Abstract
The integrin family of cell adhesion receptors plays a major role in mediating interactions between cells and the extracellular matrix. Normal adult articular chondrocytes express α1β1, α3β1, α5β1, α10β1, αVβ1, αVβ3, and αVβ5 integrins, while chondrocytes from osteoarthritic tissue also express α2β1, α4β1, α6β1. These integrins bind a host of cartilage extracellular matrix (ECM) proteins, most notably fibronectin and collagen types II and VI, which provide signals that regulate cell proliferation, survival, differentiation, and matrix remodeling. By initiating signals in response to mechanical forces, chondrocyte integrins also serve as mechanotransducers. When the cartilage matrix is damaged in osteoarthritis, fragments of fibronectin are generated that signal through the α5β1 integrin to activate a pro-inflammatory and pro-catabolic response which, if left unchecked, could contribute to progressive matrix degradation. The cell signaling pathways activated in response to excessive mechanical signals and to fibronectin fragments are being unraveled and may represent useful therapeutic targets for slowing or stopping progressive matrix destruction in arthritis.
Keywords: Integrin, Cartilage, Chondrocyte, Osteoarthritis, Cell signaling
1. Introduction
1.1. Introduction to integrins
Integrins are heterodimeric transmembrane proteins consisting of α and β subunits with large extracellular domains that cooperate in binding to matrix ligands, and short cytoplasmic domains, that lack intrinsic kinase activity but which interact with proteins that initiate kinase-mediated intracellular signaling (reviewed in Giancotti and Ruoslahti (1999), Hynes (2002), Legate et al. (2009)). The integrin cytoplasmic tails also bind to and help organize cytoskeletal proteins including talin, paxillin, vinculin, tensin and actin (Wolfenson et al., 2013). Thus, integrins function to “integrate” the extracellular matrix (ECM) with cytoskeletal structures and signaling components. Signals mediated by integrins also cross talk with signals generated by soluble factors, including growth factors and cytokines (Danen and Yamada, 2001; Legate et al., 2009; Schwartz and Ginsberg, 2002). In response to extracellular cues, integrins can therefore regulate many important cellular functions including cell proliferation, differentiation, survival, and migration as well as tissue morphogenesis and remodeling.
There are 24 different integrin heterodimers formed by the combination of 8 types of β subunits and 18 types of α subunits (Hynes, 2002). In general, the β1 and αV subfamilies mediate cell–matrix interactions and will be the focus of this review while the β2 subfamily mediates cell-cell adhesions and are mainly present on leukocytes. Each integrin heterodimer can recognize and bind one or more different ECM proteins and various ECM proteins can bind to one or more different integrin heterodimers. For example, the matrix protein fibronectin has binding sites for α3β1, α4β1, α5β1, and αVβ1 integrins while the collagen binding integrins include α1β1, α2β1, α10β1, and α11β1. The integrin repertoire expressed by a given cell is influenced by the composition of the surrounding ECM as well as by signals generated by soluble factors, such as growth factors, that regulate integrin expression.
Integrin binding of ECM proteins results in stimulation of signaling networks that include multiple tyrosine and serine kinases as well as adapter proteins (“outside-in” signaling) (Hynes, 2002; Legate et al., 2009). In many cell types, focal adhesion kinase (FAK) is a key upstream mediator of integrin signaling and serves as a major hub for integrating signals generated by integrins and growth factors (Legate et al., 2009). Many of the signaling intermediates activated by integrin ligation converge on the mitogen activated protein (MAP) kinase family which includes ERK, JNK, and p38 resulting in downstream regulation of gene transcription (Giancotti and Ruoslahti, 1999; Legate et al., 2009). Depending on the context of activation, the MAP kinase family can also mediate signals generated by anabolic factors, such as growth factors, as well as catabolic factors, such as cytokines, and by mechanical stimuli which also activate integrin signaling. In this way, integrin signals work in concert with signals generated by growth factors, cytokines and mechanical forces making them very important mediators of signaling events relevant to tissues such as articular cartilage.
1.2. Chondrocyte integrin expression in normal and arthritic cartilage
Normal adult articular chondrocytes primarily express α1β1, α3β1, α5β1, α10β1, αVβ1, αVβ3, and αVβ5 integrins (Camper et al., 1998; Loeser et al., 1995, 2000; Ostergaard et al., 1998; Salter et al., 1992; Woods et al., 1994). In OA cartilage, there appears to be an increase in levels of α1β1 and α3β1 (Loeser et al., 1995) along with the appearance of α2β1, α4β1 and perhaps some α6β1 not detected in normal cartilage (Lapadula et al., 1997; Ostergaard et al., 1998). The mechanism responsible for a change in integrin expression in OA tissue has not been determined but could relate to the effects of growth factors and cytokines that stimulate integrin expression and are present in OA issue, as well as feedback regulation from changes in the ECM and promotion of chondrocyte hypertrophy resulting in expression of integrins seen on hypertrophic chondrocytes (Arner and Tortorella, 1995; Hausler et al., 2002; Jobanputra et al., 1996; Loeser, 1997).
2. Integrin–matrix interactions in cartilage
2.1. Integrin-mediated binding of chondrocytes to extracellular matrix proteins
A major function of integrins is to mediate cell adhesion to the ECM and a number of studies have examined integrin-mediated adhesion of chondrocytes to cartilage matrix proteins. The major chondrocyte integrins and the ECM proteins to which they bind are shown in Table 1. The α5β1 and αVβ3 integrins recognize and bind to the Arg-Gly-Asp (RGD) sequence present in many ECM proteins and short synthetic RGD peptides can be used to inhibit this binding in adhesion assays. This technique was shown in an early study to inhibit adhesion of adult chondrocytes to fibronectin, osteopontin, bone sialoprotein, and vitronectin, which contain RGD sequences, and to matrix Gla protein (MGP) which does not (Loeser, 1993).
Table 1.
