Abstract
Purpose
The pathophysiology of painful bladder syndrome (PBS) is poorly understood; however, there is evidence of female predominance and comorbidity with irritable bowel syndrome (IBS). Our hypothesis is that cross-sensitization between the bladder and colon is due to altered permeability in one organ affecting the other organ.
Materials and methods
Experiments were performed in anesthetized, ovariectomized (OVX) female rats. In separate groups, protamine sulfate was infused into the bladder or TNBS was infused into the colon, with untreated rats serving as controls. Both bladder and colonic tissue were harvested for all rats at 1, 3, and 5 days post-treatment. Permeability was assessed in vitro in Ussing chambers via measurements of transepithelial electrical resistance (TEER) and macromolecular flux of Fluorescein isothiocyanate (FITC)-4 dextran.
Results
Exposing the bladder to protamine sulfate induced a significant (p<0.05) decrease in bladder TEER and an increase in the translocation of FITC across the tissue compared to controls at 1 and 3 days. Colonic tissue from rats with enhanced bladder permeability exhibited a significant (p<0.05) decrease in TEER and increase in FITC when compared to untreated controls at all time points. Conversely, when colonic permeability was increased with TNBS, we observed an increase in bladder permeability in the absence of any changes to the bladder urothelium.
Conclusions
Changes in epithelial permeability may represent a novel mechanism for visceral organ crosstalk and may explain the overlapping symptomology of PBS and IBS.
Keywords: urinary bladder, colon, inflammation, permeability, visceral organ crosstalk, rat
INTRODUCTION
Painful bladder syndrome (PBS), or interstitial cystitis (IC), is a chronic pain condition that affects nearly one million people in the United States, the vast majority of whom are women. PBS/IC is characterized by frequent urination, increased urgency, and pain associated with bladder filling.1 PBS/IC patients often suffer from a variety of other pelvic pain disorders including functional bowel disorders, such as irritable bowel syndrome (IBS). As many as 30-50% of patients diagnosed with IBS exhibit symptoms of PBS, while as many as 40% of patients diagnosed with PBS exhibit symptoms that fulfill the criteria for IBS.2, 3 In support of cross-communication between visceral organs, experiments in rodent models have demonstrated that colonic irritation is capable of producing such irregular micturition patterns as early onset of micturition and enhanced urethral sphincter activity in rats.4 In addition, there is evidence that active colonic inflammation induces abnormalities in bladder detrusor-muscle contractility5, and can result in vascular permeability in the bladder taken from female rats.6 Conversely, bladder irritation results in increased visceral sensitivity to colonic stimulation.7
The mechanisms for the overlap of symptomatology in patients with PBS and IBS are poorly understood; however, visceral organ communication may be a contributing variable. Evidence suggests that visceral organ crosstalk may be due to convergence of sensory neural pathways within the dorsal root ganglion (DRG), the spinal cord, and/or the brain.8 Specifically, dual labeling studies have revealed that approximately 3-15% of afferent nerve fibers innervating the bladder and colon are common to both structures.9 In the lumbosacral region of the spinal cord, approximately 30% of neurons respond to both bladder and colorectal stimulation.10 Current evidence also has pointed to sensitization of the afferent neurons at the DRG and lumbosacral neurons that become hyper-excitable following colonic inflammation.11 It thus is possible that afferent sensitization may play a pivotal role in visceral organ cross-communication. The possibility exists that activation of afferent nerves in response to mucosal damage may be the result of increased epithelial permeability allowing foreign substances to have direct access to the visceral sensory neurons. In support of this idea, previous studies have shown that inflammation of the urinary bladder can disrupt the integrity of the bladder within 24 hours and cause a marked reduction in transepithelial electrical resistance (TEER).12, 13 Similarly, previous studies have shown that damage to the colon via TNBS increases colonic permeability and afferent neuron activity in the colon.14, 15, 16, 17 Given that PBS and IBS are characterized by the presence of minimal- to low-grade inflammation, we performed this study to investigate the effect of an acute disruption of either the bladder urothelium or colonic mucosa on both bladder and colonic permeability to test the hypothesis that enhanced epithelial permeability represents a novel mechanism for visceral organ crosstalk, and may explain the overlapping symptomology of PBS and IBS.
