Abstract
Currently there are no viable treatment options for patients with debilitating inherited retinal degeneration. The vast variability in disease-inducing mutations and resulting phenotypes has hampered the development of therapeutic interventions. Gene therapy is a logical approach, and recent work has focused on ways to optimize vector design and packaging to promote optimized expression and phenotypic rescue after intraocular delivery. In this review, we discuss ongoing ocular clinical trials, which currently use viral gene delivery, but focus primarily on new advancements in optimizing the efficacy of non-viral gene delivery for ocular diseases. Non-viral delivery systems are highly customizable, allowing functionalization to improve cellular and nuclear uptake, bypassing cellular degradative machinery, and improving gene expression in the nucleus. Non-viral vectors often yield transgene expression levels lower than viral counterparts, however their favorable safety/immune profiles and large DNA capacity (critical for the delivery of large ocular disease genes) make their further development a research priority. Recent work on particle coating and vector engineering present exciting ways to overcome limitations of transient/low gene expression levels, but also highlight the fact that further refinements are needed before use in the clinic.
Keywords: non-viral gene therapy, eye, retina, subretinal delivery, nanoparticles
Graphical Abstract
Introduction
Inherited retinal degeneration is a primary cause for debilitating impairments in vision among working age people in the developed world(1), and development of effective therapeutics for inherited retinal disease is a primary research goal. Retinal dystrophies are categorized roughly into two categories: 1) retinitis pigmentosa (RP), a broad term encompassing phenotypes that initially affect rod photoreceptors and peripheral vision, and may later be associated with cone cell death, and 2) macular dystrophy (MD), a term encompassing widely varying phenotypes in which macular or central vision is initially affected(2, 3). Though MD can be caused by defects in cone photoreceptors, it can also be caused by defects in the retinal pigment epithelium (RPE). For example, Stargardt's MD is associated with retinal pigment epithelium (RPE) atrophy (4) and subsequent degeneration of the cones in the macular region of the retina and leads to central vision loss. There are also various syndromic forms of inherited retinal diseases (particularly leading to RP) which exhibit degenerative processes not only in the eye but also in other organs (5). The most common form of syndromic RP is Usher syndrome which comprises hearing loss in addition to visual impairment (for review see(6)).
Inherited retinal diseases are complex. Causal mutations in more than a hundred genes have been identified for inherited retinal degeneration and there are still many unidentified (7). In addition, most disease genes contain multiple pathogenic mutations leading to a wide variety in the severity and age of onset of the disease (8, 9). Mutations in certain genes like the structural protein peripherin-2 can induce RP as well as MD, depending on the site of mutation involved (10, 11) and RP can be inherited in a dominant or recessive manner (5). There is also a large degree of intra- and inter-familial phenotypic heterogeneity and often incomplete penetrance even within groups of patients carrying the same mutation. This wide variability in phenotypes has led geneticists to look for modifiers or digenic/polygenic disease, topics that are still being explored (12-14). Many patients with RP develop symptoms in the third or fourth decade of life, however, there are more severe forms of retinal degeneration, such as Leber's congenital amaurosis (LCA), which can lead to complete loss of vision before patients reach puberty (for review see (15)). Gene therapy is a desirable treatment method, but the genetic and phenotypic heterogeneity in inherited retinal degeneration makes the development of a single therapeutic approach a very challenging process.
Gene augmentation therapy is a theoretically straightforward option for the treatment of recessive disease (usually caused by loss-of-function mutations)or a dominant disease associated with haploinsufficiency phenotype (16, 17). In these cases, supplementation with a healthy copy of the gene should be effective, provided that the degeneration is not yet severe and that a sufficient amount of gene expression can be obtained from the therapeutic vector. More challenging is designing a treatment for dominantly inherited degenerations caused by gain-of-function mutations. Patients have one healthy and one mutated allele, and the mutant protein interrupts normal function leading to retinal degeneration. Multiple mechanisms (many as yet poorly understood) result in this degeneration, so many different treatment approaches must be considered. A common gain-of-function disease mechanism occurs when mutant proteins are misfolded/aggregated (18, 19)or are mislocalized (20) leading to endoplasmic reticulum (ER) stress, and ultimately cell death (21). Different pharmaceuticals can be used to alleviate ER stress and prolong the life of photoreceptors. Proof-of-principle for this therapeutic approach has been shown in multiple cases, for example, increasing the cellular unfolded protein response via pharmacological induction of the heat-shock protein rescued retinal function and morphology in a P23H rat model of rhodopsin-associated RP (22). Other dominant mutations cause cell death by other mechanisms, for example some mutations in guanylate cyclase lead to constitutive activity and overproduction of cGMP, causing LCA(23, 24). A key defining feature of these dominant diseases is that reintroduction of the WT allele is not sufficient to completely prevent retinal degeneration.
However, several gene therapy approaches have been tested for dominant diseases. For example, the administration of neurotrophic factors (either as purified proteins or as gene therapy vectors) has been beneficial in some cases (25), however, without the correction of the underlying disease mutations, these treatments merely delay the degenerative process. Thus other research has focused on ways to eliminate the undesirable transcript or mutation. Silencing RNA (siRNA) which is complementary to unwanted transcripts prevents their efficient translation,and knockdown approaches have been tested for dominant phenotypes (26). Geneknockdown in the retina has been tested using multiple modalities and multiple disease genes, including siRNA (rhodopsin, peripherin-2 and GCAP1) (27-30), and zinc-finger nucleases (for rhodopsin and USH1C)(31, 32). The recent development of additional genome editing systems such as CRISPR/Cas9 that can be used to correct the disease-causing mutation in the native allele also provide great hope for the development of effective treatments for dominant diseases (33). Preliminary results from the Bakondi group utilizing CRISPR/Cas9 genome editing to correct the RP phenotype in a rhodopsin mutant rat model (S334ter-3) are encouraging (Bakondi B, et al., Gene Editing Corrects The Retinal Dystrophy Phenotype in S334ter-3 Rats, ARVO2015, Abstract number 3183), and results from a complete study are highly anticipated. However, there are several limitations to knockdown technologies that remain to be overcome including the difficulty in designing a knockdown construct which can bind the mutated version but not the healthy sister allele, potential off-target effects, and concerns about delivery and expression levels which affect all types of gene delivery strategies. The new technology is intensely studied right now and will hopefully lead to a major breakthrough in future therapy of retinal degeneration.
However, even with the new treatment possibilities, there are still obstacles for any type of gene therapy method including, 1) successful delivery of the therapeutic DNA to the affected tissue, 2) its uptake by the target cells and 3) efficient expression of the transfected vector. All three parts will be discussed in this review for non-viral vectors with a special emphasis on their applications in the eye.
1. Therapeutic approaches for ocular diseases with gene therapy: pre-clinical testing and clinical trials
The retina is an excellent system to test newly emerging gene therapy vectors due to its relative immune-privilege, the plethora of non-invasive procedures available to assess functional and structural rescue, and the ability to deliver therapeutic agents directly to specific tissues of the eye. Thus gene supplementation therapy in the eye is a mature field and several clinical trials are either completed or underway. Thus far, the ocular gene therapy trials have used viral delivery systems, either adeno-associated virus (AAV) or lentivirus. However, concerns regarding the immune response to viral vectors, limitations in payload capacity, and production costs have spurred investigation of non-viral alternatives. Though these non-viral approaches have not yet been tested in ocular clinical trials,they have been under investigation in animal models for several years and may be poised to enter the clinical trial phase in the near future.
1.1. Current gene therapy clinical trials for retinal degeneration
So far, most clinical trials for gene therapy in inherited retinal degeneration are in phase I or II to test possible toxicity and adverse effects of the applied vectors and gather preliminary data on the efficacy(34). However, one of the earliest successful therapies which uses AAV to target LCAhas successfully completed phase I and II in several different clinical trials (NCT00481546/NCT01496040/NCT00749957/NCT01208389/NCT00643747/NCT00516477) ((see(35, 36) for the most recent information) and is now in phase III (NCT00999609). In 1997, a mutation in the RPE-specific gene RPE65 was identified as causative agent for LCA in several patients with a very early onset vision loss (37). The identification of similar phenotypes in Swedish briard dogs (38), a naturally occurring mouse model of LCA (39) with RPE65 mutations, and an RPE65 knockout mouse line (40)have facilitated the development of a feasible gene therapy vector for RPE65-associated LCA by providing the means to test it. Delivery of the RPE65 gene to RPE cells in the retina of dogs (41, 42) and mice (43, 44) showed long-term improvement of retinal function and few adverse effects leading to the initiation ofthe different clinical trials. Patients were treated with AAV containing the RPE65 cDNA and either a ubiquitously expressed promoter or the native RPE65 promoter(45-48). None of the trials revealed any severe adverse effects, and reported moderate improvement of visual function in some of the patients. Long-term follow-up studies showed that the increase in visual acuity is stable for up to 3 years after treatment(49) despite a progressive retinal degeneration (36). However, recently released follow-up for up to six years has showed continued diminishment in the areas of improvement (50, 51). Given the already modest nature of the improvement, these results highlight the need for further understanding of the mechanism underlying the disease defect in order to improve on the design of therapy.
Three other retinal disease genes are currently being delivered by AAV in clinical trials. Choroideremia is an X-linked retinal degenerative disease with a prevalence of 1 in 50,000 and is associated with early onset photoreceptor and RPE degeneration and a marked choroidal atrophy (for review see (52)). Mutations in the Rab escort protein-1 (REP-1) are responsible for this disease (53) and cause a defect in membrane association of Rab GTPases which are crucial for proper protein trafficking inside the cell (54). Two different approaches were tested in a mouse model for choroideremia: a lentiviral vector carrying the elongation factor 1 promoter known to express in ocular cells and the REP-1 cDNA(55) and an AAV2 vector with a ubiquitously expressed promoter and the REP-1 cDNA(56). Both vectors showed successful transfection of RPE cells in mouse models, but AAV2 was chosen for the phase I/II clinical trial (NCT01461213). Though the trial is ongoing, the 6 patients with advanced retinal degeneration who were initially enrolled showed increased visual acuity after treatment, and the subretinal injection did not cause any detectable damage at 6 months post-treatment, the latest time point assessed so far (57). Clinical trials for Leber hereditary optic atrophy (LHOA) caused by mutations in the mitochondrial ND4 gene (NCT02161380) (58) and for autosomal-recessive RP caused by mutations in the RPE-specific MERTK gene (NCT01482195) (59) have just been started and are currently recruiting patients. Both trials use AAV2 to subretinally deliver the wildtype version of their respective mutated genes, and results will be eagerly anticipated. AAVs are also being used in clinical trials for nonmonogenic disease. There are currently at least two open trials for the treatment of exudative age-related macular degeneration with AAV carrying a VEGF scavenger receptor (NCT01024998/NCT014948050) as an attempt to replace the costly anti-VEGF antibody injections which are currently in use.
Although AAV has been the viral vector of choice for retinal gene therapy clinical trials thus far due to its high transfection rate and its tropism toward RPE and photoreceptor cells (60), it cannot efficiently package more than 5 kb (61), a limitation when considering large disease genes. In contrast, lentiviruses have a packing capability between 8 to 10 kb (62), and have also been demonstrated to transfect the RPE cell layer with high efficiency after subretinal injection (63), though most serotypes do not transfect photoreceptors cells well (64). However, the equine infectious anemia virus (EIAV) shows modest transgene expression in rods and cones especially with the addition of photoreceptor-specific promoters (65). Two mouse models of retinal degeneration were treated effectively with EIAV-delivered transgenes: an Usher syndrome model showed rescue of light-induced photoreceptor cell death (66) and a Stargardt's disease model demonstrated a reversal in the degeneration (67). The former led to development of a drug named UshStat which is presently under investigation in a phase I/II clinical trial currently recruiting study subjects (NCT01505062). The latter showed a favorable safety profile in extensive studies in rabbits and macaques (68) and is currently in a phase I/II clinical trial in patients with Stargardt's disease under the brand name StarGen (NCT01367444) and results are pending.
1.2. Therapeutic benefits of non-viral ocular gene therapies in pre-clinical testing
As clinical trials with viral vectors are progressing, there are still concerns regarding their safety and efficacy in human patients sparking interest in non-viral gene delivery approaches. Presently, nonviral gene therapy vectorsare not part of any active clinical trials for retinal degeneration but have been tested for other diseases. For example delivery of the p53 gene with liposomal nanoparticles (NPs) showed a favorable safety profile and appreciable transgene expression in solid tumors (NCT00470613) (69). Similarly, several different types of non-viral gene delivery, including cationic liposomes(NCT00470613) (70) and polylysine compacted NPs (71) have been tested for their ability to mediate improvements in cystic fibrosis patients.
Although no ocular gene therapy trials have yet used non-viral vectors, their therapeutic benefits have been achieved in animal models. For example, polyethylene glycol-substituted polylysine (CK30PEG) NPs (further discussed below) have been used in several disease models (for review see (72)). These NPs are non-toxic, non-immunogenic and have no adverse effects on the retina after subretinal injection in wildtype mice or mice with retinal degeneration (73, 74), and importantly have a large carrying capacity (tested up to 14 kbp in the eye (75) and up to 20 kbp in the lung (76)). When subretinally delivered to neonatal or adult mice, CK30PEG NPs were capable of mediating structural and functional improvements in several different mouse models carrying mutations in photoreceptor specific genes, the rds+/− model of RP(74, 77), the Abca4−/− model of Stargardt disease (75), and the rho−/− and P23H models of rhodopsin-associated RP ((78) and Naash MI, Nanoparticle-based gene therapy for ocular diseases: an update, ARVO 2015 Abstract #3185). They have also been used to mediate improvements in the rpe65−/− LCA model (79, 80), indicating that they are suitable for both photoreceptors and RPE targeting. In each case, the improvement persisted anywhere from 8 months to 2.5 years (depending on the ages assessed). In spite of these positive outcomes, levels of gene expression from subretinal injection of NPs have yet to meet wildtype levels and drop over time. As a result they have provided incomplete rescue, highlighting the need for further refinements in nanoparticle formulation, vector content and delivery approach.
In addition to CK30PEG NPs, various other non-viral ocular gene delivery strategies have been explored. One of the latest developments in this field, and one of the few non-viral vectors to extend testing into therapeutic animal models as opposed to just expression of reporter constructs, is the use of liposome-protamine-DNA complexes (LPD) to deliver the RPE65 gene(81). These liposomes incorporate cell penetrating and nuclear targeting peptides to improve gene expression/delivery compared to untargeted liposomes and carry an RPE-specific promoter to limit ectopic expression(81). Subretinal injection of LPDs into RPE65 knockout mice at five days of age (P5) led to improvements in cone ERG function and increased cone survival. Though extensive safety studies will have to follow before a clinical trial (81), this technology represents an additional promising approach.
In the remainder of this review, we will focus on strategies that are being employed to improve the efficacy of non-viral gene therapy in the eye, including delivery approaches, vector modifications, and non-viral packaging methods.
As comparison of clinical trials applying viral versus non-viral vectors clearly shows, non-viral gene therapy vectors are still far behind their viral counterparts. Their improved safety profile gives them a huge advantage however for future applications. As the efforts into the non-viral DNA compaction methods are intensified, we can be sure that these will soon catch up in terms of transgene expression levels and transfection efficiency with the AAV vectors currently in clinical trials.
2. Delivery strategies for non-viral gene therapy in the retina
The delivery route for non-viral gene therapy vectors is crucial for the success of the treatment and should lead to the highest transfection rate for the targeted cell type and the least risk for severe adverse effects (for a summary see Table 1).
Table 1.
Non-viral therapeutic delivery methods can dictate the transduction efficiency of different ocular cell types
| Delivery Method | Target cells | Pro | Con | Ref. |
|---|---|---|---|---|
| Topical | Cornea | No injection | Inefficient penetration | (82-84) |
| Intravenous | Mostly inner retina | No adverse effects due to injection at the eye | Opsonization and phagocytosis of particles Off-target delivery to other tissues |
(85-87) |
| Periocular | Corneal epithelium Photoreceptors RPE |
Non-invasive | Low transfection rates for retina and RPE | (88, 89) |
| Intravitreal | Inner retina | Non-invasive Adverse effects rare | Vitreous humour aggregates particles. Ocular infections and retinal detachment can occur |
(90-94) |
| Subretinal | Photoreceptors RPE | Highest transfection rate | Invasive Retinal detachment can cause cell death |
(95-98) |
| Suprachoroidal | Photoreceptors RPE | No retinal detachment | Very inefficient transcleral transport | (89) |
2.1. Topical administration
Topical administration of therapeutics is by far the least invasive form of delivery, and is routinely used for pharmacological treatment of anterior segment diseases such as glaucoma. However, it is also the least efficient for retinal targeting due to the many barriers surrounding the eye. After corneal application of liposome-compacted DNA, transgene expression is scarce and only detectable in the iris, limbus and conjunctiva (82). Internalization of drugs into the corneal epithelium can be achieved (83) as well as transfection of corneal cells with AVV with therapeutic transgenes (84), but gene delivery to the retina via the topical administration route is not currently effective.
2.2 Intravenous injection
The delivery of therapeutic compounds directly into the eye is always associated with the risk of damaging the fragile structure of the retina or inducing a detrimental inflammatory reaction. To avoid any unnecessary adverse effects caused by the delivery route, compounds can be administered directly to the bloodstream. The main barrier for drugs to reach the retina after intravascular delivery is the blood-retina barrier, a structure much like the blood-brain barrier (99) which prevents diffusion of most large compounds into the retina (86). While the non-specific transport across the blood retina barrier is rather inefficient in general, particle size seems to be of minor importance, at least for diameters up to 460 nm (100). To overcome this and allow non-viral NPs to penetrate the retina after systemic administration, particles have been targeted with an antibody against the transferrin receptor present in retinal vascular cells. This strategy led to widespread expression of the transgene in ocular tissues, namely in the inner retina and the RPE (87). Though this approach is less invasive, one of the main disadvantages of delivery of genetic therapies in the blood stream is that serum proteins tend to cause opsonization and swift removal of particles from circulation (85). This massively decreases bioavailability, but can be ameliorated by chemical modification of the particle as discussed in section 5. Another concern is the volume of therapeutic needed to achieve efficacious concentrations in the eye (given the volume of the systemic circulation and potential unavoidable uptake into other tissues), but with proper targeting and vector specificity (to prevent ectopic expression) intravenous delivery could be considered.
2.3 Periocular injections
In addition to the more invasive but more common subretinal and intravitreal injections (discussed below), researchers have also developed other routes for injection of drugs into the eye. The injection of non-viral RNA particles in the subconjunctival space showed appreciable transfection of cells in the retina, but the majority of the particles migrated towards the cornea (88). It is therefore not surprising, that this method has been mainly used in the delivery of gene therapy vectors for the cornea (101, 102), especially as only very small particles (20 nm diameter) seem to be able to successfully penetrate into the inner ocular tissue (103).
2.4 Intravitreal injection
Though intraocular (intravitreal and subretinal) injections are significantly more invasive than other delivery methods, they are currently the most widely used for delivery of genetic therapies, largely due to the increased efficacy with which they are associated. The main barrier for therapeutic compounds after intravitreal injection is the vitreous itself. Despite its clear appearance, the vitreous contains a 3D meshwork of connected fibers of collagen, hyaluronan and other proteoglycans like heparin and chondroitin sulfate which gives it its jelly-like texture (104-106). This structure caninterfere with diffusion of particles both by steric hindrance and also because charged delivery vehicles can interact with the charged proteins in the vitreous. It has been shown that polystyrene nanospheres are completely immobilized in the vitreous humor and DNA-compacting lipoplexes aggregate immediately upon contact with the vitreal meshwork (91). The charge of a NP is crucial for this process, as experiments have demonstrated that cationic serum albumin particles do not show any vitreal penetration, while anionic particles of the same kind penetrate well (107). Penetration through the vitreous can be altered by coating the particles, a topic which will be discussed later.
