Abstract
Neuroimaging and neuropsychological studies suggest that in right-handed individuals, the left hemisphere plays a dominant role in praxis, relative to the right hemisphere. However hemispheric asymmetries assessed with transcranial magnetic stimulation (TMS) has not shown consistent differences in corticospinal (CS) excitability of the two hemispheres during movements. In the current study, we systematically explored hemispheric asymmetries in inhibitory processes that are manifest during movement preparation and initiation. Single-pulse TMS was applied over the left or right primary motor cortex (M1LEFT and M1RIGHT, respectively) to elicit motor-evoked potentials (MEPs) in the contralateral hand while participants performed a two-choice reaction time task requiring a cued movement of the left or right index finger. In Experiments 1 and 2, TMS probes were obtained during a delay period following the presentation of the preparatory cue that provided partial or full information about the required response. MEPs were suppressed relative to baseline regardless of whether they were elicited in a cued or uncued hand. Importantly, the magnitude of these inhibitory changes in CS excitability was similar when TMS was applied over M1LEFT or M1RIGHT, irrespective of the amount of information carried by the preparatory cue. In Experiment 3, there was no preparatory cue and TMS was applied at various time points after the imperative signal. When CS excitability was probed in the cued effector, MEPs were initially inhibited and then rose across the reaction time interval. This function was similar for M1LEFT and M1RIGHT TMS. When CS excitability was probed in the uncued effector, MEPs remained inhibited throughout the RT interval. However, MEPs in right FDI became more inhibited during selection and initiation of a left hand movement, whereas MEPs in left FDI remained relatively invariant across RT interval for the right hand. In addition to these task-specific effects, there was a global difference in CS excitability across experiments between the two hemispheres. When the intensity of stimulation was set to 115% of the resting threshold, MEPs were larger when the TMS probe was applied over the M1LEFT than over M1RIGHT. In summary, while the latter result suggests that M1LEFT is more excitable than M1RIGHT, the recruitment of preparatory inhibitory mechanisms is similar within the two cerebral hemispheres.
Keywords: Inhibition, Hemisphere, Intermanual, Competition, Corticospinal excitability, Decision making, Response selection, Transcranial magnetic stimulation
Introduction
Studies of motor control emphasize a dominant role for the left hemisphere in praxis, similar to that observed in language (Hammond, 2002; Herve et al., 2013). Apraxia, the disruption of skilled movement is much more common following lesions of the left hemisphere compared to the right hemisphere (Goldenberg, 2009; Haaland, 2006; Rushworth et al., 2003). In right-handed healthy individuals, neuroimaging studies show broad activation across parietal and frontal regions in the left hemisphere during movements of either the left or right hand, whereas activation of homologous regions in the right hemisphere tends to be more limited to contralateral actions (Johnson-Frey et al., 2005; Verstynen and Ivry, 2011). This asymmetry is observed for a range of movements (Pool et al., 2014; Verstynen et al., 2005). In addition, anatomical evidence supports the idea that, in right-handed individuals, motor areas in the left hemisphere may be thicker than in the right hemisphere (Amunts et al., 1996; Herve et al., 2009).
Several studies have used TMS to assess hemispheric asymmetries. One approach here has been to examine corticospinal (CS) excitability at rest, comparing motor-evoked potentials (MEPs) elicited by stimulation over the primary motor cortex of left or right hemisphere (M1LEFT and M1RIGHT respectively) (Davidson and Tremblay, 2013; Hammond et al., 2004). The results from this literature are inconsistent (Barber et al., 2012; Serrien et al., 2006). Several studies have reported greater CS excitability for the left hemisphere, reflected either by larger MEPs following M1LEFT stimulation compared to M1RIGHT stimulation, or by lower intensity levels required for stimulation over M1LEFT to produce MEPs of a targeted amplitude (Macdonell et al., 1991; Triggs et al., 1999). Other studies have failed to observe hemispheric differences (Cicinelli et al., 1997; Civardi et al., 2000).
A second approach has been to look at changes in CS excitability prior to the execution of unimanual movements (Hayashi et al., 2008; Ziemann and Hallett, 2001). Leocani et al (2000) showed that CS excitability changes in the selected effector are similar for M1LEFT and M1RIGHT stimulation, rising rapidly just prior to movement initiation. In contrast, MEPs elicited from non-selected effectors were more suppressed when the TMS pulse was applied over M1RIGHT compared to M1LEFT, although this effect was not statistically analyzed.
Given this state of affairs, we set out to provide a systematic comparison of changes in CS excitability in the two hemispheres. We focused on two well-described inhibitory processes observed during response selection and movement initiation (Bestmann and Duque, 2015; Duque and Ivry, 2009; Hasbroucq et al., 1999). These processes have been observed in delayed response tasks in which a cue indicates the hand for the forthcoming response and, after a delay period, is followed by an imperative signal indicating that the prepared movement should be initiated (see Fig. 1A). TMS probes applied late in the delay period (100 ms prior to the imperative) reveal a marked suppression of MEPs observed in either the selected or non-selected hand (Chambers et al., 2009; Hasbroucq et al., 1997). Based on a set of converging methods (Davranche et al., 2007; Duque et al., 2010, 2012; Prut and Fetz, 1999), this attenuation has been attributed to two distinct inhibitory processes. Inhibition of the non-selected hand is referred to as “competition resolution”, a process invoked to help sharpen response selection (see also Duque et al., 2013; Klein et al., 2014). Inhibition of the selected hand has been related to “impulse control”, a process invoked to facilitate response initiation, perhaps by inhibiting the response until the onset of the imperative.
Fig. 1.
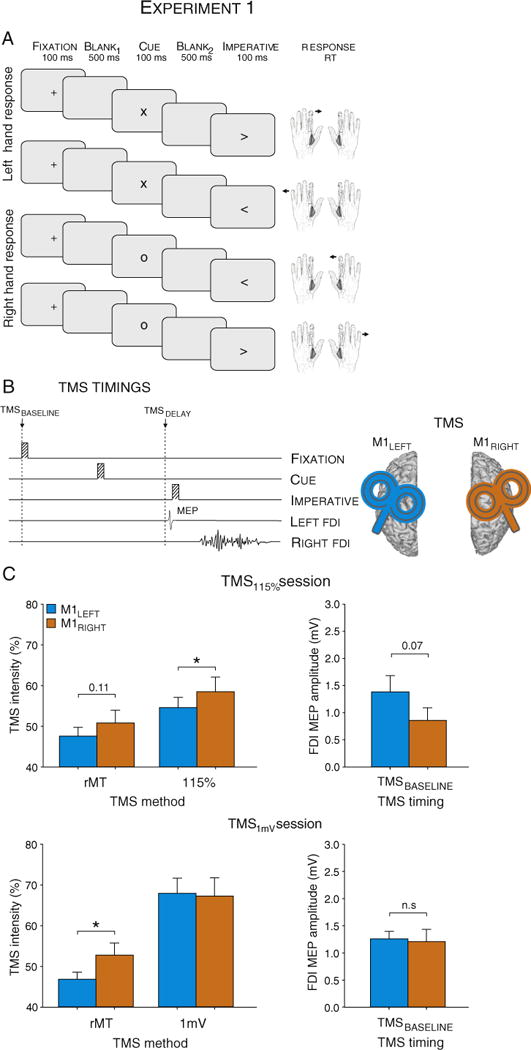
A: Trial types and sequence of events in Experiment 1. A partially informative preparatory cue (“x” or “o”) indicated the hand required for the forthcoming response (left or right respectively), with the actual response (index or pinky) specified by the imperative signal (“<” or “>”). B: Sequence and TMS stimulation timings. Each trial started with the brief presentation (100 ms) of a fixation cross. After a blank screen of 500 ms, the preparatory cue was presented for 100 ms. After a second blank screen of 500 ms, the imperative signal appeared (100 ms). A single TMS pulse was applied over primary motor cortex (M1), during one of two epochs (TMSBASELINE, TMSDELAY), with the side of stimulation varied between blocks. C: Intensity of stimulation (% of maximum stimulator output) to elicit motor evoked potentials (MEPs) following right (M1RIGHT; orange histograms) and left (M1LEFT; blue histograms) hemisphere in the TMS115% group (TMS intensity set at 115% of rMT; upper traces) and the TMS1 mV group (TMS intensity set to elicit 1 mV MEPs; lower traces, see “Method” section). rMT = rest Motor Threshold. FDI = First Dorsal Interosseous.
