Abstract
γ-tocopherol (γ-T) alone or in combination with α-tocopherol has been shown to suppress biomarkers of oxidative stress in asthamatics and human subjects with metabolic syndrome. Oxidative stress has been implicated as a key event in prostate carcinogenesis. Hence, the purpose of this study was to examine the effects of γ-tocopherol-enriched mixed tocopherol diet on prostate carcinogenesis in a murine prostate cancer model (TRAMP). 8 week old TRAMP males were fed 0.1% γ -T-enriched mixed tocopherol diet that contained 20-fold higher levels of γ-tocopherol, and roughly 3-fold higher levels of α-tocopherol. The effect of such diet on tumor and PIN development was observed. The expression of phase II detoxifying, antioxidant enzymes and Nrf2 mRNA and protein were determined by RT-PCR, immunohistochemistry and western blotting techniques. Treatment with γ-T-enriched mixed tocopherols significantly suppressed the incidence of palpable tumor and Prostate Intraepithelial Neoplasia (PIN) development without affecting the expression of the transgene (SV-40). Tumor progression occurred with a significant suppression of antioxidant enzymes such as catalase, superoxide dismutase, glutathione peroxidase, heme-oxygenase-1 and phase II detoxifying enzymes. Treatment with γ-T-enriched mixed tocopherol diet upregulated the expression of most detoxifying and antioxidant enzymes. Nrf2—a redox sensitive transcription factor known to mediate the expression of phase II detoxifying enzymes, was also significantly upregulated following treatment with γ-T-enriched mixed tocopherol diet. γ-T-enriched mixed tocopherols significantly up-regulated the expression of Nrf2 and its related detoxifying and antioxidant enzymes thereby suppressing PIN and tumor development.
Keywords: oxidative stress, Nrf2, antioxidant detoxifying enzymes, prostate cancer
Reactive oxygen species (ROS) such as superoxide radical anion, hydroxyl radical and hydrogen peroxide are generated as by-products of aerobic mitochondrial respiration. The human body has robust defense mechanisms comprising of detoxifying enzymes such as glutathione-s-transferase, UDP glucuronyl transferases and antioxidant enzymes such as superoxide dismutase, glutathione peroxidase and catalase as well as non-enzyme antioxidant species that, under physiological conditions, are known to quench these reactive moieties. However, a scenario where excessive reactive oxygen species are generated and the body's antioxidant defenses are overwhelmed leads to a state of oxidative stress. Oxidative stress can cause adducts and permanent damage to cellular macromolecules such as DNA/RNA, proteins, lipids, etc. A rich body of evidence substantiates the role of such oxidative stress in carcinogenesis. Needless to say, increased levels of ROS coupled with suppressed antioxidant defense have been reported in human prostate intraepithelial neoplasia and prostate cancer.1–4
The antioxidant activity of vitamin E has persuaded many researchers to study its ability to ameliorate chronic diseases, especially those in which oxidative stress plays a key role such as arthrosclerosis, cardiovascular diseases and cancer.5 Vitamin E is a generic name for structurally related tocopherols and tocotrienols. A standard American diet contains large amounts of γ-T compared to European diet due to high intake of soybeans and corn oil.6 Despite high intake of γ-T, plasma concentrations of γ-T are about 10 times lower than that of α-T. The reason for the plasma preference for α-T is in its specific selection by α-T transfer protein (α-TTP). α-TTP not only specifically selects the α form of all tocopherols but also has a preference for 2R-stereoisomers.6,7 As a result, the cancer chemopreventive activities of Vitamin E have been extensively studied with α-T.8,9 However the protective effects of γ-T having only recently been recognized are now the subject of active investigation. A γ-T-enriched mixed tocopherol diet (containing more than 50% γ-T) has been shown to suppress azoxymethane induced crypt foci - a precursor of colon cancer, in rats.10 A similar diet has also shown to suppress N-methyl-N-nitrosourea induced mammary tumors in rats.11 A recent study by Devaraj et al reports that in human subjects with metabolic syndrome, supplementation with γ-T alone or in combination with α-T significantly decreased TNF levels. However nitrotyrosine levels (a biomarker for oxidative stress) were decreased only by γ-T and not by α-T.12 A nested case-control study in Washington County, MD that examined the effects of αT, γ-T and selenium on the incidence of prostate cancer in human subjects found that the inverse relationship between high levels of selenium and α-T held true when the levels of γ-T were high. Interestingly, α 5-fold reduction in prostate cancer was observed in men who also had the highest plasma γ-T concentrations.13
γ-T possesses certain unique properties that are relevant to cancer chemoprevention. Jiang and coworkers demonstrated that both γ-T and its catabolic metabolite 2,7,8-trimethyl-2-(β-carbox-yethyl)-6-hydroxychroman (γ-CEHC) possess anti-inflammatory attributes. They significantly suppress cyclooxygenases and other inflammatory proteins such as interleukins and cytokines.14,15 Owing to its strong nucleophilic properties, γ-T exhibits more efficiency than α-T in trapping ROS and reactive nitrogen species (RNS).16 Cooney et al reported that γ-T was superior to α-T in suppressing the transformation of murine fibroblasts incubated with chemical carcinogen 3-methylcholanthrene.17 A study by Gysin et al reported that γ-T inhibited cell proliferation, cell cycle progression and DNA synthesis in prostate cancer cells better than α-T.18
To our knowledge the chemopreventive effects of a γ- T-enriched mixed tocopherol diet that provides more than 60% γ-T, have not been explored in genetically modified murine model for prostate cancer (TRAMP). This model recapitulates many salient features of the progressive forms of human prostate cancer. This study was aimed at observing the effects of a -γ-T rich mixed tocopherol diet on prostate carcinogenesis in TRAMP mice. The effects of such treatment on the levels of PIN (a recognized precursor for prostate cancer) and the expression of phase II detoxifying and antioxidant enzymes were determined.