In addition to charge, particle size also has a large influence on diffusion rates. Studies with fluorescently labelled polystyrene nanoparticles showed an average pore size in the vitreal mesh of around 550 nm, allowing particles of up to 500 nm in diameter almost uninhibited diffusion (108). Rare pores can show sizes up to 1000 nm, which also explains the ability of larger, uncharged particles to diffuse through the vitreous(94, 108). The diffusion paths and rates of various particles in the vitreous have been extensively studied with single-particle tracking (SPT) microscopy. This method allows the tracking of single particles and therefore also the description of specific paths instead of overall distribution (109). By teaming up SPT with an ex vivo model of intact bovine vitreous, the behavior of nanoparticles can be described in detail in relation to size and charge which is demonstrated with polystyrene beads (94). In addition, DNA compacted with polylysine or synthetic polymers like poly(amide amine) (CBA-ABOL) into nanoparticles analyzed with the same method showed unhindered passage through the vitreal meshwork when their charge was neutralized by the addition of PEG (see section 5 below) (94, 108). Intravitreal delivery of NPscan be effective for gene delivery in the retina with many different particles including liposomes or PLGA particles (110, 111). Successful diffusion of particles from 350 to 600 nm in diameter through the whole retina with subsequent transfection of photoreceptors and RPE cells was observed after intravitreal application(112, 113).However, studies show a much higher transfection rate for cells in the inner retina after intravitreal delivery, with only inefficient transfection of outer retinal cells using this method (90, 92). Delivery to the inner retina, can be beneficial, for example for the treatment of glaucomatous optic neuropathy (82), but the lack of penetration to the outer retina means that intravitreal injection has largely been avoided in favor of subretinal injection for photoreceptor and RPE delivery. Since intravitreal injections are already routinely used for ocular drug delivery and are less invasive than subretinal delivery, there is great interest in improving outer retinal targeting of genetic therapies after intravitreal delivery. Nevertheless, risks are associated with the intravitreal application including possible retinal detachment and endophthalmitis which occur, though with low incidence (93, 114).
2.5 Subretinal injection
While the risk of retinal detachment after intravitreal injection is low, detachment is a routine feature after subretinal injections, as the introduction of therapeutic liquid induces the separation of the photoreceptor from the RPE cell layer (95, 98). This process is damaging to the retina, as the photoreceptors rely on the structural support and the nutrients from the RPE cell layer especially for regeneration of photopigments (115). It is therefore not surprising that detachment of the retina is associated with photoreceptor cell death and loss of visual function (96-98), a pathology which can also be observed in patients with retinal detachment due to other reasons (116). Though the retina typically reattaches, this detachment is a critical limitation and one that is a great concern clinically. In addition to detachment, subretinal injection can also be associated with inflammatory response and recruitment of immune cells into the subretinal space. For example, one of the viral vectors currently in use in gene therapy trials, StarGen, causes a cellular inflammatory response after subretinal injections in macaque and a certain amount of retinal degeneration and atrophy is detectable (68). The first data about the safety of subretinal injections in the RPE65 clinical trials show that retinal detachment occurred in the study participants and was associated with some minor adverse effects. Nevertheless, results thus far from the clinical trials (choroideremia NCT01461213 and LCA NCT00999609) indicate thatthe improvements in function in the treated eyes seem to outweigh the detrimental effects of the delivery in most patients (57, 117). There are currently no data available on the impact of repeated injections in human eyes, which may have more severe consequences, thus underscoring the need for long-lasting therapeutics. Studies in mice have shown that subretinal delivery is by far the most efficacious for the transfection of photoreceptor and RPE cells with non-viral vectors and yields the highest transgene expression in the outer retina (74, 90). This route of application is therefore chosen for most applications in inherited retinal disease in spite of its invasiveness.
2.6 Suprachoroidal injection
An additional option is delivery to the suprachoroidal space, between choroid and sclera, which prevents the detrimental detachment seen in subretinal injections while still delivering the therapeutic compound close to the RPE. With this method, widespread transfection of RPE cells with naked DNA can be achieved, but only with the aid of electroporation (89). Suprachoroidal injections have a very beneficial safety profile, as they do not induce any retinal detachment or bleeding and with proper DNA packaging, it might be possible to overcome the scleral barrier without electroporation to make this delivery method more clinically viable (118). However, the transcleral diffusion rate of nanoparticles is very low even for small particles up to 20 nm in diameter, while larger particles (200 nm) do not diffuse across the sclera at all (119).
From a clinical point of view, the only currently feasible application methods for future ocular gene therapy in human patients are the intravitreal and the intravenous approach. The other applications are either too invasive or have been shown to be little effective. While intravitreal injections still bear a certain risk of adverse effects, intravenous applications generally have a higher toxicity profile due to the widespread distribution of the therapeutic compound and the larger doses needed to reach a significant concentration of drugs in the target tissue. Nevertheless, if the efforts for the search of tissue-specific, targeted particles (see section 3) are successful, this issuewill be reduced and intravenous injections may become the method of choice.
3. Improvement of cellular and nuclear uptake of non-viral vectors
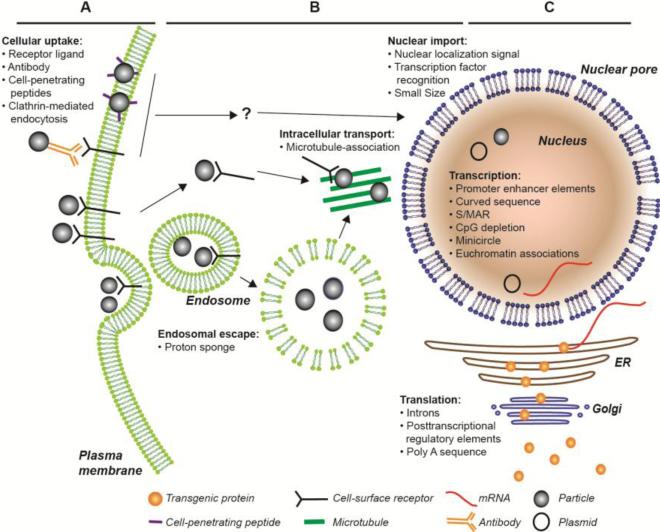
Efficient uptake of therapeutic vectors in the target cell is crucial for the success of the treatment. The requirements in this process depend very much on the nature of the vector, but in this case, we solely focus on DNA plasmids for the delivery of transgenes. Once the particle has reached the cell membrane, there are several obstacles it has to overcome to reach the nucleus and achieve expression. Modifications on the particle and the vector described in this section are designed to facilitate this process (see Figure 1).
Figure 1.
Diagram illustrating some of the ways that non-viral therapies can be taken up and expressed in cells. Bulleted lists indicate places/methods where modifications to the particle or vector can be used to improve transfection efficiency or expression. Question marks indicate trafficking pathways that remain to be elucidated.
3.1. Cellular uptake
Once delivered to the site of interest, the plasma membrane is the first barrier for effective gene therapy. RPE cells have a high rate of phagocytosis due to their involvement in photoreceptor disc renewal and can readily take up particles in their environment (120). Due to this activity, RPE cells can be easily transfected, even with naked (i.e. uncompacted) plasmid DNA(80), which partially explains the success of RPE-targeting gene therapy trials. Photoreceptors on the other hand, the main site of expression of many retinal disease genes, do not exhibit phagocytosis and are notoriously hard to transfect. Nevertheless, even non-phagocytic cells take in many different proteins to maintain their function and these endocytic systems can be taken advantage of. The first system discovered for receptor-mediated endocytosis involves clathrin-coated pits which form at the plasma membrane upon binding of the ligand to its receptor. The receptor-ligand complex is then internalized and ultimately fuses with protein-processing organelles in the cytoplasm (121). Subsequently, many clathrin-independent endocytic pathways have been described (122, 123), and this knowledge has been used to increase specificity and uptake of non-viral vectors by modifying particle envelopes to target receptors on the cell surface.
Significant progress in this direction has been made by coating drug carriers with antibodies specific for receptors on target cells. Cancer cells, for example, are known to express high levels of certain integrins and by covalently linking an anti-integrin antibody to NPs, cytotoxic compounds can be delivered specifically to target cells thus minimizing adverse effects (124). This method has also proven valuable for the delivery of therapeutic DNA in hard to transfect neurons. An antibody specific for the neurotrophin receptor p75NTR (125) was coupled to polyethylenimine NPs carrying a reporter plasmid. Neonatal rats were injected with these particles and GFP expression was reported exclusively in motor neurons expressing p75NTR while non-targeted particles yielded very little expression. These are exciting results, as the p75NTR receptors becomes highly upregulated in disease damaged motor neurons and could therefore be used as a feasible therapeutic vector (125). Incorporating such antibody-targeting into ocular therapeutics may help increase cellular uptake in hard to transfect cells like photoreceptors.
In addition to antibodies, small receptor ligands have also been shown to enhance the uptake rate of NPs. Many tumor cells express large amounts of growth factor receptors such as the EGF receptor (126). Delivering chemotherapeutic drugs like cisplatin (127) or doxorubicin (128) in EGF targeted NPs largely decreases their toxic side effects. Similarly, folate and transferrin may be useful for NP targeting to malignant tumors even after systemic administration (129, 130). Transferrin-mediated uptake has also been explored as a way to facilitate uptake from the vasculature into the retina (87). As mentioned above, intravenous delivery of transferrin-targeted particles led to transgene expression in RPE cells, as well as in neurons in the inner retina (87). An alternative approach is the use of hyaluronic acid in NPs which targets the CD44 receptor widely expressed in retinal glia cells (131) and the RPE cell layer (110). The incorporated hyaluronic acid increases the transfection efficiency in RPE cells in vitro (132) as well as in vivo in the rat eye (110). The use of the CD44 pathway not only significantly increases the efficiency of the uptake of the particles, but also decreases the intracellular degradation rate of the particles which is associated with other uptake pathways (133).
Instead of using cell-surface receptors to increase uptake rates, cell-penetrating peptides (CPP) can also be used. These peptides are coated on the outer shell of the particles and originally take their sequence from various viruses. One of the earliest CPP's identified is the Tat protein of human immunodeficiency virus 1 (HIV-1), which mediates entry into the cell without interacting with a specific receptor (134). Similarly, the structural VP22 protein from the herpes simplex virus is able to enter cells without any external aid (135). The Tat protein has been successfully incorporated into lipid NPs and used for the delivery of transgenes to retinal cells (81). However, not all of the CPPs work in vivo in the retina (136, 137), so recent studies have used synthetic peptides which were specifically engineered for ocular tissue and yielded much higher success rates (138).
3.2. Nuclear entry
After successfully entering the cell, the transgenic DNA has to reach the nucleus to escape cytoplasmic degradation and to be transcribed effectively. Nuclear delivery in actively dividing cells is straightforward, as the nuclear envelope breaks down during mitosis allowing plasmids to enter (139). However, in post-mitotic cells like photoreceptors the nucleus is only accessible via the nuclear pores, which have an opening of 9 nm in diameter allowing ions and smaller proteins to diffuse freely, but excluding molecules larger than ~50 kDa (140). Larger molecules can enter the nucleus by active transport, which depends on the presence of a nuclear localization signal (NLS) (140). Many viruses have evolved these targeting sequences, and the minimal sequence was identified from SV40 as a single seven amino acid stretch (PKKKKRKV) (141). These NLS sequences can interact with different proteins inside the cell like nuclear pore transport proteins or transcription factors which lead to active translocation into the nucleus (140, 142). Inclusion of NLSs on the envelope of the particle can be used to directly target non-viral vectors to the nucleus, an approach that has been tested successfully in the eye(81). Studies have shown that the inclusion of such a sequence can increase nuclear accumulation tremendously, while plasmids without such a signal barely enter the nucleus at all (142-144). The peptide protamine also contains a NLS and its addition to compacted DNA increases the transgene expression in cultured ARPE-19 cells by more than 6-fold (145). Another way to target non-virally delivered DNA or NPs to the nucleus is to take advantage of other cytoplasmic proteins that have the tendency to translocate to the nucleus. For example, CK30PEG NPs have been shown to traffic to the nucleus of airway epithelial cells by associating with nucleolin and glucocorticoid receptor, and stimulation of nuclear translocation of the glucocorticoid receptor with cortisone improves transfection efficiency (146).
In addition to the peptide/protein based approaches, early research with plasmids based on SV40 indicated a region of the viral genome around the origin of replication enhancingnuclear uptake of the plasmid in non-dividing cells (147). Further investigation narrowed down the necessary sequence for this process to a 72 bp repeat (148). Introduction of this sequenceinto a CMV-driven expression vector showed a 20-fold increase in transgene expression after delivery to skeletal muscles of mice (149). In addition, some plasmids can interact with transcription factors via binding sites in their promoters leading to increased rates of movement across the cytoplasm to the nucleus. This often takes advantage of the microtubule cytoskeleton and can be beneficial for effective gene transfer (142).
Another approach to increasing nuclear localization is to decrease the size of the delivered vector. Minicircle DNA (see description below) was developed to prevent gene silencing caused by the bacterial backbone, but experiments in vitro revealed that the smaller size resulting from the excision of the backbone also increases the transfection efficiency (150). To decrease the size of the DNA plasmid even further, it has been suggested that type II topoisomerase can be used to tighten the DNA into a so-called miniknot which would be unwound inside the nucleus by the host's topoisomerase enzymes (151). Similarly, the ability of CK30PEG NPs to transfect non-dividing cells has been in part attributed to their small size; CK30PEG NPs compacted with acetate as the lysine counterion are rod shaped with a minor diameter of 8-11 nm (76, 152). In the meantime, CK30PEG particles have been successfully used to transfect photoreceptor cells in the retina (75, 153).
As mentioned at the beginning of this section, we mainly discuss DNA plasmid vectors in this review.For vectors containing mRNA instead of the corresponding DNA sequence, nuclear entry is not necessary and therefore not an issue. Successful transgene expression with compacted RNA nanoparticles has been shown with high expression levels, but due to the unstable nature of RNA only for short time periods(154, 155).
Recent research has clearly shown that targeted nanoparticles have a clear advantage over their non-targeted counterparts with higher transfections efficiencies and a far better safety profile. The ability to target one specific set of cells or maybe even only diseased cells is likely to reduce the issue of off-target toxicity and will also decrease the costs of treatment by limiting the amount of therapeutic particles needed. Nuclear entry is a topic that has been addressed only minimally in retinal gene therapy so far. It is however immensely important to do so in the future, as the post-mitotic status of photoreceptors naturally has a negative effect on the transgene expression efficiency in the retina.
4. Vector engineering
Non-viral gene therapy is a desirable approach because of its safety profile, ease of production, and large payload capabilities. However, even after reaching the nucleus effective therapy requires persistent, elevated transgene expression. Coupled with insufficient transfection rates, weak transgene expression and rapid downregulation have thus far impeded successful clinical application of non-viral therapies in the eye. To improve the expression levels and longevity of these vectors, proper design of the expression vector is extremely important and has been anactive research field in recent years (see Figure 1B,C). Luckily, the availability of non-viral packaging methods which can deliver larger DNA payloads (76) has made inclusion and testing of additional DNA regulatory elements easier.
4.1. S/MARs
A number of different factors can lead to loss of gene expression over time including loss of vector DNA and silencing of the vector due to epigenetic or transcriptional changes. Transgene loss can occur due to the non-integrated state of non-virally delivered DNA. Some viruses like SV40 have the ability to replicate episomally inside the nucleus of an infected cell without integration into the host genome (156). The virus achieves this by producing the T antigen, which has the ability to transform and immortalize infected cells, which unfortunately makes it unfit for the use in human gene therapy (157). Piechaczek et al. overcame this limitation by replacing the sequence for the T antigen in the vector with the sequence for a scaffold/matrix attached region (S/MAR) of the human interferon β-gene to create the now extensively used pEPI plasmid vector (158). The S/MAR sequence has been shown to interact with proteins in the nuclear matrix thereby stabilizing the vector inside the nucleus for episomal replication in dividing cells (159). Vectors with this S/MAR sequence do not show any signs of integration, but can stay inside dividing cells for more than 100 generations (158). S/MARs can also promote increased transgene expression (80, 160, 161) apart from benefits conferred by self-replication, indicating that they can also be useful in post-mitotic cells.However, this increased expression depends on the vector and system used and is not always detectable (162). In vivo studies using the pEPI vector have shown transgene expression in the RPE cell layer of the retina up to two years after injection(80), even though decreased expression over time is still a problem(75, 80).
4.2 Epigenetic regulation
In addition to vector loss, gene silencing is often associated with the methylation of CpG islands (cluster of C and G nucleotide pairs) in the promoter region (163), and with the presence of heterochromatin. In recent years, several options have been investigated to improve the transcription rate in plasmids after transfection. The discovery of the high activity of the cytomegalovirus (CMV) promoter in mammalian cells raised hopes for swift development of effective gene therapy vectors (164), but this promoter is silenced quickly in vivo due to extensive methylation (165). Bacterial backbones are necessary for the efficient production of plasmids, however, delivering bacterial backbones in vivo can lead to silencing, both due to methylation and due to heterochromatin associations(166, 167). Several options are available to limit the adverse effects of the backbone. One is to deliver linearized DNA fragments that have been removed from the backbone. An alternative is to place phage-integrase specific recombination sites on both sides of the eukaryotic expression cassette in a plasmid which also carries the integrase gene. When expression of the integrase is induced in bacteria, the bacterial backbone is excised leaving behind a circular miniplasmid ready for delivery (150). Recent work has shown that minicircles can lead to sustained gene expression, are less susceptible to methylation, and retain an active chromatin conformation (168).The production of minicircle DNA is rather inefficient and contamination with parental plasmids and circular backbone have prevented large-scale use of this technology, but improvements like the inclusion of restriction sites leading to the degradation of the bacterial backbone have helped make the approach more practical(169). Recently, minicircles have been used to successfully deliver shRNA and rescue the phenotype in a myocardial infarct model(170). A final option for minimizing methylation is to construct CpG depleted vectors. For example, a newer version of pEPI was constructed with a CpG-depleted backbone, called pEPito (171). Experiments have shown that pEPito can drive stable transgene expression at higher levels and for a longer time period than its successor pEPI in vitro and in vivo especially in the retina (171, 172). This has the added benefit of a minimized inflammatory response (173), which is often associated with typical CpG patterns in bacterial backbones (174).
In addition to methylation, other factors can influence chromatin structure. Chromatin structure is extremely important for transcription initiation, as the proteins in the transcription complex need access to specific sites in the DNA. Studies have shown that the curvature of the DNA helix plays a major role in the accessibility of the promoter and that left-handedly curved DNA is more efficiently transcribed than right-handedly curved DNA (175). This phenomenon can be partially explained by the fact that curved DNA allows nucleosomes, which organize the chromatin into functional sections, to slide more easily along the helix and therefore can open up access to specific binding sites like the TATA box (176). The sequences causing the helix to bend are mostly A- and T-rich and have been tightly conserved among species (177), but in recent years, artificial versions have also been used to enhance vector expression in vitro and in vivo. The shortest effective sequence has been identified as a quadruple repeat of five thymidines connected by a four base pair linker aptly named T4 (178). The addition of T4 in front of the promoter sequence increases transcription rates up to ten-fold in vitro (175), but even higher rates can be achieved by increasing the repeat number (178). The magnitude of the effect is less in vivo, nevertheless, a T36 repeat addition was able to increase the transgene expression levels after transfection of a mouse liver by eight times (179). These curved sequences are fairly short even with a higher number of repeats, and the fact that the expression levels can be modulated by changing the number of repeats, makes the curved DNA sequence an excellent candidate for vector engineering strategies to enhance gene expression.
Other sequences, termed insulators, can also help prevent the plasmid DNA from adopting the detrimental heterochromatin structure(180). These sequences keep heterochromatin from forming around adjacent expression cassettes by recruiting histone acetyltransferases which prevent the methylation of lysine 9 on histone 3, a hallmark process in heterochromatin formation (181). An insulator sequence identified in the chicken genome has demonstrated great potential in inhibiting vector silencing in lentiviral transfection, but occupies a considerable amount of space in the viral genome with its 400 bp (182). However, a recent study identified several very potent and short (between 100 and 300 bp) insulators in a genome-wide search, providing potential candidates for future investigations into this topic (183) and inclusion of these sequences into ocular gene delivery vehicles may be beneficial.
