Abstract
Lipids are necessary for every step in the replication cycle of hepatitis C virus (HCV) and dengue virus (DENV), members of the family Flaviviridae. Recent studies have demonstrated that discrete steps in the replication cycles of these viruses can be inhibited by pharmacological agents that target host factors mediating lipid synthesis, metabolism, trafficking, and signal transduction. Despite this, targeting host lipid metabolism and trafficking as an antiviral strategy by blockade of entire pathways may be limited due to host toxicity. Knowledge of the molecular details of lipid structure and function in replication and the mechanisms whereby specific lipids are generated and trafficked to the relevant sites may enable more targeted antiviral strategies without global effects on the host cell. In this review, we discuss lipids demonstrated to be critical to the replication cycles of HCV and DENV and highlight potential areas for anti-viral development. This review article forms part of a symposium on flavivirus drug discovery in Antiviral Research.
1. Introduction
Specific lipids from multiple lipid classes are central to every step in the replication cycle of the family Flaviviridae, including during viral entry, translation and replication of the viral genome, and assembly and egress of progeny virions (Fig. 1). The Flaviviridae are the causative agents for various acute and chronic disease worldwide, including dengue hemorrhagic fever (dengue virus), viral hepatitis C (hepatitis C virus), yellow fever (yellow fever virus), as well as encephalitis caused by multiple members of the Flavivirus genus within the Flaviviridae family, including West Nile virus, tickborne encephalitis virus, Japanese encephalitis virus, amongst others.
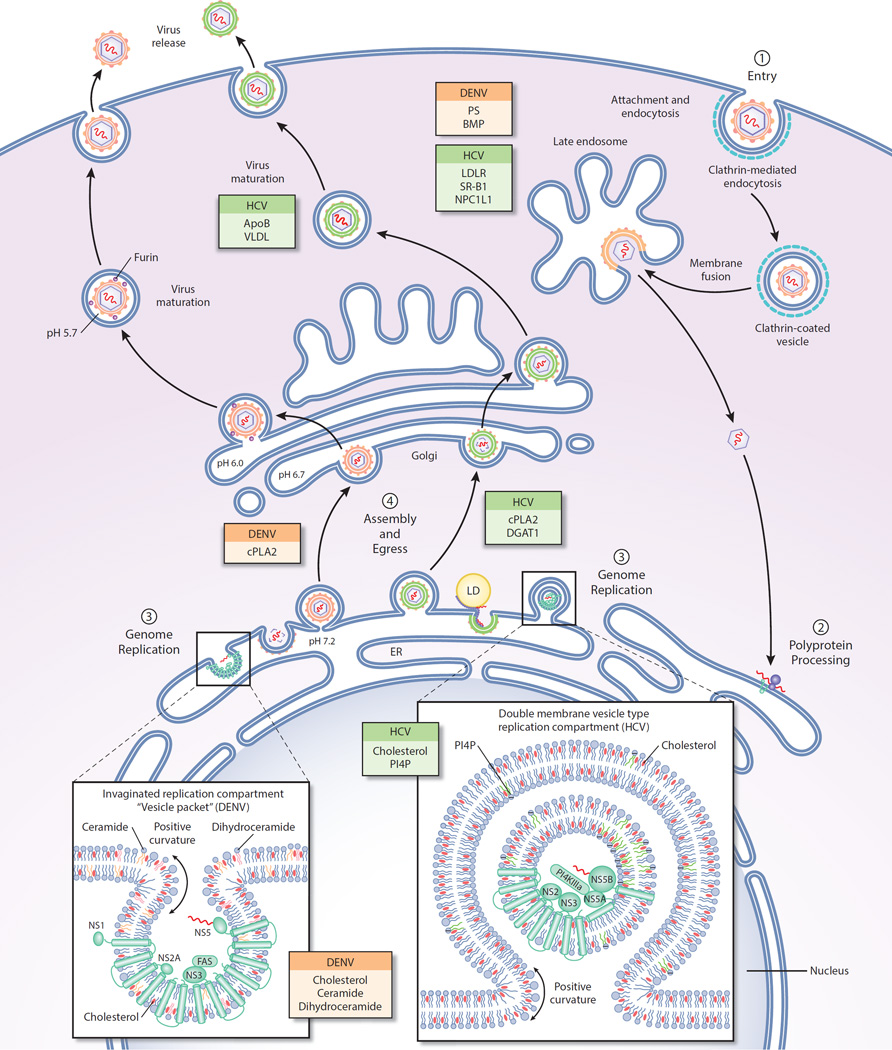
Figure 1.
Association of viral replication with subcellular membranes during replication. Colored boxes represent lipids or lipid-associated proteins shown to be important to HCV or DENV at each step in replication. (1) DENV and HCV enter cells via clathrin-mediated endocytosis. Viral genome release occurs after fusion of the viral and endosomal membranes at acidic pH in the endosome. (2) Viral genome is transported to the ER membrane where viral proteins are translated. (3) Genome replication occurs on membranous structures induced by virus-induced perturbations of the ER membrane. HCV genome replication occurs in double-membrane vesicle replication factories. DENV genome replication occurs within invaginations of the ER membrane. (4) Both HCV and DENV particles are known to associate with lipid droplets (LDs) during assembly although the role of specific lipids in assembly and egress is poorly understood. DENV and HCV acquire their lipid envelope by budding through the ER membrane. Both DENV and HCV are trafficked through the Golgi before secretion of mature virions through the plasma membrane. HCV has also been shown to acquire some of its viral membrane at the plasma membrane.
The interaction of the Flaviviridae with host lipids and host lipid metabolism is the subject of this review, with particular focus on dengue virus (DENV) and hepatitis C virus (HCV). Since an exhaustive review of current literature in this area of research is limited by space constraints, pharmacological agents approved for use in humans and that may inhibit HCV by directly or indirectly target lipid and lipid-associated pathways are summarized in Table 1. Since there are to our knowledge no clinical studies assessing the effect of host lipid-targeting agents against DENV, Table 2 summarizes work in which small molecule probes have been used to study the role of host lipid biosynthesis, metabolism, and trafficking in Flavivirus replication. These summaries together highlight the potential to target host lipid metabolism as a countermeasure against pathogens in the Flavivirus and Hepacivirus genera.
Table 1.
Inhibition of hepatitis C virus with known drugs and probes.
| Target | Drug/Probe | Viral process | references |
|---|---|---|---|
| NPC1L1, cholesterol synthesis | Ezetimibe | HCV entry | (Sainz et al., 2012) |
| PI4KIIIalpha | Compound A and B* | RNA replication via MW formation | (Vaillancourt et al., 2012) |
| HMG-CoA | Fluvastatin, atorvastatin, simvastatin | Entry | (Bader et al., 2008; Forde et al., 2009; Ikeda et al., 2006; O'Leary et al., 2007) |
| Lipid peroxidation | Polyunsaturated fatty acids | RNA replication | (Yamane et al., 2014) |
These kinase inhibitors block HCV replication in cell culture, but suitability of these probes for use in humans has not been reported.
Table 2.
Pharmacological probes of host pathways of lipid synthesis, trafficking, and metabolism with anti-flavivirus activity.
| Virus | Function | Antiviral activity of inhibitor |
References |
|---|---|---|---|
| Dengue virus, Japanese encephalitis virus | Modulation of cholesterol synthesis and trafficking affects viral entry | Cholesterol | (Lee et al., 2008a; Tani et al., 2010) |
| Japanese encephalitis virus | Plasma membrane ceramides are required for entry | Amitriptyline, imipramine (sphingomyelinase inhibitors); C6-ceramide | (Lee et al., 2008a; Tani et al., 2010) |
| Dengue virus, Japanese encephalitis virus, West Nile virus | Modulation of cholesterol synthesis and trafficking affects entry and replication | Hemaglusin (HMG CoA synthase inhibitor); fluvastatin, lovastatin, pravastatin (HMG CoA reductase inhibitors); nystatin (cholesterol chelator); zaragozic acid (Squalene synthase inhibitor); U18666A (inhibitor of squalene oxidase, desmosterol Δ24reductase, and cholesterol trafficking); AY9944 (7-dehydrocholesterol Δ7-reductase inhibitor) | (Lee et al., 2008a; Martinez-Gutierrez et al., 2014; Pena and Harris, 2012; Poh et al., 2012; Rothwell et al., 2009; Soto-Acosta et al., 2013; Tani et al., 2010) |
| Dengue virus | Cholesterol-rich rafts implicated in polyprotein processing and replication | None tested | (Garcia Cordero et al., 2014) |
| West Nile virus | protein geranylgeranylation required for viral replication | GGTI (geranylgeranylation inhibitor) | (Mackenzie, Khromykh, and Parton, 2007) |
| Dengue virus | Fatty acid synthase is required for replication and recruited via interaction with NS3 in a Rab18-dependent manner | Fatty acid synthase inhibitors: C75, cerulenin | (Heaton et al., 2010; Perera et al., 2012; Poh et al., 2012; Samsa et al., 2009; Soto-Acosta et al., 2014; Tang et al., 2014) |
| Dengue virus | Lipid droplets (LD) and association of LD with core protein in viral assembly | Fatty acid synthase inhibitor, Nordihydroguaiaretic acid (target undefined) | (Samsa et al., 2009; Soto-Acosta et al., 2014; Teoh et al., 2014) |
| West Nile Virus | Sphingolipids implicated in virion morphogenesis/release | GW4869, spiroepoxide (neutral sphingomyelinase inhibitors) | (Martin-Acebes et al., 2014) |
Note that some compounds on this list are multi-targeted, and others affect the homeostasis of multiple lipid classes; moreover, some lipids function in multiple viral processes. Unequivocal attribution of an antiviral activity to a specific molecular target and lipid (or lipid class) is therefore non-trivial. This is an on-going area of active investigation.
2. Targeting cellular lipid pathways
Strategies to inhibit viral pathogens by manipulating the host targets on which they depend are attractive due to their potential for broad spectrum activity against multiple viruses within the same genus or family and for having high barriers to resistance compared to virally-targeted agents. Rational design of selective host-targeted antivirals, while avoiding host toxicity, can conceivably be achieved by identification and targeting of lipids that are not required for host cell functions and/or by targeting steps in lipid synthesis, metabolism, or trafficking for which viruses have a greater sensitivity than the host cell, thus allowing for a reasonable therapeutic window. For example, the discovery of an increase in malonyl-coenzyme A (malonyl-CoA, precursor in fatty acid synthesis) in human cytomegalovirus (HCMV)-infected cells revealed that cellular fatty acid synthase (FAS) is necessary for HCMV replication. Pharmacological inhibition of FAS with C75 reduced HCMV replication by 2 log10 unit and was shown to be non-toxic to cells at the concentrations tested against viral infection (Munger et al., 2008), providing proof of concept that a therapeutic window can exist for even one of the most central pathways in lipid metabolism. In most cases, however, a better fundamental understanding of lipid structure and function in viral replication is likely needed to design and develop antivirals that target host pathways of lipid synthesis, metabolism, trafficking, and signal transduction.