MATERIALS AND METHODS
Animal Model
Female, OVX Sprague Dawley rats (220-250 g) were purchased from Charles River Laboratories. All animals had free access to food and water and were acclimated to facility housing for a minimum of one week before experimentation. The protocol was approved by the University of Oklahoma Health Sciences Center Institutional Animal Care and Use Committee (animal protocol − 11-162).
Study 1: Administration of Protamine Sulfate into the Bladder
Rats were anesthetized with isoflurane (3%) and oxygen for a period of approximately 10 minutes, and protamine sulfate was instilled transurethrally as previously described.13 Bladder and colon were harvested at 24 hours, 3 days, and 5 days after infusion of protamine sulfate. Non-catheterized OVX female rats served as naïve controls.
Bladder Damage Assessment
Segments of bladder were isolated, fixed, and blocked. Sections (10 μm) were histologically evaluated by a board-certified animal pathologist who was blinded to the treatment for signs of inflammation and damage.
Study 2: Administration of TNBS into the Colon
Rats were fasted overnight with free access to water, removed from the animal facility, and brought to the laboratory, where they were briefly anesthetized with isoflurane (3%) with a steady supply of oxygen. While sedated, the rats received an enema containing a solution of 50 mg/ml Trinitrobenzensulfonic acid (TNBS) diluted into 25% ethanol and 25% saline. The volume of TNBS solution infused into colon was adjusted so that each rat received 50 mg/kg TNBS. Controls were infused with 100% saline.
Disease Activity Index (DAI)
To determine the severity of TNBS-induced colonic inflammation, the rats were weighed and stools were graded for a period of five consecutive days following TNBS infusion. The DAI was calculated as previously described.18 The presence of blood within the feces was tested using an occult blood indicator test (Beckman Coulter Inc, Fullerton, CA).
Colonic Damage Assessment
Segments of colon tissue from each rat approximately, 1cm × 1 cm, were harvested. Tissues were embedded in 10% buffered formalin, processed, embedded in paraffin, and sectioned at 5μm thickness. They were then stained with haematoxylin and eosin and assessed under a light microscope for inflammation by a board-certified animal pathologist who was blinded to the treatment. Inflammatory parameters using a previously validated method were assessed.18
Bladder and Colonic Permeability Measurements
Following tissue isolation, the colon and bladder were opened longitudinally and mounted into either biopsy perfusion chambers (bladder) or modified Ussing chambers (colon). Organs were bathed in oxygenated Krebs solution at 37° C. Permeability was assessed electrophysiologically via measurement of trans-epithelial resistance (TEER). To calculate TEER, the potential difference (PD) and short circuit current (Isc) were recorded and used to calculate TEER. Ohm’s law was applied to PD and Isc.
Permeability also was assessed via the movement of a macromolecular marker, fluorescein isothiocynate-destrin (FITC-4). In these experiments, FITC-4 was added to the urothelial (bladder) or mucosal (colon) side of the preparations to determine macromolecular flux using fluorescence spectroscopy.
Experimental Design
A schematic of the experimental design is shown in Figure 1. Rats were allowed to acclimate from the shipping stress for at least 7 days. Animals were then divided into two groups: Group 1 received protamine sulfate infusions (or non-treated catheter sham controls) into the bladder, whereas Group 2 received intra-colonic TNBS, with controls receiving intra-colonic saline. Each group was then subdivided into 3 time points following either the protamine sulfate into the bladder or TNBS infusions into the colon: day 1, day 3 and day 5 post-infusion. At each time point, rats (n = 4-7/group) were euthanized and the bladder and colon removed for histology and assessments of permeability.
Figure 1.
A schematic illustration of the experimental design. Rats were acclimated to the facility for seven days and then divided into two groups. Group 1: protamine sulfate (non-treated rats served as controls) was infused into the bladder; animals were euthanized 1, 3, or 5 days later. Bladder and colon were harvested for permeability measurements and assessment of damage. Group 2: TNBS was infused into the colon (saline-treated rats served as controls). For each rat, a daily disease activity index (DAI) was calculated following the TNBS infusion. Rats were then euthanized 1, 3, or 5 days post-TNBS infusion. Bladder and colonic tissue were harvested for permeability measurements and assessment of tissue damage.