Chondrocyte integrins and their ligands.
| Integrin | Extracellular matrix proteins |
|---|---|
| α1β1 | Collagen types VI and II, matrilin-1 |
| α2β1 (OA chondrocytes) | Collagen type II and VI, chondroadherin |
| α3β1 | Fibronectin |
| α4β1 (OA chondrocytes) | Fibronectin |
| α5β1 | Fibronectin |
| α6β1 (OA chondrocytes) | Laminin |
| α10 | Collagen type II |
| αvβ1 | Fibronectin, vitronectin, osteopontin |
| αvβ3 | COMP, fibronectin, vitronectin, osteopontin |
| αvβ5 | Fibronectin, vitronectin, osteopontin |
The binding of chondrocytes to MGP was likely indirect and mediated by fibronectin which binds to MGP (Cancela et al., 1994), demonstrating the complex interactions between the ECM and integrins. A similar interaction with α5β1 has been observed with fibronectin and connective tissue growth factor (Hoshijima et al., 2006). RGD-dependent binding of chondrocytes has also been observed with thrombospondin 1 (Miller and McDevitt, 1995) and with cartilage oligomeric matrix protein (COMP), the latter of which is through the αVβ3 integrin (Chen et al., 2005). Adhesion of chondrocytes to the cut surface of articular cartilage was inhibited using antibodies to β1, α5β1, and αVβ3 under conditions of flow in order to study chondrocyte–matrix interactions that may be important in cell-based repair (Kurtis et al., 2003).
The primary type II collagen binding integrin expressed by normal adult chondrocytes is α10β1 (Camper et al., 1998) while α1β1 can bind type II collagen but may prefer type VI collagen (Loeser et al., 2000). Unlike cells from normal cartilage, OA chondrocytes express α2β1 (Lapadula et al., 1997; Ostergaard et al., 1998) which can bind type II collagen (Loeser et al., 2000) as well as chondroadherin (Haglund et al., 2011). Binding of chondrocytes to cartilage matrix protein (matrilin-1) can be mediated by α1β1 through an interaction with type II collagen (Makihira et al., 1999). Complex interactions with collagen, and perhaps other ECM proteins, also appear to mediate binding of chondrocytes to laminin via the α6β1 integrin (Durr et al., 1996).
Integrin blocking antibodies have been used to examine functions mediated by integrin–ECM interactions. Studies using isolated chick sternal chondrocytes found that blocking α1, α2, or β1 integrins reduced survival and hypertrophic differentiation (Hirsch et al., 1997) while studies in mouse limb organ culture using antibodies to α5β1 found that this integrin also plays a role in chondrocyte differentiation and joint formation (Garciadiego-Cazares et al., 2004). Similar ex vivo experiments used injections into the upper limbs of mouse embryos of antibodies to α5β1 or RGDS peptides to support a role for the α5β1 integrin in endochondral ossification (Inoue et al., 2014).
In adult articular chondrocytes, inhibition of the α5 integrin subunit reduced cell survival in serum-free culture in alginate and inhibited the ability of IGF-1, but not serum, to prevent cell death (Pulai et al., 2002). In monolayer cultures, treatment with α5β1 or αVβ5 antibodies inhibited the de-differentiation that occurs over time as the cells attach and spread. Together, these in vitro studies provided evidence that, like other cell types, integrin-mediated interactions with the ECM are important for cell survival and differentiation.
2.2. Effects of chondrocyte integrin deficiency in mice
A few studies have evaluated the effect of integrin deficiency on cartilage in knock-out mice in order to study integrin function in vivo. Mice homozygous for a null mutation in the β1 integrin subunit die at a very early embryonic stage (Sheppard, 2000) and so floxed β1 integrin mice were crossed with the Col2a1-cre mice to delete β1 integrins in chondrocytes. These mice were found to develop a chondrodysplasia due to a disorganized growth plate resulting from defects in chondrocyte proliferation and migration (Aszodi et al., 2003). As noted above, members of the β1 integrin family serve to bind collagens and fibronectin and chondrocyte adhesion to these proteins was impaired in the β1 deficient mice. These studies were consistent with results from previous in vitro experiments using chick sternal chondrocytes that found reduced chondrocyte survival and differentiation in cultures treated with β1 integrin antibodies (Hirsch et al., 1997).
The α10β1 integrin is the major collagen-binding integrin expressed by chondrocytes and mice deficient in α10 also develop a chondrodysplasia with a disorganized growth plate (Bengtsson et al., 2005). The major fibronectin binding integrin expressed by chondrocytes is α5β1 but like β1 knockout, α5 knock-out results in early embryonic lethality (Sheppard, 2000). In another study, the floxed β1 mice were crossed with Prx1-cre mice which also resulted in a chondrodysplasia with disorganized articular cartilage as well as growth plate abnormalities (Raducanu et al., 2009). These animals were evaluated in time course experiments out to 16 months of age and were found to have a decrease in cartilage cellularity and increase in arthritic changes starting by 4 months of age with loss of normal knee joint flexibility by 7 months and reduced mobility by 8 months.
Mice deficient in the α1 integrin subunit are normal appearing at birth and did not have any obvious developmental abnormalities but with age were found to have reduced cellularity and increased apoptosis in the articular cartilage and developed premature OA-like changes with significant cartilage loss at 9 months (Zemmyo et al., 2003). However, by 12 months of age there were no significant differences from the wild-type BALB/c controls. In a separate study of 10–12 week-old α1 knockouts, the ability to form a callus in a fracture model was found to be reduced which was associated with a defect in chondrocyte proliferation and reduced mesenchymal progenitors at the callus site (Ekholm et al., 2002). These studies suggest an important role for α1β1 in chondrocyte proliferation and survival.
