Abstract
Background
Ebstein-Barr virus (EBV) plays a critical role in nasopharynx cancer, which can be effectively monitored by serum levels of early antigen antibody (EA-IgA) and viral capsid antigen antibody (VCA-IgA). This study explored the diagnostic value of combined assays of sialic acid (SA), EA-IgA, and VCA-IgA via the expressional assay.
Material/Methods
A total of 42 nasopharynx cancer patients and 42 benign rhinitis and healthy controls were recruited in this study. Serum EA-IgA and VCA-IgA were tested by enzyme-linked immunosorbent assay (ELISA) and enzymatic assay of serum SA. Specificity and sensitivity of those 3 assays were compared. The diagnostic value of each parameter was evaluated by ROC curves.
Results
All 3 indexes (SA, EA-IgA and VCA-IgA) showed elevated serum levels in nasopharynx cancer patients when compared to those with rhinitis, who had higher levels than healthy individuals. Concentrations of these factors were also positively correlated with the TNM staging of cancer. The sensitivity and specificity were 30.95% and 83.33% (in SA), 57.14% and 95.24% (in EA-IgA), and 76.19% and 92.86% (in VCA-IgA), respectively. VCA-IgA had the highest sensitivity among all 3 indexes. The combined assay increased the diagnostic sensitivity to 92.86% without compromising specificity.
Conclusions
SA, EA-IgA, and VCA-IgA levels were significantly elevated in nasopharynx patients’ serum. The combined assay may have clinical value in diagnosis and monitoring.
MeSH Keywords: N-Acylneuraminate Cytidylyltransferase, Nasopharyngeal Neoplasms, Sleep Initiation and Maintenance Disorders
Background
Nasopharyngeal cancer is the most common malignant tumor of the ear, nose, and throat in Chinese [1,2]. The tumor commonly develops in the top or lateral wall of the nasopharynx cavity, making it hard to identify in routine physical examination. Such characteristics, plus the insidious onset with few significant clinical symptoms, impede the diagnosis of pharynx cancer until it has reached the late stage, causing unsatisfactory treatment efficacy and higher mortality rates [3,4]. Therefore, early diagnosis is of critical importance for improving patient survival rates. It is now widely accepted that Ebstein-Barr virus (EBV) is a cacogenic herpesvirus and is a main viral factor for nasopharynx cancer. The major EBV antigens after viral activation include intracellular early antigen (EA) and viral capsid antigen (VCA), both of which have been identified in nasopharynx cancer cells, along with their specific antibodies, EA-IgA and VCA-IgA, making all these molecules biological markers predicting cancer prognosis [5,6]. Therefore, serum levels of EA-IgA and VCA-IgA can reflect the physiological state of intracellular EBV, further predicting the stage of cancer. Sialic acid (SA) is also known to be related with the occurrence and metastasis of malignant tumors and can be used to predict the metastatic stage [7,8]. This study thus investigated the serum level of SA, VCA-IgA, and EA-IgA in nasopharynx cancer patients to elucidate the diagnostic implication of combined assays and to provide a strategy for further development of clinical diagnostic methods.
Material and Methods
General information of patients
A total of 42 nasopharynx cancer patients (20 males and 22 females; ages 20~67 years old, average=44.6±0.7 years old) admitted to the Second Municipal Hospital of Weihai between September 2012 and October 2013 were recruited in this study, along with 42 patients with benign rhinitis (23 males and 19 females; ages 21~66 years old, average=44.7±0.5 years old) and 42 healthy adults (21 males and 21 females; ages 22~68 years old, average=43.7±0.6 years old). All nasopharynx cancer patients received confirmed diagnosis by pathological examination as low-differentiation squamous cell carcinoma according to UICC standard (6th version, 2002). TNM staging revealed 8 cases at stage I, 16 at stage II patients, 13 at stage III, and 5 at stage IV cancer. All patients had no other inflammatory disease and had not received any post-operative chemotherapy or immunomodulation therapy. No difference regarding age or sex distribution was detected among all 3 groups (p>0.05), which were thus comparable. This study was pre-approved by the Ethics Committee of the Second Municipal Hospital of Weihai and we obtained written consent from all participants.
