Synopsis
Langerhans cell histiocytosis (LCH) is a diagnosis encompassing a wide spectrum of clinical manifestations, characterized by the common finding of inflammatory lesions containing clonal CD1a+ Langerin+ (CD207) histiocytes or LCH cells. Based on phenotypic similarity of LCH cells and epidermal Langerhans cells (LCs), models of pathogenesis have focused on aberrations in the differentiation of epidermal LCs. Recently, activating somatic mutations in the MAPK pathway have been discovered in the majority of cases of LCH and ERK phosphorylation appears to be universal in LCH cells. Using the BRAF V600E mutation as a lineage marker, emerging data support a model in which MAPK activation in self-renewing hematopoietic progenitors may drive disseminated high-risk disease, whereas MAPK activation in more differentiated committed myeloid precursors or peripheral tissue myeloid populations may induce multifocal or unifocal low-risk LCH. The heterogeneous clinical manifestations with shared histology may therefore represent the final common pathway of an acquired defect of differentiation, initiated at more than one point. Implications of this model include re-definition of LCH as a myeloid neoplasia and re-focusing therapeutic strategies on the cells and lineages of origin.
Keywords: Langerhans cell histiocytosis, BRAF, MAPK signaling, dendritic cell, myeloid differentiation
Introduction to LCH
The Histiocytoses
The spectrum of histiocytic diseases is characterized by collections of abnormal histiocytes or literally ‘tissue cells’ related to myeloid cells of the mononuclear phagocyte system 1-4. LCH is defined by the presence of a large pale-staining histiocyte with high expression of CD1a and Langerin (CD207) and containing Birbeck granules (Figure 1). These features, shared with Langerhans cells (LCs) of the epidermis, are the basis of classifying the disease as a ‘Langerhans cell’ histiocytosis. Prior to this, LCH was known as Histiocytosis X, a disease entity incorporating historically described syndromes: Hand-Christian-Schüller disease, characterized by lytic bone lesions and mucosal lesions; Letterer-Siwe disease, a fatal hepatosplenomegaly; and eosinophilic granuloma of bone. The discovery of the Birbeck granule in LCs in 1961 and identification of the same organelle in Histiocytosis X by Basset and colleagues in 1965 formed the basis for modern models of LCH5. Another influence driving this model of LCH at the same time, was the recently conceived ‘Mononuclear phagocyte system’ (MPS) in which peripheral macrophages were thought to be continually renewed from bone marrow derived monocytes1. LCs had recently joined the MPS, by virtue of their expression of MHC class II, complement and Fc receptors and repopulation by bone marrow derived cells after transplantation. The formation of LCH was therefore perceived to be an aberration of this development leading to the accumulation of abnormal LC-like cells in inappropriate locations.
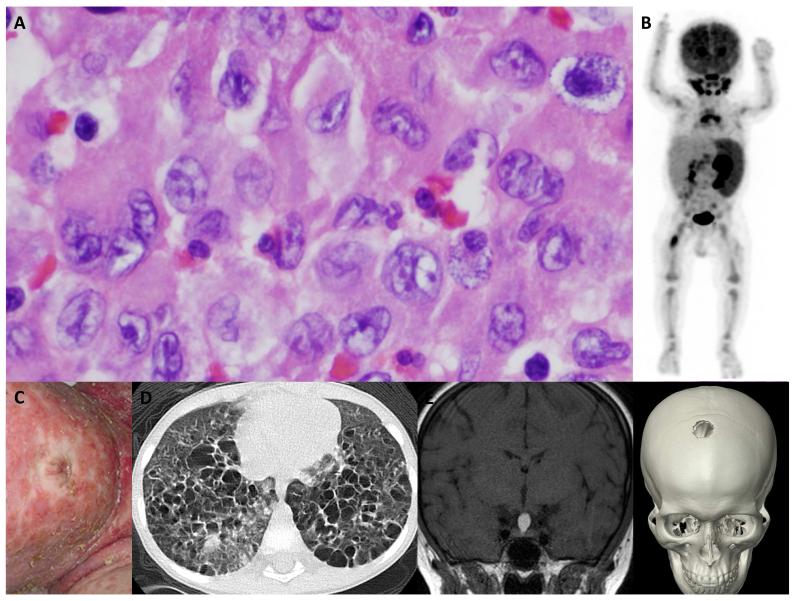
Figure 1. LCH: Common histology and clinical heterogeneity.
A. Hematoxylin and eosin staining of a typical LCH lesion, demonstrating the classic large histiocytes with grooved “coffee-bean” nuclei and abundant eosinophilic cytoplasm. The inflammatory infiltrate varies, but typically includes lymphocytes, eosinophils and macrophages. Histologic features have not been associated with specific clinical presentations. This biopsy could have come from any of the cases presented in B-F.
B. PET scan of a 1 year old with high-risk LCH: PET-avid R femur lesion, spleen, bone marrow and cervical lymph nodes are evident in this image.
C. Infant with severe LCH skin lesions. In infants, skin LCH may be self-limiting and spontaneously resolve or may be part of life-threatening multi-system high-risk disease.
D. CT scan demonstrating innumerable cysts and lung lesions in a 3 year old with LCH involving lung, pituitary and skin.
E. Brain MRI in a teenager with an isolated pituitary LCH lesion.
F. CT scan of a teenager with an isolated skull LCH lesion.
Clinical Overview
The clinical spectrum of LCH ranges from a trivial single lesion to aggressive and potentially lethal disseminated disease (Figure 1). Almost every organ system has been reported to be involved by LCH lesions. In infants, skin lesions are common and may represent skin-limited disease that resolves without therapy; alternatively, skin “rash” may be part of a constellation of lesions in disseminated multisystem disease. In older children and adults, presenting symptoms may include pain from bone lesions, dyspnea from lung lesions, failure to thrive from intestinal involvement, or uncontrolled thirst from pituitary infiltration and resulting diabetes insipidus. The range of presenting symptoms and overlapping clinical features with more common childhood conditions make LCH a challenging diagnosis. However, once biopsy is performed, the unique features of LCH cells and the inflammatory context of lymphocytes, eosinophils and macrophages are pathognomonic. While the number of pathologic histiocytes and relative contribution of infiltrating leukocyte populations may be variable, the overall pattern is conserved across the spectrum of clinical manifestations of LCH (Figure 1).
