Abstract
Data obtained from recent studies in humans, rodents, and cell culture demonstrate that circulating maternal cholesterol can be transported to the fetus. The two major cell types responsible for the transport are trophoblasts and endothelial cells of the fetoplacental vasculature. Maternal lipoprotein-cholesterol is initially taken up by trophoblasts via receptor-mediated and receptor-independent processes, is transported by any number of the sterol transport proteins expressed by cells, and is effluxed or secreted out of the basal side via protein-mediated processes or by aqueous diffusion. This cholesterol is then taken up by the endothelium and effluxed to acceptors within the fetal circulation. The ability to manipulate the mass of maternal cholesterol that is taken up by the placenta and crosses to the fetus could positively impact development of fetuses affected with the Smith-Lemli-Opitz Syndrome (SLOS) that have reduced ability to synthesize cholesterol and possibly impact growth of fetuses unaffected by SLOS but with low birthweights.
Keywords: Smith-Lemli-Opitz Syndrome, fetus, trophoblast, placenta, yolk sac
1. Introduction
Cholesterol is required for embryonic and fetal development. It is present in every cellular membrane, for membrane integrity and to maintain cholesterol-rich microdomains that are key for most membrane-associated signaling cascades. Cholesterol is also a precursor for steroid hormones and is essential for activation of various signaling pathways, including sonic hedgehog. A significant amount of cholesterol is synthesized by the fetus. The fetus appears to have an exogenous source of cholesterol as well, one that is not synthesized within the fetus itself. This cholesterol would be obtained from external sources, specifically the maternal circulation via the placenta. The maternal plasma cholesterol would need to be taken up by the placenta, transported across trophoblasts and then endothelial cells of the fetoplacental vasculature, and effluxed or secreted into the fetal circulation. This review article will discuss the routes by which cholesterol that originates in the maternal circulation can be transported to the fetus. We will also discuss the roles of cholesterol in development, including how a lack of exogenous (maternal-derived) and endogenous (de novo synthesis) cholesterol affects the fetus.
2. Transport of maternal cholesterol to the fetus by the placenta
A number of studies have been completed which have demonstrated the ability of maternal cholesterol to be transported to the fetus. Importantly, mass cholesterol is present in fetuses unable to synthesize cholesterol in humans (1, 2) as well as mice (3). Even if fetuses can synthesize cholesterol, maternal cholesterol is transported to the fetus (4, 5); studies used radiolabeled LDL- and HDL-cholesteryl ester (low density lipoprotein and high density lipoprotein) or stably labeled cholesterol. There are two layers of cells that maternal cholesterol must cross to reach the fetal circulation. The layer closest to the maternal circulation is the trophoblast. The layer between the trophoblasts and fetal circulation is the endothelium.
2.1. Uptake and transport across trophoblast
Trophoblast takes up maternal cholesterol-carrying lipoproteins. In most mammals, the majority of cholesterol is carried in the circulation as LDL or HDL. The placenta expresses the receptors that take up LDL and HDL, those being the LDL receptor and SR-BI (scavenger receptor class B type I), respectively (6–8). Not unexpectedly, both LDL- and HDL-cholesterol have been shown to be taken up by placenta or trophoblast of rodents and humans (4, 7, 9, 10). After receptor-mediated uptake of LDL, the particle is transported to the lysosome/endosome pathway where the cholesteryl ester is hydrolyzed as in any cell type. The free cholesterol is transported across the cells to metabolically active pools or membranes via Niemann-Pick C1 (NPC1) or other less well defined sterol carrier proteins. SR-BI selectively takes up cholesteryl ester primarily of HDL. The ester is hydrolyzed by a cytosolic esterase, and transported across the cells to the basal membrane via potential sterol carrier proteins. Though the precise route of transcellular transport is unknown, a number of the transporters could be involved; the placenta expresses NPC1, NPC1L1, ABCA2, and SCP-x (4).
2.2. Efflux and secretion from trophoblast
There are several routes by which cholesterol can exit cells in general and trophoblast specifically [reviewed in (11–15)]. First, lipoprotein particles can be secreted from cells as occurs in the liver and small intestine. Placental explants do indeed secrete lipoprotein particles (16). Second, cholesterol can be complexed with apolipoproteins and secreted from cells. Trophoblast does indeed secrete apoE (17). Finally, a number of cell types efflux membrane cholesterol to various acceptors, including HDL, lipid-poor apoAI, and phospholipid discs or vesicles, either via aqueous diffusion or via SR-BI, ABCA1, or ABCG1. Since the placenta expresses these three proteins, aqueous diffusion and protein-mediated export is also feasible.