Here, we compared the strength of these two inhibitory processes when elicited by TMS over M1LEFT or M1RIGHT. In Experiments 1 and 2, we used a delayed response task and focused on inhibition just prior to the imperative. In Experiment 3, we eliminated the cue and delay period, assessing hemispheric asymmetries as participants selected and initiated a response following an imperative signal. In addition to comparing the operation of preparatory inhibition processes between the two hemispheres, our design allows us to evaluate hemispheric asymmetries in CS excitability at rest.
Methods
Participants
A total of 47 participants (24 women; mean age = 22.6 ± 0.5 years old) were financially compensated for completing the study. All were right-handed based on self-reports and their scores on the Edinburgh Handedness Inventory (Oldfield, 1971). All met the inclusion criterion for TMS, involving no history of neurological disorder, psychiatric illness, or substance abuse, and none were taking medications that could influence performance or neural activity. The participants were naive to the purpose of the study. Participants provided informed consent at the start of the study, following protocols approved by the institutional review boards of the University of California, Berkeley and the Université catholique de Louvain, Brussels.
Experimental tasks
Previous studies have identified inhibitory processes that shape the activity of motor representations during movement preparation and initiation (Brown and Heathcote, 2005; Duque et al., 2010; Klein et al., 2014; Usher and McClelland, 2004). The goal of the present study was to compare the efficacy of the two cerebral hemispheres in the recruitment of these control processes. To this end, we applied TMS over the motor cortex of the right or left hemisphere as participants prepared to generate movements with either the right or left hand. This allowed us to measure CS excitability changes associated with a muscle from the dominant or non-dominant hand, when the targeted muscle was either selected or not-selected for the forthcoming response.
We performed three experiments. Experiment 1 used a delayed response task in which participants were provided with a partially informative preparatory cue in advance of an imperative signal, and CS excitability was probed during the delay period. Experiment 2 used a similar design, but CS excitability was compared between conditions in which the preparatory cue was either fully or partially informative. The preparatory cue was eliminated in Experiment 3; Here, CS excitability was probed with TMS applied after the imperative had signaled the required movement. These protocols allowed us to probe the dynamics of CS excitability during response selection and initiation across a range of preparatory contexts.
Experiment 1: inhibitory changes occurring during a delay period
Task
Participants (n = 20 [9 women]; mean age = 21.1 ± 0.6 years old) performed an instructed-delay choice reaction time task (see Fig. 1A), implemented with E-Prime 2.0 software (Psychology Software Tools, Pittsburgh, Pennsylvania, USA). The task was similar to that used in a previous study (Duque et al., 2012). Participants were required to produce a speeded response with one of four fingers (index or pinky abduction with the left or right hand). A partially informative preparatory cue indicated the hand required for the forthcoming response. The imperative signal specified the response finger (for the cued hand).
Participants sat in front of a computer screen with both hands resting on a pillow, palms down, with the elbows in a semi-flexed, comfortable position. Each trial began with the brief presentation (100 ms) of a fixation cross at the center of the screen (Fig. 1A). After a blank screen of 500 ms, a preparatory cue was presented for 100 ms. The cue was either an “x” or “o”, indicating that the forthcoming response should be produced with either the left or right hand, respectively, but not which finger will be moving. After a delay period of 500 ms, an imperative signal was presented for 100 ms. The imperative was either a leftward or rightward pointing arrow, mapped in a spatially compatible manner to the fingers on the cued hand (“<”: pinky for left hand, index for right hand; “>”: index for left hand, pinky for right hand). The participant was instructed to perform the specified finger movement as quickly as possible following the imperative signal. The preparatory cue was always valid and participants were instructed to use this information to reduce their reaction time (RT). For index finger movements, the agonist was the first dorsal interosseous (FDI); for pinky movements, the agonist was the abductor digiti minimi (ADM). EMG signals from left and right FDI and ADM were monitored continuously, and participants were reminded to restrict the response to one hand if the traces indicated activity in both hands.
Experimental design
The experiment began with a short practice period to familiarize the participants with the behavioral task. In the main phase of the experiment, participants completed four blocks of 60 trials each. In two of the blocks, motor-evoked potentials (MEPs) were elicited in the right FDI by applying TMS over left M1 (M1LEFT); in the 2 other blocks, MEPs were elicited in left FDI following TMS over right M1 (M1RIGHT). The order of these two block types was counterbalanced across participants. Each block lasted about 6 min. Participants were given a 5-minute break after every pair of blocks.
One TMS pulse was applied on every trial, with two possible timings (see Fig. 1B). To obtain a baseline measure of CS excitability, the pulse was applied at the onset of the fixation cross (TMSBASELINE; 20 MEPs/block). For the other timing, the TMS pulse was applied late in the delay period, 50 ms before the imperative (TMSDELAY, 40 MEPs/block). For half of these trials, the MEP was elicited in the hand cued for the forthcoming response (selected); for the other half, the MEP was elicited in the non-cued hand (non-selected). Hence, this procedure provided us with a measure of CS excitability associated with a selected (TMSDELAY-SEL) or non-selected (TMSDELAY-NSEL) response elicited following M1RIGHT or M1LEFT stimulation. The full data set included 40 MEPs for each of these four conditions, plus 40 MEPs for each of the baseline conditions.
TMS procedure
TMS pulses were generated with a figure-of-eight coil (wing external diameter 90 mm) connected to a Magstim 200 magnetic stimulator (Magstim, Whitland, Dyfed, UK). The coil was placed tangentially on the scalp over M1RIGHT or M1LEFT. The handle was oriented towards the back of the head and laterally at a 45° angle away from the midline, approximately perpendicular to the central sulcus. Before starting the experimental blocks, the optimal coil placements over M1RIGHT and M1LEFT for eliciting MEPs in the contralateral FDI were identified, with the order of hemispheres, counterbalanced across participants. The M1RIGHT and M1LEFT foci were marked on an electroencephalography cap fitted on the participant’s head, providing a reference point for the experimental session (Vandermeeren et al., 2009). Resting motor threshold (rMT) was defined as the minimal TMS intensity required to evoke MEPs of about 50 μV peak-to-peak in the targeted muscle in 5 out of 10 consecutive trials.
Across participants, the rMT corresponded to 47.2 ± 1.4% and 51.8 ± 2.1% of the maximum stimulator output (MSO) for M1LEFT and M1RIGHT, respectively. Consistent with previous reports, the rMT was higher for M1RIGHT compared with M1LEFT (t(19) = 2.98, p < 0.008). Our initial plan was to set the stimulation intensity in the experimental blocks to 115% of the individual rMT. However, after testing the first 12 participants, we noted that there was a considerable difference in the mean MEPs for TMSBASELINE (M1LEFT: 1.4 ± 0.9 mV and M1RIGHT 0.9 ± 0.7 mV; t(11) = 2.03, p = 0.067). Because of this discrepancy, we opted to use a different procedure for a second group of 12 participants (4 who had completed the first 115% procedure). For this group, the stimulation intensity was set to produce resting MEPs of 1 mV. Surprisingly, the required intensity here was similar for the two hemispheres, corresponding to 67.9 ± 3.7% (MEP: 1.06 ± 0.1 mV) and 67.3 ± 4.5% (1.03 ± 0.1 mV) of the MSO (t(11) = 0.10, p > 0.897) for M1LEFT and M1RIGHT, respectively (probed between the experimental blocks, n = 45 for each condition). On average in this second group, MEPs at TMSBASELINE were about 20% larger than when elicited at rest (i.e. outside the block; F(1,11) = 2.73, p < 0.127), but were comparable between the two hands (1.26 ± 0.1 mV and 1.21 ± 0.2 mV, F(1,11) = 0.04, p < 0.847; see Fig. 1C).
EMG recordings
EMG activity was recorded from surface electrodes (Delsys, Inc., Boston, Massachusetts, USA) placed over the left and right FDI and ADM muscles. EMG data were collected for 2600 ms on each trial, starting 200 ms before the timing of the TMSBASELINE pulse. The EMG signals were amplified, bandpass filtered on-line (50–2000 Hz; Delsys, Inc., Boston, Massachusetts, USA) and digitized at 2000 Hz for off-line analysis. The EMG signals were used to measure peak-to-peak amplitudes of the FDI MEPs. Trials with background EMG activity larger than 100 μV in the 200 ms window preceding the TMS pulse were excluded from the analysis. This was done to prevent contamination of the MEP measurements by significant fluctuations in background EMG (Cavallo et al., 2012; Duque et al., 2005; Sartori et al., 2011). After trimming the data for background EMG activity and outliers, a minimum of 35 MEPs remained in each condition to assess CS excitability. EMG signals were processed in the same way for Experiments 2 and 3.