4.3 Addition of intronic sequences
Due to capacity constraints for the packaging approach (e.g. ~5kb for AAV), many gene therapy vectors only contain the coding sequence of the transgene without any regulatory sequences or introns. This can have detrimental effects on the protein level of the expressed gene as research has shown that inclusion of regulatory elements in the native gene can have positive effects on expression.Introns in particular can improve gene expression by multiple mechanisms including altering transcription, translational yield, polyadenylation, etc. (184-186). These effects are highly variable, dependent on the gene, intron content, and intron position (185). The inclusion of intron A of CMV increases the expression levels of transgenic proteins in cultured mammalian cells when placed inside the coding sequence, a phenomenon named intron-mediated enhancement which was originally described in plants (187). The ability to enhance the expression can be independent of the actual intron sequence, as introns can sometimes be substituted between genes, even though there are differences in the magnitude of expression enhancement from gene to gene(188). The presence of untranslated sequences in the mRNA seems to stabilize it (189) and enhance the rate of maturation, for example by augmenting the polyadenylation of the transcript (190). Several different introns have been tested for intron mediated enhancement in gene therapy vectors so far and the results show a clear benefit from the additional sequences. Transgenic mice have shown an almost 5-fold increase in the production of human thrombopoietin upon inclusion of only a single intron of the same gene (191) and introns of mouse, rabbit and human β-globin have been shown to be potent enhancer elements (192, 193). Several viral introns including SV40 (194) or CMV (195) have also been tested in vitro and in vivo more or less successfully.
Since some non-viral NPs can deliver large genetic sequences, it has recently become possible to directly compare the expression efficiency of genomic DNA (i.e. including introns) with that of the cDNA. We have shown that in one case at least, that of NP-mediated rhodopsin gene delivery, inclusion of genomic sequences significantly improves expression levels and phenotypic rescue of the rho−/− mouse model of RP. Though the mechanism of this improvement is unknown, mRNA levels were the same for genomic and cDNA, while protein levels were improved in genomic vs. cDNA suggesting the benefits were on protein translation(153). Introns have demonstrated their ability to increase mRNA stability and enhance translation, but in many cases, even large capacity packaging methods cannot deliver the full genomic sequence in any practically applicable way as the genes are just too large (e.g. the Usher syndrome type 2A gene that spans 800 kbp). As a result, consideration must be given to inclusion of heterologous introns, or only a select number of native introns, and the relative contributions of any given intron to the overall expression level can be evaluated.
4.4 Other Enhancer Elements
Though the CMV promoter is rapidly silenced and therefore not clinically useful, in 1985 it was discovered that a small part of the promoter acts as an enhancer (196) and when added to almost any promoter, greatly increases the transcription rate (194, 197). Inclusion of this enhancer can be used to improve the expression of normally weak but tissue-specific promoters without compromising the specificity of the promoter (198), and has been implemented in the eye with the RPE65 promoter (172). This enhancer effect has also been demonstrated for regions from other promoters such as the photoreceptor-specific interphotoreceptor retinoid binding protein (IRBP), even though the effect is much less potent than in the case of CMV (65). Subsequently, a genome-wide search for enhancing elements via in vitro analysis yielded thousands of possible candidates for enhancement (199). One 37-bp leader sequence was analyzed in-depth and demonstrated an ability to increase mRNA levels of the transgene by more than ten-fold (200). Sequences of this sort are valuable because of their small size and high activity, but will have to be tested in vivo extensively to assess their capabilities. In addition, the effects of enhancer sequences can vary based on target tissue and gene/promoter so individual testing for any given application will likely be required.
Viruses like herpes simplex have evolved short sequences to boost the translation of their intronless genes in the host cell, which can be utilized in plasmids in lieu of introns(201). One of those sequences was identified in the human hepatitis B virus (HBV) and aptly named the post transcriptional regulatory element (PRE) (202). The PRE sequence does not increase the transcription rate of the gene nor does it have an influence on the mRNA stability in the cytoplasm, but rather enhances the nuclear export of the mRNA which in turn greatly improves translation rates (202). It was later discovered that a close relative of HBV, the woodchuck hepatitis virus, contains an even more potent PRE labelled WPRE (203). The HBV PRE consists of only two subelements, while the WPRE is a tripartite enhancer element which explains the difference in strength (203). The full WPRE sequence is only 600 bp in size, but in vitro transfection experiments in neurons showed that it can be shortened to 247 bp without impeding its translation enhancing ability (204). Since its discovery the WPRE sequence has been demonstrated to be one of the most successful enhancer elements and is currently under investigation for in vitro production of therapeutic proteins (205) and for gene therapy in AAV vectors ((206, 207) as examples) because of its small size and its ability to significantly increase transgene expression. The WPRE sequence has also been used as part of an AAV vector containing the cDNA for brain-derived neurotrophic factor (BDNF) which induced transgene expression and phenotypic improvement in a rodent model of glaucoma (208).
4.5 Influence of polyadenylation site on protein expression
Efficient protein translation is not possible without a proper polyadenylation signal added to the mRNA and mutations in the poly A signal sequence quickly lead to aberrant gene expression and disease (209). A short sequence (AATAAA) is the consensus signal for the polyadenylation of mRNAs, but deletion experiments have shown that additional recognition sites are necessary for the successful addition of poly A but are not conserved between genes (210). Different poly A signals can influence protein levels of transgenes very differently, as shown by the direct comparison of the signals of SV40 and the bovine growth hormone (BGH) gene. Expression of luciferase was increased two- to ten-fold with the BGH poly A sequence over the SV40 in different cell lines in vitro and in multiple tissues in vivo including kidney, lung or heart (194, 195). The differences in efficiency of the poly A signal between different genes may be an evolved mechanism to control the level of innate gene expression. Varying the polyA signal may be used as an advantage in gene therapy vectors, though such alternate polyA signals have not yet been tested in ocular gene delivery plasmids.
The discovery of countless DNA and RNA regulatory mechanisms in the last several years shows how little we actually know about regulation of gene expression in mammalian cells. These new regulatory elements will certainly pave the way for better transgenic expression vectors, even though the process is must still be evaluated using a largely trial and error approach untilwe gain a deeper understanding of the processes involved. The lack of appropriate transgene expression is one of the main issues in non-viral gene therapy and can hopefully be resolved by the insertion of a combination of any of the enhancers mentioned above.
5. NP formulations for gene therapy
Non-viral gene packaging is an active research topic, as it is considered a safer alternative to viral gene delivery. The concerns about the safety of viral vectors are based on two major incidents: in 1999, a participant of a gene therapy study died of an acute immune response after the injection of a therapeutic adenovirus (211, 212) and in 2002, children treated for severe inherited immunodeficiency with a retrovirus developed leukemia (213, 214). These two cases illustrate the two main issues with viral gene therapy: recognition of the vector by the immune system and random insertion of retroviral vectors causing mutagenesis. The use of non-integrating AAV has so far not caused any severe adverse effects; nevertheless, the development of an alternative is desirable particularly because traditional AVV cannot deliver DNA molecules larger than 5 kb (61). In addition, though AAVs are safer than older viral vectors such as adenoviruses, they can induce an immune response, in particular the production of neutralizing antibodies against the viral capsid (215, 216). This is particularly true after intravitreal delivery (217, 218), but can also occur after subretinal injection (219). Non-viral vectors show generally a very low immunogenicity, especially when DNA is compacted with innate proteins like human albumin (see below). Plasmids delivered via non-viral gene therapy can be maintained episomally (158) which removes the risk of insertional mutagenesis, but can also lead to transient instead of stable transfection. In direct comparison with AAV, most non-viral gene delivery vectors show inferior transfection efficiency and do not lead to sufficient transgene expression (220). Improvements can be achieved on the DNA level as described previously, but the formulation of the particle or packaging method itself can also be optimized.
Non-viral particles for gene therapy can be broadly divided into two groups, lipoplex particles containing lipid molecules and polyplex particles, which are based on polycations from chemical groups such as polysaccharides and proteins. Particles from both groups have been shown to benefit in some way from the modification with polyethylene glycol (PEG), therefore this very common modification and its consequences will be discussed separately (for a summary of this chapter see Table 2).
Table 2.
Packaging methods.
| Compaction agents | Target cells | Retinal model | Ref. |
|---|---|---|---|
| DOTAP | Neural retina/RPE/cornea | Wistar rats ARPE-19 (in vitro) |
(221-223) |
| DOTAP/DOPE | RPE RPE |
RPE65−/− mouse (LCA) ARPE-19 (in vitro) |
(81) (132) |
| DSPE | Inner retina/RPE/Cornea | Balb/c albino mouse | (87) |
| Albumin | RPE | ARPE-19 (in vitro) | (107, 224) |
| Polylysine | RPE | Balb/c albino mouse RPE65−/− (LCA) |
(80, 225) |
| Outer retina Outer retina |
Rho−/− mouse (RP) ABCA4−/− mouse(Stargardt's disease) |
(153) (75) |
|
| Chitosan | Inner retina/outer retina RPE | Sprague Dawley rat Balb/c albino mouse |
(92) (226) |
| Chitosan/hyaluronic acid | Cornea | Human corneal epithelium (in vitro) | (227) |
| Dextran/chondroitin sulfate | Cornea | Human corneal epithelium (in vitro) | (228) |
| PEI | Retinal Muller Glia Glia and ganglion cells Retina |
Lewis rat Human retinal cells (in vitro) White Japanese rabbit |
(229) (230) (231) |
| PLGA | Inner retina/RPE | Lewis rat | (112) |
| Gelatin* | Outer nuclear layer | RCS Rats | (232) |
Thus far these particles have only been used to deliver drugs, not genetic material, to the eye.
5.1. Lipoplex NPs
Lipid-based delivery systems have been widely utilized as a non-viral method to carry DNA molecules across the hydrophobic lipid bilayer of the plasma membrane and therefore increase the transfection efficiency immensely compared to naked DNA (233). The compaction is mediated by the interaction of the positively charged cationic head groups of the lipids and the negatively charged base pairs of the DNA leading to a small particle with a diameter of around 50nmor larger with a slightly positive charge (234). Early clinical trials in cancer patients showed no toxic adverse effects and transgene expression in tumor cells, demonstrating the viability of the approach (235). Different lipid formulations have been used for the compaction of DNA into liposomes allowing adaption of the particle to specific requirements. Phospholipids like DOPE, DOTAP and DSPE are widely used andparticles made with combinations of these lipids show no toxicity and moderate transfection efficiency in vivo (236).
Solid lipid particles consisting of DNA complexed with protamine and dextran and compacted into cationic lipids with DOTAP to a size of around 250nm showed high transfection rates in vitro in transformed RPE cells (ARPE-19),and also the ability to transfect retinal cells after intravitreal or subretinal delivery and corneal cells after topical application (90). The addition of protamine, which is a mixture of small basic peptides, can produce particles with higher transfection efficiency (237) and dextran inhibits the interaction of the particles with erythrocytes, a beneficial characteristic for intravenous applications, as it prevents fatal blood clots (238). These particles show potentialfor the efficient transfection of retinal cells and are currently under investigation for gene therapy in a mouse model of retinoschisis(223). Protamine was also used as a transfection enhancer in modified targeted liposomes formulated with a mixture of DOTAP, DOPE and cholesterol (81). The introduction of cholesterol into the outer layer of the liposomes has been shown to increase transfections rates in vitro (239). These particles were able to successfully transfect RPE and photoreceptor cells and to mediate appreciable rescue in a mouse model of LCA (81). Though several liposome formulations are effective in vitro, (e.g (132, 222)), the in vivo environment is very different making in vivo testing of potential formulations essential. One of the major drawbacks of liposome formulations is their tendency to aggregate when in contact with serum proteins which largely decreases bioavailability after intravenous administration (240). Nevertheless, with the appropriate modifications, liposomes can be delivered intravenously and lead to successful transgene expression in retinal cells (87).
5.2. Polyplex NPs
5.2.1. Peptide and protein-based NPs
Proteins and peptides can be used as an alternative to phospholipids for compaction of DNA and some formulations have shown promising results. Human serum albumin can compact DNA into particles of 150 to 280 nm via a cost-effective and highly reproducible process (241). Human albumin as a major serum component is non-immunogenic, biodegradable and easily prepared in large amounts as an ultrapure recombinant protein (242) and the safety of albumin in the retina after intravitreal application has been established (243). Compaction with albumin effectively protects the plasmid against degradation and imparts a transfection rate higher than lipofectamine in vitro in the ARPE-19 cell line (224). Cytotoxicity was detected neither in vitro nor in vivo after intravitreal injection in the mouse eye and protein expression was detectable in retinal extracts even though the exact location of expression was not established (224). Further investigations into the mobility of these particles in the vitreous revealed aggregation of positively charged formulations probably due to interaction with abundant negatively charged proteins in the vitreous humor, but altering the surface charge of the particles can resolve this issue (107). However, the influence of altering the charge on the transfection efficiency of the particles will need to be determined. Gelatin-compaction is another attempt at using innate proteins as a nonimmunogenic drug delivery vector which shows no adverse effects in the eye after intravitreal injection (232). Gelatin can also compact DNA effectively (244, 245), but it has so far not been tested in retinal gene therapy despite its potential.
NP compaction can also be achieved by peptides instead of whole proteins. The positively charged amino acid lysine is a perfect candidate for this and early experiments demonstrated high transfection efficiency for DNA compacted with polylysine (246). Since then, the compaction procedure has been refined and used in different tissues for transgene delivery(247-249). With the addition of PEG (see discussion below), polylysine NPs can effectively transfect lung epithelial cells and induce minimal immune response even when administered at high dosage (250). CK30PEG NPs can also transfect postmitotic cells, something which has historically proven difficult with non-viral delivery methods (74). These particles have been widely used in the eye to provide therapeutic improvement as described above and do not show any adverse effects after delivery to the eye even after repeated treatment (225), nor any extraocular gene expression after subretinal delivery (251). The efficiency of polylysine particles can be altered by adjusting the lysine counterion used during the compaction process. For example, switching from acetate to trifluoroacetate can change the shape of the particle form rod to sphere and influence the transfection rate both in the eye and in the lung (76, 252). Likewise, the addition of histidines to the polylysine compaction agent can increase the efficiency of the gene transfer up to 20-fold (tested in vitro). This ‘proton sponge’ effect is due to the amino nitrogens in the histidine which are able to accept protons (253). This immense buffering capability prevents the acidic degradation of the particle in the endosome after uptake and leads to rupture of the organelle and escape of the DNA into the cytoplasm (247). Polylysine can also be engineered into branched dendrimers before compaction, increasing its buffering capacity and thereby its gene delivery capabilities (254). This type of particle is still completely biodegradable and therefore exhibits much less toxicity than other dendrimer polymers like PAMAM described below. These examples show how versatile the polylysine particles are and a change of certain amino acids or the content of lysine could have immense influence on the transfection efficiency of the final particle.
5.2.2. Polysaccharide-based particles
Polysaccharides are well suited for use as drug delivery agents due to their non-immunogenic and biodegradable properties, inexpensive production and ease of modification. Hyaluronic acid can be used to target lipid NPs to the CD44 receptor as described above, but can also be used to deliver drugs on its own. Hyaluronic acid is present in large amounts in the interphotoreceptor matrix of the retina and is highly biocompatible and non-immunogenic (255). Compaction of chemotherapy drugs with hyaluronic acid has been successfully achieved (256), however, for gene therapy, a mixture of hyaluronic acid and chitosan is more generally in use as the overall negative charge of hyaluronic acid interferes with productive DNA compaction. In combination with chitosan, a cationic polysaccharide harvested from shellfish, hyaluronic acid can form small particles (100-200 nm in size) which protect DNA from degradation and can effectively transfect cells in vitro (257). These particles are also able to successfully transfect corneal epithelium cells, become internalized efficiently,and induce transgene expression (258). During production, the size and surface charge of the particles can be easily manipulated by changing the ratio of chitosan to hyaluronic acid which has a distinct influence on transfection efficiency and transgene persistence (257). Chitosan can also be used without hyaluronic acid to compact DNA and deliver NPs, either unmodified or with various alternate functional groups. The interaction of chitosan with plasma membrane proteins has been discussed as a factor for the transfection abilities of chitosanNPs though the precise uptake mechanisms are not clear (259). Whatever the mechanism, chitosanparticles can deliver plasmid DNA to a variety of different cells including intestinal and corneal epithelial cells (227, 260). In vitro transfection is inefficient without additives, but in vivo, transgene expression was observed in RPE cells (92, 226) and photoreceptors (92) after subretinal injections without any notable cytotoxicity. Together with the highly efficient compaction process (261), these results show the potential of chitosan as a non-viral gene packaging method, but no therapeutic efficacy studies have been published as yet.
Other polysaccharides like cyclodextrin or dextran have also been shown to facilitate cellular uptake of nucleic acids(262). Dextran has been tested as a carrier for siRNA and exhibits considerable transfection efficiency in vivo in lung tissue when formulated as a nanogel (263). For plasmid transfection, dextran is mostly used in combination with other polymers, but not as a compaction agent alone. Dextran can increase the transfection rate of lipid NPs after intravenous delivery (238)and decrease the cytotoxicity associated with some polymer-based particles (264). For the use in ocular gene therapy, a combination of dextran, gelatin and chondroitin has been used to condense DNA and transfect corneal cells in vitro. This approach could be developed into a topical application of therapeutic DNA for the cornea (228) but has not yet been evaluated in the retina.
5.2.3. Polymer-based NPs
There are many different compounds neither peptide- nor polysaccharide-based which have been tested for compaction of drugs, siRNA and DNA into nanoscale particles for delivery and here we briefly discuss some of the most successful ones.One of the most efficient transfection agents, polyethylenimine (PEI), was discovered in 1995 and has since been tested in many applications. The transfective quality of PEI is linked to the high amount of amino nitrogens in the macromolecule which confer the abovementioned ‘proton sponge’ effect(253) and promote endosomal release. PEI condenses into small particles (100-1000 nm) upon combination with DNA, a process which is highly dependent on pH and ionic strength of the reaction solution (253). PEI-compacted DNA has emerged as a powerful tool for non-viral transfection in notoriously hardtotransfect neurons, especially when coupled with a cell-specific receptor ligand like mannose (265, 266). The feasibility of this approach has been shown in the retina; PEI NPs successfully transfectedRPE and Müller glia cells in vitro and in vivo (229, 230), but these experiments also showed fairly high acute cytotoxicity of native PEI in vitro in differentiated and undifferentiated epithelium cells(231, 267) and ARPE-19 cells (231). This toxicity can be alleviated by the addition of PEG to the PEI particles, as described below (268), and PEI remains of great interest as a non-viral delivery strategy.
Another ‘proton sponge’ compound is polyamidoamine (PAMAM), which is synthesized as a repetitively branched dendrimer and can compact DNA into small particles (around 130 nm) (269). Successful transgene expression has been shown after in vivo deliveryof the PAMAM-DNA particles to tumors and cardiac grafts (270, 271), but the compound was associated with relatively high toxicity levels in vitro in Y79 cells and moderate pathology in mice 30 days after systemic administration from the beginning, which explains why it is not more broadly in use for gene therapy applications (272). Again, studies as recent as 2014 with PEGylated dendrimers have demonstrated reduced induction of cell death in vitro in HEK293T cells compared to unmodified PAMAM, however in this case, the PEGylation led to loss of transfection efficiency, a phenomenon described in more detail below (273). The ‘proton sponge’ effect is naturally of interest for the development of better non-viral vectors and is therefore subject of serious research. The imidazole ring of the amino acid histidine can serve the same purpose and actually shows appreciable gene transfer in vitro without associated toxicity (274).
