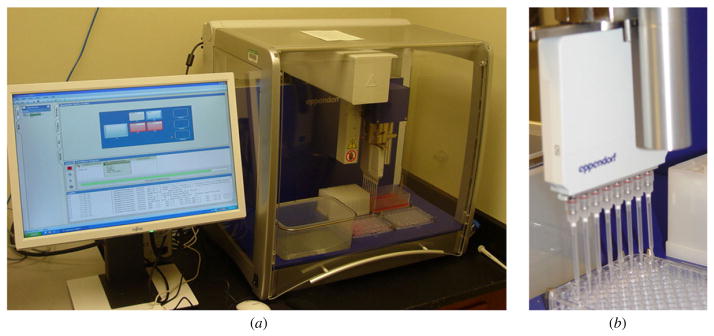
For seeding of the casted microrecessions, we used the epMotion 5070 automated pipetting system. The eight-channel dispensing tool allows seeding of eight wells in one step, speeding up the fabrication process (figure 3).
Figure 3.
Picture of the epMotion 5070 automated pipetting system used for robotic seeding of the microrecessions (a), detailed view on the eight-channel dispensing tool (b).
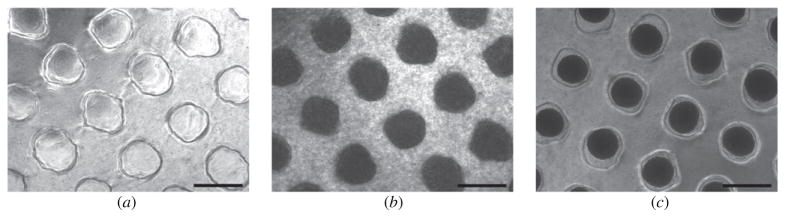
The dispensing tool is equipped with an optical sensor, which detects the level of the agarose in the wells. Therefore the level of the dispensing tool is automatically adjusted thereby avoiding disruption of the microrecessions by the tip of the dispensing pipettes. The epMotion 5070 was used to dispense 300 μl of cell suspension containing 1.9 × 106 cell ml−1 into each well. After 1 h of seeding, the microrecessions were completely filled up with cells. They appeared as loose masses with no demarcation line separating them from the edge of the microrecessions (figure 4(b)). During 48 h in culture, these loose cell masses self-assembled into compact, sharp-edged tissue spheroids at the center of the microrecessions, with visible detachment from the walls of the recessions (figure 4(c)). No cell growth was seen between the microrecessions. After seeding, some cells were observed between the microrecessions but they completely disappeared by the end of the 48 h incubation. This was likely due to washing into the recessions due to medium movement caused by repeated moving of the plate for following up the self-assembling process.
Figure 4.
Fabrication of tissue spheroids by the molded microrecession method: (a) top view of the casted microrecessions before cell seeding; (b) top view of the microrecessions 1 h post-seeding, the dark areas correspond to the loose cell masses that completely fill the microrecessions; (c) top view of the recessions after 48 h incubation. Compact tissue spheroids (black spots) are evident at the center of the recession with clear demarcation from the edge of the microrecessions. Scale bar: 500 μm.
In addition to demonstrating that the presented molding method combined with automated cell seeding is feasible for production of tissue spheroids, we additionally sought to compare this method with the widely used hanging drop method. When considering the efficiency of spheroid production processes, we evaluated uniformity of shape and size of the produced spheroids as criteria for comparison.
Regarding time, the presented method was 40 times more efficient than the hanging drop method. Our method, where 61 microrecessions are present in each well of a 96-well plate and an eight-channel automated pipetting machine is used for seeding, permits production of 488 tissue spheroids in one pipetting movement, compared with the productivity of the hanging drop method, which permits fabrication of maximum 12 tissue spheroids (if a 12-channel pipette is used) in one pipetting movement. Through introduction of the automated pipetting system, the pipetting process is made more precise and the time consuming, laborious manual dispensing is eliminated. Moreover, with more sophisticated automated dispensing machines, which can automatically change the plates too, the whole seeding process can be completely robotized. When considering efficiency, efficient use of space must also be considered. Using our method, 5856 tissue spheroids can be fabricated in one 96-well plate. This number of spheroids corresponds to 39 Petri dishes that contain hanging drops assuming that (in the best case) 150 droplets can be placed in one Petri dish.
