Abstract
Hepatocellular carcinoma (HCC) occurs most commonly secondary to cirrhosis due to chronic hepatitis C or B virus (HCV/HBV) infections. Type I interferon (IFN-α) treatment of chronic HCV/HBV infections reduces the incidence of HCC in cirrhotic patients. However, IFN-α toxicity limits its tolerability and efficacy highlighting a need for better therapeutic treatments. A recently discovered type III IFN (IFN-λ) has been shown to possess antiviral properties against HCV and HBV in vitro. In phase I clinical trials, IFN-λ treatment did not cause significant adverse reactions. Using a gene therapy approach, we compared the antitumor properties of IFN-α and IFN-λ in a transplantable hepatoma model of HCC. BALB/c mice were inoculated with syngeneic BNL hepatoma cells, or BNL cells expressing IFN-λ (BNL.IFN-λ cells) or IFN-α (BNL.IFN-α cells). Despite the lack of antiproliferative activity of IFNs on BNL cells, both BNL.IFN-λ and BNL.IFN-α cells displayed retarded growth kinetics in vivo. Depletion of NK cells from splenocytes inhibited splenocyte-mediated cytotoxicity, demonstrating that NK cells play a role in IFN-induced antitumor responses. However, isolated NK cells did not respond directly to IFN-λ. There was also a marked NK cell infiltration in IFN-λ producing tumors. In addition, IFN-λ and, to a lesser extent, IFN-α enhanced immunocytotoxicity of splenocytes primed with irradiated BNL cells. Splenocyte cytotoxicity against BNL cells was dependent on IL-12 and IFN-γ, and mediated by dendritic cells. In contrast to NK cells, isolated from spleen CD11c+ and mPDCA+ dendritic cells responded directly to IFN-λ. The antitumor activities of IFN-λ against hepatoma, in combination with HCV and HBV antiviral activities warrant further investigation into the clinical use of IFN-λ to prevent HCC in HCV/HBV-infected cirrhotic patients, as well as to treat liver cancer.
Keywords: Interferons, Hepatocellular carcinoma, Hepatitis C and B virus, Natural killer cells, Dendritic cells, Interleukin-12
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common solid tumor, the third leading cause of cancer related death worldwide, and the second most lethal cancer with the 5-year survival rate below 9% (reviewed in [1–3]). Treatment options for HCC are limited due to the low efficiency of existing anticancer drugs against HCC. Due to a lack of biomarkers and screening for HCC, most patients are diagnosed at advanced stages of the disease and are not eligible for surgical tumor resection or orthotopic liver transplantation (OLT) [4–6]. However, even after resection, the tumor recurrence is about 70% [7]. In patients with unresectable HCC and preserved liver function, transarterial chemoembolization (TACE) prolongs survival. However, TACE is rarely curative and progression-free survival beyond 24 months is infrequent [3, 8]. For patients with advanced disease, systemic chemotherapy is of limited benefit because of the low responsiveness of HCC to existing anticancer drugs and the fact that about 50% of patients with HCC die secondary to liver failure from cirrhosis [9, 10].
HCC occurs most frequently in patients with cirrhosis because of chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infections, and alcohol abuse [1, 11]. Because IFN-α is used clinically to treat chronic HCV and HBV infection, several studies evaluated the effect of IFN treatment on the incidence of HCC (reviewed in [1]). The majority of studies concluded that IFN therapy alone or in combination with ribavirin decreased the incidence of HCC, particularly in patients with sustained virological response [12–15]. Therefore, IFN alone or in combination with other drugs, can be used as a preventive therapy against the development of HCC in HCV and HBV positive patients. However, numerous adverse effects limit the overall tolerability of IFN-α, particularly in patients with cirrhosis [16, 17]. Because IFN-α (or type I IFN) is a pleiotropic cytokine with widespread effects on nearly all types of cells due to the ubiquitous expression of the IFN-α receptor [18], it is not surprising that most patients develop significant side effects. Recently, a new type of IFN was discovered and designated IFN-λ or type III IFN [19, 20]. In addition to its antiviral properties, others and we have demonstrated that IFN-λs display potent antitumor activity in murine models of cancer [21–23]. However, in contrast to IFN-α, the activity of IFN-λ appears to be tissue specific [21, 24, 25], underlining the possibility that IFN-λ treatment may cause fewer side effects.
Tissue specificity of the IFN-λ response is determined by the restricted expression of IFN-λR1, the unique chain of the IFN-λ receptor complex that also contains the IL-10R2 chain, which is shared with the IL-10, IL-22, and IL-26 receptor complexes [9, 26]. In contrast, IFN-α exerts its biological activities through the heterodimeric type I IFN receptor complex composed of IFN-αR1 and IFN-αR2 chains [18, 27]. Although IFN-α and IFN-λ engage distinct receptor complexes, they activate similar signaling pathways and induce similar set of genes and, subsequently, biological activities such as antiviral activity and upregulation of MHC class I antigen expression in cells sensitive to both types of IFN (reviewed in [26, 28]). Within the Jak-STAT (Janus kinases-signal transducers and activators of transcription) signal transduction pathway, both type I and type III IFNs stimulate activation of Jak1 and Tyk2 kinases and several STAT proteins, primarily STAT1 and STAT2 that together with IFN regulatory factor (IRF) 9 form IFN-stimulated regulatory factor 3 (ISGF3) transcription complex [19, 29]. ISGF3 regulates gene transcription by binding to an interferon-stimulated response element (ISRE), whereas activated STAT1 also forms homodimers that bind to an IFN-γ activation site (GAS) within the promoters of IFN-stimulated genes [30].
Antiviral studies performed in vitro and in vivo have shown that both IFN-α and IFN-λ contribute to the overall host antiviral defense system [19, 20, 31–34]. Several studies demonstrated that type III IFNs could inhibit replication of HCV and HBV in vitro [35–38]. However, in most cases, antiviral potency of IFN-λ against several viruses, as well as antiproliferative activity, seems to be lower than those of IFN-α [19, 20, 31, 36, 39]. In addition, although IFN-λ and IFN-α stimulate similar sets of antiviral genes, the kinetics of IFN-λ-triggered signaling events and induction of target genes were distinct from those triggered by IFN-α [36, 40]. Therefore, although signaling and activities induced by IFN-λ and IFN-α seem to be similar, different kinetics, biological potency, and particularly distinct sets of target cells sensitive to IFN-λ and IFN-α suggest that these IFNs have distinct physiological functions.
In the present study, we investigated the role of IFN-λ in a murine model of hepatoma growth to assess the potential antitumor activity of IFN-λ and compare it to IFN-α. Our results demonstrate that both IFN-λ and IFN-α were potent inducers of innate antitumor responses and displayed comparable antitumor activities in this cancer model.
Materials and methods
Expression plasmids
Expression plasmids pEF-mIFN-λ2 and pEF-mIFN-λR1 were described previously [21]. The intronless mIFN-α7 gene was amplified by PCR with primers 5′-GCGGTACCATGGCTAGGCTCTGTGCTTTCCTG-3′ and 5′-TCAGGAGAATTCTCTCTACTTTGTC-3′ and murine genomic C57BL/6 DNA. The resulting PCR product was cloned into a pcDEF3 (pEF) vector [41] with the use of KpnI and EcoRI restriction endonucleases, generating plasmid pEF-mIFN-α7. The nucleotide sequence of the mIFN-α7 gene was determined and found to be identical to GenBank accession number M13710.
Cells and transfection
BALB/c-derived murine hepatocellular carcinoma cell line BNL 1ME A.7R.1 (BNL; ATCC, Manassas, VA, USA), 4T1 mammary gland carcinoma cell line (ATCC), and C57BL6-derived murine melanoma B16 cell line were grown in DMEM supplemented with 10% heat inactivated fetal bovine serum (FBS).
pEF-mIFN-λ2 and pEF-mIFN-α7 plasmids were stably transfected into 106 BNL cells with the use of transit-LT1 reagent (Mirus, Madison, WI, USA) and G418-resistant cells were selected (500 μg/ml of Geneticin; Invitrogen, Carlsbad, CA, USA). BNL cells were transiently transfected with expression vectors pEF-mIFN-λR1 along with coexpression of EGFP using the jetPEITM reagent (Polyplus-transfection Inc., New York, NY, USA).
To assess the proliferation rate, cells were seeded at 0.5 × 105 per well in six-well plates in triplicates, grown at 37°C, and counted at days 1, 2, 3, 4, and 5.