Statistical analysis
Reaction times (RTs) were determined by detecting the onset of agonist activation in the EMG traces. RT data in the current paper were analyzed only for baseline trials and averaged across stimulation side. We imposed this restriction since the TMS pulse on these trials should have minimal effect on response preparation/initiation. A 2 × 2 ANOVARM with HAND (left, right) and FINGER (index, pinky) as factors was conducted. The analysis was performed separately for the TMS115% and TMS1 mV groups given that the two groups were not independent.
CS excitability was analyzed by means of a 2 × 3 ANOVARM with factors HEMISPHERE (M1LEFT, M1RIGHT) and TMS-CONDITION (TMSBASELINE, TMSDELAY-SEL, TMSDELAY-NSEL). In order to obtain a measure of inhibitory changes in each condition, we expressed the MEPs elicited during the delay period as a percent change with respect to those elicited at baseline [(TMSDELAY − TMSBASELINE)/TMSBASELINE * 100]. These normalized data were evaluated with a 2 × 2 ANOVARM with the factors HEMISPHERE (M1LEFT, M1RIGHT) and TMS-CONDITION (TMSDELAY-SEL, TMSDELAY-NSEL). The analyses were performed separately for the TMS115% (n = 12) and TMS1 mV (n = 12) groups.
The results are expressed as mean ± SE. All post hoc comparisons were conducted using the Fisher’s LSD procedure.
Experiment 2: inhibitory changes occurring during a delay period following a partially or fully informative preparatory cue
Task
The cue used in Experiment 1 provided partial information concerning the forthcoming response. As such, participants were limited in the extent to which they could use this information to prepare the response. We conducted a second experiment in which we compared conditions involving this partially informative cueing method to conditions in which the cue was fully informative. For the fully informative task, an “x” indicated a left index finger response and an “o” indicated a right index finger response. Thus, for this task, the imperative signal (“<” or “>”) served solely to indicate that the response should be initiated. Note that there were four possible responses for the partially informative task and only two possible responses for the fully informative task.
Experimental design
A new group of participants (n = 16 [8 women]; mean age = 22.5 ± 0.8 years old) performed a short practice block and four experimental blocks of 90 trials each, two with the partially informative task and two with the fully informative task. For each task, one block involved stimulation over M1LEFT and the other one stimulation over M1RIGHT. Both the hemisphere and the preparatory cue orders were counterbalanced across participants. Each block lasted about 8 min with breaks provided after the second experimental block.
Similar to Experiment 1, a TMS pulse was applied on each trial, either at fixation (TMSBASELINE) or at the end of the delay period, with the latter divided into trials in which the contralateral hand had been cued (TMSDELAY-SEL) or not cued (TMSDELAY-NSEL). The full data set included 30 MEPs for each of the four conditions (selected/non-selected × left/right M1), plus 30 MEPs for the two baseline conditions.
The stimulation level was set with the rMT procedure. The data for one participant was excluded from the analyses because his MEPDELAY values were more than 3 SD from the mean of all individuals. For the other 15 participants, the rMT corresponded to 39.1 ± 2.2% and 38.7 ± 2.2% of the MSO in the M1LEFT and M1RIGHT stimulation conditions, respectively. The intensity of TMS was set at 115% of the individual rMT for the experimental session. Consistent with Experiment 1, a trend was found for MEPs to be larger following M1LEFT than after M1RIGHT stimulation at TMSBASELINE (2.0 ± 0.3 mV and 1.5 ± 0.2 mV, respectively). A specific analysis was conducted on the baseline values with HEMISPHERE and TASK as factors, and the results suggests a marginally significant hemisphere main effect (F(1,14) = 3.6, p < 0.07) whereas the task factor and the interactions had no influence (all p > 0.35; see Fig. 2). EMG recording and statistical procedures were comparable to those described for Experiment 1.
Fig. 2.
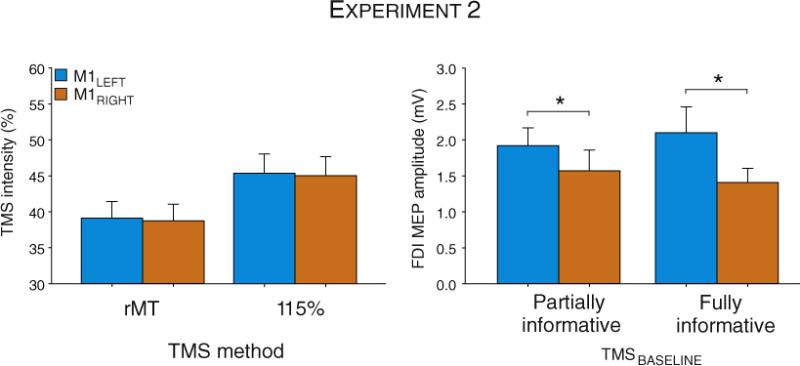
Intensity of stimulation in Experiment 2 (% of maximum stimulator output) to elicit motor evoked potentials (MEPs) following right (M1RIGHT; orange histograms) and left (M1LEFT; blue histograms) hemisphere in the Partially informative session and the Fully informative session (see “Methods” section). rMT = rest Motor Threshold. FDI = First Dorsal Interosseous.
Experiment 3: inhibitory changes occurring during a movement preparation period
Task
Experiments 1 and 2 examined the dynamics of CS excitability associated with response preparation in a delayed response task. In the final experiment, we asked a similar question but, now examined changes in CS excitability when there was no opportunity for advance preparation. The task required participants to press a button with the right or left index finger according to the color (blue or red) of an imperative signal (circle; see Fig. 3A). Importantly, the imperative signal was not preceded by a preparatory cue in this experiment; hence the participants could not prepare the required response before the imperative signal. We also eliminated the fixation cross and used a variable inter-trial interval to further minimize anticipatory effects. Twelve participants ([8 women]; mean age = 23.5 ± 0.4 years old) were tested with the task implemented using Matlab 6.5 (The Mathworks, Natick, Massachusetts, USA) and the Psychophysics Toolbox extensions (Brainard, 1997).
Fig. 3.
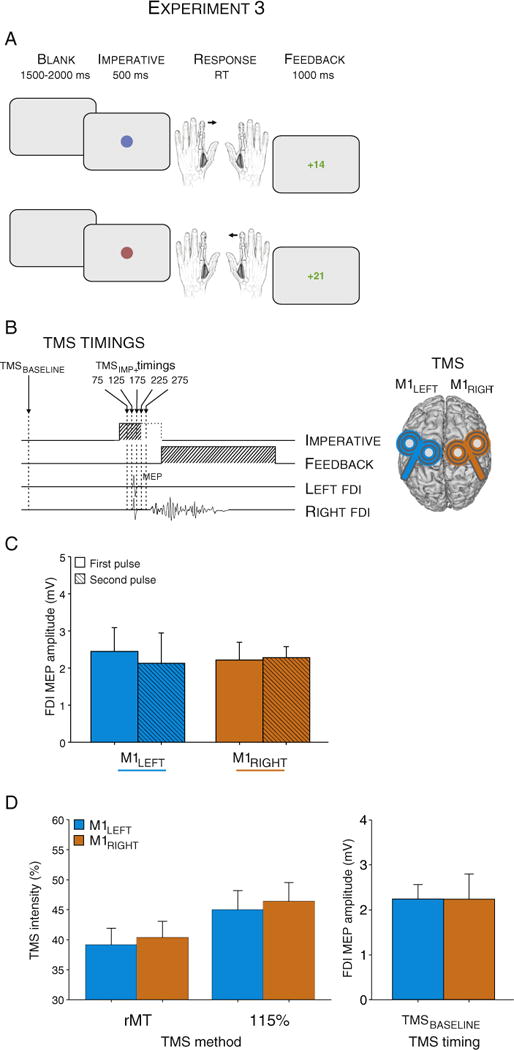
A: Trial types and sequence of events in Experiment 3. Participants were asked to press a button with the right or left index finger according to the color (blue or red) of an imperative signal. This latter was displayed until a response was performed or for a maximum of 500 ms, followed by visual feedback (1000 ms), indicating a correct (positive score) or incorrect (negative score) response. Positive scores were displayed in green and were proportional to the participants RT (k/RT5); negative scores were displayed in red and were always equal to −10. B: Sequence and TMS stimulation timings. Bilateral TMS pulses were applied concurrently over the left and right M1 at one of six possible timings (TMSBASELINE, TMSIMP+ timings). C: Amplitude of MEPs elicited by the bilateral TMS pulses at rest. Right and left M1 were stimulated with a 1 ms delay between the pulses to avoid interference between the currents generated in the two coils. The order of the pulses was counterbalanced between participants. Histograms illustrate M1LEFT (elicited following left M1 stimulation) and M1RIGHT (elicited following right M1 stimulation) amplitudes (mV) when they were elicited by the first pulse (lighter color) or by the second pulse (darker color). D: Intensity of stimulation (% of maximum stimulator output) to elicit motor evoked potentials (MEPs) following right (M1RIGHT; orange histograms) and left (M1LEFT; blue histograms) hemisphere. rMT = rest Motor Threshold. FDI = First Dorsal Interosseous. TMS = Transcranial Magnetic Stimulation. MEP = Motor Evoked Potential.