3. Cell signaling mediated by chondrocyte integrins
3.1. Integrins and chondrocyte mechanotransduction
As major players in binding cells to the ECM, it is perhaps not surprising that integrins can mediate the effects of mechanical forces that alter cell behavior via activation of cell signaling, a process known as mechanotransduction (Roca-Cusachs et al., 2012). Early studies demonstrated that activation of protein kinase C (PKC) signaling through the chondrocyte α5β1 integrin was associated with membrane hyperpolarization after cyclical pressure-induced strain (Wright et al., 1997). Subsequent studies from the same group revealed that the α5β1-mediated membrane hyperpolarization in response to mechanical strain required release of IL-4 which in turn promoted aggrecan expression and reduced expression of MMP-3 (Millward-Sadler et al., 2000, 1999). They also tied these results to integrin signaling pathways that included phosphorylation of FAK, β-catenin and paxillin (Lee et al., 2000), translocation of PKCα to the plasma membrane where it associated with RACK1 and the β1 integrin subunit (Lee et al., 2002), and a requirement for chondrocyte α5β1 interaction with the CD47/integrin-associated protein (Orazizadeh et al., 2008). A role for integrin-mediated signaling through FAX and Src in the cell death response seen after impact loading of cartilage explants was also recently suggested (Jang et al., 2014). Mechanical stimulation has also been shown to increase the expression of the α5 integrin subunit which could contribute to a positive feedback loop (Lucchinetti et al., 2004).
The MAP kinases mediate cell signaling induced not only by growth factors and cytokines but also through mechanical stimulation of integrins (Roca-Cusachs et al., 2012). Regulation of matrix gene expression and chondrocyte proliferation in response to mechanical stimulation of chondrocyte integrins involves MAP kinases, most notably ERK (Liang et al., 2013; Perera et al., 2010). As will be discussed next, activation of MAP kinases after stimulation of integrins with matrix fragments promotes catabolic signaling that results in matrix degradation. It is well known that excessive mechanical loading plays a key role in cartilage matrix destruction in OA (Andriacchi et al., 2004) and so it is likely that abnormal mechanical loading and signals from matrix fragments work together through integrins to promote progressive matrix destruction in OA.
3.2. Chondrocyte integrin signaling and cartilage matrix destruction
There has been extensive work examining the role of integrins in transmitting signals that result in expression of inflammatory cytokines, chemokines, and matrix degrading enzymes including the matrix metalloproteinases (MMPs). These studies have primarily utilized isolated chondrocytes or cartilage explants treated in vitro with either integrin antibodies, RGD peptides, or fragments of matrix proteins that bind to and activate specific integrins, most notably the α5β1 integrin. The initial studies demonstrating that integrins regulate MMP production were performed in synovial fibroblasts where an antibody to the α5β1 integrin or fibronectin fragments (FN-f) and synthetic peptides containing the RGD integrin recognition sequence but not intact fibronectin were found to increase MMP-1 and MMP-3 expression (Werb et al., 1989).
Subsequent studies in synovial fibroblasts demonstrated that intact fibronectin did not produce the signals that upregulate MMP expression seen with FN-f due to cross-talk between the α4β1 and α5β1 integrins where binding of α4β1 to the IIICS region of fibronectin inhibited the signals from α5β1 that were driving MMP expression (Huhtala et al., 1995; Tremble et al., 1992). The FN-f contained the RGD α5β1 cell binding region but not the α4β1 binding region while intact fibronectin contains both, providing a different set of signals from FN-f. It is thought that this integrin cross-talk provides a mechanism for cells to sense when the matrix is intact and when matrix fragments are present. The fragments indicate matrix damage that needs to be repaired and the first step in the process is to produce MMPs to clean out and remove the damaged matrix before new matrix is produced.
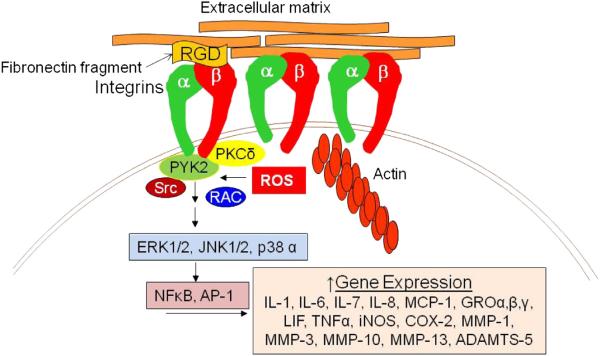
Chondrocytes have been found to have a similar catabolic response to stimulation of the α5β1 integrin with RGD peptides (Arner and Tortorella, 1995), antibodies to the α5β1 integrin (Attur et al., 2000; Forsyth et al., 2002) or FN-f (Forsyth et al., 2002; Gemba et al., 2002; Homandberg, 1999; Homandberg et al., 1992). These factors stimulate chondrocytes to produce pro-inflammatory cytokines and mediators, such as PGE2 reactive oxygen species (ROS), and nitric oxide (NO), as well as several matrix degrading enzymes including MMP-1, MMP-3, MMP-10, MMP-13, and ADAMTS-5 (Fig. 1). Like synovial fibroblasts, treatment with intact fibronectin does not induce the chondrocyte catabolic response (Forsyth et al., 2002; Homandberg, 1999). However, the mechanism must be different from the integrin cross observed with synovial fibroblasts, since, unlike synovial fibroblasts, normal chondrocytes do not express the α4β1 integrin (Loeser et al., 2000; Ostergaard et al., 1998; Woods et al., 1994). One study found that stimulation of the chondrocyte αVβ3 could inhibit production of IL-1β, NO, and PGE2 in response to α5β1 antibodies as well as in response to other cytokines (Attur et al., 2000) but this is unlikely to be the mechanism for the difference between intact fibronectin and FNfs since αVβ3, like α5β1, binds the RGD region of fibronectin and not the region recognized by α4β1. In addition, we have tested activators of αVβ3 and found that they do not inhibit the catabolic response to FN-f (unpublished results).
Fig. 1.