Material and methods
Animals
Female hemizygous C57BL/TGN TRAMP mice, line PB Tag 8247NG, and male C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME). The animals were bred on same genetic background and maintained in the Animal Care Facility of Rutgers University. Housing and care of the animals was in accordance with the guidelines established by the University's Animal Research Committee consistent with the NIH Guidelines for the Care and Use of Laboratory Animals. Transgenic males for these studies were routinely obtained as [TRAMP × C57BL/6] F1 or as [TRAMP × C57BL/6] F2 offspring. Identity of transgenic mice was established by the PCR-based DNA screening. Throughout the experiment the animals were housed in cages with wood chip bedding in a temperature-controlled room (68-72°F) with a 12-h light dark cycle, at a relative humidity of 45–55%.
Diets and study design
All animals were fed AIN-76A diet obtained from Research Dyets (Easton, PA). Treated animals were fed 0.1% mixed tocopherols incorporated in AIN-76A diet. The mixed tocopherols were obtained from Cognis Corporation (Kankakee, IL) and contained <60% γ-tocopherol, 12% α-tocopherol, 21% δ-tocopherol and also insignificant levels of (β-tocopherol. This diet contained about 20-fold higher levels of γ-tocopherol, and roughly 3-fold higher levels of α-tocopherol. All the animals were put on AIN-76A diet 1 week prior to the study. The control animals (n = 17) received AIN-76A diet throughout the experiment while the treatment group (n = 11) received 0.1% -γ-T-enriched mixed tocopherol diet for a period of 24 weeks. Fresh diets were added to the cages twice a week. The animals were weighed weekly and monitored on a regular basis for their general health. At each time point mice were killed by cervical dislocation and the genitourinary apparatus (GUT) consisting of the seminal vesicles, prostate and the bladder were isolated for further analyses.
Histopathology
The dorso-lateral prostate was excised and fixed overnight in 10% formalin and then transferred to 70% ethanol. Sections (4 μm) were cut from paraffin embedded tissue and mounted on slides. The sections were stained with hematoxylin and eosin to observe any neoplastic changes. Sections were blindly evaluated by a histopathologist to classify PIN lesions. Lesions were classified as PIN I, PIN II, PIN III and PIN IV as described by Park et al.19 For the purpose of ease, PIN I and PIN II have been grouped as low grade PIN while PIN III and PIN IV have been grouped as high grade PIN.
Immunohistochemistry staining
Sections of 4 μm thickness were cut from formalin fixed, paraffin-embedded blocks and mounted on glass slides. The slides were deparafinized in xylene and antigen retrieval enhanced by boiling in 10 mM sodium citrate buffer (pH 6.0) for 10 min in a microwave oven. Endogenous peroxidase was blocked by incubation in 3% hydrogen peroxide for 30 min. Non-specific binding was blocked by incubating slides with 5% BSA for 1 hour. Incubation with primary antibody (Nrf2–1:100, GSTM1 1:50) was carried out overnight at 4°C. For the subsequent reaction, a streptavidin-biotin complex peroxidase kit was used according to manufacturer's instructions and the slides were counterstained with hematoxylin for 5 min. For both Nrf2 and GSTM1 staining, a modified semiquantitative scoring method was used. The degree of positive staining for all antibodies was evaluated on a scale from 0 to 4 for percentage of positive cells and on a scale from 0 to 3 for strength of staining intensity. The percentage of positive cells was evaluated using the following scale: 0, no staining of the epithelial cells in any field; 1+, <25% of the epithelium stain positive; 2+, 25–50% stain positive; 3 +, 50–75% stain positive; and 4+, >75% stain positive. The strength of intensity of staining was estimated using the following scale: 0, no staining of epithelial cells; 1+, mild staining; 2 +, moderate staining; and 3 +, intense staining. The final total score was generated by adding the score for percentage of positive cells and the strength of stain intensity. Hence, the minimum and maximum score for an area were 0 and 7, respectively.
Immunoblot analysis
The dorso-lateral prostate tissues removed from each treated and control groups were pooled accordingly and homogenized with RIPA buffer (Roche, Manheim, Germany). The protein concentrations were measured by Bicinchonic Acid solution (Pierce, Rockford, IL). 20 μg of protein was loaded onto Biorad pre-cast gels (4–12%) and transferred to polyvinyl difluoride membranes. The membranes were blocked with 5% BSA in TBST for 1 h followed by overnight incubation with primary antibodies and horseradish peroxidase conjugated secondary antibody for 1 h. Protein bands were visualized using Supersignal West Femto (Pierce, Rockford, IL).
RNA extraction and semi-quantitative RT-PCR
Total RNA was isolated with a combined method using Trizol (Invitrogen, Carlsbad, CA) and RNeasy Mini Kit (Qiagen, Valencia, CA). Total RNA samples were converted to single-stranded cDNA by the Superscript First-Strand Synthesis System III (Invitrogen) and the resulting cDNA was amplified by the PCR super-mix kit (Invitrogen). PCR conditions are as follows: 94°C for 5 min followed by cycles of denaturation at 94°C for 30 s, annealing at 55°C for 30 s, extension at 72°C for 30 s, and a final extension at 72°C for 10 min. The forward and reverse primers used for amplifying UGT1A1 were 5′-GTGGCCCAGTACCTGACTGT-3′ and 5′-CGATGGTCTAGTTCCGGTGT-3′. The primers used for amplifying NQO1 were 5′-CAGATCCTGGAAGGATGGAA-3′ (forward) and 5′-AAGTTAGTCCCTCGGCCATT-3′ (reverse). The forward and reverse primers used for amplifying Nrf2 were 5′-TGAAGCTCAGCTCGCATTGATCC-3′ and 5′AAGATACAA GGTGCTGAGCCGCC-3′. Actin was used as an internal control and was amplified with the primers: 5′-TGTTACCAACTGG GACGACA-3′ and 5′-TCTCAGCTGTGGTGGT GAAG-3′. PCR products were resolved on 1.5% agarose gels and visualized under UV lamps.