Rationale for current approaches to LCH
Clinically, LCH has been categorized as having a “high-risk” of mortality when it involves bone marrow, spleen or liver or “low-risk” in any other site. Treatment regimens are empiric, as summarized by Dr. Arceci and colleagues in the last Clinics of North America issue dedicated to LCH (1998): “The variety of different treatment approaches to such patients has prompted some individuals to believe that LCH treatment “strategy” is based more on a roulette wheel than on scientifically based logic. Certainly, part of the confusion and lack of consensus is derived from a persisting ambivalence as to whether LCH is primarily a neoplastic disorder, an immunodysrgulatory disorder, or a disorder with characteristics of both” 6. Vinblastine and prednisone have been the standard induction therapy for decades, though LCH-II and LCH-III trials demonstrated improved outcomes with dose intensification and therapy prolongation7;8.
Molecular Insights into Pathogenesis of LCH
Langerhans Cell Histiocytosis: The debate
The fundamental nature of LCH as neoplastic versus reactive disorder has been an ongoing debate 6;9. The granulomatous histology with quiescent histiocytes suggested potential autoimmune or infectious etiology10 but the unique appearance of LCH cells and destructive nature of lesions hinted at dysplastic development. Although Nezelof and colleagues described LCs as the ‘stem cell’ of LCH, they also acknowledged the prevailing view that elements of the MPS including LCs, were continually replenished by the differentiation of bone marrow derived precursors. Many hypotheses emerged that LCH might arise from LC precursors in a state of arrested development, misguided to inappropriate sites by a pathological cytokine or chemokine milieu 11;12,13;14, but no unifying ‘extrinsic’ explanation for pathological LCH cell differentiation was ever achieved (Reviewed in 15). A neoplastic origin for LCH was suggested by the coincidence of LCH with myelodysplastic syndrome and other malignancies 16;17 and a major breakthrough came with the finding the LCH cells are clonal 18;19. However, persistent failure to identify genetic abnormalities in systematic analysis of LCH lesions tempered classification of LCH as a “cancer” 20-23.
Somatic MAPK mutations in LCH
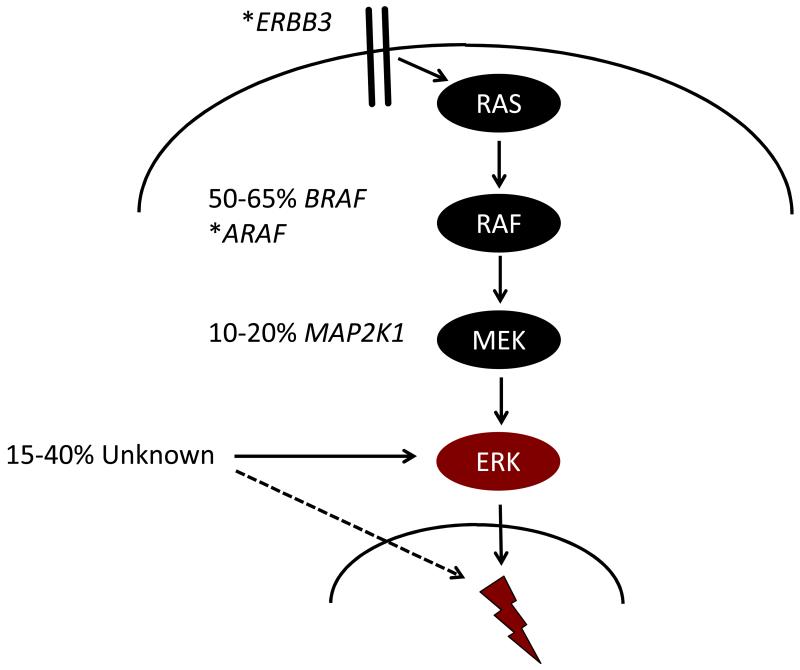
In 2010, Rollins and colleagues reported the seminal finding of recurrent BRAF V600E point mutations in approximately 60% of LCH lesions 24. BRAF is a central kinase which transduces signals through the MAPK pathway that regulates numerous essential cellular functions (Figure 2A). The BRAF mutation encoding the V600E substitution leads to constitutive activation of downstream MEK and ERK kinases25 and is observed at high frequency in melanoma, in approximately 7% of human cancers overall and also in a number of benign neoplastic conditions including epidermal nevi and colon polyps26;27. Subsequently, whole exome sequencing of LCH lesions has revealed recurrent mutations in MAP2K1 (encoding MEK1) in another 20% of patients and cases of mutations in other MAPK pathway genes ARAF and ERBB3, on a very low overall mutation rate background (0.03 mutations per Mb) (Figure 2)28-30. These mutations all induce ERK activation, a universal feature of LCH cells24;29. The genomic landscape of LCH is discussed in more detail elsewhere in this issue.
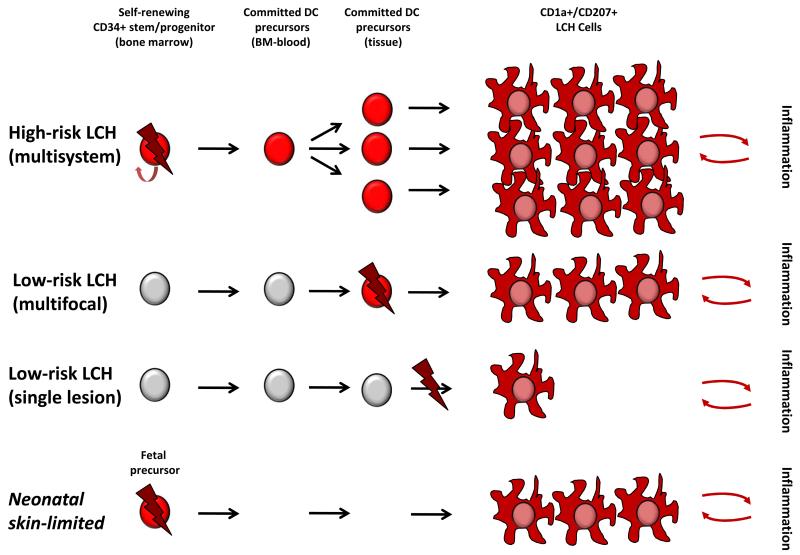
Figure 2. Model of LCH pathogenesis: ERK activation at specific stages of myelopoiesis determines clinical phenotype.
A. Mutually exclusive activating mutations in MAPK genes have been reported in most cases of LCH, and ERK activation appears to be universal in LCH lesion CD207+ cells. Lightning bolt represents the downstream impact of ERK activation that presumably is essential for LCH pathogenesis.