To isolate murine splenocytes, spleen was extracted, placed into RPMI medium with 5% FBS, and minced; large chunks of tissue were removed after gravitational sedimentation, and remaining in suspension splenocytes were pelleted by centrifugation. To isolate murine peripheral blood mononuclear cells (PBMCs), blood, drawn from anesthetized mice by cardiac puncture, underwent red blood cell (RBC) lysis by 10 min incubation with RBC lysis solution (Gentra Systems, Minneapolis, MN, USA); and remaining PBMCs were pelleted.
Flow cytometry
To detect MHC class I expression, cells were treated for 72 h with IFNs or cell-conditioned medium, harvested and incubated with mouse antibody (Ab) against H-2 kb (eBioscience, San Diego, CA, USA), followed by incubation with FITC-goat anti-mouse IgG (Sigma, St. Louis, MO, USA). To identify circulating T regulatory cells, PBMCs were stained first with CD4 APC and CD25 PE (CALTAG Laboratories, Burlingame, CA, USA), fixed with 2% paraformaldehyde, permeablized with 0.2% Triton-X, and subsequently stained with Foxp3 PE-Cy5 Ab (eBioscience). The isotype-matched rat IgG was used in a control staining. Stained cells were analyzed by flow cytometry.
Electrophoretic mobility shift assay
To evaluate IFN-induced STAT1 activation, cells were harvested and treated for 15 min with 10 ng/ml of murine IFN-γ (PeproTech, Rocky Hill, NJ, USA), IFN-λ (PeproTech) or IFN-α (R&D Systems, Minneapolis, MN, USA). Cells were lysed and STAT activation was detected in the cellular extracts by using a P32-radiolabled GAS probe in an electrophoretic mobility shift assay (EMSA) as described [21].
Mice, tumor transplantation, and histological and immunohistochemical analysis of tumor tissue
Immunocompetent female BALB/c mice, 6–8 weeks old, were obtained from Jackson Laboratory (Bar Harbor, ME, USA) and maintained in a pathogen-free barrier facility. Mice were injected subcutaneously (s.c.) on the flank with 106 cells in 0.1 ml of PBS. Tumor growth was evaluated by palpation of the injection site every 2 days. Animals with excessive tumor burden (≥1 cm3) were euthanized.
For histological evaluation, tumor tissues were extracted from mice with similar tumor size and immediately fixed in 10% phosphate-buffered formalin. Paraffin-embedded sections were stained with hematoxylin and eosin (H&E) and analyzed by an expert pathologist blinded to treatment group. The mitotic index and lymphocyte infiltration were determined as follows: 15 randomly selected fields of each slide were examined at high magnification [400× high power field (hpf)] and mitotic figures (mitoses/hpf) and the rate of lymphocyte infiltration (cells/hpf) were counted.
For immunohistochemical staining of tumors for NK cell infiltration, paraffin-embedded tumor sections were deparaffinized. Samples were subjected to 2100 Retriever (Pickcell Laboratories, Netherlands) in sodium citrate buffer 10 mM pH 6.0, followed by overnight incubation with purified anti-mouse CD49b pan-NK cell Ab (1:50; Biolegend, San Diego, CA, USA) at room temperature in a moist chamber and subsequent staining for 2 h at room temperature in a moist chamber with Alexa Fluor 488 goat anti-rat IgM (1:100; Invitrogen). The sections were also stained for nuclei with 40 μg/mL 7-aminoactinomycin D (Invitrogen), mounted in Vectashield (Vector Laboratories, Burlingame, CA, USA) and examined in a Zeiss LCM 510 confocal fluorescent microscope at 250× magnification.
Immunocytotoxicity assays, IL-12, and IFN-γ neutralization, and in vitro NK cell and DC depletion
For immunocytotoxicity assays, freshly harvested splenocytes (106 cells) were treated in culture with IFN-λ or IFN-α in the presence or absence of 105 irradiated (7500 Rad) BNL cells for 4 days at 37°C. At day 4, the stimulated splenocytes were isolated and co-cultured with alive BNL or 4T1 target cells (105 cells) for 24 h. The number of dead target cells (%) was assessed by FACS analyses of the propidium iodide stained cells in the forward and side scatter-gated tumor cell population.
In vitro depletion of NK cells was performed by incubating splenocytes for 60 min on ice with 0.25 μg of biotinylated monoclonal rat anti-mouse NK antibody (clone DX5; CALTAG Laboratories) or 0.25 μg isotype-matched rat IgM. To remove unbound antibody, cells were washed twice with PBS. Pelleted cells were then incubated on ice for 30 min with 30 μg of prewashed magnetic Dynabeads M-280 Streptavidin (Dynal Biotech ASA, Oslo, Norway) followed by magnetic pelletation of the cells. Non-pelleted cells were cultured and used in immunocytotoxicity assays.
In vitro depletion of CD11c+ dendritic cells (DCs), mPDCA+ (plasmacytoid DCs), or both populations, were performed on freshly isolated splenocytes. DCs were positively selected by two successive magnetic cell separations (MACS) using CD11c, anti-mPDCA, and Pan DC magnetic MACS microbeads (Miltenyi Biotec, Auburn, CA, USA) followed by magnetic separation with MACS columns and MACS separators according to the manufacturer’s protocol.
For IL-12 and IFN-γ in vitro neutralization, purified anti-IL-12p40 antibody (clone C17.8; BioXCell, West Lebanon, NH, USA), purified anti-mouse IFN-γ antibody (clone XMG1.2; eBioscience) or isotype-matched rat IgG was added at a final concentration of 20 μg/ml.
IL-12 and IFN-γ measurement
IL-12 and IFN-γ levels in cultured splenocyte supernatants were measured using MesoScale Discovery (MSD) multiplex kit for mouse pro-inflammatory cytokines (Mesoscale Discovery, Maryland, USA) or mouse IFN-γ ELISA kit (eBioscience), according to manufacturer’s instructions. Sensitivity of MSD for IL-12 and IFN-γ was 1.2 and 0.3 pg/ml, respectively, and for mouse IFN-γ ELISA kit, 0.7 pg/ml.
Statistics
The Kaplan–Meier estimator was used to calculate the median survival (Tumor appearance) time and to derive tumor appearance (survival) curves. Statistical analysis was performed using a one-way ANOVA or Mann–Whitney U test. Differences were considered to be statistically significant when P value was <0.05.
Results
IFN response in BNL hepatoma cells
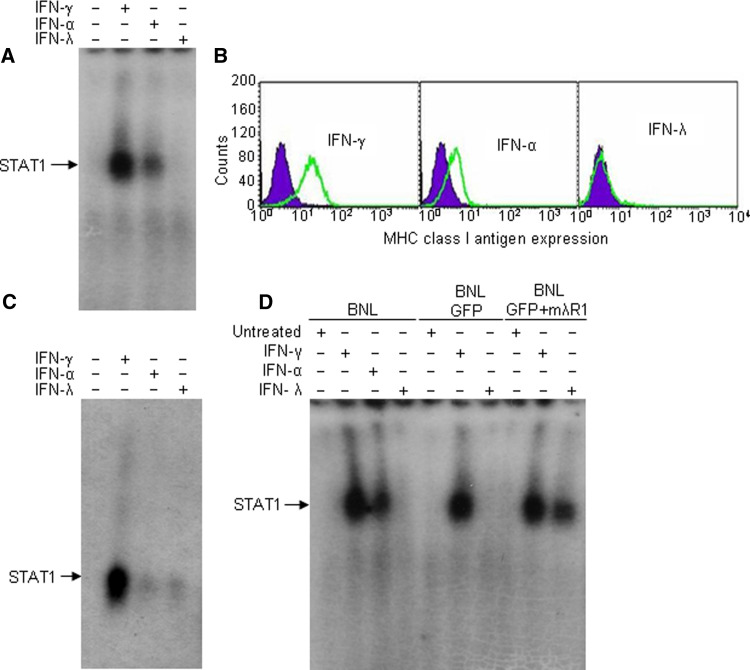
The responsiveness of mouse hepatoma BNL cells to three types of IFNs (γ, α, and λ) was determined by measuring STAT1 activation and the ability of IFNs to upregulate the level of MHC class I antigen expression. The formation of STAT1 DNA-binding complexes was strongly induced in response to mIFN-γ and to a lesser extent in response to mIFN-α (Fig. 1a). Similarly, strong upregulation of MHC class I antigen expression was detected on BNL cells in response to mIFN-γ and to a lower extent in response to mIFN-α (Fig. 1b). In contrast, treatment of BNL cells with mIFN-λ failed to induce STAT1 activation or upregulation of MHC class I antigen expression (Fig. 1a, b). Therefore, in contrast to IFN-α and IFN-γ, IFN-λ failed to induce a response in BNL cells. However, primary murine hepatocytes not only readily responded to IFN-γ, but also weakly responded to both IFN-λ and IFN-α as measured by IFN-induced activation of STAT1 DNA-binding complexes (Fig. 1c), suggesting that hepatocyte-derived BNL cells lost their responsiveness to IFN-λ. Because the IFN-λ and IFN-α receptors engage similar downstream signal transduction pathways and IFN-α-induced signaling was intact in BNL cells, resistance to IFN-λ response could be attributed to the lack of IFN-λR1 receptor expression in BNL cells. Indeed, transient expression of intact murine IFN-λR1 chain in BNL cells restored STAT1 activation upon treatment with IFN-λ (Fig. 1d), indicating that unresponsiveness of BNL cells was due to a deficiency in the IFN-λR1 expression.