GST assay
Prostate tissue homogenates were centrifuged and the supernatants were analyzed for total GST activity. GST activity was determined by the change in absorbance at 340 nm of chlorodinitrobenzene incubated in the presence of GSH.
Statistics
For a dichotomous measure like the presence or absence of a palpable tumor prior to necropsy Chi square test was used. For all other determinations statistical significance was determined using students' t test at significance (p < 0.05).
Results
General health observations
The overall health of the mice throughout the study period was found to be good. No significant changes in the body weights were observed in animals given control or tocopherol mixture supplemented diet.
γ-T-enriched mixed tocopherol diet reduces tumor incidence in TRAMP mice
A significant reduction in the tumor incidence was observed as recorded by palpation prior to necropsy (Table I). 13 of 17 animals in the control group versus 2 of 11 in the treated group developed palpable tumors. Autopsy revealed significant difference between wet weight of the genitourinary apparatus of all animals in control and γ-T-enriched mixed tocopherol treated groups. The γ-T-enriched mixed tocopherol treated group demonstrated about 60% reduced weight as compared to the control group.
Table 1. γ-T-Enriched Mixed Tocopherols Inhibit Prostate Carcinogenesis.
| Number of animals | Incidence of palpable tumor1 | Average wet weight of genitourinary apparatus (gm) | |
|---|---|---|---|
| Control | 17 | 13/172 | 3.9 ± 1.23 |
| γ-T-enriched mixed tocopherol treated | 11 | 2/112 | 1.6 ± 0.713 |
Tumors detected by palpation prior to necropsy.
Represents the number of animals that showed presence of tumor upon palpation.
Chi's square test was used to compare incidence of tumor between control and treated mice at 24 weeks of age. p values <0.05 were considered significant.
Numbers represent mean ± S.E. Student's t test was used to evaluate significance.
p values <0.05 were considered significant.
Although primary prostate tumor was not grossly palpable in 4 control animals at the time of sacrifice, histological analysis revealed a relatively well differentiated carcinoma, marked stromal thickening and hypercellularity. Hematoxylin and eosin staining further revealed multiple layers of atypical cells with significantly reduced luminal space. The epithelial cells demonstrated a characteristic cribiform or tufting growth pattern. High-grade PIN was also characterized by nuclear atypia and an increased mitotic index. Control animals demonstrated approximately 60% incidence of high grade PIN and approximately 40% incidence of low-grade PIN (Fig. 1b). Four control animals demonstrated distinct lymph node metastasis. No pulmonary or liver metastases were observed. In contrast, histological analysis of age-matched non-transgenic animals demonstrated the presence of approximately 100% normal prostatic tissue (Fig. 1b). Normal prostatic acinus was characterized by the presence of single-layered columnar epithelial cells and a distinct lumen. Surrounding stroma consisted of layers of loose connective tissue.
Figure 1.
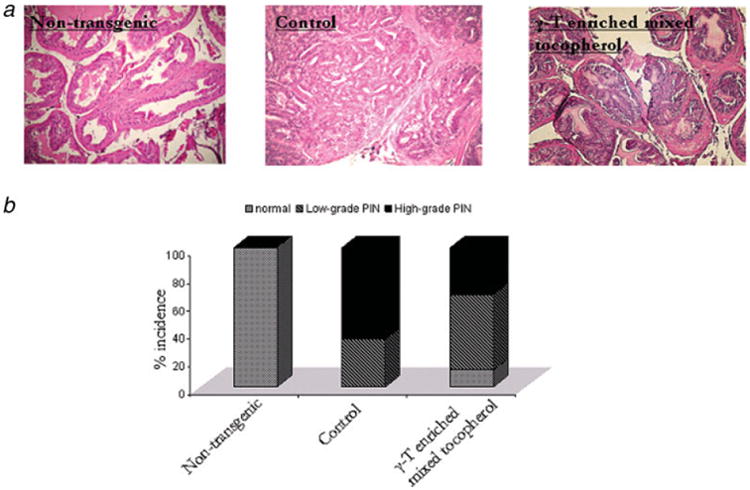
γ-T-enriched mixed tocopherol diet suppresses tumorigenesis in TRAMP mice. (a) Representative photomicrographs (×100 magnification) of H&E stained dorso-lateral section of non-transgenic, control and treated mice at 24 weeks of age. The genitourinary apparatus was removed en bloc. The dorsolateral prostate was microdissected and analyzed for PIN evaluation. (b) Percentage incidence of normal tissue, low grade and high grade PIN displayed by non-transgenic, control and γ-T-enriched mixed tocopherol treated 24-week old mice. Normal tissue was characterized by single-layered epithelial cells and a distinct lumen, low grade PIN typically displayed a couple of layers of atypical cells that did not, however, fill up the luminal space and high grade PIN characterized by marked stromal thickening and hypercellularity and significantly reduced luminal space. The bars represent mean values ± S.E. Statistical significance of the difference was analyzed by Chi's square test. p values < 0.05 were considered significant. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Histological evaluation revealed that γ-T-enriched mixed tocopherol treatment resulted in approximately 50% low grade PIN, approximately 30% high-grade PIN and 20% normal tissue. Low grade PIN was characterized by a couple of layers of atypical cells that did not fill up the luminal space. The cells displayed tufting or cribiform growth patterns though the underlying fibromuscular sheath in most cases was almost intact. Tumor incidence and decrease in PIN levels by γ-T-enriched mixed tocopherol treatment were not a result of suppression of SV-40 transgene expression (results not shown).