B. Proposed “Misguided Myeloid DC” model of LCH pathogenesis in which somatic mutation (lightning bolt) and subsequent ERK activation (red cell) at specific stages of DC development determines clinical outcome. This model reflects interpretation of data from human and mouse studies75, but leaves room for future refinement. According to this model, ERK activation in a self-renewing progenitor/stem cell in the bone marrow has the potential to form lesions in hematopoietic organs, liver, and virtually any organ system leading to MS LCH with somatic mutation detectable in BM and blood. In contrast, ERK activation in committed precursors may form multiple lesions in a limited number of organ systems, but somatic mutation is not usually detectable in the BM or blood at diagnosis, or subsequently. ERK activation at a later stages of differentiation, perhaps even in a single tissue cell, may form a single unifocal lesion. Based on recent data describing the pre-natal origin of tissue myeloid cells, it is also conceivable that ERK activating mutation may arise during fetal development. This is speculative but could explain “self-resolving” neonatal LCH (Hashimoto Pritzker syndrome) in which mutated fetal precursors would be replaced by normal myeloid cells after birth. In all models, it is suggested that LCH cells recruit and activate inflammatory cells which may provide reciprocal survival signals and clearly play a role in clinical manifestations of LCH.
Branches of DC Differentiation
The insight of Nezelof and colleagues identified commonality between LCs and LCH but by their own reckoning, could not fully explain the histogenesis of LCH31. The identification of MAPK pathway mutations provides a genetic neoplastic etiology and an important investigational tool with which to track potential LCH precursor cells. However, the problem of understanding exactly how potential LCH precursors differentiate abnormally remains unsolved.
The phenotype of LCH DCs
In order to understand how aberrant DC differentiation might give rise to LCH cells, it is necessary to consider the phenotype of LCH cells in more detail. Breathnach, Basset and Nezelof noted that in addition to Birbeck granules, acid-phosphatase, non-specific esterase, and alpha-D mannosidase were expressed by LCs and LCH cells5. The advent of monoclonal OKT6, binding CD1a was a major advance both conceptually and diagnostically, as was the description of Langerin32. Expression of CD1a is universal in LCH, and typically CD1a and Langerin are highly co-expressed. Birbeck granules are associated with high CD1a and Langerin and are most abundant in the skin but may be absent in the liver and in old ‘xanthomatous’ lesions33. Similarly, several reports describe heterogeneous states of differentiation of LCH cells within lesions, including variable CD1a+/Langerin-subpopulations33-35 Antibody recognizing BRAF V600E (VE1), has also demonstrated that the mutation is present in some Langerin-negative cells within LCH lesions36.
Despite these similarities, gene expression studies have demonstrated many differences between LCH cells and LCs: LCH cells express relatively less EpCAM, E-cadherin and CD36; they also express higher levels of CD2, CD11b, CD11c, CD13, CD33, CD66c, and CD300LF, markers associated with myeloid DCs at all states of maturation. LCH cells also express high levels of cytokines and chemokines including SPP1 (osteopontin), together with receptors CCR1, and NRP1 that influence capacity to recruit and interact with T cells37. When isolated from lesions, they are somewhat immature, promoting a Th2-rich environment enriched with eosinophils and Tregs21. These observations support a hypothesis that aberrant differentiation of a myeloid progenitor leads to a series of LC-like differentiation steps that are time and location dependent. The nature of this precursor remains undefined although the low expression of S100A8/9 indicates that LCH cells are unlikely to be recently derived from monocytes37.
Origin and homeostasis of Langerhans cells in the steady-state
It is now appreciated that LCs are unique not only in their residence in the epidermis and mucosae but in their origin and homeostasis38. Contrary to the MPS model, LCs are not continually replenished from blood-borne progenitors in the steady-date. The first LCs are derived from myeloid precursors during fetal life39;40 which differentiate under the influence of the CSF-1R cytokine IL-3441;42 and autocrine production of TGFβ43. Ultimately, fetal LC progenitors are derived from primitive waves of hematopoiesis that are c-myb and flt3-independent and precede definitive hematopoiesis in the fetal liver44-46. Once seeded in the epidermis, LCs are able to undergo homeostatic self-renewal, as first suggested in humans47 and proven by elegant experiments in mice22. The independence of LCs from monocytes and blood DCs in the steady state is also demonstrated by the preservation of these cells in patients with genetic deficiency of monocytes and DCs48;49 and in patients who have received limb transplants50. The development and subsequent self-renewing potential of a tissue macrophage/DC network during fetal life raises new possibilities for the origin of LCH cells. It is conceivable that somatic mutation could arise in these cycling cells and could result in LCH or that activation of a more widely-distributed fetal precursor could occur (Figure 2B).
Replenishment of LCs during inflammation
While fetal-derived LCs are self-renewing in epidermis during steady-state, bone marrow-derived precursors are recruited during inflammation, such as graft versus host disease after human stem cell transplantation 51;52. In mice, the epidermis is initially infiltrated by classical monocytes53, but a second wave of more long-lived LC precursors has recently been described54;55. In a recapitulation of embryonic development, IL-34 is also required to maintain long term LC repopulation54.
Dual inflammatory LC precursors
The duality of inflammatory LC precursors in mice also finds resonance with in vitro experiments in humans. A two phase kinetic was observed many years ago in serial skin biopsies of DTH reactions56. Langerin+ cells can be derived from monocytes57-59, from CD14+ cells appearing in CD34+ cultures60 and from dermal CD14+ cells that are now known to be monocyte-derived61;62. All these may represent the monocyte pathway of short term recruitment. In addition, CD1a+ CD14-negative intermediates with restricted LC potential can be generated from CD34+ progenitors63;64. This suggests potential for an alternative pathway of LC differentiation, a conclusion that was recently supported by direct comparisons of CD14 monocytes and CD1c+ blood DCs exposed to LC differentiation conditions. In these experiments, surprisingly, CD1c+ DCs expressed much higher levels of CD1a and Langerin than monocytes and only CD1c + DCs rapidly formed Birbeck granules65;66. Either GM-CSF or TSLP was able to induce CD1a expression and high Langerin was promoted by TGFβ or BMP7. The role of IL-34 was not explored. Together these results suggest that the DC differentiation pathway may contribute to long term LC precursors observed in mice and furthermore, that both bone marrow-derived monocytes and myeloid DCs can express Langerin and are candidate precursors for LCH cells.
