Abstract
The biological activities of tocotrienols are receiving increasing attention. Herein, we report the efficacy of a mixed-tocotrienol diet against prostate tumorigenesis in the transgenic adenocarcinoma mouse prostate (TRAMP) mouse model. Male TRAMP mice, 8 wk old, were fed 0.1%, 0.3%, or 1% mixed tocotrienols in AIN-76A diet up to 24 wk old. Likewise, a positive control group consisting of male TRAMP mice and a negative control group consisting of wild-type nontransgenic mice were fed regular AIN-76A diet up to 24 wk old. Our results show that mixed-tocotrienol-fed groups had a lower incidence of tumor formation along with a significant reduction in the average wet weight of genitourinary apparatus. Furthermore, mixed tocotrienols significantly reduced the levels of high-grade neoplastic lesions as compared to the positive controls. This decrease in levels of high-grade neoplastic lesions was found to be associated with increased expression of proapoptotic proteins BAD (Bcl2 antagonist of cell death) and cleaved caspase-3 and cell cycle regulatory proteins cyclin dependent kinase inhibitors p21 and p27. In contrast, the expression of cyclins A and E were found to be decreased in mixed-tocotrienol groups. Taken together, our results show that by modulating cell cycle regulatory proteins and increasing expression of proapoptotic proteins, mixed tocotrienols suppress prostate tumorigenesis in the TRAMP mice.
INTRODUCTION
Prostate cancer is the second leading cause of cancer-related death among men in the United States (1). This disease initially responds well to hormone ablation but would eventually become hormone refractory and ultimately results in death (2). Interest in the use of alternative and complementary therapies, mainly nutraceuticals as chemopreventive compounds, is increasing for prostate cancer since this disease has a long latency period and thus offers a unique window for chemoprevention (3). Chemoprevention may help to inhibit tumor formation and may also help in delaying the progression from neoplasms to a more advanced state of the disease. For the latter, the delay in progression is thought to be the result of modulation of complex signal transduction pathways that lead up to tumor formation (4).
The natural vitamin E family includes 8 chemically distinct molecules: α-, β-, γ-, and δ-tocopherol and α-, β-, γ-, and δ-tocotrienol. Structurally, vitamin E consists of a chroman ring with either a saturated side chain (tocopherols) or an unsaturated side chain (tocotrienols). Isoforms of tocotrienol differ from each other based on the methyl groups on the chroman ring (Fig. 1). For chemoprevention research, α-tocopherol is among the most studied components of vitamin E (5,6). The biological activities of the other isoforms are largely unknown. This represents a major void in vitamin E research. Significance of the void is enhanced by the observation that the biological functions of the different homologues of natural vitamin E are not identical. We and other researchers have shown that γ-tocopherols are potent against various cancers (7,8,9). Likewise, during the last couple of years, tocotrienol research has also gained substantial momentum. Palm oil is one of the richest sources of tocotrienols. Tocotrienols extracted from crude palm oil mainly consist of a mixture of α-, γ-, and δ-tocotrienols and some α-tocopherols, referred to as tocotrienol-rich fraction (TRF). TRF from palm oil has been shown to inhibit proliferation and growth of human breast cancer cells (10–12). Recent studies have shown that γ-tocotrienols inhibit cell proliferation by decreasing Akt and activation of NF-κB, implicated in the regulation of cell growth, cell cycle, and apoptosis (13). More recently, researchers have demonstrated that TRF imparts differential antiproliferative and apoptotic effects in human prostate cancer cells versus normal cells and preferentially sensitizes xenograft bearing nude mice to radiation therapy (14,15). Based on the documented anticancer efficacy of TRF, we evaluated the chemopreventive efficacy of a mixed tocotrienol diet against prostate cancer growth and progression in the transgenic adenocarcinoma mouse prostate (TRAMP) mouse model.
FIG. 1.