Signaling pathways activated in chondrocytes by fibronectin fragments. This figure only shows proteins studied to date in adult articular chondrocytes and is not a complete representation of all integrin signaling proteins. Peptides and fibronectin fragments containing the RGD cell binding sequence can bind to the α5β1 integrin and initiate a cell signaling cascade that results in increased expression of a host of pro-inflammatory mediators and matrix degrading enzymes. Fibronectin fragments such as the 29 kD N-terminal fragment which does not have an RGD sequence also appear to bind to and signal through the α5β1 integrin. Reactive oxygen species and the small GTPase Rac1 are required for the activation of gene expression but it is not clear precisely where in the pathway they act.
The pro-catabolic response to matrix fragments, such as FN-f, may be particularly relevant to promoting matrix destruction in arthritic joints. Although there are multiple mechanisms responsible for the initial upregulation of matrix degrading enzymes, once degradation starts, the generation of matrix fragments would perpetuate the process. FN-f, generated by MMPs including MMP-3 and -13 as well as by ADAM-8 (Zack et al., 2009), have been detected in the synovial fluid and cartilage from patients with OA and rheumatoid arthritis (Homandberg et al., 1998; Peters et al., 2003; Xie et al., 1992; Zack et al., 2006). When injected into rabbit knee joints, FN-f induced cartilage damage and proteoglycan loss and the RGD-containing fragment which binds the α5β1 integrin was most active (Homandberg et al., 1993).
Several different FN-f have been found to promote chondrocyte catabolic gene expression including a 45 kDa N-terminal collagen binding FN-f shown to stimulate MMP-13 and aggrecanase expression (Stanton et al., 2002) and a very potent 29 kD N-terminal fragment which does not contain the classic α5β1 RGD recognition sequence (Gemba et al., 2002; Homandberg et al., 1992; Xie and Homandberg, 1993). Despite this, it appears that α5β1 signaling mediates the effect of the 29 kD FN-f (Homandberg et al., 2002a, 2002b). This is consistent with studies in other cell types, such as fibroblasts, showing that N-terminal fragments of FN can bind α5β1 (Dzamba et al., 1994; Hocking et al., 1998)
Many of the signaling proteins downstream of chondrocyte α5β1 that are required for the catabolic response to FN-f have been identified. For the 110–120 kD fragment that contains the RGD cell binding region these include PKCδ activation of proline-rich tyrosine kinase 2 (PYK2) and downstream activation of the MAP kinases ERK1/2, JNK1/2, and p38α leading to increased activity of NFκB and AP-1 (Forsyth et al., 2002; Im et al., 2003; Loeser et al., 2003; Pulai et al., 2005). This signaling also requires the production of reactive oxygen species (Del Carlo et al., 2007) and the presence of active Rac1, a small GTPase (Long et al., 2013) (Fig. 1). Using the 29-kD N-terminal fragment as a stimulus, inhibitor studies found that MAP kinases as well as Src were required for stimulation of NO production (Gemba et al., 2002). Similarly, three different FN-f (29 kD N-terminal, 50 kD gelatin binding, and the central 140 kD fragment) activated similar signaling proteins as the 110–120 kD fragment and MAP kinases and the upstream mediators PKCδ, PYK2, and Src were required for the increase in MMP-3 and MMP-13 production (Ding et al., 2008, 2009).
Many of the signaling proteins activated by FN-f are also activated by IL-1. In studies with synovial fibroblasts treated with antibodies to the α5 integrin subunit, Rac1 activation and ROS production led to activation of NFκB and increased IL-1 expression (Kheradmand, 1998). In that study, inhibition of IL-1 with IL-1ra blocked MMP-1 expression suggesting an autocrine mechanism in response to integrin activation was responsible for the MMP production. A study with chondrocytes treated with RGD peptides that measured MMP production found that IL-1 synergized with the peptides in stimulating stromelysin activity and inhibition of IL-1 with IL-1ra blocked the RGD peptide effect (Arner and Tortorella, 1995). However, even though the 120 kD FN-f also increase IL-1 expression through NFκB, IL-1 is not necessary for production of other cytokines, chemokines or MMP-13 but did appear to contribute to activation of collagenase activity (Forsyth et al., 2002; Pulai et al., 2005).
The response to IL-1 and to TGF-β, measured by examining intracellular calcium transients, has been compared between chondrocytes from wild-type and α1 integrin deficient mice (Parekh et al., 2014). Since α1 integrin deficient mice had been shown to develop early onset OA (Zemmyo et al., 2003), it was hypothesized that chondrocytes from these mice would be more responsive to IL-1 and less responsive to TGF-β but just the opposite was seen. Because the experiments in this study focused on calcium signaling and did not provide data on downstream readouts such as MMP or matrix production the significance of the findings is not clear.
4. Conclusions
Chondrocyte integrins are important mediators of cell–matrix interactions in cartilage by regulating the response of the cells to signals from the ECM that control cell proliferation, survival, differentiation, and matrix remodeling. Integrins participate in development and maintenance of the tissue but also in pathological processes related to matrix destruction, where they likely play a role in the progression of OA. However, the evidence to date to support a role for integrins in OA is primarily from in vitro studies that have documented an extensive pro-inflammatory and catabolic response in isolated chondrocytes or cartilage explants treated with FN-f.
Defining a role for integrins in OA in vivo will be difficult since deletion or inhibition of specific integrin subunits can have pathologic effects due to the disruption of normal cell–matrix interactions. This also makes it unlikely that inhibition of a specific integrin could be used therapeutically to slow matrix destruction in OA. Because the signaling pathways and the pro-inflammatory and catabolic products produced when chondrocytes are stimulated with FN-f are very similar to those that have been found to be active in OA cartilage, studies of chondrocyte integrin signaling could still be used to discover novel therapeutic targets downstream of the integrins which mediate pathologic effects.
Acknowledgments
The work in Dr. Loeser's lab was supported by a grant from the National Institutes of Health (R37AR049003).