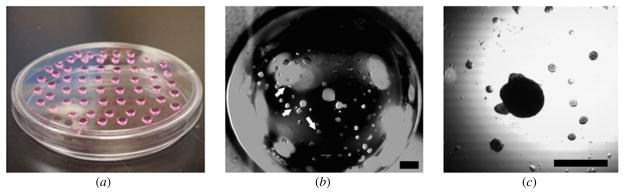
Moreover, the number of cells in the forming tissue spheroids can be more tightly controlled in our method. With the hanging drop method, even when the number of cells in the droplet is tightly controlled, we can never precisely control the number of cells in the forming tissue spheroids due to the frequent formation of undesirable smaller ‘satellite’ tissue spheroids that form on the wall of the hanging droplets. These additional ‘satellite’ tissue spheroids can fall to the bottom of the hanging droplets where their attachment will alter the uniform spherical shape of the forming main tissue spheroids (figure 5). This ‘satellite’ tissue spheroid formation is not observed in our method. All of the seeded cells fall into the casted microrecessions where they self-assemble into uniformly shaped tissue spheroids. We do not know the exact reason behind this difference in ‘satellite’ formation. We speculate that while on the solid, nonadherent surface of the microrecessions the main physical force that drives the sedimentation of the cells is the gravitational force, in the case of the hanging drop method some additional forces, like the surface tension of the liquid droplet, might impede movement of the cells to the bottom of the droplet (especially those cells that are located at the edge of the droplet).
Figure 5.
Fabrication of tissue spheroids by the traditional hanging drop method: confluent cell layers were trypsinized and the resulting cell suspension was adjusted to standard density. Droplets of 25 μl of cell suspension were pipetted onto the lid of a Petri dish that was subsequently inverted over its base (a). After 48 h of incubation, a tissue spheroid was formed at the bottom of the droplet (b) along with additional ‘satellite’ formation (white arrows). This ‘satellite’ can attach to the main tissue spheroid, altering the spherical shape (c). Scale bar: 500 μm.
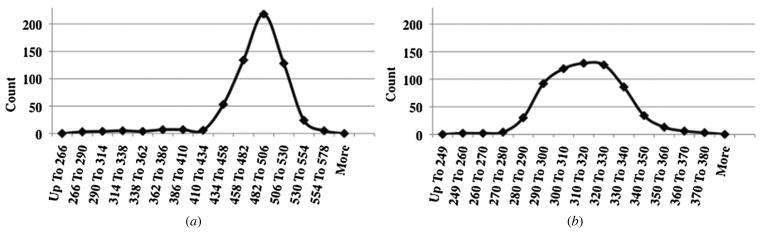
To evaluate the uniformity of diameter of the tissue spheroids, 600 tissue spheroids from both methods were measured. Although the seeding densities were not the same (seeding densities were selected based on optimal outcomes from prior experiments) we did not consider this to be problematic when comparing the two methods because our goal was to determine how uniformly shaped the spheroids were, not to determine and compare their actual diameter. The distribution curves of the collected values were plotted graphically (figure 6). When comparing the shape of the two curves, we observed that the values for the tissue spheroids fabricated by the presented method exhibit a much tighter size distribution than did the values for spheroids made by the hanging drop method. The curve corresponding to the values from the molded microrecession method is almost symmetrical, whereas the one corresponding to the hanging drop method is skewed to the left. This is well represented by the lack of symmetry (skewness value) of the data, which is −2.15 for the hanging drop method compared to only 0.18 for the molding method.
Figure 6.
Distribution of the diameter of tissue spheroids fabricated by the hanging drop (a) and molded microrecession (b) methods.
Representative values from statistical analysis (table 1) also support the efficacy of our method. Because we had two different series of measurements with different means, the absolute values for standard deviation, range and difference between the median and mean could not be compared. To overcome this, we represented these values as percentage of the corresponding mean and will subsequently refer to them simply as percentage (%). The standard deviation (SD), which corresponds to the coefficient of variance (CV) when presented as percentage of the mean, in the case of the molding method is 5.68% compared to 8.36% calculated for the hanging drop method. The same difference can be observed with the other values such as the range of distribution (41.33% compared to 63.8%) and the difference between the median and mean (0.33% compared to 1.2%).
Table 1.