Serum assays
Peripheral venous blood samples were collected and centrifuged at 2000 g for 10 min to extract the serum. EA-IgA and VCA-IgA levels were quantified using enzyme-linked immunosorbent assay (ELISA) using test kits (Zhongshan Biotech, China) following the manual instructions. Enzymatic method was used to examine serum SA levels using a test kit (Zhongshan Biotech, China). When any 2 factors were tested together, the result was defined as positive when any index showed positive values, but was defined as negative only if both factors showed negative results. When all 3 indexes were checked altogether, positive results occurred when any factor showed a positive value, while negative results were identified only when all 3 indexes showed negative results. ROC curve analysis was used to determine the threshold of SA, EA-IgA, and VCA-IgA levels.
Polymerase chain reaction (PCR)
Paraffin-embedded blocks were cut into 10-μm-thick sections. These microdissected tumor tissues were put into 1.5-mL EP tubes and treated with 0.5 mL of TaKaRa DEXPAT™ (TaKaRa, Japan) to extract DNA according to the manufacturer’s instructions. The samples were incubated at 100°C for 10 min and centrifuged at 12 000 rpm for 10 min. The supernatant containing DNA was transferred to new EP tubes and stored at −20°C until used as the template for subsequent PCR. The template DNA (1 μg) was mixed with 10× buffer (100 mM Tris-HCl, 500 mM KCl, 15 mM MgCl2), 10 mM dNTP mixture, 1.5 μU Taq polymerase (TaKaRa, Japan), and the primers EBVF 5′-CCAGACAGCAGCCAATTGTC-3′, and EBVR 5′-GGTAGAAGACCCCCTCTTAC-3′. The targeted PCR product was 129 base pairs long. The PCR reaction conditions were as follows: initial denaturation at 95ºC for 5 min; 35 cycles of denaturation at 95ºC for 30 s; annealing at 57ºC for 30 s and extension at 72ºC for 1 min, and a step of final extension at 72ºC for 7 min. The amplification products were electrophoresed in a 2% agarose gel and then stained with ethidium bromide.
Statistical analysis
The SPSS 19.0 software package was used to analyze all collected data, of which ratios were compared by chi-square test. Data are presented as mean ± standard deviation (SD) and were compared by analysis of variance (ANOVA) or Student’s t-test. ROC curve was used to evaluate the diagnostic implication of SA, EA-IgA, and VCA-IgA. A statistical significance was defined when p<0.05.
Results
Serum levels of SA, EA-IgA and VCA-IgA
Our results (Table 1) showed higher SA, EA-IgA, and VCA-IgA serum levels in nasopharynx cancer patients compared to rhinitis patients, who also had higher serum levels than healthy individuals (p<0.05 in all cases compared).
Table 1.
Serum levels of SA, EA-IgA and VCA-IgA.
| Group | SA (mg/L) | EA-IgA (against standard) | VCA-IgA (against standard) |
|---|---|---|---|
| Control | 564.51±41.42 | 0.55±0.11 | 0.64±0.22 |
| Rhinitis | 590.32±42.65* | 0.79±0.17* | 0.86±0.26* |
| Cancer | 695.17±62.14*,# | 1.21±0.38*,# | 2.52±0.44*,# |
| F value | 12.613 | 36.354 | 32.396 |
| P value | 0.01 | 0.00 | 0.00 |
p<0.05 compared to the control group;
p<0.05 compared to the rhinitis patients.
Serum levels of SA, EA-IgA, and VCA-IgA across different TNM stages
We further compared the serum expression levels of these 3 factors across different TNM stages of pharynx cancer patients and found higher serum levels of all indexes in late-stage cases (Table 2).
Table 2.