Participants sat in front of a computer screen and positioned their hands such that the left and right index fingers rested on two small yellow pads positioned lateral to the response keys. Responses in Experiment 3 thus required participants to perform a brisk abduction then flexion of the index finger.
After a variable inter-trial interval of 1500–2000 ms, a red or blue circle was displayed at the center of the screen. This signal remained on the screen until a response was detected or for 500 ms, whichever came first. Following the response, a feedback screen was displayed for 1000 ms, indicating if the response was correct or incorrect and the current tally of points earned. In the case of a correct response, the score incremented according to the formula (k/RT2 [in ms] with k = 15 × 105) such that faster responses produced larger increments. Following an incorrect response, participants received a fixed negative score (−10).
Experimental design
After a short practice period, participants performed three blocks of 96 trials each. We used a novel TMS protocol in this experiment (see below) in which two coils were positioned over M1RIGHT and M1LEFT, allowing us to elicit MEPs in the two hands simultaneously. Each block lasted about 8 min with breaks as in the previous two experiments.
Bilateral TMS pulses were applied on each trial with the pulses applied at one of six possible timings (see Fig. 3B). To obtain a baseline measure of CS excitability (TMSBASELINE), the pulses were applied during the inter-trial interval, randomly occurring 1000 to 1400 ms before the onset of the imperative signal (8 MEPs/block; 24 MEPs total). To probe the dynamics of response preparation and initiation, five postimperative timings were used: 75, 125, 175, 225 and 275 ms, with the same timing used for both coils on a given trial. A total of 48 MEPs were elicited at each timing (16 MEPs/block). In half of these trials, the imperative signal had indicated a left hand response whereas in the other half, the imperative signal had indicated a right hand response.
Given that MEPs in the agonist muscle show a dramatic rise in amplitude just before the onset of the volitional EMG response, we computed the latency between the TMS pulse and EMG onset for each trial (Klein et al., 2012). As a first-pass analysis, the MEPs were categorized into two pre-movement epochs. The “early” epoch included all trials in which the TMS pulse was applied between 225 and 125 ms prior to EMG onset (TMSPREP-EARLY). The “late” epoch included trials in which the TMS pulse was applied between 125 and 25 ms prior to EMG onset (TMSPREP-LATE). At the latter timing, we expected MEPs to be facilitated with respect to baseline when they were elicited from the responding hand (TMSPREP-SEL) and to be suppressed when elicited in the non-selected hand (TMSPREP-NSEL) (Chen and Hallett, 1999; Michelet et al., 2010). However, three participants failed to show inhibition in the non-selected hand, irrespective of whether the TMS pulse was applied over the left or right M1. Given our focus on examining hemispheric asymmetries in preparatory inhibition, we opted to exclude these participants from the analysis and focus on the data from remaining nine participants. This decision is conservative in that we did not want to bias the results such that an absence of a hemispheric effect in preparatory inhibition arises because some participants show no inhibition.
The preceding analysis provides a broad characterization of changes in CS excitability, using epochs that contain sufficient data for each participant in the four conditions (selected/non-selected × left/right M1). To provide a finer-grained analysis of CS excitability changes over time, we performed a second analysis in which we pooled the normalized MEPs from all participants (% of Baseline), and then assigned each MEP to one of five epochs: [310–240 ms]; [240–190 ms], [190–140 ms], [140–90 ms] and [90–20 ms] prior to EMG onset. We will refer to these epochs by their midpoint values (TMSPREP-275, TMSPREP-215, TMSPREP-165, TMSPREP-115 and TMSPREP-55, respectively). Note that this procedure does not normalize with respect to individual differences in RT. However, since each trial is binned with respect to RT on the individual trial, the bins consistently define epochs with respect to a common event, the participant’s response. The duration of these epochs was chosen to ensure (1) that there were at least 50 MEPs per epoch and (2) that there was a reasonably balanced contribution from each participant.
TMS procedure
We employed a TMS protocol in which two small figure-of-eight coils (70 mm external diameter, internal wing diameter = 35 mm) were each connected to separate Magstim 200 stimulators, with one coil positioned over the hand area of M1RIGHT and the other over the hand area of M1LEFT (see Fig. 4). We did not counterbalance the assignment of coil to hemisphere, opting to keep the assignment constant to reduce within-hemisphere variability. The relatively small diameter of these coils allowed us to simultaneously position each one over the optimal hotspot for its associated hemisphere. Once the hotspots were identified, the rMT was defined by adjusting the intensity of the two stimulators in parallel (but with intensities adjusted independently). Note that by using the two-coil configuration during the hotspot and rMT procedures, we ensured that the coils were positioned/oriented in the same way as in the subsequent experimental blocks. We found a match in all participant of a pilot study between hotspot and rMT when assessed with a single- or double-coil methodology.
Fig. 4.

Two small coils were used in Experiment 3 (70 mm refers to the outer circle of the butterfly-coil: internal wing diameter = 35 mm). Such coils can be positioned simultaneously on the subjects head without having to choose suboptimal coil positions or to make major adaptations to their orientation. The hotspot was defined for each hemisphere and corresponded to the location at which the coil was at the best position and orientation to elicit the largest MEP amplitude in the contralateral first dorsal interosseous (FDI) muscle.
With this two-coil procedure, we were able to collect MEPs from both FDIs by applying TMS over M1LEFT and M1RIGHT on each trial. The pulses were triggered with a 1 ms inter-stimulus interval (ISI), with the order of the pulses counterbalanced between participants (M1RIGHT TMS first for five participants; M1LEFT TMS first in the other four). Importantly, a 1 ms ISI should eliminate interference that could arise between the currents generated in the two coils, and avoid interference from interhemispheric projections through the corpus callosum (Ferbert et al., 1992; Matsunami and Hamada, 1984; Salerno and Georgesco, 1996).
The stimulation intensity during the experiment was fixed to 115% of the individual rMT. The rMT was 39.1 ± 0.7% and 40.3 ± 0.7% of MSO for M1LEFT and M1RIGHT respectively. Contrary to Experiments 1 and 2, MEPs at TMSBASELINE during the experimental session were similar for M1LEFT and M1RIGHT (2.2 ± 0.3 mV and 2.2 ± 0.2 mV, respectively; t(8) = 0.01, p = 0.99; see Fig. 3D). This lack of an effect should be considered cautiously given that MEPs were elicited by simultaneously stimulating both motor cortices using two different TMS devices. We did not find any order effect on MEP amplitudes (averaged across TMS timings, see Fig. 3C).
Statistical analysis
In addition to the EMG-derived RTs, we also obtained RT measures based on when the response keys were pressed. As in Experiments 1 and 2, the RT data from baseline trials were analyzed by means of 2 × 2 ANOVARM with HAND (left, right) and RESPONSE (EMG onset, button press) as factors.
To assess CS excitability, mean MEP amplitudes (mV) were analyzed using a 2 × 2 × 3 ANOVARM with HEMISPHERE (M1LEFT, M1RIGHT), SELECTION (selected, non-selected) and TMS-CONDITION (TMSBASELINE, TMSPREP-EARLY, TMSPREP-LATE) as factors. The pooled MEPs were analyzed using a factorial ANOVA for the selected or non-selected hands, with the factors HEMISPHERE (M1LEFT, M1RIGHT), SELECTION (selected, non-selected) and TMS-EPOCH (TMSPREP-275, TMSPREP-215, TMSPREP-165, TMSPREP-115, TMSPREP-55).
