Abstract
Recent studies suggest that latexin (Lxn) expression is involved in stem cell regulation and that it plays significant roles in tumor cell migration and invasion. The clinicopathological significance of Lxn expression and its possible correlation with CD133 expression in pancreatic ductal adenocarcinoma (PDAC) is currently unknown. In the present study, immunohistochemical analysis was performed to determine Lxn and CD133 expression in 43 PDAC patient samples and in 32 corresponding adjacent non-cancerous samples. The results were analyzed and compared with patient age, gender, tumor site and size, histological grade, clinical stage and overall mean survival time. Lxn expression was clearly decreased in the PDAC tissues compared with that in the adjacent non-cancerous tissues, while CD133 expression was increased. Low Lxn expression in the PDAC tissues was significantly correlated with tumor size (P=0.002), histological grade (P=0.000), metastasis (P=0.007) and clinical stage (P=0.018), but not with age (P=0.451), gender (P=0.395) or tumor site (P=0.697). Kaplan-Meier survival analysis revealed that low Lxn expression was significantly correlated with reduced overall survival time (P=0.000). Furthermore, Lxn expression was found to be inversely correlated with CD133 expression (r=−0.485, P=0.001). Furthermore, CD133-positive MIA PaCa-2 pancreatic tumor cells were sorted by magnetic-activated cell sorting (MACS), and those that overexpressed Lxn exhibited a significantly higher rate of apoptosis and lower proliferative activity. Our findings suggest that Lxn may function as a tumor suppressor that targets CD133-positive pancreatic cancer cells.
Keywords: pancreatic ductal adenocarcinoma, cancer stem cells, latexin, CD133, proliferation
Introduction
Pancreatic ductal adenocarcinoma (PDAC) has a high mortality rate due to its aggressive growth and high rates of metastasis (1) and chemoresistance (2). Increasing evidence supports the roles of cancer stem cells (CSCs) in the initiation, maintenance, proliferation, metastasis and resistance of malignant tumors to therapy (3). Numerous studies have demonstrated the existence of CSC fractions in PDAC (4), which have been detected by CSC-specific markers, including CD24 (5–7), CD44 (5,6,8,9), CD133 (10–13), CXCR4 (10,14), epithelial cell adhesion molecule (EpCAM; epithelial-specific antigen, ESA) (5), nestin (15) and combinations of these markers (5,10). There is an urgent need for the discovery of novel regulatory molecules of CSCs and for effective approaches that target this specific cancer cell population.
As the only known mammalian carboxypeptidase inhibitor, latexin (Lxn) shares 30% sequence similarity with tazarotene-induced gene 1 (TIG1), which is downregulated or absent in many types of tumors (16). Several studies have reported that Lxn has important roles in stem cell proliferation and tumor suppression. Liang et al revealed that Lxn functions in the negative regulation of the hematopoietic stem cell (HSC) population in mice by causing a decrease in cell replication, and an increase in apoptosis (17). Lxn-deficient HSCs have been shown to possess enhanced colony-forming ability (18). Muthusamy et al reported that the increased expression of Lxn in melanoma cell lines is correlated with a reduction in expression of the stem cell transcription factors, such as octamer-binding transcription factor 4 (OCT4), sex determining region Y-box 2 (SOX2), kruppel-like factor 4 (KLF4), NANOG and MYCN, indicating that Lxn may exert its tumor-suppressive function by altering the stem cell-like properties of melanoma cells (19). Our previous study also found that Lxn induced apoptosis and inhibited the proliferation of CD133+ MIA PaCa-2 pancreatic cancer stem-like cells (20). Lxn may thus be a negative regulator of CSCs.
CD133 is one of the most important cancer-initiating (stem) cell markers (21–23), and its expression has been confirmed in solid cancers, such as colon (24), brain (25,26) and pancreatic cancers (27–29), and cholangiocarcinoma (30). Studies of CD133 expression in PDAC have been conducted in Korea (27) and China (28,29), and the results indicate that CD133 expression tends to be associated with a higher incidence of metastasis. Moreover, CD133 is independently related to worse prognosis in PDAC patients (27). However, an investigation of the relationship between Lxn and CD133 expression in PDAC has not yet been conducted, and it would be interesting to determine whether an association exists. Therefore, we performed immunohistochemical analysis to investigate the possible roles of Lxn in the pathology and prognosis of 43 PDAC patients. We then analyzed the possible relationship between Lxn and CD133 expression. Furthermore, we studied the effect of Lxn gene overexpression on the proliferation and apoptosis of CD133-positive pancreatic cancer cells, which were sorted by magnetic-activated cell sorting (MACS). We hope that the present study provides information on the targeting of CSCs for development of an effective therapy for pancreatic cancer.
Materials and methods
Patients and tissue samples
Forty-three formalin-fixed paraffin-embedded pancreatic tumor samples and 32 corresponding adjacent non-cancerous samples were obtained from the Department of Pathology of the Second Affiliated Hospital of Wenzhou Medical University, China. None of the patients who provided samples received radiotherapy or chemotherapy prior to surgery. Each tissue specimen was histologically evaluated by at least two experienced pathologists. Tumor grading and staging were based on the American Joint Committee on Cancer/International Union Against Cancer staging manual (2009). The carcinoma patients included 18 men and 25 women who ranged in age from 43 to 78 years (mean age of 59.6 years). The primary clinicopathological features of the patients are shown in Table I. All PDAC tissues samples were collected using protocols approved by the Ethics Committee of the Second Affiliated Hospital of Wenzhou Medical University, and informed consent was obtained from every patient.
Table I.
Relationship between latexin or CD133 expression and clinicopathological features of the pancreatic cancer samples.