Serum levels of SA, EA-IgA and VCA-IgA across different TNM stages.
| Group | N | SA (mg/L) | EA-IgA (against standard) | VCA-IgA (against standard) |
|---|---|---|---|---|
| Stage I | 8 | 608.22±32.15 | 0.83±0.17* | 0.89±0.13* |
| Stage II | 16 | 635.11±32.08* | 1.14±0.16* | 1.21±0.16* |
| Stage III | 13 | 676.16±24.16* | 1.20±0.18* | 2.44±0.13* |
| Stage IV | 5 | 705.05±22.34* | 1.45±0.21* | 2.56±0.21* |
| F value | – | 12.315 | 12.528 | 14.403 |
| P value | – | 0.01 | 0.000 | 0.00 |
p<0.05 with statistical significance.
ROC curve analysis
We plotted ROC curves using both healthy controls and nasopharynx cancer patients as the subjects. The areas under the curve (AUC), which represents the diagnostic efficacy, were 0.807, 0.932, and 0.989 for SA, EA-IgA, and VCA-IgA, respectively (Figure 1, Table 3). Therefore, all these 3 factors had satisfactory diagnostic efficacy for nasopharynx cancer, with better efficacy in VCA-IgA.
Figure 1.
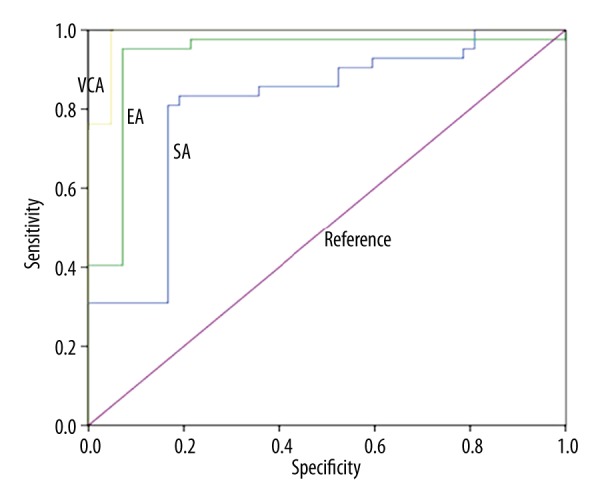
ROC curves of SA, EA-IgA, and VCA-IgA.
Table 3.
Area under curve (AUC) of ROC analysis.
| Index | AUC | SD* | Significance** | 95% confidence interval | |
|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||
| SA | .807 | .049 | .000 | .711 | .904 |
| EA-IgA | .932 | .033 | .000 | .868 | .996 |
| VCA- IgA | .989 | .009 | .000 | .972 | .999 |
Under the non-parametric hypothesis;
null hypothesis: AUC=0.5.
Sensitivity and specificity of all indexes
With reference to serum levels and ROC curves for SA, EA-IgA, and VCA-IgA in both normal and nasopharynx cancer patients, we defined positive signals as SA >650 mg/L, or EA-IgA >1.1, or VCA-IgA >1.1. Thus, the sensitivity and specificity of SA, VCA-IgA, and EA-IgA in diagnosis of nasopharynx cancer were 30.95% and 83.33%, 76.19% and 92.86%, and 57.14% and 95.24%, respectively. The omission diagnostic rate and misdiagnosis rates were 69.05% and 16.67%, 23.81% and 7.14%, and 42.86% and 4.76% for SA, VCA-IgA, and EA-IgA, respectively. In summary, when only 1 index is used for diagnosis, VCA-IgA has the highest sensitivity and EA-IgA has the highest specificity. There were statistically significant sensitivity and false-positive rates among the 3 indexes (Table 4).
Table 4.
