To the Editor:
On-therapy decline or normalization of liver enzyme levels is often observed following initiation of interferon-based hepatitis C virus (HCV) antiviral treatment and may reflect response to therapy and decreasing HCV RNA levels (1,2). On-treatment alanine aminotransferase (ALT) level elevation may be associated with persistent viremia (3–5). The value of enzymatic response in predicting sustained virological response (SVR) and relapse is unclear.
We evaluated the role of baseline and week 4 ALT and aspartate aminotransferase (AST) levels in predicting HCV response to interferon-based antiviral therapy. A retrospective database analysis of 434 adults with HCV initiating therapy at The Ottawa Hospital Viral Hepatitis Clinic (Ottawa, Ontario) between January 1, 2000 and December 31, 2009 was conducted (Research Ethics Board number 2004–196).
Patients were predominately Caucasian (81%) and male (72%), with a mean (± SD) age of 44.3±9.1 years. HCV risk factors included intravenous drug use history (56%) and contaminated blood product exposure (26%). Nine percent were HIV coinfected. The population was predominantly infected with HCV genotype 1 (56.1%), genotype 3 (22.4%) and genotype 2 (15.8%). Advanced fibrosis (F3 to F4) was identified in 35% of patients. Thirty-nine percent had normal ALT (<63 U/L) and 26% had normal AST (<37 U/L) levels at baseline.
At week 4, 83% of patients had normal ALT levels and 57% had normal AST levels. Fifty-two patients (62%) achieved rapid virological response, 182 (88%) early virological response and 154 (68%) SVR. Twenty-four (11%) patients relapsed post-treatment.
Baseline ALT and AST levels were higher in patients not achieving SVR. According to multivariate analysis, baseline AST (OR 0.99 [95% CI 0.98 to 0.99]) was a predictor of SVR. A ROC curve of this model generated an area under the curve (AUC) of 0.739. In a model including week 4 variables, baseline ALT and normal ALT levels at week 4 predicted SVR. The ROC AUC for this model was 0.627.
Among the subset of patients with elevated ALT levels at baseline, genotype (2/3), low baseline viral load (<800,000 U/L) and baseline platelet levels were associated with SVR. The ROC AUC for this model was 0.531. In a model considering week 4 variables, normalized ALT levels at week 4 resulted in an AUC of 0.734 (OR 17.04 [95% CI 6.17 to 47.09]).
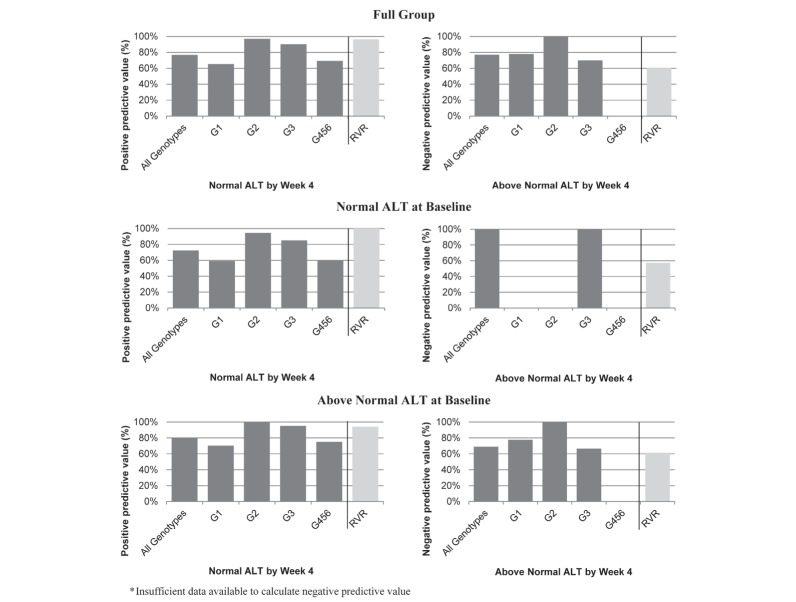
In an evaluation of the entire population, ALT levels at week 4 (above/below normal) yielded a 77% positive predictive value and a 77% negative predictive value for SVR (Figure 1). This suggests that normal ALT level by week 4, irrespective of baseline ALT level, correctly predicted SVR achievement in 77% of the study population and that above-normal ALT values at week 4 correctly predicted failure to achieve SVR in 77% of the study population.
Figure 1).
Positive predictive value and negative predictive value of alanine aminotransferase (ALT) level at week 4 in the full sample, and stratified according to baseline ALT levels, with virological response at week 4 (rapid virological response [RVR]) as a reference
Normalization of ALT levels by week 4 predicted SVR in interferon-based HCV antiviral treatment recipients. Although not as predictive as on-therapy viral kinetics, ALT is a simple, inexpensive, widely available and commonly performed test that may be underutilized in determining treatment efficacy. We are currently evaluating the utility of on-treatment liver enzyme response in interferon-free direct-acting antiviral treatment recipients.
Footnotes
This letter was peer-reviewed.
REFERENCES
- 1.Aoki YH, Ohkoshi S, Yamagiwa S, et al. Characterization of elevated alanine aminotransferase levels during pegylated-interferon alpha-2b plus ribavirin treatment for chronic hepatitis C. Hepatol Res. 2011;41:118–25. doi: 10.1111/j.1872-034X.2010.00749.x. [DOI] [PubMed] [Google Scholar]
- 2.Butt AA, Justice AC, Skanderson M, Rigsby MO, Good CB, Kwoh CK. Rate and predictors of treatment prescription for hepatitis C. Gut. 2007;56:385–9. doi: 10.1136/gut.2006.099150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basso M, Giannini EG, Torre F, Blanchi S, Savarino V, Picciotto A. Elevations in alanine aminotransferase levels late in the course of antiviral therapy in hepatitis C virus RNA-negative patients are associated with virological relapse. Hepatology. 2009;49:1442–8. doi: 10.1002/hep.22810. [DOI] [PubMed] [Google Scholar]
- 4.Hung CH, Lee CM, Lu SN, et al. Is delayed normalization of alanine aminotransferase a poor prognostic predictor in chronic hepatitis C patients treated with a combined interferon and ribavirin therapy? J Gastroenterol Hepatol. 2002;17:1307–11. doi: 10.1046/j.1440-1746.2002.02874.x. [DOI] [PubMed] [Google Scholar]
- 5.Thurairajah PH, Thorburn D, Hubscher S, et al. Incidence and characterization of serum transaminases elevations in pegylated interferon and ribavirin treated patients with chronic hepatitis C. Aliment Pharmacol Ther. 2007;25:1293–300. doi: 10.1111/j.1365-2036.2007.03322.x. [DOI] [PubMed] [Google Scholar]