Although many host lipid metabolic pathways were first identified and characterized over 70 years ago, only recently have they been examined in the context of viral infection to reveal novel host-virus interactions that are essential for viral replication. There are several known functions that host lipids serve in the replication of many viruses. First, lipid membranes are structural components of all enveloped virions. Second, lipid bilayers often serve as platforms for the chemical reactions that comprise viral replication, including viral gene expression, replication of the viral genome, and viral assembly. Localization of these viral processes to membranes compartmentalizes them, thus protecting them from host defense systems. In addition, membranes increase the efficiency of viral processes by concentrating reactants and catalysts and also by scaffolding molecular interactions. The functions of membranes in biological processes are dictated by the physicochemical properties of their component lipids, including but not limited to membrane curvature and fluidity (Fig. 2). Third, lipid-mediated signal transduction is an important regulator of the host-response to viral infection as well as viral pathogenesis.
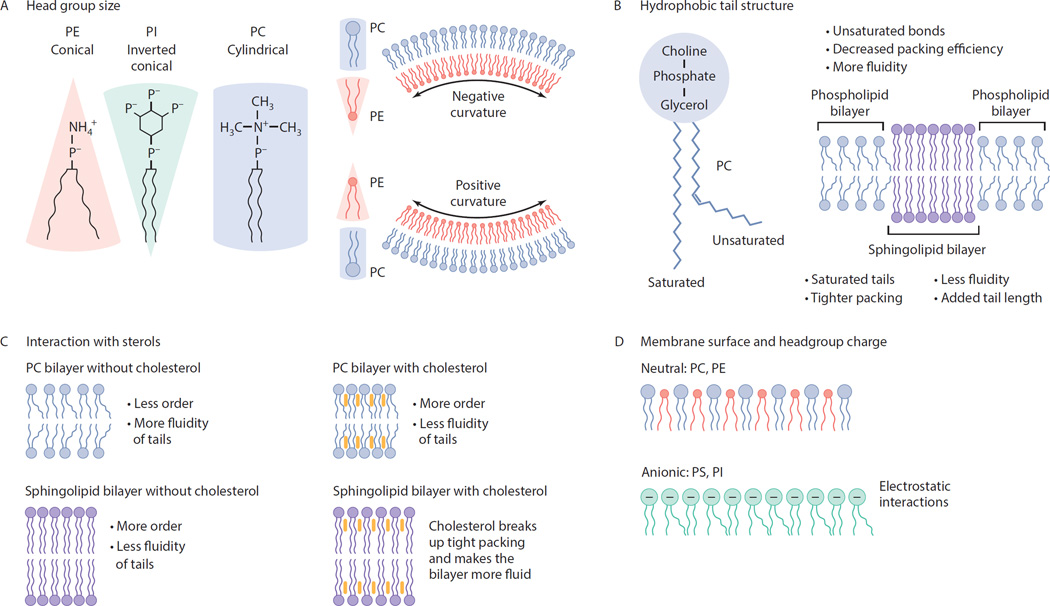
Figure 2.
How lipid structure alters intrinsic membrane properties. (A) Headgroup size and tail saturation influence the surface area that a lipid occupies in a membrane. A lipid with a small headgroup (PE) and unsaturated tails forms a conical shape. Large headgroups (PI) with saturated tails form an inverted cone. Large headgroups with saturated or singly unsaturated side chains have a cylindrical shape (PC). Lipids with small headgroups will tend to localize in regions of negative curvature, whereas species with large headgroups localize in regions of positive curvature. (B) Acyl chain saturation and tail length alter membrane mobility, membrane thickness, and domain formation. Double bonds decrease packing efficiency and increase membrane mobility. Sphingolipids with saturated tails decrease mobility, increase packing efficiency, and promote formation of ordered domains. Mixtures of sphingolipids and phospholipids can phase separate into highly ordered and disordered domains within cellular membranes. Increasing acyl chain length increases membrane thickness and can lead to hydrophobic mismatch between domains, thus affecting protein localization. (C) Interaction with sterols. Cholesterol alters membrane mobility and packing. Increasing cholesterol content reduces fluidity and increases membrane ordering in the presence of phospholipids, but has the opposite effect upon interaction with the longer, saturated tail groups of sphingolipids. (D) Membrane surface and headgroup charge. Surface charge is determined by the properties of the headgroup. Zwitterionic lipid headgroups (PC, PE) compromise a high percentage of cellular membranes, while anionic lipids (PS, PI) induce electrostatic interactions but are present in membranes in lower quantities. Charged surfaces in turn affect membrane curvature as well as interaction with proteins (e.g., PS-binding proteins, PI-binding proteins).
To develop antiviral strategies that inhibit these viral processes via more narrowly focused effects on specific host lipid synthesis, metabolism, and trafficking targets, we must first define the specific lipids that function in viral processes. Virologists have traditionally inferred functional significance of lipids via detection of virus-induced changes in the expression of host factors that mediate lipid synthesis, metabolism, and trafficking. Since these proteins generally act on entire classes of lipid molecules, studies that interpret changes in host gene expression have generally been unable to provide details about the specific lipid molecules involved in viral replication. It simply is not possible to infer how changes in host gene expression or protein abundance affect the specific lipid structures produced. Likewise, RNA interference (RNAi) studies showing the antiviral effects caused by depletion of relevant host factors do not provide structural data on the specific lipid molecules required for viral replication.
Since direct methods for examining how viral pathogens affect lipid homeostasis or correlating changes in lipid abundance with antiviral activity have not been widely accessible, most of our knowledge has been limited to how classes of lipids (e.g., fatty acids, sterols, sphingolipids) may function in viral replication. As with other types of proteins and other biomolecules, however, even small changes in structure can result in significant changes in biological function (Fig. 3). For example, phosphatidylinositol (4, 5) bisphosphate is an activator of several sodium channels (Hilgemann, Feng, and Nasuhoglu, 2001), while phosphatidylinositol (3, 4, 5) triphosphate, which differs by one additional phosphate group, is a second messenger molecule that activates the AKT and other signaling pathways (Newton, 2009). These two molecules, while structurally very similar, cannot substitute for each other functionally. Likewise, cis versus trans isomers of the same fatty acid side chain can have significantly different effects on lipid bilayer mobility (Fig. 2). The significant impact of even subtle structural changes on function makes it necessary to resolve and examine specific lipid molecules rather than groups of lipids or entire classes of lipids in order to study how they function in viral replication.
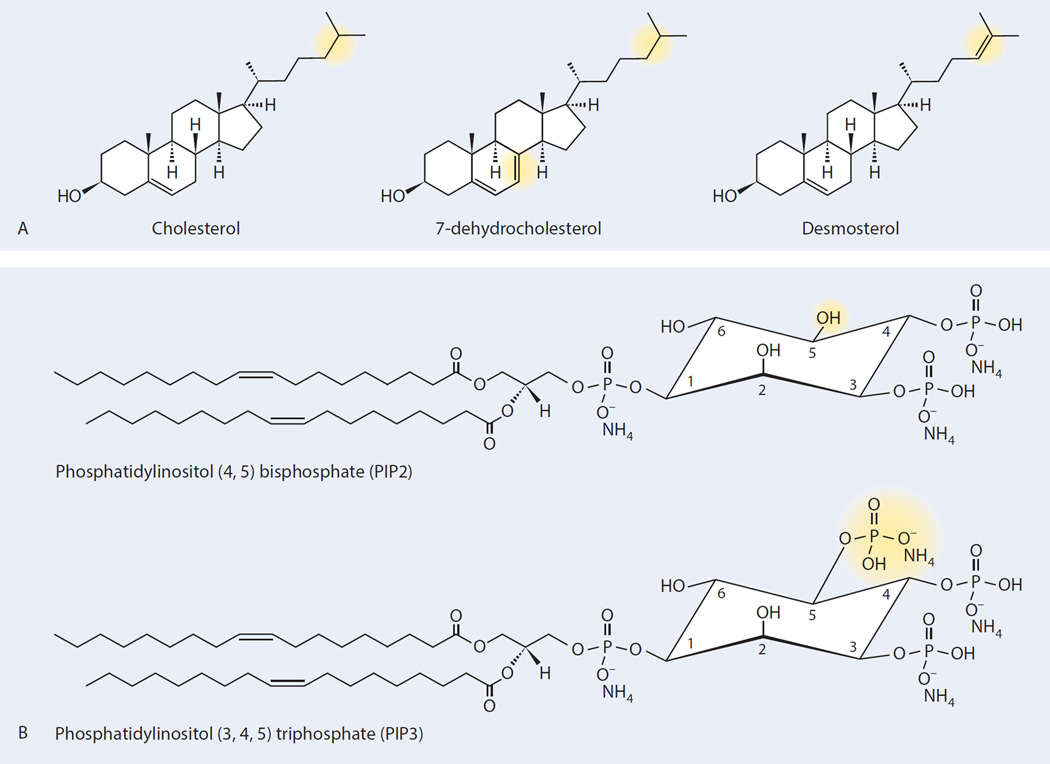
Figure 3.
Subtle structural differences in lipid molecules (highlighted in yellow) affect biological function. (A) Desmosterol differs from cholesterol by the presence of a double bond in the tail of the molecule. In cell culture, HCV JFH1 infection increases steady-state desmosterol levels in Huh 7.5 cells 10-fold without changes in cholesterol or other sterols (Munger et al., 2008; Rodgers et al., 2012). Inhibition of desmosterol synthesis blocks viral replication. Rescue of this antiviral effect occurs to differing extents upon the addition of exogenous desmosterol versus exogenous cholesterol or 7-dehydrocholesterol even though these molecules have similar (cholesterol) or identical (desmosterol, 7-dehydrocholesterol), molecular weights and differ only by location of a double bond. (B) Phosphatidylinositol (4,5) bisphosphate (PIP2) differs from phosphatidylinositol (3,4,5) triphosphate (PIP3) by the addition of a phosphate group. The additional phosphorylation imparts markedly different biological functions. PIP2 activates sodium channels, while PIP3 is involved in many signally pathways including the AKT pathway.