| Clinicopathological features | Parameter | CD133
|
P-value | Latexin
|
P-value | ||
|---|---|---|---|---|---|---|---|
| Low | High | Low | High | ||||
| Age (years) | ≥60 | 10 | 12 | 0.864 | 13 | 9 | 0.451 |
| <60 | 9 | 12 | 10 | 11 | |||
| Gender | Male | 8 | 10 | 0.977 | 11 | 7 | 0.395 |
| Female | 11 | 14 | 12 | 13 | |||
| Tumor site | Head | 12 | 13 | 0.553 | 14 | 11 | 0.697 |
| body, tail | 7 | 11 | 9 | 9 | |||
| Size | >3 cm | 7 | 17 | 0.026 | 18 | 6 | 0.002 |
| ≤3 cm | 12 | 7 | 5 | 14 | |||
| Histologic grade | High to moderate | 15 | 9 | 0.016 | 6 | 18 | 0.000 |
| Low | 4 | 15 | 17 | 2 | |||
| Metastasis | Yes | 5 | 14 | 0.036 | 15 | 4 | 0.007 |
| No | 14 | 10 | 8 | 16 | |||
| TNM stage | 1–2 | 16 | 8 | 0.003 | 9 | 15 | 0.018 |
| 3–4 | 3 | 16 | 14 | 5 | |||
| Survival | Mean (months) | 27.4 | 16.2 | 0.000 | 14.7 | 28.5 | 0.000 |
Immunohistochemical analysis
Tissue sections (4-μm thick) of the paraffin-embedded pancreatic cancer samples were cut and placed onto slides coated with polylysine. The Lxn and CD133 expression levels were examined in the pancreatic cancer samples following a standard immunohistochemistry protocol. Briefly, after deparaffinization and rehydration, the slides were immersed in boiling ethylenediamine tetraacetic acid (EDTA) buffer (pH 8.0) for antigen retrieval. Then, the sections were incubated in 3% hydrogen peroxide for 10 min at room temperature to quench endogenous peroxidase activity. This step was followed by incubation in 5% normal bovine serum albumin for 20 min to block non-specific conjugation. Subsequently, the slides were incubated with an anti-Lxn rabbit polyclonal antibody (ab103485, diluted 1:100; Abcam, London, UK) or with an anti-CD133 rabbit polyclonal antibody (orb18124, diluted 1:200; Biorbyt, San Francisco, CA, USA) at 4°C overnight. All of the sections were treated with a biotinylated secondary antibody for 2 h and were then incubated with a streptavidin-horseradish peroxidase (HRP) complex. After washes in phosphate-buffered saline (PbS), the sections were stained with 3,3′-diaminobenzidine (DAb). Finally, they were rinsed with distilled water, counterstained with Mayer's hematoxylin, dehydrated and mounted. The Lxn and CD133 expression levels in the pancreatic tumor samples were evaluated by two independent, experienced experts in clinical pathology. For immunohistochemical evaluation of Lxn and CD133 protein expression, both the extent and intensity of immunopositivity were considered, according to the method of Hao et al (31). The degree of intensity was scored as follows: 0, negative; 1, weak; 2, moderate; and 3, strong. The extent of positivity was scored as follows: 0, <5%; 1, >5–25%; 2, >25–50%; 3, >50–75%; and 4, >75% of the cells in the respective lesions. The final scores were determined by multiplying the scores for intensity by those for the extent of positivity, which yielded values ranging from 0 to 12. High expression was defined by a score of ≥4, and low expression was defined by a score of <4.
Cell sorting and culturing
The human pancreatic cancer cell line MIA PaCa-2 was purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA) and was maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FbS), 100 U/ml penicillin G and 10 μg/ml streptomycin (both from Gibco, Grand Island, NY, USA). CD133+ cells of the human pancreatic carcinoma cell line MIA PaCa-2 were isolated by MACS. A total of 5×107 MIA PaCa-2 cells were trypsinized, washed and resuspended in 300 μl PbS. After an addition of 100 μl FcR blocking reagent, the cells were incubated for 30 min at 4°C in 100 μl of a monoclonal CD133 antibody labeled with Microbeads (Miltenyi Biotec Ltd., Bergisch Gladbach, Germany: CD133/1; clone AC133, order no. 130-050-801). CD133+ cells were enriched with a MiniMACS magnet and MS columns (Miltenyi Biotech Ltd.) and were flushed out via application of a plunger that was supplied with the column. CD133+ cells that were isolated by MACS were seeded at a density of 1,000 cells/ml and cultured in serum-free medium (SFM) containing DMEM/F12 (Gibco), 20 ng/ml epidermal growth factor (EGF; PeproTech Asia, Rehovot, Israel), 1% B27 (Gibco), 0.4% FbS, 5 μg/ml bovine insulin (both from Sigma-Aldrich, St. Louis, MO, USA), and 100 U/ml penicillin-streptomycin at 37°C in a humidified atmosphere with 5% CO2.
Cell preparation and transfection
CD133+ MIA PaCa-2 cells in the logarithmic growth phase were collected and combined with 1 ml trypsin-EDTA. The cell suspension was then aspirated for 2 min, and 2 ml complete culture medium was added. After vigorous mixing, the cells were centrifuged at 1,200 × g, and the supernatant was discarded. Next, the cells were resuspended in 2 ml SFM. A volume of 200 μl of cell suspension (~1×105 cells) was seeded in a 10-cm culture dish with 8 ml SFM. Forty-eight hours later, ~5×105 cells were prepared for transfection.
Transfection was performed with Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. Briefly, 250 μl SFM was added to each 1.5 ml extreme pressure (EP) tube, followed by the addition of different doses of pCMV6-AC-GFP-LXN (1, 3 and 5 μg) [Lxn (NM_020169) Human cDNA ORF Clone; OriGene, Rockville, MD, USA] or pCMV6-AC-GFP empty vector. The resulting solution (A solution) was mixed for 5 min. Next, 250 μl SFM and 10 μl Lipofectamine 2000 were added to each 1.5 ml EP tube and mixed for 5 min (B solution). The B solution was added to the A solution and mixed for 20 min at room temperature. After the transfection complex formed, it was added to the prepared cells, which were cultured for 24 h.
qRT-PCR
Total RNA was isolated with TRIzol (Invitrogen) according to the manufacturer's instructions. Reverse transcription was performed with a RevertAid™ First Strand cDNA Synthesis kit (Fermentas, Burlington, ON, Canada) using 2 μg of total RNA in a final reaction volume of 20 μl. qRT-PCR was performed with a SYbR® Premix Ex Taq™ (Perfect Real-Time) PCR kit (Takara, Dalian, China) and a LightCycler 480 (Roche Biochemicals, Indianapolis, IN, USA). The primer sequences were as follows: Lxn sense, 5′-GAAGGTCAAACAAGCCAGCA-3′ and antisense, 5′-AACCCAGGCTAAATGTAGAACG-3′; β-actin sense, 5′-CGTGGACATCCGCAAAGAC-3′ and antisense, 5′-AAGAAAGGGTGTAACGCAACTAAG-3′. The qRT-PCR conditions were 30 sec at 95°C, followed by 45 cycles at 95°C for 10 sec and 60°C for 20 sec. Melting curve analysis was performed to verify the purity of the products and to exclude undesired primer dimers. All analyses were performed in triplicate in three independent experiments. The relative amount of target gene mRNA was normalized to that of the control (β-actin).
Western blot analysis
Harvested cells were washed with cold PbS and then lysed with radioimmunoprecipitation assay (RIPA) lysis buffer (Beyotime Bio, Haimen, China) containing 50 mM Tris (pH 7.4), 15 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS) and protease inhibitors. Cell lysates were centrifuged at 12,000 × g at 4°C for 20 min, and the total protein level in the supernatants was measured with a Beyotime BCA protein assay kit (Beyotime Bio) according to the manufacturer's protocol. Equal amounts (30 μg) of protein were electrophoresed on a 10% SDS-PAGE gel and transferred to a polyvinylidene fluoride (PVDF) membrane (Beyotime Bio), which was incubated overnight at 4°C with the following primary antibodies: anti-Lxn antibody (ab103485, diluted 1:500; Abcam) and anti-β-actin antibody (aa128, diluted 1:1,000; Beyotime Bio). Subsequently, the membrane was incubated at room temperature for 2 h with the following HRP-labeled secondary antibodies: HRP-labeled goat anti-mouse IgG (a0216, diluted 1:1,000) and HRP-labeled goat anti-rabbit IgG (aa208, diluted 1:1,000) (both from Beyotime Bio). Protein bands were detected using enhanced chemiluminescence detection reagents (Applygen Technologies, Beijing, China) and were documented with AlphaEaseFC 4.0 software (Alpha Innotech Co., San Leandro, CA, USA). All analyses were performed in triplicate in three independent experiments. The relative amount of target protein was normalized to that of the control (β-actin).