Test power of serum SA, EA-IgA and VCA-IgA in nasopharynx cancer diagnosis.
| Index | Positive | Sensitivity (%) | Specificity (%) | Omission diagnosis (%) | Misdiagnosis (%) |
|---|---|---|---|---|---|
| SA | ≥650 mg/L | 30.95 (13/42) | 83.33 (35/42) | 69.05 (29/42) | 16.67 (7/42) |
| VCA-IgA | >1.1 | 76.19 (32/42) | 92.86 (39/42) | 23.81 (10/42) | 7.14 (3/42) |
| EA-IgA | >1.1 | 57.14 (24/42) | 95.24 (40/42) | 42.86 (18/42) | 4.76 (2/42) |
| χ2 | – | 17.4920 | 3.8684 | 17.4920 | 3.8684 |
| P | – | 0.00 | 0.14 | 0.00 | 0.14 |
Diagnostic efficacy of combined assays
Using 2 or 3 of these indexes, combined assays had higher sensitivity (92.86%) and negative prediction power (91.18%) without compromising specificity (Table 5).
Table 5.
Combined assay.
| Indexes tested | Sensitivity (%) | Specificity (%) | Positive power (%) | Negative power (%) |
|---|---|---|---|---|
| SA+EA-IgA | 71.43 (30/42) | 76.19 (32/42) | 75.00 | 72.73 |
| SA+VCA-IgA | 83.33 (35/42) | 78.57 (33/42) | 79.55 | 82.50 |
| EA-IgA+ VCA-IgA | 90.48 (38/42) | 88.10 (37/42) | 88.37 | 90.24 |
| SA+ EA-IgA+ VCA-IgA | 92.86 (39/42) | 73.81 (31/42) | 78.00 | 91.18 |
EBV PCR
EBV was detected in 38 of 42 cases (90%) of nasopharynx cancer by PCR, but not in the benign cases or the control group.
Discussion
Various factors, including genetics, EBV infection, and environmental toxicity, underlie the pathogenesis of nasopharynx cancer [9,10]. The expression products of EBV may interact with related genes of epithelial cells to initiate a cascade of molecular events leading to the occurrence of nasopharynx cancer, thus making the level of EA-IgA and VCA-IgA good clinical indexes for cancer condition [11–13]. Due to the insidious onset and difficulty of identification in routine examination, serological indexes are of critical importance for large-scale screening and early diagnosis for nasopharynx cancer [14,15]. Currently used indexes, such as SA, VCA-IgA, and EA-IgA, had advantages over classical pathological examination after biopsy [16,17]. This study investigated the utility of SA, VCA-IgA, and EA-IgA in a combined assay for diagnosis of nasopharynx cancer.
EBV genome has been identified in most populations affected by nasopharynx cancer in southern China [18,19]. In the occurrence of cancer, the EBV genome initiates the transcription of EBV antigens in epithelial cells. Therefore, serum levels of EA-IgA and VCA-IgA may effectively reflect the proliferation of EBV and the condition of the nasopharynx at an early stage. SA, on the other hand, is widely distributed in body tissues, where they are conjugated with glycoproteins or carbohydrate chains of glycolipid molecules and participate in cell surface physiological functions. The dynamic change of cell surface SA levels is correlated with malignant tumor progression and thus is recognized as a tumor marker [20]. This study revealed higher serum levels of SA, EA-IgA, and VCA-IgA in nasopharynx cancer patients compared to rhinitis patients, who had higher serum levels of those 3 factors than control adults. Moreover, cancer patients with late-stage tumors had further elevated serum indexes. TNM stage is a major factor predicting prognosis of cancer. Current studies have not proved the efficacy of using serum EA-IgA and VCA-IgA levels in determining TNM stage of cancer, probably due to the relatively longer half-life of those 2 IgA molecules. However, the consistency between serum indexes and TNM stage in this study suggest the potential value in reflecting the condition of cancer.