Suppression of phase II detoxifying enzymes during tumor progression
The 3 enzymes UGT1A1, NQO-1 and GST-M1 were consistently suppressed at 24 weeks compared with 8-week old prostate tissue. Both UGT1A1 and GSTM1 showed a slight peak in the expression levels at 12 weeks followed by reduced expression at 16 and 24 weeks. Conversely, the expression of NQO-1 was relatively high even at 16 weeks but eventually was reduced at 24 weeks (Fig. 2a). Similarly, the mRNA levels of both UGT1A1 and GSTM1 were reduced at 24 weeks compared to 8 week old samples (Fig. 2b). However the mRNA levels of NQO-1 were not significantly reduced.
Figure 2.
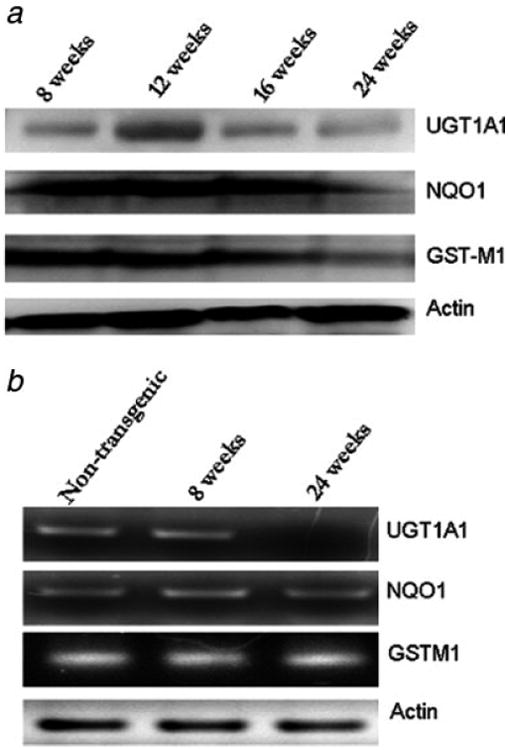
Phase II detoxifying enzymes are suppressed during prostate tumorigenesis in TRAMP mice. (a) Western blot analysis of UGT1A1, NQO-1 and GSTM1 in 8, 12, 16 and 24-week old TRAMP mice. (b) RT PCR analysis of UGT1A1 (30 cycles), NQO-1 (30 cycles) and GSTM1 (30 cycles) in 24-week old non-transgenic, 8 week and 24-week-old TRAMP mice. The forward and reverse primers are as described in material and methods.
γ-T-enriched mixed tocopherol diet increases the expression of phase II detoxifying enzymes
The γ-T-enriched mixed tocopherol treatment significantly increased the expression levels of UGT1A1 and GSTM1. Results are as shown in Figure 3a. No effect was observed on the expression levels of NQO-1 (results not shown). Furthermore, immunohistochemical staining with GSTM1 antibody confirmed the results from western blotting. The results of the immunohistochemistry staining are as depicted in Figure 3b. Tocopherol treatment increased the expression of GSTM1 as compared to the control animals. The mean total score for control and tocopherol mixture treated slides was 4.9 ± 1.1 and 6 ± 0.6. This difference was not found to be statistically significant.
Figure 3.
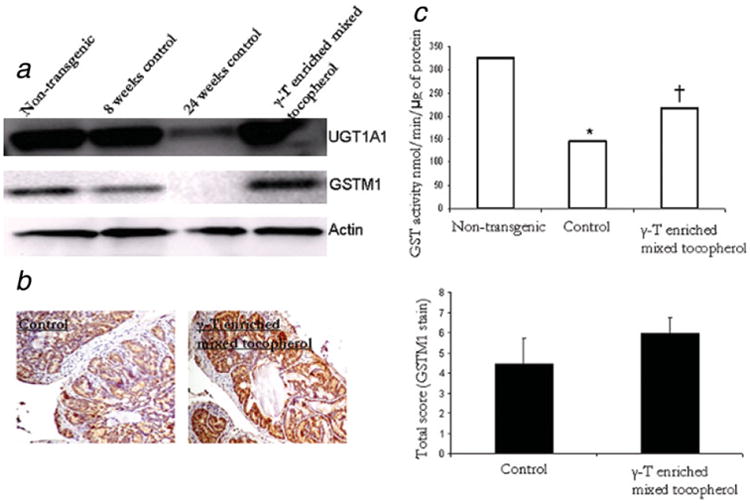
γ-T-enriched mixed tocopherol diet increases expression of phase II detoxifying enzymes. (a) Western blot analysis of UGT1A1 and GSTM1 in 24-week old non-transgenic, 8 week and 24-week old TRAMP control and γ-T-enriched mixed tocopherol diet treated samples. (b) Immunohistochemical analysis of GSTM1 in control and γ-T-enriched mixed tocopherol diet treated 24-week old mice. Bars represent mean ± S.E. of relative total score of GSTM1 positively stained cells in control and treated 24-week old mice. No statistically significant difference was observed between control and treated samples. (c) Catalytic activity of GST in non-transgenic, control and treated 24-week old mice. GST activity was determined using 1-chloro-2,4-dinitrobenzene as a substrate. The reaction product, GSH-DNB conjugate absorbs at 340 nm. The rate of increase in absorption is directly proportional to the GST activity in the sample. Bars represent mean values. Student's t test was used to evaluate significance. *Significantly different compared to non-transgenic. ‡Significantly different compared to control. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Furthermore, an in vitro assay to determine the catalytic activity of GST was also performed to further corroborate the results from expression data. The results are as depicted in Figure 3c. A comparison with age-matched non-transgenic animals revealed the catalytic activity of GST was significantly suppressed in control animals. Treatment with γ-T-enriched mixed tocopherols restored the activity significantly.