Detection of apoptosis
CD133+ MIA PaCa-2 cells were prepared and transfected (see the above paragraph entitled 'Cell preparation and transfection'). The experimental cells were divided into four groups according to the different doses of pCMV6-AC-GFP-LXN (0.1, 0.2 and 0.4 μg) or pCMV6-AC-GFP empty vector. Forty-eight hours after transfection, cells were collected from the treated and control groups and digested with EDTA trypsinase. The collected cells were washed twice with ice-cold PbS and were then precipitated by centrifugation at 500 × g for 10 min. Cell pellets were resuspended in 1X Annexin V binding buffer. Then, 5 μl Annexin V (20 μg/ml) and 5 μl propidium iodide (PI; 50 μg/ml) (both from Beyotime, Jiangsu, China) were added to a 100-μl aliquot of cell suspension, and the cells were incubated in the dark for 15 min at room temperature (25°C). Flow cytometry was performed with a FACSCalibur system (Becton-Dickinson, San Jose, CA, USA). Data from a total of 10,000 events were analyzed with CellQuest software (Becton-Dickinson Immunocytometry Systems, San Jose, CA, USA). Finally, the percentage of Annexin V-positive or PI-positive cells was calculated.
Cell proliferation assay
CD133+ MIA PaCa-2 cells were prepared and transfected (see the above paragraph entitled 'Cell preparation and transfection'). The experimental cells were divided into five groups according to the different doses of pCMV6-AC-GFP-LXN (0.1, 0.2, 0.3 and 0.4 μg) or pCMV6-AC-GFP empty vector. A Cell Counting Kit-8 (CCK-8; Dojindo, Kumamoto, Japan) was used to assay the antiproliferative activity of Lxn. Briefly, cells were plated at a density of 5,000 cells/well into 96-well plates containing SFM. Then, the transfection complex, which included different doses of pCMV6-AC-GFP-LXN (0.1, 0.2, 0.3 and 0.4 μg) or the control (pCMV6-AC-GFP empty vector), was added to the wells at a final volume of 100 μl/well. The cells were then incubated for 24, 48 and 72 h. Subsequently, 10 μl CCK-8 reagent was added to each well, and the cells were incubated for another 4 h. Optical density (OD) was measured at a wavelength of 450 nm using a microplate reader (ELx800; Bio-Tek, Shoreline, WA, USA). All assays were performed in triplicate in three independent experiments.
Statistical analysis
Statistical analysis was performed with IbM SPSS statistics 22.0 software program (IbM Corp., New York, NY, USA). Differences between the treated groups were analyzed by one-way analysis of variance (ANOVA). Fisher's exact, Pearson's Chi-square tests or Spearman's correlation coefficient test (for trends in proportions), and the Kaplan-Meier method with the log-rank test or Cox regression method (for univariate or multivariate overall survival analysis) were used to assess the associations between Lxn or CD133 expression and pathological indices. A P<0.05 was considered to indicate a statistically significant result.
Results
Lxn and CD133 expression in pancreatic carcinoma and adjacent non-cancerous pancreatic tissues
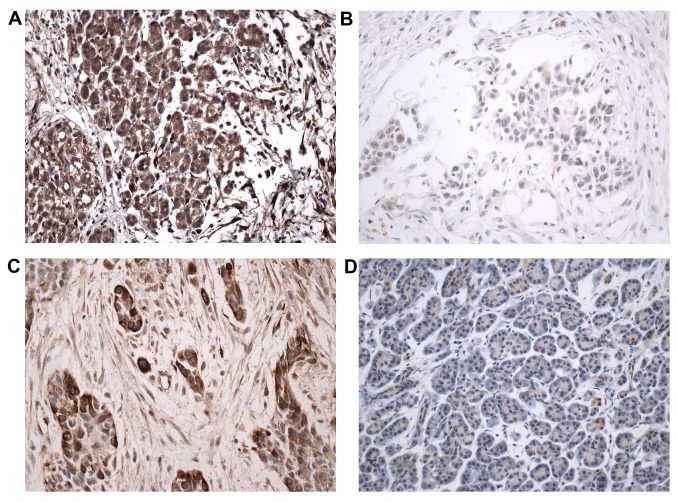
The Lxn protein was highly expressed or was diffusely positive in the cytoplasm and membrane of the pancreatic cells in 29 (29/32, 90.6%) adjacent non-cancerous pancreatic tissues (Fig. 1A). Lxn showed diffuse expression in the cytoplasm and membrane of the cancer cells in the carcinoma samples (20, 46.5%). Its expression was low or negative in 23 (53.5%) tumor samples. Most of the poorly differentiated cancer cells were negative for the Lxn protein (Fig. 1B). A significant difference in Lxn expression was observed between the pancreatic carcinoma and adjacent non-cancerous pancreatic tissues (P=0.000).
Figure 1.
Lxn and CD133 protein expression in pancreatic ductal adenocarcinoma (PDAC) and the adjacent non-cancerous tissues. Lxn protein was highly expressed or was diffusely positive in the cytoplasm and membrane of pancreatic cells from the adjacent non-cancerous pancreatic tissues (A). Low Lxn protein expression was observed in the PDAC tissues (B). CD133 protein was highly expressed or was diffusely positive in the cytoplasm and membrane of the PDAC cells (C). CD133 protein expression was negative in the adjacent non-cancerous pancreatic tissues (D) (Lxn, CD33; magnification, ×400).
High CD133 expression was observed in 24 (55.8%) carcinoma samples, including diffuse and strong expression in the membrane and cytoplasm of the cancer cells. Most cancer cells in the pancreatic carcinoma tissues demonstrated diffuse and strong positive staining for the CD133 protein in the membrane and cytoplasm (Fig. 1C). CD133 protein expression was low or negative in all 32 adjacent non-cancerous tissues (Fig. 1D). A significant difference in CD133 expression was observed between the pancreatic carcinoma and adjacent non-cancerous pancreatic tissues (P=0.000).
Relationship between Lxn expression and age, gender, tumor site and size, histological grade, clinical stage and prognosis
Lxn expression was correlated with tumor size, histological grade, metastasis, TNM stage and survival but not with age, gender or tumor site in the 43 pancreatic tumor samples (Table I). The overall mean survival time between the individuals with tumors with low Lxn expression (14.7 months) and those with tumors with high Lxn expression (28.5 months) was significantly different (P=0.000) (Fig. 2). These results revealed that low Lxn expression was significantly related to a shorter mean survival time (P=0.000) of the cancer patients. According to multivariate analysis performed using Cox regression, Lxn expression was an independent prognostic factor for PDAC (P=0.000).