The International Committee for the Classification and Nomenclature of Lipids (ICCNL) has named 8 lipid classes in biology (Table 3) (Fahy et al., 2011; Fahy et al., 2005; Fahy et al., 2009). “Global” lipidomic analysis of all eight lipid classes has posed a significant challenge versus comparable analyses of other biopolymers (e.g., nucleic acids, proteins) due to diversity in the structure and biosynthetic mechanisms of lipids, the number of distinct lipid substances within cells, the dynamic range of lipid concentrations in biological samples (bioactive lipids present at attomolar concentrations to fatty acids present at nanomolar concentrations), and the inability to define the absolute structure of a lipid molecule by simply measuring its molecular weight. Typically, lipids are extracted from biological samples into an organic solvent chosen based on the type of biological sample and the metabolite to be quantified in downstream analysis. Traditional methods of analysis have included separation and analysis of retention times on chromatography substrates, thin layer chromatography, or nuclear magnetic resonance. The most powerful tool for lipid analysis today is mass spectrometry (MS) coupled with liquid or gas chromatography (Han and Gross, 2005; Ivanova et al., 2009; Rolim et al., 2015), and recent studies demonstrate the power of this approach in studying how viral replication and host lipids intersect (Perera et al., 2012; Rodgers, Saghatelian, and Yang, 2009; Rodgers et al., 2012; Tanner et al., 2014).
Table 3.
Classification of lipids.
| Lipid class | Basic features |
|---|---|
| Fatty acids (FA) | Fatty acids (FAs) are long hydrophobic carbon chains with a polar head group (Figure 2.) that are further categorized by the number of carbon atoms comprising the aliphatic tail (short: <6 carbon atoms, medium: 6–12 carbon atoms, or long: >12) and whether the aliphatic tail contains C-C double bonds. As an energy source for the cell, their synthesis and oxidation of the β-carbon (β-oxidation) are highly regulated. However, FAs can also be obtained via metabolic by-products, directly through dietary intake, as well as through the catabolism of glycerolipids. The diverse FAs available in the cell hint at the various roles FAs play. In addition to their role as a crucial energy source, FAs have been shown to be important for signaling, prostaglandin formation, and membrane fluidity. Notably, a major constituent of plasma and organelle membranes is the phosphorylated fatty acid (phospholipid). |
| Glycerolipids (GL) and glycero-phospholipids (GP) | Glycerolipids and glycerophospholipids share a common glycerol backbone; however, GLs contain one to three fatty acids covalently attached to the glycerol backbone via an ester bond (mono-, di-, and triglycerides). Hydrolysis of these ester bonds yields free FAs that can undergo β-oxidation and produce ATP in the mitochondria. For GPs, one alcohol of the core glycerol molecule forms phosphate ester linkage. The simplest GP is phosphatidic acid. Additional substituents on the phosphate yield, respectively, phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine (PS), and phosphatidylinositol (PI). Mammalian membranes primarily contain other common GPs: PC, PE, PS, and PI. |
| Sphingolipids (SP) | Sphingolipids are fatty acids that are amide-linked to a sphingoid base that bears a long-chain hydrocarbon. The archetype sphingolipid is ceramide, which is also the central precursor for other sphingolipids such as sphingosines and sphingomyelin. Sphingosines are important for cell to cell signaling and cellular adherence to matrices. Sphingomyelins are critical for protection of nerve cells via formation of the axon-insulator, the myelin sheath, and have been shown to be reduced in multiple sclerosis. Sphingomyelins have also been shown to interact with cholesterol to modulate lipid bilayer fluidity, particularly at the plasma membrane. |
| Sterol lipids (ST) | Sterol lipids are tetracyclic (A, B, C, D rings) in structure, with a hydroxyl group attached to the A ring and an aliphatic chain attached to the D ring. Derived from condensation of isoprene subunits, sterol lipids are critical for membrane architecture and formation of lipid-ordered domains (“lipid rafts”), which are important for signaling and dynamic movement in the cell. Additionally, the sterol lipid backbone is the precursor to steroid hormones and vitamin D. The archetype sterol found in most cellular membranes cholesterol, which has been shown to be critical for the entry and replication of many viruses. In addition, biosynthetic precursors of cholesterol, such as desmosterol, have also been shown to be important for sterol regulation by binding to and activating liver X receptors, and these signal transducing functions may also affect other viral processes |
| Prenols (PR), saccharolipids (SL) and polyketide (PK) lipids | Similar to sterol lipids, prenol lipids are derived from condensation of isoprene subunits. Notably, prenol lipids are precursors to vitamins A, E, and K, and also function as antioxidants. Polyprenols have been shown to be important for shuttling oligosaccharides across cell membranes. Polyketides are derived from condensation of ketoacyl units and are commonly found in bacteria and fungi. They are synthesized by multi-domain protein complexes composed of polyketide synthases, which bear similarities to fatty acid synthases (FAS). Many polyketides have anti-bacterial or anti-fungal properties. Saccharolipids are similiar to glycerolipids, however the sugar backbone has replaced the glycerol backbone. There is minimal literature regarding the antiviral activity of prenols, saccharolipids, and polyketides; however, some polyketides have been reported to inhibit influenza virus. |
| Bioactive lipids | Lipids are generally identified as sources of energy or components to define cellular membranes. However, some lipids are important regulators or mediators of cellular pathways and signaling events. Bioactive lipids include members of the aforementioned lipid classes and generally contain fatty acids. Identified bioactive lipids have been categorized into three groups: diacylglycerols (DAG), eicosanoids, and sphingolipids. DAGs, an intermediate in the catabolism of glycerolipids, have been shown to be important for protein kinase C regulation. Eicosanoids are metabolites of twenty-carbon polyunsaturated fatty acids. Arachidonic acid (AA), which is metabolized to prostaglandins, the archetype eicosanoid, is of particular importance because prostaglandins can stimulate inflammation, thereby inducing an immune response. Additional bioactive lipids derived from AA and other polyunsaturated fatty acids also have pro- and anti-inflammatory activities important in the host response to viral infection. Finally, sphingolipids participate in wide array of cellular pathways. Notably, sphingosine-1-phosphate and ceramide have been shown to be important for inflammation and apoptosis, respectively. |
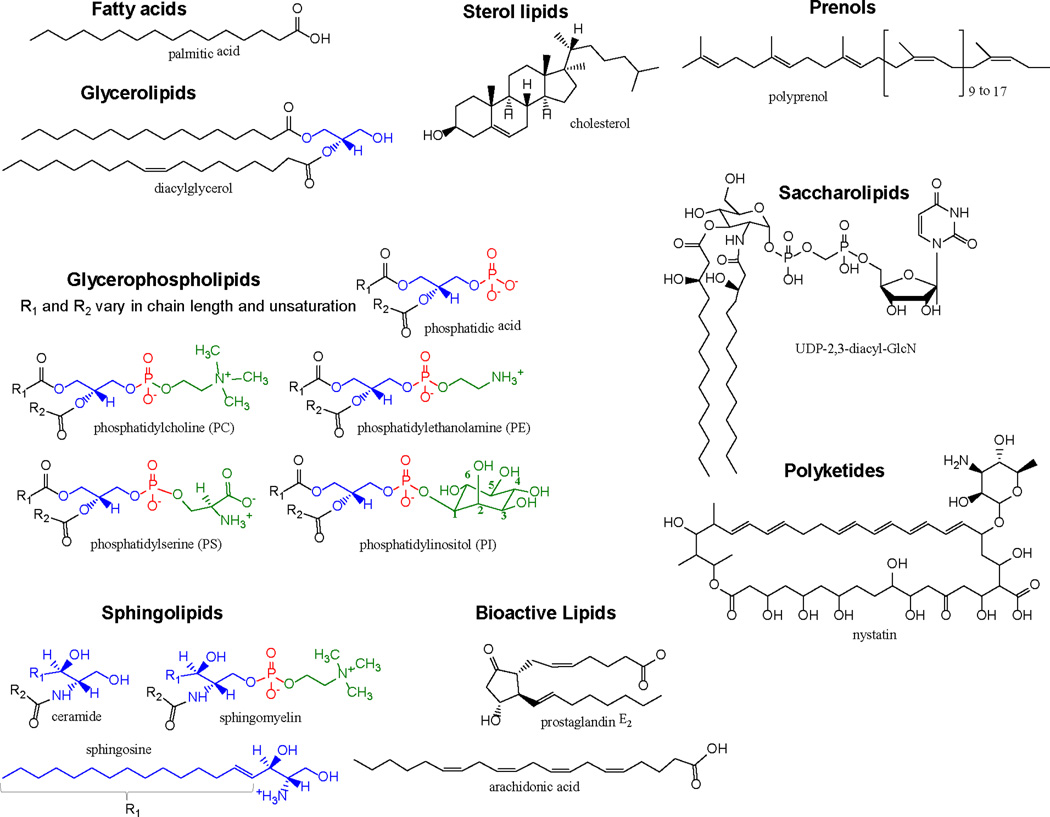
The International Lipid Classification and Nomenclature Committee (ILCNC) developed a “Comprehensive Classification System for Lipids” to include specific chemical characteristics for their widely-accepted definition of lipids. For brevity, lipids are hydrophobic or amphipathic molecules that are generally insoluble in aqueous solution. The ILCNC divided lipids into eight major classes by the chemical condensation of ketoacyl thioesters or isoprene groups. Representative structures for each lipid class are presented in Fig. 4.
3. Lipids involved in the DENV and HCV replication cycles
Viral entry
DENV, a member of the genus Flavivirus, and HCV, a member of the genus Hepacivirus, have plus-sense RNA genomes of 10- to 11-kilobases in length that share a common genomic organization and that are protected by a lipid membrane. The first step in the replication cycle is entry of the virus and delivery of the viral genome to the host cytosol. For DENV and other members of the Flavivirus genus, this entails clathrin-mediated uptake of the virion following contact with attachment factors on the cell surface and then subsequent fusion of the viral lipid membrane with the host endosomal membrane.
As with other enveloped viruses, the efficiency of the fusion process is likely affected by the lipid content of the entering virion as well as by the host target membrane (Zaitseva et al., 2010). Membrane fusion is catalyzed by structural changes in the viral surface glycoprotein, E, in response to exposure to acidic pH. While the early endosomal pH is sufficient to trigger the structural changes in E that catalyze fusion of DENV virions with liposomes in vitro, live cell imaging experiments (van der Schaar et al., 2008; van der Schaar et al., 2007) indicate that the nucleocapsid is released from Rab7-positive late endosomes in vivo. This suggests that fusion during infection is more complex and likely requires additional physiological triggers, including, perhaps, the direct involvement of specific lipids in the target membrane. Consistent with this idea, negatively charged lipids like phosphatidylserine or bis(monoacylglycero)phosphate (BMP) increase the fusion of dengue virus with synthetic liposomes (Nour et al., 2013; Zaitseva et al., 2010), and these lipids are abundant within the internal membranes of the late endosome and lysosome but lacking in the internal membranes of the early endosome. Delaying viral fusion until anionic lipids are encountered in the late endosome may ensure that viral RNA is efficiently delivered to the translation and replication machinery.