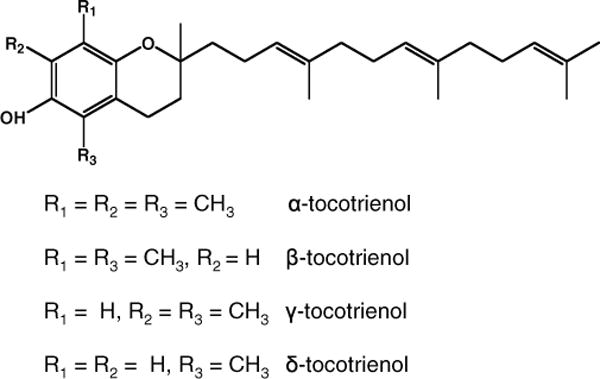
Structure of the different analogs of tocotrienols.
The TRAMP mouse model was developed in C57BL/6J mice using minimal rat probasin promoter to drive the expression of SV40 early genes specifically in prostatic epithelium. These mice demonstrate the progression of the disease from early prostatic intraepithelial neoplasia (PIN) lesions to a more aggressive metastatic adenocarcinoma that closely mimics the various stages in human prostate cancer (16). Since this model bears close relevance to the human form of the disease, we evaluated the efficacy of mixed tocotrienols in this mouse model. Our results demonstrate a dose-dependent increase in chemopreventive efficacy of mixed tocotrienols against prostate cancer in the TRAMP mice.
MATERIALS AND METHODS
Animals and Study Design
Female hemizygous C57BL/TGN TRAMP mice, line PB Tag 8247NG, and male C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME). The animals were bred on the same genetic background and maintained in the Animal Care Facility of Rutgers University. Housing and care of the animals was in accordance with the guidelines established by Rutgers University’s Animal Research Committee consistent with the NIH Guidelines for the Care and Use of Laboratory Animals. Transgenic males for these studies were routinely obtained as [TRAMP × C57BL/6] F1 or as [TRAMP × C57BL/6] F2 offspring. Identity of transgenic mice was established by the PCR-based DNA screening. Throughout the experiment, the animals were housed in cages with wood chip bedding in a temperature-controlled room (68°F to 72°F) with a 12 h light/dark cycle at a relative humidity of 45% to 55% as we reported previously (9).
Diets and Study Design
All animals were fed AIN-76A diet obtained from Research Dyets (Easton, PA). Treated animals were fed 0.1%, 0.3%, or 1% mixed tocotrienols incorporated in AIN-76A diet. The mixed tocotrienols (Tocomin) were a kind gift from Carotech Bhd (Perak, Malaysia) and contained D-α-tocotrienol (12–14%), D-β-tocotrienol (1%), D-γ-tocotrienol (18–20%), D-δ-tocotrienol (4–6%), and D-α-tocopherol (12–14%). The 0.1%, 0.3%, or 1% mixed tocotrienol diets were designed to deliver dosages of ≈ 1.7, 5.2, and 17.1 mg, respectively, of D-γ-tocotrienol/day. All the animals were put on AIN-76A diet 1 wk prior to the study. The control animals (n = 11) received AIN-76A diet throughout the experiment, whereas the treatment group received 0.1% (n = 8), 0.3% (n = 9), or 1% (n = 9) mixed-tocotrienol diet for a period of 24 wk. Fresh diets were added to the cages twice a week. The animals were weighed weekly and monitored on a regular basis for their general health. At each time point, mice were killed by cervical dislocation; and the genitourinary apparatus (GU) consisting of the seminal vesicles, prostate, and the bladder were isolated for further analyses.
Histopathology
The dorso-lateral prostate was excised and fixed overnight in 10% formalin and then transferred to 70% ethanol. Sections (4 μm) were cut from paraffin-embedded tissue and mounted on slides. The sections were stained with hematoxylin and eosin (H&E) to observe any neoplastic changes. Sections were blindly evaluated by a histopathologist to classify PIN lesions. Lesions were classified as PIN I, PIN II, PIN III, and PIN IV as described by Park and coworkers (17). For the purpose of ease, PIN I and PIN II have been grouped as low-grade PIN, whereas PIN III and PIN IV have been grouped as high-grade PIN.