Fig. 1.
IFN response in BNL cells. STAT activation in murine hepatoma BNL cells (a) and in primary mouse hepatocytes (c) in response to mouse IFN-γ, IFN-α or IFN-λ was evaluated by EMSA with a GAS probe. Cells were treated for 15 min with IFNs (10 ng/ml), lysed and STAT activation was detected by EMSA. Position of STAT DNA-binding-complexes in EMSA is indicated by the arrow. The results are representative of three independent experiments. b The level of MHC class I antigen expression was determined by flow cytometry in BNL cells untreated (black filled histograms) or treated for 72 h with recombinant mIFN-α, mIFN-λ2 or mIFN-γ (10 ng/ml, gray open histograms). The data shown are representative of three independent experiments. d BNL cells were transiently transfected with mouse IFN-λR1, treated with IFNs and STAT1 activation was determined by EMSA
Constitutive expression of IFN-λ and IFN-α and effects on tumor growth
In the murine B16 melanoma model, IFN-λ demonstrated a marked antitumor activity independent of whether B16 cells were sensitive or resistant to IFN-λ response [21], implying that IFN-λ antitumor effects are mediated by host mechanisms. As previously described [21], a gene therapy approach was utilized to study IFN-λ antitumor potential and compare it to that of IFN-α in the murine BNL hepatoma model. BNL cells were stably transfected with either pEF-mIFN-λ2 or pEF-mIFN-α7 plasmid, or empty vector. G418-resistant cells were selected, pooled together and designated BNL.IFN-λ, BNL.IFN-α, or BNL.vector cells, respectively.
To assess IFN-λ and IFN-α production, we tested whether conditioned media from BNL.IFN-λ and BNL.IFN-α cells can upregulate MHC class I antigen expression in B16 cells that are sensitive to the IFN treatment [21] (Fig. 2). The expression level of MHC class I antigen was upregulated in B16 cells after treatment with conditioned media from BNL.IFN-λ or BNL.IFN-α cells, but not from control BNL.vector cells (Fig. 2a). These results demonstrate that BNL.IFN-λ and BNL.IFN-α stable transfectants constitutively secrete mIFN-λ and mIFN-α, respectively. The amounts of IFNs in the conditioned media of BNL.IFN-λ and BNL.IFN-α cells were determined by comparing biological activity of the media with those of recombinant mouse IFN-λ and IFN-α with known concentrations. By measuring IFN-induced MHC class I antigen upregulation in IFN-sensitive B16 melanoma cells, we found that BNL.IFN-λ and BNL.IFN-α cells secrete an average of 1.4 and 1 ng/ml from 0.4 × 106 cells/ml in 24 h, respectively (data not shown).
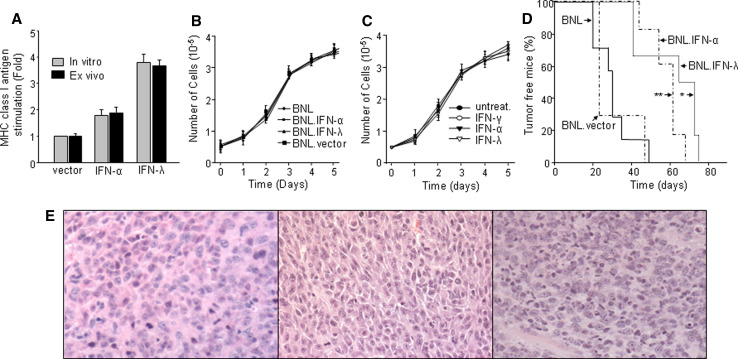
Fig. 2.
Constitutive expression of IFNs in BNL cells and its effects on tumor growth. a B16 melanoma cells were treated for 72 h with either conditioned medium from BNL.vector, BNL.IFN-λ or BNL.IFN-α cells (the pre-implantation cell cultures; gray bars), or with conditioned medium from BNL.vector, BNL.IFN-λ or BNL.IFN-α ex vivo cell cultures [BNL.vector, BNL.IFN-λ or BNL.IFN-α tumors were extracted from mice (n = 5), tumor cells were isolated and grown ex vivo; black bars]. The level of MHC class I antigen expression was determined by flow cytometry and the fold increase over the basal level of MHC class I antigen expression in untreated B16 cells was calculated. Data shown are representative of two independent experiments and presented as the mean ± SD (n = 5 per data point). b Growth rate of parental BNL, BNL.vector, BNL.IFN-α, or BNL.IFN-λ cells was assessed by growing an equal number of cells (5 × 104) in each well and counting the cells at days 1, 2, 3, 4, and 5. These experiments were performed in triplicate. Data are presented as the mean ± SD. c Antiproliferative activity of recombinant murine IFNs was evaluated on BNL cells. An equal number of BNL cells (5 × 104) was plated in all wells, the cells were left untreated or treated with either mIFN-γ, mIFN-α or mIFN-λ2 (10 ng/ml). Every day for 5 days, cells were collected and counted. These experiments were performed in triplicate. Data are presented as the mean ± SD. d Tumorigenicity of parental and modified IFN-producing BNL cells was evaluated in immunocompetent syngeneic BALB/c mice. Mice (n = 8) were injected s.c. in the flank with 106 BNL, BNL.vector, BNL.IFN-λ or BNL.IFN-α cells, and tumor development was monitored every other day by palpation of the injection site. The results are representative of two independent experiments. Data are shown as percentage of tumor free mice. BNL.vector versus BNL.IFN-λ *P < 0.05; BNL.vector versus BNL.IFN-α **P = 0.09. e H&E staining followed by light microscopy (×10) was performed on BNL.parental (left), BNL.IFN-α (middle) or BNL.IFN-λ tumors (right)
To determine whether secreted IFNs had an effect on cell proliferation, we cultured BNL, BNL.vector, BNL.IFN-λ, and BNL.IFN-α cells under identical conditions. Parental BNL cells were also treated with exogenous IFNs. As indicated in Fig. 2b, we did not observe any significant difference in the in vitro growth rate between BNL.IFN-λ, BNL.IFN-α, BNL.vector or parental BNL cells. Moreover, treatment with exogenous IFNs did not affect parental BNL cell proliferation (Fig. 2c).
Since the constitutive expression of IFN-λ at the tumor site was found to affect the tumorigenicity of B16 melanoma cells in vivo [21], we examined whether similar effects of IFN-λ would be displayed in the case of BNL hepatoma. One million parental or modified BNL cells were injected subcutaneously into immunocompetent syngeneic BALB/c mice. Mice injected with BNL.vector or parental BNL cells developed tumors in 4–6 weeks, whereas the appearance of BNL.IFN-λ tumors was significantly delayed (Fig. 2d). Similar effects were obtained in mice inoculated with BNL.IFN-α cells. These experiments demonstrated that constitutive expression of IFNs at the tumor site resulted in the delay of the in vivo tumor growth.
To examine whether the BNL.IFN-λ and BNL.IFN-α tumors, that demonstrated delayed growth in vivo, still preserved their pre-implantation IFN-λ and IFN-α expression, tumors from five mice injected with BNL.IFN-λ and BNL.IFN-α cells were extracted and analyzed ex vivo. In a similar fashion as described previously, we collected the conditioned media after 48 h of in vitro culture and assessed for the presence of IFN-λ or IFN-α by testing the ability of conditioned media to upregulate MHC class I antigen expression in B16 cells. As indicated in Fig. 2a, stimulation of MHC class I antigen expression was observed in B16 cells treated with conditioned media obtained from ex vivo BNL.IFN-λ tumors. Similar effects were also observed for BNL.IFN-α tumors but not from BNL.vector tumors. Therefore, the injected tumor cells retained IFN expression in vivo.