HCV virions have been shown to associate with several host lipoproteins. Interactions with apolipoprotein A-I (apoA-I), apoB-48, apoB-100, apoC-I, and apoE (Benga et al., 2010; Catanese et al., 2013; Diaz et al., 2006; Dreux et al., 2007; Hishiki et al., 2010; Mancone et al., 2011), lead to lower buoyant densities due to the formation of “lipoviral particles” that protect virions from the humoral immune response of the host while also mediating viral entry, as evidenced by the high specific infectivity of virions in lipoviral particles (Catanese et al., 2013). Although HCV’s entry mechanism(s) are complex and still not fully elucidated, host lipid trafficking proteins including the low-density lipoprotein receptor (LDLR) (Albecka et al., 2012; Monazahian et al., 1999), scavenger receptor class B member 1 (SRB1) (Bartosch et al., 2005; Kapadia et al., 2007), and Niemann-Pick C1-like protein (NPC1L1) (Sainz et al., 2012) are clearly required. DENV infectivity is similarly enhanced when the virus is preincubated with apoA-1 and is decreased upon knockdown of SRB1(Li et al., 2013); however, the mechanistic basis of these effects has been less studied. LDLR’s interaction with HCV-associated apoE provides an initial low-affinity interaction that concentrates virions on the cell surface (Owen et al., 2009). SRB1 functions at multiple points in HCV entry: the interaction of SRB1 with the viral E2 glycoprotein contributes to the attachment step, while its lipid transfer activity is thought to facilitate subsequent release of the virion from the lipoviral particle and its interaction with the HVR region of E2, thereby inducing conformational changes that enable E2’s interaction with CD81, a cell-surface protein identified as a co-receptor for HCV entry (Bartosch et al., 2005; Cormier et al., 2004; Pileri et al., 1998). The precise function of the cholesterol-binding protein NPC1L1 in HCV entry is not understood; however, NPC1L1’s natural function is in cholesterol absorpation and uptake (Zhang et al., 2011), and pharmacological agents that block cholesterol-trafficking by NPC1L1 inhibit HCV entry. Together, these observations suggest that NPC1L1 may be involved in trafficking of HCV to the endosomal compartment. Thus, although ezetimibe was originally approved by the Federal Drug Administration (FDA) to treat high cholesterol levels, repurposing of this NPC1L1 inhibitor shows promise as a pan-genotype anti-HCV therapeutic (Sainz et al., 2012).
Viral gene expression and genome replication
Translation and co-translational processing of viral polyproteins occur on the ER membrane or membranes derived from the ER, which is the site of biosynthesis of glycerophospholipids, sterols, ceramides, and glycosphingolipids (Table 3). The ER membrane itself has relatively low cholesterol and sphingolipid content due to rapid transport of these lipid classes to other subcellular locations. This contributes to relatively loose packing of lipids in the ER membrane, which likely has functional significance since newly synthesized proteins and lipids insert into the ER membrane before being transported to other subcellular locations. These attributes of relatively loose lipid packing and high mobility may likewise be important for efficient co-translational processing of the DENV and HCV polyproteins by host peptidases and the DENV NS2B/NS3 and HCV NS3/NS4A proteases, respectively, although we are unaware of experiments that have explicitly examined this possibility.
For DENV, convoluted membranes (CMs) in infected Huh7 cells have been shown to contain the viral NS2B/3 protease but no other detectable viral proteins (Junjhon et al., 2014). While this suggests that CMs may be the site of polyprotein processing, the absence of CMs in DENV-infected mosquito cells (Junjhon et al., 2014) makes it unclear whether these structures play a specialized role in DENV translation or polyprotein processing. Regardless of whether CMs are a functional structure for DENV polyprotein processing or a by-product of viral infection, the distinct morphological differences between CM and the adjacent ER membrane must reflect differences in their respective lipid and/or protein content. Thus, characterization of the chemical composition of CMs may provide insights into their function, the biochemical mechanisms leading to their formation, and whether specific inhibition of enzymes leading to their formation might mediate antiviral activity.
Replication of DENV and HCV RNA genomes occurs in specialized organelles derived from the ER or Golgi membranes (Figure 1). For DENV, the compartments – dubbed “vesicle packets” (VP) – appear as invaginations of the ER membrane, approximately 90 nm-wide and containing NS5, the RNA-dependent RNA polymerase, as well as nonstructural proteins 2B, 3, 4A, and 4B (NS2B, NS3, NS4A, NS4B) along with double-stranded RNA (Grief et al., 1997; Welsch et al., 2009). A pore of approximately 10-nm width appears to connect the interior of the VP with the cytosol, and presumably permits trafficking of NTPs and newly synthesized viral genomes into and out of the VP, respectively. For HCV, the replication compartments appear as double-membrane vesicles (DMVs) with an average diameter of 150 nm (Romero-Brey et al., 2012). In contrast to DENV VPs, DMVs appear to be protrusions of the ER membrane into the cytosol, and it remains unclear whether RNA replication occurs on the interior of the DMVs or on the outer surface.
Formation of both DENV VPs and HCV DMVs requires regions of both negative and positive membrane curvature, which may be induced by proteins embedded in the membrane and/or by the physicochemical properties of the lipids forming the bilayer (Figure 2). Notably, biochemically isolated replication membranes for both DENV and HCV are enriched in sphingolipids and sterols relative to the ER membrane from which they are derived (Merz et al., 2011; Perera et al., 2012). These lipids may function in inducing curvature, formation of ordered domains, and/or in specific recruitment of host or viral proteins to the site of genome replication. Indeed, cholesterol may be important for assembly of the DENV RNA replication complex, perhaps due to the formation of cholesterol-rich micro domains within the ER. Consistent with this, pharmacological inhibition of squalene synthase and HMG-CoA synthase, two enzymes necessary for cholesterol biosynthesis, inhibited live DENV in cell culture (Rothwell et al., 2009). It is also worth noting, however, that inhibition of squalene synthase and HMG-CoA synthase perturb isoprenoid biosynthesis. Consequently, the antiviral activity of compounds targeting these enzymes may also be due to their effects on protein lipidation, perhaps by interfering with recruitment of necessary factors to the membranes where DENV genome replication occurs.
Indeed, since inhibition of a DENV replicon by RNAi against mevalonate diphospho decarboxylase (MVD) was not rescued by the individual addition of cholesterol, farnesyl pyrophosphate, or geranylgeranyl pyrophosphate, this suggests that multiple branches of isoprenoid biosynthesis and metabolism have distinct roles in DENV RNA replication. In addition, mass spectrometry-based lipidomic analysis DENV replication membranes isolated from infected mosquito cell line C6/36 revealed that these membranes are enriched in long-chain ceramides and dihydroceramides (Perera et al., 2012), which are sphingolipid-class lipids (Table 3). Whether ceramide production modulates DENV replication in vivo remains to be seen since inhibition of ceramide biosynthesis was recently reported to enhance DENV replication in cell culture (Aktepe, Pham, and Mackenzie, 2015). In model membranes, these lipids have been shown to induce negative membrane curvature and to alter the size and shape of lipid-ordered domains, leading to the formation of much larger rafts that protrude up to two nanometers above the surrounding phospholipid bilayer (Ira and Johnston, 2008; Ira et al., 2009; Johnston and Johnston, 2006). An understanding of how the physico-chemical properties of these lipids are important for the formation of VPs and/or the assembly of the macromolecular replicase complex therein remains to be determined.
HCV has, in turn, been shown to cause significant perturbations of cellular lipid trafficking, leading to budding of ER vesicles collectively known as a membranous web (MW) (Egger et al., 2002; Romero-Brey et al., 2012). The MW is thought to be a vesicular scaffold from which functional RCs are generated (Romero-Brey et al., 2012). Formation of RCs requires synthesis of phosphatidylinositol-4-phosphates (PI4P lipids), members of the glycerophospholipid class (Table 3) (Berger et al., 2009; Reiss et al., 2011). The intracellular abundance of PI4P lipids is enriched three-fold in HCV-infected cells and is specifically enriched at sites where replication of the HCV genome occurs (Reiss et al., 2011). Notably, the HCV NS5a protein is sufficient to relocalize PI4KIIIalpha to HCV RCs and to increase enzymatic activity of this complex (Reiss et al., 2011), and replicon resistance to inhibitors of PI4KIIIalpha maps to the C-terminus of NS4b and N-terminus of NS5a. While the presence of PI4KIIIalpha alone could be necessary and sufficient for HCV replication, the finding that a concomitant decrease in viral protein with a PI4KIIIalpha inhibitor suggests that its product, a PI4P lipid, is necessary (Berger et al., 2009).
Consistent with models in which NS5a-mediated recruitment of PI4KIIIalpha and synthesis of PI4P lipids are critical for HCV RNA replication, the MW does not form in the absence of PI4KIIIalpha, and HCV RNA replication is inhibited upon RNAi-mediated depletion, pharmacological inhibition, or mutation of the active site of PI4KIIIalpha, but not the closely related PI4KIIIbeta enzyme (Berger et al., 2009; Reiss et al., 2011). While NS5a’s biochemical function(s) remain poorly understood, modulation of its phosphorylation state (phosphorylated versus hyperphosphorylated) has been shown to regulate HCV replication (Evans, Rice, and Goff, 2004; Masaki et al., 2008; Quintavalle et al., 2007) as well as its interaction with PI4KIIIalpha, suggesting a relationship between these two phenomena. Pharmacological inhibition of NS5a hyperphosphorylation reduces its interaction with PI4KIIIalpha, resulting in NS5a clustering and relocalization, and a reduction in PI4P lipids in replication-associated membranes (Berger et al., 2014; Chukkapalli et al., 2015; Lee et al., 2011; Reghellin et al., 2014).
Although attempts to pharmacologically inhibit PI4KIIIalpha as an antiviral strategy appear to have stalled due to toxicity (Vaillancourt et al., 2012), the mechanism(s) of action of NS5a inhibitors may include their downstream effects on the recruitment of PI4KIIIalpha to replication membranes and synthesis of PI4P lipids at this site. Consistent with this, abrogation of HCV replication achieved by blocking formation of the MW (Berger et al., 2014) was observed with daclatasvir (formerly BMS-790052, currently in Phase 3 trials and approved for the European market) (Chukkapalli et al., 2015) and BMS-553 (a daclatasvir-derivative) (Berger et al., 2014).