Langerhans cells are not the only fruit: other human dendritic cells
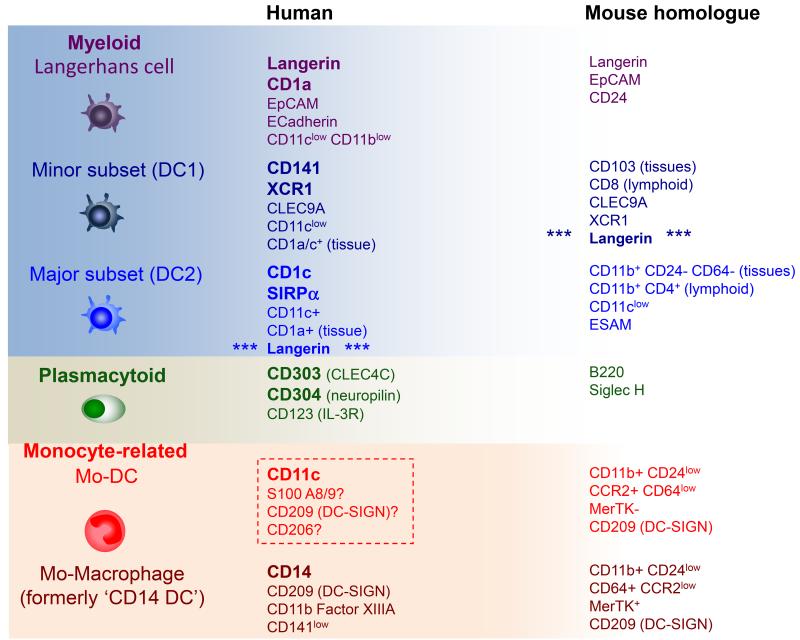
LCs are the paradigmatic migratory DC, but blood and interstitial tissues contain two other populations of myeloid DCs: a minor subset of CD141+ cells and a major subset of CD1c+ cells67-69. (Table 1) Representatives of both are found in the blood and lymph nodes and are evolutionarily conserved in mammals, corresponding to the two subsets of classical or conventional DCs described in mice2;4. The term ‘myeloid’ is quite specific in humans and refers to the expression of antigens typically seen on granulocytes or monocytes including CD13, CD33, CD11b and CD11c. Plasmacytoid DCs, typically lack myeloid antigens and are morphologically and functionally quite distinct, providing a major source type 1 interferon in response to viral infection (Reviewed in70). They are typically only found in lymph nodes and inflamed tissues. In addition, a number of monocyte-derived DCs and macrophages are found in human tissues61;71;72.
Table 1. Steady-state human dendritic cells.
The principal DC subsets found in human blood, tissues and lymphoid tissues, together with their murine homologues. LCs are restricted only to stratified squamous epithelia (skin and mucosae). Plasmacytoid DCs are only found in lymphoid tissue in the steady state. The existence of a human monocyte-derived DC (mo-DC) in the steady state is unconfirmed (broken line box).
Langerin-positive dendritic cells
It is now known that other DC lineage cells expression Langerin. A small population of CD1c+ DCs (the major myeloid DC), distinct from LCs and from CD141+ DCs, expresses a low level of Langerin in vivo 68,73. Confusingly, Langerin is also found on mouse DCs but restricted to the minor classical DC population which is not the direct homologue of human CD1c+ DCs74 (Table 1). The presence of Langerin+ CD1c+ DCs in human tissues is consistent with the ability of blood CD1c+ DCs to up-regulate Langerin in vitro and reinforces the idea that CD1c DCs could function as LC precursors during inflammation and potentially give rise to LCH cells. Intriguingly, some of the key differences in gene expression profile between LCH cells and LCs are lost when LCH cells are compared with Langerin+ CD1c+ DCs. For example, Langerin+ DCs are EpCAM and E-Cadherin negative and have higher CD11b and CD11c, unlike LCs 73. Direct comparison of the transcriptional profiles of LCH with blood CD1c+ DCs and epidermal LCs indicates similar proximity59 but further studies are required to compare LCH with tissue CD1c+ DCs and Langerin+ in vitro-derived CD1c+ DCs in detail.
Revisiting the Cell of Origin in LCH
Lineage tracing LCH
Somatic mutations in LCH have provided a foothold with which to study the cell of origin of LCH. From the previous discussion it is evident that LCH cells could arise from more than one source. In an attempt to more precisely identify candidate precursor populations, we asked whether the mutation encoding BRAF V600E was present in circulating mononuclear cells in patients with active LCH. In our series of 100 patients, peripheral blood mononuclear cells with the BRAF V600E point mutation were identified in all patients with high-risk LCH in whom BRAF V600E was detected in lesion LCH cells 75. No circulating cells with BRAF V600E were identified in patients with single system disease, and only in a small number of patients with presumed multifocal low-risk disease. Furthermore, the presence of circulating cells in high-risk patients correlated with disease activity. Lineage analysis of the circulating cells in high-risk patients identified cells with BRAF V600E in CD14+ monocyte and CD11c+ myeloid DCs (which includes CD1c+ DCs, CD141+ DCs, CD16+ non-classical monocytes) fractions in all cases, suggesting the somatic mutation arose in a myeloid progenitor.
Continuing the search upstream for the hematopoietic origin of LCH, analysis of bone marrow aspirate demonstrated that the BRAF V600E mutation was present in CD34+ hematopoietic stem and progenitor cells (HSPC) in some, but not all, cases of high-risk patients. It should be noted that in half of the cases where BRAF V600E was identified in bone marrow aspirate, histology was reportedly normal, suggesting the mutation arose in morphologically normal precursor cells. Interestingly, in cases where BRAF V600E was identified in CD34+ HSPCs, it could also be identified in circulating CD19+ B cells, suggesting the involvement of a pluripotent stem cells. CD3+ T cells were not affected in keeping with their relative independence from the bone marrow75. BRAF V600E was also recently identified in CD34+ cells of patients with hairy cell leukemia76. It is not clear why the same presumed driver mutation occurring in HSC leads to distinct conditions. Potential explanations include epigenetic imprinting of individual stem cells towards different fates or additional modifying somatic mutations77 or acquisition of mutations at different stages of hematopoietic development.
An updated model of LCH
These data suggest a revised model of LCH pathogenesis in which the developmental stage at which an ERK activating mutation arises determines the clinical manifestations (Figure 2B). The common histology observed by Lichtenstein could represent a common final pathway from many different starting points. This hypothesis is further supported by the observation that enforced expression of BRAF V600E in Langerin+ cells in mice resulted in formation of localized LCH-like lesions with no BRAF V600E expressed in cells in circulation, where enforced expression of BRAF V600E in CD11c+ cells resulted in a more aggressive phenotype similar to disseminated high-risk LCH75.
JXG and ECD: Cousins or siblings of LCH?