To determine whether potential histological modifications have occurred in vivo and if they may be associated with the tumor growth delay, tumors were removed from several mice in each experimental group and examined by light microscopy (Fig. 1e). Histology analysis did not reveal any significant difference in the vasculature between BNL.IFN-λ, BNL.IFN-α and parental tumors, suggesting that IFN-induced antitumor activity was not associated with an anti-angiogenic response. Although the mitotic rate of BNL.IFN-α tumors (3–8/hpf) was higher than that of BNL.IFN-λ (2–4/hpf) or parental BNL tumors (1–5/hpf), the apoptotic profile was more pronounced in the tumors obtained from mice injected with BNL.IFN-λ cells. In addition, BNL.IFN-λ tumors were extensively infiltrated with immune cells (2–5/hpf; vs. 0–1/hpf in parental or BNL.IFN-α tumors), implying a potential role of these cells in controlling tumor growth.
IFN treatment in vitro: effect on immunocytotoxicity and the production of IL-12 and IFN-γ
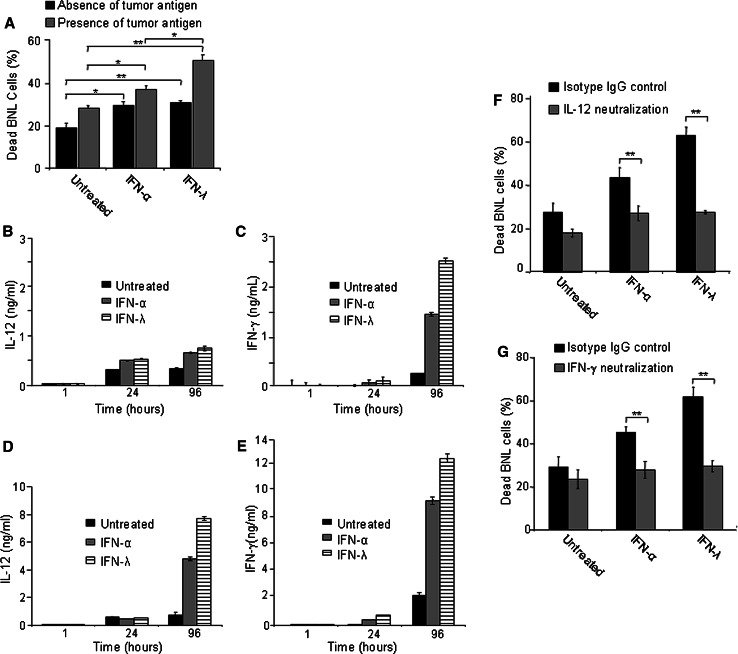
Since BNL cells were resistant to IFN-λ, the observed antitumor activities induced by IFN-λ should depend on host mechanisms. To study potential immune mechanisms underlying the delayed growth of BNL.IFN-λ tumors, splenocytes harvested from naive mice were cultured in vitro with or without exogenous IFN and in the presence or absence of tumor antigens (irradiated BNL cells). After the 4-day treatment, cytotoxicity of stimulated splenocytes against BNL cells was measured by co-culturing the splenocytes with live BNL cells for 24 h and determining the percentage of dead target BNL cells in the culture. Treatment of splenocytes with IFN-λ or IFN-α enhanced the cytotoxicity of splenocytes against parental BNL cells (Fig. 3a). Preincubation of splenocytes with irradiated BNL cells further augmented killing of BNL cells (Fig. 3a), suggesting that the presence of tumor antigens enhanced IFN-stimulated cytotoxicity of splenocytes. In combination with antigen, IFN-λ stimulated higher immunocytotoxicity of splenocytes against tumor cells than IFN-α, suggesting that IFN-λ may be an important player in the antitumor immune response. We next investigated the IFN-induced antitumor mechanisms by assessing the potential modulation of the expression of IL-12 and IFN-γ, cytokines well known for their role in antitumor immune responses [42, 43]. To test whether IFN-λ or IFN-α have any effect on IL-12 and/or IFN-γ secretion, splenocytes were treated with either IFN-λ or IFN-α and the levels of IL-12 and IFN-γ were assessed. Increased secretion of IL-12 (Fig. 3b) and IFN-γ (Fig. 3c) were detected following treatment with IFN-α or IFN-λ. However, the kinetics of secretion and the effect of IFN-λ and IFN-α were different. The level of IL-12 secretion increased at 24 h of IFN treatment (Fig. 3b), while the secretion of IFN-γ was not significantly changed at 24 h, but strongly increased at 96 h of the treatment with IFNs (Fig. 3c). There is a correlation between higher level of IFN-γ production (Fig. 3c) and stronger cytotoxicity (Fig. 3a) observed in the presence of IFN-λ, suggesting that the immunocytotoxicity induced by IFN-λ may be mediated by IFN-γ. Higher levels of IL-12 (Fig. 3d) and IFN-γ (Fig. 3e) secretion were observed after pretreatment of splenocytes with irradiated BNL cells, indicating that the effect of IFNs, particularly IFN-λ was enhanced in the presence of tumor antigens. To examine whether IL-12 and IFN-γ play a role in the IFN-induced immunocytotoxicity, we used neutralizing antibodies against IL-12 and IFN-γ. Blocking the action of either IL-12 or IFN-γ inhibited the splenocyte immunotoxicity against BNL cells (Fig. 3f, g), demonstrating that both cytokines serve as important mediators of IFN-α- or IFN-λ-induced cytotoxicity of splenocytes against BNL hepatoma cells.
Fig. 3.
IFN-induced immunocytotoxicity and production of IL-12 and IFN-γ. a Splenocytes (106) were isolated from naive mice (n = 3) and treated with IFNs in the presence or absence of irradiated (105) BNL cells (4 day culture). On day 4, splenocytes were collected and co-cultured with fresh (105) target BNL cells for 24 h. The cells were harvested, stained with propidium iodide, and the amount of dead target BNL tumor cells was assessed by FACS. Dead parental BNL cell numbers (%) are shown as the mean ± SD (n = 3 per data point). Results are representative of two independent experiments. *P < 0.05; **P < 0.01. b–e Conditioned media of splenocytes treated with IFNs in the absence (b, c) or presence (d, e) of tumor antigens were collected at 1, 24, and 96 h of the treatment and the levels of IL-12 (b, d) and IFN-γ (c, e) in the conditioned media were analyzed. Data are presented as the mean value of triplicate wells ± SD. f, g Untreated or IFN-treated splenocyte cultures were incubated in the presence of irradiated BNL cells with 20 μg/mL of purified anti-IL-12p40 (f), purified anti-mouse IFN-γ (g) or isotype-matched rat IgG and splenocyte immunocytotoxicity was measured after the 4 day treatment as described in a. Dead parental BNL cell numbers (%) are shown as the mean ± SD (n = 3 per data point). All results are representative of two independent experiments. F and G: **P < 0.01
In vivo effect of IFN on immunocytotoxicity and the involvement of NK cells
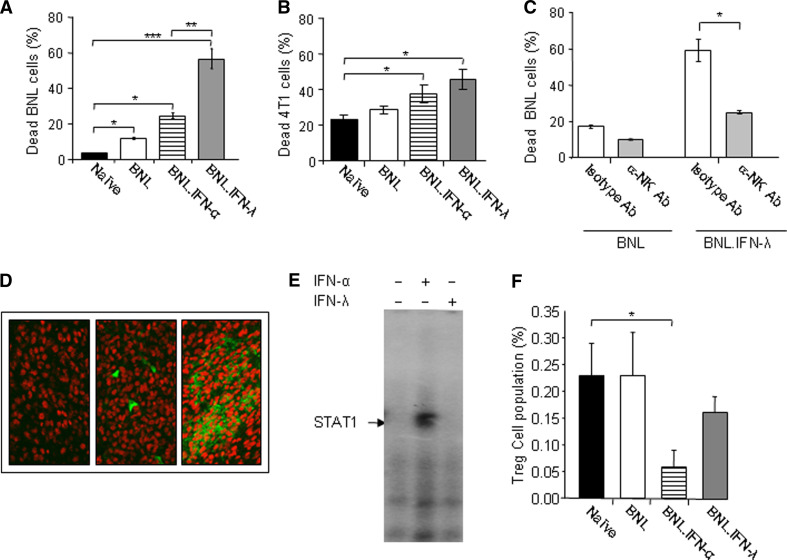
To study the in vivo effect of IFN on immunocytotoxicity, groups of mice (n = 5) were injected with either parental BNL, BNL.IFN-λ or BNL.IFN-α. After 2 weeks, mice were sacrificed, spleens extracted, and splenocytes were immediately co-cultured for 24 h with parental BNL target cells (Fig. 4a) or heterologous 4T1 breast carcinoma target cells (Fig. 4b). As shown in Fig. 4a, a marked immune response was observed in mice injected with BNL.IFN-λ in comparison to naive mice or mice injected with parental BNL or BNL.IFN-α cells. Splenocytes obtained from mice injected with IFN-producing BNL cells also demonstrated increased immunocytotoxicity against unrelated 4T1 cells, implying that IFNs stimulate innate antitumor immunity.