γ-T-enriched mixed tocopherols induce the expression of antioxidant enzymes
The expression of glutathione peroxidase, heme-oxygenase 1, superoxide dismutase 1 and catalase were significantly suppressed in 24 weeks compared with the age-matched non transgenic prostate tissue samples. The expression of most of these enzymes with the exception of glutathione peroxidase showed a slight increase in expression at 8 weeks followed by complete suppression at 24 weeks. Expression of glutathione peroxidase was completely abolished even in the 8 week old TRAMP prostate samples. However treatment with γ-T-enriched mixed tocopherol diet significantly increased the expression levels of all antioxidant enzymes (Fig. 4).
Figure 4.
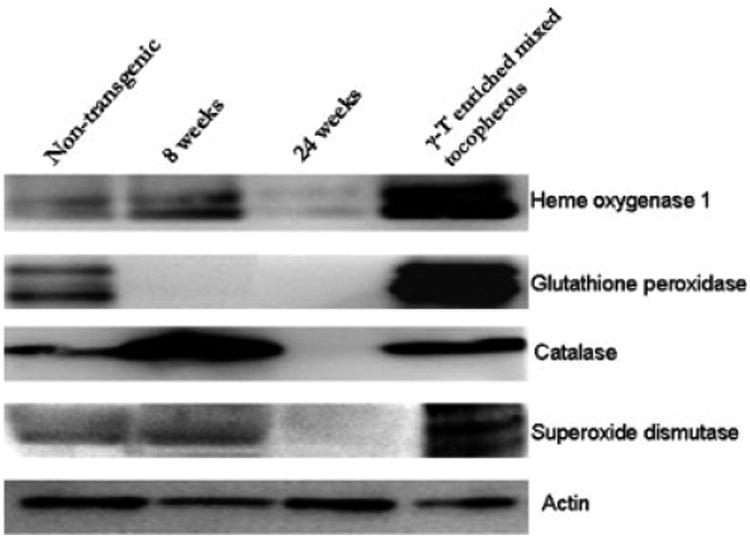
γ-T-enriched mixed tocopherol diet increases expression of antioxidant enzymes—hemeoxygenase -1, glutathione peroxidase, catalase, superoxide dismutase. The expression of the enzymes was compared between non-transgenic 24 week old, 8-week old TRAMP, 24-week old TRAMP and treated samples. Actin was used as a loading control.
γ-T-enriched mixed tocopherols induce the expression of Nrf2
Tumorigenesis in TRAMP mice progresses with significant suppression of Nrf2 protein and mRNA as depicted in Figure 5a and 5b. Of note, the enhanced expression level of Nrf2 at 12 weeks of age corresponds well with the increased expression of UGT1A1 and GSTM1 at 12 weeks as described above. Age advancement in control animals clearly demonstrates low expression of Nrf2 compared to age-matched non transgenic animals. γ-T-enriched mixed tocopherols significantly upregulated the expression of Nrf2 as demonstrated by western blotting and immunohistochemistry staining (Fig. 4c and 4d). The treated mice clearly demonstrated a stronger staining for Nrf2, indicative of its increased expression levels of the same. The mean scores for control and treated slides were 3.1± 0.8 and 5.2 ± 0.5. This difference was found to be statistically significant (p < 0.05).
Figure 5.
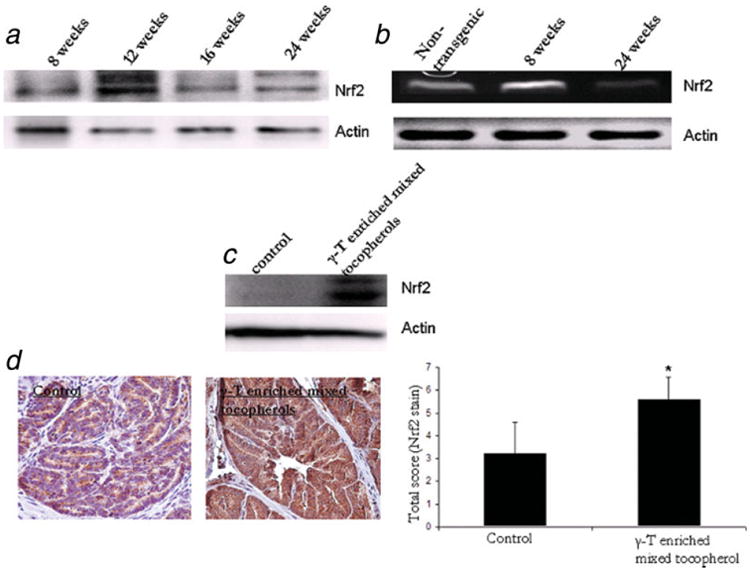
γ-T-enriched mixed tocopherol diet upregulates the expression redox-sensitive transcription factor Nrf2 that is known to mediate the expression pf phase II detoxifying and antioxidant enzymes. (a) Western blot analysis of Nrf2 in 8, 12, 16 and 24-week old TRAMP mice. (b) RT PCR analysis of Nrf2 (32 cycles) in 24-week old non-transgenic, 8 week and 24-week old TRAMP mice. The forward and reverse primers are described in material and methods. (c) Western blot analysis of Nrf2 in control and γ-T-enriched mixed tocopherol diet treated 24-week old TRAMP mice. (d) Immunohistochemical analysis of Nrf2 in control and γ-T-enriched mixed tocopherol diet treated 24-week old TRAMP mice. Bars represent mean ± S.D. of the relative total score of Nrf2 postively stained cells in control and treated sections. *Significantly different from control using Students' t test. p value < 0.05 was considered significant. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Discussion
The efficacy of a 0.1% γ-T-enriched mixed tocopherol diet against azoxymethane- induced aberrant crypt foci and N-methyl-N-nitrosourea induced mammary tumors has been recently reported.11 The present study was undertaken to examine the chemopreventive properties of a similar γ-T-enriched mixed tocopherol diet on prostate tumor progression in TRAMP mice - a mouse model that closely mimics and captures most pathological and biochemical events leading to human prostate cancer. γ-T rich diet significantly attenuated PIN levels and the development of