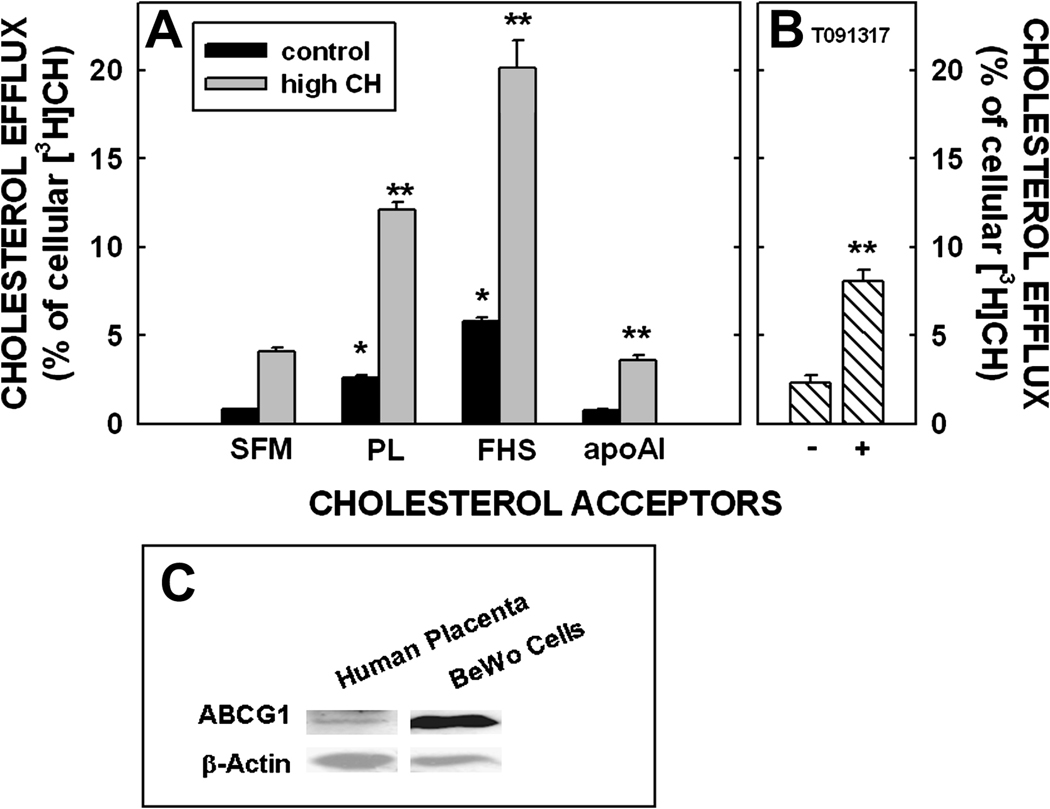
To study sterol efflux from trophoblasts, we used a cultured choriocarcinoma cell line (BeWo cells) as our model. BeWo cells synthesize placental hormones and express trophoblast proteins [reviewed in (18, 19)]. Subclone 30 of BeWo cells becomes a confluent monolayer that is polarized when grown on transwells allowing for the study of unilateral transport across a polarized cell. In all studies, cells were grown to confluence, and incubated with LDL radiolabeled with cholesteryl ester. After removal of apical LDL, various acceptors were added to the basal side as described (18). The percent cholesterol effluxed is the radiolabel in the basal media as a percentage of radiolabel taken up by the cells. Cells were incubated with serum-free media (SFM), with phospholipid discs, and with apoAI; ABCA1 and ABCG1 efflux cholesterol to lipid poor apoAI and phospholipid discs, respectively. A small amount of cholesterol was always exported to serum-free media, which may indicate secretion of cholesterol from cells. More cholesterol was effluxed to phospholipid discs as compared to that secreted to SFM only. In contrast, a similar amount of cholesterol was effluxed/secreted to apoAI as to SFM. When incubated with LDL to increase cholesterol concentration (18), the amount of cholesterol effluxed/secreted to each acceptor increased significantly (Fig. 1A). Though direct incubation with oxysterols, a downstream product of cholesterol catabolism, did not affect efflux from cells (18), there was an increase in efflux to phospholipid discs after 6 h when cells were preincubated with 5 µM LXR agonist (T091317) for 24 h (Fig. 1B). As expected from these data, ABCG1 was present in BeWo cells (Fig. 1C); we did not test if ABCA1 was present in cells since there was no transfer of cholesterol to apoAI greater than that secreted to SFM. Since efflux was more pronounced when phospholipid discs were used as acceptors and since SR-BI is expressed on the apical side of cells (20), the efflux from the membranes on the basal side of this trophoblast model appears to be diffusional or mediated by ABCG1, which is localized on the basal side of trophoblast (21) (Fig. 2). It might be surprising that there was no increase in efflux of cholesterol to apoAI in BeWo cells, an ABCAI-mediated process, since ABCA1 is expressed within membranes on the fetal-facing side of trophoblast (22), is involved with sterol efflux from primary trophoblasts (not polarized) (21), and since mice lacking ABCA1 have reduced transfer to the placenta (23). Though the BeWo cells have many features similar to human trophoblasts and they have the added benefit of becoming polarized on transwells, they are not the same as placenta-derived trophoblasts and can differ in some aspects of metabolism, as any cell culture system. More studies will need to be completed to verify the involvement or lack of involvement of ABCA1 in cholesterol efflux from the basal side of trophoblasts and the expression of ABCA1 by BeWo cells.