Fig. 4.
IFN-λ triggers a substantial immunocytotoxic response against target tumor cells and the involvement of NK cells. a, b Splenocytes were isolated from naive mice or mice (n = 5) injected with either parental BNL, BNL.IFN-α, or BNL.IFN-λ cells, and their cytotoxicity was tested against parental BNL tumor cells (a) or heterologous 4T1 breast carcinoma cells (b). Dead parental BNL (a) or 4T1 (b) cell numbers (%) are shown as the mean ± SD [n = 5 (a) and n = 3 (b) per data point]. Results in (a) are representative of two independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001. c NK cells were depleted from splenocytes isolated from mice injected with parental BNL or BNL.IFN-λ cells with the use of a biotinylated rat monoclonal Ab against NK or with isotype-matched rat IgM as a control. Cytotoxicity of splenocytes against parental BNL cells was evaluated in vitro as described above. Dead parental BNL cell numbers (%) are shown as the mean ± SD (n = 3 per data point). Results are representative of three independent experiments. *P < 0.05. d Immunohistochemical staining of CD49b+ NK cells (green fluorescence) was performed in BNL.parental (left), BNL.IFN-α (middle) or BNL.IFN-λ tumors (right). Samples were examined in a confocal fluorescent microscope at ×250. e NK cells were purified from spleen of mice which received BNL.IFN-λ cells with the use of magnetic beads as described in “Materials and methods”. NK cells were treated with IFN-α or IFN-λ (10 ng/ml) for 15 min, lysed and STAT1 activation was detected in cellular extracts by EMSA. The EMSA is representative of two independent experiments. f PBMCs were isolated from naive mice or mice injected with either parental BNL cells, BNL.IFN-α, or BNL.IFN-λ cells, and the number of circulating CD4+ CD25+ Foxp3+ Tregs in the lymphoid gate population (%) was determined by flow cytometry and shown as the mean ± SD (n = 3 per data point). *P < 0.05
To determine effector cells of IFN-λ-induced antitumor immunity, we tested the potential involvement of NK cells. By using an antibody specific to NK cells, we depleted NK cells from cultured splenocytes obtained from mice injected with either parental BNL or BNL.IFN-λ cells and assessed their killing ability against parental BNL cells. Depletion of NK cells from splenocytes of mice injected with BNL.IFN-λ cells resulted in a marked decrease in their cytotoxicity against BNL cells (Fig. 4c). Therefore, results indicated that NK cells are important mediators of the innate antitumor immunity induced by IFN-λ.
To determine whether IFN treatment affects tumor infiltration of NK cells, we performed immunohistochemical staining for NK cells in the tumor tissue extracted from mice injected with parental or modified BNL cells. As indicated in Fig. 4d, in contrast to IFN-α, a marked infiltration of NK cells was observed in IFN-λ-producing tumors, suggesting that IFN-λ may be more potent than IFN-α in the recruitment or induction of the proliferation of NK cells at the tumor site. However, when we assessed the response of NK cells to IFNs by measuring IFN-induced STAT1 activation, only IFN-α was able to activate STAT1 DNA-binding complexes in NK cells (Fig. 4e). In contrast, NK cells did not respond directly to IFN-λ (Fig. 4e), implying that NK cells were activated by IFN-λ indirectly. Therefore, the effect of IFN-λ on NK cells may be mediated through other molecules such as IL-12 and IFN-γ (Fig. 3b–g).
We next asked whether IFN-λ and IFN-α may modulate the number of circulating CD4+ CD25+ Foxp3+ T regulatory cells (Tregs). As shown in Fig. 4f, the amount of Tregs was unchanged in mice inoculated with parental BNL cells in comparison with naive mice. However, a significant decrease in CD4+ CD25+ Foxp3+ Tregs was observed in mice receiving BNL.IFN-α, whereas a moderate decrease in Tregs observed in mice receiving BNL.IFN-λ was not statistically significant (Fig. 4f). Therefore, antitumor mechanisms activated by IFN-α and IFN-λ may differ; IFN-α had a stronger effect on Tregs, whereas IFN-λ increased the number of NK cells at the tumor site.
Involvement of DCs in IFN-λ-induced immunocytotoxicity
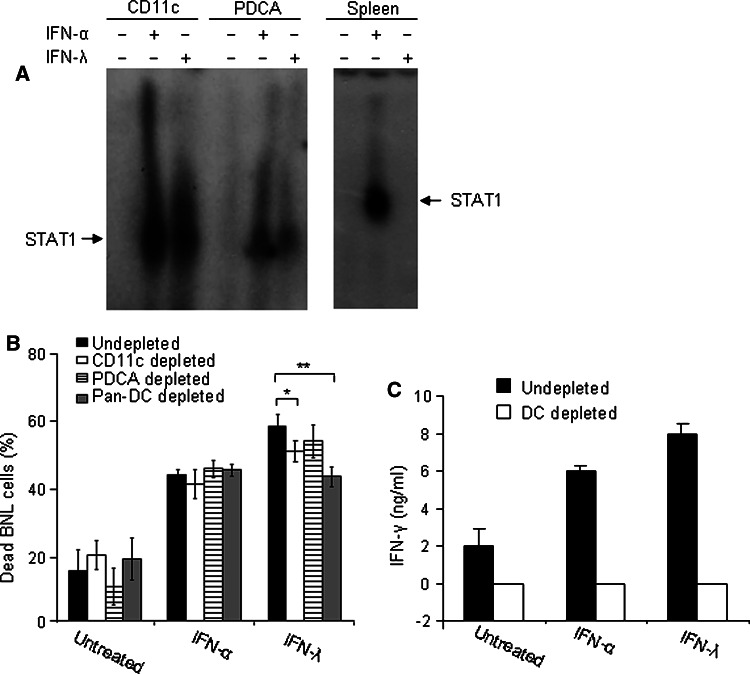
IFN-α can directly regulate immunomodulatory functions of various immune cells [44, 45]. In contrast, due to restricted pattern of IFN-λR1 expression, NK and T cells do not respond directly to IFN-λ ([21, 24, 46]; and Fig. 4e). To investigate whether DCs are involved in mediating effects of IFN-λ on NK cells, we first demonstrated that DCs, isolated from mouse splenocytes, are directly responsive to IFN-λ treatment. IFN-λ and to a similar extent IFN-α were able to induce STAT activation in both myeloid and plasmacytoid DCs (Fig. 5a). In contrast, splenocytes responded only to IFN-α ([21] and Fig. 5a). We next analyzed the involvement of DCs in the IFN-induced immunocytoxicity by testing the cytotoxic ability of DC-depleted splenocytes against target BNL tumor cells. As shown in Fig. 5b, depletion of DCs caused a decrease in splenocyte cytotoxicity in the presence of IFN-λ, but not IFN-α, demonstrating for the first that DCs are important mediator of IFN-λ-induced antitumor response. The strongest effect was observed after the depletion of both CD11c+ and PDCA+ DCs (Fig. 5b). The production of IFN-γ induced by either IFN-α or IFN-λ was completely abolished after DCs depletion (Fig. 5c). The suppression of IFN-γ production in DC-depleted splenocytes inhibited only IFN-λ- but not IFN-α-induced immunocytotoxicity, demonstrating that splenocytes respond differently to IFN-λ and IFN-α.
Fig. 5.