Juvenile xanthogranuloma (JXG) and Erdheim-Chester disease (ECD) are histologically similar diseases characterized by CD14+, CD68+, fascin+, factor XIII+, CD1a−, CD207− histiocytes. JXG is a disease of childhood that, like LCH, ranges from trivial skin-limited lesions to life-threatening disseminated disease. JXG has been reported to co-present with juvenile myelomonocytic leukemia as well as with germline mutations in NF1 that implicate potential role for MAPK hyperactivity in pathogenesis (Reviewed in 78). ECD is a very rare disease of adults that typically presents with more clinically ominous systemic lesions. Like LCH, BRAF V600E and other activating MAPK mutations are reported in the majority of cases79;80. Furthermore, both JXG and ECD have been reported to co-present with LCH, either with mixed histology in the same lesion or with distinct histology in different lesions (Reviewed in 78). Together, the common trigger of MAPK hyperactivity and consistent mutations across lesions in patients with mixed histiocytic disorders29;75;81 suggest potential for a common origin and blurs the distinction between the neoplastic histiocytoses.
LCH as a myeloproliferative disorder
Activating somatic mutations in hematopoietic stem cells and myeloid precursors in LCH patients, coupled with the formation of lesions driven by BRAF V600E in mouse models, support the classification of LCH as a myeloid neoplasia. Transformed DC clones presumably recruit inflammatory cells, resulting in a cytokine rich milieu that promotes LCH cell survival. We therefore propose that LCH and related JXG and ECD represent a class of histiocytic inflammatory myeloproliferative neoplastic (MPN) disorder. Other MPNs, such as chronic myelogenous leukemia and myelofibrosis, also share the features of a genetically quiet landscape with single driver mutations in kinase pathways and a prominent inflammatory component to disease.
Re-branding LCH as a bona fide myeloid neoplasia has some practical as well as political implications. First, understanding LCH as a “stem cell” disease may explain the very high rate of relapse in patients treated with local or minimally myelotoxic therapeutic strategies82. If approached as a stem cell disease, optimal therapy for patients with systemic disease would focus on eradicating the transformed precursor rather than treating individual lesions or tolerating frequent “reactivations”. Further, chemotherapy agents with efficacy against myleloid lineages may have superior efficacy in LCH, compared with traditional ALL-based therapies. In keeping with this, small cohort studies report that clofarabine, a nucleoside analogue with efficacy in AML, has cured some patients with aggressive, refractory LCH83-85. Finally, direct targeted therapy with RAF inhibitors such as vemurafenib has shown early promise in adults with ECD and LCH, further demonstrating the critical role of MAPK activation in driving pathogenesis86.
Summary/Future Directions
LCH is a complex disease with a fascinating history. Individual reports of highly variable intriguing presentations merged into eponymous classifications that were later unified as Histiocytosis X, due to common histology. Subsequently, “Langerhans cell” histiocytosis shifted focus to the aberrant development of LCs. New data support a model that once again deconstructs this single diagnosis into its many facets each caused by a repertoire of mutations acting at multiple stages of myeloid cell development. The common molecular pathway is MAPK hyperactivation and the common endpoint is a CD1a+ CD1c+ neoplastic cell, but it now seems that every patient has their own private route to developing LCH.
The pace of progress since the last LCH edition of Clinics has accelerated, though patients continue to have suboptimal outcomes, and many fundamental aspects of pathogenesis remain unanswered. Politically, new understanding of the nature of LCH is highly significant in placing LCH in the disease family embraced by funding agencies including the National Cancer Institute as well as by cooperative clinical trial and translational research groups that advocate for patients with cancer and related disorders. Scientifically, the mechanisms by which MAPK activation drives differentiation of the precursor and subsequent recruitment of inflammatory infiltrate are not known. Improved understanding of mechanisms of pathogenesis will further inform general clinical approaches as well as opportunities for personalized approaches to risk stratification, targeted therapy and monitoring disease burden. While much remains to be learned, we believe that there is now a more rational path to develop “strategy” to improve outcomes for patients with LCH beyond the luck of the roulette wheel.
Key Points.
Dendritic cells are immune cells that arise from different lineages with the shared function of presenting antigen and activating adaptive immunity.
LCH arises from myeloid dendritic cell precursors. MAPK activation is a universal feature of CD1a+ Langerin+ LCH cells. The clinical extent of LCH is related to the stage of development in which somatic MAPK mutations arise; either self-renewing progenitors or committed precursors.
Activating MAPK mutations in hematopoietic stem cells and committed myeloid precursors support classification of LCH as a myeloid neoplasia.
Acknowledgements
The Texas Children’s Cancer Center Histiocytosis Program (CEA and KLM) is supported by the HistioCure Foundation. Grant support includes NIH R01 (CA154489) (CEA, KLM), NIH SPORE in Lymphoma (P50CA126752) (CEA), and the St. Baldrick’s Consortium Grant for the North American Consortium for Histiocytosis Research (CEA, KLM). MC is supported by the Histiocytosis Research Trust (UK), the Leventis Foundation and the Histiocytosis Association. VB is a Wellcome Trust Intermediate Fellow (WT088555MA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicting financial interests.
