Abstract
Routine incorporation of blood-based biomarker measurements in population studies has been hampered by challenges in obtaining samples suitable for biomarker assessment outside of laboratory settings. Here, we assessed the suitability of venous blood left unprocessed for four, 24 or 48 hours post-collection at either room temperature or 4°C for quantification of two biomarkers, Interleukin-6 (IL-6) and C-Reactive Protein (CRP). Blood samples were collected in both K2EDTA tubes and a dedicated plasma-preservation tube, P100. Dried Blood Spot (DBS) samples from the same subjects were also collected in order to compare delayed-processing plasma performance against a popular alternative collection method. K2EDTA mean plasma concentrations of both IL-6 and CRP were not significantly different from concentrations in plasma processed immediately; this was observed for tubes stored up to 48 hours pre-processing at either temperature. Concentrations of IL-6 measured in P100 tubes showed significant time-dependent increases when stored at room temperature; otherwise, levels of IL-6 and CRP were similar to those processed immediately. Levels of CRP in DBS were correlated with plasma CRP levels, even when pre-processed blood was stored for up to 48 hours. These data indicate that plasma is suitable for IL-6 and CRP estimation under data-collection conditions that involve processing delays.
Introduction
Biomarker measurement is of great interest, as research progressively demonstrates the importance of biomolecules as both predictors and consequences of health outcomes (Crimmins, et al., 2010). Of particular interest are biomolecules related to inflammation, such as Interleukins and C-Reactive Protein (CRP), which are measured in isolated blood and their products, including plasma. Historically, the handling and processing requirements of whole blood samples destined for plasma-based analyses have proven a barrier to incorporation of blood collection in large population-based studies. A key concern is that separation of the plasma fraction soon after blood draw is necessary to prevent stimulation and/or degradation of biomarkers. Best practices suggest that blood should be processed into plasma and other blood products within 4 hours of collection for optimal preservation of biomarker profiles (e.g. (Tuck, et al., 2009)). Since many population-based studies are conducted remotely to a processing laboratory, it is difficult or impossible to deliver blood samples for processing in a timely fashion. Alternative strategies, such as use of mobile processing laboratories or blood collection in central clinical facilities, are logistically challenging and prohibitively costly. As such, identification and validation of a blood collection strategy that prolongs this pre-processing timeframe could open up the possibility of assessing biomarkers in large, population-representative studies.
Plasma is usually derived from blood collected in blood tubes containing preservatives such as K2EDTA and citrate. These tubes are popular as they are widely available and relatively inexpensive. Contrary to the ‘best-practice’ notion discussed above, a variety of research suggests that certain plasma biomarkers derived from these tubes show temporal and thermal stability under conditions outside of the optimal processing timeframe (Flower, et al., 2000, Skogstrand, et al., 2008). However, this is not universally applicable across the complete range of available biomarkers, and this inconsistency has prompted researchers to develop alternative strategies for collection.
One strategy is to rely on specialized collection tubes. Currently, there are no commercial products available that can preserve unprocessed blood intended for plasma isolation over prolonged periods. However, there do exist products intended to preserve plasma profiles in processed blood; i.e. blood samples require some immediate post-blood draw processing, usually centrifugation. One such product, the P100 tube available from Becton Dickinson (BD), contains K2EDTA plus propriety additives intended to stabilize plasma biomarker profiles in post-centrifuged blood for up to five days at room temperature. The stability of biomarker profiles in blood left un-processed for this period of time has not been assessed, but these tubes could be an attractive alternative if demonstrated to preserve profiles comparably.
A second strategy is to collect substrates other than plasma. A popular alternative method of collecting blood under field conditions is via dried bloodspots (DBS) (McDade, et al., 2007, Danese, et al., 2011, Crimmins, et al., 2014, Danese, et al., 2014). The main advantages of this method is that a) it is minimally invasive, and b) DBS are easier to handle than whole blood, and purport to preserve biomarker profiles under non-restrictive time and temperature conditions. The main disadvantage is that a smaller sample volume is obtained, which can limit the number of biomarkers that can be analyzed.
We sought to assess the stability of two commonly measured plasma biomarkers, IL-6 and CRP, across a variety of pre-processing blood handling conditions. We aimed to mimic common conditions encountered in a field-based population study; that is 1) time periods between collection and processing ranging from 0–48 hours; and 2) sample storage temperatures of either 4°C or room temperature. These conditions were executed in parallel in both K2EDTA and P100 tubes. In addition, we compared CRP levels in DBS to the levels in plasma samples from the same subjects in order to confirm the applicability of this method as an alternative collection strategy.