Although much remains to be learned regarding the relationship between lipid structure and function in DENV and HCV replication membranes, the idea that genome replication compartments have a functionally specialized lipid composition is consistent with the observation that formation of these compartments often coincides with specific perturbations of the lipid biosynthesis and trafficking machinery of the host. Our understanding of the specific lipids required for biogenesis of replication membranes, RCs or MWs, will be greatly improved as technological advances arise to distinguish similar lipids within a given class (e.g., PI4P lipids).
Viral assembly
Budding of DENV particles is thought to occur at ER sites proximal to VPs, which have been visualized via ultrastructural methods (Welsch et al., 2009). Lipid droplets – cytoplasmic organelles that store neutral lipids such as cholesteryl and other sterol esters— have been known to play a critical function in HCV particle assembly for some time (McLauchlan et al., 2002; Miyanari et al., 2007). In contrast, evidence for their role in DENV replication has only recently been forthcoming (Carvalho et al., 2012; Martins et al., 2012; Samsa et al., 2009). Trafficking of the HCV core protein to lipid droplets requires MAP kinase-regulated cytosolic phospholipase A2 (cPLA2) (Menzel et al., 2012) and diacylglycerol O-acetyltransferase (Herker et al., 2010). Pharmacological inhibition of either enzyme leads to inhibition of HCV assembly. cPLA2 catalyzes the lipolysis of arachidonic acid (AA), a fatty acid-class lipid (Table 3). While both HCV and DENV appear to differ in their requirement for AA, inhibition of cPLA2 with pyrrolidine-2 significantly reduced the infectivity of both DENV and HCV (Menzel et al., 2012).
Pyrrolidine-2 appears to block HCV assembly by inhibiting the association of core protein with lipid droplets (Menzel et al., 2012). Although the effect of pyrrolidine-2 on the analogous association of DENV core with lipid droplets was not assessed, evidence suggesting an analogous mechanism of inhibition includes the observation of impaired DENV particle assembly but not impaired viral RNA synthesis when the interaction of DENV core protein with lipid droplets is disrupted using a peptide mimic (Martins et al., 2012).
Viral budding/egress
HCV particle secretion is linked to cholesterol secretion by host lipoproteins. The heterogeneous pool of HCV virions in patient serum have a wide range of buoyant densities; only the low density viral particles are infectious (Agnello et al., 1999; Andre et al., 2002). These particles are characterized by very low density lipoprotein (VLDL) markers. The detergent-resistant interaction between HCV and one of these markers, ApoB, suggests that the virion is physically associated with VLDL particles and does not simply copurify with VLDL particles (Nielsen et al., 2006). Virions and VLDL are likely secreted from the cell by a common pathway because inhibition of VLDL assembly also blocks HCV secretion from cells; moreover, the VLDL assembly machinery colocalizes with HCV RNA replication complexes (Gastaminza et al., 2008; Huang et al., 2007).
Interestingly, lipid droplets are the major source of triglycerides, a class of lipids marked by a glycerol core esterified to three fatty acids that are used for energy storage and transport and that are also present in VLDL assembly (Chao, Stiers, and Ontko, 1986). This makes it tempting to hypothesize that HCV increases the rate of its own particle secretion via association with VLDL particles by enhancing the accumulation of VLDL cargo in lipid droplets. Consistent with the notion that the roles of lipid droplets and VLDL in Flaviviridae assembly are intertwined, the analogous interaction of the DENV core protein with VLDL was inhibited in vitro by the same amidated peptide used to inhibit its association with lipid droplets (Faustino et al., 2014; Martins et al., 2012). It appears that DENV may also use a strategy similar to HCV’s for secretion into the extracellular milieu; however, the antiviral potential of disrupting of this interaction remains to be demonstrated (Faustino et al., 2014).
Lipid analyses of HCV suggest that part of its envelope may be derived from the plasma membrane; however, data acquired from cryo-electron tomography suggests that budding from the ER contributes to the formation of the viral lipid envelope (Romero-Brey et al., 2012). Indeed, a mixture of host lipids, including, cholesteryl esters, cholesterol, sphingomyelin, and phosphatidylcholine were the major lipid species identified in the virion (Merz et al., 2011). Interestingly, although the ER membrane is known to be rich in phosphatidylcholines but low in cholesterol and sphingolipids (Glaumann and Dallner, 1968), HCV virions have been shown to be enriched in all three classes of lipids. Sterols and sphingomyelin in the viral lipid bilayer appear to be functionally important, since depletion of cholesterol or hydrolysis of virion-associated sphingomyelin causes a significant reduction in particle infectivity (Aizaki et al., 2008).
One potential explanation consistent with these observations is that HCV buds from the ER membrane at ordered sites that are sphingolipid- and cholesterol-rich sites. Although phosphatidylcholine is abundant in the plasma membrane and was detected in HCV, phosphatidylethanolamine, another abundant plasma membrane lipid, was detected only at low levels in HCV (Merz et al., 2011). While this could indicate that HCV particles can actually bud from the plasma membrane, it more likely shows that the lipid content of the virion is dynamic and may change as the viral particle traffics through the cell. The presence of cholesteryl esters in HCV virions is likely another manifestation of this. Viral egress may thus reflect and require a concerted series of modulations in lipid composition and localization that occur specifically and concomitantly with changes in viral or cellular protein localization. Whether this is an active or passive mechanism is not known.
The DENV particle acquires its lipid bilayer and also associates with its respective glycoproteins -- E with its chaperone prM -- during budding into the ER lumen (Junjhon et al., 2014; Romero-Brey et al., 2012; Welsch et al., 2009). The lipid composition of the viral particle therefore has functional significance although the mechanism whereby virions achieve a lipid composition that appears to differ significantly from the general composition of the ER remains to be elucidated (Heaton et al., 2010; Perera et al., 2012). Although DENV and HCV both appear to use the conventional secretory pathway for particle egress, the extent to which trafficking affects the lipid content of viral particles and, conversely, the extent to which lipid content directs particle trafficking, have not been reported.
4. Supportive mechanisms that promote viral replication
Membrane proliferation and energy availability
DENV perturbs fatty acid synthesis by altering localization of fatty acid synthase (FAS). The DENV NS3 protein binds FAS and enhances its activity upon relocalization to DENV replication complexes (Heaton et al., 2010). This likely explains the observed increase in fatty acids upon DENV infection (Heaton et al., 2010) and suggests that the de novo synthesis of fatty acids is important for DENV replication. Autophagy, which is elevated upon DENV infection, also contributes to the increasing pool of fatty acids during DENV infection, although inhibition of autophagy has a smaller effect on DENV replication (~1 log10 unit) than inhibition of FAS (>2 log10 units)(Heaton and Randall, 2010; Lee et al., 2013; Lee et al., 2008b; Mateo et al., 2013). Increasing fatty acids in the host cell may support DENV replication by providing a source of ATP in the cell since inhibition of the beta-oxidation of fatty acids also reduced DENV replication by approximately 1 log (Heaton and Randall, 2010). C75, a small molecule inhibitor of FAS, has been shown to inhibit DENV replication in cell culture, illustrating that inhibitors of FAS may represent a promising avenue for anti-DENV therapeutics (Heaton et al., 2010; Perera et al., 2012; Poh et al., 2012).
Interestingly, orlistat, an FDA-approved drug to combat obesity, has also been shown to inhibit FAS (Kridel et al., 2004); however, we are unaware of published studies examining the effect of orlistat on DENV replication. Additional roles for FAS and specific fatty acids in DENV replication – for example, as structural components of the replication compartments or as components of host cell signal transduction regulating viral processes -- remain to be explored. Further investigation is required to determine the structural and biochemical mechanisms responsible for NS3’s enhancement of FAS activity and whether similar mechanisms exist for HCV and other members of the Flaviviridae.
5. Concluding remarks
The importance of cellular lipid metabolism for a variety of human viral pathogens suggests that we may someday be able to exploit these pathways to selectively inhibit viral pathogens with minimal host toxicity and also increased barriers to viral resistance. Studies to date on DENV and HCV illustrate the rich potential for this, but also demonstrate that a more sophisticated understanding of specific lipids and their functions in viral processes will be required to engineer therapeutic strategies that avoid host toxicity or that inadvertantly enhance the replication of other viral pathogens.
For example, statin drugs are commonly used as tools to probe the roles of sterols in viral replication. These drugs specifically inhibit the main rate-determining step in isoprenoid biosynthesis, the synthesis of mevalonate by 3-hydroxy-3-methyl-glutaryl-CoA (HMG CoA) reductase. The effect of this on cholesterol synthesis is the basis for the use of these drugs in cardiovascular disease, but inhibition of HMG CoA reductase can affect multiple processes in the HCV and DENV replication cycles via inhibition of cholesterol biosynthesis and can also can exert profound effects on protein lipidation, which is functionally important for viral replication (Kapadia and Chisari, 2005; Ye et al., 2003). Statin treatment blocked HCV RNA synthesis in a stable HCV replicon cell line, but this effect could be reversed by treatment with downstream metabolites in the pathway (Ikeda et al., 2006; Ye et al., 2003). Interestingly, some members of the statin family had greater effects than others, and one member, pravastatin, had no effect at all (Ikeda et al., 2006).
Although the mechanisms underlying these observations made in vitro are still not fully understood, the antiviral activity of HMG CoA reductase inhibitors prompted immediate clinical trials. To date, these have reported contradictory results. The first study found no change in serum viral RNA after 12 weeks of treatment of ten HCV patients with 20 mg/day atorvastatin (O'Leary et al., 2007). These patients all had high serum cholesterol levels that were reduced after atorvastatin treatment, suggesting that the drug had an effect on its target but not on HCV. A subsequent study found a significant reduction in serum viral RNA after 2–12 weeks of treatment of thirty-one HCV patients with 20–320 mg/day fluvastatin (Bader et al., 2008). This reduction in viral RNA was as high as 1.75 log10 for two of the subjects and was sustained throughout treatment. The most recent epidemiological study compared the medical records of 200 patients and found no difference in HCV RNA levels with any statin treatment (Forde et al., 2009). As illustrated by this example, pharmacological approaches that have complex (or potentially complex) mechanisms due to their effects on the synthesis and metabolism of multiple lipid classes complicates mechanistic studies and may be subject to greater variability when translated to humans.
