Abstract
OBJECTIVE
The purpose of this study is to perform and evaluate baseline abdominal ultrasound in infants with sickle cell anemia who participated in the BABY HUG multiinstitutional randomized placebo-controlled trial of hydroxyurea therapy and to examine the potential relationships among ultrasound results and clinical, nuclear medicine, and laboratory data.
SUBJECTS AND METHODS
After local institutional review board approval and with informed guardian consent, 116 girls and 87 boys (age range, 7.5–18 months) with sickle cell anemia underwent standardized abdominal sonography at 14 institutions. Imaging was centrally reviewed by one radiologist who assessed and measured the spleen, kidneys, gallbladder, and common bile duct. Baseline physical assessment of spleen size, serum alanine aminotransferase and bilirubin levels, 99mTc sulfur colloid liver-spleen scans, and 99mTc diethylenetriaminepentaacetic acid clearance glomerular filtration rates (GFRs) were obtained. Analysis of variance and the Student test were performed to compare sonographic findings to published results in healthy children and to clinical and laboratory findings.
RESULTS
The mean (± SD) spleen volume (108 ± 47 mL) was significantly greater than published normal control values (30 ± 14 mL; p < 0.0001). There was no correlation between spleen volume and function assessed by liver-spleen scan. The mean GFR (125 ± 34 mL/min/1.73 m2) was elevated compared with control GFRs (92 ± 18 mL/min/1.73 m2). Renal volumes (right kidney, 29 ± 8 mL; left kidney, 31 ± 9 mL) were significantly greater than control volumes (right kidney, 27 ± 3 mL; left kidney, 27 ± 3 mL; p < 0.0001) and were positively correlated with GFR (p = 0.0009). Five percent of patients had sonographic biliary abnormalities (sludge, n = 6; dilated common bile duct, n = 2; and cholelithiasis and thickened gallbladder wall, n = 1 each). There was no correlation between biliary sonographic findings and laboratory results.
CONCLUSION
In infants with sickle cell anemia, sonographic spleen volume does not reflect function, but increased renal volume correlates with GFR and is consistent with hyperfiltration. Sonographic biliary abnormalities can occur early in life, while remaining clinically silent.
Keywords: abdominal ultrasound, children, kidneys, sickle cell anemia, spleen
Individuals with sickle cell hemoglobinopathies experience clinical conditions related to low erythrocyte oxygen content, erythrocyte sickling, abnormal cellular adhesion, acute and chronic inflammation, and hemolysis [1–3]. Beginning in infancy, and coincident with the physiologic decline in fetal hemoglobin, ischemia and inflammation cause impairment of the spleen, kidneys, and biliary system [4–8]. The spleen initially becomes congested and enlarged then undergoes infarction. Renal glomerular enlargement and hyperfiltration occur early, ultimately leading to glomerulosclerosis and renal insufficiency [4, 9]. The chronic hemolysis associated with sickle cell anemia (SCA) contributes to the development of pigmented gallstones and biliary sludge, which can lead to biliary obstruction [10, 11].
Hydroxyurea (HU) therapy has shown efficacy in children and adults with SCA by stimulating fetal hemoglobin production and reducing the number of vasoocclusive episodes, transfusions, and hospitalizations [12, 13]. Although the efficacy of HU has been increasingly recognized for individuals with SCA, important questions regarding its effect on organ dysfunction and use in infants and young children remain. The Pediatric Hydroxyurea Phase 3 Clinical Trial, BABY HUG, is a multiinstitutional double-blind randomized placebo-controlled trial of infants (7.5–18 months old at screening) with SCA. Two hundred three infants underwent abdominal sonography during the pre-randomization screening to document spleen and renal size and parenchymal abnormalities and to determine the presence of biliary disease. The purpose of our study was to describe baseline ultrasound findings and to examine the potential relationships among these results and baseline clinical, nuclear medicine, and laboratory data.
Subjects and Methods
Patient Selection
After local institutional review board approval and with HIPAA compliance, informed consent for participation in BABY HUG was obtained from the legal guardians of infants with known SCA. Clinical severity was not a factor in determining eligibility [14].