References
- Andriacchi TP, Mundermann A, Smith RL, Alexander EJ, Dyrby CO, Koo S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann. Biomed. Eng. 2004;32:447–457. doi: 10.1023/b:abme.0000017541.82498.37. [DOI] [PubMed] [Google Scholar]
- Arner EC, Tortorella MD. Signal transduction through chondrocyte integrin receptors induces matrix metalloproteinase synthesis and synergizes with interleukin-1. Arthritis Rheum. 1995;38:1304–1314. doi: 10.1002/art.1780380919. [DOI] [PubMed] [Google Scholar]
- Aszodi A, Hunziker EB, Brakebusch C, Fassler R. β1 integrins regulate chondrocyte rotation, G1 progression, and cytokinesis. Genes Dev. 2003;17:2465–2479. doi: 10.1101/gad.277003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attur MG, Dave MN, Clancy RM, Patel IR, Abramson SB, Amin AR. Functional genomic analysis in arthritis-affected cartilage: yin-yang regulation of inflammatory mediators by α5β1 and αVβ3 integrins. J. Immunol. 2000;164:2684–2691. doi: 10.4049/jimmunol.164.5.2684. [DOI] [PubMed] [Google Scholar]
- Bengtsson T, Aszodi A, Nicolae C, Hunziker EB, Lundgren-Akerlund E, Fassler R. Loss of α10β1 integrin expression leads to moderate dysfunction of growth plate chondrocytes. J. Cell Sci. 2005;118:929–936. doi: 10.1242/jcs.01678. [DOI] [PubMed] [Google Scholar]
- Camper L, Hellman U, Lundgren-Akerlund E. Isolation, cloning, and sequence analysis of the integrin subunit α10, a β1-associated collagen binding integrin expressed on chondrocytes. J. Biol. Chem. 1998;273:20383–20389. doi: 10.1074/jbc.273.32.20383. [DOI] [PubMed] [Google Scholar]
- Cancela ML, Williamson MK, Price PA. The putative RGD-dependent cell adhesion activity of matrix Gla protein is due to higher molecular weight contaminants. J. Biol. Chem. 1994;269:12185–12189. [PubMed] [Google Scholar]
- Chen FH, Thomas AO, Hecht JT, Goldring MB, Lawler J. Cartilage oligomeric matrix protein/thrombospondin 5 supports chondrocyte attachment through interaction with integrins. J. Biol. Chem. 2005;280:32655–32661. doi: 10.1074/jbc.M504778200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danen EH, Yamada KM. Fibronectin, integrins, and growth control. J. Cell. Physiol. 2001;189:1–13. doi: 10.1002/jcp.1137. [DOI] [PubMed] [Google Scholar]
- Del Carlo M, Schwartz D, Erickson EA, Loeser RF. Endogenous production of reactive oxygen species is required for stimulation of human articular chondrocyte matrix metalloproteinase production by fibronectin fragments. Free Radic. Biol. Med. 2007;42:1350–1358. doi: 10.1016/j.freeradbiomed.2007.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Guo D, Homandberg GA. The cartilage chondrolytic mechanism of fibronectin fragments involves MAP kinases: comparison of three fragments and native fibronectin. Osteoarthritis Cartilage. 2008;16:1253–1262. doi: 10.1016/j.joca.2008.02.015. [DOI] [PubMed] [Google Scholar]
- Ding L, Guo D, Homandberg GA. Fibronectin fragments mediate matrix metalloproteinase upregulation and cartilage damage through proline rich tyrosine kinase 2, c-src, NF-kappaB and protein kinase Cδ. Osteoarthritis Cartilage. 2009;17:1385–1392. doi: 10.1016/j.joca.2009.03.024. [DOI] [PubMed] [Google Scholar]
- Durr J, Lammi P, Goodman SL, Aigner T, von der Mark K. Identification and immunolocalization of laminin in cartilage. Exp. Cell Res. 1996;222:225–233. doi: 10.1006/excr.1996.0028. [DOI] [PubMed] [Google Scholar]
- Dzamba BJ, Bultmann H, Akiyama SK, Peters DM. Substrate-specific binding of the amino terminus of fibronectin to an integrin complex in focal adhesions. J. Biol. Chem. 1994;269:19646–19652. [PubMed] [Google Scholar]
- Ekholm E, Hankenson KD, Uusitalo H, Hiltunen A, Gardner H, Heino J, Penttinen R. Diminished callus size and cartilage synthesis in α1β1 integrin-deficient mice during bone fracture healing. Am. J. Pathol. 2002;160:1779–1785. doi: 10.1016/s0002-9440(10)61124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth CB, Pulai J, Loeser RF. Fibronectin fragments and blocking antibodies to α2β1 and α5β1 integrins stimulate mitogen-activated protein kinase signaling and increase collagenase 3 (matrix metalloproteinase 13) production by human articular chondrocytes. Arthritis Rheum. 2002;46:2368–2376. doi: 10.1002/art.10502. [DOI] [PubMed] [Google Scholar]
- Garciadiego-Cazares D, Rosales C, Katoh M, Chimal-Monroy J. Coordination of chondrocyte differentiation and joint formation by α5β1 integrin in the developing appendicular skeleton. Development. 2004;131:4735–4742. doi: 10.1242/dev.01345. [DOI] [PubMed] [Google Scholar]
- Gemba T, Valbracht J, Alsalameh S, Lotz M. Focal adhesion kinase and mitogen-activated protein kinases are involved in chondrocyte activation by the 29-kDa amino-terminal fibronectin fragment. J. Biol. Chem. 2002;277:907–911. doi: 10.1074/jbc.M109690200. [DOI] [PubMed] [Google Scholar]
- Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- Haglund L, Tillgren V, Addis L, Wenglen C, Recklies A, Heinegard D. Identification and characterization of the integrin α2β1 binding motif in chondroadherin mediating cell attachment. J. Biol. Chem. 2011;286:3925–3934. doi: 10.1074/jbc.M110.161141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausler G, Helmreich M, Marlovits S, Egerbacher M. Integrins and extracellular matrix proteins in the human childhood and adolescent growth plate. Calcif. Tissue Int. 2002;71:212–218. doi: 10.1007/s00223-001-2083-x. [DOI] [PubMed] [Google Scholar]
- Hirsch MS, Lunsford LE, Trinkaus-Randall V, Svoboda KK. Chondrocyte survival and differentiation in situ are integrin mediated. Dev. Dyn. 1997;210:249–263. doi: 10.1002/(SICI)1097-0177(199711)210:3<249::AID-AJA6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Hocking DC, Sottile J, McKeown-Longo PJ. Activation of distinct α5β1-mediated signaling pathways by fibronectin's cell adhesion and matrix assembly domains. J. Cell Biol. 1998;141:241–253. doi: 10.1083/jcb.141.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homandberg GA. Potential regulation of cartilage metabolism in osteoarthritis by fibronectin fragments. Front. Biosci. 1999;4:D713–D730. doi: 10.2741/homandberg. [DOI] [PubMed] [Google Scholar]
- Homandberg GA, Meyers R, Xie DL. Fibronectin fragments cause chondrolysis of bovine articular cartilage slices in culture. J. Biol. Chem. 1992;267:3597–3604. [PubMed] [Google Scholar]
- Homandberg GA, Meyers R, Williams JM. Intraarticular injection of fibronectin fragments causes severe depletion of cartilage proteoglycans in vivo. J. Rheumatol. 1993;20:1378–1382. [PubMed] [Google Scholar]
- Homandberg GA, Wen C, Hui F. Cartilage damaging activities of fibronectin fragments derived from cartilage and synovial fluid. Osteoarthritis Cartilage. 1998;6:231–244. doi: 10.1053/joca.1998.0116. [DOI] [PubMed] [Google Scholar]
- Homandberg GA, Costa V, Ummadi V, Pichika R. Antisense oligonucleotides to the integrin receptor subunit α5 decrease fibronectin fragment mediated cartilage chondrolysis. Osteoarthritis Cartilage. 2002a;10:381–393. doi: 10.1053/joca.2002.0524. [DOI] [PubMed] [Google Scholar]
- Homandberg GA, Costa V, Wen C. Fibronectin fragments active in chondrocytic chondrolysis can be chemically cross-linked to the α5 integrin receptor subunit. Osteoarthritis Cartilage. 2002b;10:938–949. doi: 10.1053/joca.2002.0854. [DOI] [PubMed] [Google Scholar]
- Hoshijima M, Hattori T, Inoue M, Araki D, Hanagata H, Miyauchi A, Takigawa M. CT domain of CCN2/CTGF directly interacts with fibronectin and enhances cell adhesion of chondrocytes through integrin alpha5beta1. FEBS Lett. 2006;580:1376–1382. doi: 10.1016/j.febslet.2006.01.061. [DOI] [PubMed] [Google Scholar]
- Huhtala P, Humphries MJ, McCarthy JB, Tremble PM, Werb Z, Damsky CH. Cooperative signaling by α5β1 and α4β1 integrins regulates metalloproteinase gene expression in fibroblasts adhering to fibronectin. J. Cell Biol. 1995;129:867–879. doi: 10.1083/jcb.129.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Im HJ, Pacione C, Chubinskaya S, Van Wijnen AJ, Sun Y, Loeser RF. Inhibitory effects of insulin-like growth factor-1 and osteogenic protein-1 on fibronectin fragment- and interleukin-1beta-stimulated matrix metalloproteinase-13 expression in human chondrocytes. J. Biol. Chem. 2003;278:25386–25394. doi: 10.1074/jbc.M302048200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Hashimoto R, Matsumoto A, Jahan E, Rafiq AM, Udagawa J, Hatta T, Otani H. In vivo analysis of Arg-Gly-Asp sequence/integrin α5β1-mediated signal involvement in embryonic enchondral ossification by exo utero development system. J. Bone Miner. Res. 2014;29:1556–1563. doi: 10.1002/jbmr.2166. [DOI] [PubMed] [Google Scholar]
- Jang KW, Buckwalter JA, Martin JA. Inhibition of cell-matrix adhesions prevents cartilage chondrocyte death following impact injury. J. Orthop. Res. 2014;32:448–454. doi: 10.1002/jor.22523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobanputra P, Lin H, Jenkins K, Bavington C, Brennan FR, Nuki G, Salter DM, Godolphin JL. Modulation of human chondrocyte integrins by inflammatory synovial fluid. Arthritis Rheum. 1996;39:1430–1432. doi: 10.1002/art.1780390826. [DOI] [PubMed] [Google Scholar]
- Kheradmand F. Role of Rac1 and oxygen radicals in collagenase-1 expression induced by cell shape change. Science. 1998;280:898–902. doi: 10.1126/science.280.5365.898. [DOI] [PubMed] [Google Scholar]
- Kurtis MS, Schmidt TA, Bugbee WD, Loeser RF, Sah RL. Integrin-mediated adhesion of human articular chondrocytes to cartilage. Arthritis Rheum. 2003;48:110–118. doi: 10.1002/art.10704. [DOI] [PubMed] [Google Scholar]
- Lapadula G, Iannone F, Zuccaro C, Grattagliano V, Covelli M, Patella V, Lo Bianco G, Pipitone V. Integrin expression on chondrocytes: correlations with the degree of cartilage damage in human osteoarthritis. Clin. Exp. Rheumatol. 1997;15:247–254. [PubMed] [Google Scholar]
- Lee HS, Millward-Sadler SJ, Wright MO, Nuki G, Salter DM. Integrin and mechanosensitive ion channel-dependent tyrosine phosphorylation of focal adhesion proteins and beta-catenin in human articular chondrocytes after mechanical stimulation. J. Bone Miner. Res. 2000;15:1501–1509. doi: 10.1359/jbmr.2000.15.8.1501. [DOI] [PubMed] [Google Scholar]