tumors. We report that antitumor efficacy of γ-T rich diet is in part due to its antioxidant properties. Nrf2 is a redox-sensitive transcription factor that is involved in the transcriptional regulation of many detoxifying and antioxidant genes. Under normal unstimulated conditions, Nrf2 remains sequestered in the cytoplasm due to actin-bound Keap1. It has been shown that treatment with oxidants such as H2O2, oxidative stress or electrophiles results in conformational changes due to oxidation of thiol groups present on Nrf2/ Keap1 and/or activate upstream signaling cascades causing dissociation of Nrf2 from Keap1 and nuclear translocation of Nrf2 molecule leading to enhanced expression of phase II detoxifying and antioxidant enzyme expression.20–22 In a recent study, Kode and coworkers demonstrated that short term treatment with oxidants from cigarette smoke caused dissociation of Nrf2 from Keap1 and nuclear translocation of Nrf2. Long term treatment with oxidants, on the other hand, formed protein carbonyl adducts with the sulfhydryl groups of Nrf2/Keap1 thereby modulating them in a manner such that Nrf2 failed to translocate into the nucleus and thus did not enhance the transcription of detoxifying and antioxidant enzymes.23
Since oxidative stress has been shown to be implicated in prostate carcinogenesis, we believe that sustained oxidative stress for long periods may suppress instead of enhance the expression of Nrf2 as evident from our study. Indeed, we observed that although the expression of Nrf2 and certain phase II detoxifying and antioxidant enzymes were enhanced in the prostates of TRAMP mice upto 12 weeks of age, the neoplasms in 24-week old mice expressed low to almost nil levels of Nrf2 and its regulated enzymes. Thus sustained oxidative stress may lead to concerted suppression of Nrf2 and detoxifying enzymes, leading to high-grade PIN and tumor progression in TRAMP mice. Figure 6 portrays this dual role played by ROS in modulating Nrf2 protein and thereby carcinogenesis in TRAMP mice. The exact mechanism by which enhanced oxidative stress suppresses Nrf2 is currently being investigated.
Figure 6.
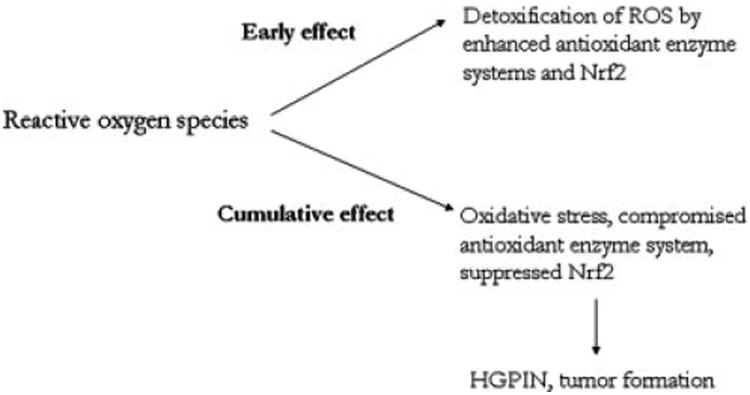
A schematic representation of the putative role of reactive oxygen species/oxidative stress and its modulation of Nrf2 and related phase II antioxidant and detoxifying enzymes in prostate carcinogenesis in TRAMP mice.
Tissues expressing high levels of GST, UGT1A1 and NQO-1 are often protected from cytotoxic damage by electrophiles.24,25 The expression and activities of these enzymes and their modulation in tissues that are subjected to physiological or environmental stimuli offers an important tool for monitoring the detoxification potential of cellular systems. The expression of these detoxifying enzymes was significantly suppressed during PIN development and tumor formation in TRAMP mice. On the other hand, treatment with γ-T-enriched mixed tocopherol diet restored the activity and expression levels of these enzymes, perhaps, enhancing the detoxification of ROS and effectively retarding PIN and tumor formation. The exact mechanism by which γ-tocopherol exerts these effects is not known. However, in light of the knowledge that γ-tocopherol possesses chain breaking properties, it may be speculated that it can break oxidative stress induced protein carbonyl adducts thereby breaking the Nrf2/Keap1 dissociation and increase translocation of Nrf2 and thereby transcription/translation of its related genes.26,27 However detailed biochemical assays are necessary to prove such interaction.