Figure 1.
Efflux of cholesterol from the basal surface of BeWo cells to various acceptors under different conditions. The apical side of the cells were labeled with [3H]cholesteryl ester-labeled LDL for 24 h. A). After labeling, the cells were washed and the basal side was incubated with serum free media (SFM), phospholipid discs (PL; 100 µg/ml), fetal human serum (FHS; 10%) or lipid-poor apoAI (50 µg/ml) for 24 h. The amount of [3H] effluxed was presented as a percentage of cellular [3H] at 0 h. Data are presented for cells with normal cholesterol concentrations (open bar) and concentrations ≈2-fold greater (hatched bar). This figure has been modified and redrawn (18) with permission of the publisher. B). After labeling, cells were incubated with an LXR agonist (T091317) and efflux measured using phospholipid discs; - (without T091317) and + (with T091317). C). Western blot for ABCG1 using equal amounts of protein from human placentas or confluent BeWo cells and using β-actin as a loading control; differences in expression likely due to multiple cell types in the human placentas.
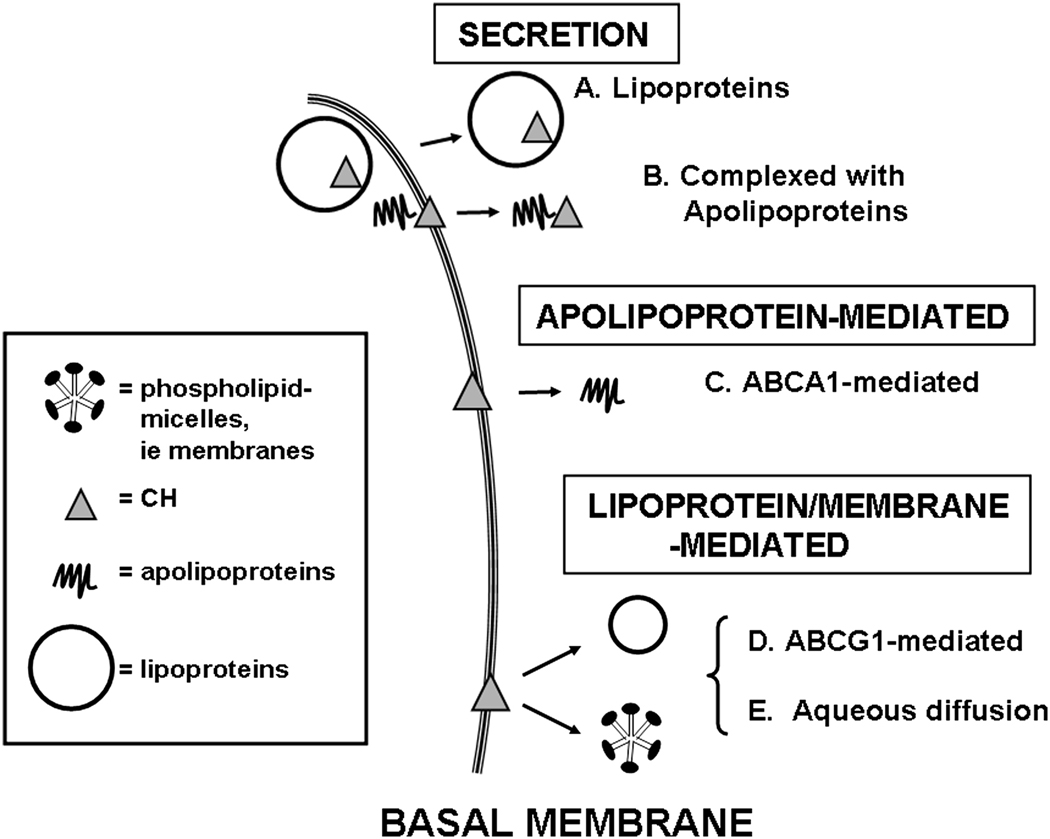
Figure 2.