Methods
Blood collection and processing
Venous blood samples were collected from six volunteers by a trained phlebotomist using standard blood collection apparatus. Blood was collected into either 3ml K2EDTA Vacutainer tubes (BD, Franklin Lakes, NJ) or P100 tubes (BD) in series and anonymized. After the necessary initial inversion, tubes were incubated, without further agitation, according to the experimental protocol outlined in Figure 1. After the appropriate incubation period, tubes were processed by centrifugation at 2,000g for 10 minutes (K2EDTA) or 2,500g for 20 minutes (P100), and the plasma fraction was transferred into 2ml cryovials. Tubes selected for 0 hour processing (hereafter termed ‘baseline’) were centrifuged immediately upon collection. All plasma was stored at −80°C until analysis.
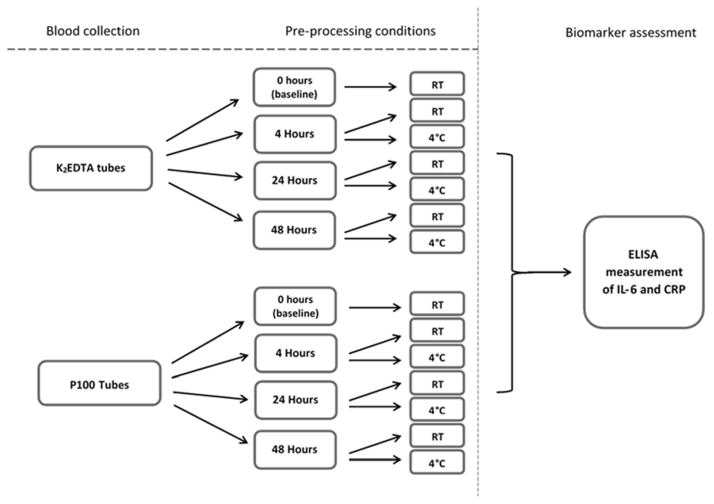
Figure 1.
Diagrammatic representation of blood pre-processing conditions employed in the current investigation. Fourteen separate experimental conditions (tubes of whole blood) were collected per subject. Processing consisted of centrifugation of whole blood and collection of plasma fraction for downstream biomarker measurement. Briefly, tubes selected for room temperature (RT) incubation were placed in racks within the laboratory at ambient temperature (22–23°C) for the allotted period of time. Tubes selected for 4°C incubation were placed within a laboratory refrigerator for the allotted time. The designated incubation periods were 4, 24 and 48 hours.
In addition, DBS samples were collected from each volunteer via the use of Whatman Protein Saver Cards (GE Healthcare, Pittsburgh, PA), as described previously (Danese, et al., 2011). Briefly, a lancet was used to pierce the side of the middle fingertip, and a series of five blood spots was collected. After collection, cards were air-dried until dry (minimum of 30 minutes) before being transferred to an airtight box and placed at −20°C until analysis. The DBS samples were prepared for analysis as follows: one 3mm punch of the fourth spot on the Protein Saver card was taken per subject; these spots were placed into 250 μl of DBS elution buffer (1xPBS (Sigma Aldrich, St. Louis, MO), 0.05% Tween 20 (Sigma Aldrich)). Spots were allowed to elute for 18 hours at 4°C. Prior to assaying, eluted DBS samples were placed on a shaker for 1 hour.
Biomarker measurement
Levels of IL-6 and CRP were determined using human IL-6 and CRP Quantikine ELISA kits (R&D, Minneapolis, MN) following manufacturer’s instructions. Briefly, 100 μL (IL-6) or 50μL (CRP) of sample were used per reaction. For CRP analysis, plasma was diluted 1 in 100 in calibrator diluent prior to assaying as recommended by the manufacturer, whilst eluted DBS samples remained undiluted. For IL-6 analysis, plasma samples were used neat. All ELISA reactions were performed in duplicate. A 2-fold serial dilution of a manufacturer-provided standard was included, the lowest being a sample blank. Upon completion of the reactions, OD readings at 450nm (correcting for background at 540nm) were read using a Spectramax-384 spectrophotometer (Molecular Devices, Sunnydale, CA) and data acquired using SoftMax Pro software (Molecular Devices). Raw OD units were transformed by subtracting the mean OD value of the sample blank, and a standard curve was calculated using 4-parameter polynomial regression. The resulting equation was used to extrapolate sample OD values into concentrations. For plasma CRP, these concentrations were multiplied by the initial dilution factor (1 in 100) to derive an estimate of plasma concentration. Duplicate values were then averaged to derive a mean concentration per sample. DBS samples were assayed for CRP only due to hypothesized levels of circulating IL-6 falling below the limit of detection of our assay. Plasma equivalent concentrations of CRP in DBS samples were calculated by regressing uncorrected values against the concentration measured in plasma at baseline (Stockl, et al., 1998, McDade, 2014). The mean within-assay coefficient of variation (%CV) for IL-6 measurements was 4.13 percent, and for CRP measurements 3.34 percent.