Immunoblot Analysis
The dorso-lateral prostate tissues removed from each treated and control group were pooled accordingly and homogenized with RIPA buffer (Roche, Manheim, Germany). The protein concentrations were measured by Bicinchonic Acid solution (Pierce, Rockford, IL). A total of 20 μg of protein was loaded onto Biorad precast gels (4–12%) and transferred to polyvinyl difluoride membranes. The membranes were blocked with 5% BSA in TBST for 1 h followed by overnight incubation with primary antibodies and horseradish peroxidase conjugated secondary antibody for 1 h. Protein bands were visualized using Supersignal West Femto (Pierce, Rockford, IL).
Statistics
The chi-square test was used to assess the significance of incidence of palpable tumor. For all other determinations, statistical significance was determined using Student’s t-test at significance (P < 0.05 or P < 0.01).
RESULTS
General Health Observations
The overall health of the mice throughout the study period was found to be good. No significant changes in the body weights were observed in animals given control or mixed tocotrienol supplemented diet.
Mixed Tocotrienols Reduce Tumor Incidence in TRAMP Mice
A significant reduction in the tumor incidence was observed as recorded by palpation prior to necropsy (Fig. 2A). A total of 8 out of 11 animals in the control group vs. 3 out of 8, 3 out of 9, and 2 out of 9 animals in the 0.1%, 0.3%, and 1% mixed tocotrienols treated groups, respectively, developed palpable tumors. Autopsy revealed significant difference between wet weight of the GU of all animals in control and mixed tocotrienol treated groups (Fig. 2B). The 0.3% and 1% mixed tocotrienols significantly decreased the weight as compared to the control group (P < 0.05). The decrease in GU weight by 0.1% mixed tocotrienols was not statistically significant.
FIG. 2.
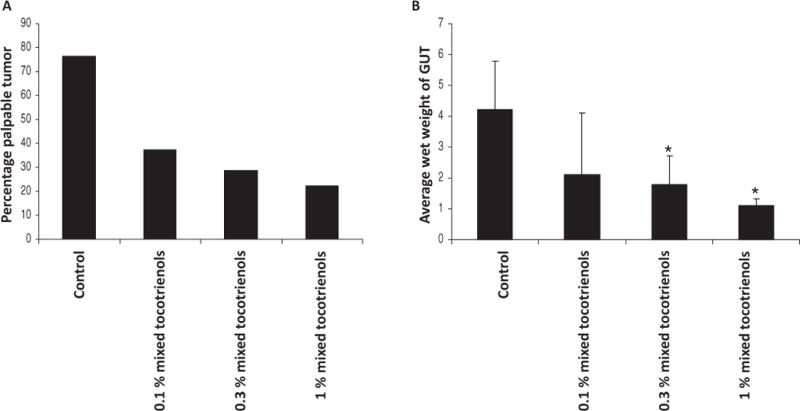
Mixed tocotrienols suppress tumor incidence in TRAMP mice. A: Percentage incidence of palpable tumor in the control and mixed tocotrienol treated groups. The tumor was detected by palpation prior to necropsy. B: Average wet weight of the genitourinary apparatus of control and tocotrienol-treated groups. At the time of necropsy, the genitourinary apparatus consisting of seminal vesicles, prostate, and bladder was removed en bloc and weighed. The bars represent mean values ± SE. Statistical significance of the difference between control and treated groups was analyzed by Student’s t-test. P values < 0.05 were considered significant. GUT, genitourinary apparatus.
Histological analysis was performed to determine percentage levels of PIN in the animals (Fig. 3). A brief description of the histology displayed by normal prostatic tissue and low-grade and high-grade PIN is as follows: Normal prostatic acinus was characterized by the presence of single layered columnar epithelial cells and a distinct lumen. Surrounding stroma consisted of layers of loose connective tissue. Low-grade PIN was characterized by a couple of layers of atypical cells that did not fill up the luminal space. The cells displayed tufting or cribiform growth patterns, although the underlying fibromuscular sheath in most cases was almost intact. High-grade PIN was characterized by multiple layers of atypical cells with significantly reduced luminal space. The epithelial cells demonstrated a characteristic cribiform or tufting growth pattern.