Figure 2.
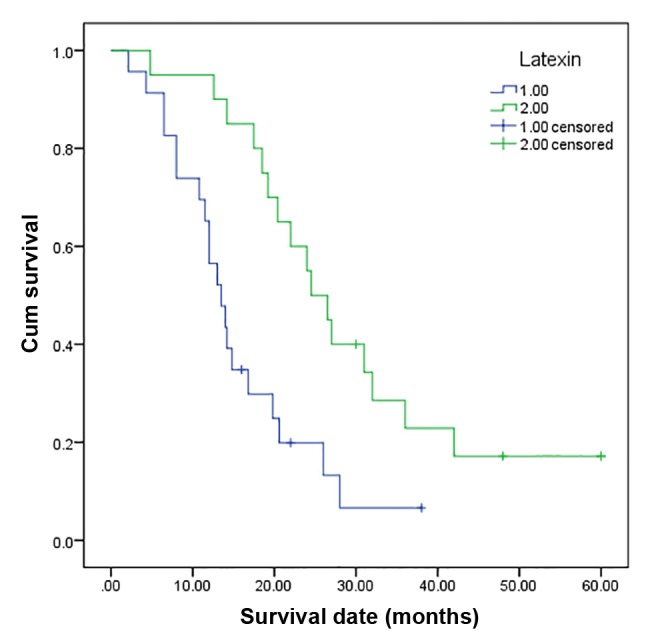
Kaplan-Meier survival analysis according to latexin status (n=43). The y-axis indicates the percentage of patients, and the x-axis depicts their survival in months. The green line represents the patients with high latexin expression, who showed a trend of better survival than the patients with low latexin expression, indicated by the blue line (P=0.000). The mean overall survival (OS) time was 28.5 months for the high latexin expression group and 14.7 months for the low latexin expression group.
Relationship between CD133 expression and age, gender, tumor site, tumor size, histological grade, clinical stage and prognosis
CD133 expression was correlated with histological grade, metastasis, TNM stage and survival but not with age, gender or tumor site in the group of 43 pancreatic tumor samples (Table I). The follow-up data revealed a significant difference in the overall mean survival time between the patients with a tumor with CD133 high expression (16.2 months) and those with a tumor with low CD133 expression (27.4 months) (P=0.000) (Fig. 3). These findings revealed that high CD133 expression was significantly related to a shorter mean survival time of the cancer patients. Multivariate analysis conducted using Cox regression revealed that CD133 expression was also an independent prognostic factor for PDAC (P=0.000).
Figure 3.
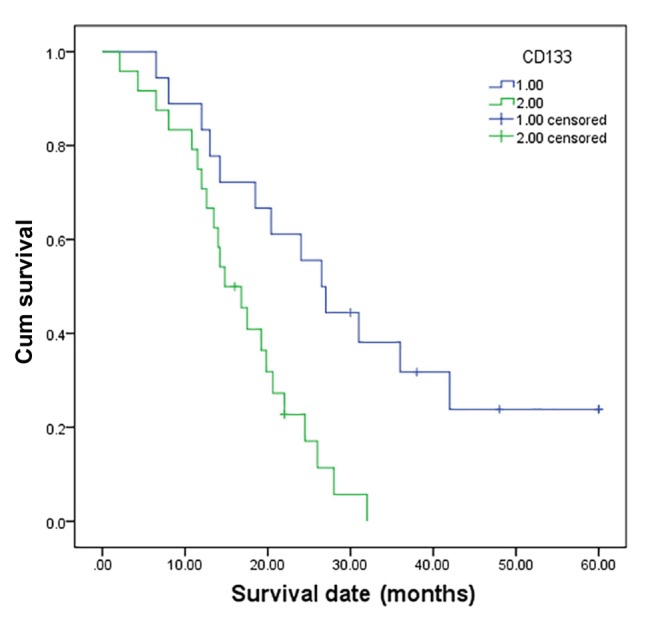
Kaplan-Meier survival analysis according to CD133 status (n=43). The y-axis indicates the percentage of patients, and the x-axis depicts their survival in months. The green line represents the patients with high CD133 expression, who showed a trend of worse survival than those with low CD133 expression, indicated by the blue line. The mean overall survival (OS) time was 16.2 months for the high CD133 expression group and 27.4 months for the low CD133 expression group (P=0.000).
Relationship between Lxn and CD133 expression
When Lxn was not expressed or was expressed at a low level (Fig. 4A), CD133 was often found to be highly expressed in cancer cells from the pancreatic tumors (Fig. 4B). Lxn expression was detected in only 25% (6/24) of the pancreatic tumors with high CD133 expression, whereas it was highly expressed in up to 73.7% (14/19) of the tumors with low CD133 expression. A significant inverse relationship was found between the expression of Lxn and CD133 (r=−0.485, P=0.001; Fig. 5).
Figure 4.
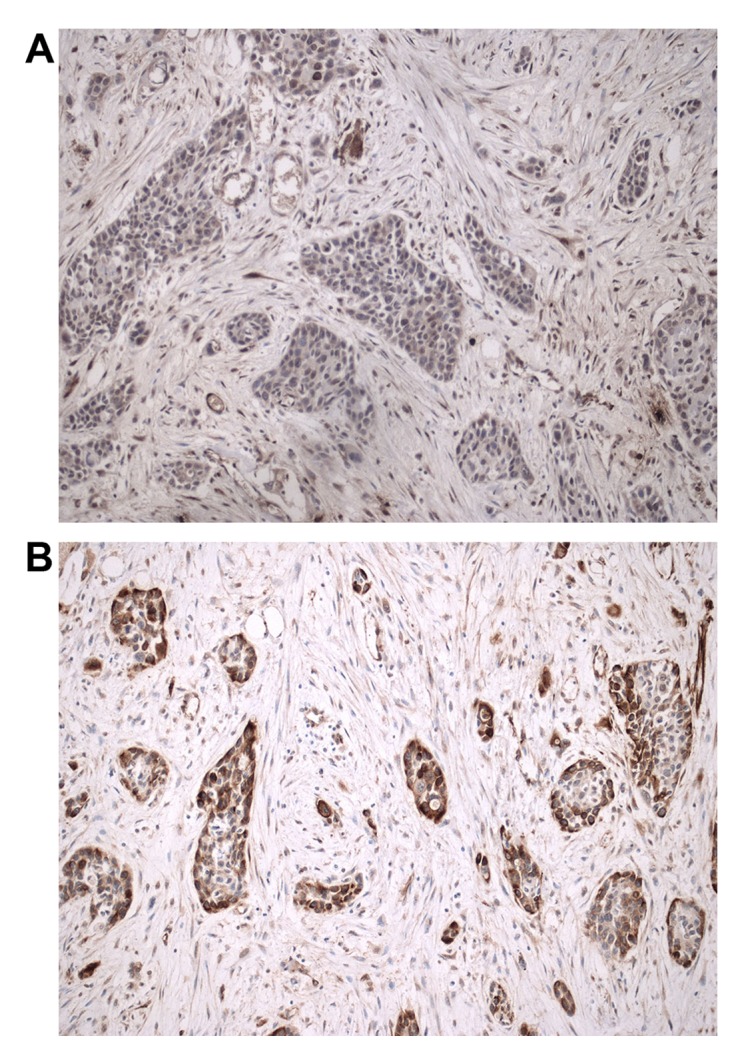
Lxn and CD133 protein expression in pancreatic ductal adenocarcinoma (PDAC). Lxn was not expressed (A) but CD133 was highly expressed (B) in the same cancerous, poorly differentiated PDAC tissues (Lxn, CD33; magnification, ×200).