Imaging Procedures, Physical Assessment, and Laboratory Examinations
Screening sonograms were performed at 14 participating institutions using a standard scanning procedure developed for BABY HUG. Patients were kept on nothing by mouth status (except clear liquids) for 6 hours before the ultrasound, to prevent gallbladder contraction. Imaging was submitted as hard copy film or in DICOM format on CD and was centrally reviewed by one pediatric radiologist, with 8 years of attending-level experience, who determined the adequacy of images, obtained organ measurements, and assessed the spleen and renal parenchyma and biliary system. Because imaging was reviewed after patients were randomized and began receiving study drug, examinations that were inadequate could not be repeated. Imaging included the maximum longitudinal, anteroposterior, and transverse measurements of the spleen (Fig. 1) and kidneys (Fig. 2). The spleen was imaged in the supine position only. The kidneys were imaged in both supine and prone positions; whichever image provided the best visualization of renal margins was used for measurement. Organs were remeasured as needed by the central reviewer using the methods shown in Figures 1 and 2. Spleen and renal volumes were calculated in milliliters, using the formula for an ellipsoid model (longitudinal × anteroposterior × transverse) × 0.523, similar to that used in previous reports [15, 16]. The spleen was assessed for parenchymal homogeneity and focal abnormalities. Kidneys were considered abnormally echogenic if the renal cortex was equal to or more echogenic than adjacent spleen or liver. Renal corticomedullary differentiation was considered abnormal when it was difficult to distinguish cortex from medulla visually [17]. Kidneys were also assessed for renal calculi, cysts, or other focal parenchymal abnormalities. The presence of gallstones, gallbladder sludge, gallbladder wall thickening (> 3 mm), and common bile duct dilatation (> 2 mm) was recorded [18, 19].
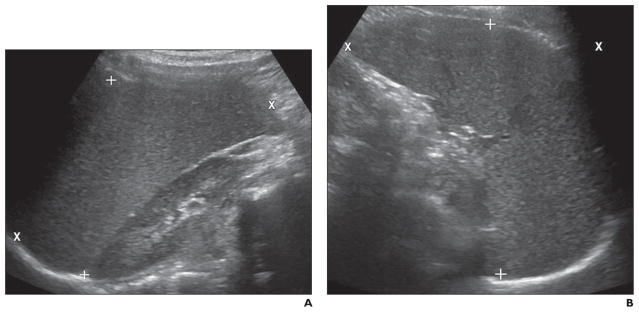
Fig. 1. Spleen measurement technique.
A, Image shows placement of cursors for longitudinal (X) and anteroposterior (+) diameter measurements.
B, Image shows placement of cursors for transverse (X) and anteroposterior (+) diameter measurements. Sonographic image plane that best depicted anteroposterior margins was used for anteroposterior measurement.
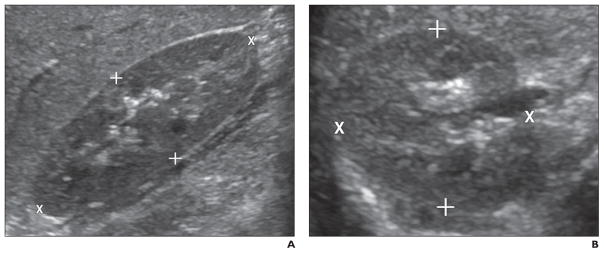
Fig. 2. Kidney measurement technique.
A, Image shows placement of cursors for length (X) and anteroposterior (+) diameter measurements.
B, Image shows placement of cursors for transverse (X) and anteroposterior (+) diameter measurements. Sonographic image plane that best depicted anteroposterior margins was used for anteroposterior measurement.
Splenic function was assessed by standardized 99mTc-labeled sulfur colloid liver-spleen scan, pitted erythrocyte count, and quantitation by flow cytometry of Howell-Jolly (HJ) bodies within 2 months of the ultrasound examination. Pitted erythrocyte count was considered normal if fewer than 3.5% of 500 RBCs had abnormal “pocks,” and a normal HJ body count was less than 300 HJ bodies/106 RBCs [20–22]. Liver-spleen scans were interpreted qualitatively by two central reviewers (three in discrepant cases) as follows: 1, spleen present with radiotracer uptake equal to liver; 2, spleen present but radiotracer uptake decreased relative to liver; or 3, splenic radiotracer uptake absent. Spleen size was estimated by palpation at the anterior axillary and midclavicular lines on physical examination.