Reference List
- 1.Van Furth R, Cohn ZA. The origin and kinetics of mononuclear phagocytes. J.Exp.Med. 1968;128:415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guilliams M, Ginhoux F, Jakubzick C, et al. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat.Rev.Immunol. 2014;14:571–578. doi: 10.1038/nri3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geissmann F, Manz MG, Jung S, et al. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collin M, McGovern N, Haniffa M. Human dendritic cell subsets. Immunology. 2013;140:22–30. doi: 10.1111/imm.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nezelof C, Basset F, Rousseau MF. Histiocytosis × histogenetic arguments for a Langerhans cell origin. Biomedicine. 1973;18:365–371. [PubMed] [Google Scholar]
- 6.Arceci RJ, Brenner MK, Pritchard J. Controversies and new approaches to treatment of Langerhans cell histiocytosis. Hematol.Oncol.Clin.North Am. 1998;12:339–357. doi: 10.1016/s0889-8588(05)70514-1. [DOI] [PubMed] [Google Scholar]
- 7.Gadner H, Grois N, Arico M, et al. A randomized trial of treatment for multisystem Langerhans’ cell histiocytosis. J.Pediatr. 2001;138:728–734. doi: 10.1067/mpd.2001.111331. [DOI] [PubMed] [Google Scholar]
- 8.Gadner H, Grois N, Potschger U, et al. Improved outcome in multisystem Langerhans cell histiocytosis is associated with therapy intensification. Blood. 2008;111:2556–2562. doi: 10.1182/blood-2007-08-106211. [DOI] [PubMed] [Google Scholar]
- 9.Degar BA, Rollins BJ. Langerhans cell histiocytosis: malignancy or inflammatory disorder doing a great job of imitating one? Dis.Model.Mech. 2009;2:436–439. doi: 10.1242/dmm.004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeziorski E, Senechal B, Molina TJ, et al. Herpes-virus infection in patients with Langerhans cell histiocytosis: a case-controlled sero-epidemiological study, and in situ analysis. PLoS.One. 2008;3:e3262. doi: 10.1371/journal.pone.0003262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geissmann F, Lepelletier Y, Fraitag S, et al. Differentiation of Langerhans cells in Langerhans cell histiocytosis. Blood. 2001;97:1241–1248. doi: 10.1182/blood.v97.5.1241. [DOI] [PubMed] [Google Scholar]
- 12.Nezelof C, Basset F. An hypothesis Langerhans cell histiocytosis: the failure of the immune system to switch from an innate to an adaptive mode. Pediatr.Blood Cancer. 2004;42:398–400. doi: 10.1002/pbc.10463. [DOI] [PubMed] [Google Scholar]
- 13.Fleming MD, Pinkus JL, Fournier MV, et al. Coincident expression of the chemokine receptors CCR6 and CCR7 by pathologic Langerhans cells in Langerhans cell histiocytosis. Blood. 2003;101:2473–2475. doi: 10.1182/blood.V101.7.2473. [DOI] [PubMed] [Google Scholar]
- 14.Annels NE, da Costa CE, Prins FA, et al. Aberrant chemokine receptor expression and chemokine production by Langerhans cells underlies the pathogenesis of Langerhans cell histiocytosis. J Exp.Med. 2003;197:1385–1390. doi: 10.1084/jem.20030137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laman JD, Leenen PJ, Annels NE, Hogendoorn PC, Egeler RM. Langerhans-cell histiocytosis ‘insight into DC biology’. Trends Immunol. 2003;24:190–196. doi: 10.1016/s1471-4906(03)00063-2. [DOI] [PubMed] [Google Scholar]
- 16.Egeler RM, Neglia JP, Arico M, et al. The LCH-Malignancy Study Group of the Histiocyte Society The relation of Langerhans cell histiocytosis to acute leukemia, lymphomas, and other solid tumors. Hematol.Oncol.Clin.North Am. 1998;12:369–378. doi: 10.1016/s0889-8588(05)70516-5. [DOI] [PubMed] [Google Scholar]
- 17.Surico G, Muggeo P, Rigillo N, Gadner H. Concurrent Langerhans cell histiocytosis and myelodysplasia in children. Med.Pediatr.Oncol. 2000;35:421–425. doi: 10.1002/1096-911x(20001001)35:4<421::aid-mpo5>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 18.Willman CL, Busque L, Griffith BB, et al. Langerhans’-cell histiocytosis (histiocytosis X)--a clonal proliferative disease. N.Engl.J.Med. 1994;331:154–160. doi: 10.1056/NEJM199407213310303. [DOI] [PubMed] [Google Scholar]
- 19.Yu RC, Chu C, Buluwela L, Chu AC. Clonal proliferation of Langerhans cells in Langerhans cell histiocytosis. Lancet. 1994;343:767–768. doi: 10.1016/s0140-6736(94)91842-2. [DOI] [PubMed] [Google Scholar]
- 20.Waskow C, Liu K, Darrasse-Jeze G, et al. The receptor tyrosine kinase Flt3 is required for dendritic cell development in peripheral lymphoid tissues. Nat.Immunol. 2008;9:676–683. doi: 10.1038/ni.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Senechal B, Elain G, Jeziorski E, et al. Expansion of regulatory T cells in patients with Langerhans cell histiocytosis. PLoS.Med. 2007;4:e253. doi: 10.1371/journal.pmed.0040253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merad M, Manz MG, Karsunky H, et al. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat.Immunol. 2002;3:1135–1141. doi: 10.1038/ni852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.da Costa CE, Szuhai K, van ER, et al. No genomic aberrations in Langerhans cell histiocytosis as assessed by diverse molecular technologies. Genes Chromosomes. Cancer. 2009;48:239–249. doi: 10.1002/gcc.20634. [DOI] [PubMed] [Google Scholar]
- 24.Badalian-Very G, Vergilio JA, Degar BA, et al. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010;116:1919–1923. doi: 10.1182/blood-2010-04-279083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maurer G, Tarkowski B, Baccarini M. Raf kinases in cancer-roles and therapeutic opportunities. Oncogene. 2011;30:3477–3488. doi: 10.1038/onc.2011.160. [DOI] [PubMed] [Google Scholar]
- 26.Michaloglou C, Vredeveld LC, Mooi WJ, Peeper DS. BRAF(E600) in benign and malignant human tumours. Oncogene. 2008;27:877–895. doi: 10.1038/sj.onc.1210704. [DOI] [PubMed] [Google Scholar]
- 27.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 28.Nelson DS, Quispel W, Badalian-Very G, et al. Somatic activating ARAF mutations in Langerhans cell histiocytosis. Blood. 2014;123:3152–3155. doi: 10.1182/blood-2013-06-511139. [DOI] [PubMed] [Google Scholar]
- 29.Chakraborty R, Hampton OA, Shen X, et al. Mutually exclusive recurrent somatic mutations in MAP2K1 and BRAF support a central role for ERK activation in LCH pathogenesis. Blood. 2014;124:3007–3015. doi: 10.1182/blood-2014-05-577825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown NA, Furtado LV, Betz BL, et al. High prevalence of somatic MAP2K1 mutations in BRAF V600E-negative Langerhans cell histiocytosis. Blood. 2014;124:1655–1658. doi: 10.1182/blood-2014-05-577361. [DOI] [PubMed] [Google Scholar]
- 31.Nezelof C, Basset F. From histiocytosis × to Langerhans cell histiocytosis: a personal account. Int.J Surg.Pathol. 2001;9:137–146. doi: 10.1177/106689690100900208. [DOI] [PubMed] [Google Scholar]