Representative values from descriptive statistical analysis of diameter measurements.
| Standard deviation
|
Range
|
Median
|
||||||
|---|---|---|---|---|---|---|---|---|
| Skewness | Mean (μm) | Absolute (μm) | % of the mean | Absolute (μm) | % of the mean | Absolute (μm) | % of the mean | |
| Hanging drop | −2.15 | 484.54 | 40.51 | 8.36 | 309.454 | 63.8 | 490.18 | 101.2 |
| Molded recession | 0.18 | 316.04 | 17.95 | 5.68 | 130.64 | 41.33 | 314.97 | 99.66 |
Taken together we conclude that the values for diameter of tissue spheroids fabricated by the presented molding method not only exhibit a more symmetrical redistribution, but have less variability than the values obtained from tissue spheroids made by the hanging drop method.
4. Discussion
Organ printing is a biomedical variant of rapid prototyping technology or layer-by-layer additive biomanufacturing. It could ultimately surpass the traditional solid scaffold-based tissue engineering through its use of self-assembling tissue spheroids as building blocks [1–5, 21]. The concept of using tissue spheroids as building blocks raises two major questions: What cell type is optimal and what is the most efficient method for fabrication of the tissue spheroids?
The ideal cell source for tissue engineering must be: (1) autologous, in order to eliminate immunological response after implantation of the fabricated construct; (2) easy to isolate in huge numbers with minimal invasiveness; (3) easy to expand in vitro and (4) multipotent, in order to allow fabrication of different types of tissues using the single cell source [22, 23]. For years, bone marrow mesenchymal stem cells (BM-MSCs) were considered to be the most ideal cell source for tissue engineering and regenerative medicine. However, since 2001, when presence of multipotent cells within the stromal vascular fraction of adipose tissue was first reported, ADSCs have received increasing attention as an alternative cell source for regenerative medicine and tissue engineering. Based on reports to date, these cells satisfy the above-mentioned criteria for ideal cell source and in some aspects may be a better cell source than BM-MSCs [22–28]. Adipose progenitors can be isolated from adipose tissue harvested by liposuction, which is a less invasive and painful procedure than bone marrow aspiration. The number of stem cells isolated from 1 g adipose tissue is 500-fold greater than the number of stem cells isolated from the same amount of bone marrow [23]. Moreover, ADSCs show a higher proliferation rate than BM-MSCs, allowing easier expansion in vitro [25, 26]. Numerous studies demonstrate the multipotency of ADSCs. This multipotential character suggests that ADSCs may be an ideal cell source for regenerative medicine and tissue engineering. Indeed, several human clinical trials have already suggested that ADSCs may be useful for treatment of disorders that include: diabetes mellitus, Crohn’s disease, coronary artery disease, liver cirrhosis and others [24, 27]. For organ printing, spheroids biofabricated from differentiated ADSCs could potentially be bioprinted in any type of configuration, depending on what tissue type is needed. For instance, for fabrication of a vascular tube, spheroids from ADSCs can be differentiated toward smooth muscle lineage and deposited in a three-dimensional circular pattern through layer-by-layer bioprinting. After spheroid fusion, a tubular construct will be formed. Subsequent to maturation, these structures could be used for replacement of diseased or damaged vascular segments.
The most efficacious method of spheroid biofabrication for organ printing must satisfy well-defined criteria [5]. Straightforward arithmetic calculations, based on the volume of tissue spheroids and different human organ volumes, strongly indicate that in order to print human organs it will be necessary to generate millions of tissue spheroids. Therefore, one of the most important criteria is scalability [5, 16, 20]. The method of spheroid biofabrication for organ printing must allow production of tissue spheroids in large number and in a time-efficient manner. The presented method completely satisfies these criteria by allowing production of 5856 spheroids in only 12 pipetting movements. By introducing the automated pipetting machine for cell seeding, we made the first step toward automated spheroid production. By designing an automated system for molding and use of a more advanced automated dispensing machine (which can automatically change the plates too), the whole method can be completely automated. This would eliminate time consuming, laborious manual dispensing and possible human errors, making the whole production process more efficient.