Statistical analysis
The effects of average time to processing, temperature and tube-type on biomarker concentrations were calculated and tested using paired-sample t-tests and repeated measures ANOVA in SPSS version 22 (IBM, New York, NY).
Results
Measurement of IL-6 and CRP
In K2EDTA-derived plasma, baseline concentrations of IL-6 and CRP were 0.99±0.50 pg/mL (mean±SD) and 0.87±0.46 mg/L, respectively. In P100-derived plasma, those values were 1.00±0.47 pg/mL and 0.87±0.44 mg/L. For both analytes, baseline levels were highly correlated between the two tube types (r = 0.99, p <0.01), suggesting no initial differences in concentrations relating to blood collection procedures.
Does length of time between blood collection and plasma processing influence IL-6 and CRP concentrations?
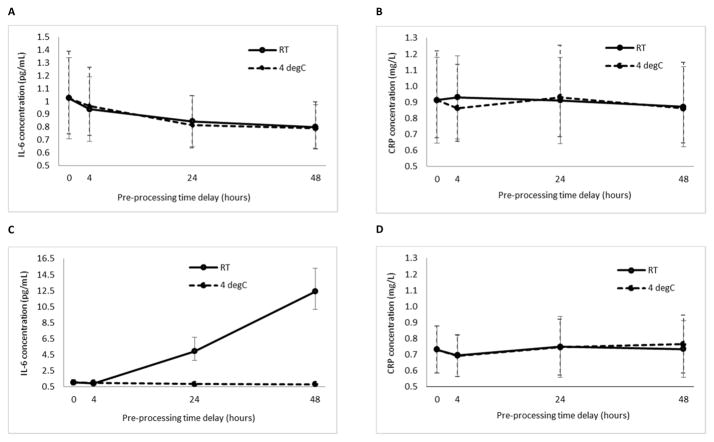
Concentrations of both IL-6 and CRP in plasma derived from K2EDTA tubes were not significantly affected by time-course whether stored at room temperature (RT; 22–23°C) or 4°C (Figs. 2A and 2B). Concentrations of both biomarkers in plasma derived from P100 tubes were not significantly affected by time-course when stored at 4°C. At RT, however, there was a significant rise in IL-6 concentration over the time-course (Fig. 2C). This was not observed for CRP (Fig. 2D).
Figure 2.
Timecourse profile of mean IL-6 and CRP concentrations in plasma from blood collected in K2EDTA (A and B) and P100 (C and D) tubes. For blood stored in K2EDTA tubes, a repeated measures ANOVA (with Greenhouse-Geisser correction) showed that time-course did not significantly affect mean IL-6 concentrations at either RT (F(1.01,3.03) = 1.45, p =0.32) or 4°C (F(1.12,4.49) = 3.63, p = 0.12; fig. A). Similarly, mean CRP concentrations were not significantly affected by time-course at either RT (F(3,9) = 0.75, p = 0.55) or 4°C (F(3,12) = 1.31, p = 0.32; Fig. B). For blood stored in P100 tubes at 4°C, the same analysis showed that time-course did not affect IL-6 concentrations (F(1.92,9.60) = 1.79, p = 0.22; Fig. C). However, storage at RT results in significantly elevated estimates of IL-6 concentrations (note the y-axis scale of Fig. C); mean concentrations differed significantly between time points (F(1.26,6.32) = 25.77, p < 0.01). The same analysis determined that for P100 tubes, time-course did not affect mean concentrations of CRP at either RT (F(3,15) = 0.27, p = 0.85) or 4°C (F(3,12) = 0.99, p = 0.43; Fig. D). Error bars represent SEM. Plots are derived from subjects with full complement of data points for comparison purposes.
Does storage temperature have an effect on IL-6 and CRP concentrations?
For this analysis we compared blood stored at RT and 4°C, within the same tube type and for the same length of time pre-processing. For blood stored in K2EDTA tubes, mean differences in IL-6 concentration did not differ significantly between RT and 4°C incubation (Table 1, Panel A; Fig. 2A). In contrast, IL-6 concentrations in blood stored in P100 tubes were significantly different between incubation temperature conditions (Fig. 2C), explained by the time-course profile described above.