Proposed model for secretion and efflux of cholesterol out of trophoblasts. Cholesterol can be secreted from cells as lipoproteins (A) or complexed with apolipoproteins (B). There can be apolipoprotein-mediated efflux via ABCA1 (C) as well as lipoprotein/membrane-mediated efflux via ABCG1 (D) and aqueous diffusion (E).
2.3. Efflux and secretion from endothelium of fetoplacental vasculature
Once the cholesterol is secreted/effluxed from the basal side of the trophoblast, it must reach and then cross the endothelial cells of the fetoplacental vasculature prior to entering the fetal circulation. Late in gestation, the transport of cholesterol between vasculosyncytial membranes is likely. Earlier in gestation, cholesterol would be effluxed or secreted into stroma and then transverse to the endothelial cells where it would be taken up. Using human placental endothelial cells (HPEC), it has also been shown that exogenous cholesterol is secreted/effluxed to various acceptors (24). The process is tightly regulated by cellular cholesterol and LXR activity through mediation of ABCA1 and ABCG1, and is most efficient when fetal-like apoE-rich HDL is used as acceptors (24). A possible other route of export from the PEC is based on the fact that other endothelial cells transport lipoproteins across cells (25). It seems that this could occur in HPEC since the human placenta does indeed secrete newly synthesized lipoproteins (16).
3. Role of cholesterol in fetal development
The developmental processes affected by cholesterol throughout gestation are numerous. Very early in gestation, cholesterol is needed to activate sonic hedgehog, a protein involved in neural patterning and forebrain development (26). A lack of sonic hedgehog activity leads to abnormal mid-line facial features and other major congenital defects. Though it was shown that holoprosencephaly can be associated with abnormal sonic hedgehog (27), holoprosencephaly is now also associated with abnormal sterol metabolism (28). Cholesterol is critical to development throughout pregnancy as well due to the fact that it is a part of every membrane and is enriched in lipid rafts, the origin of a number of signaling cascades. Since most signaling pathways are needed for growth, proliferation, and metabolism, membrane function is essential for normal development to proceed.
4. Impact on human health
Knowing that the fetus synthesizes cholesterol at relatively elevated rates (29), why is it important to study cholesterol transport across the placenta? First, not all fetuses synthesize cholesterol. There are seven known defects in the cholesterol biosynthetic pathway [reviewed in (30)]. Six of the defects are extremely rare, and are often lethal. The seventh and most common defect leads to the Smith-Lemli-Opitz Syndrome (SLOS), also referred to as the RSH Syndrome. The defect occurs in the last step of the pathway where 7-dehydrocholesterol is converted to cholesterol; the enzyme that catalyzes this reaction is Δ7-dehydrocholesterol reductase (Δ7 reductase) (31). The carrier frequency of this mutation is 1 in 30 (32). Since the number of reported cases (1 in 20,000 to 60,000) is much less than the expected 1 in 1,590 to 13,500, either an increased number of early spontaneous abortions from an extreme phenotype and/or the misdiagnosing of individuals with a very mild phenotype must occur.
Fetuses with SLOS have a spectrum of defects from mild to severe. In fact, the severity of the syndrome can be correlated to plasma cholesterol concentrations (33–35). Newborns can have subtle learning disorders, such as autism, and minor dysmorphic features, to severe mental retardation and major congenital abnormalities [reviewed in (30)]. Though some of the defects are consistent with those of abnormal sonic hedgehog activity (26), other signaling defects also come into play (36), especially those affected by abnormal membrane structure and function detected in tissues of SLOS individuals (37–40) which includes most signaling cascades.
Since the severity of the syndrome appears to be correlated to plasma cholesterol levels, it seems likely that the ability to increase the transport of maternal cholesterol to the fetus would improve the outcome of pregnancy. In humans, heterozygous mothers with an apoE2 allele have infants with a more severe SLOS phenotype as compared to mothers without the apoE2 allele (41); apoE2 binds defectively to the LDL receptor (42), which is abundant in the placenta (7, 10). The paternal genotype has no impact upon severity of SLOS. These data strongly suggest a decrease in lipoprotein uptake by the placenta leads to a more severe phenotype. Likewise, in SLOS rodents, the ability to increase fetal plasma cholesterol will improve fetal development (43) and when maternal cholesterol uptake by the placenta is reduced, abnormal fetal development is more pronounced (44). Increases in transport must occur early in gestation when sonic hedgehog is initially expressed as well as throughout gestation to assure proper membrane-mediated signaling. Though it is not a simple task to increase plasma maternal LDL- and HDL-cholesterol levels, changes can be accomplished with diet and exercise. Chylomicron cholesterol changes readily with dietary cholesterol, however. Since the placenta expresses a number of apoE receptors [reviewed in (13)], it is possible that cholesterol-carrying apoE-containing remnants are taken up by the placenta and are an additional source of exogenous cholesterol. A second route to increase cholesterol transport would be to change the expression of lipoprotein receptors and/or ABC transporters.