Another important criterion is standardization of the shape and size of the produced spheroids. Production of uniformly shaped and sized tissue spheroids is important in the printing step. During this step, the tissue spheroids are dispensed through a nozzle. In order that this process is undisrupted and to avoid destruction of the spheroids, it is essential that the spheroids be of the same size and shape as the printer’s nozzle [5]. The size (diameter) to which the spheroids are fabricated is optional and can be determined by the design of the printer’s nozzle. However, there is a size limit for the individual tissue spheroids based on cell viability at the center of the spheroids. The oxygen diffusion limit in tissues is around 150–200 μm, so cells inside tissue spheroids with diameter of greater than 300–400 μm could undergo hypoxia-induced cell death [16, 29–31]. Based on this concept, our microrecessions have a diameter of 400 μm to limit the diameter of the fabricated spheroids to this value. The presence of hipoxia-related cell death in the center of the spheroids depends not only on the oxygen diffusion but also on oxygen consumption (which is cell dependent). Therefore further investigations must be done with this specific cell type (adipose tissue derived stem cell) to determine the optimal diameter and seeding density. The method presented in this study generates relatively uniform tissue spheroids and from this point of view is much better than the broadly used hanging drop method. Our method is not perfect either; variations in size are still observed. We attribute these variations to the resolution limits of the printing machine used for fabrication of the mold. The small variations in size of the micropillars on the mold translate into variations in size of the casted microrecessions. This variability leads to variation in cell seeding number in each microrecession, which ultimately leads to variation in size of the fabricated spheroids. Another possible explanation for the variation in size of the spheroids could be the ‘meniscus’ problem. When the wells of the 96-well plates are filled with agarose, the surface of the agarose (due to the small diameter of the wells) is not completely horizontal as a meniscus is formed. The micropillars from the mold are in the same plane, so when the mold is applied on the concave surface of the agarose, the casted microrecessions will not be completely uniform in depth. We strongly believe that improvement of the mold design respective use of more precise resin technologies for printing the mold will help producing even more uniform tissue spheroids.
The existing methods of tissue spheroid biofabrication can be further improved by using a droplet microfluidics device (droplet generator), which allows production of thousand of droplets (spheroids) per second. However, microfluidic technology could potentially compromise the level of cell density in the hydrogel-based droplets. Development of non-toxic sacrificial hydrogels would enable development of digital or droplet-based microfluidic methods for scalable biofabrication tissue spheroids.
Tissue spheroids, sometimes referred to as multicellular spheroids (MCS), are not used in tissue engineering alone. They have been widely used as a three-dimensional (3D) cell culture model in cancer research, tumor therapy, developmental and cell biology, drug discovery, etc [32–37]. Researchers prefer spheroid cultures over the conventional 2D monolayer cultures because 3D cultures more closely mimic the in vivo cell arrangements. Therefore cell behaviors (proliferation, migration, apoptosis, response to different stimuli) may more closely model in vivo physiology. Thus, conclusions from experiments conducted using 3D cultures may be more easily translated into animal or human models. Based on this wide range of applications for tissue spheroids, we consider that robotic scalable tissue spheroid biofabricators will be an integral element not only of future industrial organ biofabrication, but also of high-throughput screening assays for drug testing, cancer cell biology and stem cell biology. It is reasonable to predict that new, more effective robotic methods of scalable tissue spheroid biofabrication will be developed. Cost-effectiveness of the biofabrication process will emerge as a most critical factor in the design of novel tissue spheroid biofabricators.
Acknowledgments
This work was supported by NSF R-II grant ‘South Carolina Project for Organ Biofabrication’ and EPSCoR GEAR grant.