Chemicals and Solutions
All chemicals were obtained from SIGMA-ALDRICH (St. Louis, MO) and were prepared fresh prior to use. Protamine sulfate was dissolved in saline at 1 mg/ml. TNBS was diluted in ethanol and saline as previously described. FITC-4 (4kDa) at a concentration of 1 mg/ml was dissolved in Krebs solution. Krebs solution was made as follows: 120 mM NaCl, 6 mM KCl, 1.2 mM MgCl2, 1.2 mM H2PO4, 2.5 mM CaCl2, 14.4 mM NaHCO3, and 11.5 mM glucose.
Statistical Analysis
Data was collected manually from MacLab software and transferred to a Microsoft Excel spreadsheet. Values were expressed as mean ± standard error of the mean (SEM). Analyses were performed using GraphPad Prism (GraphPad Software, USA), and for all data a 95% confidence interval was used as a measure of statistical significance. Statistical significance was determined using One-Way ANOVA with Tukey’s post-hoc multi-comparison test for the protamine sulfate treated experiments and unpaired t-test for the TNBS treated experiments. A *p <0.05 was considered statistically significant in all tests.
RESULTS
Study 1: Effects of protamine sulfate infusion into the urinary bladder
Acute urinary bladder permeability was induced by exposure of the urinary bladder to protamine sulfate. We observed minimal urothelial damage as assessed histologically at all time points (day 1, day 3, and day 5 post protamine sulfate infusion (Figure 2A-D). Protamine sulfate infusion into the bladder was not associated with any histological changes in the colonic mucosa (Figure 2E-H).
Figure 2.
Bladder histology at 1 day (A), 3 days (B) and 5 days (C) following protamine sulfate infusion into the urinary bladder. The bladder from protamine sulfate-treated rats showed signs of edema at 1-5 days, but the urothelium remained intact with no damage to the umbrella cells. Bladder tissue from untreated control rats also is shown (D). Reduced from ×40. Colonic histology from protamine sulfate-treated rats showed no damage to the colon at any time point compared to controls (E-H). Reduced from ×20.
Urinary Bladder Permeability
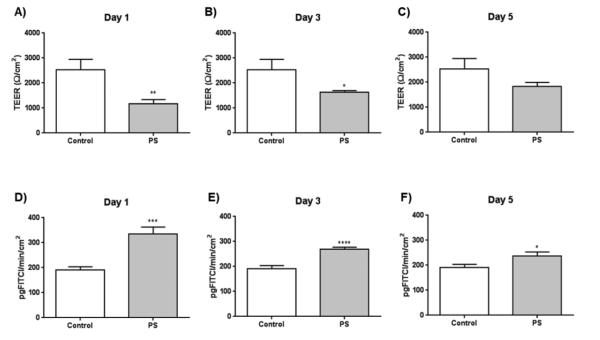
At 1 and 3 days post-infusion, a significant decrease in urinary bladder TEER in protamine sulfate-treated urinary bladders was observed as compared to controls (Figure 3A and B). At 1, 3, and 5-day post-infusion, there was a significant increase of macromolecular flux of FITC dextran in the protamine sulfate-infused urinary bladder compared to that observed in the urinary bladder from control rats (Figure 3D-F). However, at 5 days post-infusion, there was no significant difference between the TEER (Figure 3C) across the urinary bladder.
Figure 3.
Bladder permeability assessments were collected at 1-5 days after urinary-bladder infusion of protamine sulfate. The urinary bladder TEER was significantly decreased from protamine sulfate-treated rats on day 1 (A) and day 3 (B), but was not significantly different from controls by day 5 (C). The macromolecular flux of FITC-dextran across the urinary bladder from protamine sulfate-treated rats was significantly increased at all time points (D-F) (*p<0.05; **p<0.01; ***p<0.001; ****p<0.0001 ) compared to controls. At each time point, rats n = 5-7/group were used.