Figure 5.
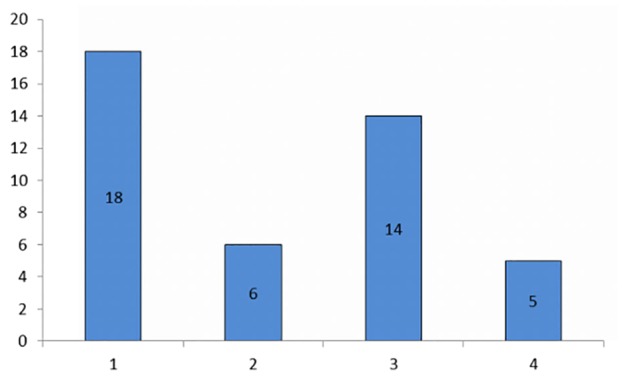
Inverse correlation between Lxn and CD133 expression. From left to right, expression of latexin compared with that of CD133 (n=43), classified as low to high (1, n=18), high to high (2, n=6), high to low (3, n=14) and low to low (4, n=5) (r=−0.485, P=0.001).
Effect of pCMV6-AC-GFP-LXN transfection on Lxn gene expression in the CD133+ MIA PaCa-2 pancreatic cancer cells
As shown in Fig. 6, the western blotting and qRT-PCR results indicated that Lxn expression in transfected CD133+ MIA PaCa-2 pancreatic cancer cells was significantly higher than that in the control cells and that its expression increased with the pCMV6-AC-GFP-LXN dose in a concentration-dependent manner (P<0.05). The Lxn gene was successfully transfected into the CD133+ MIA PaCa-2 pancreatic cancer cells.
Figure 6.

Lxn expression in CD133-positive MIA PaCa-2 pancreatic tumor cells. Lxn protein expression was increased in the CD133+ cells transfected with pCMV6-AC-GFP-LXN compared with that in the control cells (A and B). Lxn mRNA expression was significantly higher in the CD133+ cells transfected with pCMV6-AC-GFP-LXN compared with that in the control cells (C). *P<0.05.
Effects of pCMV6-AC-GFP-LXN transfection on apoptosis and cell growth in the CD133+ MIA PaCa-2 pancreatic cancer cells
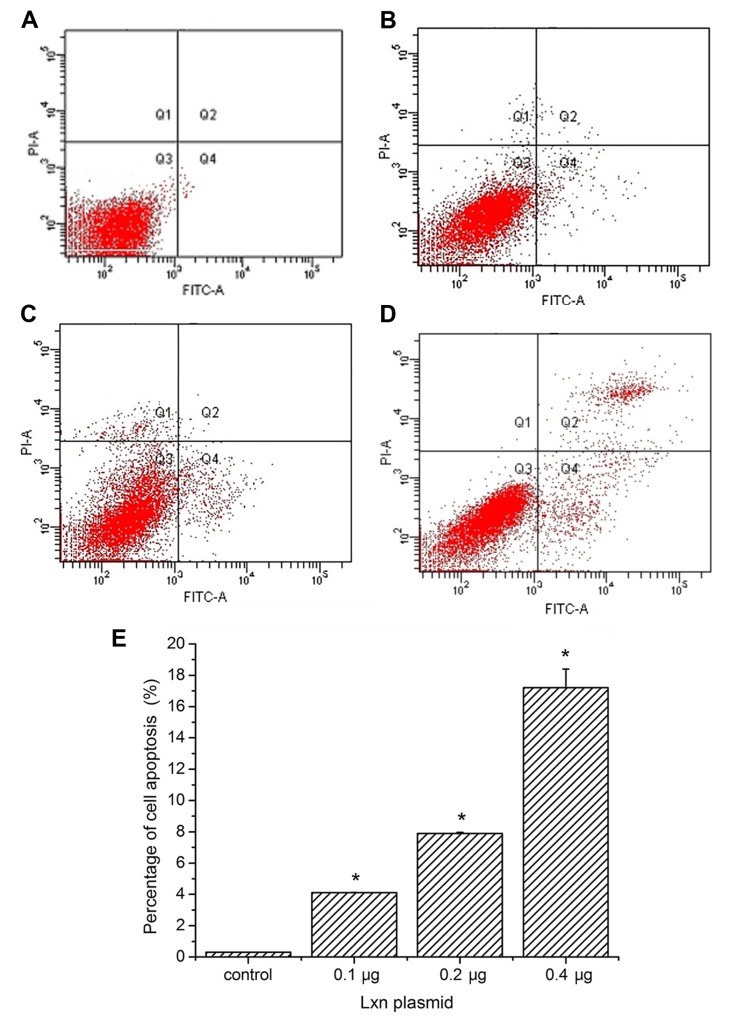
We determined the effects of pCMV6-AC-GFP-LXN transfection on the rate of apoptosis of the CD133+ MIA PaCa-2 pancreatic cancer cells relative to that of the control cells. As shown in Fig. 7B–D, transfection of the cells with pCMV6-AC-GFP-LXN for 48 h increased the percentage of early and late apoptotic cells as well as necrotic cells in a dose-dependent manner.
Figure 7.
Analysis of CD133+ cell death after transfection with the latexin gene. (A–D) Apoptosis of CD133+ MIA PaCa-2 cells treated with 0 μg (control) (A), 0.1 μg (B), 0.2 μg (C) and 0.4 μg (D) Lxn plasmid. The lower left quadrant shows live cells (Annexin V-FITC-negative/PI-negative); the lower right quadrant depicts early apoptotic cells (Annexin V-FITC-positive/PI-negative). The upper left quadrant shows damaged cells (Annexin V-FITC-negative/PI-positive), while the upper right quadrant depicts necrotic and late apoptotic cells (Annexin V-FITC-positive/PI-positive). The numbers represent the percentages of early apoptotic, necrotic and late apoptotic cells under each condition (right quadrant). (E) The percentages of apoptotic, necrotic and late apoptotic CD133+ MIA PaCa-2 cells treated with 0.1, 0.2 and 0.4 μg Lxn plasmid and control. Compared with the control, *P<0.05. FITC, fluorescein isothiocyanate; PI, propidium iodide.