Table 1.
Comparison of mean IL-6 concentrations between different tube types stored at different temperatures. Concentrations of IL-6 are not significantly different between room temperature (RT) and 4°C incubated K2EDTA tubes at any time period; however, there is a significant increase in IL-6 in RT incubated P100 tubes compared with 4°C tubes over time (Panel A; significant differences are marked in bold). At 4°C, concentrations of IL-6 do not differ significantly between K2EDTA and P100 tubes (Panel B).
| PANEL A | PANEL B | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IL-6 | K2EDTA | P100 | 4°C | |||||||
| Comparison | 4 hrs incubation, RT vs 4°C | 24 hrs incubation, RT vs 4°C | 48 hrs incubation, RT vs 4°C | 4 hrs incubation, RT vs 4°C | 24 hrs incubation, RT vs 4°C | 48 hrs incubation, RT vs 4°C | baseline, K2EDTA vs P100 | 4 hrs incubation, K2EDTA vs P100 | 24 hrs incubation, K2EDTA vs P100 | 48 hrs incubation, K2EDTA vs P100 |
| Mean fold change (SEM) | 0.98 (0.02) | 1.04 (0.03) | 1.01 (0.04) | 0.85 (0.05) | 4.70 (0.72) | 15.18 (1.94) | 0.99 (0.05) | 0.95 (0.04) | 0.95 (0.04) | 0.91 (0.05) |
| Mean difference (SEM) | −0.02 (0.02) | 0.03 (0.03) | 0.00 (0.04) | −0.17 (0.08) | 3.44 (1.08) | 11.72 (2.16) | 0.00 (0.03) | −0.04 (0.04) | −0.03 (0.02) | −0.06 (0.04) |
| 95% CI of difference (lower, upper) | −0.06, 0.03 | −0.07, 0.13 | −0.10, 0.10 | −0.36, 0.03 | 0.66, 6.21 | 6.17, 17.23 | −0.09, 0.08 | −0.15, 0.07 | −0.09, 0.02 | −0.18, 0.05 |
| Paired t (df) | −0.98 (4) | 0.96 (3) | 0.70 (4) | −2.22 (5) | 3.18 (5) | 5.42 (5) | −0.06 (5) | −1.08 (4) | −1.67 (4) | −1.50 (4) |
| P | 0.38 | 0.41 | 0.95 | 0.08 | 0.03 | <0.01 | 0.96 | 0.34 | 0.17 | 0.21 |
Regardless of pre-processing incubation time, there were no significant mean differences in CRP concentration between blood stored at RT and 4°C within either tube type (Table 2, Panel A). All paired sample comparisons were highly correlated (p < 0.01; Fig. 2B and D).
Table 2.
Comparison of mean CRP concentrations between different tube types stored at different temperatures. Concentrations of CRP are not significantly different between room temperature (RT) and 4°C incubated K2EDTA or P100 tubes at any time period (Panel A). Additionally, concentrations of CRP do not differ significantly between K2EDTA and P100 tubes whether stored at RT or 4°C (Panel B).
| PANEL A | PANEL B | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CRP | K2EDTA | P100 | RT | 4°C | |||||||||
| Comparison | 4 hrs incubation, RT vs 4°C | 24 hrs incubation, RT vs 4°C | 48 hrs incubation, RT vs 4°C | 4 hrs incubation, RT vs 4°C | 24 hrs incubation, RT vs 4°C | 48 hrs incubation, RT vs 4°C | baseline, K2EDTA vs P100 | 4 hrs incubation, K2EDTA vs P100 | 24 hrs incubation, K2EDTA vs P100 | 48 hrs incubation, K2EDTA vs P100 | 4 hrs incubation, K2EDTA vs P100 | 24 hrs incubation, K2EDTA vs P100 | 48 hrs incubation, K2EDTA vs P100 |
| Mean fold change (SEM) | 1.06 (0.04) | 0.99 (0.02) | 1.02 (0.02) | 1.01 (0.01) | 0.98 (0.04) | 0.98 (0.03) | 1.01 (0.04) | 1.05 (0.04) | 1.21 (0.2) | 0.93 (0.04) | 1.03 (0.03) | 0.98 (0.05) | 0.90 (0.06) |
| Mean difference (SEM) | 0.06 (0.04) | −0.02 (0.02) | 0.01 (0.02) | 0.00 (0.01) | −0.02 (0.05) | −0.01 (0.04) | 0.01 (0.03) | 0.03 (0.06) | 0.11 (0.13) | −0.10 (0.05) | 0.02 (0.02) | −0.02 (0.05) | −0.12 (0.07) |
| 95% CI of difference (lower, upper) | −0.05, 0.17 | −0.10, 0.06 | −0.06, 0.08 | −0.02, 0.02 | −0.15, 0.10 | −0.12, 0.09 | −0.08, 0.09 | −0.12, 0.17 | −0.27, 0.48 | −0.24, 0.04 | −0.05, 0.08 | −0.17, 0.13 | −0.32, 0.07 |
| Paired t (df) | 1.56 (4) | −0.77 (3) | 0.40 (4) | 0.53 (4) | −0.52 (5) | −0.35 (5) | 0.16 (5) | 0.47 (5) | 0.79 (4) | −2.06 (4) | 0.82 (3) | −0.32 (4) | −1.74 (4) |
| P | 0.19 | 0.50 | 0.71 | 0.63 | 0.62 | 0.74 | 0.88 | 0.66 | 0.48 | 0.11 | 0.47 | 0.77 | 0.16 |
Does collection tube type have an effect on IL-6 and CRP concentrations?