Colonic Permeability
At 1, 3, and 5 days post urinary-bladder infusion, a significant decrease of colonic TEER was observed in the colon isolated from rats treated intravesically with protamine sulfate when compared to naïve control rats (Figure 4A-C). A significant increase in macromolecular flux was observed on days 1, 3, and 5 across the colon isolated from protamine sulfate treated rats as compared to control rats (Figure 4D-F).
Figure 4.
Permeability assessments were collected from the undamaged colon at 1-5 days after urinary bladder infusion of protamine sulfate. The colonic TEER was significantly decreased at all time points (A-C), and the colonic macromolecular flux significantly increased at all time points (E-F) (*p<0.05; **p<0.01; ***p<0.001; ****p<0.0001) compared to controls. At each time point, rats n = 5-7/group were used.
Study 2: Effects of TNBS infusion into the colon
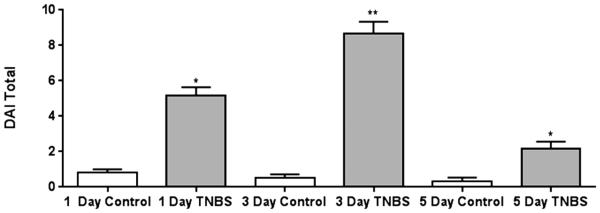
A significant increase in DAI following the TNBS enema was observed due to the presence of blood in the feces, a decrease in body weight gain, and altered stool consistency. TNBS-treated rats showed a significantly higher DAI (p<0.05) compared to controls (Figure 5).
Figure 5.
Daily Disease Activity Index (DAI) following intra-colonic TNBS. Filled bars indicate rats treated with TNBS. Open bars represent control mice. There was a significant increase in the DAI in TNBS-treated rats compared to paired controls at all time points, with the highest level of severity occurring at 3 days post-TNBS infusion *p<0.05,**p<0.01 compared to controls.
At the first and second time points assessed after intra-rectal administration of TNBS (1- and 3-day post-TNBS), histological analysis revealed damage of the colon characterized by increased ulceration, fibrosis, lesion depth, and production of inflammatory infiltrates (Figure 6A and B) compared to controls (Figure 6D). At day 5 post-TNBS administration, the amount of damage had decreased to a lesser degree, but was still present (Figure 6C) compared to controls (Figure 6D). Following TNBS infusion and the activation of colonic inflammation, no changes in urinary bladder histology were observed (Figure 6E-H).
Figure 6.
Colonic histology at 1 day (A), 3 days (B), and 5 days (C) following TNBS infusion into the colon compared to controls (D). Reduced from ×20. On days 1 and 3, there was a significant increase in inflammatory infiltrate, and by day 5 there was still a slight increase in colonic damage as seen by areas of surface epithelial disruption, ulceration, fibrosis, and increased inflammatory infiltrate. Histological assessment from TNBS-treated rats showed no damage to the bladder at any time point compared to controls (E-H). Reduced from ×20.
Colonic Permeability
In the colon from TNBS-treated rats, there was a significant decrease in TEER after 1 day (p<.01) and 3 days (p<.0001) compared to saline-treated controls (Fig. 7A and B), but not at day 5 (Fig. 7C). Concurrently, a significant increase in the macromolecular flux of FITC-dextran across the colonic membrane was seen following TNBS administration at all time points (Fig. 7D-F).
Figure 7.
Colon permeability assessments were collected at 1-5 days post-infusion of intra-colonic TNBS. The colon TEER was significantly decreased from TNBS treated rats on day 1 (A) and day 3 (B), but was not significantly different from controls by day 5 (C). The macromolecular flux of FITC-dextran across the colon from TNBS-treated rats was significantly increased at all time points (D-F) *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 compared to controls. At each time point, rats n = 4-7/group were used.