It is unclear what the role of maternal cholesterol might have when the fetus is able to synthesize its own cholesterol. Since at least some of the cholesterol transport and efflux is protein-mediated, cholesterol will still cross the placenta in humans, as shown in rodents. When maternal plasma cholesterol is low (<160 mg/dl), birthweights are lower than normal and there is a trend for microcephaly (45). Mice with low plasma cholesterol also have fetuses with reduced growth rates (46). In addition, women with infants that have intrauterine growth restriction have lower plasma cholesterol levels (47, 48). Thus, even if the infant is synthesizing cholesterol, a lack of transfer of maternal cholesterol may impact growth. The effect could be directly on the fetus since cholesterol can affect the cell cycle and is required for membrane formation and cell signaling of various growth factors, including insulin. The effect could also be indirect by affecting placental nutrient transport via a change in membrane signaling pathways. Transport of too much cholesterol can have adverse consequences, however. In humans, fetuses of hypercholesterolemic mothers have an increase of aortic fatty streaks (49). In fetal rodents exposed to maternal hypercholesterolemia, newborns mature to animals with an increased risk to develop coronary artery disease (50).
Thus, while it seems beneficial to enhance sterol transport in infants affected by SLOS that are unable to make cholesterol, it may be advisable to limit transport in unaffected infants exposed to maternal hypercholesterolemia. There also appears to be a role of maternal cholesterol in fetal growth of unaffected fetuses as well, though the mechanism is still unknown and recommendations can not be made. Much work is still needed to delineate the mechanism responsible for the movement of cholesterol from the maternal to fetal cholesterol, and to define any additional roles of maternal cholesterol in fetal development.
Acknowledgements
I would like to thank the past and present individuals in my laboratory who were involved in these studies, especially Dr. Kara Schmid. I also would like to thank the NIH for their support of these studies (HD34089).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
There was no conflict of interest with relation to these studies.
References
- 1.Linck LM, Hayflick SJ, Lin DS, Battalie KP, Ginat S, Burlingame T, Gibson KM, Honda M, Honda A, Salen G, Tint GS, Connor WE, Steiner RD . Fetal demise with Smith-Lemli-Opitz syndrome confirmed by tissue sterol analysis and the absence of measurable 7-dehydrocholesterol Δ7-reductase activity in chorionic villi. Prenat. Diagn. 2000;20:238–240. [PubMed] [Google Scholar]
- 2.Nowaczyk MJM, Farrell SA, Sirkin WL, Velsher L, Krakowiak PA, Waye JS, Porter FD. Smith-Lemli-Opitz (RHS) syndrome: Holoprosencephaly and homozygous IVS8-1G to C genotype. Am. J. Med. Genet. 2001;103:75–80. doi: 10.1002/1096-8628(20010915)103:1<75::aid-ajmg1502>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 3.Tint GS, Yu H, Shang Q, Xu G, Patel SB. The use of the Dhcr7 knockout mouse to accurately determine the origin of fetal sterols. J. Lipid Res. 2006;47:1535–1541. doi: 10.1194/jlr.M600141-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burke KT, Colvin PL, Myatt L, Graf GA, Schroeder F, Woollett LA. Transport of maternal cholesterol to the fetus is affected by maternal plasma cholesterol concentrations in the Golden Syrian hamster. J. Lipid Res. 2009;50:1146–1155. doi: 10.1194/jlr.M800538-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshida S, Wada Y. Transfer of maternal cholesterol to embryo and fetus in pregnant mice. J. Lipid Res. 2005;46:2168–2174. doi: 10.1194/jlr.M500096-JLR200. [DOI] [PubMed] [Google Scholar]
- 6.Wadsack C, Hirschmugl B, Maier A, Hiden U, Desoye G. The placental scavenger receptor class B type-I (SR-BI) undergoes spatio-developmental changes in human pregnancy. Placenta. 2005;26:A49. [Google Scholar]