Across-experiment comparison of baseline MEPs
To investigate global hemispheric difference regarding CS excitability at rest, we compared MEP amplitudes at TMSBASELINE across experiments. We included the MEP data from Experiment 1 (115%) and Experiment 2 (partially and fully informative), as these were obtained with the same threshold procedure and both hemispheres were assessed with the same coil. A factorial ANOVA was conducted with HEMISPHERE (M1LEFT, M1RIGHT) and EXPERIMENT (Experiment 1, Experiment 2 fully informative, Experiment 2 partially informative).
Results
Experiment 1: inhibitory changes occurring during a delay period
EMG onset occurred on average 347 ± 18 ms and 341 ± 20 ms after the appearance of the imperative signal in the TMS115% and TMS1 mV groups respectively. In both groups, the effect of FINGER was significant (both F > 5.86, both p < 0.03), revealing that index finger responses were faster (317 ± 10 ms) than pinky responses (377 ± 11 ms; Fig. 5A). The factor HAND was not significant in either group (all F < 1.8, all p > 0.20).
Fig. 5.
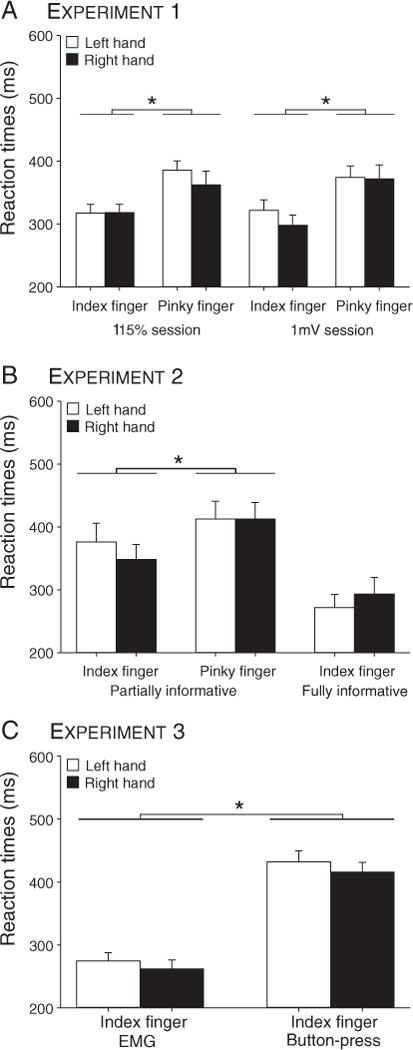
RTs for left (light bars) and right hands (dark bars) in the three experiments. A, B: Index finger responses were faster than pinky responses in Experiments 1 & 2. In Experiment 2, RTs were faster when the cue was fully informative compared to when it was partially informative. C: In Experiment 3, button-press occurred approximately 150 ms after EMG RTs. * = significantly different (p-value < 0.05). EMG = electromyographic. RT = reaction time.
In terms of the TMS data, a marked reduction in MEPs was observed 100 ms before the onset of the imperative signal, relative to baseline. For the subgroup stimulated at 115% of rMT (n = 12), there was a main-effect of TMS-CONDITION (F(1,11) = 16.5, p < 0.0001), with MEPs lower than baseline when the targeted finger was either selected for the forthcoming response or not selected (both TMSDELAY-SEL and TMSDELAY-NSEL p < 0.003; see Fig. 6A – left panel). Moreover, the MEP suppression was larger in the selected condition compared to the non-selected condition (p < 0.03). Thus, consistent with previous reports, we observed significant preparatory inhibition, regardless of whether the probed finger was selected or not selected for the forthcoming response, and the former was greater than the latter (Duque et al., 2009; Duque et al., 2010).
Fig. 6.
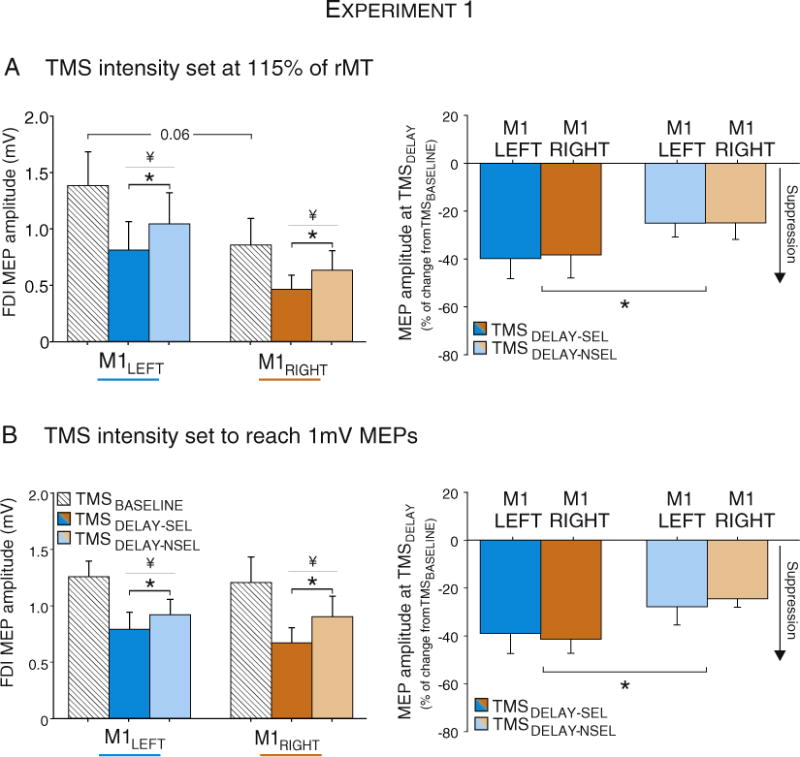
MEP amplitudes following right (M1RIGHT) and left hemisphere stimulation (M1LEFT) recorded at TMSDELAY in the TMS115% (Panel A, n = 12) or the TMS1 mV (Panel B, n = 12) groups of Experiment 1. MEPs are shown in mV (left panel) and expressed as a percent change with respect to MEPs elicited at TMSBASELINE (right panel). * = significantly different (p-value < 0.05). MEP suppression at TMSDELAY was more pronounced in a selected muscle (TMSDELAY-SEL) than in a non-selected muscle (TMSDELAY-NSEL). No differences were found between M1RIGHT and M1LEFT conditions. ¥ = significantly different (p-value < 0.05) from MEPs elicited at TMSBASELINE.
Our primary interest in this experiment was the comparison of preparatory inhibition when probed from M1LEFT or M1RIGHT. The main effect of HEMISPHERE was marginally significant (F(1,11) = 4.34, p < 0.06), reflecting a trend for MEPs to be larger after M1LEFT stimulation. However, this pattern was evident in both the baseline and delay periods, with no evidence of an interaction (HEMISPHERE × TMS-CONDITION F(1,11) = 0.4, p > 0.68). Thus, the trend for a hemisphere effect suggests that increasing the stimulation level by 15% above threshold has a larger effect on M1LEFT MEPs compared to M1RIGHT MEPs.
A more focused comparison of hemispheric differences in inhibitory effects comes from the normalized data, obtained by expressing the MEPs elicited during the delay period with respect to those evoked at baseline (Fig. 6A – right panel). The effect of TMS-CONDITION was significant (F(1,11) = 5.4, p < 0.04): MEPs were reduced by 39% when the targeted finger was selected for the forthcoming response, whereas the reduction was only 25% when the finger was not selected. We did not observe any difference in the magnitude of these inhibitory effects between the two hemispheres (HEMISPHERE F(1,11) < 0.01, p > 0.95), nor did this factor interact with TMS-CONDITION (F(1,11) < 0.02, p > 0.89).