Urinary Bladder Permeability
In response to TNBS-induced infusion into the colon, the TEER in the urinary bladder significantly decreased after 1 day (p<.0001), 3 days (p<.0001), and 5 days (p<.0001) (Fig. 8A-C). Further, the bladder FITC-dextran flux significantly increased after 1 day (p<.05) and 3 days (p<.01) when compared to saline-treated controls (Fig. 8D and E), but there was not a significant increase after 5 days (Figure 8F).
Figure 8.
Permeability assessments were collected from the undamaged bladder at 1-5 days post-infusion of intra-colonic TNBS. The bladder TEER was significantly decreased at all time points (A-C), and the colonic macromolecular flux significantly increased at day 1 (D) and day 3 (E), but showed recovery by day 5 (F) (*p<0.05; **p<0.01; ***p<0.001; ****p<0.0001) compared to controls. At each time point, rats n = 4-7/group were used.
DISCUSSION
IC/PBS and IBS share overlapping symptoms, that may be attributable to visceral organ cross-sensitization.2, 4, 8 Given that there is growing evidence suggesting that increases in permeability play a pathogenic role in functional bowel disorders such as IBS, it was our hypothesis that changes in epithelial permeability represent a novel mechanism for visceral organ crosstalk. In support, the current study provides compelling evidence that pathology in one visceral organ is capable of producing functional changes in another. Specifically, we found that damage to one visceral organ can lead to changes in permeability not only in the damaged organ, but also in a visceral organ that is remote from the damaging insult. Key findings from the present study are include: 1) protamine sulfate infusion into the urinary bladder increases bladder permeability, at the same time causing a significant increase in colonic permeability in the absence of histological damage to the colon; 2) colonic infusion of TNBS caused significant increases in both colonic and bladder permeability in the absence of any histological damage to the urinary bladder. This new data suggests that changes in epithelial permeability represent a novel mechanism for visceral organ crosstalk that may explain the overlapping symptomology of PBS and IBS. However, a note of clarification when extrapolating our findings to the clinical situation is their they relevance not only to IBS patients with a low grade colonic inflammation but also to inflammatory bowel disease (IBD) since we found a significant increase in urinary bladder permeability in the presence of colonic inflammation.
In the current study, permeability in the colon was assessed in Ussing chambers as previously described by our and many others’ laboratories19; however, to assess urinary bladder permeability, we developed a technique using small, biopsy-sized tissue samples placed in modified Ussing chamber preparations based upon original work of Negrete et al., (1996)20 and Lavelle et al. in 199812. In both preparations, permeability was rapidly assessed electrophysiologically via transepithelial potential difference (PD) and short circuit current (Isc) measurements to calculate transepithelial electrical resistance (TEER) via Ohm’s law. We found that TEER could directly be correlated with the flux across the bladder (and colonic) tissue of a macromolecular marker (FITC-dextran). Employing both approaches we found that acute urinary bladder permeability was induced by exposing the urinary bladder to low concentrations of protamine sulfate. This methodology is unique because it induced minimal damage to the urothelium; specifically, the umbrella cells remained intact and only a mild edema was induced by 24 hours after protamine sulfate treatment. In a recent study in which protamine sulfate infusion into the bladder led to only modest tissue damage, the authors reported that protamine treatment of the bladder was analgesic upon bladder distension in mice, and even suggested that protamine sulfate may be protective to the bladder.21 In the current study, we observed modest urothelial damage and a transient urinary bladder permeability that resolved within five days. A novel and important aspect to our study was that the induction of urinary bladder permeability following protamine sulfate infusion was at the same time associated with a marked disruption in colonic barrier function in the absence of any alterations to the histological appearance of the colon. This finding is supported by a recent study where we used contrast-enhanced magnetic resonance imaging (CE-MRI) to monitor a loss of permeability in rat bladder urothelium and visceral organ crosstalk, which was measured as secondary enhanced contrast of the colon mucosa within 24 hours following exposure of the bladders to protamine sulfate.22
Conversely, in another series of experiments, we investigated the reverse effect of an acute colonic inflammation on bladder function. Colonic inflammation was induced by infusion of TNBS into the colon, which is an established technique for inducing a transient colitis, which lasts between five and 10 days.23 In this model, we have previously reported that colonic inflammation induced by TNBS insult leads to hyperexcitability of convergent colonic and bladder dorsal root ganglion neurons,11 attenuates the amplitude of detrusor muscle contractions in vitro,5 and (as shown in the current study) increases bladder permeability. Of significance, these changes in urinary bladder function are reversible following recovery from the colonic inflammation.5 However, the effect of long-term colonic inflammation on urinary bladder function is unknown, and is the focus of our ongoing studies.