A better strategy for targeting host lipid synthesis/metabolism might be to identify specific lipid species (not classes of lipids) that are required for viral replication but that are not required by the host due to redundancy in synthetic pathways and also uptake from nutritional sources. Steady-state lipidomic analyses comparing infected and non-infected lipidomes provide an approach for identifying these lipids that may be rare in uninfected cells due to metabolic flux, but that are important for viral replication and that become much more abundant due to viral perturbations (Munger et al., 2008; Rodgers et al., 2012). For example, lipidomic profiling of HCV JFH1 infection in cell culture identified a ten-fold increase in intracellular desmosterol, a lipid relatively non-abundant in uninfected cells. No change in intracellular cholesterol was observed (Rodgers et al., 2012). Inhibition of desmosterol biosynthesis was found to block HCV replication in vitro and rescue of this antiviral effect through the addition of exogenous sterol varied with lipid structure: even the subtle difference of a single alkene in desmosterol and cholesterol (Figure 3) was associated with a difference in the extent of rescue. While the function of desmosterol in HCV replication remains unclear, this illustrates a potential antiviral strategy that more narrowly targets lipid molecules that appear to be specific for viral replication while avoiding global perturbation of other cellular pathways. Key to testing this approach is elucidation of the structural and functional specificity of host lipids in viral replication.
Figure 4.
Representative structures for major lipid classes discussed in Table 3.
Highlights.
We review lipid synthetic, signaling, and trafficking pathways as potential drug targets for the treatment of dengue and hepatitis C.
Lipids serve critical functions in viral replication.
The relationship between lipid structure and specific function in viral processes is still poorly understood.
The diversity of lipid structures and functions poses an analytical challenge compared to other classes of biomolecules.
Understanding lipid structure-function in viral processes may enable selective antiviral therapies.
Acknowledgements
VAV was supported by NIH Diversity Supplement to NIH R01AI076442 and by NRSA Fellowship F32AI106178. PLY and DAC also gratefully acknowledge support from a John and Virginia Kaneb Fellowship and NIH awards R01AI076442 (P.L.Y.) and U19AI109740 (Whelan) during preparation of this manuscript. M.A.R. was a Manfred Karnovsky Predoctoral Fellow and was supported by an NIH F32 NRSA Postdoctoral Fellowship (F32-AI096698). The authors thank Wendy Beth Jackelow for expert help with figure preparation, and Editors Mike Bray and Subhash Vasuvedan for patience and feedback during writing of this review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agnello V, Abel G, Elfahal M, Knight GB, Zhang QX. Hepatitis C virus and other flaviviridae viruses enter cells via low density lipoprotein receptor. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:12766–12771. doi: 10.1073/pnas.96.22.12766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizaki H, Morikawa K, Fukasawa M, Hara H, Inoue Y, Tani H, Saito K, Nishijima M, Hanada K, Matsuura Y, Lai MM, Miyamura T, Wakita T, Suzuki T. Critical role of virion-associated cholesterol and sphingolipid in hepatitis C virus infection. Journal of virology. 2008;82:5715–5724. doi: 10.1128/JVI.02530-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aktepe TE, Pham H, Mackenzie JM. Differential utilisation of ceramide during replication of the flaviviruses West Nile and dengue virus. Virology. 2015;484:241–250. doi: 10.1016/j.virol.2015.06.015. [DOI] [PubMed] [Google Scholar]
- Albecka A, Belouzard S, Op de Beeck A, Descamps V, Goueslain L, Bertrand-Michel J, Terce F, Duverlie G, Rouille Y, Dubuisson J. Role of low-density lipoprotein receptor in the hepatitis C virus life cycle. Hepatology. 2012;55:998–1007. doi: 10.1002/hep.25501. [DOI] [PubMed] [Google Scholar]
- Andre P, Komurian-Pradel F, Deforges S, Perret M, Berland JL, Sodoyer M, Pol S, Brechot C, Paranhos-Baccala G, Lotteau V. Characterization of low- and very-low-density hepatitis C virus RNA-containing particles. Journal of virology. 2002;76:6919–6928. doi: 10.1128/JVI.76.14.6919-6928.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader T, Fazili J, Madhoun M, Aston C, Hughes D, Rizvi S, Seres K, Hasan M. Fluvastatin inhibits hepatitis C replication in humans. The American journal of gastroenterology. 2008;103:1383–1389. doi: 10.1111/j.1572-0241.2008.01876.x. [DOI] [PubMed] [Google Scholar]
- Bartosch B, Verney G, Dreux M, Donot P, Morice Y, Penin F, Pawlotsky JM, Lavillette D, Cosset FL. An interplay between hypervariable region 1 of the hepatitis C virus E2 glycoprotein, the scavenger receptor BI, high-density lipoprotein promotes both enhancement of infection and protection against neutralizing antibodies. Journal of virology. 2005;79:8217–8229. doi: 10.1128/JVI.79.13.8217-8229.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benga WJ, Krieger SE, Dimitrova M, Zeisel MB, Parnot M, Lupberger J, Hildt E, Luo G, McLauchlan J, Baumert TF, Schuster C. Apolipoprotein E interacts with hepatitis C virus nonstructural protein 5A and determines assembly of infectious particles. Hepatology. 2010;51:43–53. doi: 10.1002/hep.23278. [DOI] [PubMed] [Google Scholar]
- Berger C, Romero-Brey I, Radujkovic D, Terreux R, Zayas M, Paul D, Harak C, Hoppe S, Gao M, Penin F, Lohmann V, Bartenschlager R. Daclatasvir-like inhibitors of NS5A block early biogenesis of hepatitis C virus-induced membranous replication factories, independent of RNA replication. Gastroenterology. 2014;147:1094–1105. e25. doi: 10.1053/j.gastro.2014.07.019. [DOI] [PubMed] [Google Scholar]
- Berger KL, Cooper JD, Heaton NS, Yoon R, Oakland TE, Jordan TX, Mateu G, Grakoui A, Randall G. Roles for endocytic trafficking and phosphatidylinositol 4-kinase III alpha in hepatitis C virus replication. Proceedings of the National Academy of Sciences. 2009;106:7577–7582. doi: 10.1073/pnas.0902693106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho FA, Carneiro FA, Martins IC, Assuncao-Miranda I, Faustino AF, Pereira RM, Bozza PT, Castanho MA, Mohana-Borges R, Da Poian AT, Santos NC. Dengue virus capsid protein binding to hepatic lipid droplets (LD) is potassium ion dependent and is mediated by LD surface proteins. Journal of virology. 2012;86:2096–2108. doi: 10.1128/JVI.06796-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catanese MT, Uryu K, Kopp M, Edwards TJ, Andrus L, Rice WJ, Silvestry M, Kuhn RJ, Rice CM. Ultrastructural analysis of hepatitis C virus particles. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:9505–9510. doi: 10.1073/pnas.1307527110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao FF, Stiers DL, Ontko JA. Hepatocellular triglyceride synthesis and transfer to lipid droplets and nascent very low density lipoproteins. Journal of lipid research. 1986;27:1174–1181. [PubMed] [Google Scholar]
- Chukkapalli V, Berger KL, Kelly SM, Thomas M, Deiters A, Randall G. Daclatasvir inhibits hepatitis C virus NS5A motility and hyper-accumulation of phosphoinositides. Virology. 2015;476:168–179. doi: 10.1016/j.virol.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormier EG, Tsamis F, Kajumo F, Durso RJ, Gardner JP, Dragic T. CD81 is an entry coreceptor for hepatitis C virus. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:7270–7274. doi: 10.1073/pnas.0402253101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz O, Delers F, Maynard M, Demignot S, Zoulim F, Chambaz J, Trepo C, Lotteau V, Andre P. Preferential association of Hepatitis C virus with apolipoprotein B48-containing lipoproteins. The Journal of general virology. 2006;87:2983–2991. doi: 10.1099/vir.0.82033-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreux M, Boson B, Ricard-Blum S, Molle J, Lavillette D, Bartosch B, Pecheur EI, Cosset FL. The exchangeable apolipoprotein ApoC-I promotes membrane fusion of hepatitis C virus. The Journal of biological chemistry. 2007;282:32357–32369. doi: 10.1074/jbc.M705358200. [DOI] [PubMed] [Google Scholar]
- Egger D, Wolk B, Gosert R, Bianchi L, Blum HE, Moradpour D, Bienz K. Expression of hepatitis C virus proteins induces distinct membrane alterations including a candidate viral replication complex. Journal of virology. 2002;76:5974–5984. doi: 10.1128/JVI.76.12.5974-5984.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MJ, Rice CM, Goff SP. Phosphorylation of hepatitis C virus nonstructural protein 5A modulates its protein interactions and viral RNA replication. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:13038–13043. doi: 10.1073/pnas.0405152101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahy E, Cotter D, Sud M, Subramaniam S. Lipid classification, structures and tools. Biochimica et biophysica acta. 2011;1811:637–647. doi: 10.1016/j.bbalip.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahy E, Subramaniam S, Brown HA, Glass CK, Merrill AH, Jr, Murphy RC, Raetz CR, Russell DW, Seyama Y, Shaw W, Shimizu T, Spener F, van Meer G, VanNieuwenhze MS, White SH, Witztum JL, Dennis EA. A comprehensive classification system for lipids. Journal of lipid research. 2005;46:839–861. doi: 10.1194/jlr.E400004-JLR200. [DOI] [PubMed] [Google Scholar]
- Fahy E, Subramaniam S, Murphy RC, Nishijima M, Raetz CR, Shimizu T, Spener F, van Meer G, Wakelam MJ, Dennis EA. Update of the LIPID MAPS comprehensive classification system for lipids. Journal of lipid research. 2009;50(Suppl):S9–S14. doi: 10.1194/jlr.R800095-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faustino AF, Carvalho FA, Martins IC, Castanho MA, Mohana-Borges R, Almeida FC, Da Poian AT, Santos NC. Dengue virus capsid protein interacts specifically with very low-density lipoproteins. Nanomedicine : nanotechnology, biology, and medicine. 2014;10:247–255. doi: 10.1016/j.nano.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Forde KA, Law C, O'Flynn R, Kaplan DE. Do statins reduce hepatitis C RNA titers during routine clinical use? World journal of gastroenterology : WJG. 2009;15:5020–5027. doi: 10.3748/wjg.15.5020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia Cordero J, Leon Juarez M, Gonzalez YMJA, Cedillo Barron L, Gutierrez Castaneda B. Caveolin-1 in lipid rafts interacts with dengue virus NS3 during polyprotein processing and replication in HMEC-1 cells. PloS one. 2014;9:e90704. doi: 10.1371/journal.pone.0090704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastaminza P, Cheng G, Wieland S, Zhong J, Liao W, Chisari FV. Cellular determinants of hepatitis C virus assembly, maturation, degradation, and secretion. Journal of virology. 2008;82:2120–2129. doi: 10.1128/JVI.02053-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaumann H, Dallner G. Lipid composition and turnover of rough and smooth microsomal membranes in rat liver. Journal of lipid research. 1968;9:720–729. [PubMed] [Google Scholar]