Involvement of DCs in IFN-λ-induced immunocytotoxic response. a CD11c+ and mPDCA+ DCs were purified from mouse spleens using magnetic MACS microbeads (as described in the “Materials and methods”). DCs and total splenocytes were treated with IFN-α or IFN-λ (10 ng/ml) for 15 min, lysed and STAT1 activation was detected in cellular extracts by EMSA. b In vitro depletion of CD11c+, mPDCA+, or both populations in splenocytes (106) was performed (as described in the “Materials and methods”). Splenocytes were harvested from naive mice (n = 3) and treated with IFNs in the presence or absence of irradiated (105) BNL cells (4 day culture). On day 4, splenocytes were collected and co-cultured with fresh (105) target BNL cells for 24 h. The cells were harvested, stained with propidium iodide, and the amount of dead target BNL tumor cells was assessed by FACS. Dead parental BNL cell numbers (%) are shown as the mean ± SD (n = 3 per data point). *P < 0.05, **P < 0.01. c Conditioned media of splenocytes (n = 3) treated with IFNs in (a) were collected after 4 days of treatment and the levels of IFN-γ in the conditioned media were analyzed. Data are presented as the mean value of triplicate wells ± SD
Discussion
In addition to their potent antiviral activity, type I IFNs are also recognized for their antitumor properties. IFN-α is used clinically to treat various malignancies, including hairy cell and myelogenous leukemias, multiple myeloma, lymphomas, renal cell carcinoma, Kaposi sarcoma and metastatic melanoma [18, 47, 48]. In addition, clinical targets of type I IFN therapy include chronic viral infections, particularly HCV and HBV infections. Importantly, chronic HCV and HBV infections are the main cause of HCC [49, 50]. Consequently, preventive treatment against HCC may be highly valuable, particularly in patients with HCV and HBV-associated liver injury. Several studies reported that IFN-α therapy decreased the incidence of HCC in patients with HCV or HBV cirrhosis [12–15]. However, the use of IFN-α is limited by numerous severe side effects including inhibition of hematopoiesis, neuropsychiatric effects (depression, anxiety) and influenza-like symptoms (myalgia, fever, fatigue) [51–54]. Therefore, there is a great need for alternative biological agents that elicit fewer or less severe side effects. There is a possibility that type III IFNs or IFN-λs [19, 20] may be an attractive treatment compared to type I IFNs. Type III IFNs share biological activities with type I IFNs but their action is tissue-specific due to the restricted expression of the IFN-λ receptor. Therefore, IFN-λ therapy may cause fewer and/or milder side effects and improve tolerance of IFN therapy. Indeed, a recently conducted Phase 1b open-label dose- and schedule-escalation study with subcutaneously delivered pegylated IFN-λ to HCV patients revealed that the treatment was well-tolerated [55]. Interestingly, it was recently demonstrated that a polymorphism near the IFN-λ3 gene, which seems to affect IFN-λ expression levels [56], was not only correlated and with the spontaneous clearance of HCV [57], but was also associated with the sustained virologic response in patients with chronic HCV undergoing pegylated IFN-α/ribavirin combination therapy [56, 58, 59].
In the present study, we investigated the antitumor properties of IFN-λ in comparison with those of IFN-α in a murine hepatoma model. Subcutaneously injected hepatoma BNL cells form tumors in syngeneic BALB/c mice (Fig. 2d). However, injection of BNL cells constitutively producing either IFN-α or IFN-λ resulted in delayed tumor appearance and retarded growth kinetics (Fig. 2d). Interestingly, although the production of either IFN-α or IFN-λ at the tumor site reduced the tumorigenicity of BNL cells to a similar extent, only IFN-α had a direct effect on BNL cells (Fig. 1a, b), whereas BNL cells were resistant to IFN-λ treatment due to the lack of IFN-λR1 expression (Fig. 1d). Nevertheless, none of the IFNs demonstrated an antiproliferative effect on BNL cells (Fig. 2b, c), suggesting that both IFNs activate host mechanisms to suppress BNL tumor growth in vivo.
Although histological analysis of the hepatoma tumors did not reveal significant differences in the vasculature between the parental tumors and tumors secreting IFN-λ or IFN-α, tumors from mice inoculated with BNL.IFN-λ cells had extensive infiltration of immune cells (Fig. 2e). Immunohistochemical staining of the tumors revealed a marked infiltration of NK cells in IFN-λ-producing tumors (Fig. 4d), suggesting that IFN-λ may be more potent than IFN-α in the recruitment or induction of the proliferation of NK cells at the tumor site. Moreover, IFN-λ appeared to be more potent than IFN-α in inducing splenocyte cytotoxicity against BNL hepatoma cells (Figs. 3a, 4a). In addition, treatment of splenocytes with either IFN-α or IFN-λ resulted in the secretion of IL-12 and IFN-γ (Fig. 3b, c) that was strongly augmented by co-treatment of splenocytes with tumor antigens (Fig. 3d, e). Moreover, neutralization of either IL-12 or IFN-γ inhibited IFN-induced splenocyte cytotoxicity (Fig. 3f, g). Because IL-12 is a major inducer of IFN-γ production from NK and T cells [60, 61], it is likely that the effect of IFN-α and IFN-λ on IFN-γ secretion is mediated by IL-12. Using antibody-mediated NK cell depletion, we also demonstrated that the antitumor activity induced by IFN-λ was mediated by NK cells (Fig. 4c). All these results correlate with recent reports describing the involvement of NK cells in antitumor activities of IFN-λ in melanoma, colon cancer, and fibrosarcoma tumor models [22, 23]. Interestingly, NK cells isolated from naive mice or tumor-bearing mice responded well to IFN-α treatment, but we were unable to detect responsiveness of NK cells to IFN-λ treatment (Fig. 4e and data not shown), demonstrating that NK cells are not a direct target of IFN-λ, and suggesting that effects of IFN-λ on NK cells are mediated by other IFN-λ-responsive cells such as DCs (Fig. 5a). Nevertheless, NK cells seem to be important mediators of IFN-λ-induced antitumor responses.
We also observed a strong reduction in circulating CD4+ CD25+ Foxp3+ Tregs only in mice injected with BNL.IFN-α tumors, whereas a moderate decrease in Tregs observed in mice bearing BNL.IFN-λ cells was not statistically significant (Fig. 4f). Although, it has been recently reported that IFN-λ has the ability to reduce Treg population in mice [62], it was previously reported that in mixed lymphocyte reaction cultures, IFN-λ-treated DCs specifically induced IL-2-dependent proliferation of a CD4+ CD25+ Foxp3+ T-cell subset [63]. In our experimental model, we did not observe statistically significant effect of IFN-λ on the number of Tregs (Fig. 4f). However, we demonstrated that DCs are indeed directly responsive to IFN-λ treatment (Fig. 5a) and serve as critical mediators of IFN-α- and IFN-λ-induced IFN-γ production from splenocytes (Fig. 5c). This observation correlates with a role of DC-produced IL-12 as a master regulator of IFN-γ expression in T and NK cells [60, 63]. Interestingly, depletion of DCs resulted in a significantly decreased cytotoxicity of splenocytes in response to IFN-λ, but not IFN-α (Fig. 5b). Therefore, although production of IFN-γ by splenocytes in response to either IFN-λ or IFN-α is mediated by DCs, IFN-α-induced cytotoxicity is unaffected by the depletion of DCs, whereas IFN-λ- induced immunocytotoxicity is mediated by DCs.
In conclusion, this study demonstrated that IFN-λ has antitumor activity that is comparable to or exceeds that of IFN-α in the murine BNL hepatoma model. However, different effects observed on NK cell recruitment, Treg reduction and the role of DCs in splenocyte cytotoxicity, suggest that IFN-α and IFN-λ activate overlapping but distinct antitumor mechanisms. Therefore, IFN-λ treatment should be considered as an alternative or additive to currently existing IFN-α therapy in the prevention and treatment of liver cancer.
Acknowledgments
We thank George Yap, Deborah Lazzarino, David Lagunoff, Lauriston Prescott and Edward Azzam for reagents, technical support and helpful suggestions. This study was supported in part by the US Public Health Services Grant RO1 AI057468 (SVK) from the NIAID, and P30 ES005022 (KR) from NIEHS for the Molecular Pathology Core. Author Statement: AL and SVK designed research; WA and AL performed research; MB, IC, AD and YY provided technical assistance; IC performed statistical analysis; WA, AD, KR, ESR, AL and SVK analyzed data; and WA, AL and SVK wrote the manuscript. AL and SVK contributed equally to this study.
Contributor Information
Ahmed Lasfar, Phone: +1-973-9723134, FAX: +1-973-9725594, Email: lasfarah@umdnj.edu.