- Lee HS, Millward-Sadler SJ, Wright MO, Nuki G, Al-Jamal R, Salter DM. Activation of Integrin-RACK1/PKCα signalling in human articular chondrocyte mechanotransduction. Osteoarthritis Cartilage. 2002;10:890–897. doi: 10.1053/joca.2002.0842. [DOI] [PubMed] [Google Scholar]
- Legate KR, Wickstrom SA, Fassler R. Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev. 2009;23:397–418. doi: 10.1101/gad.1758709. [DOI] [PubMed] [Google Scholar]
- Liang W, Ren K, Liu F, Cui W, Wang Q, Chen Z, Fan W. Periodic mechanical stress stimulates the FAK mitogenic signal in rat chondrocytes through ERK1/2 activity. Cell. Physiol. Biochem. 2013;32:915–930. doi: 10.1159/000354495. [DOI] [PubMed] [Google Scholar]
- Loeser RF. Integrin-mediated attachment of articular chondrocytes to extracellular matrix proteins. Arthritis Rheum. 1993;38:1103–1110. doi: 10.1002/art.1780360811. [DOI] [PubMed] [Google Scholar]
- Loeser RF. Growth factor regulation of chondrocyte integrins. Differential effects of insulin-like growth factor 1 and transforming growth factorβ on α1β1 integrin expression and chondrocyte adhesion to type VI collagen. Arthritis Rheum. 1997;40:270–276. doi: 10.1002/art.1780400211. [DOI] [PubMed] [Google Scholar]
- Loeser RF, Carlson CS, McGee MP. Expression of β1 integrins by cultured articular chondrocytes and in osteoarthritic cartilage. Exp. Cell Res. 1995;217:248–257. doi: 10.1006/excr.1995.1084. [DOI] [PubMed] [Google Scholar]
- Loeser RF, Sadiev S, Tan L, Goldring MB. Integrin expression by primary and immortalized human chondrocytes: evidence of a differential role for α1β1 and α2β1 integrins in mediating chondrocyte adhesion to types II and VI collagen. Osteoarthritis Cartilage. 2000;8:96–105. doi: 10.1053/joca.1999.0277. [DOI] [PubMed] [Google Scholar]
- Loeser RF, Forsyth CB, Samarel AM, Im HJ. Fibronectin fragment activation of proline-rich tyrosine kinase PYK2 mediates integrin signals regulating collagenase-3 expression by human chondrocytes through a protein kinase C-dependent pathway. J. Biol. Chem. 2003;278:24577–24585. doi: 10.1074/jbc.M304530200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long DL, Willey JS, Loeser RF. Rac1 is required for matrix metalloproteinase 13 production by chondrocytes in response to fibronectin fragments. Arthritis Rheum. 2013;65:1561–1568. doi: 10.1002/art.37922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchinetti E, Bhargava MM, Torzilli PA. The effect of mechanical load on integrin subunits α5 and β1 in chondrocytes from mature and immature cartilage explants. Cell Tissue Res. 2004;315:385–391. doi: 10.1007/s00441-003-0836-8. [DOI] [PubMed] [Google Scholar]
- Makihira S, Yan W, Ohno S, Kawamoto T, Fujimoto K, Okimura A, Yoshida E, Noshiro M, Hamada T, Kato Y. Enhancement of cell adhesion and spreading by a cartilage-specific noncollagenous protein, cartilage matrix protein (CMP/Matrilin-1), via integrin α1β1. J. Biol. Chem. 1999;274:11417–11423. doi: 10.1074/jbc.274.16.11417. [DOI] [PubMed] [Google Scholar]
- Miller RR, McDevitt CA. Thrombospondin 1 binds to the surface of bovine articular chondrocytes by a linear RGD-dependent mechanism. FEBS Lett. 1995;363:214–216. doi: 10.1016/0014-5793(95)00311-v. [DOI] [PubMed] [Google Scholar]
- Millward-Sadler SJ, Wright MO, Lee H, Nishida K, Caldwell H, Nuki G, Salter DM. Integrin-regulated secretion of interleukin 4: a novel pathway of mechanotransduction in human articular chondrocytes. J. Cell Biol. 1999;145:183–189. doi: 10.1083/jcb.145.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millward-Sadler SJ, Wright MO, Davies LW, Nuki G, Salter DM. Mechanotransduction via integrins and interleukin-4 results in altered aggrecan and matrix metalloproteinase 3 gene expression in normal, but not osteoarthritic, human articular chondrocytes. Arthritis Rheum. 2000;43:2091–2099. doi: 10.1002/1529-0131(200009)43:9<2091::AID-ANR21>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Orazizadeh M, Lee HS, Groenendijk B, Sadler SJ, Wright MO, Lindberg FP, Salter DM. CD47 associates with α5 integrin and regulates responses of human articular chondrocytes to mechanical stimulation in an in vitro model. Arthritis Res. Ther. 2008;10:R4. doi: 10.1186/ar2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostergaard K, Salter DM, Petersen J, Bendtzen K, Hvolris J, Andersen CB. Expression of alpha and beta subunits of the integrin superfamily in articular cartilage from macroscopically normal and osteoarthritic human femoral heads. Ann. Rheum. Dis. 1998;57:303–308. doi: 10.1136/ard.57.5.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh R, Lorenzo MK, Shin SY, Pozzi A, Clark AL. Integrin α1β1 differentially regulates cytokine-mediated responses in chondrocytes. Osteoarthritis Cartilage. 2014;22:499–508. doi: 10.1016/j.joca.