Renal function was examined using 99mTc-labeled diethylenetriaminepentaacetic acid clearance to determine glomerular filtration rate (GFR). A GFR of 92 ± 18 mL/min/1.73 m2 (10–90% range of 60–120 mL/min/1.73 m2) has been reported in hematologically healthy age-matched children [23]. Liver and gallbladder function were assessed with serum alanine aminotransferase (ALT) and bilirubin levels that were obtained within 2 months of the ultrasound examination. An ALT level less than 41 U/L was considered normal [24]. Physical examination and laboratory tests were performed at well-child visits within 2 months of the ultrasound.
Statistical Analysis
Analysis of variance was undertaken to assess the association between spleen volume and spleen function by liver-spleen scan. The Student t test was performed to compare spleen volumes of groups with normal or abnormal HJ body or pitted erythrocyte counts and to perform comparisons between the study cohort and height-matched hematologically normal spleen volumes and weight-matched predicted normal kidney volumes [15, 16]. The Student t test was also used to detect potential differences in biliary ultrasound findings between subjects with and without abnormal ALT and serum bilirubin levels. All analyses were performed using SAS software (version 9.1, SAS Institute).
Results
Two hundred three infants (age range, 7.5–18 months; mean, 12.9 months) with SCA underwent screening sonography. Fifty-seven percent (116/203) were female, 97% (197/203) had the hemoglobin SS (HbSS) genotype, and 3% (6/203) had hemoglobin Sβ (HbSβ)–negative thalassemia. A small number of sonograms were inadequate for assessment of some organs, resulting in a variable number of measurements for different organs. Also, because not all sonographically screened patients were enrolled, some laboratory investigations and nuclear scans were not obtained.
Compared with published data for height-matched hematologically healthy children [15], the mean spleen volume in our cohort was increased (BABY HUG cohort, 108 ± 47 mL; healthy children, 30 ± 14 mL; p < 0.0001; Table 1). By assessment with nuclear liver-spleen scan (n = 199), 23 patients (12%) had normal splenic uptake, 148 (74%) had present but decreased uptake, and 28 (14%) had absent uptake. There was no correlation between spleen volume and liver-spleen scan findings whether patients were categorized in three groups (normal, decreased, and absent splenic uptake; p = 0.13; Fig. 3) or in two groups (normal and abnormal [decreased plus absent] uptake; p = 0.19). Of note, patients with decreased spleen uptake had slightly larger spleen volumes than those with normal or absent uptake. There was no correlation between pitted erythrocyte count (n = 197; p = 0.27) or HJ body count (n = 177; p = 0.71) and spleen volume. As expected, spleen volume was significantly associated with palpated spleen size at the anterior axillary line (n = 196; p = 0.0005) and the midclavicular line (n = 196; p < 0.0001). On average, spleen volume increased by 14.1 mL for every 1-cm increase at the anterior axillary line and 15.8 mL for every 1-cm increase at the midclavicular line. One patient had a focal peripheral hypoechoic area in the spleen, of unknown significance. There was no corresponding abnormality on this subject’s liver-spleen scan.
TABLE 1.
Spleen Volume Measurements of Children With Sickle Cell Anemia, 7.5–18 Months Old, Compared With Height-Matched Hematologically Healthy Children
| Group | No. of Children | Spleen Volume (mL)
|
|
|---|---|---|---|
| Mean (SD) | Range | ||
|
| |||
| BABY HUG full cohort | 199 | 105 (46) | 28–281 |
| BABY HUG subset (with body length 71–85 cm) | 180 | 108 (47) | 28–281 |
| Height-matched healthy children | 18 | 30 (14)a | Not applicableb |
p < 0.0001 for BABY HUG cohort and subset vs healthy children.
Range was not provided in reference article [25].
Fig. 3.