Given the global hemispheric difference (larger MEPs at 115% rMT following M1LEFT stimulation), we tested a second group (n = 12), using a stimulation intensity that produced 1 mV MEPs at rest. For this group, the MEPs elicited at TMSBASELINE were comparable for the two hemispheres (Fig. 6B – left panel). We again observed profound inhibition of the MEPs at the end of the delay period, relative to baseline (raw MEPs: TMS-CONDITION F(1,11) = 31.8, p < 0.0001), with the MEP suppression again greater when the targeted effect was selected for the forthcoming response compared to when it was not selected (p < 0.009, Fig. 6B – left panel). There was no effect of HEMISPHERE, (F(1,11) = 0.09, p < 0.77). The same picture was observed in the analysis of the normalized data (percent of baseline, Fig. 6B – right panel). While inhibition was greater when the targeted finger was selected for the forthcoming response (TMS-CONDITION: F(1,11) = 20, p < 0.0001), the effect of HEMISPHERE was not significant (F(1,11) = 0.003, p > 0.952) nor was the interaction of these factors (F(1,11) = 1.9, p > 0.20).
In sum, the results from Experiment 1 fail to identify hemispheric differences in the operation of inhibitory processes observed during response preparation. As shown in the normalized results, inhibition attributed to impulse control (TMSDELAY-SEL) and competition resolution (TMSDELAY-NSEL) was similar following M1LEFT and M1RIGHT stimulation. While caution is always required when considering null results, the absence of an effect here was observed with two different protocols, one based on a stimulation intensity of 115% rMT and the other using a fixed MEP amplitude value.
Experiment 2: inhibitory changes occurring during a delay period following a partially or fully informative preparatory cue
RTs (obtained from the EMG traces) from the fully informative condition (282 ± 26 ms) were faster than in the partially informative task (337 ± 29 ms; see Fig. 5B). Given that pinky responses were not employed in the fully informative task, we performed separate analyses of the RT data for the two tasks. The effect of FINGER was significant in the partially informative task (F(1,14) > 7.1, p < 0.02), with index finger responses (375 ± 23 ms) faster than pinky responses (399 ± 27 ms). The main effect of HAND and FINGER × HAND interaction were not significant in either task (partially-informative: all p > 0.17; fully-informative task: p > 0.18).
Similar to Experiment 1, there was a marked reduction in the amplitude of MEPs just prior to the onset of the imperative signal. The effect of TMS-CONDITION was significant (F(2,28) = 11.8, p < 0.0001), with MEPs significantly lower than baseline when the targeted hand was either selected or not selected for the forthcoming response (both TMSDELAY-SEL and TMSDELAY-NSEL p < 0.002; see Fig. 7 – left panel). This pattern was comparable for conditions in which the cue provided partial or full information about the forthcoming response (PREPARATORY CUE: F(1,14) = 0.1, p > 0.79, n = 15).
Fig. 7.
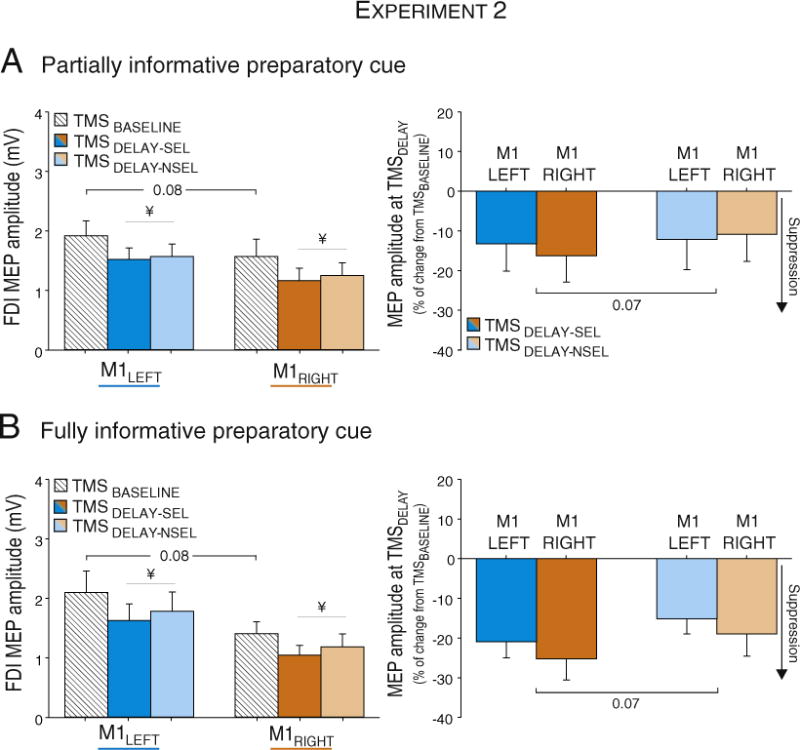
MEP amplitudes following right (M1RIGHT) and left hemisphere (M1LEFT) stimulation at TMSDELAY. MEPs are shown in mV (left side) and expressed as a percent change with respect to MEPs elicited at TMSBASELINE (right side). * = significantly different (p-value < 0.05). MEP suppression at TMSDELAY was more pronounced in a selected muscle (TMSDELAY-SEL) than in a non-selected muscle (TMSDELAY-NSEL). No differences were found between M1RIGHT and M1LEFT conditions. ¥ = significantly different (p-value < 0.05) from MEPs elicited at TMSBASELINE.
The effect of HEMISPHERE was marginally significant (F(1,14) = 4.1, p < 0.06). In line with the global hemispheric effect observed in Experiment 1, MEP amplitudes following M1LEFT stimulation were larger (1.8 ± 0.3 mV on average) than after M1RIGHT (1.3 ± 0.2 mV on average). This difference was similar for the baseline and delay periods (HEMISPHERE × TMS-CONDITION: F(2,28) = 0.2, p > 0.79). Moreover, the main effect of PREPARATORY CUE was not significant (F(1,14) = 0.1, p > 0.79) nor did this factor interact with TMS-CONDITION (F(1,14) = 0.4, p > 0.67). Thus, in terms of the raw MEP data, TMS over either hemisphere revealed a similar reduction in CS excitability during the delay period, and the effect was independent of whether full or partial information had been provided concerning the forthcoming response.
When looking at normalized values (percent of baseline; see Fig. 6 – right panel), MEPs elicited from the selected hand (TMSDELAY-SEL) showed more inhibition (19 ± 4.6% with respect to MEPs at TMSBASELINE) than MEPs evoked in the non-selected hand (TMSDELAY-NSEL: 14.3 ± 4.1% suppression). This effect was independent of the side of stimulation as well as the level of information provided by the preparatory cue (HEMISPHERE, HEMISPHERE × TMS-CONDITION or PREPARATORY CUE × HEMISPHERE: all F < 2.4, p > 0.15).
Taken together, the results from Experiments 1 and 2 provide a consistent picture showing that M1LEFT and M1RIGHT are associated with comparable inhibitory changes during response preparation in a delayed response task.
Experiment 3: inhibitory changes occurring during a movement preparation period
In the final experiment, we examine preparatory inhibition in the absence of a delay period, again comparing stimulation over M1LEFT and M1RIGHT. RTs, when measured based on the time of EMG onset, were relatively fast, occurring on average 268 ± 13 ms after the onset of the imperative signal. When measured behaviorally, the mean latency for the button press was 424 ± 16 after the imperative signal (see Fig. 5C). The large latency between EMG onset and button press is due to the fact that producing a finger response required participants to move from a start position to a lateral response key (see Methods section). The HAND effect was not significant (all F = 2.3, all p > 0.15). Surprisingly, EMG onsets were faster in Experiment 3 than in Experiments 1 and 2, despite the absence of the pre-cue in the former. There are various methodological changes that might account for this difference including the bonus payment scheme, the feedback provided in Experiment 3 to emphasize speed, and the use of different imperative cues.
MEPs following M1LEFT and M1RIGHT stimulation at TMSBASELINE averaged 2.24 ± 0.5 mV and 2.24 ± 0.3 mV, respectively. Interestingly, the hemisphere difference in baseline CS excitability observed in Experiments 1 and 2 was not obtained in Experiment 3.
Following the imperative signal (TMSPREP-EARLY), the amplitude of MEPs initially decreased below baseline, an effect observed regardless of whether the muscle was selected or not selected for the forthcoming response (relative to TMSBASELINE: selected p < 0.029; non-selected p < 0.062; see Fig. 8A). The MEPs for these two conditions diverged as the TMS probe occurred closer to movement onset. MEPs at TMSPREP-LATE were facilitated relative to baseline when the muscle was the agonist for the forthcoming response (p < 0.0001) and remained suppressed when the muscle was not selected (p < 0.002; Fig. 8A). There was no effect of HEMISPHERE for MEPs at TMSPREP-EARLY or TMSPRE-LATE (all p > 0.330).