Our studies using TEER and the macromolecular flux of FITC-dextran revealed that the colon is more permeable than the bladder. Such differences in permeability reflect the function of the organ, with the colon being required to absorb water and electrolytes across the permeable barrier, whereas the bladder is a much tighter urothelial barrier to prevent the flux of urinary solutes. Movement across the intestinal barrier is complex and includes a transcellular route for lipophilic and small hydrophilic compounds, another transcellular route via aqueous pores as well as active carrier-mediated absorption for nutrients and electrolytes and endocytosis, followed by transcytosis and exocytosis for peptides. Between intestinal epithelial cells tight junction proteins such as the claudins, occludins, and zonula occludens play a pivotal role in permeability. 24 This complexity in colonic permeability may explain the discrepancy between the FITC-dextran and TEER on day 5 post TNBS when there was a significant increase in the macromolecular flux of FITC-dextran but no change in TEER. In the urinary bladder-barrier function, the urothelium forms a complex barrier. Key to this barrier are the umbrella cells.25 In the urinary bladder, uroplakins located on the apical surface of the umbrella cells restrict trans-cellular permeability, whereas tight junction proteins between umbrella cells control paracellular permeability. 25 In the current study, we have demonstrated that enhanced permeability in one visceral organ can affect permeability in another; however, due to the complexity of each barrier, characterizing the barrier defect in each organ is beyond the scope of the current study, but represents an area of active investigation for our laboratory.
IC/PBS and IBS are more commonly seen in women; thus in the current study we selected to use female rats. However, in pilot experiments (data not shown), we discovered that in cycling females, the permeability measurements were extremely variable. In a desire to reduce the number of rats used in the study, we opted to use OVX female rats. However, a note of caution in the interpretation of our data is that previous studies have shown increases in bladder permeability in OVX rabbits26, which may be due to a reduction in estrogen levels. In support, in vivo bladder permeability measurement showed significantly higher bladder permeability in female estrogen β receptor deficient mice compared to their male counterparts.27 In the current study, although we have shown that inducing an increase in urinary bladder or colon permeability rapidly induces permeability in the other, the potential mechanisms responsible for the increase in permeability and the pathways for visceral organ crosstalk remain to be resolved. However, it is likely that estrogen plays a role is cross-organ sensitization via a mechanism involving P13K, which is required for the downstream activation of estrogen-receptor signaling in a model of intra-colonic mustard oil-induced visceral organ cross-sensitization in female rats.28
CONCLUSIONS
There is well-known comorbidity between PBS/IC and IBS; however, there is limited knowledge of the mechanisms that link these conditions. In the current study, we investigated visceral organ crosstalk between the urinary bladder and the colon. We demonstrated that induction of permeability in the bladder induces increased permeability in the colon, and that inflammation of the colon likewise induces permeability in the urinary bladder. The finding that inducing an increase in urinary bladder or colon permeability rapidly induces permeability in the other organ supports the notion that altered permeability may be responsible for visceral organ cross-talk.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the thoughtful comments and suggestions on the final drafts of this manuscript from Dr. Amy Wisniewski, Tara Malone and Dr. Brad Kropp. Financial support for the research was provided in part by an NIH P20 Grant to RH.