For IL-6, we restricted this analysis to blood stored at 4°C, since we had already observed that the profile from RT stored P100 plasma is too dissimilar from that of RT K2EDTA plasma to make any meaningful comparison. Regardless of pre-processing incubation time, were no significant mean differences in concentrations of either biomarker whether collected in K2EDTA and P100 tubes (Table 2, Panel B).
Are DBS CRP concentrations predictive of plasma CRP concentration?
First, we compared the concentration of DBS CRP against the baseline plasma concentration of CRP (Stockl, et al., 1998, McDade, 2014). The reasoning was a) we aimed to mathematically determine the dilution factor for DBS samples, and b) that the baseline plasma concentrations should reflect subject’s CRP levels before the introduction of any bias due to the effects of pre-processing conditions. Correlations between DBS CRP concentrations and both K2EDTA and P100 CRP concentrations at baseline were high (r = 0.97 for both), which allowed us to use the resulting regression equation (y = 256.18x − 0.0665) to correct DBS values to estimated plasma-equivalent concentrations. The observation that uncorrected concentrations of CRP from DBS samples are an order of 256 times lower than those measured in neat plasma suggests that elution of one DBS punch dilutes CRP levels approximately 256 times; values measured using 256 μl of DBS elute would approximate those made using 1μl of neat plasma. This suggests our single-punch DBS samples have a plasma-equivalent volume of 0.98μl (250 μl/256).
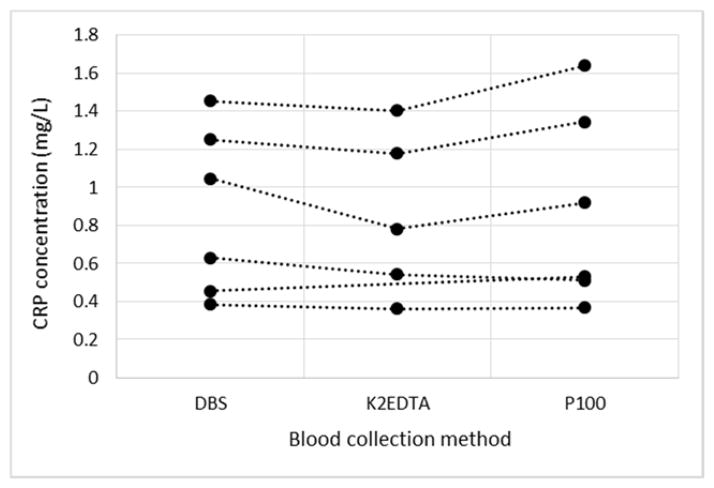
We then sought to determine whether there were any mean differences between estimated DBS CRP levels and those in K2EDTA- and p100-derived plasma stored for 48 hours at RT. This analysis would determine whether ‘worst-case scenario’ venous blood collection strategies yielded significantly different values from those derived using DBS. The intra-individual estimates of CRP under each condition can be seen in Fig. 3. Within-individual plasma equivalent DBS values were similar to values measured in plasma from either tube-type (r = 0.98, p < 0.01), suggesting both methods of blood collection yield good approximations of baseline plasma CRP concentrations.