Fig. 8.
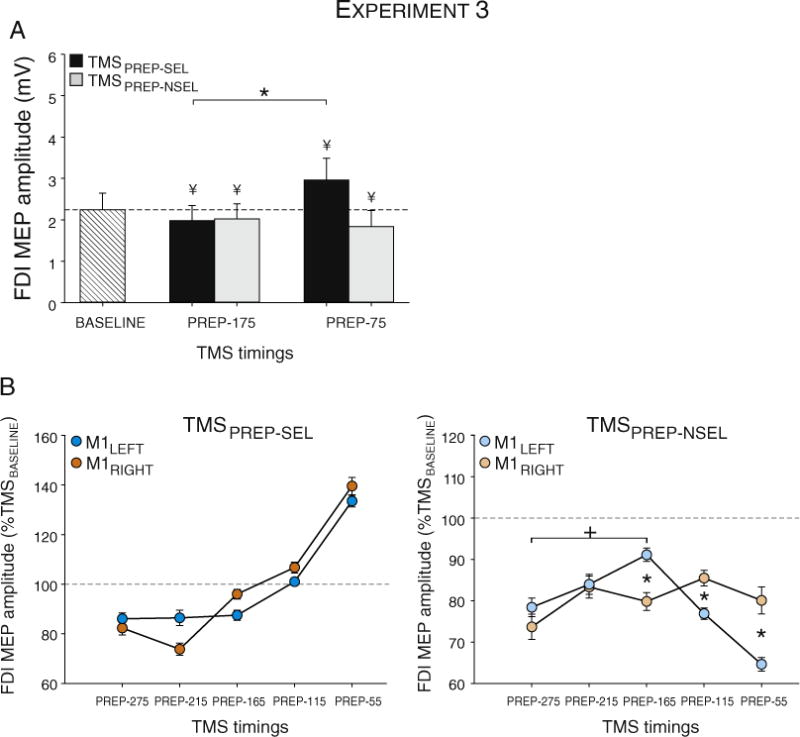
MEP amplitudes in Experiment 3. A: Evolution of MEPs elicited from a selected (MEPPREP-SEL) or non-selected (MEPPREP-NSEL) hand at TMSBASELINE and in pre-movement windows (TMSPREP-EARLY, TMSPREP-LATE). MEPs are suppressed at TMSPREP-175. At TMSPREP-LATE, excitability increased in the selected hand but remains suppressed in the non-selected hand. B: MEP amplitudes following M1RIGHT (filled dots) and M1LEFT stimulation (empty dots) for the different TMS epochs (see Methods section) in the selected (left) and non-selected hands (right). Note the absence of a HEMISPHERE effect for the selected handSEL. For the non-selected hand, inhibition in the left hand was relatively constant (M1RIGHT stimulation), but showed a non-monotonic profile in the right hand (M1LEFT stimulation). * and + = significantly different (p-value < 0.05). ¥ = significantly different (p-value < 0.05) from MEPs elicited at TMSBASELINE.
To obtain a more fine-grained comparison of the temporal dynamics of CS excitability during movement preparation when TMS was applied over M1LEFT or M1RIGHT, we pooled the MEPs from all participants (normalized on an individual basis) and assigned them to one of five pre-movement epochs (see Methods section). We did not find any hemispheric difference when the MEPs were elicited in the selected finger (see Fig. 7B – left panel). In contrast, there was a significant HEMISPHERE × SELECTION × TMS-EPOCH interaction for the TMSPREP-NSEL condition (F(4,3080) = 2.39, p < 0.048; see Fig. 7B – right panel). MEPs elicited by TMS over the M1RIGHT remained similarly inhibited across the preparation period (all post-hoc p > 0.245) whereas MEPs from M1LEFT stimulation initially increased (reaching 91.1 ± 3% at TMSPREP-165, p < 0.0005 when compared to TMSPREP-275) and then decreased closer to movement onset (dropping to 64.6 ± 3% at TMSPREP-55, p < 0.0001 when compared to TMSPREP-165). As a consequence, MEPs were larger for M1LEFT than M1RIGHT at TMSPREP-165 (p < 0.0001) but smaller for M1LEFT than M1RIGHT at TMSPREP-115 (p < 0.054) and at TMSPREP-55 (p < 0.0001).
Across-experiment comparison of baseline MEPs
In all three experiments, stimulation intensity was established separately for M1LEFT and M1RIGHT, based on identifying rMT in each individual and then increasing the stimulation level to 115% of rMT (excluding those tested with 1 mV in Experiment 1). We performed a post-hoc between-experiment analysis to assess hemispheric differences in sensitivity to the 15% increase in stimulation intensity above rMT. While the effect of EXPERIMENT (p = 0.73) was not significant, there was a highly significant effect of HEMISPHERE during the task, even when measured at baseline (F(1,39) = 8.32, p < 0.006). MEPs from M1LEFT stimulation at TMSBASELINE elicited were larger from M1LEFT stimulation (1.83 ± 0.2 mV) than M1RIGHT stimulation (1.31 ± 0.2 mV). That is, a 15% increase in intensity had a much more pronounced effect on MEPs for M1LEFT stimulation when tested in the context of the experimental task.
Discussion
Similar to all domains of human behavior, asymmetries in function between the two cerebral hemispheres have been the subject of considerable study in the field of motor control (Schluter et al., 2001; Serrien et al., 2006). Neuropsychological studies of disorders such as apraxia make clear there are pronounced asymmetries (Goldenberg, 2014; Haaland, 2006) and process-based models have inspired a wide range of hypotheses to capture functional differences (Rushworth et al., 2003). TMS offers a powerful tool to make physiological comparisons between the hemispheres (Bestmann and Duque, 2015; Leocani et al., 2000). We systematically examined this issue in the current study, using TMS to assess changes in corticospinal excitability as right-handers prepared to produce a unimanual movement with either the left or right hand.
The results of Experiments 1 and 2 showed pronounced preparatory inhibition during a delayed response period. This inhibition was evident when the targeted muscle was not involved in the forthcoming response and even more pronounced when the targeted muscle was the agonist for the forthcoming response (see also, Davranche et al., 2007; Hasbroucq et al., 1997). These effects have been attributed to the operation of distinct preparatory mechanisms (Duque et al., 2010), the former associated with competition resolution and the latter associated with impulse control.
In a comparison of the two hemispheres, the overall picture here is one of hemispheric symmetry concerning the recruitment and operation of inhibitory mechanisms for response preparation during a delayed response task. We found no differences between M1LEFT and M1RIGHT stimulation when CS excitability was probed just prior to the imperative signal. The symmetrical pattern of inhibition was observed when participants were provided with partial or fully informative information about the forthcoming response. While caution is always required when considering null results, the absence of a laterality effect was replicated with two different protocols in Experiment 1, one based on stimulation intensity of 115% rMT and the other using an intensity designed to produce MEPs of a targeted amplitude.
In Experiment 3, we eliminated the delay period, investigating the dynamics of CS excitability after the presentation of an imperative signal. Initially, MEPs recorded from either a selected or non-selected muscle were suppressed below baseline (Duque et al., 2014). As the preparation interval approached movement onset, MEPs became larger when the muscle was selected for the forthcoming response, but remained suppressed if the muscle was not selected (Klein et al., 2012; Michelet et al., 2010).
With this protocol, we observed consistent, yet subtle, differences between M1LEFT and M1RIGHT stimulation. When the data were pooled into “early” and “late” epochs to have sufficient data for a standard statistical analysis (using participants as a repeated measure), the pattern was similar for the two hemispheres. However, when the data were pooled to capture the dynamics of response preparation at a finer temporal resolution, a laterality effect was observed. Whereas the pattern of increasing excitability in the selected muscle was similar for M1LEFT and M1RIGHT stimulation, the hemispheres differed in how the muscle was inhibited when not selected for the forthcoming response. M1RIGHT stimulation revealed relatively constant inhibition of the left hand. In contrast, M1LEFT stimulation showed a more complex pattern: CS excitability initially increased (release of inhibition) and then showed a strong reduction (larger inhibition) late in the RT interval.