Other polymers have also had noteworthy success for gene transfer. The use of poly(lactic acid) (PLA) as a DNA-compacting agent is quite common, as the compound is simple to synthesize and releases its cargo easily (275). The particles features very low short-term toxicity levels in vitro in liver carcinoma cells(276) and high stability in the presence of serum when PEGylated (277). Poly(D,L-lactide-co-glycolide) (PLGA), a derivative of PLA, forms stable nanospheres of less than 200 nm together with DNA (278). The particles achieve rapid endosomal escape (279) and can efficiently induce transgene expression in RPE cells and ganglion cells in vivo upon intravitreal administration(111, 112). Cationic hyperbranched poly(amino ester) (PAGA) was described as a possible non-toxic DNA carrier as well (280), but the transfection efficiency is clearly inferior to other particles compacted with PAMAM or PEI (281).
It is conceivable due to their large variety that there are many more possible synthetic, degradable polymers which could provide non-toxic, highly efficient non-viral gene delivery. In an early study, a high-throughput approach used to test the transfection qualities of a library of polymers yielded 140 possible candidates (282) and experiments like this could lead to the next-generation of polymer vectors.
5.3. Modification of NPs with polyethylene glycol (PEG)
All the NP formulations described above have distinct transfection efficiency, toxicity and bioavailability profiles and they have virtually all been conjugated with PEG to help overcome some of their deficiencies. Hydrophobic and electrostatic interactions of NPs with serum proteins in the blood, a process called opsonization, leads to a swift removal of particles from the circulation by phagocytic cells (85, 283). For intravenous delivery this is an issue, as it decreases the availability of the therapeutic compound at the target location. One way to decrease the opsonization rate of particles by serum proteins is the addition of PEG to the outer shell. The PEG molecules form a hydrophilic, neutral cover around the particle which creates a protein-rejecting buffer, also called stealth coating, preventing opsonization, increasing the half-life of the particles in the bloodstream, and decreasing macrophage uptake significantly (284-286). Plasmid DNA compacted with polylysine showed an increased stability in the presence of serum proteins when the particles were PEGylated (287) and siRNA compacted into liposomes demonstrated much better tissue delivery with the addition of PEG after intravenous application (288). This can lead to significantly higher transgene expression rates in the target tissue as shown with PEGylated polylysine particles in the lung (250). The modification of gelatin-based nanovectors with PEG also showed a significant increase of tissue accumulation of plasmid DNA after systemic administration which is due to the increased stability of the particles in the blood stream (289). The effect of PEGylation on macrophage uptake can be quite dramatic, a decrease up to three-fold, as research on drug-carriers formulated with PLGA has shown (290). This leads to a prolonged circulation time of up to 72 hours while non-conjugated drugs are cleared within 6 hours (290). There is also evidence of increased stability of PLA-PEG particles in the digestive tract after oral administration (291) and reduced aggregation of PEGylated liposomes in the vitreous (222).Another issue for many NP formulations is their considerable cytotoxicity. The addition of PEG can be beneficial in this respect as well, decreasing the negative effect of compounds like the PAMAM dendrimer (292), dextran (293), and PEI (268) and therefore increasing their potential feasibility for clinical application.
Despite all the positive results on the use of PEG for NP formulations, there is a huge disadvantage. PEGylation masks the positive charge on NPs, so it can also decrease the transfection efficiency since that depends on the same cationic charge (294). The added stability of PEGylated particles can also interfere with the endosomal escape of particles after uptake and can therefore lead to rapid degradation of the carrier and its cargo in the endosome (295). This issue has been described by many researchers in the field and is commonly called the ‘PEG dilemma’, as the increased stability of the particles is desirable for delivery, but comes at the cost of hugely decreased transfection rates (222, 293, 296). Different solutions to this problem have been proposed, one suggestion is the post-PEGylation of particles with PEG-ceramide which would dissociate from the inner particle upon contact with the target cell membrane or in the endosomal compartment. This would increase the mobility of the particles in the vitreous just like covalently linked PEG without hampering the transfection (222). Another approach takes advantage of the specific environment found in target tissues, in one case cancer cells. Tumors have a slightly acidic pH due to their high metabolic rate which can be used to cleave off the PEG coating after delivery to the site of interest if pH-sensitive hydrazine bonds were used to link the PEG to the particle (297). As an alternative to PEG, particles can also be stealth coated with peptides changing charge from negative to positive upon encountering low pH values (298). Another approach which may be more valuable for gene therapy vectors in the eye is the use of chemical bonds cleavable by matrix-metalloproteinases (MMP) abundant in the extracellular matrix of certain cells (299). This is particularly applicable since the retinal detachment that occurs during subretinal injection leads to upregulation of MMP-12 and MMP-13 (300). In any case, the “PEG dilemma”remains to be fully resolved and remains a topic of interest to researchers.
Liposome-mediated transfection of cells was initially described over 30 years ago and researchers have discovered many other compounds suitable for compaction of DNA in small particles capable of penetrating the cell membrane in the meantime. Unfortunately for the whole field of non-viral gene therapy, compounds with very high transfection efficiency like PEI are also the ones with the highest toxicity levels and are therefore unsuitable for application in patients. The toxicity issue is partially alleviated with the addition of PEG, but that also reduces the transfection efficiency to a level comparable with the non-toxic compounds. This predicament needs to be resolved before successful non-viral gene therapy in patients can become a reality, but high-throughput synthesis and testing of new compounds for example described by Lynn et al. 2001 will certainly accelerate the process.
6. Conclusions
Since the development of liposomes in the 1980 as transfection agents, non-viral vectors have come a long way. Recent breakthroughs in particle compaction, surface coating and vector engineering have shown that these vectors have large potential in the future of gene therapy. The introduction of peptides like polylysine which are more biocompatible and less toxic (75) make the delivery of larger, therapeutically relevant doses possible. The advent of intravitreal injections of anti-angiogenic drugs in the past decades has also led to improved surgical techniques and reduced the probability of injection-related adverse effects (114). The intravitreal method of delivery is also gaining traction with the invention of stealth coating with PEG or hyaluronic acid as the bioavailability of intravitreally delivered particles in the retina increases (222, 301). When coupled with receptor-targeting of particles, this may lead to vectors which can be injected intravenously or intravitreally with high efficacy and with minimal risk of off-target adverse effects (87). Together with the advances in vector engineering, non-viral vectors have the capability to be a clinically relevant complement to viral vectors in gene therapy, particularly for the delivery of large or precisely engineered genes.
Since viral vectors have already been used in clinical trials in the eye, there arenow data available on human efficacy and limitations for ocular gene therapy, which are informing the next generation of viral trials. Though much of what has been learned from early ocular viral gene therapy trials can be applied to non-viral gene delivery as well (namely the dangers associated with subretinal injection, the need for higher levels of expression and improved distribution), non-viral gene delivery still has a long way to go reach to wide application in patients.However, with the innovations seen in the last couple of years and the fast evolution of gene therapy vectors, both viral and non-viral, there is hope that a viable treatment optionat least for some patients with retinal degeneration will be available in the next decade.
Acknowledgements
This work was supported by the NIH (EY018656, EY22778, MIN) and the Knights Templar Eye Foundation (SMC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Retina international; www.retina-international.org. [Google Scholar]
- 2.Hamel C. Retinitis pigmentosa. Orphanet J Rare Dis. 2006;1:40. doi: 10.1186/1750-1172-1-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liutkeviciene R, Lesauskaite V, Asmoniene V, Gelzinis A, Zaliuniene D, Jasinskas V. Inherited macular dystrophies and differential diagnostics. Medicina (Kaunas) 2012;48:485–495. [PubMed] [Google Scholar]
- 4.Gemenetzi M, Lotery AJ. Phenotype/genotype correlation in a case series of Stargardt's patients identifies novel mutations in the ABCA4 gene. Eye (Lond) 2013;27:1316–1319. doi: 10.1038/eye.2013.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- 6.Mathur P, Yang J. Usher syndrome: Hearing loss, retinal degeneration and associated abnormalities. Biochim Biophys Acta. 2015;1852:406–420. doi: 10.1016/j.bbadis.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daiger SP, Sullivan LS, Bowne SJ. Genes and mutations causing retinitis pigmentosa. Clin Genet. 2013;84:132–141. doi: 10.1111/cge.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malanson KM, Lem J. Rhodopsin-mediated retinitis pigmentosa. Prog Mol Biol Transl Sci. 2009;88:1–31. doi: 10.1016/S1877-1173(09)88001-0. [DOI] [PubMed] [Google Scholar]
- 9.Chen H, Chen Y, Horn R, Yang Z, Wang C, Turner MJ, Zhang K. Clinical features of autosomal dominant retinitis pigmentosa associated with a Rhodopsin mutation. Ann Acad Med Singapore. 2006;35:411–415. [PubMed] [Google Scholar]
- 10.Francis PJ, Schultz DW, Gregory AM, Schain MB, Barra R, Majewski J, Ott J, Acott T, Weleber RG, Klein ML. Genetic and phenotypic heterogeneity in pattern dystrophy. Br J Ophthalmol. 2005;89:1115–1119. doi: 10.1136/bjo.2004.062695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wroblewski JJ, Wells JA, 3rd, Eckstein A, Fitzke FW, Jubb C, Keen TJ, Inglehearn CF, Bhattacharya SS, Arden GB, Jay MR, et al. Ocular findings associated with a 3 base pair deletion in the peripherin-RDS gene in autosomal dominant retinitis pigmentosa. Br J Ophthalmol. 1994;78:831–836. doi: 10.1136/bjo.78.11.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Venturini G, Rose AM, Shah AZ, Bhattacharya SS, Rivolta C. CNOT3 is a modifier of PRPF31 mutations in retinitis pigmentosa with incomplete penetrance. PLoS Genet. 2012;8:e1003040. doi: 10.1371/journal.pgen.1003040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fahim AT, Bowne SJ, Sullivan LS, Webb KD, Williams JT, Wheaton DK, Birch DG, Daiger SP. Allelic heterogeneity and genetic modifier loci contribute to clinical variation in males with X-linked retinitis pigmentosa due to RPGR mutations. PLoS One. 2011;6:e23021. doi: 10.1371/journal.pone.0023021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poloschek CM, Bach M, Lagreze WA, Glaus E, Lemke JR, Berger W, Neidhardt J. ABCA4 and ROM1: implications for modification of the PRPH2-associated macular dystrophy phenotype. Invest Ophthalmol Vis Sci. 2010;51:4253–4265. doi: 10.1167/iovs.09-4655. [DOI] [PubMed] [Google Scholar]
- 15.Chacon-Camacho OF, Zenteno JC. Review and update on the molecular basis of Leber congenital amaurosis. World J Clin Cases. 2015;3:112–124. doi: 10.12998/wjcc.v3.i2.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davidson AE, Schwarz N, Zelinger L, Stern-Schneider G, Shoemark A, Spitzbarth B, Gross M, Laxer U, Sosna J, Sergouniotis PI, et al. Mutations in ARL2BP, encoding ADP-ribosylation-factor-like 2 binding protein, cause autosomal-recessive retinitis pigmentosa. Am J Hum Genet. 2013;93:321–329. doi: 10.1016/j.ajhg.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng T, Peachey NS, Li S, Goto Y, Cao Y, Naash MI. The effect of peripherin/rds haploinsufficiency on rod and cone photoreceptors. Journal of Neuroscience. 1997;17:8118–8128. doi: 10.1523/JNEUROSCI.17-21-08118.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conley SM, Stuck MW, Burnett JL, Chakraborty D, Azadi S, Fliesler SJ, Naash MI. Insights into the mechanisms of macular degeneration associated with the R172W mutation in RDS. Human Molecular Genetics. 2014;23:3102–3114. doi: 10.1093/hmg/ddu014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stuck MW, Conley SM, Naash MI. The Y141C knockin mutation in RDS leads to complex phenotypes in the mouse. Human Molecular Genetics. 2014;23:6260–6274. doi: 10.1093/hmg/ddu345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hollingsworth TJ, Gross AK. The severe autosomal dominant retinitis pigmentosa rhodopsin mutant Ter349Glu mislocalizes and induces rapid rod cell death. J Biol Chem. 2013;288:29047–29055. doi: 10.1074/jbc.M113.495184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kroeger H, Messah C, Ahern K, Gee J, Joseph V, Matthes MT, Yasumura D, Gorbatyuk MS, Chiang WC, LaVail MM, et al. Induction of endoplasmic reticulum stress genes, BiP and chop, in genetic and environmental models of retinal degeneration. Invest Ophthalmol Vis Sci. 2012;53:7590–7599. doi: 10.1167/iovs.12-10221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parfitt DA, Aguila M, McCulley CH, Bevilacqua D, Mendes HF, Athanasiou D, Novoselov SS, Kanuga N, Munro PM, Coffey PJ, et al. The heat-shock response co-inducer arimoclomol protects against retinal degeneration in rhodopsin retinitis pigmentosa. Cell Death Dis. 2014;5:e1236. doi: 10.1038/cddis.2014.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dizhoor AM, Boikov SG, Olshevskaya EV. Constitutive activation of photoreceptor guanylate cyclase by Y99C mutant of GCAP-1. Possible role in causing human autosomal dominant cone degeneration. Journal of Biological Chemistry. 1998;273:17311–17314. doi: 10.1074/jbc.273.28.17311. [DOI] [PubMed] [Google Scholar]
- 24.Zulliger R, Naash MI, Rajala RV, Molday RS, Azadi S. Impaired association of retinal degeneration-3 with guanylate cyclase-1 and guanylate cyclase-activating protein-1 leads to leber congenital amaurosis-1. Journal of Biological Chemistry. 2015;290:3488–3499. doi: 10.1074/jbc.M114.616656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGee Sanftner LH, Abel H, Hauswirth WW, Flannery JG. Glial cell line derived neurotrophic factor delays photoreceptor degeneration in a transgenic rat model of retinitis pigmentosa. Mol Ther. 2001;4:622–629. doi: 10.1006/mthe.2001.0498. [DOI] [PubMed] [Google Scholar]
- 26.Xia H, Mao Q, Paulson HL, Davidson BL. siRNA-mediated gene silencing in vitro and in vivo. Nature Biotechnology. 2002;20:1006–1010. doi: 10.1038/nbt739. [DOI] [PubMed] [Google Scholar]
- 27.Jiang L, Li TZ, Boye SE, Hauswirth WW, Frederick JM, Baehr W. RNAi-mediated gene suppression in a GCAP1(L151F) cone-rod dystrophy mouse model. PLoS One. 2013;8:e57676. doi: 10.1371/journal.pone.0057676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teusner JT, Lewin AS, Hauswirth WW. Down-regulation of rhodopsin gene expression by AAV-vectored short interfering RNA. Advances in Experimental Medicine and Biology. 2006;572:233–238. doi: 10.1007/0-387-32442-9_33. [DOI] [PubMed] [Google Scholar]
- 29.Mao H, Gorbatyuk MS, Rossmiller B, Hauswirth WW, Lewin AS. Long-term rescue of retinal structure and function by rhodopsin RNA replacement with a single adeno-associated viral vector in P23H RHO transgenic mice. Human Gene Therapy. 2012;23:356–366. doi: 10.1089/hum.2011.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petrs-Silva H, Yasumura D, Matthes MT, LaVail MM, Lewin AS, Hauswirth WW. Suppression of rds expression by siRNA and gene replacement strategies for gene therapy using rAAV vector. Advances in Experimental Medicine and Biology. 2012;723:215–223. doi: 10.1007/978-1-4614-0631-0_29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Overlack N, Goldmann T, Wolfrum U, Nagel-Wolfrum K. Gene repair of an Usher syndrome causing mutation by zinc-finger nuclease mediated homologous recombination. Investigative Ophthalmology and Visual Science. 2012;53:4140–4146. doi: 10.1167/iovs.12-9812. [DOI] [PubMed] [Google Scholar]
- 32.Greenwald DL, Cashman SM, Kumar-Singh R. Engineered zinc finger nuclease-mediated homologous recombination of the human rhodopsin gene. Investigative Ophthalmology and Visual Science. 2010;51:6374–6380. doi: 10.1167/iovs.10-5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arranz V, Kress M, Ernoult-Lange M. The gene encoding the MOK-2 zinc-finger protein: characterization of its promoter and negative regulation by mouse Alu type-2 repetitive elements. Gene. 1994;149:293–298. doi: 10.1016/0378-1119(94)90164-3. [DOI] [PubMed] [Google Scholar]
- 35.Cideciyan AV, Aguirre GK, Jacobson SG, Butt OH, Schwartz SB, Swider M, Roman AJ, Sadigh S, Hauswirth WW. Pseudo-fovea formation after gene therapy for RPE65-LCA. Invest Ophthalmol Vis Sci. 2015;56:526–537. doi: 10.1167/iovs.14-15895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cideciyan AV, Jacobson SG, Beltran WA, Sumaroka A, Swider M, Iwabe S, Roman AJ, Olivares MB, Schwartz SB, Komaromy AM, et al. Human retinal gene therapy for Leber congenital amaurosis shows advancing retinal degeneration despite enduring visual improvement. Proc Natl Acad Sci U S A. 2013;110:E517–525. doi: 10.1073/pnas.1218933110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gu SM, Thompson DA, Srikumari CR, Lorenz B, Finckh U, Nicoletti A, Murthy KR, Rathmann M, Kumaramanickavel G, Denton MJ, et al. Mutations in RPE65 cause autosomal recessive childhood-onset severe retinal dystrophy. Nat Genet. 1997;17:194–197. doi: 10.1038/ng1097-194. [DOI] [PubMed] [Google Scholar]