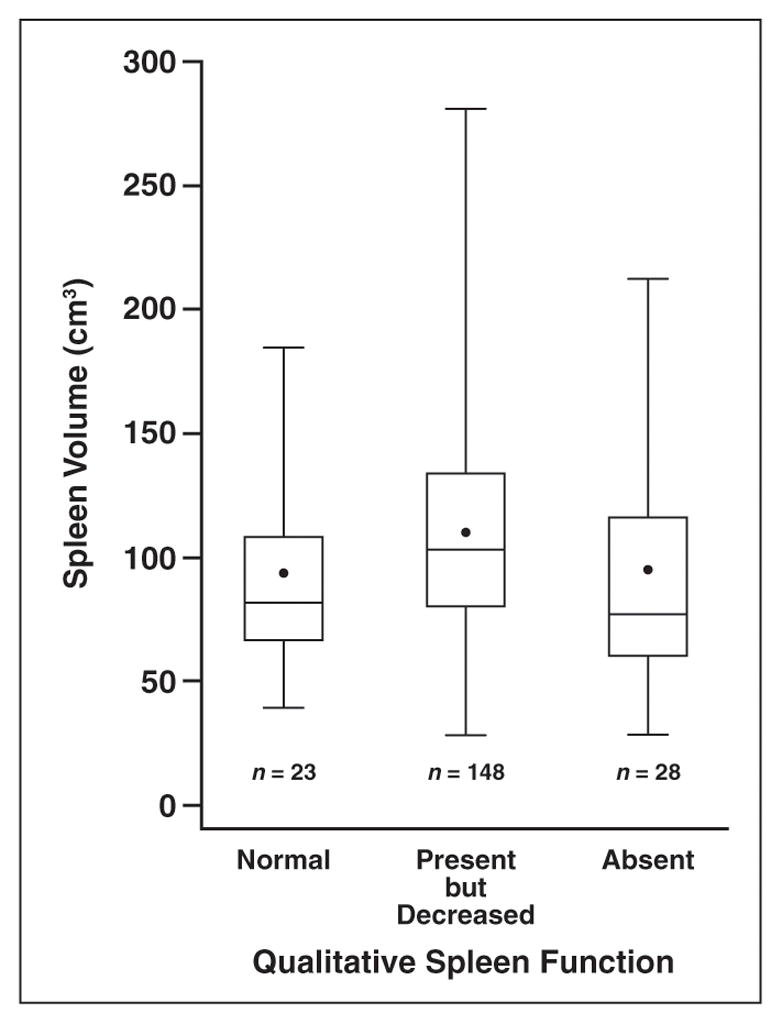
Correlation of spleen volume and 99mTc sulfur-colloid spleen uptake in 199 infants with sickle cell anemia, 7.5–18 months old. “Normal” refers to splenic uptake present and equal to that in liver. “Present but Decreased” refers to splenic uptake present but less than that in liver. “Absent” refers to no splenic uptake.
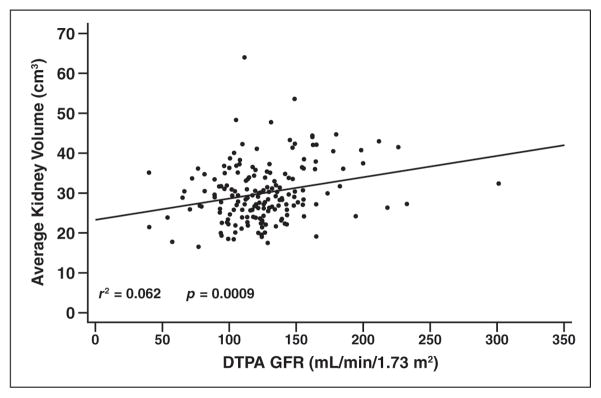
Of 201 patients with adequate renal imaging, eight (4%) had renal abnormalities: six had diffusely increased renal echogenicity or loss of corticomedullary differentiation bilaterally and two had increased medullary echogenicity (one bilaterally and one in the left kidney only). No patient had a renal calculus, cyst, or other abnormality. Right and left renal volumes were significantly larger in the study cohort (right kidney, 29 ± 8 mL; left kidney, 31 ± 9 mL) compared with predicted weight-matched volumes for hematologically healthy children (right kidney, 27 ± 3 mL; left kidney, 27 ± 3 mL; p < 0.0001 for both kidneys; Table 2) [16] and were positively associated with GFR (Fig. 4; p = 0.0009). The average GFR measurement was 125 ± 34 mL/min/1.73 m2 (range, 40–301 mL/min/1.73 m2) [4], which was elevated compared with the published normal value for this age group (10–90% normal range, 60–120 mL/min/1.73 m2).
TABLE 2.
Left and Right Kidney Volumes of Children With Sickle Cell Anemia, 7.5–18 Months Old, Compared With Predicted Volumes for Weight-Matched Hematologically Healthy Children
| Kidney | BABY HUG Cohort | Predicted for Weight-Matched Healthy Childrena | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| No. of Children | Kidney Volume (mL), Mean (SD) | Kidney Volume (mL), Range | No. of Childrenb | Kidney Volume (mL), Mean (SD)c | Kidney Volume (mL), Range | |
|
| ||||||
| Left | 197d | 31 (9) | 13–57 | 195 | 27 (3) | 21–35 |
| Right | 199 | 29 (8) | 16–72 | 197 | 27 (3) | 21–35 |
Note—p < 0.0001 for both kidneys.