FIG. 3.
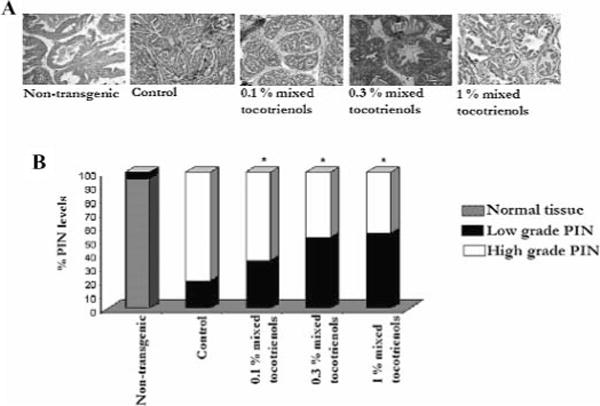
Mixed tocotrienols inhibit tumorigenesis in TRAMP mice. A: Representative photomicrographs (×100 magnification) of H&E stained dorso-lateral section of nontransgenic, control, and mixed tocotrienol fed mice at 24 wk of age. B: Percentage levels of normal tissue, low-grade, and high-grade PIN displayed by nontransgenic, control, and mixed tocotrienol fed 24–wk-old mice. At the time of necropsy, the genitourinary apparatus was removed en bloc. The dorso-lateral prostate was microdissected and analyzed for PIN evaluation. Normal tissue was characterized by single-layered epithelial cells, and a distinct lumen, low-grade PIN typically displayed a couple of layers of atypical cells that did not, however, fill up the luminal space and high-grade PIN characterized by marked stromal thickening and hypercellularity and significantly reduced luminal space. The bars represent mean values ± SE. Statistical significance of the difference between control and treated groups was analyzed by Student’s t-test. P values <0.05 were considered significant.
All nontransgenic animals displayed approximately >95% normal prostatic tissue and <5% low-grade PIN. Histological analysis of the all-control animals including the ones without a palpable tumor revealed well-differentiated carcinoma, marked stromal thickening, and hypercellularity. Control animals demonstrated ≈ 75–80% incidence of high-grade PIN and ≈ 25–30% incidence of low-grade PIN (Fig. 3B). Lymph node metastasis was evident in control mice bearing tumors. No pulmonary or liver metastases were observed; 0.1% mixed tocotrienol treatment decreased the percentage of high-grade PIN by 40%. On the other hand, 0.3% and 1% mixed tocotrienol treatment decreased the percentage of high-grade PIN, resulting in higher percentages of low-grade PIN, significantly (P < 0.05). Immunoblot analysis for SV-40 transgene expression revealed that tumor incidence and decrease in PIN levels by mixed tocotrienols were not a result of suppression of transgene expression (results not shown).
Mixed Tocotrienols Effect Apoptosis and Cell Cycle Control Proteins
The suppression of tumor formation by mixed tocotrienols led us to investigate their effects on proapoptotic and cell cycle regulatory proteins. Figure 4 demonstrates the immunoblot analysis of proapoptotic proteins BAD and cleaved caspase 3 as well as cyclins and cyclin-dependent kinase (cdk) inhibitors with the densitometric data after adjusting with β-actin as a loading control (note that the β-actin blot is not shown).
FIG. 4.