- 38.Aguirre GD, Baldwin V, Pearce-Kelling S, Narfstrom K, Ray K, Acland GM. Congenital stationary night blindness in the dog: common mutation in the RPE65 gene indicates founder effect. Molecular Vision. 1998;4:23. [PubMed] [Google Scholar]
- 39.Pang JJ, Chang B, Hawes NL, Hurd RE, Davisson MT, Li J, Noorwez SM, Malhotra R, McDowell JH, Kaushal S, et al. Retinal degeneration 12 (rd12): a new, spontaneously arising mouse model for human Leber congenital amaurosis (LCA). Molecular Vision. 2005;11:152–162. [PubMed] [Google Scholar]
- 40.Redmond TM, Hamel CP. Genetic analysis of RPE65: from human disease to mouse model. Methods Enzymol. 2000;316:705–724. doi: 10.1016/s0076-6879(00)16758-8. [DOI] [PubMed] [Google Scholar]
- 41.Acland GM, Aguirre GD, Bennett J, Aleman TS, Cideciyan AV, Bennicelli J, Dejneka NS, Pearce-Kelling SE, Maguire AM, Palczewski K, et al. Long-term restoration of rod and cone vision by single dose rAAV-mediated gene transfer to the retina in a canine model of childhood blindness. Mol Ther. 2005;12:1072–1082. doi: 10.1016/j.ymthe.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Narfstrom K, Katz ML, Bragadottir R, Seeliger M, Boulanger A, Redmond TM, Caro L, Lai CM, Rakoczy PE. Functional and structural recovery of the retina after gene therapy in the RPE65 null mutation dog. Invest Ophthalmol Vis Sci. 2003;44:1663–1672. doi: 10.1167/iovs.02-0595. [DOI] [PubMed] [Google Scholar]
- 43.Bennicelli J, Wright JF, Komaromy A, Jacobs JB, Hauck B, Zelenaia O, Mingozzi F, Hui D, Chung D, Rex TS, et al. Reversal of blindness in animal models of leber congenital amaurosis using optimized AAV2-mediated gene transfer. Mol Ther. 2008;16:458–465. doi: 10.1038/sj.mt.6300389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Y, Moiseyev G, Takahashi Y, Ma JX. RPE65 gene delivery restores isomerohydrolase activity and prevents early cone loss in Rpe65−/− mice. Investigative Ophthalmology and Visual Science. 2006;47:1177–1184. doi: 10.1167/iovs.05-0965. [DOI] [PubMed] [Google Scholar]
- 45.Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, Viswanathan A, Holder GE, Stockman A, Tyler N, et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. New England Journal of Medicine. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 46.Banin E, Bandah-Rozenfeld D, Obolensky A, Cideciyan AV, Aleman TS, Marks-Ohana D, Sela M, Boye S, Sumaroka A, Roman AJ, et al. Molecular anthropology meets genetic medicine to treat blindness in the North African Jewish population: human gene therapy initiated in Israel. Human Gene Therapy. 2010;21:1749–1757. doi: 10.1089/hum.2010.047. [DOI] [PubMed] [Google Scholar]
- 47.Cideciyan AV, Aleman TS, Boye SL, Schwartz SB, Kaushal S, Roman AJ, Pang JJ, Sumaroka A, Windsor EA, Wilson JM, et al. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:15112–15117. doi: 10.1073/pnas.0807027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maguire AM, Simonelli F, Pierce EA, Pugh EN, Jr., Mingozzi F, Bennicelli J, Banfi S, Marshall KA, Testa F, Surace EM, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. New England Journal of Medicine. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Testa F, Maguire AM, Rossi S, Pierce EA, Melillo P, Marshall K, Banfi S, Surace EM, Sun J, Acerra C, et al. Three-year follow-up after unilateral subretinal delivery of adeno-associated virus in patients with Leber congenital Amaurosis type 2. Ophthalmology. 2013;120:1283–1291. doi: 10.1016/j.ophtha.2012.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bainbridge JW, Mehat MS, Sundaram V, Robbie SJ, Barker SE, Ripamonti C, Georgiadis A, Mowat FM, Beattie SG, Gardner PJ, et al. Long-term effect of gene therapy on Leber's congenital amaurosis. New England Journal of Medicine. 2015;372:1887–1897. doi: 10.1056/NEJMoa1414221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jacobson SG, Cideciyan AV, Roman AJ, Sumaroka A, Schwartz SB, Heon E, Hauswirth WW. Improvement and decline in vision with gene therapy in childhood blindness. New England Journal of Medicine. 2015;372:1920–1926. doi: 10.1056/NEJMoa1412965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kalatzis V, Hamel CP, MacDonald IM. Choroideremia: towards a therapy. Am J Ophthalmol. 2013;156:433–437. e433. doi: 10.1016/j.ajo.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 53.van den Hurk JA, Hendriks W, van de Pol DJ, Oerlemans F, Jaissle G, Ruther K, Kohler K, Hartmann J, Zrenner E, van Bokhoven H, et al. Mouse choroideremia gene mutation causes photoreceptor cell degeneration and is not transmitted through the female germline. Hum Mol Genet. 1997;6:851–858. doi: 10.1093/hmg/6.6.851. [DOI] [PubMed] [Google Scholar]
- 54.Seabra MC, Mules EH, Hume AN. Rab GTPases, intracellular traffic and disease. Trends Mol Med. 2002;8:23–30. doi: 10.1016/s1471-4914(01)02227-4. [DOI] [PubMed] [Google Scholar]
- 55.Tolmachova T, Tolmachov OE, Wavre-Shapton ST, Tracey-White D, Futter CE, Seabra MC. CHM/REP1 cDNA delivery by lentiviral vectors provides functional expression of the transgene in the retinal pigment epithelium of choroideremia mice. J Gene Med. 2012;14:158–168. doi: 10.1002/jgm.1652. [DOI] [PubMed] [Google Scholar]
- 56.Tolmachova T, Tolmachov OE, Barnard AR, de Silva SR, Lipinski DM, Walker NJ, Maclaren RE, Seabra MC. Functional expression of Rab escort protein 1 following AAV2-mediated gene delivery in the retina of choroideremia mice and human cells ex vivo. J Mol Med. 2013;91:825–837. doi: 10.1007/s00109-013-1006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.MacLaren RE, Groppe M, Barnard AR, Cottriall CL, Tolmachova T, Seymour L, Clark KR, During MJ, Cremers FP, Black GC, et al. Retinal gene therapy in patients with choroideremia: initial findings from a phase 1/2 clinical trial. Lancet. 2014;383:1129–1137. doi: 10.1016/S0140-6736(13)62117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lam BL, Feuer WJ, Schiffman JC, Porciatti V, Vandenbroucke R, Rosa PR, Gregori G, Guy J. Trial end points and natural history in patients with G11778A Leber hereditary optic neuropathy : preparation for gene therapy clinical trial. JAMA Ophthalmol. 2014;132:428–436. doi: 10.1001/jamaophthalmol.2013.7971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Conlon TJ, Deng WT, Erger K, Cossette T, Pang JJ, Ryals R, Clement N, Cleaver B, McDoom I, Boye SE, et al. Preclinical potency and safety studies of an AAV2-mediated gene therapy vector for the treatment of MERTK associated retinitis pigmentosa. Hum Gene Ther Clin Dev. 2013;24:23–28. doi: 10.1089/humc.2013.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Manfredi A, Marrocco E, Puppo A, Cesi G, Sommella A, Della Corte M, Rossi S, Giunti M, Craft CM, Bacci ML, et al. Combined rod and cone transduction by adeno-associated virus 2/8. Hum Gene Ther. 2013;24:982–992. doi: 10.1089/hum.2013.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lai Y, Yue Y, Duan D. Evidence for the failure of adeno-associated virus serotype 5 to package a viral genome > or = 8.2 kb. Mol Ther. 2010;18:75–79. doi: 10.1038/mt.2009.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Azzouz M, Kingsman SM, Mazarakis ND. Lentiviral vectors for treating and modeling human CNS disorders. J Gene Med. 2004;6:951–962. doi: 10.1002/jgm.600. [DOI] [PubMed] [Google Scholar]
- 63.Balaggan KS, Binley K, Esapa M, Iqball S, Askham Z, Kan O, Tschernutter M, Bainbridge JW, Naylor S, Ali RR. Stable and efficient intraocular gene transfer using pseudotyped EIAV lentiviral vectors. J Gene Med. 2006;8:275–285. doi: 10.1002/jgm.845. [DOI] [PubMed] [Google Scholar]
- 64.Puppo A, Cesi G, Marrocco E, Piccolo P, Jacca S, Shayakhmetov DM, Parks RJ, Davidson BL, Colloca S, Brunetti-Pierri N, et al. Retinal transduction profiles by high-capacity viral vectors. Gene Ther. 2014;21:855–865. doi: 10.1038/gt.2014.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nicoud M, Kong J, Iqball S, Kan O, Naylor S, Gouras P, Allikmets R, Binley K. Development of photoreceptor-specific promoters and their utility to investigate EIAV lentiviral vector mediated gene transfer to photoreceptors. J Gene Med. 2007;9:1015–1023. doi: 10.1002/jgm.1115. [DOI] [PubMed] [Google Scholar]
- 66.Zallocchi M, Binley K, Lad Y, Ellis S, Widdowson P, Iqball S, Scripps V, Kelleher M, Loader J, Miskin J, et al. EIAV-based retinal gene therapy in the shaker1 mouse model for usher syndrome type 1B: development of UshStat. PLoS One. 2014;9:e94272. doi: 10.1371/journal.pone.0094272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kong J, Kim SR, Binley K, Pata I, Doi K, Mannik J, Zernant-Rajang J, Kan O, Iqball S, Naylor S, et al. Correction of the disease phenotype in the mouse model of Stargardt disease by lentiviral gene therapy. Gene Therapy. 2008;15:1311–1320. doi: 10.1038/gt.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Binley K, Widdowson P, Loader J, Kelleher M, Iqball S, Ferrige G, de Belin J, Carlucci M, Angell-Manning D, Hurst F, et al. Transduction of photoreceptors with equine infectious anemia virus lentiviral vectors: safety and biodistribution of StarGen for Stargardt disease. Invest Ophthalmol Vis Sci. 2013;54:4061–4071. doi: 10.1167/iovs.13-11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Senzer N, Nemunaitis J, Nemunaitis D, Bedell C, Edelman G, Barve M, Nunan R, Pirollo KF, Rait A, Chang EH. Phase I study of a systemically delivered p53 nanoparticle in advanced solid tumors. Mol Ther. 2013;21:1096–1103. doi: 10.1038/mt.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gill DR, Southern KW, Mofford KA, Seddon T, Huang L, Sorgi F, Thomson A, MacVinish LJ, Ratcliff R, Bilton D, et al. A placebo-controlled study of liposome-mediated gene transfer to the nasal epithelium of patients with cystic fibrosis. Gene Therapy. 1997;4:199–209. doi: 10.1038/sj.gt.3300391. [DOI] [PubMed] [Google Scholar]
- 71.Konstan MW, Davis PB, Wagener JS, Hilliard KA, Stern RC, Milgram LJ, Kowalczyk TH, Hyatt SL, Fink TL, Gedeon CR, et al. Compacted DNA nanoparticles administered to the nasal mucosa of cystic fibrosis subjects are safe and demonstrate partial to complete cystic fibrosis transmembrane regulator reconstitution. Human Gene Therapy. 2004;15:1255–1269. doi: 10.1089/hum.2004.15.1255. [DOI] [PubMed] [Google Scholar]
- 72.Adijanto J, Naash MI. Nanoparticle-based technologies for retinal gene therapy. Eur J Pharm Biopharm. 2015 doi: 10.1016/j.ejpb.2014.12.028. Epub ahead of print 01/17/2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ding XQ, Quiambao AB, Fitzgerald JB, Cooper MJ, Conley SM, Naash MI. Ocular delivery of compacted DNA-nanoparticles does not elicit toxicity in the mouse retina. PLoS ONE. 2009;4:e7410. doi: 10.1371/journal.pone.0007410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cai X, Conley SM, Nash Z, Fliesler SJ, Cooper MJ, Naash MI. Gene delivery to mitotic and postmitotic photoreceptors via compacted DNA nanoparticles results in improved phenotype in a mouse model of retinitis pigmentosa. FASEB Journal. 2010;24:1178–1191. doi: 10.1096/fj.09-139147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Han Z, Conley SM, Makkia RS, Cooper MJ, Naash MI. DNA nanoparticle-mediated ABCA4 delivery rescues Stargardt dystrophy in mice. Journal of Clinical Investigation. 2012;122:3221–3226. doi: 10.1172/JCI64833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fink TL, Klepcyk PJ, Oette SM, Gedeon CR, Hyatt SL, Kowalczyk TH, Moen RC, Cooper MJ. Plasmid size up to 20 kbp does not limit effective in vivo lung gene transfer using compacted DNA nanoparticles. Gene Therapy. 2006;13:1048–1051. doi: 10.1038/sj.gt.3302761. [DOI] [PubMed] [Google Scholar]
- 77.Cai X, Nash Z, Conley SM, Fliesler SJ, Cooper MJ, Naash MI. A partial structural and functional rescue of a retinitis pigmentosa model with compacted DNA nanoparticles. PLoS One. 2009;4:e5290. doi: 10.1371/journal.pone.0005290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Han Z, Banworth MJ, Makkia R, Conley SM, Al-Ubaidi MR, Cooper MJ, Naash MI. Genomic DNA nanoparticles rescue rhodopsin-associated retinitis pigmentosa phenotype. FASEB Journal. 2015;29:2535–2544. doi: 10.1096/fj.15-270363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Koirala A, Conley SM, Makkia R, Liu Z, Cooper MJ, Sparrow JR, Naash MI. Persistence of non-viral vector mediated RPE65 expression: case for viability as a gene transfer therapy for RPE-based diseases. J Control Release. 2013;172:745–752. doi: 10.1016/j.jconrel.2013.08.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Koirala A, Makkia RS, Conley SM, Cooper MJ, Naash MI. S/MAR-containing DNA nanoparticles promote persistent RPE gene expression and improvement in RPE65-associated LCA. Human Molecular Genetics. 2013;22:1632–1642. doi: 10.1093/hmg/ddt013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rajala A, Wang Y, Zhu Y, Ranjo-Bishop M, Ma JX, Mao C, Rajala RV. Nanoparticle-assisted targeted delivery of eye-specific genes to eyes significantly improves the vision of blind mice in vivo. Nano Lett. 2014;14:5257–5263. doi: 10.1021/nl502275s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alqawlaq S, Sivak JM, Huzil JT, Ivanova MV, Flanagan JG, Beazely MA, Foldvari M. Preclinical development and ocular biodistribution of gemini-DNA nanoparticles after intravitreal and topical administration: towards non-invasive glaucoma gene therapy. Nanomedicine. 2014;10:1637–1647. doi: 10.1016/j.nano.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 83.Kim DW, Lee SH, Ku SK, Cho SH, Cho SW, Yoon GH, Hwang HS, Park J, Eum WS, Kwon OS, et al. Transduced PEP-1-FK506BP ameliorates corneal injury in Botulinum toxin A-induced dry eye mouse model. BMB Rep. 2013;46:124–129. doi: 10.5483/BMBRep.2013.46.2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mohan RR, Tandon A, Sharma A, Cowden JW, Tovey JC. Significant inhibition of corneal scarring in vivo with tissue-selective, targeted AAV5 decorin gene therapy. Invest Ophthalmol Vis Sci. 2011;52:4833–4841. doi: 10.1167/iovs.11-7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chaudhari KR, Ukawala M, Manjappa AS, Kumar A, Mundada PK, Mishra AK, Mathur R, Monkkonen J, Murthy RS. Opsonization, biodistribution, cellular uptake and apoptosis study of PEGylated PBCA nanoparticle as potential drug delivery carrier. Pharm Res. 2012;29:53–68. doi: 10.1007/s11095-011-0510-x. [DOI] [PubMed] [Google Scholar]
- 86.Shakib M, Cunha-Vaz JG. Studies on the permeability of the blood-retinal barrier. IV. Junctional complexes of the retinal vessels and their role in the permeability of the blood-retinal barrier. Exp Eye Res. 1966;5:229–234. doi: 10.1016/s0014-4835(66)80011-8. [DOI] [PubMed] [Google Scholar]
- 87.Zhu C, Zhang Y, Pardridge WM. Widespread expression of an exogenous gene in the eye after intravenous administration. Invest Ophthalmol Vis Sci. 2002;43:3075–3080. [PubMed] [Google Scholar]
- 88.Feng L, Li SK, Liu H, Liu CY, LaSance K, Haque F, Shu D, Guo P. Ocular delivery of pRNA nanoparticles: distribution and clearance after subconjunctival injection. Pharm Res. 2014;31:1046–1058. doi: 10.1007/s11095-013-1226-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Touchard E, Berdugo M, Bigey P, El Sanharawi M, Savoldelli M, Naud MC, Jeanny JC, Behar-Cohen F. Suprachoroidal electrotransfer: a nonviral gene delivery method to transfect the choroid and the retina without detaching the retina. Mol Ther. 2012;20:1559–1570. doi: 10.1038/mt.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Delgado D, del Pozo-Rodriguez A, Solinis MA, Aviles-Triqueros M, Weber BH, Fernandez E, Gascon AR. Dextran and protamine-based solid lipid nanoparticles as potential vectors for the treatment of X-linked juvenile retinoschisis. Hum Gene Ther. 2012;23:345–355. doi: 10.1089/hum.2011.115. [DOI] [PubMed] [Google Scholar]
- 91.Peeters L, Sanders NN, Braeckmans K, Boussery K, Van de Voorde J, De Smedt SC, Demeester J. Vitreous: a barrier to nonviral ocular gene therapy. Investigative Ophthalmology and Visual Science. 2005;46:3553–3561. doi: 10.1167/iovs.05-0165. [DOI] [PubMed] [Google Scholar]
- 92.Puras G, Zarate J, Diaz-Tahoces A, Aviles-Trigueros M, Fernandez E, Pedraz JL. Oligochitosan polyplexes as carriers for retinal gene delivery. Eur J Pharm Sci. 2013;48:323–331. doi: 10.1016/j.ejps.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 93.Storey P, Dollin M, Pitcher J, Reddy S, Vojtko J, Vander J, Hsu J, Garg SJ. The role of topical antibiotic prophylaxis to prevent endophthalmitis after intravitreal injection. Ophthalmology. 2014;121:283–289. doi: 10.1016/j.ophtha.2013.08.037. [DOI] [PubMed] [Google Scholar]
- 94.Martens TF, Vercauteren D, Forier K, Deschout H, Remaut K, Paesen R, Ameloot M, Engbersen JF, Demeester J, De Smedt SC, et al. Measuring the intravitreal mobility of nanomedicines with single-particle tracking microscopy. Nanomedicine (Lond) 2013;8:1955–1968. doi: 10.2217/nnm.12.202. [DOI] [PubMed] [Google Scholar]
- 95.Bruewer AR, Mowat FM, Bartoe JT, Boye SL, Hauswirth WW, Petersen-Jones SM. Evaluation of lateral spread of transgene expression following subretinal AAV-mediated gene delivery in dogs. PLoS One. 2013;8:e60218. doi: 10.1371/journal.pone.0060218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hisatomi T, Sakamoto T, Murata T, Yamanaka I, Oshima Y, Hata Y, Ishibashi T, Inomata H, Susin SA, Kroemer G. Relocalization of apoptosis-inducing factor in photoreceptor apoptosis induced by retinal detachment in vivo. Am J Pathol. 2001;158:1271–1278. doi: 10.1016/S0002-9440(10)64078-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nakazawa T, Hisatomi T, Nakazawa C, Noda K, Maruyama K, She H, Matsubara A, Miyahara S, Nakao S, Yin Y, et al. Monocyte chemoattractant protein 1 mediates retinal detachment-induced photoreceptor apoptosis. Proc Natl Acad Sci U S A. 2007;104:2425–2430. doi: 10.1073/pnas.0608167104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nour M, Quiambao AB, Peterson WM, Al-Ubaidi MR, Naash MI. P2Y(2) receptor agonist INS37217 enhances functional recovery after detachment caused by subretinal injection in normal and rds mice. Invest Ophthalmol Vis Sci. 2003;44:4505–4514. doi: 10.1167/iovs.03-0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Palm E. On the occurrence in the retina of conditions corresponding to the blood-brain barrier. Acta Ophthalmol (Copenh) 1947;25:29–35. doi: 10.1111/j.1755-3768.1947.tb07542.x. [DOI] [PubMed] [Google Scholar]