Reference data are from Allon [26].
Weight was not obtained for two patients.
Normal left kidney = 4.214 × weight0.823; normal right kidney = 4.456 × weight0.795.
Imaging of the left kidney was inadequate for two patients.
Fig. 4.
Scatterplot shows correlation between average kidney volume and diethylene-triaminepentaacetic acid (DTPA) glomerular filtration rate (GFR) in 174 infants with sickle cell anemia, 7.5–18 months old.
Of 200 patients with adequate gallbladder and common bile duct imaging, one (0.5%) patient, who was 17.9 months old, had numerous small gallstones; six patients (3%), ranging in age from 9.4 to 18.0 months, had gallbladder sludge; one (0.5%) had a thick gallbladder wall; and two (1%) had a dilated common bile duct. Thus, at a mean age of 12.9 months, 5% (10/200) of infants had abnormal biliary sonograms. Among these patients, two had abnormal ALT values. One patient, with a dilated common bile duct, had an ALT level of 100 IU/L, and the other patient, who had gallbladder sludge, had an ALT level of 45 IU/L. Although there were no significant differences in ALT (p = 0.19) or bilirubin levels (total bilirubin, p = 0.38; direct bilirubin, p = 0.84) between patients with and without sonographic biliary abnormalities, the only infant with gallstones had a markedly elevated total bilirubin level of 5.6 mg/dL.
Discussion
In our study, most infants with SCA had a greater spleen volume than did height-matched hematologically healthy children. Sonographic spleen volume did not predict splenic function as assessed by liver-spleen scan. In fact, there was substantial overlap in spleen volumes among patients with normal, decreased, and absent spleen function. However, patients with decreased spleen uptake had slightly larger spleen volumes than did the other patients. This finding is consistent with the natural evolution of spleen changes in SCA because spleen size increases with increasing congestion (which may result in decreased function) until autoinfarction occurs, resulting in spleen atrophy and involution with absent spleen uptake. We also found no correlation between spleen volume and surrogate markers of spleen function (i.e., HJ body and pitted erythrocyte count) [25]. Furthermore, only one of our patients had abnormal splenic echotexture. These findings suggest that sonographic spleen volume and parenchymal findings, when compared with 99mTc liver-spleen scan and laboratory investigations, are not helpful in estimating the severity of splenic injury in infants with SCA.
In our cohort, renal volumes were significantly larger than those predicted for weight-matched hematologically healthy children and were significantly correlated with GFR. Children with SCA are known to develop glomerular hyperfiltration, as indicated by elevated GFR [4, 26–28]. Also, large renal size is known to develop in older children and adults with sickle cell disease, and, eventually, glomerular hyperfiltration may result in glomerulosclerosis and renal insufficiency [29, 30]. Our findings suggest that increased sonographic renal volume may indicate glomerular hyperfiltration and that this process begins in infancy.
Eight patients in our study had additional sonographic findings suggesting renal disease, including diffusely increased renal echogenicity or loss of corticomedullary differentiation and increased medullary echogenicity. Other researchers have shown that, among older children and young adults with severe (i.e., HbSS or HbSβ-negative thalassemia) and milder (i.e., hemoglobin SC or HbSβ positive) forms of sickle cell disease, diffuse loss of corticomedullary differentiation was most common in patients with the HbSS genotype (25/315 [8%]) [17]. Conversely, increased medullary echogenicity was more frequent in patients with milder hemoglobin SC disease and was thought to be due to medullary nephrocalcinosis. The authors [17] postulated that medullary scarring in severe sickle cell disease diminished blood flow to the renal pyramids and may have protected them from calcium deposition. The diffusely increased renal echogenicity in patients with HbSS disease, both in previous studies [30–32] and the present study, likely results from the generalized glomerular and interstitial changes that occur in these patients. Our findings suggest that such renal abnormalities may begin in infancy in patients with severe forms of sickle cell disease, before they are clinically evident.