We also determined the cell death-promoting effects of pCMV6-AC-GFP-LXN transfection on the CD133+ cells. As shown in Fig. 8, with increasing doses of transfected pCMV6-AC-GFP-LXN, the inhibitory effects on CD133+ cells was also increased. Treatment with 0.1 and 0.4 μg pCMV6-AC-GFP-LXN for 48 h resulted in cell growth inhibition rates of 4.7 and 26.4%, respectively. Similarly, the inhibitory effects on CD133+ cells increased as a function of the incubation time. For example, treatment with 0.4 μg pCMV6-AC-GFP-LXN for 24 and 72 h resulted in cell inhibition rates of 15 and 36.2%, respectively. Therefore, Lxn suppressed the proliferation of CD133+ cells in a time- and concentration-dependent manner (Table II).
Figure 8.
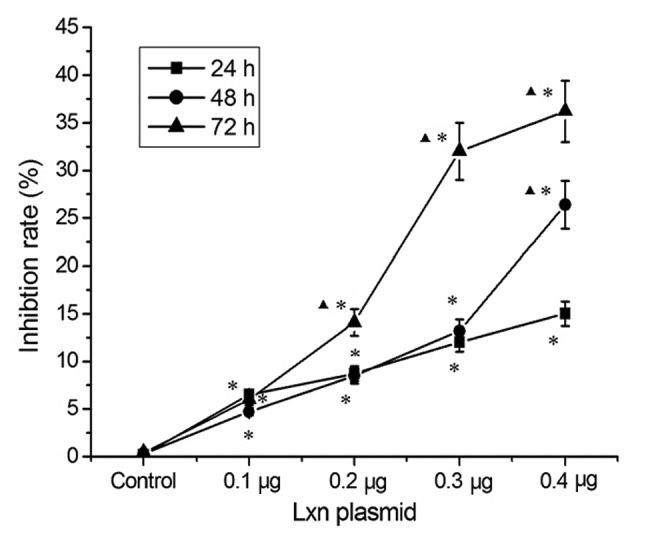
Cytotoxic effects of CD133+ MIA PaCa-2 cells trasfected with the Lxn gene at various doses of Lxn plasmid. The inhibitory ratio was calculated according to the following formula: Inhibitory ratio = (OD450 nm value of control group - OD450 nm value of Lxn treatment group)/(OD450 nm value of control group - OD450 nm value of blank group). Compared with the control, *P<0.05; compared with the 24 h group, ▲P<0.05.
Table II.
Effect of Lxn gene overexpression on the proliferation of CD133-positive MIA PaCa-2 cells (OD450, mean ± SD).
| Lxn μmol/l | OD at 24 h | Inhibition ratio (%) | OD at 48 h | Inhibition ratio (%) | OD at 72 h | Inhibition ratio (%) |
|---|---|---|---|---|---|---|
| Con | 0.92±0.013 | 0.2±0.00 | 0.46±0.012 | 0.3±0.01 | 0.30±0.011 | 0.4±0.01 |
| 0.1 | 0.86±0.019 | 6.5±0.14a | 0.40±0.019 | 4.7±0.22a | 0.27±0.011 | 6±0.22a |
| 0.2 | 0.81±0.142 | 8.7±1.53a | 0.38±0.0208 | 8.5±0.47a | 0.25±0.012 | 14.1±0.68a,b |
| 0.3 | 0.76±0.0318 | 12±0.5a | 0.33±0.022 | 13.2±0.88a | 0.19±0.002 | 32±0.34a,b |
| 0.4 | 0.69±0.0218 | 15±0.47a | 0.27±0.022 | 26.4±2.2a,b | 0.17±0.008 | 36.2±1.7a,b |
Compared with the control,
P<0.05; compared with the 24 h group,
P<0.05. Lxn, latexin; OD optical density.
Discussion
Lxn has primarily been studied in the nervous system since it is involved in the specification of cortical brain regions during development (32), as well as in the speed of nerve transmission in the adult peripheral nervous system (33). Lxn has ~30% sequence homology, but has much greater structural homology, with TIG1 or retinoic acid receptor responder 1 (RARRES1), the expression of which is downregulated in a variety of tumor types in humans (16,34–37). Lxn and TIG1 are closely linked genetically and may represent members of a family of functionally related genes. Liang et al identified the Lxn locus as the primary determinant of HSC frequency variation between two inbred mouse strains. Exogenous Lxn expression in the hematopoietic compartment has been demonstrated to negatively regulate the number of HSCs. Muthusamy et al also demonstrated that Lxn was downregulated in >50% of melanomas by promoter hypermethylation. Furthermore, they showed that Lxn has a tumor-suppressive role and that it negatively regulates the expression of tumor-sustaining stem cell factors (19). Recently, Lxn has been demonstrated to be a tumor-suppressor gene in gastric cancer (38), hepatocellular carcinoma (39), melanoma (19), leukemia (40), prostate (41) and breast cancer (42). Our previous study revealed that Lxn induced the apoptosis and inhibited the proliferation of CD133+ MIA PaCa-2 pancreatic cancer cells (20). However, to the best of our knowledge, little is known concerning the role of Lxn in PDAC. To investigate the relationship between Lxn and the clinicopathological indices in PDAC patients, we analyzed the relationship between Lxn and CD133 expression in PDAC tissues by immunohistochemistry. For the PDAC patients with follow-up data, our results indicated that low Lxn expression was correlated with tumor size (P=0.002), histological grade (P=0.000), metastasis (P=0.007) and clinical stage (P=0.018). Furthermore, our data showed that low Lxn protein expression was highly associated with overall survival (P=0.000), suggesting that its expression may be a potential prognostic factor for PDAC. Thus, Lxn may have a regulatory role in PDAC as a tumor suppressor.
Currently, CD133 is one of the most frequently studied markers in pancreatic tumor stem cell research (43–45). However, some inconsistent results have been obtained with regard to the relationship between CD133 expression and the clinicopathological features of PDAC (23,27,43–45). Nomura et al reported that CD133 expression contributes to 'stemness,' tumorigenicity, epithelial-mesenchymal transition (EMT) induction, invasion and metastasis in pancreatic cancer (23). Chen et al reported that co-expression of CD133, CD44v6 and human tissue factor is associated with metastasis and poor prognosis in pancreatic carcinoma patients (28). A study conducted in Korea revealed that upregulation of CD133 expression in PDAC is related to poor prognosis (27), while Immervoll et al reported no correlation between CD133 expression and patient survival (43). One study showed that CD133 expression is positively associated with venous invasion but not with tumor grade (44), while another study demonstrated that CD133 expression is significantly inversely correlated with tumor grade, but not with disease stage (45). The present study investigated CD133 protein expression in 43 PDAC patients with follow-up data, and the results indicated that its expression was correlated with histological grade (P=0.016) and clinical stage (P=0.003). Furthermore, our data showed that CD133 protein expression was highly associated with overall survival (P=0.000), suggesting that its expression may be a potential prognostic factor for PDAC. We found that 55.8% of the PDAC samples were CD133-positive. Our results are consistent with those of a previous study of PDAC conducted in Korea (27) and with other studies performed by two research groups in Chongqing (28) and Taiwan (29), China, reporting rates of CD133 positivity of 66.7, 59.6 and 43.7%, respectively; in addition, these studies considered CD133 expression to be a potential prognostic indicator.