References
- 1.Mironov V, Boland T, Trusk T, Forgacs G, Markwald RR. Organ printing: computer-aided jet-based 3D tissue engineering. Trends Biotechnol. 2003;21:157–61. doi: 10.1016/S0167-7799(03)00033-7. [DOI] [PubMed] [Google Scholar]
- 2.Jakab K, Neagu A, Mironov V, Markwald RR, Forgacs G. Engineering biological structures of prescribed shape using self-assembling multicellular systems. Proc Natl Acad Sci USA. 2004;101:2864–69. doi: 10.1073/pnas.0400164101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelm JM, Djonov V, Ittner LM, Fluri D, Born W, Hoerstrup SP, Fussenegger M. Design of custom-shaped vascularized tissues using microtissue spheroids as minimal building units. Tissue Eng. 2006;12:2151–60. doi: 10.1089/ten.2006.12.2151. [DOI] [PubMed] [Google Scholar]
- 4.Mironov V, Kasyanov V, Drake C, Markwald RR. Organ printing: promises and challenges. Regen Med. 2008;3:93–103. doi: 10.2217/17460751.3.1.93. [DOI] [PubMed] [Google Scholar]
- 5.Mironov V, Visconti RP, Kasyanov V, Forgacs G, Drake CJ, Markwald RR. Organ printing: tissue spheroids as building blocks. Biomaterials. 2009;30:2164–74. doi: 10.1016/j.biomaterials.2008.12.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelm JM, Fussenegger M. Microscale tissue engineering using gravity-enforced cell assembly. Trends Biotechnol. 2004;22:195–202. doi: 10.1016/j.tibtech.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Lazar A, Mann HJ, Remmel RP, Shatford RA, Cerra FB, Hu WS. Extended liver-specific functions of porcine hepatocyte spheroids entrapped in collagen gel. In Vitro Cell Dev Biol Anim. 1995;31:340–46. doi: 10.1007/BF02634282. [DOI] [PubMed] [Google Scholar]
- 8.Sakai Y, Naruse K, Nagashima I, Muto T, Suzuki M. Large-scale preparation and function of porcine hepatocyte spheroids. Int J Artif Organs. 1996;19:294–301. [PubMed] [Google Scholar]
- 9.Nyberg SL, Hardin J, Amiot B, Argikar UA, Remmel RP, Rinaldo P. Rapid, large-scale formation of porcine hepatocyte spheroids in a novel spheroid reservoir bioartificial liver. Liver Transpl. 2005;11:901–10. doi: 10.1002/lt.20446. [DOI] [PubMed] [Google Scholar]
- 10.Song H, David O, Clejan S, Giordano CL, Pappas-Lebeau H, Xu L, O’Connor KC. Spatial composition of prostate cancer spheroids in mixed and static cultures. Tissue Eng. 2004;10:1266–76. doi: 10.1089/ten.2004.10.1266. [DOI] [PubMed] [Google Scholar]
- 11.Landry J, Bernier D, Ouellet C, Goyette R, Marceau N. Spheroidal aggregate culture of rat liver cells: histotypic reorganization, biomatrix deposition, and maintenance of functional activities. J Cell Biol. 1985;101:914–23. doi: 10.1083/jcb.101.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuhas JM, Li AP, Martinez AO, Ladman AJ. A simplified method for production and growth of multicellular tumor spheroids. Cancer Res. 1977;37:3639–43. [PubMed] [Google Scholar]
- 13.Qihao Z, Xigu C, Guanghui C, Weiwei Z. Spheroid formation and differentiation into hepatocyte-like cells of rat mesenchymal stem cell induced by co-culture with liver cells. DNA Cell Biol. 2007;26:497–503. doi: 10.1089/dna.2006.0562. [DOI] [PubMed] [Google Scholar]
- 14.Akiyama M, Nonomura H, Kamil SH, Ignotz RA. Periosteal cell pellet culture system: a new technique for bone engineering. Cell Transplant. 2006;15:521–32. doi: 10.3727/000000006783981765. [DOI] [PubMed] [Google Scholar]
- 15.Kelm JM, Timmins NE, Brown CJ, Fussenegger M, Nielsen LK. Method for generation of homogeneous multicellular tumor spheroids applicable to a wide variety of cell types. Biotechnol Bioeng. 2003;83:173–80. doi: 10.1002/bit.10655. [DOI] [PubMed] [Google Scholar]
- 16.Lin RZ, Chang HY. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol J. 2008;3:1172–84. doi: 10.1002/biot.200700228. [DOI] [PubMed] [Google Scholar]
- 17.Karp JM, et al. Controlling size, shape and homogeneity of embryoid bodies using poly(ethylene glycol) microwells. Lab Chip. 2007;7:786–94. doi: 10.1039/b705085m. [DOI] [PubMed] [Google Scholar]