Abbreviations
- PBS
painful bladder syndrome
- IBS
irritable bowel syndrome
- IC
interstitial cystitis
- TNBS
trinitrobenzene sulfonic acid
- FITC
Fluorescein isothiocyanate
- TEER
transepithelial electrical resistance
- PD
potential difference
- Isc
Short circuit current
- R
resistance
- OVX
ovariectomized
Footnotes
Authorship Contribution
Participated in research design: BGVM, RH, RT
Conducted experiments: EM, KT, SVG, AP
Contributed new reagents or analytic tools: EM
Performed data analysis: KT, EM, BGVM, AP
Wrote or contributed to the writing of the manuscript: BGVM, EM, AP
REFERENCES
- 1.Held PJ, Hanno PM, Wein AJ, et al. Epidemiology of interstitial cystitis: 2. In: Hanno PM, Staskin DR, Krane RJ, et al., editors. Interstitial Cystitis. Springer; London, UK: 1990. p. 29. [Google Scholar]
- 2.Ustinova EE, Fraser MO, Pezzone MA. Colonic irritation in the rat sensitizes urinary bladder afferents to mechanical and chemical stimuli: an afferent origin of pelvic organ cross-sensitization. Am J Physiol Renal Physiol. 2006;290:1478. doi: 10.1152/ajprenal.00395.2005. [DOI] [PubMed] [Google Scholar]
- 3.Nickel JC, Tripp DA, Pontari M, et al. Interstitial Cystitis/painful bladder syndrome and associated medical conditions with an emphasis on irritable bowel syndrome, fibromyalgia and chronic fatigue syndrome. J Urol. 2010;184:1358. doi: 10.1016/j.juro.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Pezzone MA, Liang R, Fraser MO. A Model of Neural Cross-Talk and Irritation in the Pelvis: Implications for the Overlap of Chronic Pelvic Pain Disorders. Gastroenterology. 2005;128:1953. doi: 10.1053/j.gastro.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Noronha R, Akbarali H, Malykhina A, et al. Changes in urinary bladder smooth muscle function in response to colonic inflammation. Am J Physiol Renal Physiol. 2007;293:1461. doi: 10.1152/ajprenal.00311.2007. [DOI] [PubMed] [Google Scholar]
- 6.Winnard KP, Dmitrieva N, Berkley KJ. Cross-organ interactions between reproductive, gastrointestinal, and urinary tracts: modulation by estrous stage and involvement of the hypogastric nerve. Am J Physiol Regul Integr Comp Physiol. 2006;291:1592. doi: 10.1152/ajpregu.00455.2006. [DOI] [PubMed] [Google Scholar]
- 7.Bielefeldt K, Lamb K, Gebhart GF. Convergence of sensory pathways in the development of somatic and visceral hypersensitivity. Am J Physiol Gastrointest Liver Physiol. 2006;291:658. doi: 10.1152/ajpgi.00585.2005. [DOI] [PubMed] [Google Scholar]
- 8.Malykhina AP. Neural mechanisms of pelvic organ cross-sensitization. Neuroscience. 2007;149:660. doi: 10.1016/j.neuroscience.2007.07.053. [DOI] [PubMed] [Google Scholar]
- 9.Christianson JA, Liang R, Ustinova EE, et al. Convergence of bladder and colon sensory innervation occurs at the primary afferent level. Pain. 2007;128(3):235–43. doi: 10.1016/j.pain.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qin C, Foreman RD. Viscerovisceral convergence of urinary bladder and colorectal inputs to lumbosacral spinal neurons in rats. Neuroreport. 2004;15(3):467. doi: 10.1097/00001756-200403010-00017. [DOI] [PubMed] [Google Scholar]
- 11.Malykhina AP, Qin C, Greenwood-van Meerveld B, et al. Hyperexcitability of convergent colon and bladder dorsal root ganglion neurons after colonic inflammation: mechanism for pelvic organ cross-talk. Neurogastroenterol Motil. 2006;18:936. doi: 10.1111/j.1365-2982.2006.00807.x. [DOI] [PubMed] [Google Scholar]
- 12.Lavelle JP, Apodaca G, Meyers SA, et al. Disruption of guinea pig urinary bladder permeability barrier in noninfectious cystitis. Am J physiol. 1998;274:205. doi: 10.1152/ajprenal.1998.274.1.F205. [DOI] [PubMed] [Google Scholar]
- 13.Lavelle J, Meyers S, Ramage R, et al. Bladder permeability barrier: recovery from selective injury of surface epithelial cells. Am J Physiol Renal Physiol. 2002;283:242. doi: 10.1152/ajprenal.00307.2001. [DOI] [PubMed] [Google Scholar]