- 32.Valladeau J, Ravel O, Dezutter-Dambuyant C, et al. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity. 2000;12:71–81. doi: 10.1016/s1074-7613(00)80160-0. [DOI] [PubMed] [Google Scholar]
- 33.Chikwava K, Jaffe R. Langerin (CD207) staining in normal pediatric tissues, reactive lymph nodes, and childhood histiocytic disorders. Pediatr.Dev.Pathol. 2004;7:607–614. doi: 10.1007/s10024-004-3027-z. [DOI] [PubMed] [Google Scholar]
- 34.Peters TL, McClain KL, Allen CE. Neither IL-17A mRNA nor IL-17A protein are detectable in Langerhans cell histiocytosis lesions. Mol.Ther. 2011;19:1433–1439. doi: 10.1038/mt.2011.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coury F, Annels N, Rivollier A, et al. Langerhans cell histiocytosis reveals a new IL-17A-dependent pathway of dendritic cell fusion. Nat.Med. 2008;14:81–87. doi: 10.1038/nm1694. [DOI] [PubMed] [Google Scholar]
- 36.Sahm F, Capper D, Preusser M, et al. BRAFV600E mutant protein is expressed in cells of variable maturation in Langerhans cell histiocytosis. Blood. 2012;120:e28–e34. doi: 10.1182/blood-2012-06-429597. [DOI] [PubMed] [Google Scholar]
- 37.Allen CE, Li L, Peters TL, et al. Cell-specific gene expression in Langerhans cell histiocytosis lesions reveals a distinct profile compared with epidermal Langerhans cells. J.Immunol. 2010;184:4557–4567. doi: 10.4049/jimmunol.0902336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romani N, Clausen BE, Stoitzner P. Langerhans cells and more: langerin-expressing dendritic cell subsets in the skin. Immunol.Rev. 2010;234:120–141. doi: 10.1111/j.0105-2896.2009.00886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schuster C, Vaculik C, Fiala C, et al. HLA-DR+ leukocytes acquire CD1 antigens in embryonic and fetal human skin and contain functional antigen-presenting cells. J Exp.Med. 2009;206:169–181. doi: 10.1084/jem.20081747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoeffel G, Wang Y, Greter M, et al. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J Exp.Med. 2012;209:1167–1181. doi: 10.1084/jem.20120340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y, Szretter KJ, Vermi W, et al. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat.Immunol. 2012;13:753–760. doi: 10.1038/ni.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greter M, Lelios I, Pelczar P, et al. Stroma-derived interleukin-34 controls the development and maintenance of langerhans cells and the maintenance of microglia. Immunity. 2012;37:1050–1060. doi: 10.1016/j.immuni.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borkowski TA, Letterio JJ, Farr AG, Udey MC. A role for endogenous transforming growth factor beta 1 in Langerhans cell biology: the skin of transforming growth factor beta 1 null mice is devoid of epidermal Langerhans cells. J Exp.Med. 1996;184:2417–2422. doi: 10.1084/jem.184.6.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schulz C, Gomez PE, Chorro L, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 45.Boiers C, Carrelha J, Lutteropp M, et al. Lymphomyeloid contribution of an immune-restricted progenitor emerging prior to definitive hematopoietic stem cells. Cell Stem Cell. 2013;13:535–548. doi: 10.1016/j.stem.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 46.Gomez PE, Klapproth K, Schulz C, et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518:547–551. doi: 10.1038/nature13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Czernielewski JM, Demarchez M. Further evidence for the self-reproducing capacity of Langerhans cells in human skin. J Invest Dermatol. 1987;88:17–20. doi: 10.1111/1523-1747.ep12464659. [DOI] [PubMed] [Google Scholar]
- 48.Hambleton S, Salem S, Bustamante J, et al. IRF8 mutations and human dendritic-cell immunodeficiency. N.Engl.J Med. 2011;365:127–138. doi: 10.1056/NEJMoa1100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bigley V, Haniffa M, Doulatov S, et al. The human syndrome of dendritic cell, monocyte, B and NK lymphoid deficiency. J Exp.Med. 2011;208:227–234. doi: 10.1084/jem.20101459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kanitakis J. Transmission of rosacea from the graft in facial allotransplantation. Am.J Transplant. 2011;11:1338–1339. doi: 10.1111/j.1600-6143.2011.03507.x. [DOI] [PubMed] [Google Scholar]
- 51.Collin MP, Hart DN, Jackson GH, et al. The fate of human Langerhans cells in hematopoietic stem cell transplantation. J Exp.Med. 2006;203:27–33. doi: 10.1084/jem.20051787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mielcarek M, Kirkorian AY, Hackman RC, et al. Langerhans cell homeostasis and turnover after nonmyeloablative and myeloablative allogeneic hematopoietic cell transplantation. Transplantation. 2014;98:563–568. doi: 10.1097/TP.0000000000000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ginhoux F, Tacke F, Angeli V, et al. Langerhans cells arise from monocytes in vivo. Nat.Immunol. 2006;7:265–273. doi: 10.1038/ni1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sere KM, Lin Q, Felker P, et al. Dendritic cell lineage commitment is instructed by distinct cytokine signals. Eur.J Cell Biol. 2012;91:515–523. doi: 10.1016/j.ejcb.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 55.Nagao K, Kobayashi T, Moro K, et al. Stress-induced production of chemokines by hair follicles regulates the trafficking of dendritic cells in skin. Nat.Immunol. 2012;13:744–752. doi: 10.1038/ni.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaplan G, Nusrat A, Witmer MD, Nath I, Cohn ZA. Distribution and turnover of Langerhans cells during delayed immune responses in human skin. J Exp.Med. 1987;165:763–776. doi: 10.1084/jem.165.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Geissmann F, Prost C, Monnet JP, et al. Transforming growth factor beta1, in the presence of granulocyte/macrophage colony-stimulating factor and interleukin 4, induces differentiation of human peripheral blood monocytes into dendritic Langerhans cells. J Exp.Med. 1998;187:961–966. doi: 10.1084/jem.187.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoshino N, Katayama N, Shibasaki T, et al. A novel role for Notch ligand Delta-1 as a regulator of human Langerhans cell development from blood monocytes. J Leukoc.Biol. 2005;78:921–929. doi: 10.1189/jlb.1204746. [DOI] [PubMed] [Google Scholar]
- 59.Hutter C, Kauer M, Simonitsch-Klupp I, et al. Notch is active in Langerhans cell histiocytosis and confers pathognomonic features on dendritic cells. Blood. 2012;120:5199–5208. doi: 10.1182/blood-2012-02-410241. [DOI] [PubMed] [Google Scholar]