- 7.Wyne KL, Woollett LA. Transport of maternal LDL and HDL to the fetal membranes and placenta of the Golden Syrian hamster is mediated by receptor-dependent and receptor-independent processes. J. Lipid Res. 1998;39:518–530. [PubMed] [Google Scholar]
- 8.Shi W, Swan KF, Lear SR, O'Neil JS, Erickson SK, Henson MC. Regulation of pathways determining cholesterol availability in the baboon placenta with advancing gestation. Biol. Reprod. 1999;61:1499–1505. doi: 10.1095/biolreprod61.6.1499. [DOI] [PubMed] [Google Scholar]
- 9.Wadsack C, Hammer A, Levak-Frank S, Desoye G, Kozarsky KF, Hirschmugl B, Sattler W, Malle E. Selective cholesteryl ester uptake from high density lipoprotein by human first trimester and term villous trophoblast cells. Placenta. 2003;24:131–143. doi: 10.1053/plac.2002.0912. [DOI] [PubMed] [Google Scholar]
- 10.Winkel CA, MacDonald PC, Simpson ER. The role of receptor-mediated low-density lipoprotein uptake and degradation in the regulation of progesterone biosynthesis and cholesterol metabolism by human trophoblasts. Placenta. 1981;6(suppl):133–143. [PubMed] [Google Scholar]
- 11.Oram JF, Vaughan AM. ATP-Binding cassette cholesterol transporters and cardiovascular disease. Circ. Res. 2006;99:1031–1043. doi: 10.1161/01.RES.0000250171.54048.5c. [DOI] [PubMed] [Google Scholar]
- 12.Zhao Y, Van Berkel TJ, Van Eck M. Relative roles of various efflux pathways in net cholesterol efflux from macrophage foam cells in atherosclerotic lesions. Curr. Opin. Lipidol. 2010;21:441–453. doi: 10.1097/MOL.0b013e32833dedaa. [DOI] [PubMed] [Google Scholar]
- 13.Woollett LA. Maternal cholesterol in fetal development: transport of cholesterol from the maternal to the fetal circulation. Am. J. Clin. Nutr. 2005;82:1155–1161. doi: 10.1093/ajcn/82.6.1155. [DOI] [PubMed] [Google Scholar]
- 14.Duong M, Collins HL, Jin W, Zanotti I, Favari E, Rothblat GH. Relative contributions of ABCA1 and SR-BI to cholesterol efflux to serum from fibroblasts and macrophages. Arter. Thromb. Vasc. Biol. 2006;26:541–547. doi: 10.1161/01.ATV.0000203515.25574.19. [DOI] [PubMed] [Google Scholar]
- 15.Adorni MP, Zimetti F, Bilheimer JT, Wang N, Rader DJ, Phillips MC, Rothblat GH. The roles of different pathways in the release of cholesterol from macrophages. J. Lipid Res. 2007;48:2453–2462. doi: 10.1194/jlr.M700274-JLR200. [DOI] [PubMed] [Google Scholar]
- 16.Madsen EM, Lindegaard MLS, Andersen CB, Damm Pl, Nielsen LB. Human placenta secretes apolipoprotein B-100-containing lipoproteins. J. Biol. Chem. 2004;279:55271–55276. doi: 10.1074/jbc.M411404200. [DOI] [PubMed] [Google Scholar]
- 17.Rindler MJ, Traber MG, Esterman AL, Bersinger NA, Dancis J. Synthesis and secretion of apolipoprotein E by human placenta and choriocarcinoma cell lines. Placenta. 1991;12:615–624. doi: 10.1016/0143-4004(91)90496-3. [DOI] [PubMed] [Google Scholar]
- 18.Schmid KE, Davidson WS, Myatt L, Woollett LA. The transport of cholesterol across a BeWo cell monolayer: Implications for net transport of sterol from the maternal to fetal circulation. J. Lipid Res. 2003;44:1909–1918. doi: 10.1194/jlr.M300126-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Liu F, Soares MJ, Audus KL. Permeability properties of monolayers of the human trophoblast cell line BeWo. Am. J. Physiol. 1997;273:C1596–C1604. doi: 10.1152/ajpcell.1997.273.5.C1596. [DOI] [PubMed] [Google Scholar]
- 20.Hatzopoulos AK, Rigotti A, Rosenberg RD, Krieger M. Temporal and spatial pattern of expression of the HDL receptor SR-BI during murine embryogenesis. J. Lipid Res. 1998;39:495–508. [PubMed] [Google Scholar]