- 14.Han X, Ren X, Jurickova I, et al. Regulation of intestinal barrier function by signal transducer and activator of transcription 5b. Gut. 2009;58:49. doi: 10.1136/gut.2007.145094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suckow SK, Caudle RM. NMDA subunit expression and PAR2 receptor activation in colospinal afferent neurons (CANs) during inflammation induced visceral hypersensitivity. Mol Pain. 2009;5:54. doi: 10.1186/1744-8069-5-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu SJ, Grider JR, Gulick MA, et al. Up-regulation of brain derived neurotrophic factor is regulated by extracellular signal-regulated protein kinase 5 and nerve growth factor retrograde signaling in colonic afferent neurons in colitis. Exp Neurol. 2012;238:209. doi: 10.1016/j.expneurol.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Welch MG, Margolis KG, Zhishan L, et al. Oxytocin regulates gastrointestinal motility, inflammation, macromolecular permeability, and mucosal maintenance in mice. Am J Physiol Gastrointest Liver Physiol. 2014;307:848. doi: 10.1152/ajpgi.00176.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenwood-Van Meerveld B, Tyler KR. CPI-1189 protects against dextran sulfate sodium-induced colitis in mice. Am J Pharma and Tox. 2006;1:54. [Google Scholar]
- 19.Braniste V, Leveque M, Buisson-Brenac C, et al. Oestradiol decreases colonic permeability through oestrogen receptor beta-mediated up-regulation of occludin and junctional adhesion molecule-A in epithelial cells. J Physiol. 2009;587:3317. doi: 10.1113/jphysiol.2009.169300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Negrete HO, Lavelle JP, Berg J, et al. Permeability properties of the intact mammalian bladder epithelium. Am J Physiol. 1996;271:886. doi: 10.1152/ajprenal.1996.271.4.F886. [DOI] [PubMed] [Google Scholar]
- 21.Stemler KM, Crock LW, Lai HH, et al. Protamine Sulfate-induced Bladder Injury is Protective from Distention-induced Bladder Pain. J Urol. 2013;189:343. doi: 10.1016/j.juro.2012.08.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Towner RA, Smith N, Saunders D, et al. Contrast-enhanced magnetic resonance imaging (CE-MRI) as a diagnostic tool for assessing bladder permeability and associated colon cross-talk: pre-clinical studies in a rat model. J Urology. 2014 doi: 10.1016/j.juro.2014.10.120. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deiteren A, Vermeulen W, Moreels TG, et al. The effect of chemically induced colitis, psychological stress and their combination on visceral pain in female Wistar rats. Stress. 2014;17:431. doi: 10.3109/10253890.2014.951034. [DOI] [PubMed] [Google Scholar]
- 24.Camilleri M, Madsen K, Spiller R, et al. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol Motil. 2012;24:503. doi: 10.1111/j.1365-2982.2012.01921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewis SA. Everything you wanted to know about the bladder epithelium but were afraid to ask. Am J Physiol Renal Physiol. 2000;278:867. doi: 10.1152/ajprenal.2000.278.6.F867. [DOI] [PubMed] [Google Scholar]
- 26.Hass MA, Nichol P, Lee L, et al. Estrogen modulates permeability and prostaglandin levels in the rabbit urinary bladder. Prostaglandins Leukot Essent Fatty Acids. 2009;80:125. doi: 10.1016/j.plefa.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 27.Imamov O, Yakimchuk K, Morani A, et al. Estrogen receptor beta-deficient female mice develop a bladder phenotype resembling human interstitial cystitis. Proc Natl Acad Sci U S A. 2007;104:9806. doi: 10.1073/pnas.0703410104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng H-Y, Chen G-D, Lai C-Y, et al. PI3K modulates estrogen-dependent facilitation of colon-to-urethra cross-organ sensitization in ovariectomized female rats. J. Neurochem. 2010;113:54. doi: 10.1111/j.1471-4159.2010.06577.x. [DOI] [PubMed] [Google Scholar]