There is limited literature, to our knowledge, regarding the prevalence of cholelithiasis and gallbladder sludge in infants with SCA. Sarnaik et al. [33] found a 12% (4/33) incidence of gallstones in 2–4-year-old children with SCA screened with ultrasound. The prevalence of gallstones in children with SCA increases with age and can be as high as 43% in adolescents [10, 11, 33, 34]. Other investigators have shown that gallbladder sludge in children with SCA progresses to the formation of gallstones [10, 11, 33]. We found that sonographic findings of biliary disease can occur at a very early age in children with SCA; 3% of our patients had gallbladder sludge, and one patient (0.5%) had numerous small gallstones. Interestingly, we found no correlation between serum chemistry levels and sonographic biliary abnormalities. Thus, sonography is the only useful method of detecting biliary tract abnormalities in infants with SCA.
A potential limitation of our study was that, although specific ultrasound scanning guidelines were provided to local institutions, the large number of participating sites resulted in variable image quality and differences in scanning techniques. To standardize measurements, the central reviewer remeasured organ dimensions as needed. Differences between organ measurements obtained for this study and those previously reported may be due, in part, to interobserver variability in the published literature and differences in methods.
This is the largest report, to our knowledge, of the sonographic appearance of the spleen, kidneys, and biliary system in infants with SCA. We have shown that the effects of SCA on these structures can be detected sonographically very early in life. In infants, ultrasound is useful in assessing renal and biliary disease but has limited value, if any, in predicting spleen function. Follow-up sonograms of these patients, after 2 years of HU therapy or placebo, will provide insight into the efficacy of HU in halting, reversing, or preventing the sonographically evident sequelae of SCA in these organs.
Acknowledgments
This work was funded by the National Heart, Lung, and Blood Institute, National Institutes of Health (contracts N01-HB-07150 to N01-HB-07160) with partial support of the Best Pharmaceuticals for Children Act, the National Institute of Child Health and Human Development, and the American, Lebanese, and Syrian Associated Charities. This work is registered under ClinicalTrials.gov number NCT00006400.
We acknowledge the efforts of the BABY HUG subjects and their families. We thank the following BABY HUG personnel at the participating Clinical Centers and the Medical Coordinating Center: Children’s National Medical Center (Catherine Driscoll, Caterina Minniti, Brenda Martin, Barbara Speller-Brown, and Romuladus Azuine), Duke University Medical Center (Courtney Thornburg, Sherri Zimmerman, and Tracy Kelly), Howard University College of Medicine (Caroline K. Reed, Erin Yeagley, Patricia Houston-Yu, and Connie Nguyen), Johns Hopkins University School of Medicine (James Casella, Phillip Seaman, Jeffrey Keefer, Sue Dixon, and Patrice Sharp), Medical University of South Carolina (Sherron Jackson, Betsy Rackoff, Lisa Kuisel, and Deborah Disco), St. Jude Children’s Research Hospital (William Schultz, Lane Faughnan, and Lynn Wynn), SUNY Downstate Medical Center/Kings County Hospital Center (Kathy Rey, Sreedhar P. Rao, and Sandra Graham), University of Miami (Stuart Toledano, Julie Barredo, Tally Hustace, Noeline Lewis, and Ofelia Alvarez), University of Mississippi Medical Center (Glenda Thomas), University of Texas Southwestern Medical Center at Dallas (Cindy Cochran, Nicole Corrigan, Jennifer Marshall, and Leah Adix), University of Alabama at Birmingham (Thomas Howard, Jennifer McDuffie, Kimberly Whelan, and Roy Mc-Donald), Drexel University (Carlton Dampier, Lori Luck, Mary Lou MacDermott, Maureen Meier, and Michele Cahill), Emory University School of Medicine (R. Clark Brown, Ifeyinwa Osunkwo, Ellen Debenham, Leann Hassen, and Terrell Faircloth), Children’s Hospital of Michigan (Sharada Sarniak, Wanda Whitten-Shurney, Mary Murphy, Madhvi Pajpurkar, and Kristin Storie-Kennedy), and Clinical Trials & Surveys (Zhibao Mi, Billie Fish, and Anne Martien). A full list of contributors to BABY HUG is available at http://www.c-tasc.com/sites/default/files/StudySites/babyhug_test.html.