- 21.Aye IL, Waddell BJ, Mark PJ, Keelan JA. Placental ABCA1 and ABCG1 transporters efflux cholesterol and protect trophoblasts from oxysterol induced toxicity. Biochim. Biophys. Acta. 2010;1801:1013–1024. doi: 10.1016/j.bbalip.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 22.Bhattacharjee J, Ietta F, Giacomello E, Bechi N, Romagnoli R, Fava A, Paulesu L. Expression and localization of ATP binding cassette transporter A1 (ABCA1) in first trimester and term human placenta. Placenta. 2010;31:423–430. doi: 10.1016/j.placenta.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 23.Lindegaard ML, Wassif CA, Varisman B, Amar M, Wasmuth EV, Shamburek R, Nielsen LB, Remaley AT, Porter FD. Characterization of placental cholesterol transport: ABCA1 is a potential target for in utero therapy of Smith-Lemli-Opitz syndrome. Hum. Mol. Genet. 2008;17:3806–3813. doi: 10.1093/hmg/ddn278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sfefuji J, Panzenboeck U, Becker T, Hirschmugl B, Schweinzer C, Lang I, Marsche G, Sadjak A, Lang U, Desoye G, Wadsack C. Human endothelial cells of the placental barrier efficiently deliver cholesterol to the fetal circulation via ABCA1 and ABCG1. Circ. Res. 2009;104:600–608. doi: 10.1161/CIRCRESAHA.108.185066. [DOI] [PubMed] [Google Scholar]
- 25.Cavelier C, Rohrer L, von Eckardstein A. ATP-Binding cassette transporter A1 modulates apolipoprotein A-I transcytosis through aortic endothelial cells. Circ. Res. 2006;99:1060–1066. doi: 10.1161/01.RES.0000250567.17569.b3. [DOI] [PubMed] [Google Scholar]
- 26.Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Wesphal H, Beachy PA. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- 27.Roessler E, Belloni E, Gaudenz K, Jay P, Berta P, Scherer SW, Tsui LC, Muenke M. Mutations in the human Sonic Hedgehog gene cause holoprosencephaly. Nature Genet. 1996;14:357–360. doi: 10.1038/ng1196-357. [DOI] [PubMed] [Google Scholar]
- 28.Haas D, Morgenthaler J, Lacbawan F, Long B, Runz H, Garbade SF, Zschocke J, Kelley RI, Okun JG, Hoffmann GF, Muenke M. Abnormal sterol metabolism in holoprosencephaly: studies in cultured lymphoblasts. J. Med. Genet. 2007;44:298–305. doi: 10.1136/jmg.2006.047258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dietschy JM, Turley SD, Spady DK. Role of liver in the maintenance of cholesterol and low density lipoprotein homeostasis in different animal species, including humans. J. Lipid Res. 1993;34:1637–1659. [PubMed] [Google Scholar]
- 30.Porter FD. Human malformation syndromes due to inborn errors of cholesterol synthesis. Curr. Opin. Pediatr. 2003;15:607–613. doi: 10.1097/00008480-200312000-00011. [DOI] [PubMed] [Google Scholar]
- 31.Tint GS, Irons M, Elias ER, Batta AK, Frieden R, Chen TS, Salen G. Defective cholesterol biosynthesis associated with the Smith-Lemli-Opitz syndrome. N. Eng. J. Med. 1994;330:107–113. doi: 10.1056/NEJM199401133300205. [DOI] [PubMed] [Google Scholar]
- 32.Battaile KP, Battaile BC, Merkens LS, Maslen CL, Steiner RD. Carrier frequency of the common mutation IVS8-1G>C in DHCR7 and estimate of the expected incidence of Smith-Lemli-Opitz syndrome. Mol. Genet. Metab. 2001;72:67–71. doi: 10.1006/mgme.2000.3103. [DOI] [PubMed] [Google Scholar]
- 33.Cunniff C, Kratz LE, Moser A, Natowicz MR, Kelley RI. Clinical and biochemical spectrum of patients with RSH/Smith-Lemli-Opitz syndrome and abnormal cholesterol metabolism. Am. J. Med. Genet. 1997;68:263–269. [PubMed] [Google Scholar]
- 34.Tint GS, Salen G, Batta AK, Shefer S, Irons M, Elias ER, Abuelo DN, Johnson VP, Lambert M, Lutz R. Correlation of severity and outcome with plasma sterol levels in variants of the Smith-Lemli-Opitz syndrome. J. Pediatr. 1995;127:82–87. doi: 10.1016/s0022-3476(95)70261-x. [DOI] [PubMed] [Google Scholar]
- 35.Witsch-Baumgartner M, Fitzky BU, Ogorelkova M, Kraft HG, Moebius FF, Glossmann H, Seedorf U, Gillessen-Kaesbach G, Hoffmann GF, Clayton P, Kelley RI, Utermann G. Mutational spectrum in the Δ7-sterol reductase gene and genotype-phenotype correlation in 84 patients with Smith-Lemli-Opitz syndrome. Am. J. Hum. Genet. 2000;66:402–412. doi: 10.1086/302760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lanoue L, Dehart DB, Hinsdale ME, Maeda N, Tint GS, Sulik KK. Limb, genital, CNS, and facial malformations result from gene/environment-induced cholesterol deficiency: Further evidence for a link to sonic hedgehog. Am J. Med. Genet. 1997;73:24–31. [PubMed] [Google Scholar]