- 100.Voigt N, Henrich-Noack P, Kockentiedt S, Hintz W, Tomas J, Sabel BA. Surfactants, not size or zeta-potential influence blood-brain barrier passage of polymeric nanoparticles. Eur J Pharm Biopharm. 2014;87:19–29. doi: 10.1016/j.ejpb.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 101.Ge HY, Xiao N, Yin XL, Fu SB, Ge JY, Shi Y, Liu P. Comparison of the antiangiogenic activity of modified RGDRGD-endostatin to endostatin delivered by gene transfer in vivo rabbit neovascularization model. Mol Vis. 2011;17:1918–1928. [PMC free article] [PubMed] [Google Scholar]
- 102.Iriyama A, Usui T, Yanagi Y, Amano S, Oba M, Miyata K, Nishiyama N, Kataoka K. Gene transfer using micellar nanovectors inhibits corneal neovascularization in vivo. Cornea. 2011;30:1423–1427. doi: 10.1097/ICO.0b013e318206c893. [DOI] [PubMed] [Google Scholar]
- 103.Amrite AC, Kompella UB. Size-dependent disposition of nanoparticles and microparticles following subconjunctival administration. J Pharm Pharmacol. 2005;57:1555–1563. doi: 10.1211/jpp.57.12.0005. [DOI] [PubMed] [Google Scholar]
- 104.Brewton RG, Mayne R. Mammalian vitreous humor contains networks of hyaluronan molecules: electron microscopic analysis using the hyaluronan-binding region (G1) of aggrecan and link protein. Exp Cell Res. 1992;198:237–249. doi: 10.1016/0014-4827(92)90376-j. [DOI] [PubMed] [Google Scholar]
- 105.Bishop PN, Crossman MV, McLeod D, Ayad S. Extraction and characterization of the tissue forms of collagen types II and IX from bovine vitreous. Biochem J. 1994;299(Pt 2):497–505. doi: 10.1042/bj2990497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Aretz S, Krohne TU, Kammerer K, Warnken U, Hotz-Wagenblatt A, Bergmann M, Stanzel BV, Kempf T, Holz FG, Schnolzer M, et al. In-depth mass spectrometric mapping of the human vitreous proteome. Proteome Sci. 2013;11:22. doi: 10.1186/1477-5956-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim H, Robinson SB, Csaky KG. Investigating the movement of intravitreal human serum albumin nanoparticles in the vitreous and retina. Pharmaceutical Research. 2009;26:329–337. doi: 10.1007/s11095-008-9745-6. [DOI] [PubMed] [Google Scholar]
- 108.Xu Q, Boylan NJ, Suk JS, Wang YY, Nance EA, Yang JC, McDonnell PJ, Cone RA, Duh EJ, Hanes J. Nanoparticle diffusion in, and microrheology of, the bovine vitreous ex vivo. J Control Release. 2013;167:76–84. doi: 10.1016/j.jconrel.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zagato E, Forier K, Martens T, Neyts K, Demeester J, De Smedt S, Remaut K, Braeckmans K. Single-particle tracking for studying nanomaterial dynamics: applications and fundamentals in drug delivery. Nanomedicine (Lond) 2014;9:913–927. doi: 10.2217/nnm.14.43. [DOI] [PubMed] [Google Scholar]
- 110.Gan L, Wang J, Zhao Y, Chen D, Zhu C, Liu J, Gan Y. Hyaluronan-modified core-shell liponanoparticles targeting CD44-positive retinal pigment epithelium cells via intravitreal injection. Biomaterials. 2013;34:5978–5987. doi: 10.1016/j.biomaterials.2013.04.035. [DOI] [PubMed] [Google Scholar]
- 111.Park K, Chen Y, Hu Y, Mayo AS, Kompella UB, Longeras R, Ma JX. Nanoparticle-mediated expression of an angiogenic inhibitor ameliorates ischemia-induced retinal neovascularization and diabetes-induced retinal vascular leakage. Diabetes. 2009;58:1902–1913. doi: 10.2337/db08-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bejjani RA, BenEzra D, Cohen H, Rieger J, Andrieu C, Jeanny JC, Gollomb G, Behar-Cohen FF. Nanoparticles for gene delivery to retinal pigment epithelial cells. Molecular Vision. 2005;11:124–132. [PubMed] [Google Scholar]
- 113.Bourges JL, Gautier SE, Delie F, Bejjani RA, Jeanny JC, Gurny R, BenEzra D, Behar-Cohen FF. Ocular drug delivery targeting the retina and retinal pigment epithelium using polylactide nanoparticles. Invest Ophthalmol Vis Sci. 2003;44:3562–3569. doi: 10.1167/iovs.02-1068. [DOI] [PubMed] [Google Scholar]
- 114.Meyer CH, Michels S, Rodrigues EB, Hager A, Mennel S, Schmidt JC, Helb HM, Farah ME. Incidence of rhegmatogenous retinal detachments after intravitreal antivascular endothelial factor injections. Acta Ophthalmol. 2011;89:70–75. doi: 10.1111/j.1755-3768.2010.02064.x. [DOI] [PubMed] [Google Scholar]
- 115.Bridges CD. Vitamin A and the role of the pigment epithelium during bleaching and regeneration of rhodopsin in the frog eye. Exp Eye Res. 1976;22:435–455. doi: 10.1016/0014-4835(76)90182-2. [DOI] [PubMed] [Google Scholar]
- 116.Pandey AN, Kakde A. A Retrospective Clinical Study of the Etiology and Post-operative Visual Outcome of Rhegmatogenous Retinal Detachment. J Clin Diagn Res. 2014;8:VC01–VC03. doi: 10.7860/JCDR/2014/8303.4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jacobson SG, Cideciyan AV, Ratnakaram R, Heon E, Schwartz SB, Roman AJ, Peden MC, Aleman TS, Boye SL, Sumaroka A, et al. Gene therapy for leber congenital amaurosis caused by RPE65 mutations: safety and efficacy in 15 children and adults followed up to 3 years. Arch Ophthalmol. 2012;130:9–24. doi: 10.1001/archophthalmol.2011.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Einmahl S, Savoldelli M, D'Hermies F, Tabatabay C, Gurny R, Behar-Cohen F. Evaluation of a novel biomaterial in the suprachoroidal space of the rabbit eye. Invest Ophthalmol Vis Sci. 2002;43:1533–1539. [PubMed] [Google Scholar]
- 119.Amrite AC, Edelhauser HF, Singh SR, Kompella UB. Effect of circulation on the disposition and ocular tissue distribution of 20 nm nanoparticles after periocular administration. Mol Vis. 2008;14:150–160. [PMC free article] [PubMed] [Google Scholar]
- 120.Young RW, Bok D. Participation of the retinal pigment epithelium in the rod outer segment renewal process. J Cell Biol. 1969;42:392–403. doi: 10.1083/jcb.42.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Willingham MC, Maxfield FR, Pastan IH. alpha 2 Macroglobulin binding to the plasma membrane of cultured fibroblasts. Diffuse binding followed by clustering in coated regions. J Cell Biol. 1979;82:614–625. doi: 10.1083/jcb.82.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chaudhary N, Gomez GA, Howes MT, Lo HP, McMahon KA, Rae JA, Schieber NL, Hill MM, Gaus K, Yap AS, et al. Endocytic crosstalk: cavins, caveolins, and caveolae regulate clathrin-independent endocytosis. PLoS Biol. 2014;12:e1001832. doi: 10.1371/journal.pbio.1001832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Malaba L, Smeland S, Senoo H, Norum KR, Berg T, Blomhoff R, Kindberg GM. Retinol-binding protein and asialo-orosomucoid are taken up by different pathways in liver cells. J Biol Chem. 1995;270:15686–15692. doi: 10.1074/jbc.270.26.15686. [DOI] [PubMed] [Google Scholar]
- 124.Wagner S, Rothweiler F, Anhorn MG, Sauer D, Riemann I, Weiss EC, Katsen-Globa A, Michaelis M, Cinatl J, Jr., Schwartz D, et al. Enhanced drug targeting by attachment of an anti alphav integrin antibody to doxorubicin loaded human serum albumin nanoparticles. Biomaterials. 2010;31:2388–2398. doi: 10.1016/j.biomaterials.2009.11.093. [DOI] [PubMed] [Google Scholar]
- 125.Rogers ML, Smith KS, Matusica D, Fenech M, Hoffman L, Rush RA, Voelcker NH. Non-viral gene therapy that targets motor neurons in vivo. Front Mol Neurosci. 2014;7:80. doi: 10.3389/fnmol.2014.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rusch V, Klimstra D, Venkatraman E, Pisters PW, Langenfeld J, Dmitrovsky E. Overexpression of the epidermal growth factor receptor and its ligand transforming growth factor alpha is frequent in resectable non-small cell lung cancer but does not predict tumor progression. Clin Cancer Res. 1997;3:515–522. [PubMed] [Google Scholar]
- 127.Tseng CL, Su WY, Yen KC, Yang KC, Lin FH. The use of biotinylated-EGF-modified gelatin nanoparticle carrier to enhance cisplatin accumulation in cancerous lungs via inhalation. Biomaterials. 2009;30:3476–3485. doi: 10.1016/j.biomaterials.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 128.Long JT, Cheang TY, Zhuo SY, Zeng RF, Dai QS, Li HP, Fang S. Anticancer drug-loaded multifunctional nanoparticles to enhance the chemotherapeutic efficacy in lung cancer metastasis. J Nanobiotechnology. 2014;12:37. doi: 10.1186/s12951-014-0037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Davis ME, Zuckerman JE, Choi CH, Seligson D, Tolcher A, Alabi CA, Yen Y, Heidel JD, Ribas A. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464:1067–1070. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Werner ME, Karve S, Sukumar R, Cummings ND, Copp JA, Chen RC, Zhang T, Wang AZ. Folate-targeted nanoparticle delivery of chemo- and radiotherapeutics for the treatment of ovarian cancer peritoneal metastasis. Biomaterials. 2011;32:8548–8554. doi: 10.1016/j.biomaterials.2011.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shinoe T, Kuribayashi H, Saya H, Seiki M, Aburatani H, Watanabe S. Identification of CD44 as a cell surface marker for Muller glia precursor cells. J Neurochem. 2010;115:1633–1642. doi: 10.1111/j.1471-4159.2010.07072.x. [DOI] [PubMed] [Google Scholar]
- 132.Apaolaza PS, Delgado D, del Pozo-Rodriguez A, Gascon AR, Solinis MA. A novel gene therapy vector based on hyaluronic acid and solid lipid nanoparticles for ocular diseases. Int J Pharm. 2014;465:413–426. doi: 10.1016/j.ijpharm.2014.02.038. [DOI] [PubMed] [Google Scholar]
- 133.Yamada Y, Hashida M, Harashima H. Hyaluronic acid controls the uptake pathway and intracellular trafficking of an octaarginine-modified gene vector in CD44 positive- and CD44 negative-cells. Biomaterials. 2015;52:189–198. doi: 10.1016/j.biomaterials.2015.02.027. [DOI] [PubMed] [Google Scholar]
- 134.Mann DA, Frankel AD. Endocytosis and targeting of exogenous HIV-1 Tat protein. EMBO J. 1991;10:1733–1739. doi: 10.1002/j.1460-2075.1991.tb07697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Elliott G, O'Hare P. Intercellular trafficking and protein delivery by a herpesvirus structural protein. Cell. 1997;88:223–233. doi: 10.1016/s0092-8674(00)81843-7. [DOI] [PubMed] [Google Scholar]
- 136.Cashman SM, Morris DJ, Kumar-Singh R. Evidence of protein transduction but not intercellular transport by proteins fused to HIV tat in retinal cell culture and in vivo. Mol Ther. 2003;8:130–142. doi: 10.1016/s1525-0016(03)00131-x. [DOI] [PubMed] [Google Scholar]
- 137.Cashman SM, Sadowski SL, Morris DJ, Frederick J, Kumar-Singh R. Intercellular trafficking of adenovirus-delivered HSV VP22 from the retinal pigment epithelium to the photoreceptors--implications for gene therapy. Mol Ther. 2002;6:813–823. doi: 10.1006/mthe.2002.0806. [DOI] [PubMed] [Google Scholar]
- 138.Johnson LN, Cashman SM, Read SP, Kumar-Singh R. Cell penetrating peptide POD mediates delivery of recombinant proteins to retina, cornea and skin. Vision Res. 2010;50:686–697. doi: 10.1016/j.visres.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Smoyer CJ, Jaspersen SL. Breaking down the wall: the nuclear envelope during mitosis. Curr Opin Cell Biol. 2014;26:1–9. doi: 10.1016/j.ceb.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 140.Nigg EA. Nucleocytoplasmic transport: signals, mechanisms and regulation. Nature. 1997;386:779–787. doi: 10.1038/386779a0. [DOI] [PubMed] [Google Scholar]
- 141.Kalderon D, Roberts BL, Richardson WD, Smith AE. A short amino acid sequence able to specify nuclear location. Cell. 1984;39:499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- 142.Badding MA, Vaughan EE, Dean DA. Transcription factor plasmid binding modulates microtubule interactions and intracellular trafficking during gene transfer. Gene Ther. 2012;19:338–346. doi: 10.1038/gt.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wilson GL, Dean BS, Wang G, Dean DA. Nuclear import of plasmid DNA in digitonin-permeabilized cells requires both cytoplasmic factors and specific DNA sequences. Journal of Biological Chemistry. 1999;274:22025–22032. doi: 10.1074/jbc.274.31.22025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Young JL, Benoit JN, Dean DA. Effect of a DNA nuclear targeting sequence on gene transfer and expression of plasmids in the intact vasculature. Gene Therapy. 2003;10:1465–1470. doi: 10.1038/sj.gt.3302021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Delgado D, del Pozo-Rodriguez A, Solinis MA, Rodriguez-Gascon A. Understanding the mechanism of protamine in solid lipid nanoparticle-based lipofection: the importance of the entry pathway. Eur J Pharm Biopharm. 2011;79:495–502. doi: 10.1016/j.ejpb.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 146.Chen X, Shank S, Davis PB, Ziady AG. Nucleolin-mediated cellular trafficking of DNA nanoparticle is lipid raft and microtubule dependent and can be modulated by glucocorticoid. Mol Ther. 2011;19:93–102. doi: 10.1038/mt.2010.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dean DA. Import of plasmid DNA into the nucleus is sequence specific. Exp Cell Res. 1997;230:293–302. doi: 10.1006/excr.1996.3427. [DOI] [PubMed] [Google Scholar]
- 148.Dean DA, Dean BS, Muller S, Smith LC. Sequence requirements for plasmid nuclear import. Experimental Cell Research. 1999;253:713–722. doi: 10.1006/excr.1999.4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Li S, MacLaughlin FC, Fewell JG, Gondo M, Wang J, Nicol F, Dean DA, Smith LC. Muscle-specific enhancement of gene expression by incorporation of SV40 enhancer in the expression plasmid. Gene Ther. 2001;8:494–497. doi: 10.1038/sj.gt.3301419. [DOI] [PubMed] [Google Scholar]
- 150.Darquet AM, Cameron B, Wils P, Scherman D, Crouzet J. A new DNA vehicle for nonviral gene delivery: supercoiled minicircle. Gene Therapy. 1997;4:1341–1349. doi: 10.1038/sj.gt.3300540. [DOI] [PubMed] [Google Scholar]
- 151.Tolmachov OE. Tightly-wound miniknot vectors for gene therapy: a potential improvement over supercoiled minicircle DNA. Med Hypotheses. 2010;74:702–704. doi: 10.1016/j.mehy.2009.10.035. [DOI] [PubMed] [Google Scholar]
- 152.Liu G, Li D, Pasumarthy MK, Kowalczyk TH, Gedeon CR, Hyatt SL, Payne JM, Miller TJ, Brunovskis P, Fink TL, et al. Nanoparticles of compacted DNA transfect postmitotic cells. Journal of Biological Chemistry. 2003;278:32578–32586. doi: 10.1074/jbc.M305776200. [DOI] [PubMed] [Google Scholar]
- 153.Han Z, Banworth MJ, Makkia R, Conley SM, Al-Ubaidi MR, Cooper MJ, Naash MI. Genomic DNA nanoparticles rescue rhodopsin-associated retinitis pigmentosa phenotype. FASEB J. 2015 doi: 10.1096/fj.15-270363. Epub ahead of print 02/26/2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kim H, Park Y, Lee JB. Self-assembled Messenger RNA Nanoparticles (mRNA-NPs) for Efficient Gene Expression. Sci Rep. 2015;5:12737. doi: 10.1038/srep12737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Palama IE, Cortese B, D'Amone S, Gigli G. mRNA delivery using non-viral PCL nanoparticles. Biomater Sci. 2015;3:144–151. doi: 10.1039/c4bm00242c. [DOI] [PubMed] [Google Scholar]
- 156.Cooper MJ, Lippa M, Payne JM, Hatzivassiliou G, Reifenberg E, Fayazi B, Perales JC, Morrison LJ, Templeton D, Piekarz RL, et al. Safety-modified episomal vectors for human gene therapy. Proc Natl Acad Sci U S A. 1997;94:6450–6455. doi: 10.1073/pnas.94.12.6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Eckhart W. Cell transformation by polyoma virus and SV40. Nature. 1969;224:1069–1071. doi: 10.1038/2241069a0. [DOI] [PubMed] [Google Scholar]
- 158.Piechaczek C, Fetzer C, Baiker A, Bode J, Lipps HJ. A vector based on the SV40 origin of replication and chromosomal S/MARs replicates episomally in CHO cells. Nucleic Acids Res. 1999;27:426–428. doi: 10.1093/nar/27.2.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jenke BH, Fetzer CP, Stehle IM, Jonsson F, Fackelmayer FO, Conradt H, Bode J, Lipps HJ. An episomally replicating vector binds to the nuclear matrix protein SAF-A in vivo. EMBO Rep. 2002;3:349–354. doi: 10.1093/embo-reports/kvf070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kim JM, Kim JS, Park DH, Kang HS, Yoon J, Baek K, Yoon Y. Improved recombinant gene expression in CHO cells using matrix attachment regions. J Biotechnol. 2004;107:95–105. doi: 10.1016/j.jbiotec.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 161.Klehr D, Maass K, Bode J. Scaffold-attached regions from the human interferon beta domain can be used to enhance the stable expression of genes under the control of various promoters. Biochemistry. 1991;30:1264–1270. doi: 10.1021/bi00219a015. [DOI] [PubMed] [Google Scholar]
- 162.Chancham P, van Ijperen T, McDoom I, Hughes JA. Nucleic acid-matrix attachment recognition regions--as facilitators in plasmid transfer. J Drug Target. 2003;11:205–213. doi: 10.1080/10611860310001603823. [DOI] [PubMed] [Google Scholar]
- 163.Boyes J, Bird A. Repression of genes by DNA methylation depends on CpG density and promoter strength: evidence for involvement of a methyl-CpG binding protein. EMBO J. 1992;11:327–333. doi: 10.1002/j.1460-2075.1992.tb05055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tsan MF, White JE, Shepard B. Lung-specific direct in vivo gene transfer with recombinant plasmid DNA. Am J Physiol. 1995;268:L1052–1056. doi: 10.1152/ajplung.1995.268.6.L1052. [DOI] [PubMed] [Google Scholar]
- 165.Brooks AR, Harkins RN, Wang P, Qian HS, Liu P, Rubanyi GM. Transcriptional silencing is associated with extensive methylation of the CMV promoter following adenoviral gene delivery to muscle. J Gene Med. 2004;6:395–404. doi: 10.1002/jgm.516. [DOI] [PubMed] [Google Scholar]
- 166.Wang J, Lawry ST, Cohen AL, Jia S. Chromosome boundary elements and regulation of heterochromatin spreading. Cell Mol Life Sci. 2014;71:4841–4852. doi: 10.1007/s00018-014-1725-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Riu E, Chen ZY, Xu H, He CY, Kay MA. Histone modifications are associated with the persistence or silencing of vector-mediated transgene expression in vivo. Mol Ther. 2007;15:1348–1355. doi: 10.1038/sj.mt.6300177. [DOI] [PubMed] [Google Scholar]