References
- 1.Platt OS, Thorington BD, Brambilla DJ, et al. Pain in sickle cell disease: rates and risk factors. N Engl J Med. 1991;325:11–16. doi: 10.1056/NEJM199107043250103. [DOI] [PubMed] [Google Scholar]
- 2.Platt OS. Sickle cell anemia as an inflammatory disease. J Clin Invest. 2000;106:337–338. doi: 10.1172/JCI10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hebbel RP, Boogaerts MA, Eaton JW, Steinberg MH. Erythrocyte adherence to endothelium in sickle-cell anemia: a possible determinant of disease severity. N Engl J Med. 1980;302:992–995. doi: 10.1056/NEJM198005013021803. [DOI] [PubMed] [Google Scholar]
- 4.Ware RE, Rees RC, Sarnaik SA, et al. Renal function in infants with sickle cell anemia: baseline data from the BABY HUG trial. J Pediatr. 2010;156:66–70. doi: 10.1016/j.jpeds.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khatib R, Rabah R, Sarnaik SA. The spleen in the sickling disorders: an update. Pediatr Radiol. 2009;39:17–22. doi: 10.1007/s00247-008-1049-9. [DOI] [PubMed] [Google Scholar]
- 6.Powars DR. Natural history of sickle cell disease: the first ten years. Semin Hematol. 1975;12:267–285. [PubMed] [Google Scholar]
- 7.Mason KP, Grandison Y, Hayes RJ, et al. Postnatal decline of fetal haemoglobin in homozygous sickle cell disease: relationship to parenteral Hb F levels. Br J Haematol. 1982;52:455–463. doi: 10.1111/j.1365-2141.1982.tb03915.x. [DOI] [PubMed] [Google Scholar]
- 8.Stevens MC, Hayes RJ, Vaidya S, Serjeant GR. Fetal hemoglobin and clinical severity of homozygous sickle cell disease in early childhood. J Pediatr. 1981;98:37–41. doi: 10.1016/s0022-3476(81)80529-x. [DOI] [PubMed] [Google Scholar]
- 9.Pitcock JA, Muirhead EE, Hatch FE, Johnson JG, Kelly BJ. Early renal changes in sickle cell anemia. Arch Pathol. 1970;90:403–410. [PubMed] [Google Scholar]
- 10.Al-Salem AH, Qaisruddin S. The significance of biliary sludge in children with sickle cell disease. Pediatr Surg Int. 1998;13:14–16. doi: 10.1007/s003830050233. [DOI] [PubMed] [Google Scholar]
- 11.Winter SS, Kinney TR, Ware RE. Gallbladder sludge in children with sickle cell disease. J Pediatr. 1994;125:747–749. doi: 10.1016/s0022-3476(94)70069-9. [DOI] [PubMed] [Google Scholar]
- 12.Charache S, Terrin ML, Moore RD, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multi-center Study of Hydroxyurea in Sickle Cell Anemia. N Engl J Med. 1995;332:1317–1322. doi: 10.1056/NEJM199505183322001. [DOI] [PubMed] [Google Scholar]
- 13.Hoppe C, Vichinsky E, Quirolo K, van Warmerdam J, Allen K, Styles L. Use of hydroxyurea in children ages 2 to 5 years with sickle cell disease. J Pediatr Hematol Oncol. 2000;22:330–334. doi: 10.1097/00043426-200007000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Thompson BW, Miller ST, Rogers ZR, et al. The pediatric hydroxyurea phase III clinical trial (BABY HUG): challenges of study design. Pediatr Blood Cancer. 2010;54:250–255. doi: 10.1002/pbc.22269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dittrich M, Milde S, Dinkel E, Baumann W, Weitzel D. Sonographic biometry of liver and spleen size in childhood. Pediatr Radiol. 1983;13:206–211. doi: 10.1007/BF00973157. [DOI] [PubMed] [Google Scholar]
- 16.Dinkel E, Ertel M, Dittrich M, Peters H, Berres M, Schulte-Wissermann H. Kidney size in childhood: sonographical growth charts for kidney length and volume. Pediatr Radiol. 1985;15:38–43. doi: 10.1007/BF02387851. [DOI] [PubMed] [Google Scholar]
- 17.Walker TM, Serjeant GR. Increased renal reflectivity in sickle cell disease: prevalence and characteristics. Clin Radiol. 1995;50:566–569. doi: 10.1016/s0009-9260(05)83194-0. [DOI] [PubMed] [Google Scholar]