Superoxide dismutases are a family of enzymes responsible for the detoxification of superoxide free radicals. SODs are expressed in many human tissues including the prostate. It has been shown that individuals with homozygous Ala genotype are at a greater risk for prostate cancer. Further, this elevation of prostate cancer risk associated with Ala allele was particularly observed in smokers with low Vitamin E intake indicating that individuals with low antioxidant status are at higher risk for developing PCa.28 It has been shown that malignant epithelial cells in prostatic adenocarcinoma express no SOD, GPx and catalase.1,3 Similarly, Aydin and coworkers have also shown that levels of Cu-Zn SOD and GPx in the erythrocytes of prostate cancer subjects were lower than in benign prostate hyperplasia and control subjects.29 We have now shown that as age advances in TRAMP mice, SOD, catalase and GPx expression is suppressed. With low GPx activity, catalase alone may be incapable of detoxifying hydrogen peroxide resulting in an accumulation of the same leading to higher production of hydroxy radical. Additionally suppressed SOD causes accumulation of superoxide radicals that are notorious in causing deleterious effects at sites distant from the tumor. Thus, suppression of SOD, catalase and GPx causes the prostate to be predisposed to oxidative insults leading to PIN and tumor formation. Administering γ-T-enriched mixed tocopherol diet perhaps shifts the redox balance within the prostatic mileu ultimately resulting in tumor inhibition.
From the current study, we can infer that tumorigenesis in TRAMP mice occurs with progressive suppression of Nrf2 and related phase II detoxifying enzymes UGT1A1 and GSTM1 and the antioxidant enzymes heme oxygenase 1, catalase, superoxide dismutase 1 and glutathione peroxidase. By exerting an effect on Nrf2 and the antioxidant and detoxifying enzymes, γ-T-enriched mixed tocopherol diet perhaps enhanced the detoxification of reactive moieties leading ultimately to suppression of PIN and tumor development. Based on these findings, we believe that mixed tocopherols, and especially γ-tocopherol, may have potential as a chemopreventive agent against the development of prostate cancer.
References
- 1.Pathak SK, Sharma RA, Steward WP, Mellon JK, Griffiths TR, Gescger AJ. Oxidative stress and cyclooxygenase activity in prostate carcinogenesis: targets for chemopreventive strategies. Eur J Cancer. 2005;41:61–70. doi: 10.1016/j.ejca.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 2.Bostwick DG, Burke HB, Djakiew D, Euling S, Ho SM, Landolph J, Morrison H, Sonawane B, Shifflett B, Waters DJ, Tims B. Human prostate cancer risk factors. Cancer. 2004;101:2371–490. doi: 10.1002/cncr.20408. [DOI] [PubMed] [Google Scholar]
- 3.Chen JZ, Kadlubar FF. Mitochondrial mutagenesis and oxidative stress in human prostate cancer. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2004;22:1–12. doi: 10.1081/GNC-120037931. [DOI] [PubMed] [Google Scholar]
- 4.De Marzo AM, Meeker AK, Zha S, Luo J, Nakayama M, Platz EA, Isaacs WB, Nelson WG. Human prostate cancer precursors and patho-biology. Urology. 2003;62:55–62. doi: 10.1016/j.urology.2003.09.053. [DOI] [PubMed] [Google Scholar]
- 5.Thompson IM. Chemoprevention of prostate cancer: agents and study designs. J Urol. 2007;178:S9–13. doi: 10.1016/j.juro.2007.03.138. [DOI] [PubMed] [Google Scholar]
- 6.Liebler DC. The role of metabolism in the antioxidant function of vitamin E. Crit Rev Toxicol. 1993;23:147–69. doi: 10.3109/10408449309117115. [DOI] [PubMed] [Google Scholar]
- 7.Wen XQ, Li XJ, Su ZL, Liu Y, Zhou XF, Cai YB, Huang WT, Gao X. Reduced expression of alpha-tocopherol-associated protein is associated with tumor cell proliferation and the increased risk of prostate cancer recurrence. Asian J Androl. 2007;9:206–12. doi: 10.1111/j.1745-7262.2007.00236.x. [DOI] [PubMed] [Google Scholar]
- 8.Ni J, Wen X, Yao J, Chang HC, Yin Y, Zhang M, Xie S, Chen M, Simons B, Chang P, di Sant'Agnese A, Messign EM, et al. Tocopherol-associated protein suppresses prostate cancer cell growth by inhibition of the phosphoinositide 3-kinase pathway. Cancer Res. 2005;65:9807–16. doi: 10.1158/0008-5472.CAN-05-1334. [DOI] [PubMed] [Google Scholar]
- 9.Kim J, Sun P, Lam YW, Troncoso P, Sabichi AL, Babaian RJ, Pisters LL, Pettaway CA, Wood CG, Lippman SM, McDonnell TJ, Lie-berman RJ, et al. Changes in serum proteomic patterns by presurgical alpha-tocopherol and L-selenomethionine supplementation in prostate cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1697–702. doi: 10.1158/1055-9965.EPI-04-0679. [DOI] [PubMed] [Google Scholar]
- 10.Newmark HL, Huang MT, Reddy BS. Mixed tocopherols inhibit azoxymethane-induced aberrant crypt foci in rats. Nutr Cancer. 2006;56:82–5. doi: 10.1207/s15327914nc5601_11. [DOI] [PubMed] [Google Scholar]