Figure 3.
plots of CRP levels measured in a) DBS, b) K2EDTA plasma from blood stored 48 hours at RT pre-processing and c) P100 plasma from blood stored 48 hours at RT. Each point represents an individual subject with lines linking within-individual measures for comparison purposes. DBS CRP levels were scaled to plasma equivalent values using the regression equation of baseline CRP values vs. uncorrected DBS values. A paired t-test analysis determined there were no significant differences between estimated plasma-equivalent DBS concentrations and those of K2EDTA derived plasma (Mean difference = −0.10, SE = 0.04, t(4) = −2.33, p = 0.08), or P100 derived plasma (Mean difference = 0.02, SE = 0.05, t(5) = 0.31, p = 0.77).
Discussion
Our data suggest that 1) blood stored in K2EDTA tubes for up to 48 hours pre-processing is suitable for estimation of plasma IL-6 and CRP levels; 2) for K2EDTA tubes, profiles of plasma biomarkers are similar between refrigerated and room temperature-stored blood; 3) the effectiveness of P100 tubes in preservation of plasma biomarkers is temperature and biomarker-specific; and 4) DBS can be used as an non-invasive alternative to whole blood for measuring CRP.
These findings reinforce the view that it is possible to collect venous blood intended for biomarker measurement in field-collection settings, and that prolonged pre-processing times are not significantly detrimental to their estimation. Furthermore, our findings confirm that, at least for the biomarkers examined, refrigerated pre-processing conditions do not improve this estimation. Our results support those of others demonstrating that IL-6 shows temporal stability in plasma derived from K2EDTA tubes (De Jongh, et al., 1997, Jackman, et al., 2011, Riches, et al., 1992, Thavasu, et al., 1992), although there have also been reports of significant time-dependent increases (Skogstrand, et al., 2008) and decreases (Flower, et al., 2000). Our study is the first to show stability of CRP in unprocessed K2EDTA-derived plasma over a 48-hour timescale. Interestingly, neither time, temperature nor tube type affect CRP plasma profiles, suggesting CRP is relatively resilient to stimulation or degradation in unprocessed blood. This is a phenomenon observed previously in serum-measured CRP (Aziz, et al., 2003) and in plasma stored up to 36 hours pre-processing (Pai, et al., 2002). To our knowledge, only one other study has assessed the stability of CRP under conditions the same as those we report here, though that study demonstrated significant increases over time (1.3-fold and 1.9-fold under 4°C and RT conditions, respectively; (Skogstrand, et al., 2008)). One difference between the two studies is the method by which the analytes were measured. In our study, CRP levels were comfortably within the working range of the assay, whilst those reported in Skogstrand et al. were below the limit of detection. Further experiments are clearly needed, and we recommend all researchers to confirm the feasibility of this method within the framework of their own collection protocols prior to implementation.
Comparison of K2EDTA tubes and P100 tubes as collection vessels suggests that, under RT conditions, P100 tubes are not a suitable product for assessing levels of IL-6. To our knowledge, this is the first report in the literature of these particular commercially-available tubes being tested as an option for collection and preservation of plasma biomarkers with pre-processing delay. Given the relatively stable levels of IL-6 in K2EDTA tubes under the same conditions, and the stability in P100 tubes at 4°C, our findings suggest that the proprietary preservative employed in the tubes has a stimulatory effect upon IL-6 levels at RT. The reason for this de novo production of IL-6 is unknown; given that this response is seen at RT only, it is unlikely to be a direct effect of the preservative per se. Since the effect is not seen for CRP, it is unlikely this effect is related to a non-specific biological and/or physical event, such as an acute phase response. Clearly, these tubes are not intended to be used in the way in which we tested them; they are designed to preserve profiles post-processing. Although we can conclude that P100 tubes can be used ‘off-label’ under certain circumstances, they are a more expensive option that K2EDTA tubes and any benefit observed in biomarker estimation needs to be weighed against the financial disadvantage related to their use. As we only assayed two biomarkers, we cannot rule out the possibility that P100 tubes perform better than K2EDTA tubes for other biomarkers.
Finally, we observed that DBS estimation of CRP levels was similar to that obtained by venous blood. Our findings confirm previous observations (McDade, et al., 2007, Danese, et al., 2011, Crimmins, et al., 2014) that DBS can be used as an alternative substrate for estimating levels of certain biomarkers. DBS samples have some advantages over venous blood samples for in-field collections, most notably the fact that biohazard risks during collection and transportation are much lower. The disadvantage is that plasma-equivalent volume in DBS samples is low; in our experiment, we calculated that DBS samples had a plasma-equivalent volume of approximately 1μL per 3mm punch, which is consistent with previous observations of serum-equivalent volumes estimated in DBS samples (Mei, et al., 2001, Brindle, et al., 2010). This fact potentially restricts the range of biomarkers that can be assayed using this method to those that are detectable in small volumes of blood. Here, we chose to assay CRP since normal levels are well above the limit of detection of our assay method (ELISA). Other assay methods might allow greater flexibility due to increased sensitivity to detect low amounts of circulating biomarkers.