- 37.Keller RK, Arnold TP, Fliesler SJ. Formation of 7- dehydrocholesterol-containing membrane rafts in vitro and in vivo, with relevance to the Smith-Lemli-Opitz syndrome. J. Lipid Res. 2004;45:347–355. doi: 10.1194/jlr.M300232-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tulenko TM, Boeze-Battaglia K, Mason RP, Tint GS, Steiner RD, Connor WE, Labelle EF. A membrane defect in the pathogenesis of the Smith-Lemli-Opitz syndrome. J. Lipid Res. 2006;47:134–143. doi: 10.1194/jlr.M500306-JLR200. [DOI] [PubMed] [Google Scholar]
- 39.Jiang X-S, Wassif CA, Backlund PS, Long L, Holtzclaw LA, Li Z, ergey AL, Porter FD. Activation of Rho GTPases in Smith-Lemli-Opitz synrome: patholphysiological and clinical implications. Hum. Mol. Genet. 2010;19:1347–1357. doi: 10.1093/hmg/ddq011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gondré-Lewis MC, Petrache HI, Wassif CA, Harrles D, Parsegian A, Porter FD, Loh YP. Abnormal sterols in cholesterol-deficiency diseases cause secretory granule malformation and decreased membrane curvature. J. Cell. Sci. 2006;119:1876–1885. doi: 10.1242/jcs.02906. [DOI] [PubMed] [Google Scholar]
- 41.Witsch-Baumgartner M, Gruber M, Kraft HG, Rossi M, Clayton P, Giros M, Haas D, Kelley RI, Krajewska-Walasek M, Utermann G. Maternal apo E genotype is a modifier of the Smith-Lemli-Opitz syndrome. J. Med. Genet. 2004;41:577–584. doi: 10.1136/jmg.2004.018085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Utermann G. Apolipoprotein E polymorphism in health and disease. Am. Heart. J. 1987;113:433–440. doi: 10.1016/0002-8703(87)90610-7. [DOI] [PubMed] [Google Scholar]
- 43.Yu H, Li M, Tint GS, Chen J, Xu G, Patel SB. Selective reconstitution of liver cholesterol biosynthesis promotes lung maturation but does not prevent neonatal lethality in Dhcr7 null mice. BMC Dev. Biol. 2007;7:27. doi: 10.1186/1471-213X-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Solcà C, Pandit B, Yu H, Tint GS, Patel SB. Loss of apolipoprotein E exacerbates the neonatal lethality of the Smith-Lemli-Opitz syndrome mouse. Mol. Genet. Metab. 2007;91:7–14. doi: 10.1016/j.ymgme.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edison RJ, Berg K, Remaley A, Kelley R, Rotimi C, Stevenson RE, Muenke M. Adverse birth outcome among mothers with low serum cholesterol. Pediatrics. 2007;120:723–733. doi: 10.1542/peds.2006-1939. [DOI] [PubMed] [Google Scholar]
- 46.McConihay JA, Honkomp AM, Granholm NA, Woollett LA. Maternal high density lipoproteins affect fetal mass and extra-embryonic fetal tissue sterol metabolism in the mouse. J. Lipid Res. 2000;41:424–432. [PubMed] [Google Scholar]
- 47.Sattar N, Greer IA, Galloway PJ, Packard CJ, Shepherd J, Kelly T, Mathers A. Lipid and lipoprotein concentrations in pregnancies complicated by intrauterine growth restriction. J. Clin. Endocrinol. Metab. 1999;84:128–130. doi: 10.1210/jcem.84.1.5419. [DOI] [PubMed] [Google Scholar]
- 48.Wadsack C, Tabano S, Maier A, Hiden U, Alvino G, Cozzi V, Huttinger M, Schneider WJ, Lang U, Cetin I, Desoye G. Intrauterine growth restriction (IUGR) is associated with alterations in placental lipoprotein receptors and maternal lipoprotein composition. Am. J. Physiol. 2007;292:E476–E484. doi: 10.1152/ajpendo.00547.2005. [DOI] [PubMed] [Google Scholar]
- 49.Napoli C, D'Armiento FP, Mancini FP, Postiglione A, Wiztum JL, Palumbo G, Palinski W. Fatty streak formation occurs in human fetal aortas and is greatly enhanced by maternal hypercholesterolemia. Intimal accumulation of LDL and its oxidation precede monocyte recruitment into early atherosclerotic lesions. J. Clin. Invest. 1997;100:2680–2690. doi: 10.1172/JCI119813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palinski W, D'Armiento FP, Witztum JL, deNigris F, Casanada F, Condorelli M, Silvestre M, Napoli C. Maternal hypercholesterolemia and treatment during pregnancy influence the long-term progression of atherosclerosis in offspring of rabbits. Cir. Res. 2001;89:991–996. doi: 10.1161/hh2301.099646. [DOI] [PubMed] [Google Scholar]