Currently, our knowledge of the relationship between Lxn and CD133 is still lacking. We reported that Lxn expression in CD133+ MIA PaCa-2 pancreatic tumor cells was significantly lower than that in CD133− MIA PaCa-2 cells. Moreover, exogenous Lxn inhibits the proliferation of CD133+ MIA PaCa-2 pancreatic cancer stem-like cells (20). Our findings suggest that expression of Lxn is inversely correlated with that of CD133 (P=0.001), implying that the loss of Lxn function may occur in cancer cells with high CD133 expression. Furthermore, we found that CD133-positive MIA PaCa-2 cells overexpressing Lxn exhibited significantly increased apoptosis and low proliferative activity, indicating that Lxn may act as a tumor suppressor that targets CD133-positive pancreatic cancer cells.
Acknowledgments
The present study received research funding from the Zhejiang Provincial Natural Science Foundation of China (Y2100546), the Wenzhou Municipal Science and Technology Bureau of Zhejiang Province, China (Y20090086). The statistical methods used in the present study were reviewed by Zuo-Kai Xie from the General Statistics Office of The Second Affiliated Hospital and Yuying Children's Hospital of Wenzhou Medical University.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Sultana A, Smith CT, Cunningham D, Starling N, Neoptolemos JP, Ghaneh P. Meta-analyses of chemotherapy for locally advanced and metastatic pancreatic cancer. J Clin Oncol. 2007;25:2607–2615. doi: 10.1200/JCO.2006.09.2551. [DOI] [PubMed] [Google Scholar]
- 3.Tan BT, Park CY, Ailles LE, Weissman IL. The cancer stem cell hypothesis: A work in progress. Lab Invest. 2006;86:1203–1207. doi: 10.1038/labinvest.3700488. [DOI] [PubMed] [Google Scholar]
- 4.Hruban RH, Maitra A, Goggins M. Update on pancreatic intraepithelial neoplasia. Int J Clin Exp Pathol. 2008;1:306–316. [PMC free article] [PubMed] [Google Scholar]
- 5.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 6.Huang P, Wang CY, Gou SM, Wu HS, Liu T, Xiong JX. Isolation and biological analysis of tumor stem cells from pancreatic adenocarcinoma. World J Gastroenterol. 2008;14:3903–3907. doi: 10.3748/wjg.14.3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ikenaga N, Ohuchida K, Mizumoto K, Yu J, Kayashima T, Hayashi A, Nakata K, Tanaka M. Characterization of CD24 expression in intraductal papillary mucinous neoplasms and ductal carcinoma of the pancreas. Hum Pathol. 2010;41:1466–1474. doi: 10.1016/j.humpath.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Hong SP, Wen J, Bang S, Park S, Song SY. CD44-positive cells are responsible for gemcitabine resistance in pancreatic cancer cells. Int J Cancer. 2009;125:2323–2331. doi: 10.1002/ijc.24573. [DOI] [PubMed] [Google Scholar]
- 9.Li C, Wu JJ, Hynes M, Dosch J, Sarkar B, Welling TH, Pasca di Magliano M, Simeone DM. c-Met is a marker of pancreatic cancer stem cells and therapeutic target. Gastroenterology. 2011;141:2218–2227.e5. doi: 10.1053/j.gastro.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Olempska M, Eisenach PA, Ammerpohl O, Ungefroren H, Fandrich F, Kalthoff H. Detection of tumor stem cell markers in pancreatic carcinoma cell lines. Hepatobiliary Pancreat Dis Int. 2007;6:92–97. [PubMed] [Google Scholar]
- 12.Kim MP, Fleming JB, Wang H, Abbruzzese JL, Choi W, Kopetz S, McConkey DJ, Evans DB, Gallick GE. ALDH activity selectively defines an enhanced tumor-initiating cell population relative to CD133 expression in human pancreatic adenocarcinoma. PLoS One. 2011;6:e20636. doi: 10.1371/journal.pone.0020636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maeda S, Shinchi H, Kurahara H, Mataki Y, Maemura K, Sato M, Natsugoe S, Aikou T, Takao S. CD133 expression is correlated with lymph node metastasis and vascular endothelial growth factor-C expression in pancreatic cancer. Br J Cancer. 2008;98:1389–1397. doi: 10.1038/sj.bjc.6604307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maréchal R, Demetter P, Nagy N, Berton A, Decaestecker C, Polus M, Closset J, Devière J, Salmon I, Van Laethem JL. High expression of CXCR4 may predict poor survival in resected pancreatic adenocarcinoma. Br J Cancer. 2009;100:1444–1451. doi: 10.1038/sj.bjc.6605020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawamoto M, Ishiwata T, Cho K, Uchida E, Korc M, Naito Z, Tajiri T. Nestin expression correlates with nerve and retro-peritoneal tissue invasion in pancreatic cancer. Hum Pathol. 2009;40:189–198. doi: 10.1016/j.humpath.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Youssef EM, Chen XQ, Higuchi E, Kondo Y, Garcia-Manero G, Lotan R, Issa JP. Hypermethylation and silencing of the putative tumor suppressor Tazarotene-induced gene 1 in human cancers. Cancer Res. 2004;64:2411–2417. doi: 10.1158/0008-5472.can-03-0164. [DOI] [PubMed] [Google Scholar]
- 17.Liang Y, Jansen M, Aronow B, Geiger H, Van Zant G. The quantitative trait gene latexin influences the size of the hematopoietic stem cell population in mice. Nat Genet. 2007;39:178–188. doi: 10.1038/ng1938. [DOI] [PubMed] [Google Scholar]
- 18.Mitsunaga K, Kikuchi J, Wada T, Furukawa Y. Latexin regulates the abundance of multiple cellular proteins in hematopoietic stem cells. J Cell Physiol. 2012;227:1138–1147. doi: 10.1002/jcp.22834. [DOI] [PubMed] [Google Scholar]