We acknowledge limitations. First, we assessed only a small number of subjects. Although our findings replicate other reports, validation in larger sample pools would be valuable. Second, we assayed only two out of a large range of biomarkers currently of interest to the research community. Previous findings have demonstrated that stability is highly biomarker-dependent (Flower, et al., 2000, Skogstrand, et al., 2008). Third, levels of IL-6 and CRP within our test subjects were within the normal range for healthy adults; we were not able to determine the effects of pre-processing conditions on samples with high levels of inflammation. Fourth, we tested the stability of samples under room temperature conditions; we did not test the profiles of samples incubated at elevated temperatures. Previous findings have demonstrated that incubation of plasma samples at 35°C for up to 48 hours has no effect on IL-6 concentration, but CRP shows evidence of induced levels (Skogstrand, et al., 2008). Researchers intending to analyze 1) biomolecules other than IL-6 or CRP, 2) samples whose levels of inflammation are anticipated to be elevated, or 3) where transport temperatures might significantly exceed normal room temperature would be advised to independently test the stability profiles prior to collection. In this regard, we hope that the design of the present study offers a paradigm for conducting systematic pre-data-collection evaluations.
In conclusion, we report that plasma collected from blood stored for up to 48 hours pre-processing can be used to estimate normal levels of IL-6 and CRP, and that blood can be transported at RT without significant detriment to biomarker estimations. In addition, we confirm the utility of DBS as a minimally invasive alternative for pre-validated biomarkers. The use of these two methods of blood collection, in complement or isolation, can facilitate wider incorporation of biomarkers into biodemographic data collection protocols.
Acknowledgments
We thank the study subjects for their participation. This research received support from US-National Institute on Aging grant AG032282, UK Medical Research Council grant G0601483, and US-NICHD grant HD077482. The study protocol was approved by the Duke University institutional review board, and subjects gave written informed consent before participating.
Contributor Information
Karen Sugden, Department of Psychology & Neuroscience, Duke University, USA; Center for Genomic and Computational Biology, Duke University, USA.
Andrea Danese, Institute of Psychiatry, King’s College London, United Kingdom.
Idan Shalev, Department of Biobehavioral Health; The Network on Child Protection and Well-Being, Social Science Research Institute, The Pennsylvania State University, University Park, PA, USA.
Benjamin S. Williams, Department of Psychology & Neuroscience, Duke University, USA; Center for Genomic and Computational Biology, Duke University, USA
Avshalom Caspi, Department of Psychology & Neuroscience, Duke University, USA; Center for Genomic and Computational Biology, Duke University, USA. Department of Psychiatry & Behavioral Sciences, Duke University Medical Center, USA and Social, Genetic, and Developmental Psychiatry Centre, Institute of Psychiatry, King’s College London, United Kingdom.