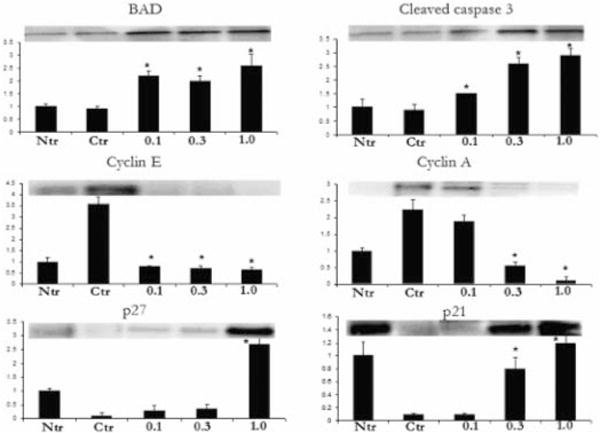
Mixed tocotrienols alter the expression levels of apoptotic proteins BAD and cleaved caspase 3 and cell cycle regulatory proteins cyclin E, cyclin A, p21, and p27. Densitometric analysis of the band intensity of each protein was adjusted with β-actin (loading control) and is shown as histograms. Note that the β-actin band is not shown. The bars represent mean values ±SE. Statistical significance of the difference between control and treated groups was analyzed by Student’s t-test. P values < 0.01 were considered significant. Ntr, nontransgenic; Ctr, control.
Results show that the mixed tocotrienols diet enhanced expression of proapoptotic proteins BAD and cleaved caspase 3 (≈ 1.5-fold to threefold and twofold to threefold over control, respectively). The prostatic samples from control animals demonstrated significantly increased expression of cyclin E and cyclin A ≈ 3.5- and 2.2-fold, respectively, over the nontransgenic controls. Likewise, the suppression of cdk inhibitor p21 and p27 were significantly suppressed ≈ 0.2-fold below the nontransgenic control animals. Interestingly, the nontransgenic prostatic samples demonstrated low expression of p27 as compared to p21. Mixed tocotrienol diet significantly increased the expression of p21 and p27 and suppressed the expression of both cyclin A and cyclin E. Together, these results suggest that tocotrienols modulate the expression of cyclins, cdk inhibitors, and proapoptotic proteins, ultimately leading to increased apoptosis and tumor suppression.
DISCUSSION
A direct link between the incidence of cancer and nutrition has been long established. Cancer chemoprevention entails the use of select agents preferably of a dietary source to prevent cancer. Chemoprevention may be most useful to prevent cancers that do not have a remedial effective chemotherapeutic agent and those that have long latency periods like prostate cancer (2,3). Prostate cancer is the second leading cause of cancer-related deaths among men in the United States. Prostate cancer patients usually respond well to hormone ablation; but in the later stages, this disease becomes hormone refractory. Unfortunately, this hormone-refractory stage of the disease has no effective treatment options. In prostate cancer patients, the progression from benign to neoplastic lesions to fully developed tumors may take several years; and hence, this disease provides a window for intervention by chemopreventive compounds.
Vitamin E, an important micronutrient in our diets, exists as 8 isoforms. Owing to its unique structure (a chroman head with a phytyl side chain), it can behave both as a pro-oxidant and an antioxidant. Emerging evidence in the literature suggests vitamin E as a potential chemopreventive agent (18,19). Support for the possible use of vitamin E as a chemopreventive agent came from a study that demonstrated high incidence of prostate cancer in subjects with low nutritional intake of vitamin E (20). However, most of the vitamin E chemoprevention research has involved the efficacy of α-tocopherol. The recently completed SELECT (Selenium and Vitamin E Cancer Prevention Trial) was one of the largest human cancer prevention trials that assessed the role of selenium and vitamin E in prostate cancer prevention. This was a phase III, double-blind study in which human subjects were administered 200 μg of pure-L-selenomethionine; 400 IU of D, L-α-tocopherol; or a combination of the two agents. No significant differences in prostate cancer incidence were observed in any of the groups. As a matter of fact, a slight but statistically insignificant increase in prostate cancer risk within the vitamin E group was observed. Whether the high dose of D, L-α-tocopherol used in the study caused suppression of the more active forms of vitamin E is not yet known (21–24). Nevertheless, this important finding compels us to continue research in order to determine the most effective isoform of vitamin E against prostate cancer. In that regard, we and others have shown that γ-tocopherols are effective against prostate, breast, as well as colon cancer (7–9). Likewise, only recently, the potency of tocotrienols against cancer has been established (11–15).