- Grief C, Galler R, Cortes LM, Barth OM. Intracellular localisation of dengue-2 RNA in mosquito cell culture using electron microscopic in situ hybridisation. Archives of virology. 1997;142:2347–2357. doi: 10.1007/s007050050247. [DOI] [PubMed] [Google Scholar]
- Han X, Gross RW. Shotgun lipidomics: electrospray ionization mass spectrometric analysis and quantitation of cellular lipidomes directly from crude extracts of biological samples. Mass Spectrom Rev. 2005;24:367–412. doi: 10.1002/mas.20023. [DOI] [PubMed] [Google Scholar]
- Heaton NS, Perera R, Berger KL, Khadka S, Lacount DJ, Kuhn RJ, Randall G. Dengue virus nonstructural protein 3 redistributes fatty acid synthase to sites of viral replication and increases cellular fatty acid synthesis. Proceedings of the National Academy of Sciences. 2010;107:17345–17350. doi: 10.1073/pnas.1010811107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton NS, Randall G. Dengue virus-induced autophagy regulates lipid metabolism. Cell Host and Microbe. 2010;8:422–432. doi: 10.1016/j.chom.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herker E, Harris C, Hernandez C, Carpentier A, Kaehlcke K, Rosenberg AR, Farese RV, Jr, Ott M. Efficient hepatitis C virus particle formation requires diacylglycerol acyltransferase-1. Nature medicine. 2010;16:1295–1298. doi: 10.1038/nm.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgemann DW, Feng S, Nasuhoglu C. The complex and intriguing lives of PIP2 with ion channels and transporters. Science's STKE Signal Transduc. Knowl. Environ. 2001;111:re19. doi: 10.1126/stke.2001.111.re19. [DOI] [PubMed] [Google Scholar]
- Hishiki T, Shimizu Y, Tobita R, Sugiyama K, Ogawa K, Funami K, Ohsaki Y, Fujimoto T, Takaku H, Wakita T, Baumert TF, Miyanari Y, Shimotohno K. Infectivity of hepatitis C virus is influenced by association with apolipoprotein E isoforms. Journal of virology. 2010;84:12048–12057. doi: 10.1128/JVI.01063-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Sun F, Owen DM, Li W, Chen Y, Gale M, Jr, Ye J. Hepatitis C virus production by human hepatocytes dependent on assembly and secretion of very low-density lipoproteins. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:5848–5853. doi: 10.1073/pnas.0700760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M, Abe K, Yamada M, Dansako H, Naka K, Kato N. Different anti-HCV profiles of statins and their potential for combination therapy with interferon. Hepatology. 2006;44:117–125. doi: 10.1002/hep.21232. [DOI] [PubMed] [Google Scholar]
- Ira, Johnston LJ. Sphingomyelinase generation of ceramide promotes clustering of nanoscale domains in supported bilayer membranes. Biochimica et biophysica acta. 2008;1778:185–197. doi: 10.1016/j.bbamem.2007.09.021. [DOI] [PubMed] [Google Scholar]
- Ira, Zou S, Ramirez DM, Vanderlip S, Ogilvie W, Jakubek ZJ, Johnston LJ. Enzymatic generation of ceramide induces membrane restructuring: Correlated AFM and fluorescence imaging of supported bilayers. Journal of structural biology. 2009;168:78–89. doi: 10.1016/j.jsb.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Ivanova PT, Milne SB, Myers DS, Brown HA. Lipidomics: a mass spectrometry based systems level analysis of cellular lipids. Curr Opin Chem Biol. 2009;13:526–531. doi: 10.1016/j.cbpa.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston I, Johnston LJ. Ceramide promotes restructuring of model raft membranes. Langmuir : the ACS journal of surfaces and colloids. 2006;22:11284–11289. doi: 10.1021/la061636s. [DOI] [PubMed] [Google Scholar]
- Junjhon J, Pennington JG, Edwards TJ, Perera R, Lanman J, Kuhn RJ. Ultrastructural characterization and three-dimensional architecture of replication sites in dengue virus-infected mosquito cells. Journal of virology. 2014;88:4687–4697. doi: 10.1128/JVI.00118-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapadia SB, Barth H, Baumert T, McKeating JA, Chisari FV. Initiation of hepatitis C virus infection is dependent on cholesterol and cooperativity between CD81 and scavenger receptor B type I. Journal of virology. 2007;81:374–383. doi: 10.1128/JVI.01134-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapadia SB, Chisari FV. Hepatitis C virus RNA replication is regulated by host geranylgeranylation and fatty acids. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:2561–2566. doi: 10.1073/pnas.0409834102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kridel SJ, Axelrod F, Rozenkrantz N, Smith JW. Orlistat is a novel inhibitor of fatty acid synthase with antitumor activity. Cancer research. 2004;64:2070–2075. doi: 10.1158/0008-5472.can-03-3645. [DOI] [PubMed] [Google Scholar]
- Lee C, Ma H, Hang JQ, Leveque V, Sklan EH, Elazar M, Klumpp K, Glenn JS. The hepatitis C virus NS5A inhibitor (BMS-790052) alters the subcellular localization of the NS5A non-structural viral protein. Virology. 2011;414:10–18. doi: 10.1016/j.virol.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CJ, Lin HR, Liao CL, Lin YL. Cholesterol effectively blocks entry of flavivirus. Journal of virology. 2008a;82:6470–6480. doi: 10.1128/JVI.00117-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YR, Hu HY, Kuo SH, Lei HY, Lin YS, Yeh TM, Liu CC, Liu HS. Dengue virus infection induces autophagy: an in vivo study. J Biomed Sci. 2013;20:65. doi: 10.1186/1423-0127-20-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YR, Lei HY, Liu MT, Wang JR, Chen SH, Jiang-Shieh YF, Lin YS, Yeh TM, Liu CC, Liu HS. Autophagic machinery activated by dengue virus enhances virus replication. Virology. 2008b;374:240–248. doi: 10.1016/j.virol.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Kakinami C, Li Q, Yang B, Li H. Human apolipoprotein A-I is associated with dengue virus and enhances virus infection through SR-BI. PloS one. 2013;8:e70390. doi: 10.1371/journal.pone.0070390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie JM, Khromykh AA, Parton RG. Cholesterol manipulation by West Nile virus perturbs the cellular immune response. Cell host & microbe. 2007;2:229–239. doi: 10.1016/j.chom.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Mancone C, Steindler C, Santangelo L, Simonte G, Vlassi C, Longo MA, D'Offizi G, Di Giacomo C, Pucillo LP, Amicone L, Tripodi M, Alonzi T. Hepatitis C virus production requires apolipoprotein A-I and affects its association with nascent low-density lipoproteins. Gut. 2011;60:378–386. doi: 10.1136/gut.2010.211292. [DOI] [PubMed] [Google Scholar]
- Martin-Acebes MA, Merino-Ramos T, Blazquez AB, Casas J, Escribano-Romero E, Sobrino F, Saiz JC. The composition of West Nile virus lipid envelope unveils a role of sphingolipid metabolism in flavivirus biogenesis. Journal of virology. 2014;88:12041–12054. doi: 10.1128/JVI.02061-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Gutierrez M, Correa-Londono LA, Castellanos JE, Gallego-Gomez JC, Osorio JE. Lovastatin delays infection and increases survival rates in AG129 mice infected with dengue virus serotype 2. PloS one. 2014;9:e87412. doi: 10.1371/journal.pone.0087412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins IC, Gomes-Neto F, Faustino AF, Carvalho FA, Carneiro FA, Bozza PT, Mohana-Borges R, Castanho MA, Almeida FC, Santos NC, Da Poian AT. The disordered N-terminal region of dengue virus capsid protein contains a lipid-droplet-binding motif. The Biochemical journal. 2012;444:405–415. doi: 10.1042/BJ20112219. [DOI] [PubMed] [Google Scholar]
- Masaki T, Suzuki R, Murakami K, Aizaki H, Ishii K, Murayama A, Date T, Matsuura Y, Miyamura T, Wakita T, Suzuki T. Interaction of hepatitis C virus nonstructural protein 5A with core protein is critical for the production of infectious virus particles. Journal of virology. 2008;82:7964–7976. doi: 10.1128/JVI.00826-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo R, Nagamine CM, Spagnolo J, Mendez E, Rahe M, Gale M, Jr, Yuan J, Kirkegaard K. Inhibition of cellular autophagy deranges dengue virion maturation. Journal of virology. 2013;87:1312–1321. doi: 10.1128/JVI.02177-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLauchlan J, Lemberg MK, Hope G, Martoglio B. Intramembrane proteolysis promotes trafficking of hepatitis C virus core protein to lipid droplets. The EMBO journal. 2002;21:3980–3988. doi: 10.1093/emboj/cdf414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel N, Fischl W, Hueging K, Bankwitz D, Frentzen A, Haid S, Gentzsch J, Kaderali L, Bartenschlager R, Pietschmann T. MAP-kinase regulated cytosolic phospholipase A2 activity is essential for production of infectious hepatitis C virus particles. PLoS pathogens. 2012;8:e1002829. doi: 10.1371/journal.ppat.1002829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz A, Long G, Hiet MS, Brugger B, Chlanda P, Andre P, Wieland F, Krijnse-Locker J, Bartenschlager R. Biochemical and morphological properties of hepatitis C virus particles and determination of their lipidome. The Journal of biological chemistry. 2011;286:3018–3032. doi: 10.1074/jbc.M110.175018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyanari Y, Atsuzawa K, Usuda N, Watashi K, Hishiki T, Zayas M, Bartenschlager R, Wakita T, Hijikata M, Shimotohno K. The lipid droplet is an important organelle for hepatitis C virus production. Nature cell biology. 2007;9:1089–1097. doi: 10.1038/ncb1631. [DOI] [PubMed] [Google Scholar]