- 11.Suh N, Paul S, Lee HJ, Ji Y, Lee MJ, Yang CS, Reddy BS, Newmark HL. Mixed tocopherols inhibit N-methyl-N-nitrosourea-induced mammary tumor growth in rats. Nutr Cancer. 2007;59:76–81. doi: 10.1080/01635580701419022. [DOI] [PubMed] [Google Scholar]
- 12.Devaraj S, Leonard S, Traber MG, Jialal I. Gamma-tocopherol supplementation alone and in combination with alpha-tocopherol alters biomarkers of oxidative stress and inflammation in subjects with metabolic syndrome. Free Radic Biol Med. 2008;44:1203–8. doi: 10.1016/j.freeradbiomed.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Helzlsouer KJ, Huang HY, Alberg AJ, Hoffmann S, Burke A, Norkus EP, Morris JS, Comstock GW. Association between alpha-tocopherol, gamma-tocopherol, selenium, and subsequent prostate cancer. J Natl Cancer Inst. 2000;92:2018–23. doi: 10.1093/jnci/92.24.2018. [DOI] [PubMed] [Google Scholar]
- 14.Jiang Q, Ames BN. Gamma-tocopherol, but not alpha-tocopherol, decreases proinflammatory eicosanoids and inflammation damage in rats. Faseb J. 2003;17:816–22. doi: 10.1096/fj.02-0877com. [DOI] [PubMed] [Google Scholar]
- 15.Jiang Q, Elson-Schwab I, Courtemanche C. Ames BN gamma-tocopherol and its major metabolite, in contrast to alpha-tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells. Proc Natl Acad Sci USA. 2000;97:11494–9. doi: 10.1073/pnas.200357097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell S, Stone W, Whaley S, Krishnan K. Development of gamma (gamma)-tocopherol as a colorectal cancer chemopreventive agent. Crit Rev Oncol Hematol. 2003;47:249–59. doi: 10.1016/s1040-8428(03)00042-8. [DOI] [PubMed] [Google Scholar]
- 17.Cooney RV, Franke AA, Harwood PJ, Hatch- Pigott V, Custer LJ, Mordan LJ. Gamma-tocopherol detoxification of nitrogen dioxide: superiority to alpha-tocopherol. Proc Natl Acad Sci USA. 1993;90:1771–5. doi: 10.1073/pnas.90.5.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gysin R, Azzi A, Visarius T. Gamma-tocopherol inhibits human cancer cell cycle progression and cell proliferation by down-regulation of cyclins. Faseb J. 2002;16:1952–4. doi: 10.1096/fj.02-0362fje. [DOI] [PubMed] [Google Scholar]
- 19.Park JH, Walls JE, Galvez JJ, Kim M, Abate-Shen C, Shen MM, Cardiff RD. Prostatic intraepithelial neoplasia in genetically engineered mice. Am J Pathol. 2002;161:727–35. doi: 10.1016/S0002-9440(10)64228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwak MK, Wakabayashi N, Kensler TW. Chemoprevention through the Keap1-Nrf2 signaling pathway by phase 2 enzyme inducers. Mutat Res. 2004;555:133–48. doi: 10.1016/j.mrfmmm.2004.06.041. [DOI] [PubMed] [Google Scholar]
- 21.Yu X, Kensler T. Nrf2 as a target for cancer chemoprevention. Mutat Res. 2005;591:93–102. doi: 10.1016/j.mrfmmm.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 22.Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med. 2004;36:1199–207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
- 23.Kode A, Rajendrasozhan S, Caito S, Yang SR, Megson IL, Rahman I. Resveratrol induces glutathione synthesis by activation of Nrf2 and protects against cigarette smoke-mediated oxidative stress in human lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2008;294:L478–88. doi: 10.1152/ajplung.00361.2007. [DOI] [PubMed] [Google Scholar]
- 24.Bostwick DG, Meiers I, Shanks JH. Glutathione S-transferase: differential expression of alpha, mu, and pi isoenzymes in benign prostate, prostatic intraepithelial neoplasia, and prostatic adenocarcinoma. Hum Pathol. 2007;38:1394–401. doi: 10.1016/j.humpath.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 25.Canada AT, Roberson KM, Vessella RL, Trump DL, Robertson CN, Fine RL. Glutathione and glutathione S-transferase in benign and malignant prostate cell lines and prostate tissues. Biochem Pharmacol. 1996;51:87–90. doi: 10.1016/0006-2952(95)02157-4. [DOI] [PubMed] [Google Scholar]
- 26.Bruno RS, Traber MG, Vitamin E. biokinetics, oxidative stress and cigarette smoking. Pathophysiology. 2006;13:143–9. doi: 10.1016/j.pathophys.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Gao L, Wang J, Sekhar KR, Yin H, Yared NF, Schneider SN, Sasi S, Dalton TP, Anderson ME, Chan JY, Morrow JD, Freeman ML. Novel n-3 fatty acid oxidation products activate Nrf2 by destabilizing the association between Keap1 and Cullin3. J Biol Chem. 2007;282:2529–37. doi: 10.1074/jbc.M607622200. [DOI] [PubMed] [Google Scholar]
- 28.Kang D, Lee KM, Park SK, Berndt SI, Peters U, Reding D, Chatterjee N, Welch R, Chanock S, Huang WY, Hayes RB. Functional variant of manganese superoxide dismutase (SOD2 V16A) polymorphism is associated with prostate cancer risk in the prostate, lung, colorectal, and ovarian cancer study. Cancer Epidemiol Biomarkers Prev. 2007;16:1581–6. doi: 10.1158/1055-9965.EPI-07-0160. [DOI] [PubMed] [Google Scholar]
- 29.Aydin A, Arsova-Sarafinovska Z, Sayal A, Eken A, Erdem O, Erten K, Ozgok Y, Dimovski A. Oxidative stress and antioxidant status in non-metastatic prostate cancer and benign prostatic hyperplasia. Clin Biochem. 2006;39:176–9. doi: 10.1016/j.clinbiochem.2005.11.018. [DOI] [PubMed] [Google Scholar]