- 18.Carroll BA, Oppenheimer DA, Muller HH. High-frequency real-time ultrasound of the neonatal biliary system. Radiology. 1982;145:437–440. doi: 10.1148/radiology.145.2.7134449. [DOI] [PubMed] [Google Scholar]
- 19.Matos C, Avni EF, Van GD, Pardou A, Struyven J. Total parenteral nutrition (TPN) and gallbladder diseases in neonates: sonographic assessment. J Ultrasound Med. 1987;6:243–248. doi: 10.7863/jum.1987.6.5.243. [DOI] [PubMed] [Google Scholar]
- 20.Rogers Z, Wang WC, Luo Z, et al. Biomarkers of splenic function in infants with sickle cell anemia: baseline data from the BABY HUG trial. Blood. doi: 10.1182/blood-2010-04-278747. Epub 2011 Jan 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pearson HA, Gallagher D, Chilcote R, et al. Developmental pattern of splenic dysfunction in sickle cell disorders. Pediatrics. 1985;76:392–397. [PubMed] [Google Scholar]
- 22.Ishiguro A, Nakahata T, Matsubara K, et al. Age-related changes in thrombopoietin in children: reference interval for serum thrombopoietin levels. Br J Haematol. 1999;106:884–888. doi: 10.1046/j.1365-2141.1999.01641.x. [DOI] [PubMed] [Google Scholar]
- 23.Piepsz A, Tondeur M, Ham H. Revisiting normal (51)Cr-ethylenediaminetetraacetic acid clearance values in children. Eur J Nucl Med Mol Imaging. 2006;33:1477–1482. doi: 10.1007/s00259-006-0179-2. [DOI] [PubMed] [Google Scholar]
- 24.Lockitch G, Halstead AC, Albersheim S, Mac-Callum C, Quigley G. Age- and sex-specific pediatric reference intervals for biochemistry analytes as measured with the Ektachem-700 analyzer. Clin Chem. 1988;34:1622–1625. [PubMed] [Google Scholar]
- 25.William BM, Corazza GR. Hyposplenism: a comprehensive review. Part I. Basic concepts and causes. Hematology. 2007;12:1–13. doi: 10.1080/10245330600938422. [DOI] [PubMed] [Google Scholar]
- 26.Allon M. Renal abnormalities in sickle cell disease. Arch Intern Med. 1990;150:501–504. [PubMed] [Google Scholar]
- 27.Allon M, Lawson L, Eckman JR, Delaney V, Bourke E. Effects of nonsteroidal antiinflammatory drugs on renal function in sickle cell anemia. Kidney Int. 1988;34:500–506. doi: 10.1038/ki.1988.209. [DOI] [PubMed] [Google Scholar]
- 28.Ataga KI, Orringer EP. Renal abnormalities in sickle cell disease. Am J Hematol. 2000;63:205–211. doi: 10.1002/(sici)1096-8652(200004)63:4<205::aid-ajh8>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 29.Walker TM, Beardsall K, Thomas PW, Serjeant GR. Renal length in sickle cell disease: observations from a cohort study. Clin Nephrol. 1996;46:384–388. [PubMed] [Google Scholar]
- 30.Tejani A, Phadke K, Adamson O, Nicastri A, Chen CK, Sen D. Renal lesions in sickle cell nephropathy in children. Nephron. 1985;39:352–355. doi: 10.1159/000183404. [DOI] [PubMed] [Google Scholar]
- 31.Alleyne GA. The kidney in sickle cell anemia. Kidney Int. 1975;7:371–379. doi: 10.1038/ki.1975.54. [DOI] [PubMed] [Google Scholar]
- 32.Bernstein J, Whitten CF. A histologic appraisal of the kidney in sickle cell anemia. Arch Pathol. 1960;70:407–418. [PubMed] [Google Scholar]
- 33.Sarnaik S, Slovis TL, Corbett DP, Emami A, Whitten CF. Incidence of cholelithiasis in sickle cell anemia using the ultrasonic gray-scale technique. J Pediatr. 1980;96:1005–1008. doi: 10.1016/s0022-3476(80)80626-3. [DOI] [PubMed] [Google Scholar]
- 34.Rennels MB, Dunne MG, Grossman NJ, Schwartz AD. Cholelithiasis in patients with major sickle hemoglobinopathies. Am J Dis Child. 1984;138:66–67. doi: 10.1001/archpedi.1984.02140390054016. [DOI] [PubMed] [Google Scholar]