- 60.Schaerli P, Willimann K, Ebert LM, Walz A, Moser B. Cutaneous CXCL14 targets blood precursors to epidermal niches for Langerhans cell differentiation. Immunity. 2005;23:331–342. doi: 10.1016/j.immuni.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 61.McGovern N, Schlitzer A, Gunawan M, et al. Human dermal CD14(+) cells are a transient population of monocyte-derived macrophages. Immunity. 2014;41:465–477. doi: 10.1016/j.immuni.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Larregina AT, Morelli AE, Spencer LA, et al. Dermal-resident CD14+ cells differentiate into Langerhans cells. Nat.Immunol. 2001;2:1151–1158. doi: 10.1038/ni731. [DOI] [PubMed] [Google Scholar]
- 63.Caux C, Vanbervliet B, Massacrier C, et al. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to GM-CSF+TNF alpha. J Exp.Med. 1996;184:695–706. doi: 10.1084/jem.184.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Strunk D, Rappersberger K, Egger C, et al. Generation of human dendritic cells/Langerhans cells from circulating CD34+ hematopoietic progenitor cells. Blood. 1996;87:1292–1302. [PubMed] [Google Scholar]
- 65.Martinez-Cingolani C, Grandclaudon M, Jeanmougin M, et al. Human blood BDCA-1 dendritic cells differentiate into Langerhans-like cells with thymic stromal lymphopoietin and TGF-beta. Blood. 2014;124:2411–2420. doi: 10.1182/blood-2014-04-568311. [DOI] [PubMed] [Google Scholar]
- 66.Milne P, Bigley V, Gunawan M, Haniffa M, Collin M. CD1c+ blood dendritic cells have Langerhans cell potential. Blood. 2015;125:470–473. doi: 10.1182/blood-2014-08-593582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jardine L, Barge D, Ames-Draycott A, et al. Rapid detection of dendritic cell and monocyte disorders using CD4 as a lineage marker of the human peripheral blood antigen-presenting cell compartment. Front Immunol. 2013;4:495. doi: 10.3389/fimmu.2013.00495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Haniffa M, Shin A, Bigley V, et al. Human tissues contain CD141hi cross-presenting dendritic cells with functional homology to mouse CD103+ nonlymphoid dendritic cells. Immunity. 2012;37:60–73. doi: 10.1016/j.immuni.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu CI, Becker C, Wang Y, et al. Human CD1c+ dendritic cells drive the differentiation of CD103+ CD8+ mucosal effector T cells via the cytokine TGF-beta. Immunity. 2013;38:818–830. doi: 10.1016/j.immuni.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reizis B, Colonna M, Trinchieri G, Barrat F, Gilliet M. Plasmacytoid dendritic cells: one-trick ponies or workhorses of the immune system? Nat.Rev.Immunol. 2011;11:558–565. doi: 10.1038/nri3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Angel CE, Lala A, Chen CJ, et al. CD14+ antigen-presenting cells in human dermis are less mature than their CD1a+ counterparts. Int.Immunol. 2007;19:1271–1279. doi: 10.1093/intimm/dxm096. [DOI] [PubMed] [Google Scholar]
- 72.Chu CC, Ali N, Karagiannis P, et al. Resident CD141 (BDCA3)+ dendritic cells in human skin produce IL-10 and induce regulatory T cells that suppress skin inflammation. J Exp.Med. 2012;209:935–945. doi: 10.1084/jem.20112583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bigley V, McGovern N, Milne P, et al. Langerin-expressing dendritic cells in human tissues are related to CD1c+ dendritic cells and distinct from Langerhans cells and CD141high XCR1+ dendritic cells. J Leukoc.Biol. 2014 doi: 10.1189/jlb.1HI0714-351R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Merad M, Ginhoux F, Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat.Rev.Immunol. 2008;8:935–947. doi: 10.1038/nri2455. [DOI] [PubMed] [Google Scholar]
- 75.Berres ML, Lim KP, Peters T, et al. BRAF-V600E expression in precursor versus differentiated dendritic cells defines clinically distinct LCH risk groups. J.Exp.Med. 2014;211:669–683. doi: 10.1084/jem.20130977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chung SS, Kim E, Park JH, et al. Hematopoietic stem cell origin of BRAFV600E mutations in hairy cell leukemia. Sci.Transl.Med. 2014;6:238ra71. doi: 10.1126/scitranslmed.3008004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Naik SH, Perie L, Swart E, et al. Diverse and heritable lineage imprinting of early haematopoietic progenitors. Nature. 2013;496:229–232. doi: 10.1038/nature12013. [DOI] [PubMed] [Google Scholar]
- 78.Berres ML, Allen CE, Merad M. Pathological consequence of misguided dendritic cell differentiation in histiocytic diseases. Adv.Immunol. 2013;120:127–161. doi: 10.1016/B978-0-12-417028-5.00005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Haroche J, Charlotte F, Arnaud L, et al. High prevalence of BRAF V600E mutations in Erdheim-Chester disease but not in other non-Langerhans cell histiocytoses. Blood. 2012;120:2700–2703. doi: 10.1182/blood-2012-05-430140. [DOI] [PubMed] [Google Scholar]
- 80.Emile JF, Diamond EL, Helias-Rodzewicz Z, et al. Recurrent RAS and PIK3CA mutations in Erdheim-Chester disease. Blood. 2014;124:3016–3019. doi: 10.1182/blood-2014-04-570937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hervier B, Haroche J, Arnaud L, et al. Association of both Langerhans cell histiocytosis and Erdheim-Chester disease linked to the BRAFV600E mutation. Blood. 2014;124:1119–1126. doi: 10.1182/blood-2013-12-543793. [DOI] [PubMed] [Google Scholar]
- 82.Minkov M, Steiner M, Potschger U, et al. Reactivations in multisystem Langerhans cell histiocytosis: data of the international LCH registry. J.Pediatr. 2008;153:700–5. 705. doi: 10.1016/j.jpeds.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 83.Simko SJ, Tran HD, Jones J, et al. Clofarabine salvage therapy in refractory multifocal histiocytic disorders, including Langerhans cell histiocytosis, juvenile xanthogranuloma and Rosai-Dorfman disease. Pediatr.Blood Cancer. 2014;61:479–487. doi: 10.1002/pbc.24772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rodriguez-Galindo C, Jeng M, Khuu P, McCarville MB, Jeha S. Clofarabine in refractory Langerhans cell histiocytosis. Pediatr.Blood Cancer. 2008;51:703–706. doi: 10.1002/pbc.21668. [DOI] [PubMed] [Google Scholar]
- 85.Abraham A, Alsultan A, Jeng M, Rodriguez-Galindo C, Campbell PK. Clofarabine salvage therapy for refractory high-risk langerhans cell histiocytosis. Pediatr.Blood Cancer. 2013;60:E19–E22. doi: 10.1002/pbc.24436. [DOI] [PubMed] [Google Scholar]
- 86.Haroche J, Cohen-Aubart F, Emile JF, et al. Dramatic efficacy of vemurafenib in both multisystemic and refractory Erdheim-Chester disease and Langerhans cell histiocytosis harboring the BRAF V600E mutation. Blood. 2013;121:1495–1500. doi: 10.1182/blood-2012-07-446286. [DOI] [PubMed] [Google Scholar]