Sergei V. Kotenko, Phone: +1-973-9723134, FAX: +1-973-9725594, Email: kotenkse@umdnj.edu
References
- 1.Sherman M. Hepatocellular carcinoma: epidemiology, risk factors, and screening. Semin Liver Dis. 2005;25:143–154. doi: 10.1055/s-2005-871194. [DOI] [PubMed] [Google Scholar]
- 2.Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6:674–687. doi: 10.1038/nrc1934. [DOI] [PubMed] [Google Scholar]
- 3.Lau WY, Lai EC. Hepatocellular carcinoma: current management and recent advances. Hepatobiliary Pancreat Dis Int. 2008;7:237–257. [PubMed] [Google Scholar]
- 4.Taketomi A, Soejima Y, Yoshizumi T, Uchiyama H, Yamashita Y, Maehara Y. Liver transplantation for hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2008;15:124–130. doi: 10.1007/s00534-007-1296-4. [DOI] [PubMed] [Google Scholar]
- 5.Mazzaferro V, Chun YS, Poon RT, Schwartz ME, Yao FY, Marsh JW, Bhoori S, Lee SG. Liver transplantation for hepatocellular carcinoma. Ann Surg Oncol. 2008;15:1001–1007. doi: 10.1245/s10434-007-9559-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL, Adam R, Neuhaus P, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35–43. doi: 10.1016/S1470-2045(08)70284-5. [DOI] [PubMed] [Google Scholar]
- 7.Sherman M. Recurrence of hepatocellular carcinoma. N Engl J Med. 2008;359:2045–2047. doi: 10.1056/NEJMe0807581. [DOI] [PubMed] [Google Scholar]
- 8.Georgiades CS, Hong K, Geschwind JF. Radiofrequency ablation and chemoembolization for hepatocellular carcinoma. Cancer J. 2008;14:117–122. doi: 10.1097/PPO.0b013e31816a0fac. [DOI] [PubMed] [Google Scholar]
- 9.Di Bisceglie AM, Carithers RL, Jr, Gores GJ. Hepatocellular carcinoma. Hepatology. 1998;28:1161–1165. doi: 10.1002/hep.510280436. [DOI] [PubMed] [Google Scholar]
- 10.Nagai H, Sumino Y. Therapeutic strategy of advanced hepatocellular carcinoma by using combined intra-arterial chemotherapy. Recent Pat Anticancer Drug Discov. 2008;3:220–226. doi: 10.2174/157489208786242296. [DOI] [PubMed] [Google Scholar]
- 11.El-Serag HB. Hepatocellular carcinoma: an epidemiologic view. J Clin Gastroenterol. 2002;35:S72–S78. doi: 10.1097/00004836-200211002-00002. [DOI] [PubMed] [Google Scholar]
- 12.Fattovich G, Giustina G, Sanchez-Tapias J, Quero C, Mas A, Olivotto PG, Solinas A, Almasio P, Hadziyannis S, Degos F, de Moura MC, Krogsgaard K, et al. Delayed clearance of serum HBsAg in compensated cirrhosis B: relation to interferon alpha therapy and disease prognosis. European Concerted Action on Viral Hepatitis (EUROHEP) Am J Gastroenterol. 1998;93:896–900. doi: 10.1111/j.1572-0241.1998.00272.x. [DOI] [PubMed] [Google Scholar]
- 13.Omata M, Yoshida H, Shiratori Y. Prevention of hepatocellular carcinoma and its recurrence in chronic hepatitis C patients by interferon therapy. Clin Gastroenterol Hepatol. 2005;3:S141–S143. doi: 10.1016/S1542-3565(05)00713-5. [DOI] [PubMed] [Google Scholar]
- 14.Lin SM, Yu ML, Lee CM, Chien RN, Sheen IS, Chu CM, Liaw YF. Interferon therapy in HBeAg positive chronic hepatitis reduces progression to cirrhosis and hepatocellular carcinoma. J Hepatol. 2007;46:45–52. doi: 10.1016/j.jhep.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 15.Yu ML, Lin SM, Chuang WL, Dai CY, Wang JH, Lu SN, Sheen IS, Chang WY, Lee CM, Liaw YF. A sustained virological response to interferon or interferon/ribavirin reduces hepatocellular carcinoma and improves survival in chronic hepatitis C: a nationwide, multicentre study in Taiwan. Antivir Ther. 2006;11:985–994. [PubMed] [Google Scholar]
- 16.Llovet JM, Sala M, Castells L, Suarez Y, Vilana R, Bianchi L, Ayuso C, Vargas V, Rodes J, Bruix J. Randomized controlled trial of interferon treatment for advanced hepatocellular carcinoma. Hepatology. 2000;31:54–58. doi: 10.1002/hep.510310111. [DOI] [PubMed] [Google Scholar]
- 17.Lo CM, Liu CL, Chan SC, Lam CM, Poon RT, Ng IO, Fan ST, Wong J. A randomized, controlled trial of postoperative adjuvant interferon therapy after resection of hepatocellular carcinoma. Ann Surg. 2007;245:831–842. doi: 10.1097/01.sla.0000245829.00977.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pestka S, Krause CD, Walter MR. Interferons, interferon-like cytokines, and their receptors. Immunol Rev. 2004;202:8–32. doi: 10.1111/j.0105-2896.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- 19.Kotenko SV, Gallagher G, Baurin VV, Lewis-Antes A, Shen M, Shah NK, Langer JA, Sheikh F, Dickensheets H, Donnelly RP. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat Immunol. 2003;4:69–77. doi: 10.1038/ni875. [DOI] [PubMed] [Google Scholar]
- 20.Sheppard P, Kindsvogel W, Xu W, Henderson K, Schlutsmeyer S, Whitmore TE, Kuestner R, Garrigues U, Birks C, Roraback J, Ostrander C, Dong D, et al. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat Immunol. 2003;4:63–68. doi: 10.1038/ni873. [DOI] [PubMed] [Google Scholar]
- 21.Lasfar A, Lewis-Antes A, Smirnov SV, Anantha S, Abushahba W, Tian B, Reuhl K, Dickensheets H, Sheikh F, Donnelly RP, Raveche E, Kotenko SV. Characterization of the mouse IFN-lambda ligand-receptor system: IFN-lambdas exhibit antitumor activity against B16 melanoma. Cancer Res. 2006;66:4468–4477. doi: 10.1158/0008-5472.CAN-05-3653. [DOI] [PubMed] [Google Scholar]
- 22.Sato A, Ohtsuki M, Hata M, Kobayashi E, Murakami T. Antitumor activity of IFN-lambda in murine tumor models. J Immunol. 2006;176:7686–7694. doi: 10.4049/jimmunol.176.12.7686. [DOI] [PubMed] [Google Scholar]
- 23.Numasaki M, Tagawa M, Iwata F, Suzuki T, Nakamura A, Okada M, Iwakura Y, Aiba S, Yamaya M. IL-28 elicits antitumor responses against murine fibrosarcoma. J Immunol. 2007;178:5086–5098. doi: 10.4049/jimmunol.178.8.5086. [DOI] [PubMed] [Google Scholar]
- 24.Doyle SE, Schreckhise H, Khuu-Duong K, Henderson K, Rosler R, Storey H, Yao L, Liu H, Barahmand-pour F, Sivakumar P, Chan C, Birks C, et al. Interleukin-29 uses a type 1 interferon-like program to promote antiviral responses in human hepatocytes. Hepatology. 2006;44:896–906. doi: 10.1002/hep.21312. [DOI] [PubMed] [Google Scholar]
- 25.Sommereyns C, Paul S, Staeheli P, Michiels T. IFN-lambda (IFN-lambda) is expressed in a tissue-dependent fashion and primarily acts on epithelial cells in vivo. PLoS Pathog. 2008;4:e1000017. doi: 10.1371/journal.ppat.1000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kotenko SV, Donnelly RP. Type III IFNs: the Interferon-λ family. In: Meager A, editor. The interferons: characterization and application. Weinheim: Wiley-VCH; 2006. pp. 141–163. [Google Scholar]
- 27.Huang J, Smirnov SV, Lewis-Antes A, Balan M, Li W, Tang S, Silke GV, Putz MM, Smith GL, Kotenko SV. Inhibition of type I and type III interferons by a secreted glycoprotein from Yaba-like disease virus. Proc Natl Acad Sci USA. 2007;104:9822–9827. doi: 10.1073/pnas.0610352104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kotenko SV (2007) IFN-λs. In: Zdanov A (ed) Class II cytokines, Transworld Research Network
- 29.Fu XY, Kessler DS, Veals SA, Levy DE, Darnell JEJ. ISGF3, the transcriptional activator induced by interferon alpha, consists of multiple interacting polypeptide chains. Proc Natl Acad Sci USA. 1990;87:8555–8559. doi: 10.1073/pnas.87.21.8555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shuai K, Schindler C, Prezioso VR, Darnell JEJ. Activation of transcription by IFN-gamma: tyrosine phosphorylation of a 91-kD DNA binding protein. Science. 1992;258:1808–1812. doi: 10.1126/science.1281555. [DOI] [PubMed] [Google Scholar]