- 19.Muthusamy V, Premi S, Soper C, Platt J, Bosenberg M. The hematopoietic stem cell regulatory gene latexin has tumor-suppressive properties in malignant melanoma. J Invest Dermatol. 2013;133:1827–1833. doi: 10.1038/jid.2013.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xue ZX, Zheng JH, Zheng ZQ, Cai JL, Ye XH, Wang C, Sun WJ, Zhou X, Lu MD, Li PH, et al. Latexin inhibits the proliferation of CD133+ miapaca-2 pancreatic cancer stem-like cells. World J Surg Oncol. 2014;12:404. doi: 10.1186/1477-7819-12-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shmelkov SV, St Clair R, Lyden D, Rafii S. AC133/CD133/Prominin-1. Int J Biochem Cell Biol. 2005;37:715–719. doi: 10.1016/j.biocel.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 22.Banerjee S, Nomura A, Sangwan V, Chugh R, Dudeja V, Vickers SM, Saluja A. CD133+ tumor initiating cells in a syngenic murine model of pancreatic cancer respond to Minnelide. Clin Cancer Res. 2014;20:2388–2399. doi: 10.1158/1078-0432.CCR-13-2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nomura A, Banerjee S, Chugh R, Dudeja V, Yamamoto M, Vickers SM, Saluja AK. CD133 initiates tumors, induces epithelial-mesenchymal transition and increases metastasis in pancreatic cancer. Oncotarget. 2015;6:8313–8322. doi: 10.18632/oncotarget.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 25.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 26.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 27.Kim HS, Yoo SY, Kim KT, Park JT, Kim HJ, Kim JC. Expression of the stem cell markers CD133 and nestin in pancreatic ductal adenocarcinoma and clinical relevance. Int J Clin Exp Pathol. 2012;5:754–761. [PMC free article] [PubMed] [Google Scholar]
- 28.Chen K, Li Z, Jiang P, Zhang X, Zhang Y, Jiang Y, He Y, Li X. Co-expression of CD133, CD44v6 and human tissue factor is associated with metastasis and poor prognosis in pancreatic carcinoma. Oncol Rep. 2014;32:755–763. doi: 10.3892/or.2014.3245. [DOI] [PubMed] [Google Scholar]
- 29.Hou YC, Chao YJ, Tung HL, Wang HC, Shan YS. Coexpression of CD44-positive/CD133-positive cancer stem cells and CD204-positive tumor-associated macrophages is a predictor of survival in pancreatic ductal adenocarcinoma. Cancer. 2014;120:2766–2777. doi: 10.1002/cncr.28774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chu X, Zhao P, Lv Y, Liu L. Decreased expression of TFPI-2 correlated with increased expression of CD133 in cholangiocarcinoma. Int J Clin Exp Pathol. 2015;8:328–336. [PMC free article] [PubMed] [Google Scholar]
- 31.Hao XP, Willis JE, Pretlow TG, Rao JS, MacLennan GT, Talbot IC, Pretlow TP. Loss of fragile histidine triad expression in colorectal carcinomas and premalignant lesions. Cancer Res. 2000;60:18–21. [PubMed] [Google Scholar]
- 32.Arimatsu Y. Latexin: A molecular marker for regional specification in the neocortex. Neurosci Res. 1994;20:131–135. doi: 10.1016/0168-0102(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 33.Jin M, Ishida M, Katoh-Fukui Y, Tsuchiya R, Higashinakagawa T, Ikegami S, Arimatsu Y. Reduced pain sensitivity in mice lacking latexin, an inhibitor of metallocarboxypeptidases. Brain Res. 2006;1075:117–121. doi: 10.1016/j.brainres.2005.12.099. [DOI] [PubMed] [Google Scholar]
- 34.Aagaard A, Listwan P, Cowieson N, Huber T, Ravasi T, Wells CA, Flanagan JU, Kellie S, Hume DA, Kobe B, et al. An inflammatory role for the mammalian carboxypeptidase inhibitor latexin: Relationship to cystatins and the tumor suppressor TIG1. Structure. 2005;13:309–317. doi: 10.1016/j.str.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 35.Jing C, El-Ghany MA, Beesley C, Foster CS, Rudland PS, Smith P, Ke Y. Tazarotene-induced gene 1 (TIG1) expression in prostate carcinomas and its relationship to tumorigenicity. J Natl Cancer Inst. 2002;94:482–490. doi: 10.1093/jnci/94.7.482. [DOI] [PubMed] [Google Scholar]
- 36.Kwong J, Lo KW, Chow LS, Chan FL, To KF, Huang DP. Silencing of the retinoid response gene TIG1 by promoter hyper-methylation in nasopharyngeal carcinoma. Int J Cancer. 2005;113:386–392. doi: 10.1002/ijc.20593. [DOI] [PubMed] [Google Scholar]
- 37.Zhang J, Liu L, Pfeifer GP. Methylation of the retinoid response gene TIG1 in prostate cancer correlates with methylation of the retinoic acid receptor beta gene. Oncogene. 2004;23:2241–2249. doi: 10.1038/sj.onc.1207328. [DOI] [PubMed] [Google Scholar]
- 38.Li Y, Basang Z, Ding H, Lu Z, Ning T, Wei H, Cai H, Ke Y. Latexin expression is downregulated in human gastric carcinomas and exhibits tumor suppressor potential. BMC Cancer. 2011;11:121. doi: 10.1186/1471-2407-11-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ni QF, Tian Y, Kong LL, Lu YT, Ding WZ, Kong LB. Latexin exhibits tumor suppressor potential in hepatocellular carcinoma. Oncol Rep. 2014;31:1364–1372. doi: 10.3892/or.2014.2966. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y, Howard D, Rector K, Swiderski C, Brandon J, Schook L, Mehta J, Bryson JS, Bondada S, Liang Y. Latexin is downregulated in hematopoietic malignancies and restoration of expression inhibits lymphoma growth. PLoS One. 2012;7:e44979. doi: 10.1371/journal.pone.0044979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oldridge EE, Walker HF, Stower MJ, Simms MS, Mann VM, Collins AT, Pellacani D, Maitland NJ. Retinoic acid represses invasion and stem cell phenotype by induction of the metastasis suppressors RARRES1 and LXN. Oncogenesis. 2013;2:e45. doi: 10.1038/oncsis.2013.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang H, Ren Y, Pang D, Liu C. Clinical implications of AGbL2 expression and its inhibitor latexin in breast cancer. World J Surg Oncol. 2014;12:142. doi: 10.1186/1477-7819-12-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Immervoll H, Hoem D, Sakariassen PØ, Steffensen OJ, Molven A. Expression of the 'stem cell marker' CD133 in pancreas and pancreatic ductal adenocarcinomas. BMC Cancer. 2008;8:48. doi: 10.1186/1471-2407-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kure S, Matsuda Y, Hagio M, Ueda J, Naito Z, Ishiwata T. Expression of cancer stem cell markers in pancreatic intraepithelial neoplasias and pancreatic ductal adenocarcinomas. Int J Oncol. 2012;41:1314–1324. doi: 10.3892/ijo.2012.1565. [DOI] [PubMed] [Google Scholar]
- 45.Vizio B, Mauri FA, Prati A, Trivedi P, Giacobino A, Novarino A, Satolli MA, Ciuffreda L, Camandona M, Gasparri G, et al. Comparative evaluation of cancer stem cell markers in normal pancreas and pancreatic ductal adenocarcinoma. Oncol Rep. 2012;27:69–76. doi: 10.3892/or.2011.1461. [DOI] [PubMed] [Google Scholar]