In this report, we showed that mixed tocotrienols are effective in preventing prostate cancer growth in a transgenic mouse model (TRAMP). Prostate cancer progression in this mouse model closely resembles that in humans and therefore provides a useful tool to test efficacy of chemopreventive compounds.
At 24 wk of age, mice that were fed mixed tocotrienols demonstrated low incidence of tumor detected by palpation compared to control mice. A neuroendocrine switch, characterized by synaptophysin positive staining, is considered a probable significant event in the TRAMP mouse model and is often predictive of high-grade or end-stage prostate cancer (25,26). The inhibitory efficacy of tocotrienols against synaptophysin-positive carcinomas or synaptophysin-negative dorso-lateral prostate lesions and seminal vesicle hypertrophy was not evaluated. However, histopathology revealed that mixed tocotrienol treatment significantly decreased the levels of high-grade neoplastic lesions, whereas increasing the levels of low-grade PIN. No characteristics of normal tissue were detected in the tocotrienol-treated groups.
The antiprostate cancer effect of tocotrienols was accompanied by significant increase in expression of proapoptotic proteins BAD and cleaved caspase-3 as well as cdk inhibitors p21 and p27. Tocotrienol treatment significantly suppressed the expression of cyclins E and A. The expression of cyclin D1 did not vary much with tocotrienol treatment.
The cyclins are an integral part of the cell cycle process. Cell cycle progression is regulated by a series of cdks. The cdks are regulated by activating (cdk-activating kinases) and inhibitory (Wee) phosphorylations as well as cdk inhibitors. The cdk inhibitors are known to bind to the cyclin-cdk complexes and inhibit their activity. The cip/kip family of cdk inhibitors includes p21, p27Kip1, and p57 (27). We observed enhanced expression of the cyclins and suppression of p21 and p27 in the prostatic lysates of control animals, and this demonstrates unstoppable cell cycle leading to vast increase in cell numbers, ultimately leading to tumor formation. The expression of p21 and p27 has been shown to be significantly downregulated in various cancers including prostate cancer (28). Recent studies demonstrated that human subjects maintaining a high expression of p27 had significantly longer disease-free intervals and longer survival as compared to subjects with low p27 expression (29–31). In the present study, we observed that tocotrienol treatment reversed this effect by significantly enhancing the expression of p21 and p27. Increased levels of p21 and p27 inhibit the kinases thereby in capacitating the cyclins. This cell cycle arrest may drive the cell into apoptosis by recruiting caspases and BAD. Thus, the anticancer effects of tocotrienols, at least in part, could be the result of mediation of the cdk inhibitors/cyclin axis.
In summary, the novel findings we report here are that mixed tocotrienols inhibited prostate tumor growth in TRAMP mice without any signs of toxicity or affecting the expression of the transgene SV-40. Mixed tocotrienols significantly suppressed the progression of high-grade prostatic neoplastic lesions to fully developed tumors by modulating the cell cycle and affecting the expression of proapoptotic proteins. These findings further support the potential use of tocotrienols as prostate cancer chemopreventive agents in humans.
Acknowledgments
This study is supported in part by Institutional Funds to A.-N. T. Kong.