- 31.Mordstein M, Kochs G, Dumoutier L, Renauld JC, Paludan SR, Klucher K, Staeheli P. Interferon-lambda contributes to innate immunity of mice against influenza A virus but not against hepatotropic viruses. PLoS Pathog. 2008;4:e1000151. doi: 10.1371/journal.ppat.1000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kugel D, Kochs G, Obojes K, Roth J, Kobinger GP, Kobasa D, Haller O, Staeheli P, von MV. Intranasal administration of interferon-alpha reduces seasonal influenza A virus morbidity in ferrets. J Virol. 2009;83:3843–3851. doi: 10.1128/JVI.02453-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ank N, Iversen MB, Bartholdy C, Staeheli P, Hartmann R, Jensen UB, gnaes-Hansen F, Thomsen AR, Chen Z, Haugen H, Klucher K, Paludan SR. An important role for type III interferon (IFN-lambda/IL-28) in TLR-induced antiviral activity. J Immunol. 2008;180:2474–2485. doi: 10.4049/jimmunol.180.4.2474. [DOI] [PubMed] [Google Scholar]
- 34.Ank N, West H, Bartholdy C, Eriksson K, Thomsen AR, Paludan SR. Lambda interferon (IFN-lambda), a type III IFN, is induced by viruses and IFNs and displays potent antiviral activity against select virus infections in vivo. J Virol. 2006;80:4501–4509. doi: 10.1128/JVI.80.9.4501-4509.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lazaro CA, Chang M, Tang W, Campbell J, Sullivan DG, Gretch DR, Corey L, Coombs RW, Fausto N. Hepatitis C virus replication in transfected and serum-infected cultured human fetal hepatocytes. Am J Pathol. 2007;170:478–849. doi: 10.2353/ajpath.2007.060789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marcello T, Grakoui A, Barba-Spaeth G, Machlin ES, Kotenko SV, MacDonald MR, Rice CM. Interferons alpha and lambda inhibit hepatitis C virus replication with distinct signal transduction and gene regulation kinetics. Gastroenterology. 2006;131:1887–1898. doi: 10.1053/j.gastro.2006.09.052. [DOI] [PubMed] [Google Scholar]
- 37.Hong SH, Kim K, Shin HJ, Kotenko SV, Park S. The different effect of Interferon-lambda on Hepatitis B Virus replication in two human hepatoma cell lines. Virus Res. 2007;162:245–249. doi: 10.1016/j.virusres.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 38.Robek MD, Boyd BS, Chisari FV. Lambda interferon inhibits hepatitis B and C virus replication. J Virol. 2005;79:3851–3854. doi: 10.1128/JVI.79.6.3851-3854.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meager A, Visvalingam K, Dilger P, Bryan D, Wadhwa M. Biological activity of interleukins-28 and -29: comparison with type I interferons. Cytokine. 2005;31:109–118. doi: 10.1016/j.cyto.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 40.Maher SG, Sheikh F, Scarzello AJ, Romero-Weaver AL, Baker DP, Donnelly RP, Gamero AM. IFNalpha and IFNlambda differ in their antiproliferative effects and duration of JAK/STAT signaling activity. Cancer Biol Ther. 2008;7:1109–1115. doi: 10.4161/cbt.7.7.6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goldman LA, Cutrone EC, Kotenko SV, Krause CD, Langer JA. Modifications of vectors pEF-BOS, pcDNA1 and pcDNA3 result in improved convenience and expression. Biotechniques. 1996;21:1013–1015. doi: 10.2144/96216bm10. [DOI] [PubMed] [Google Scholar]
- 42.Segal JG, Lee NC, Tsung YL, Norton JA, Tsung K. The role of IFN-gamma in rejection of established tumors by IL-12 : source of production and target. Cancer Res. 2002;62:4696–4703. [PubMed] [Google Scholar]
- 43.Rosenthal FM, Cronin K, Bannerji R, Golde DW, Gansbacher B. Augmentation of antitumor immunity by tumor cells transduced with a retroviral vector carrying the interleukin-2 and interferon-gamma cDNAs. Blood. 1994;83:1289–1298. [PubMed] [Google Scholar]
- 44.Decker T, Muller M, Stockinger S. The yin and yang of type I interferon activity in bacterial infection. Nat Rev Immunol. 2005;5:675–687. doi: 10.1038/nri1684. [DOI] [PubMed] [Google Scholar]
- 45.Stetson DB, Medzhitov R. Type I interferons in host defense. Immunity. 2006;25:373–381. doi: 10.1016/j.immuni.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 46.Witte K, Gruetz G, Volk HD, Looman AC, Asadullah K, Sterry W, Sabat R, Wolk K. Despite IFN-lambda receptor expression, blood immune cells, but not keratinocytes or melanocytes, have an impaired response to type III interferons: implications for therapeutic applications of these cytokines. Genes Immun. 2009;10:702–714. doi: 10.1038/gene.2009.72. [DOI] [PubMed] [Google Scholar]
- 47.Meager A. The interferons: characterization and application. Weinheim: Wiley; 2006. [Google Scholar]
- 48.Belardelli F, Ferrantini M, Proietti E, Kirkwood JM. Interferon-alpha in tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13:119–134. doi: 10.1016/S1359-6101(01)00022-3. [DOI] [PubMed] [Google Scholar]
- 49.bou-Alfa GK. Hepatocellular carcinoma: molecular biology and therapy. Semin Oncol. 2006;33:S79–S83. doi: 10.1053/j.seminoncol.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thorgeirsson SS, Lee JS, Grisham JW. Molecular prognostication of liver cancer: end of the beginning. J Hepatol. 2006;44:798–805. doi: 10.1016/j.jhep.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 51.Lai CL, Lau JY, Wu PC, Ngan H, Chung HT, Mitchell SJ, Corbett TJ, Chow AW, Lin HJ. Recombinant interferon-alpha in inoperable hepatocellular carcinoma: a randomized controlled trial. Hepatology. 1993;17:389–394. doi: 10.1002/hep.1840170307. [DOI] [PubMed] [Google Scholar]
- 52.Fattovich G, Giustina G, Favarato S, Ruol A. A survey of adverse events in 11,241 patients with chronic viral hepatitis treated with alfa interferon. J Hepatol. 1996;24:38–47. doi: 10.1016/S0168-8278(96)80184-X. [DOI] [PubMed] [Google Scholar]
- 53.Okanoue T, Sakamoto S, Itoh Y, Minami M, Yasui K, Sakamoto M, Nishioji K, Katagishi T, Nakagawa Y, Tada H, Sawa Y, Mizuno M, et al. Side effects of high-dose interferon therapy for chronic hepatitis C. J Hepatol. 1996;25:283–291. doi: 10.1016/S0168-8278(96)80113-9. [DOI] [PubMed] [Google Scholar]
- 54.Dusheiko G. Side effects of alpha interferon in chronic hepatitis C. Hepatology. 1997;26:112S–121S. doi: 10.1002/hep.510260720. [DOI] [PubMed] [Google Scholar]
- 55.Lawitz EJ, Zaman A, Muir AJ, Shiffman ML, Yoffe B, Zhang T, Souza S, Hausman DF. Interim results from a phase 1b dose-escalation study of 4 weeks of PEG-interferon lambda (PEG-rIL-29) treatment in subjects with hepatitis C virus (HCV) genotype 1 with prior virologic response and relapse to peginterferon alpha and ribavirin. Abstract. 2009;170:385A. [Google Scholar]
- 56.Tanaka Y, Nishida N, Sugiyama M, Kurosaki M, Matsuura K, Sakamoto N, Nakagawa M, Korenaga M, Hino K, Hige S, Ito Y, Mita E, et al. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet. 2009;41:1105–1109. doi: 10.1038/ng.449. [DOI] [PubMed] [Google Scholar]
- 57.Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, Heinzen EL, Qiu P, Bertelsen AH, Muir AJ, Sulkowski M, McHutchison JG, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 58.Suppiah V, Moldovan M, Ahlenstiel G, Berg T, Weltman M, Abate ML, Bassendine M, Spengler U, Dore GJ, Powell E, Riordan S, Sheridan D, et al. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat Genet. 2009;41:1100–1104. doi: 10.1038/ng.447. [DOI] [PubMed] [Google Scholar]
- 59.Thomas DL, Thio CL, Martin MP, Qi Y, Ge D, O’huigin C, Kidd J, Kidd K, Khakoo SI, Alexander G, Goedert JJ, Kirk GD, et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461:798–801. doi: 10.1038/nature08463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 61.Watford WT, Moriguchi M, Morinobu A, O’Shea JJ. The biology of IL-12: coordinating innate and adaptive immune responses. Cytokine Growth Factor Rev. 2003;14:361–368. doi: 10.1016/S1359-6101(03)00043-1. [DOI] [PubMed] [Google Scholar]
- 62.Morrow MP, Pankhong P, Laddy DJ, Schoenly KA, Yan J, Cisper N, Weiner DB. Comparative ability of IL-12 and IL-28B to regulate Treg populations and enhance adaptive cellular immunity. Blood. 2009;113:5868–5877. doi: 10.1182/blood-2008-11-190520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mennechet FJ, Uze G. Interferon-lambda-treated dendritic cells specifically induce proliferation of FOXP3-expressing suppressor T cells. Blood. 2006;107:4417–4423. doi: 10.1182/blood-2005-10-4129. [DOI] [PubMed] [Google Scholar]