When a single index was used in diagnosis, VCA-IgA had higher sensitivity compared to EA-IgA, but EA-IgA had higher specificity. These results suggest that EA may be produced when EBV initiates its replication, causing its higher specificity but lower sensitivity. The omission diagnostic rate of all 3 factors, when used singly, is relatively higher. Even double-negative status for VCA-IgA and EA-IgA may not completely exclude nasopharynx cancer. Our results showed that the combined assays had higher sensitivity (92.86%) and negative prediction power (91.18%) without compromising its specificity, which was comparable to the detection rate of PCR analysis (90%). Therefore, clinical diagnosis should be made in conjunction with symptoms. ROC curves showed satisfactory diagnostic efficacy of all 3 of these serum indexes, among which VCA-IgA had the highest efficacy. A single index, however, may not result in confirmed diagnosis, making the combined assay of all 3 indexes preferable due to improved sensitivity and negative power without compromising specificity. The combined assay has its inherent complementary benefits in the early-diagnosis of nasopharynx cancer, therefore improving the diagnostic power of serological examination. In clinical practice, parallel assays including serological assays and pathological examinations are required to overcome the limit of a single index, thus minimizing the diagnostic omission or misdiagnosis. Such laboratory results should be considered together with clinical symptoms, such as nose bleeding without known reason, unilateral progressive obstruction of Eustachian tube, insidious headache, or neck lymph node swelling. Only by the comprehensive consideration of both clinical and laboratory results can one obtain accurate diagnosis.
Conclusions
Serum levels of SA, EA-IgA, and VCA-IgA were higher in nasopharynx patients, and the combined assay of these 3 factors has clinical utility for diagnosis and prognostic prediction for cancer patients.
Footnotes
Source of support: Departmental sources
References
- 1.Peng YH, Xu YW, Huang LS, et al. Autoantibody signatures combined with Epstein-Barr virus capsid antigen-IgA as a biomarker panel for the detection of nasopharyngeal carcinoma. Cancer Prev Res (Phila) 2015;8(8):729–36. doi: 10.1158/1940-6207.CAPR-14-0397. [DOI] [PubMed] [Google Scholar]
- 2.Huang S, Li S, Peng T, et al. [Detection of cytokeratin18 and cytokeratin19 gene expression in blood and tumor tissue of nasopharyngeal carcinoma patients by RT-PCR]. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2015;29(2):111–13. [in Chinese] [PubMed] [Google Scholar]
- 3.Chen J, Zong J, Wu J, Pan J. [Prognostic analysis of nasopharyngeal carcinoma patients with distant metastasis after curative radiotherapy]. Zhonghua Zhong Liu Za Zhi. 2015;37(3):216–21. [in Chinese] [PubMed] [Google Scholar]
- 4.Lin Y, Fu Y, Xu M, et al. Evaluation of a PCR/ESI-MS platform to identify respiratory viruses from nasopharyngeal aspirates. J Med Virol. 2015;87(11):1867–71. doi: 10.1002/jmv.24262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen G, Zhou L, Jia Z, et al. [Meta-analysis of PET/CT for diagnosis of residual/recurrent nasopharyngeal carcinoma]. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2015;29(1):61–67. [in Chinese] [PubMed] [Google Scholar]
- 6.Blochmann U, Kuhnel [Nasopharyngeal carcinoma. Brush biopsy with EBV-PCR is suitable as a screening test]. Laryngorhinootologie. 2015;94(3):146–47. doi: 10.1055/s-0034-1369662. [in German] [DOI] [PubMed] [Google Scholar]
- 7.Lian S, Ji M, Wu B, Yu X. [The following-up study of high-risk and moderate-risk groups defined by EB virus serology test at the nasopharyngeal carcinoma screening programme]. Zhonghua Yu Fang Yi Xue Za Zhi. 2015;49(1):26–30. [in Chinese] [PubMed] [Google Scholar]