- Monazahian M, Bohme I, Bonk S, Koch A, Scholz C, Grethe S, Thomssen R. Low density lipoprotein receptor as a candidate receptor for hepatitis C virus. Journal of medical virology. 1999;57:223–229. doi: 10.1002/(sici)1096-9071(199903)57:3<223::aid-jmv2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Munger J, Bennett BD, Parikh A, Feng XJ, McArdle J, Rabitz HA, Shenk T, Rabinowitz JD. Systems-level metabolic flux profiling identifies fatty acid synthesis as a target for antiviral therapy. Nature biotechnology. 2008;26:1179–1186. doi: 10.1038/nbt.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton AC. Lipid activation of protein kinases. Journal of lipid research. 2009;50(Suppl):S266–S271. doi: 10.1194/jlr.R800064-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen SU, Bassendine MF, Burt AD, Martin C, Pumeechockchai W, Toms GL. Association between hepatitis C virus and very-low-density lipoprotein (VLDL)/LDL analyzed in iodixanol density gradients. Journal of virology. 2006;80:2418–2428. doi: 10.1128/JVI.80.5.2418-2428.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nour AM, Li Y, Wolenski J, Modis Y. Viral membrane fusion and nucleocapsid delivery into the cytoplasm are distinct events in some flaviviruses. PLoS pathogens. 2013;9:e1003585. doi: 10.1371/journal.ppat.1003585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary JG, Chan JL, McMahon CM, Chung RT. Atorvastatin does not exhibit antiviral activity against HCV at conventional doses: a pilot clinical trial. Hepatology. 2007;45:895–898. doi: 10.1002/hep.21554. [DOI] [PubMed] [Google Scholar]
- Owen DM, Huang H, Ye J, Gale M., Jr Apolipoprotein E on hepatitis C virion facilitates infection through interaction with low-density lipoprotein receptor. Virology. 2009;394:99–108. doi: 10.1016/j.virol.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena J, Harris E. Early dengue virus protein synthesis induces extensive rearrangement of the endoplasmic reticulum independent of the UPR and SREBP-2 pathway. PloS one. 2012;7:e38202. doi: 10.1371/journal.pone.0038202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera R, Riley C, Isaac G, Hopf-Jannasch AS, Moore RJ, Weitz KW, Pasa-Tolic L, Metz TO, Adamec J, Kuhn RJ. Dengue virus infection perturbs lipid homeostasis in infected mosquito cells. PLoS pathogens. 2012;8:e1002584. doi: 10.1371/journal.ppat.1002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pileri P, Uematsu Y, Campagnoli S, Galli G, Falugi F, Petracca R, Weiner AJ, Houghton M, Rosa D, Grandi G, Abrignani S. Binding of hepatitis C virus to CD81. Science. 1998;282:938–941. doi: 10.1126/science.282.5390.938. [DOI] [PubMed] [Google Scholar]
- Poh MK, Shui G, Xie X, Shi PY, Wenk MR, Gu F. U18666A, an intracellular cholesterol transport inhibitor, inhibits dengue virus entry and replication. Antiviral research. 2012;93:191–198. doi: 10.1016/j.antiviral.2011.11.014. [DOI] [PubMed] [Google Scholar]
- Quintavalle M, Sambucini S, Summa V, Orsatti L, Talamo F, De Francesco R, Neddermann P. Hepatitis C virus NS5A is a direct substrate of casein kinase I-alpha, a cellular kinase identified by inhibitor affinity chromatography using specific NS5A hyperphosphorylation inhibitors. The Journal of biological chemistry. 2007;282:5536–5544. doi: 10.1074/jbc.M610486200. [DOI] [PubMed] [Google Scholar]
- Reghellin V, Donnici L, Fenu S, Berno V, Calabrese V, Pagani M, Abrignani S, Peri F, De Francesco R, Neddermann P. NS5A inhibitors impair NS5A-phosphatidylinositol 4-kinase IIIalpha complex formation and cause a decrease of phosphatidylinositol 4-phosphate and cholesterol levels in hepatitis C virus-associated membranes. Antimicrobial agents and chemotherapy. 2014;58:7128–7140. doi: 10.1128/AAC.03293-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss S, Rebhan I, Backes P, Romero-Brey I, Erfle H, Matula P, Kaderali L, Poenisch M, Blankenburg H, Hiet M-S, Longerich T, Diehl S, Ramirez F, Balla T, Rohr K, Kaul A, Bühler S, Pepperkok R, Lengauer T, Albrecht M, Eils R, Schirmacher P, Lohmann V, Bartenschlager R. Recruitment and activation of a lipid kinase by hepatitis C virus NS5A is essential for integrity of the membranous replication compartment. Cell Host and Microbe. 2011;9:32–45. doi: 10.1016/j.chom.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers MA, Saghatelian A, Yang PL. Identification of an overabundant cholesterol precursor in hepatitis B virus replicating cells by untargeted lipid metabolite profiling. Journal of the American Chemical Society. 2009;131:5030–5031. doi: 10.1021/ja809949r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers MA, Villareal VA, Schaefer EA, Peng LF, Corey KE, Chung RT, Yang PL. Lipid metabolite profiling identifies desmosterol metabolism as a new antiviral target for hepatitis C virus. J Am Chem Soc. 2012;134:6896–6899. doi: 10.1021/ja207391q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolim AE, Henrique-Araujo R, Ferraz EG, de Araujo Alves Dultra FK, Fernandez LG. Lipidomics in the study of lipid metabolism: Current perspectives in the omic sciences. Gene. 2015;554:131–139. doi: 10.1016/j.gene.2014.10.039. [DOI] [PubMed] [Google Scholar]
- Romero-Brey I, Merz A, Chiramel A, Lee JY, Chlanda P, Haselman U, Santarella-Mellwig R, Habermann A, Hoppe S, Kallis S, Walther P, Antony C, Krijnse-Locker J, Bartenschlager R. Three-dimensional architecture and biogenesis of membrane structures associated with hepatitis C virus replication. PLoS pathogens. 2012;8:e1003056. doi: 10.1371/journal.ppat.1003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell C, Lebreton A, Young Ng C, Lim JY, Liu W, Vasudevan S, Labow M, Gu F, Gaither LA. Cholesterol biosynthesis modulation regulates dengue viral replication. Virology. 2009;389:8–19. doi: 10.1016/j.virol.2009.03.025. [DOI] [PubMed] [Google Scholar]
- Sainz B, Jr, Barretto N, Martin DN, Hiraga N, Imamura M, Hussain S, Marsh KA, Yu X, Chayama K, Alrefai WA, Uprichard SL. Identification of the Niemann-Pick C1-like 1 cholesterol absorption receptor as a new hepatitis C virus entry factor. Nature medicine. 2012;18:281–285. doi: 10.1038/nm.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samsa MM, Mondotte JA, Iglesias NG, Assuncao-Miranda I, Barbosa-Lima G, Da Poian AT, Bozza PT, Gamarnik AV. Dengue virus capsid protein usurps lipid droplets for viral particle formation. PLoS pathogens. 2009;5:e1000632. doi: 10.1371/journal.ppat.1000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto-Acosta R, Bautista-Carbajal P, Syed GH, Siddiqui A, Del Angel RM. Nordihydroguaiaretic acid (NDGA) inhibits replication and viral morphogenesis of dengue virus. Antiviral research. 2014;109:132–140. doi: 10.1016/j.antiviral.2014.07.002. [DOI] [PubMed] [Google Scholar]
- Soto-Acosta R, Mosso C, Cervantes-Salazar M, Puerta-Guardo H, Medina F, Favari L, Ludert JE, del Angel RM. The increase in cholesterol levels at early stages after dengue virus infection correlates with an augment in LDL particle uptake and HMG-CoA reductase activity. Virology. 2013;442:132–147. doi: 10.1016/j.virol.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Tang WC, Lin RJ, Liao CL, Lin YL. Rab18 facilitates dengue virus infection by targeting fatty acid synthase to sites of viral replication. Journal of virology. 2014;88:6793–6804. doi: 10.1128/JVI.00045-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani H, Shiokawa M, Kaname Y, Kambara H, Mori Y, Abe T, Moriishi K, Matsuura Y. Involvement of ceramide in the propagation of Japanese encephalitis virus. Journal of virology. 2010;84:2798–2807. doi: 10.1128/JVI.02499-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner LB, Chng C, Guan XL, Lei Z, Rozen SG, Wenk MR. Lipidomics identifies a requirement for peroxisomal function during influenza virus replication. Journal of lipid research. 2014;55:1357–1365. doi: 10.1194/jlr.M049148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teoh PG, Huang ZS, Pong WL, Chen PC, Wu HN. Maintenance of dimer conformation by the dengue virus core protein alpha4-alpha4' helix pair is critical for nucleocapsid formation and virus production. Journal of virology. 2014;88:7998–8015. doi: 10.1128/JVI.00940-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillancourt FH, Brault M, Pilote L, Uyttersprot N, Gaillard ET, Stoltz JH, Knight BL, Pantages L, McFarland M, Breitfelder S, Chiu TT, Mahrouche L, Faucher AM, Cartier M, Cordingley MG, Bethell RC, Jiang H, White PW, Kukolj G. Evaluation of phosphatidylinositol-4-kinase IIIalpha as a hepatitis C virus drug target. Journal of virology. 2012;86:11595–11607. doi: 10.1128/JVI.01320-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Schaar HM, Rust MJ, Chen C, van der Ende-Metselaar H, Wilschut J, Zhuang X, Smit JM. Dissecting the cell entry pathway of dengue virus by single-particle tracking in living cells. PLoS pathogens. 2008;4:e1000244. doi: 10.1371/journal.ppat.1000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Schaar HM, Rust MJ, Waarts BL, van der Ende-Metselaar H, Kuhn RJ, Wilschut J, Zhuang X, Smit JM. Characterization of the early events in dengue virus cell entry by biochemical assays and single-virus tracking. Journal of virology. 2007;81:12019–12028. doi: 10.1128/JVI.00300-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsch S, Miller S, Romero-Brey I, Merz A, Bleck CK, Walther P, Fuller SD, Antony C, Krijnse-Locker J, Bartenschlager R. Composition and three-dimensional architecture of the dengue virus replication and assembly sites. Cell host & microbe. 2009;5:365–375. doi: 10.1016/j.chom.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane D, McGivern DR, Wauthier E, Yi M, Madden VJ, Welsch C, Antes I, Wen Y, Chugh PE, McGee CE, Widman DG, Misumi I, Bandyopadhyay S, Kim S, Shimakami T, Oikawa T, Whitmire JK, Heise MT, Dittmer DP, Kao CC, Pitson SM, Merrill AH, Jr, Reid LM, Lemon SM. Regulation of the hepatitis C virus RNA replicase by endogenous lipid peroxidation. Nature medicine. 2014;20:927–935. doi: 10.1038/nm.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Wang C, Sumpter R, Jr, Brown MS, Goldstein JL, Gale M., Jr Disruption of hepatitis C virus RNA replication through inhibition of host protein geranylgeranylation. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:15865–15870. doi: 10.1073/pnas.2237238100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitseva E, Yang ST, Melikov K, Pourmal S, Chernomordik LV. Dengue virus ensures its fusion in late endosomes using compartment-specific lipids. PLoS pathogens. 2010;6:e1001131. doi: 10.1371/journal.ppat.1001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JH, Ge L, Qi W, Zhang L, Miao HH, Li BL, Yang M, Song BL. The N-terminal domain of NPC1L1 protein binds cholesterol and plays essential roles in cholesterol uptake. The Journal of biological chemistry. 2011;286:25088–25097. doi: 10.1074/jbc.M111.244475. [DOI] [PMC free article] [PubMed] [Google Scholar]