- 18.Fukuda J, Khademhosseini A, Yeo Y, Yang X, Yeh J, Eng G, Blumling J, Wang CF, Kohane DS, Langer R. Micromolding of photocrosslinkable chitosan hydrogel for spheroid microarray and co-cultures. Biomaterials. 2006;27:5259–67. doi: 10.1016/j.biomaterials.2006.05.044. [DOI] [PubMed] [Google Scholar]
- 19.Napolitano AP, Chai P, Dean DM, Morgan JR. Dynamics of the self-assembly of complex cellular aggregates on micromolded nonadhesive hydrogels. Tissue Eng. 2007;13:2087–94. doi: 10.1089/ten.2006.0190. [DOI] [PubMed] [Google Scholar]
- 20.Napolitano AP, Dean DM, Man AJ, Youssef J, Ho DN, Rago AP, Lech MP, Morgan JR. Scaffold-free three-dimensional cell culture utilizing micromolded nonadhesive hydrogels. Biotechniques. 2007;43:494–500. doi: 10.2144/000112591. [DOI] [PubMed] [Google Scholar]
- 21.Kelm JM, Lorber V, Snedeker JG, Schmidt D, Broggini-Tenzer A, Weisstanner M, Odermatt B, Mol A, Zund G, Hoerstrup SP. A novel concept for scaffold-free vessel tissue engineering: self-assembly of microtissue building blocks. J Biotechnol. 2010;148:46–55. doi: 10.1016/j.jbiotec.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–60. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mizuno H. Adipose-derived stem cells for tissue repair and regeneration: ten years of research and a literature review. J Nippon Med Sch. 2009;76:56–66. doi: 10.1272/jnms.76.56. [DOI] [PubMed] [Google Scholar]
- 24.Witkowska-Zimny M, Walenko K. Stem cells from adipose tissue. Cell Mol Biol Lett. 2011;16:236–57. doi: 10.2478/s11658-011-0005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helder MN, Knippenberg M, Klein-Nulend J, Wuisman PIJM. Stem cells from adipose tissue allow challenging new concepts for regenerative medicine. Tissue Eng. 2007;13:1799–808. doi: 10.1089/ten.2006.0165. [DOI] [PubMed] [Google Scholar]
- 26.Zhu Y, Liu T, Song K, Fan X, Ma X, Cui Z. Adipose-derived stem cells: a better stem cell than BMSC. Cell Biochem Funct. 2008;26:664–75. doi: 10.1002/cbf.1488. [DOI] [PubMed] [Google Scholar]
- 27.Tobita M, Orbay H, Mizuno H. Adipose-derived stem cells: current findings and future perspectives. Discov Med. 2011;11:160–70. [PubMed] [Google Scholar]
- 28.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;2:211–28. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 29.Lee J, Cuddihy MJ, Cater GM, Kotov NA. Engineering liver tissue spheroids with inverted colloidal crystal scaffolds. Biomaterials. 2009;30:4687–94. doi: 10.1016/j.biomaterials.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 30.Groebe K, Mueller-Klieser W. On the relation between size of necrosis and diameter of tumor spheroids. Int J Radiat Oncol Biol Phys. 1996;34:395–401. doi: 10.1016/0360-3016(95)02065-9. [DOI] [PubMed] [Google Scholar]
- 31.Acker H, Carlsson J, Mueller-Klieser W, Sutherland RM. Comparative pO2 measurements in cell spheroids cultured with different techniques. Br J Cancer. 1987;56:325–27. doi: 10.1038/bjc.1987.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mueller-Klieser W. Three-dimensional cell cultures: from molecular mechanisms to clinical applications. Am J Physiol. 1997;273:1109–23. doi: 10.1152/ajpcell.1997.273.4.C1109. [DOI] [PubMed] [Google Scholar]
- 33.Pampaloni F, Reynaud EG, Stelzer EH. The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol. 2007;8:839–45. doi: 10.1038/nrm2236. [DOI] [PubMed] [Google Scholar]
- 34.Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol. 2006;7:211–24. doi: 10.1038/nrm1858. [DOI] [PubMed] [Google Scholar]
- 35.Yamada KM, Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell. 2007;130:601–10. doi: 10.1016/j.cell.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 36.Hirschhaeuser F, Menne H, Dittfeld C, West J, Mueller-Klieser W, Kunz-Schughart LA. Multicellular tumor spheroids: an underestimated tool is catching up again. J Biotechnol. 2010;148:3–15. doi: 10.1016/j.jbiotec.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 37.Kunz-Schughart LA, Freyer JP, Hofstaedter F, Ebner R. The use of 3D cultures for high-throughput screening: the multicellular spheroid model. J Biomol Screen. 2004;9:273–85. doi: 10.1177/1087057104265040. [DOI] [PubMed] [Google Scholar]