- 168.Gracey Maniar LE, Maniar JM, Chen ZY, Lu J, Fire AZ, Kay MA. Minicircle DNA vectors achieve sustained expression reflected by active chromatin and transcriptional level. Mol Ther. 2013;21:131–138. doi: 10.1038/mt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kay MA, He CY, Chen ZY. A robust system for production of minicircle DNA vectors. Nat Biotechnol. 2010;28:1287–1289. doi: 10.1038/nbt.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Huang M, Nguyen P, Jia F, Hu S, Gong Y, de Almeida PE, Wang L, Nag D, Kay MA, Giaccia AJ, et al. Double knockdown of prolyl hydroxylase and factor-inhibiting hypoxia-inducible factor with nonviral minicircle gene therapy enhances stem cell mobilization and angiogenesis after myocardial infarction. Circulation. 2011;124:S46–54. doi: 10.1161/CIRCULATIONAHA.110.014019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Haase R, Argyros O, Wong SP, Harbottle RP, Lipps HJ, Ogris M, Magnusson T, Vizoso Pinto MG, Haas J, Baiker A. pEPito: a significantly improved non-viral episomal expression vector for mammalian cells. BMC Biotechnol. 2010;10:20. doi: 10.1186/1472-6750-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Calado SM, Oliveira AV, Machado S, Haase R, Silva GA. Sustained gene expression in the retina by improved episomal vectors. Tissue Eng Part A. 2014;20:2692–2698. doi: 10.1089/ten.tea.2013.0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hyde SC, Pringle IA, Abdullah S, Lawton AE, Davies LA, Varathalingam A, Nunez-Alonso G, Green AM, Bazzani RP, Sumner-Jones SG, et al. CpG-free plasmids confer reduced inflammation and sustained pulmonary gene expression. Nature Biotechnology. 2008;26:549–551. doi: 10.1038/nbt1399. [DOI] [PubMed] [Google Scholar]
- 174.Bauer S, Kirschning CJ, Hacker H, Redecke V, Hausmann S, Akira S, Wagner H, Lipford GB. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc Natl Acad Sci U S A. 2001;98:9237–9242. doi: 10.1073/pnas.161293498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Nishikawa J, Amano M, Fukue Y, Tanaka S, Kishi H, Hirota Y, Yoda K, Ohyama T. Left-handedly curved DNA regulates accessibility to cis-DNA elements in chromatin. Nucleic Acids Res. 2003;31:6651–6662. doi: 10.1093/nar/gkg854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tanase J, Morohashi N, Fujita M, Nishikawa J, Shimizu M, Ohyama T. Highly efficient chromatin transcription induced by superhelically curved DNA segments: the underlying mechanism revealed by a yeast system. Biochemistry. 2010;49:2351–2358. doi: 10.1021/bi901950w. [DOI] [PubMed] [Google Scholar]
- 177.Wanapirak C, Kato M, Onishi Y, Wada-Kiyama Y, Kiyama R. Evolutionary conservation and functional synergism of curved DNA at the mouse epsilon- and other globin-gene promoters. J Mol Evol. 2003;56:649–657. doi: 10.1007/s00239-002-2432-z. [DOI] [PubMed] [Google Scholar]
- 178.Sumida N, Nishikawa J, Kishi H, Amano M, Furuya T, Sonobe H, Ohyama T. A designed curved DNA segment that is a remarkable activator of eukaryotic transcription. FEBS J. 2006;273:5691–5702. doi: 10.1111/j.1742-4658.2006.05557.x. [DOI] [PubMed] [Google Scholar]
- 179.Fukunaga S, Kanda G, Tanase J, Harashima H, Ohyama T, Kamiya H. A designed curved DNA sequence remarkably enhances transgene expression from plasmid DNA in mouse liver. Gene Ther. 2012;19:828–835. doi: 10.1038/gt.2011.127. [DOI] [PubMed] [Google Scholar]
- 180.Pikaart MJ, Recillas-Targa F, Felsenfeld G. Loss of transcriptional activity of a transgene is accompanied by DNA methylation and histone deacetylation and is prevented by insulators. Genes Dev. 1998;12:2852–2862. doi: 10.1101/gad.12.18.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Emery DW. The use of chromatin insulators to improve the expression and safety of integrating gene transfer vectors. Hum Gene Ther. 2011;22:761–774. doi: 10.1089/hum.2010.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Aker M, Tubb J, Groth AC, Bukovsky AA, Bell AC, Felsenfeld G, Kiem HP, Stamatoyannopoulos G, Emery DW. Extended core sequences from the cHS4 insulator are necessary for protecting retroviral vectors from silencing position effects. Hum Gene Ther. 2007;18:333–343. doi: 10.1089/hum.2007.021. [DOI] [PubMed] [Google Scholar]
- 183.Liu M, Maurano MT, Wang H, Qi H, Song CZ, Navas PA, Emery DW, Stamatoyannopoulos JA, Stamatoyannopoulos G. Genomic discovery of potent chromatin insulators for human gene therapy. Nat Biotechnol. 2015;33:198–203. doi: 10.1038/nbt.3062. [DOI] [PubMed] [Google Scholar]
- 184.O'Connor TP, Crystal RG. Genetic medicines: treatment strategies for hereditary disorders. Nat Rev Genet. 2006;7:261–276. doi: 10.1038/nrg1829. [DOI] [PubMed] [Google Scholar]
- 185.Nott A, Meislin SH, Moore MJ. A quantitative analysis of intron effects on mammalian gene expression. RNA. 2003;9:607–617. doi: 10.1261/rna.5250403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Whitlock PR, Hackett NR, Leopold PL, Rosengart TK, Crystal RG. Adenovirus-mediated transfer of a minigene expressing multiple isoforms of VEGF is more effective at inducing angiogenesis than comparable vectors expressing individual VEGF cDNAs. Mol Ther. 2004;9:67–75. doi: 10.1016/j.ymthe.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 187.Chapman BS, Thayer RM, Vincent KA, Haigwood NL. Effect of intron A from human cytomegalovirus (Towne) immediate-early gene on heterologous expression in mammalian cells. Nucleic Acids Res. 1991;19:3979–3986. doi: 10.1093/nar/19.14.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rose AB, Beliakoff JA. Intron-mediated enhancement of gene expression independent of unique intron sequences and splicing. Plant Physiol. 2000;122:535–542. doi: 10.1104/pp.122.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Huang MT, Gorman CM. Intervening sequences increase efficiency of RNA 3' processing and accumulation of cytoplasmic RNA. Nucleic Acids Res. 1990;18:937–947. doi: 10.1093/nar/18.4.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Niwa M, Rose SD, Berget SM. In vitro polyadenylation is stimulated by the presence of an upstream intron. Genes Dev. 1990;4:1552–1559. doi: 10.1101/gad.4.9.1552. [DOI] [PubMed] [Google Scholar]
- 191.Li Y, Zhou M, Zhou H, Ning Y. The last intron of the human thrombopoietin gene enhances expression in milk of transgenic mice. Funct Integr Genomics. 2014;14:229–236. doi: 10.1007/s10142-013-0348-x. [DOI] [PubMed] [Google Scholar]
- 192.Haddad-Mashadrizeh A, Zomorodipour A, Izadpanah M, Sam MR, Ataei F, Sabouni F, Hosseini SJ. A systematic study of the function of the human beta-globin introns on the expression of the human coagulation factor IX in cultured Chinese hamster ovary cells. J Gene Med. 2009;11:941–950. doi: 10.1002/jgm.1367. [DOI] [PubMed] [Google Scholar]
- 193.Wu Z, Sun J, Zhang T, Yin C, Yin F, Van Dyke T, Samulski RJ, Monahan PE. Optimization of self-complementary AAV vectors for liver-directed expression results in sustained correction of hemophilia B at low vector dose. Mol Ther. 2008;16:280–289. doi: 10.1038/sj.mt.6300355. [DOI] [PubMed] [Google Scholar]
- 194.Yew NS, Wysokenski DM, Wang KX, Ziegler RJ, Marshall J, McNeilly D, Cherry M, Osburn W, Cheng SH. Optimization of plasmid vectors for high-level expression in lung epithelial cells. Hum Gene Ther. 1997;8:575–584. doi: 10.1089/hum.1997.8.5-575. [DOI] [PubMed] [Google Scholar]
- 195.Xu ZL, Mizuguchi H, Ishii-Watabe A, Uchida E, Mayumi T, Hayakawa T. Strength evaluation of transcriptional regulatory elements for transgene expression by adenovirus vector. J Control Release. 2002;81:155–163. doi: 10.1016/s0168-3659(02)00059-7. [DOI] [PubMed] [Google Scholar]
- 196.Boshart M, Weber F, Jahn G, Dorsch-Hasler K, Fleckenstein B, Schaffner W. A very strong enhancer is located upstream of an immediate early gene of human cytomegalovirus. Cell. 1985;41:521–530. doi: 10.1016/s0092-8674(85)80025-8. [DOI] [PubMed] [Google Scholar]
- 197.Magnusson T, Haase R, Schleef M, Wagner E, Ogris M. Sustained, high transgene expression in liver with plasmid vectors using optimized promoter-enhancer combinations. J Gene Med. 2011;13:382–391. doi: 10.1002/jgm.1585. [DOI] [PubMed] [Google Scholar]
- 198.Li Y, Yang Y, Wang S. Neuronal gene transfer by baculovirus-derived vectors accommodating a neurone-specific promoter. Exp Physiol. 2005;90:39–44. doi: 10.1113/expphysiol.2004.028217. [DOI] [PubMed] [Google Scholar]
- 199.Wellensiek BP, Larsen AC, Stephens B, Kukurba K, Waern K, Briones N, Liu L, Snyder M, Jacobs BL, Kumar S, et al. Genome-wide profiling of human cap-independent translation-enhancing elements. Nat Methods. 2013;10:747–750. doi: 10.1038/nmeth.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wellensiek BP, Larsen AC, Flores J, Jacobs BL, Chaput JC. A leader sequence capable of enhancing RNA expression and protein synthesis in mammalian cells. Protein Sci. 2013;22:1392–1398. doi: 10.1002/pro.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Liu X, Mertz JE. HnRNP L binds a cis-acting RNA sequence element that enables intron-dependent gene expression. Genes Dev. 1995;9:1766–1780. doi: 10.1101/gad.9.14.1766. [DOI] [PubMed] [Google Scholar]
- 202.Huang ZM, Yen TS. Hepatitis B virus RNA element that facilitates accumulation of surface gene transcripts in the cytoplasm. J Virol. 1994;68:3193–3199. doi: 10.1128/jvi.68.5.3193-3199.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Donello JE, Loeb JE, Hope TJ. Woodchuck hepatitis virus contains a tripartite posttranscriptional regulatory element. J Virol. 1998;72:5085–5092. doi: 10.1128/jvi.72.6.5085-5092.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Choi JH, Yu NK, Baek GC, Bakes J, Seo D, Nam HJ, Baek SH, Lim CS, Lee YS, Kaang BK. Optimization of AAV expression cassettes to improve packaging capacity and transgene expression in neurons. Mol Brain. 2014;7:17. doi: 10.1186/1756-6606-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Mariati, Ho SC, Yap MG, Yang Y. Evaluating post-transcriptional regulatory elements for enhancing transient gene expression levels in CHO K1 and HEK293 cells. Protein Expr Purif. 2010;69:9–15. doi: 10.1016/j.pep.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 206.Paterna JC, Moccetti T, Mura A, Feldon J, Bueler H. Influence of promoter and WHV post-transcriptional regulatory element on AAV-mediated transgene expression in the rat brain. Gene Ther. 2000;7:1304–1311. doi: 10.1038/sj.gt.3301221. [DOI] [PubMed] [Google Scholar]
- 207.Xu R, Janson CG, Mastakov M, Lawlor P, Young D, Mouravlev A, Fitzsimons H, Choi KL, Ma H, Dragunow M, et al. Quantitative comparison of expression with adeno-associated virus (AAV-2) brain-specific gene cassettes. Gene Ther. 2001;8:1323–1332. doi: 10.1038/sj.gt.3301529. [DOI] [PubMed] [Google Scholar]
- 208.Martin KR, Quigley HA, Zack DJ, Levkovitch-Verbin H, Kielczewski J, Valenta D, Baumrind L, Pease ME, Klein RL, Hauswirth WW. Gene therapy with brain-derived neurotrophic factor as a protection: retinal ganglion cells in a rat glaucoma model. Investigative Ophthalmology and Visual Science. 2003;44:4357–4365. doi: 10.1167/iovs.02-1332. [DOI] [PubMed] [Google Scholar]
- 209.Higgs DR, Goodbourn SE, Lamb J, Clegg JB, Weatherall DJ, Proudfoot NJ. Alpha-thalassaemia caused by a polyadenylation signal mutation. Nature. 1983;306:398–400. doi: 10.1038/306398a0. [DOI] [PubMed] [Google Scholar]
- 210.Fitzgerald M, Shenk T. The sequence 5'-AAUAAA-3'forms parts of the recognition site for polyadenylation of late SV40 mRNAs. Cell. 1981;24:251–260. doi: 10.1016/0092-8674(81)90521-3. [DOI] [PubMed] [Google Scholar]
- 211.Assessment of adenoviral vector safety and toxicity: report of the National Institutes of Health Recombinant DNA Advisory Committee. Hum Gene Ther. 2002;13:3–13. doi: 10.1089/10430340152712629. [DOI] [PubMed] [Google Scholar]
- 212.Marshall E. Gene therapy death prompts review of adenovirus vector. Science. 1999;286:2244–2245. doi: 10.1126/science.286.5448.2244. [DOI] [PubMed] [Google Scholar]
- 213.Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, Gross F, Yvon E, Nusbaum P, Selz F, Hue C, Certain S, Casanova JL, et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288:669–672. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- 214.Hacein-Bey-Abina S, von Kalle C, Schmidt M, Le Deist F, Wulffraat N, McIntyre E, Radford I, Villeval JL, Fraser CC, Cavazzana-Calvo M, et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. New England Journal of Medicine. 2003;348:255–256. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- 215.Stroes ES, Nierman MC, Meulenberg JJ, Franssen R, Twisk J, Henny CP, Maas MM, Zwinderman AH, Ross C, Aronica E, et al. Intramuscular administration of AAV1-lipoprotein lipase S447X lowers triglycerides in lipoprotein lipase-deficient patients. Arterioscler Thromb Vasc Biol. 2008;28:2303–2304. doi: 10.1161/ATVBAHA.108.175620. [DOI] [PubMed] [Google Scholar]
- 216.Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, Ozelo MC, Hoots K, Blatt P, Konkle B, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 217.Li Q, Miller R, Han PY, Pang J, Dinculescu A, Chiodo V, Hauswirth WW. Intraocular route of AAV2 vector administration defines humoral immune response and therapeutic potential. Mol Vis. 2008;14:1760–1769. [PMC free article] [PubMed] [Google Scholar]
- 218.Kotterman MA, Yin L, Strazzeri JM, Flannery JG, Merigan WH, Schaffer DV. Antibody neutralization poses a barrier to intravitreal adeno-associated viral vector gene delivery to non-human primates. Gene Ther. 2015;22:116–126. doi: 10.1038/gt.2014.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Simonelli F, Maguire AM, Testa F, Pierce EA, Mingozzi F, Bennicelli JL, Rossi S, Marshall K, Banfi S, Surace EM, et al. Gene therapy for Leber's congenital amaurosis is safe and effective through 1.5 years after vector administration. Mol Ther. 2010;18:643–650. doi: 10.1038/mt.2009.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Stiehler M, Duch M, Mygind T, Li H, Ulrich-Vinther M, Modin C, Baatrup A, Lind M, Pedersen FS, Bunger CE. Optimizing viral and non-viral gene transfer methods for genetic modification of porcine mesenchymal stem cells. Adv Exp Med Biol. 2006;585:31–48. doi: 10.1007/978-0-387-34133-0_3. [DOI] [PubMed] [Google Scholar]
- 221.Delgado D, Gascon AR, Del Pozo-Rodriguez A, Echevarria E, Ruiz de Garibay AP, Rodriguez JM, Solinis MA. Dextran-protamine-solid lipid nanoparticles as a non-viral vector for gene therapy: in vitro characterization and in vivo transfection after intravenous administration to mice. International Journal of Pharmaceutics. 2012;425:35–43. doi: 10.1016/j.ijpharm.2011.12.052. [DOI] [PubMed] [Google Scholar]
- 222.Sanders NN, Peeters L, Lentacker I, Demeester J, De Smedt SC. Wanted and unwanted properties of surface PEGylated nucleic acid nanoparticles in ocular gene transfer. J Control Release. 2007;122:226–235. doi: 10.1016/j.jconrel.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 223.Solinis MA, Del Pozo-Rodriguez A, Apaolaza PS, Rodriguez-Gascon A. Treatment of ocular disorders by gene therapy. European Journal of Pharmaceutics and Biopharmaceutics. 2014 doi: 10.1016/j.ejpb.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 224.Mo Y, Barnett ME, Takemoto D, Davidson H, Kompella UB. Human serum albumin nanoparticles for efficient delivery of Cu, Zn superoxide dismutase gene. Mol Vis. 2007;13:746–757. [PMC free article] [PubMed] [Google Scholar]
- 225.Han Z, Koirala A, Makkia R, Cooper MJ, Naash MI. Direct gene transfer with compacted DNA nanoparticles in retinal pigment epithelial cells: expression, repeat delivery and lack of toxicity. Nanomedicine. 2012;7:521–539. doi: 10.2217/nnm.11.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Mitra RN, Han Z, Merwin M, Al Taai M, Conley SM, Naash MI. Synthesis and characterization of glycol chitosan DNA nanoparticles for retinal gene delivery. ChemMedChem. 2014;9:189–196. doi: 10.1002/cmdc.201300371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Klausner EA, Zhang Z, Chapman RL, Multack RF, Volin MV. Ultrapure chitosan oligomers as carriers for corneal gene transfer. Biomaterials. 2010;31:1814–1820. doi: 10.1016/j.biomaterials.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 228.Zorzi GK, Parraga JE, Seijo B, Sanchez A. Hybrid nanoparticle design based on cationized gelatin and the polyanions dextran sulfate and chondroitin sulfate for ocular gene therapy. Macromol Biosci. 2011;11:905–913. doi: 10.1002/mabi.201100005. [DOI] [PubMed] [Google Scholar]
- 229.Gomes dos Santos AL, Bochot A, Tsapis N, Artzner F, Bejjani RA, Thillaye-Goldenberg B, de Kozak Y, Fattal E, Behar-Cohen F. Oligonucleotide-polyethylenimine complexes targeting retinal cells: structural analysis and application to anti-TGFbeta-2 therapy. Pharm Res. 2006;23:770–781. doi: 10.1007/s11095-006-9748-0. [DOI] [PubMed] [Google Scholar]
- 230.Horbinski C, Stachowiak MK, Higgins D, Finnegan SG. Polyethyleneimine-mediated transfection of cultured postmitotic neurons from rat sympathetic ganglia and adult human retina. BMC Neurosci. 2001;2:2. doi: 10.1186/1471-2202-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Kurosaki T, Uematsu M, Shimoda K, Suzuma K, Nakai M, Nakamura T, Kitahara T, Kitaoka T, Sasaki H. Ocular gene delivery systems using ternary complexes of plasmid DNA, polyethylenimine, and anionic polymers. Biol Pharm Bull. 2013;36:96–101. doi: 10.1248/bpb.b12-00728. [DOI] [PubMed] [Google Scholar]
- 232.Sakai T, Kuno N, Takamatsu F, Kimura E, Kohno H, Okano K, Kitahara K. Prolonged protective effect of basic fibroblast growth factor-impregnated nanoparticles in royal college of surgeons rats. Invest Ophthalmol Vis Sci. 2007;48:3381–3387. doi: 10.1167/iovs.06-1242. [DOI] [PubMed] [Google Scholar]
- 233.Fraley R, Subramani S, Berg P, Papahadjopoulos D. Introduction of liposome-encapsulated SV40 DNA into cells. J Biol Chem. 1980;255:10431–10435. [PubMed] [Google Scholar]