- 8.Chen H, Chi P, Wang W, et al. Evaluation of a semi-quantitative ELISA for IgA antibody against Epstein-Barr virus capsid antigen in the serological diagnosis of nasopharyngeal carcinoma. Int J Infect Dis. 2014;25:110–15. doi: 10.1016/j.ijid.2014.03.1373. [DOI] [PubMed] [Google Scholar]
- 9.Cai YL, Li J, Lu AY, et al. Diagnostic significance of combined detection of Epstein-Barr virus antibodies, VCA/IgA, EA/IgA, Rta/IgG and EBNA1/IgA for nasopharyngeal carcinoma. Asian Pac J Cancer Prev. 2014;15(5):2001–6. doi: 10.7314/apjcp.2014.15.5.2001. [DOI] [PubMed] [Google Scholar]
- 10.Luo YL, Chen H, Peng SG, et al. [Assessment of detection assays of Epstein-Barr viral Rta-IgG, VCA-IgA, EA-IgA and Epstein-Barr viral DNA at different clinical stages in the diagnosis of nasopharyngeal carcinoma]. Zhonghua Yi Xue Za Zhi. 2013;93(44):3516–19. [in Chinese] [PubMed] [Google Scholar]
- 11.Chen H, Luo YL, Zhang L, et al. EA-D p45-IgG as a potential biomarker for nasopharyngeal carcinoma diagnosis. Asian Pac J Cancer Prev. 2013;14(12):7433–38. doi: 10.7314/apjcp.2013.14.12.7433. [DOI] [PubMed] [Google Scholar]
- 12.Sun P, Chen C, Cheng YK, et al. Serologic biomarkers of Epstein-Barr virus correlate with TNM classification according to the seventh edition of the UICC/AJCC staging system for nasopharyngeal carcinoma. Eur Arch Otorhinolaryngol. 2014;271(9):2545–54. doi: 10.1007/s00405-013-2805-5. [DOI] [PubMed] [Google Scholar]
- 13.Cai YL, Li J, Lu AY, et al. [Prognostic significance of serum anti-Epstein-Barr virus antibodies in nasopharyngeal carcinoma]. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2013;27(2):119–22. [PubMed] [Google Scholar]
- 14.Xing Y, Song HM, Wei M, et al. Clinical significance of variations in levels of Epstein-Barr Virus (EBV) antigen and adaptive immune response during chronic active EBV infection in children. J Immunotoxicol. 2013;10(4):387–92. doi: 10.3109/1547691X.2012.758199. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Huang Q, Liu W, et al. Establishment of VCA and EBNA1 IgA-based combination by enzyme-linked immunosorbent assay as preferred screening method for nasopharyngeal carcinoma: a two-stage design with a preliminary performance study and a mass screening in southern China. Int J Cancer. 2012;131(2):406–16. doi: 10.1002/ijc.26380. [DOI] [PubMed] [Google Scholar]
- 16.Cao SM, Liu Z, Jia WH, et al. Fluctuations of epstein-barr virus serological antibodies and risk for nasopharyngeal carcinoma: a prospective screening study with a 20-year follow-up. PLoS One. 2011;6(4):e19100. doi: 10.1371/journal.pone.0019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai YL, Zheng YM, Wang W, et al. [Combined detection of Epstein-Barr virus antibodies for serodiagnosis of nasopharyngeal carcinoma]. Nan Fang Yi Ke Da Xue Xue Bao. 2010;30(12):2746–48. [in Chinese] [PubMed] [Google Scholar]
- 18.Cai YL, Zheng YM, Cheng JR, et al. [Evaluation of combined determinations of Epstein-Barr virus antibodies for nasopharyngeal carcinoma assessed with receiver operating characteristic curve based on logistic regression]. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2009;23(5):384–87. [in Chinese] [PubMed] [Google Scholar]
- 19.Cai YL, Zheng YM, Cheng JR, et al. [Relationship between clinical stages of nasopharyngeal carcinoma and Epstein-Barr virus antibodies Rta/IgG, EBNA1/IgA, VCA/IgA and EA/IgA]. Nan Fang Yi Ke Da Xue Xue Bao. 2010;30(3):509–11. [in Chinese] [PubMed] [Google Scholar]
- 20.Luo YL, Ou GP, Chi PD, et al. Combined determination of Epstein-Barr virus-related antibodies and antigens for diagnosis of nasopharyngeal carcinoma. Ai Zheng. 2009;28(1):76–78. [PubMed] [Google Scholar]