References
- 1.Jemal A, Seigel R, Ward E, Hao Y, Xu J, et al. Cancer statistics. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Petrylak DP. The current role of chemotherapy in metastatic hormone-refractory prostate cancer. Urology. 2005;65:3–7. doi: 10.1016/j.urology.2005.03.053. [DOI] [PubMed] [Google Scholar]
- 3.Klein EA. Can prostate cancer be prevented? Nat Clin Pract Urol. 2005;2:24–31. doi: 10.1038/ncpuro0072. [DOI] [PubMed] [Google Scholar]
- 4.Singh RP, Agarwal R. Mechanisms of action of novel agents for prostate cancer chemoprevention. Endocr Relat Cancer. 2006;13:751–778. doi: 10.1677/erc.1.01126. [DOI] [PubMed] [Google Scholar]
- 5.Wen XQ, Li XJ, Su ZL, Liu Y, Zhou XF, et al. Reduced expression of alpha-tocopherol-associated protein is associated with tumor cell proliferation and the increased risk of prostate cancer recurrence. Asian J Androl. 2007;9:206–212. doi: 10.1111/j.1745-7262.2007.00236.x. [DOI] [PubMed] [Google Scholar]
- 6.Ni J, Wen X, Yao J, Chang HC, Yin Y, et al. Tocopherol-associated protein suppresses prostate cancer cell growth by inhibition of the phosphoinositide 3-kinase pathway. Cancer Res. 2005;65:9807–9816. doi: 10.1158/0008-5472.CAN-05-1334. [DOI] [PubMed] [Google Scholar]
- 7.Suh N, Paul S, Lee HJ, Ji Y, Lee MJ, et al. Mixed tocopherols inhibit N-methyl-N-nitrosourea-induced mammary tumor growth in rats. Nutr Cancer. 2007;59:76–81. doi: 10.1080/01635580701419022. [DOI] [PubMed] [Google Scholar]
- 8.Newmark HL, Huang MT, Reddy BS. Mixed tocopherols inhibit azoxymethane-induced aberrant crypt foci in rats. Nutr Cancer. 2006;56:82–85. doi: 10.1207/s15327914nc5601_11. [DOI] [PubMed] [Google Scholar]
- 9.Barve A, Khor TO, Nair S, Reuhl K, Suh N, et al. γ-tocopherol enriched mixed tocopherols inhibit prostate carcinogenesis in TRAMP mice. Int J Cancer. 2009;124:1693–1699. doi: 10.1002/ijc.24106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nesaretnam K, Ambra R, Selvaduray KR, Radhakrishnan A, Reimann K, et al. Tocotrienol-rich fraction from palm oil and gene expression in human breast cancer cells. Ann NY Acad Sci. 2004;1031:143–157. doi: 10.1196/annals.1331.014. [DOI] [PubMed] [Google Scholar]
- 11.Shun MC, Yu W, Gapor A, Parsons R, Atkinson J, et al. Pro-apoptotic mechanisms of action of a novel vitamin E analog (alpha-TEA) and a naturally occurring form of vitamin E (delta-tocotrienol) in MDA-MB-435 human breast cancer cells. Nutr Cancer. 2004;48:95–105. doi: 10.1207/s15327914nc4801_13. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi K, Loo G. Disruption of mitochondria during tocotrienol-induced apoptosis in MDA-MB-231 human breast cancer cells. Biochem Pharmacol. 2004;67:315–324. doi: 10.1016/j.bcp.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 13.Shah SJ, Sylvester PW. Gamma-tocotrienol inhibits neoplastic mammary epithelial cell proliferation by decreasing Akt and nuclear factor kappaB activity. Exp Biol Med (Maywood) 2005;230:235–241. doi: 10.1177/153537020523000402. [DOI] [PubMed] [Google Scholar]
- 14.Srivastava JK, Gupta S. Tocotrienol-rich fraction of palm oil induces cell cycle arrest and apoptosis selectively in human prostate cancer cells. Biochem Biophys Res Commun. 2006;346:447–453. doi: 10.1016/j.bbrc.2006.05.147. [DOI] [PubMed] [Google Scholar]