- 234.Pitard B, Aguerre O, Airiau M, Lachages AM, Boukhnikachvili T, Byk G, Dubertret C, Herviou C, Scherman D, Mayaux JF, et al. Virus-sized self-assembling lamellar complexes between plasmid DNA and cationic micelles promote gene transfer. Proc Natl Acad Sci U S A. 1997;94:14412–14417. doi: 10.1073/pnas.94.26.14412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Nabel GJ, Nabel EG, Yang ZY, Fox BA, Plautz GE, Gao X, Huang L, Shu S, Gordon D, Chang AE. Direct gene transfer with DNA-liposome complexes in melanoma: expression, biologic activity, and lack of toxicity in humans. Proc Natl Acad Sci U S A. 1993;90:11307–11311. doi: 10.1073/pnas.90.23.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.San H, Yang ZY, Pompili VJ, Jaffe ML, Plautz GE, Xu L, Felgner JH, Wheeler CJ, Felgner PL, Gao X, et al. Safety and short-term toxicity of a novel cationic lipid formulation for human gene therapy. Hum Gene Ther. 1993;4:781–788. doi: 10.1089/hum.1993.4.6-781. [DOI] [PubMed] [Google Scholar]
- 237.Sorgi FL, Bhattacharya S, Huang L. Protamine sulfate enhances lipid-mediated gene transfer. Gene Ther. 1997;4:961–968. doi: 10.1038/sj.gt.3300484. [DOI] [PubMed] [Google Scholar]
- 238.Delgado D, Gascon AR, Del Pozo-Rodriguez A, Echevarria E, Ruiz de Garibay AP, Rodriguez JM, Solinis MA. Dextran-protamine-solid lipid nanoparticles as a non-viral vector for gene therapy: in vitro characterization and in vivo transfection after intravenous administration to mice. Int J Pharm. 2012;425:35–43. doi: 10.1016/j.ijpharm.2011.12.052. [DOI] [PubMed] [Google Scholar]
- 239.Bennett MJ, Nantz MH, Balasubramaniam RP, Gruenert DC, Malone RW. Cholesterol enhances cationic liposome-mediated DNA transfection of human respiratory epithelial cells. Biosci Rep. 1995;15:47–53. doi: 10.1007/BF01200214. [DOI] [PubMed] [Google Scholar]
- 240.Li S, Tseng WC, Stolz DB, Wu SP, Watkins SC, Huang L. Dynamic changes in the characteristics of cationic lipidic vectors after exposure to mouse serum: implications for intravenous lipofection. Gene Ther. 1999;6:585–594. doi: 10.1038/sj.gt.3300865. [DOI] [PubMed] [Google Scholar]
- 241.Langer K, Balthasar S, Vogel V, Dinauer N, von Briesen H, Schubert D. Optimization of the preparation process for human serum albumin (HSA) nanoparticles. Int J Pharm. 2003;257:169–180. doi: 10.1016/s0378-5173(03)00134-0. [DOI] [PubMed] [Google Scholar]
- 242.Chuang VT, Otagiri M. Recombinant human serum albumin. Drugs Today (Barc) 2007;43:547–561. doi: 10.1358/dot.2007.43.8.1067343. [DOI] [PubMed] [Google Scholar]
- 243.Irache JM, Merodio M, Arnedo A, Camapanero MA, Mirshahi M, Espuelas S. Albumin nanoparticles for the intravitreal delivery of anticytomegaloviral drugs. Mini Rev Med Chem. 2005;5:293–305. doi: 10.2174/1389557053175335. [DOI] [PubMed] [Google Scholar]
- 244.Hallaj-Nezhadi S, Valizadeh H, Baradaran B, Dobakhti F, Lotfipour F. Preparation and characterization of gelatin nanoparticles containing pDNA encoding IL-12 and their expression in CT-26 carcinoma cells. Future Oncol. 2013;9:1195–1206. doi: 10.2217/fon.13.82. [DOI] [PubMed] [Google Scholar]
- 245.Xu J, Amiji M. Therapeutic gene delivery and transfection in human pancreatic cancer cells using epidermal growth factor receptor-targeted gelatin nanoparticles. J Vis Exp. 2012:e3612. doi: 10.3791/3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Vitiello L, Chonn A, Wasserman JD, Duff C, Worton RG. Condensation of plasmid DNA with polylysine improves liposome-mediated gene transfer into established and primary muscle cells. Gene Ther. 1996;3:396–404. [PubMed] [Google Scholar]
- 247.Boylan NJ, Kim AJ, Suk JS, Adstamongkonkul P, Simons BW, Lai SK, Cooper MJ, Hanes J. Enhancement of airway gene transfer by DNA nanoparticles using a pH-responsive block copolymer of polyethylene glycol and poly-l-lysine. Biomaterials. 2012;33:2361–2371. doi: 10.1016/j.biomaterials.2011.11.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Yurek DM, Flectcher AM, Kowalczyk TH, Padegimas L, Cooper MJ. Compacted DNA nanoparticle gene transfer of GDNF to the rat striatum enhances the survival of grafted fetal dopamine neurons. Cell Transplantation. 2009;18:1183–1196. doi: 10.3727/096368909X12483162196881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Walsh M, Tangney M, O'Neill MJ, Larkin JO, Soden DM, McKenna SL, Darcy R, O'Sullivan GC, O'Driscoll CM. Evaluation of cellular uptake and gene transfer efficiency of pegylated poly-L-lysine compacted DNA: implications for cancer gene therapy. Mol Pharm. 2006;3:644–653. doi: 10.1021/mp0600034. [DOI] [PubMed] [Google Scholar]
- 250.Ziady AG, Gedeon CR, Muhammad O, Stillwell V, Oette SM, Fink TL, Quan W, Kowalczyk TH, Hyatt SL, Payne J, et al. Minimal toxicity of stabilized compacted DNA nanoparticles in the murine lung. Mol Ther. 2003;8:948–956. doi: 10.1016/j.ymthe.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 251.Han Z, Conley SM, Makkia R, Guo J, Cooper MJ, Naash MI. Comparative Analysis of DNA Nanoparticles and AAVs for Ocular Gene Delivery. PLoS One. 2012;7:e52189. doi: 10.1371/journal.pone.0052189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Farjo R, Skaggs J, Quiambao AB, Cooper MJ, Naash MI. Efficient non-viral ocular gene transfer with compacted DNA nanoparticles. PLoS One. 2006;1:e38. doi: 10.1371/journal.pone.0000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Boussif O, Lezoualc'h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Kodama Y, Nakamura T, Kurosaki T, Egashira K, Mine T, Nakagawa H, Muro T, Kitahara T, Higuchi N, Sasaki H. Biodegradable nanoparticles composed of dendrigraft poly-L-lysine for gene delivery. Eur J Pharm Biopharm. 2014;87:472–479. doi: 10.1016/j.ejpb.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 255.Bernstein MH. The interphotoreceptor matrix and the interphotoreceptor space of the vertebrate retina. Scan Electron Microsc. 1985:859–868. [PubMed] [Google Scholar]
- 256.Jeong YI, Kim ST, Jin SG, Ryu HH, Jin YH, Jung TY, Kim IY, Jung S. Cisplatin-incorporated hyaluronic acid nanoparticles based on ion-complex formation. J Pharm Sci. 2008;97:1268–1276. doi: 10.1002/jps.21103. [DOI] [PubMed] [Google Scholar]
- 257.de la Fuente M, Seijo B, Alonso MJ. Novel hyaluronic acid-chitosan nanoparticles for ocular gene therapy. Investigative Ophthalmology and Visual Science. 2008;49:2016–2024. doi: 10.1167/iovs.07-1077. [DOI] [PubMed] [Google Scholar]
- 258.de la Fuente M, Seijo B, Alonso MJ. Bioadhesive hyaluronan-chitosan nanoparticles can transport genes across the ocular mucosa and transfect ocular tissue. Gene Therapy. 2008;15:668–676. doi: 10.1038/gt.2008.16. [DOI] [PubMed] [Google Scholar]
- 259.Venkatesh S, Smith TJ. Chitosan-membrane interactions and their probable role in chitosan-mediated transfection. Biotechnol Appl Biochem. 1998;27(Pt 3):265–267. [PubMed] [Google Scholar]
- 260.Roy K, Mao HQ, Huang SK, Leong KW. Oral gene delivery with chitosan--DNA nanoparticles generates immunologic protection in a murine model of peanut allergy. Nat Med. 1999;5:387–391. doi: 10.1038/7385. [DOI] [PubMed] [Google Scholar]
- 261.Carrillo C, Sune JM, Perez-Lozano P, Garcia-Montoya E, Sarrate R, Fabregas A, Minarro M, Tico JR. Chitosan nanoparticles as non-viral gene delivery systems: determination of loading efficiency. Biomed Pharmacother. 2014;68:775–783. doi: 10.1016/j.biopha.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 262.Raemdonck K, Naeye B, Hogset A, Demeester J, De Smedt SC. Biodegradable dextran nanogels as functional carriers for the intracellular delivery of small interfering RNA. J Control Release. 2010;148:e95–96. doi: 10.1016/j.jconrel.2010.07.070. [DOI] [PubMed] [Google Scholar]
- 263.De Backer L, Braeckmans K, Demeester J, De Smedt SC, Raemdonck K. The influence of natural pulmonary surfactant on the efficacy of siRNA-loaded dextran nanogels. Nanomedicine (Lond) 2013;8:1625–1638. doi: 10.2217/nnm.12.203. [DOI] [PubMed] [Google Scholar]
- 264.Sun K, Wang J, Zhang J, Hua M, Liu C, Chen T. Dextran-g-PEI nanoparticles as a carrier for co-delivery of adriamycin and plasmid into osteosarcoma cells. Int J Biol Macromol. 2011;49:173–180. doi: 10.1016/j.ijbiomac.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 265.Abdallah B, Hassan A, Benoist C, Goula D, Behr JP, Demeneix BA. A powerful nonviral vector for in vivo gene transfer into the adult mammalian brain: polyethylenimine. Hum Gene Ther. 1996;7:1947–1954. doi: 10.1089/hum.1996.7.16-1947. [DOI] [PubMed] [Google Scholar]
- 266.Diebold SS, Kursa M, Wagner E, Cotten M, Zenke M. Mannose polyethylenimine conjugates for targeted DNA delivery into dendritic cells. J Biol Chem. 1999;274:19087–19094. doi: 10.1074/jbc.274.27.19087. [DOI] [PubMed] [Google Scholar]
- 267.Florea BI, Meaney C, Junginger HE, Borchard G. Transfection efficiency and toxicity of polyethylenimine in differentiated Calu-3 and nondifferentiated COS-1 cell cultures. AAPS PharmSci. 2002;4:E12. doi: 10.1208/ps040312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Liang B, He ML, Xiao ZP, Li Y, Chan CY, Kung HF, Shuai XT, Peng Y. Synthesis and characterization of folate-PEG-grafted-hyperbranched-PEI for tumor-targeted gene delivery. Biochem Biophys Res Commun. 2008;367:874–880. doi: 10.1016/j.bbrc.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 269.Tang MX, Szoka FC. The influence of polymer structure on the interactions of cationic polymers with DNA and morphology of the resulting complexes. Gene Ther. 1997;4:823–832. doi: 10.1038/sj.gt.3300454. [DOI] [PubMed] [Google Scholar]
- 270.Navarro G, Maiwald G, Haase R, Rogach AL, Wagner E, de Ilarduya CT, Ogris M. Low generation PAMAM dendrimer and CpG free plasmids allow targeted and extended transgene expression in tumors after systemic delivery. J Control Release. 2010;146:99–105. doi: 10.1016/j.jconrel.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 271.Qin L, Pahud DR, Ding Y, Bielinska AU, Kukowska-Latallo JF, Baker JR, Jr., Bromberg JS. Efficient transfer of genes into murine cardiac grafts by Starburst polyamidoamine dendrimers. Hum Gene Ther. 1998;9:553–560. doi: 10.1089/hum.1998.9.4-553. [DOI] [PubMed] [Google Scholar]
- 272.Roberts JC, Bhalgat MK, Zera RT. Preliminary biological evaluation of polyamidoamine (PAMAM) Starburst dendrimers. J Biomed Mater Res. 1996;30:53–65. doi: 10.1002/(SICI)1097-4636(199601)30:1<53::AID-JBM8>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 273.Sun Y, Jiao Y, Wang Y, Lu D, Yang W. The strategy to improve gene transfection efficiency and biocompatibility of hyperbranched PAMAM with the cooperation of PEGylated hyperbranched PAMAM. Int J Pharm. 2014;465:112–119. doi: 10.1016/j.ijpharm.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 274.Pack DW, Putnam D, Langer R. Design of imidazole-containing endosomolytic biopolymers for gene delivery. Biotechnol Bioeng. 2000;67:217–223. [PubMed] [Google Scholar]
- 275.Perez C, Sanchez A, Putnam D, Ting D, Langer R, Alonso MJ. Poly(lactic acid)-poly(ethylene glycol) nanoparticles as new carriers for the delivery of plasmid DNA. J Control Release. 2001;75:211–224. doi: 10.1016/s0168-3659(01)00397-2. [DOI] [PubMed] [Google Scholar]
- 276.Liu C, Chen Z, Yu W, Zhang N. Novel cationic 6-lauroxyhexyl lysinate modified poly(lactic acid)-poly(ethylene glycol) nanoparticles enhance gene transfection. J Colloid Interface Sci. 2011;354:528–535. doi: 10.1016/j.jcis.2010.10.062. [DOI] [PubMed] [Google Scholar]
- 277.Zou W, Liu C, Chen Z, Zhang N. Preparation and Characterization of Cationic PLA-PEG Nanoparticles for Delivery of Plasmid DNA. Nanoscale Res Lett. 2009;4:982–992. doi: 10.1007/s11671-009-9345-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Ravi Kumar MN, Bakowsky U, Lehr CM. Preparation and characterization of cationic PLGA nanospheres as DNA carriers. Biomaterials. 2004;25:1771–1777. doi: 10.1016/j.biomaterials.2003.08.069. [DOI] [PubMed] [Google Scholar]
- 279.Panyam J, Zhou WZ, Prabha S, Sahoo SK, Labhasetwar V. Rapid endo-lysosomal escape of poly(DL-lactide-co-glycolide) nanoparticles: implications for drug and gene delivery. FASEB Journal. 2002;16:1217–1226. doi: 10.1096/fj.02-0088com. [DOI] [PubMed] [Google Scholar]
- 280.Lim YB, Han SO, Kong HU, Lee Y, Park JS, Jeong B, Kim SW. Biodegradable polyester, poly[alpha-(4-aminobutyl)-L-glycolic acid], as a non-toxic gene carrier. Pharm Res. 2000;17:811–816. doi: 10.1023/a:1007552007765. [DOI] [PubMed] [Google Scholar]
- 281.Lim Y, Kim SM, Lee Y, Lee W, Yang T, Lee M, Suh H, Park J. Cationic hyperbranched poly(amino ester): a novel class of DNA condensing molecule with cationic surface, biodegradable three-dimensional structure, and tertiary amine groups in the interior. J Am Chem Soc. 2001;123:2460–2461. doi: 10.1021/ja005715g. [DOI] [PubMed] [Google Scholar]
- 282.Lynn DM, Anderson DG, Putnam D, Langer R. Accelerated discovery of synthetic transfection vectors: parallel synthesis and screening of a degradable polymer library. J Am Chem Soc. 2001;123:8155–8156. doi: 10.1021/ja016288p. [DOI] [PubMed] [Google Scholar]
- 283.Lunov O, Syrovets T, Loos C, Beil J, Delacher M, Tron K, Nienhaus GU, Musyanovych A, Mailander V, Landfester K, et al. Differential uptake of functionalized polystyrene nanoparticles by human macrophages and a monocytic cell line. ACS Nano. 2011;5:1657–1669. doi: 10.1021/nn2000756. [DOI] [PubMed] [Google Scholar]
- 284.Peracchia MT, Harnisch S, Pinto-Alphandary H, Gulik A, Dedieu JC, Desmaele D, d'Angelo J, Muller RH, Couvreur P. Visualization of in vitro protein-rejecting properties of PEGylated stealth polycyanoacrylate nanoparticles. Biomaterials. 1999;20:1269–1275. doi: 10.1016/s0142-9612(99)00021-6. [DOI] [PubMed] [Google Scholar]
- 285.Torchilin VP, Omelyanenko VG, Papisov MI, Bogdanov AA, Jr., Trubetskoy VS, Herron JN, Gentry CA. Poly(ethylene glycol) on the liposome surface: on the mechanism of polymer-coated liposome longevity. Biochim Biophys Acta. 1994;1195:11–20. doi: 10.1016/0005-2736(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 286.Zahr AS, Davis CA, Pishko MV. Macrophage uptake of core-shell nanoparticles surface modified with poly(ethylene glycol). Langmuir. 2006;22:8178–8185. doi: 10.1021/la060951b. [DOI] [PubMed] [Google Scholar]
- 287.Rimann M, Luhmann T, Textor M, Guerino B, Ogier J, Hall H. Characterization of PLL-g-PEG-DNA nanoparticles for the delivery of therapeutic DNA. Bioconjug Chem. 2008;19:548–557. doi: 10.1021/bc7003439. [DOI] [PubMed] [Google Scholar]
- 288.Li SD, Chono S, Huang L. Efficient gene silencing in metastatic tumor by siRNA formulated in surface-modified nanoparticles. J Control Release. 2008;126:77–84. doi: 10.1016/j.jconrel.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Kommareddy S, Amiji M. Antiangiogenic gene therapy with systemically administered sFlt-1 plasmid DNA in engineered gelatin-based nanovectors. Cancer Gene Ther. 2007;14:488–498. doi: 10.1038/sj.cgt.7701041. [DOI] [PubMed] [Google Scholar]
- 290.Parveen S, Sahoo SK. Long circulating chitosan/PEG blended PLGA nanoparticle for tumor drug delivery. Eur J Pharmacol. 2011;670:372–383. doi: 10.1016/j.ejphar.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 291.Tobio M, Sanchez A, Vila A, Soriano II, Evora C, Vila-Jato JL, Alonso MJ. The role of PEG on the stability in digestive fluids and in vivo fate of PEG-PLA nanoparticles following oral administration. Colloids Surf B Biointerfaces. 2000;18:315–323. doi: 10.1016/s0927-7765(99)00157-5. [DOI] [PubMed] [Google Scholar]
- 292.Kim TI, Seo HJ, Choi JS, Jang HS, Baek JU, Kim K, Park JS. PAMAM-PEG-PAMAM: novel triblock copolymer as a biocompatible and efficient gene delivery carrier. Biomacromolecules. 2004;5:2487–2492. doi: 10.1021/bm049563j. [DOI] [PubMed] [Google Scholar]
- 293.Naeye B, Raemdonck K, Remaut K, Sproat B, Demeester J, De Smedt SC. PEGylation of biodegradable dextran nanogels for siRNA delivery. Eur J Pharm Sci. 2010;40:342–351. doi: 10.1016/j.ejps.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 294.Mishra S, Webster P, Davis ME. PEGylation significantly affects cellular uptake and intracellular trafficking of non-viral gene delivery particles. Eur J Cell Biol. 2004;83:97–111. doi: 10.1078/0171-9335-00363. [DOI] [PubMed] [Google Scholar]
- 295.Remaut K, Lucas B, Braeckmans K, Demeester J, De Smedt SC. Pegylation of liposomes favours the endosomal degradation of the delivered phosphodiester oligonucleotides. J Control Release. 2007;117:256–266. doi: 10.1016/j.jconrel.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 296.Shi F, Wasungu L, Nomden A, Stuart MC, Polushkin E, Engberts JB, Hoekstra D. Interference of poly(ethylene glycol)-lipid analogues with cationic-lipid-mediated delivery of oligonucleotides; role of lipid exchangeability and non-lamellar transitions. Biochem J. 2002;366:333–341. doi: 10.1042/BJ20020590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Kale AA, Torchilin VP. Enhanced transfection of tumor cells in vivo using “Smart” pH-sensitive TAT-modified pegylated liposomes. J Drug Target. 2007;15:538–545. doi: 10.1080/10611860701498203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Hama S, Itakura S, Nakai M, Nakayama K, Morimoto S, Suzuki S, Kogure K. Overcoming the polyethylene glycol dilemma via pathological environment-sensitive change of the surface property of nanoparticles for cellular entry. J Control Release. 2015;206:67–74. doi: 10.1016/j.jconrel.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 299.Hatakeyama H, Akita H, Kogure K, Oishi M, Nagasaki Y, Kihira Y, Ueno M, Kobayashi H, Kikuchi H, Harashima H. Development of a novel systemic gene delivery system for cancer therapy with a tumor-specific cleavable PEG-lipid. Gene Ther. 2007;14:68–77. doi: 10.1038/sj.gt.3302843. [DOI] [PubMed] [Google Scholar]
- 300.Kim B, Abdel-Rahman MH, Wang T, Pouly S, Mahmoud AM, Cebulla CM. Retinal MMP-12, MMP-13, TIMP-1, and TIMP-2 expression in murine experimental retinal detachment. Invest Ophthalmol Vis Sci. 2014;55:2031–2040. doi: 10.1167/iovs.13-13374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Martens TF, Remaut K, Deschout H, Engbersen JF, Hennink WE, van Steenbergen MJ, Demeester J, De Smedt SC, Braeckmans K. Coating nanocarriers with hyaluronic acid facilitates intravitreal drug delivery for retinal gene therapy. J Control Release. 2015;202:83–92. doi: 10.1016/j.jconrel.2015.01.030. [DOI] [PubMed] [Google Scholar]