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera PM, Wypasek E, Madhavan S, Rath-Deschner B, Liu J, Nam J, Rath B, Huang Y, Deschner J, Piesco N, Wu C, Agarwal S. Mechanical signals control SOX-9, VEGF, and c-Myc expression and cell proliferation during inflammation via integrin-linked kinase, B-Raf, and ERK1/2-dependent signaling in articular chondrocytes. Arthritis Res. Ther. 2010;12:R106. doi: 10.1186/ar3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JH, Carsons S, Yoshida M, Ko F, McDougall S, Loredo GA, Hahn TJ. Electrophoretic characterization of species of fibronectin bearing sequences from the N-terminal heparin-binding domain in synovial fluid samples from patients with osteoarthritis and rheumatoid arthritis. Arthritis Res. Ther. 2003;5:R329–R339. doi: 10.1186/ar1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulai JI, Del Carlo M, Jr., Loeser RF. The α5β1 integrin provides matrix survival signals for normal and osteoarthritic human articular chondrocytes in vitro. Arthritis Rheum. 2002;46:1528–1535. doi: 10.1002/art.10334. [DOI] [PubMed] [Google Scholar]
- Pulai JI, Chen H, Im HJ, Kumar S, Hanning C, Hegde PS, Loeser RF. NF-kappa B mediates the stimulation of cytokine and chemokine expression by human articular chondrocytes in response to fibronectin fragments. J. Immunol. 2005;174:5781–5788. doi: 10.4049/jimmunol.174.9.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raducanu A, Hunziker EB, Drosse I, Aszodi A. β1 integrin deficiency results in multiple abnormalities of the knee joint. J. Biol. Chem. 2009;284:23780–23792. doi: 10.1074/jbc.M109.039347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca-Cusachs P, Iskratsch T, Sheetz MP. Finding the weakest link: exploring integrin-mediated mechanical molecular pathways. J. Cell Sci. 2012;125:3025–3038. doi: 10.1242/jcs.095794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter DM, Hughes DE, Simpson R, Gardner DL. Integrin expression by human articular chondrocytes. Br. J. Rheumatol. 1992;31:231–234. doi: 10.1093/rheumatology/31.4.231. [DOI] [PubMed] [Google Scholar]
- Schwartz MA, Ginsberg MH. Networks and crosstalk: integrin signalling spreads. Nat. Cell Biol. 2002;4:E65–E68. doi: 10.1038/ncb0402-e65. [DOI] [PubMed] [Google Scholar]
- Sheppard D. In vivo functions of integrins: lessons from null mutations in mice. Matrix Biol. 2000;19:203–209. doi: 10.1016/s0945-053x(00)00065-2. [DOI] [PubMed] [Google Scholar]
- Stanton H, Ung L, Fosang AJ. The 45 kDa collagen-binding fragment of fibronectin induces matrix metalloproteinase-13 synthesis by chondrocytes and aggrecan degradation by aggrecanases. Biochem. J. 2002;364:181–190. doi: 10.1042/bj3640181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremble PM, Damsky CH, Werb Z. Fibronectin fragments, but not intact fibronectin, signalling through the fibronectin receptor induce metalloproteinase gene expression in fibroblasts. Matrix Suppl. 1992;1:212–214. [PubMed] [Google Scholar]
- Werb Z, Tremble PM, Behrendtsen O, Crowley E, Damsky CH. Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J. Cell Biol. 1989;109:877–889. doi: 10.1083/jcb.109.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfenson H, Lavelin I, Geiger B. Dynamic regulation of the structure and functions of integrin adhesions. Dev. Cell. 2013;24:447–458. doi: 10.1016/j.devcel.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods VL, Jr., Schreck PJ, Gesink DS, Pacheco HO, Amiel D, Akeson WH, Lotz M. Integrin expression by human articular chondrocytes. Arthritis Rheum. 1994;37:537–544. doi: 10.1002/art.1780370414. [DOI] [PubMed] [Google Scholar]
- Wright MO, Nishida K, Bavington C, Godolphin JL, Dunne E, Walmsley S, Jobanputra P, Nuki G, Salter DM. Hyperpolarisation of cultured human chondrocytes following cyclical pressure-induced strain: evidence of a role for α5β1 integrin as a chondrocyte mechanoreceptor. J. Orthop. Res. 1997;15:742–747. doi: 10.1002/jor.1100150517. [DOI] [PubMed] [Google Scholar]
- Xie D, Homandberg GA. Fibronectin fragments bind to and penetrate cartilage tissue resulting in proteinase expression and cartilage damage. Biochim. Biophys. Acta. 1993;1182:189–196. doi: 10.1016/0925-4439(93)90140-v. [DOI] [PubMed] [Google Scholar]
- Xie DL, Meyers R, Homandberg GA. Fibronectin fragments in osteoarthritic synovial fluid. J. Rheumatol. 1992;19:1448–1452. [PubMed] [Google Scholar]
- Zack MD, Arner EC, Anglin CP, Alston JT, Malfait AM, Tortorella MD. Identification of fibronectin neoepitopes present in human osteoarthritic cartilage. Arthritis Rheum. 2006;54:2912–2922. doi: 10.1002/art.22045. [DOI] [PubMed] [Google Scholar]
- Zack MD, Malfait AM, Skepner AP, Yates MP, Griggs DW, Hall T, Hills RL, Alston JT, Nemirovskiy OV, Radabaugh MR, Leone JW, Arner EC, Tortorella MD. ADAM-8 isolated from human osteoarthritic chondrocytes cleaves fibronectin at Ala(271) Arthritis Rheum. 2009;60:2704–2713. doi: 10.1002/art.24753. [DOI] [PubMed] [Google Scholar]
- Zemmyo M, Meharra EJ, Kuhn K, Creighton-Achermann L, Lotz M. Accelerated, aging-dependent development of osteoarthritis in α1 integrin-deficient mice. Arthritis Rheum. 2003;48:2873–2880. doi: 10.1002/art.11246. [DOI] [PubMed] [Google Scholar]