Bibliography
- Aziz N, Fahey JL, Detels R, Butch AW. Analytical Performance of a Highly Sensitive C-Reactive Protein-Based Immunoassay and the Effects of Laboratory Variables on Levels of Protein in Blood. Clin Diagn Lab Immunol. 2003 Jul;10(4):652–7. doi: 10.1128/CDLI.10.4.652-657.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brindle E, Fujita M, Shofer J, O’Connor KA. Serum, Plasma, and Dried Blood Spot High-Sensitivity C-Reactive Protein Enzyme Immunoassay for Population Research. J Immunol Methods. 2010 Oct 31;362(1–2):112–20. doi: 10.1016/j.jim.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins E, Kim JK, McCreath H, Faul J, Weir D, Seeman T. Validation of Blood-Based Assays Using Dried Blood Spots for Use in Large Population Studies. Biodemography Soc Biol. 2014;60(1):38–48. doi: 10.1080/19485565.2014.901885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins E, Kim JK, Vasunilashorn S. Biodemography: New Approaches to Understanding Trends and Differences in Population Health and Mortality. Demography. 2010;47(Suppl):S41–64. doi: 10.1353/dem.2010.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Caspi A, Williams B, Ambler A, Sugden K, Mika J, Werts H, et al. Biological Embedding of Stress through Inflammation Processes in Childhood. Mol Psychiatry. 2011 Mar;16(3):244–6. doi: 10.1038/mp.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Dove R, Belsky DW, Henchy J, Williams B, Ambler A, Arseneault L. Leptin Deficiency in Maltreated Children. Transl Psychiatry. 2014;4:e446. doi: 10.1038/tp.2014.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jongh R, Vranken J, Vundelinckx G, Bosmans E, Maes M, Heylen R. The Effects of Anticoagulation and Processing on Assays of Il-6, Sil-6r, Sil-2r and Soluble Transferrin Receptor. Cytokine. 1997 Sep;9(9):696–701. doi: 10.1006/cyto.1997.0217. [DOI] [PubMed] [Google Scholar]
- Flower L, Ahuja RH, Humphries SE, Mohamed-Ali V. Effects of Sample Handling on the Stability of Interleukin 6, Tumour Necrosis Factor-Alpha and Leptin. Cytokine. 2000 Nov;12(11):1712–6. doi: 10.1006/cyto.2000.0764. [DOI] [PubMed] [Google Scholar]
- Jackman RP, Utter GH, Heitman JW, Hirschkorn DF, Law JP, Gefter N, Busch MP, Norris PJ. Effects of Blood Sample Age at Time of Separation on Measured Cytokine Concentrations in Human Plasma. Clin Vaccine Immunol. 2011 Feb;18(2):318–26. doi: 10.1128/CVI.00465-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade TW. Development and Validation of Assay Protocols for Use with Dried Blood Spot Samples. Am J Hum Biol. 2014 Jan-Feb;26(1):1–9. doi: 10.1002/ajhb.22463. [DOI] [PubMed] [Google Scholar]
- McDade TW, Williams S, Snodgrass JJ. What a Drop Can Do: Dried Blood Spots as a Minimally Invasive Method for Integrating Biomarkers into Population-Based Research. Demography. 2007 Nov;44(4):899–925. doi: 10.1353/dem.2007.0038. [DOI] [PubMed] [Google Scholar]
- Mei JV, Alexander JR, Adam BW, Hannon WH. Use of Filter Paper for the Collection and Analysis of Human Whole Blood Specimens. The Journal of Nutrition. 2001 May 1;131(5):1631S–36S. doi: 10.1093/jn/131.5.1631S. [DOI] [PubMed] [Google Scholar]
- Pai JK, Curhan GC, Cannuscio CC, Rifai N, Ridker PM, Rimm EB. Stability of Novel Plasma Markers Associated with Cardiovascular Disease: Processing within 36 Hours of Specimen Collection. Clin Chem. 2002 Oct;48(10):1781–4. [PubMed] [Google Scholar]
- Riches P, Gooding R, Millar BC, Rowbottom AW. Influence of Collection and Separation of Blood Samples on Plasma Il-1, Il-6 and Tnf-Alpha Concentrations. J Immunol Methods. 1992 Aug 30;153(1–2):125–31. doi: 10.1016/0022-1759(92)90314-j. [DOI] [PubMed] [Google Scholar]
- Skogstrand K, Ekelund CK, Thorsen P, Vogel I, Jacobsson B, Norgaard-Pedersen B, Hougaard DM. Effects of Blood Sample Handling Procedures on Measurable Inflammatory Markers in Plasma, Serum and Dried Blood Spot Samples. J Immunol Methods. 2008 Jul 20;336(1):78–84. doi: 10.1016/j.jim.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Stockl D, Dewitte K, Thienpont LM. Validity of Linear Regression in Method Comparison Studies: Is It Limited by the Statistical Model or the Quality of the Analytical Input Data? Clin Chem. 1998 Nov;44(11):2340–6. [PubMed] [Google Scholar]
- Thavasu PW, Longhurst S, Joel SP, Slevin ML, Balkwill FR. Measuring Cytokine Levels in Blood. Importance of Anticoagulants, Processing, and Storage Conditions. J Immunol Methods. 1992 Aug 30;153(1–2):115–24. doi: 10.1016/0022-1759(92)90313-i. [DOI] [PubMed] [Google Scholar]
- Tuck MK, Chan DW, Chia D, Godwin AK, Grizzle WE, Krueger KE, Rom W, et al. Standard Operating Procedures for Serum and Plasma Collection: Early Detection Research Network Consensus Statement Standard Operating Procedure Integration Working Group. J Proteome Res. 2009 Jan;8(1):113–7. doi: 10.1021/pr800545q. [DOI] [PMC free article] [PubMed] [Google Scholar]