- 15.Kumar KS, Raghavan M, Hieber K, Ege C, Mog S, et al. Preferential radiation sensitization of prostate cancer in nude mice by nutraceutical antioxidant gamma-tocotrienol. Life Sci. 2006;78:2099–2104. doi: 10.1016/j.lfs.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, et al. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci USA. 1995;92:3439–3443. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park JH, Walls JE, Galvez JJ, Kim M, Abate-Shen C, et al. Prostatic intraepithelial neoplasia in genetically engineered mice. Am J Pathol. 2002;161:727–735. doi: 10.1016/S0002-9440(10)64228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee IM, Gaziano JM, Buring JE. Vitamin E in the prevention of prostate cancer: where are we today? J Natl Cancer Inst. 2006;98:225–227. doi: 10.1093/jnci/djj066. [DOI] [PubMed] [Google Scholar]
- 19.Heinonen OP, Albanes D, Virtamo J, Taylor PR, Huttunen JK, et al. Prostate cancer and supplementation with alpha-tocopherol and beta-carotene: incidence and mortality in a controlled trial. J Natl Cancer Inst. 1998;90:440–446. doi: 10.1093/jnci/90.6.440. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez C, Jacobs EJ, Mondul AM, Calle EE, McCullough ML, et al. Vitamin E supplements and risk of prostate cancer in U.S. men. Cancer Epidemiol Biomarkers Prev. 2004;13:378–382. [PubMed] [Google Scholar]
- 21.Klein EA. Selenium and vitamin E cancer prevention trial. Ann NY Acad Sci. 2004;1031:234–241. doi: 10.1196/annals.1331.023. [DOI] [PubMed] [Google Scholar]
- 22.Klein EA, Thompson IM, Lippman SM, Goodman PJ, Albanes D, et al. SELECT: The next prostate cancer prevention trial. Selenium and Vitamin E Cancer prevention trial. J Urology. 2001;166:1311–1315. doi: 10.1016/s0022-5347(05)65759-x. [DOI] [PubMed] [Google Scholar]
- 23.Hoffman RM. ACP Journal Club: Selenium and vitamin E, alone or together, did not prevent prostate cancer. Ann Intern Med. 2009;150:JC3-10–11. doi: 10.7326/0003-4819-150-6-200903170-02010. [DOI] [PubMed] [Google Scholar]
- 24.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaplan-Lefko PJ, Chen TM, Ittmann MM, Barrios RJ, Ayala GE, et al. Pathobiology of autochthonous prostate cancer in a pre-clinical transgenic mouse model. Prostate. 2003;55:219–237. doi: 10.1002/pros.10215. [DOI] [PubMed] [Google Scholar]
- 26.Huss WJ, Gray DR, Tavakoli K, Marmillion ME, Durham LE, et al. Origin of androgen-insensitive poorly differentiated tumors in transgenic adenocarcinoma of mouse prostate model. Neoplasia. 2007;9:938–950. doi: 10.1593/neo.07562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DelSal G, Loda M, Pagano M. Cell cycle and cancer: critical events at the G1 restriction point. Crit Rev Oncog. 1996;7:127–142. doi: 10.1615/critrevoncog.v7.i1-2.80. [DOI] [PubMed] [Google Scholar]
- 28.Claudio PP, Zamparelli A, Garcia FU, Claudio L, Ammirati G, et al. Expression of cell-cycle-regulated proteins pRb2/p130, p107, p27(kip1), p53, mdm-2, and Ki-67 (MIB-1) in prostatic gland adenocarcinoma. Clin Cancer Res. 2002;8:1808–1815. [PubMed] [Google Scholar]
- 29.Cote RJ, Shi Y, Groshen S, Feng AC, Cordon-Cardo C, et al. Association of p27Kip1 levels with recurrence and survival in patients with stage C prostate carcinoma. J Natl Cancer Inst. 1998;90:916–920. doi: 10.1093/jnci/90.12.916. [DOI] [PubMed] [Google Scholar]
- 30.Cordon-Cardo C, Koff A, Drobjnak M, Capodieci M, Osman I, et al. Distinct altered patterns of p27KIP1 gene expression in benign prostatic hyperplasia and prostatic carcinoma. J Natl Cancer Inst. 1998;90:1284–1291. doi: 10.1093/jnci/90.17.1284. [DOI] [PubMed] [Google Scholar]
- 31.Yang RM, Naitoh J, Murphy M, Wang HJ, Phillipson J, et al. Low p27 expression predicts poor disease-free survival in patients with prostate cancer. J Urol. 1998;159:941–945. [PubMed] [Google Scholar]