Previous studies have also observed greater inhibition of MEPs from muscles in a non-selected hand following M1RIGHT stimulation compared to M1LEFT stimulation. Indeed, this difference was first reported in the study of Leocani et al. (2000), and has been the central finding motivating researchers (including us) to focus on M1RIGHT stimulation in TMS studies of motor preparation (see also, Ziemann and Hallett, 2001). The asymmetry might reflect an overall bias in the functional contribution of the right hemisphere, relative to the left, for inhibitory control (Aron et al., 2014; Swann et al., 2009). Alternatively, greater inhibition of left hand response representations might reflect a left hemisphere dominance in action planning (Haaland, 2006; Haaland et al., 2004; Rushworth et al., 2007). For example, many models of action selection posit a competitive process in which the accumulation of evidence for one response is accompanied by reciprocal inhibition of response alternatives (Domenech and Dreher, 2010; Gold and Shadlen, 2007; Kim and Basso, 2010). Given the assumption of stronger left hemisphere motor representations, one would expect relatively stronger inhibition of right hemisphere motor representations.
Interestingly, the early release in M1LEFT inhibition was followed by a pronounced increase in inhibition of right FDI close to movement onset of the left hand. Previous studies have consistently shown greater bilateral activation during the planning and execution of left hand movements compared to right hand movements (Hammond, 2002; Schluter et al., 2001). While this effect is especially pronounced with sequential, complex, and transitive movements (Haaland et al., 2004; Johnson-Frey et al., 2005; Verstynen and Ivry, 2011), it is also observed during simple, unimanual movements. The functional significance of this bilateral activation remains unclear. One hypothesis is that left hemisphere activation during left hand movement reflects a dominant role for the left hemisphere in motor planning. Considered from the perspective of accumulation models of response selection, the increased inhibition associated with M1LEFT stimulation might reflect the demands to inhibit motor representations in the right hand, enabling left hand movement. Alternatively, it may be that, at least for right-handers, left hemisphere motor representations have a lower threshold than right hemisphere motor representations. When producing left hand movements, M1LEFT may require greater inhibition to minimize inadvertent activation of right hand muscles.
This last hypothesis is also relevant when considering the other hemispheric asymmetry observed in the current study. Specifically, we consistently observed greater CS excitability in the left hemisphere that was independent of the task requirements. In Experiment 1, the intensity required to reach rMT was lower for M1LEFT compared to M1RIGHT. Moreover, in both Experiments 1 and 2, MEPs were larger at baseline (during the inter-trial interval) when the stimulation intensity was set to 115% of rMT. This latter result suggests different recruitment curves for each hemisphere (Macdonell et al., 1991; Triggs et al., 1999), with a faster rate of recruitment in the left hemisphere compared to the right hemisphere. This effect may reflect more focal motor representations in the left hemisphere or asymmetries in the anatomical and functional organization of the left motor cortex (Amunts et al., 1996), at least in right-handed participants. An alternative account for the baseline differences might be related to global task demands. Our task required response selection based on symbolic cues and the deciphering of these may have produced an overall engagement of the left hemisphere, one that persisted between trials (Corina et al., 1992). This hypothesis seems unlikely given TMS excitability studies that have observed similar results at rest with more direct stimulus cues (Brouwer et al., 2001; Dassonville et al., 1997; Solodkin et al., 2001).
Taken together, the results presented here indicate an overall picture of hemispheric symmetry regarding inhibitory processes during movement preparation and initiation in a delay-response task. In contrast, we observed a hemispheric asymmetry in inhibition of the uncued response in a reaction time task when the preparatory delay was eliminated. We have suggested that the latter may reflect asymmetrical contributions of the two hemispheres to action selection. This asymmetry may not be manifest in the delay-response condition given that there is less time constraint on planning processes, or perhaps greater separation of planning and implementation. Moreover, the present results do not provide insight into the mechanisms that underlie this asymmetry. The use of other TMS protocols such as measures of intra- and intercortical inhibitions (Opie and Semmler, 2014) should prove useful in understanding intra- and inter-hemispheric dynamics during action selection.
It remains to be seen if our results would also be observed in a left-handed population. On the one hand, there appears to be a left hemisphere dominance regarding praxis in left-handers (Hammond, 2002; Sainburg, 2014). However, various reports indicate that left-handers have reduced hemispheric lateralization and hence, the differences between populations varying in handedness might be complex (Solodkin et al., 2001). Regarding CS excitability at rest, for example, it seems unlikely that left-handers will show increased excitability in the right hemisphere since previous studies have reported a lower intensity required to reach rMT in M1LEFT, relative to M1RIGHT, in left- and right-handers (Davidson and Tremblay, 2013). Future assessment of left handers would allow a fuller characterization of the hemispheric similarities and differences in motor preparation and implementation.
Acknowledgments
This work was supported by grants from the National Institute of Health (NS0085570, NS074917), the FSR (« Fonds Spéciaux de Recherche » – Université catholique de Louvain) and the FRSM (« Fonds de la Recherche Scientifique Médicale » – Fonds National de la Recherche Scientifique). P.A. Klein was a doctoral research fellow at the Belgian National Funds for Scientific Research (FRS – FNRS) and was supported by a Wallonie-Bruxelles International grant.
References
- Amunts K, Schlaug G, Schleicher A, Steinmetz H, Dabringhaus A, et al. Asymmetry in the human motor cortex and handedness. NeuroImage. 1996;4:216–222. doi: 10.1006/nimg.1996.0073. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Right inferior frontal cortex: addressing the rebuttals. Front Hum Neurosci. 2014;8:905. doi: 10.3389/fnhum.2014.00905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber AD, Srinivasan P, Joel SE, Caffo BS, Pekar JJ, Mostofsky SH. Motor “dexterity”?: evidence that left hemisphere lateralization of motor circuit connectivity is associated with better motor performance in children. Cereb Cortex. 2012;22:51–59. doi: 10.1093/cercor/bhr062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestmann S, Duque J. Transcranial magnetic stimulation: decomposing the processes underlying action preparation. Neuroscientist. 2015 doi: 10.1177/1073858415592594. in press. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10:433–436. [PubMed] [Google Scholar]
- Brouwer B, Sale MV, Nordstrom MA. Asymmetry of motor cortex excitability during a simple motor task: relationships with handedness and manual performance (Experimental brain research Experimentelle Hirnforschung Experimentation cerebrale) Exp Brain Res. 2001;138:467–476. doi: 10.1007/s002210100730. [DOI] [PubMed] [Google Scholar]
- Brown S, Heathcote A. A ballistic model of choice response time. Psychol Rev. 2005;112:117–128. doi: 10.1037/0033-295X.112.1.117. [DOI] [PubMed] [Google Scholar]
- Cavallo A, Becchio C, Sartori L, Bucchioni G, Castiello U. Grasping with tools: corticospinal excitability reflects observed hand movements. Cereb Cortex. 2012;22:710–716. doi: 10.1093/cercor/bhr157. [DOI] [PubMed] [Google Scholar]
- Chambers CD, Garavan H, Bellgrove MA. Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neurosci Biobehav Rev. 2009;33:631–646. doi: 10.1016/j.neubiorev.2008.08.016. [DOI] [PubMed] [Google Scholar]
- Chen R, Hallett M. The time course of changes in motor cortex excitability associated with voluntary movement. Can J Neurol Sci. 1999;26:163–169. doi: 10.1017/s0317167100000196. [DOI] [PubMed] [Google Scholar]
- Cicinelli P, Traversa R, Bassi A, Scivoletto G, Rossini PM. Interhemispheric differences of hand muscle representation in human motor cortex. Muscle Nerve. 1997;20:535–542. doi: 10.1002/(sici)1097-4598(199705)20:5<535::aid-mus1>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Civardi C, Cavalli A, Naldi P, Varrasi C, Cantello R. Hemispheric asymmetries of cortico-cortical connections in human hand motor areas. Clin Neurophysiol. 2000;111:624–629. doi: 10.1016/s1388-2457(99)00301-6. [DOI] [PubMed] [Google Scholar]
- Corina DP, Vaid J, Bellugi U. The linguistic basis of left hemisphere specialization. Science. 1992;255:1258–1260. doi: 10.1126/science.1546327. [DOI] [PubMed] [Google Scholar]
- Dassonville P, Zhu XH, Uurbil K, Kim SG, Ashe J. Functional activation in motor cortex reflects the direction and the degree of handedness. Proc Natl Acad Sci U S A. 1997;94:14015–14018. doi: 10.1073/pnas.94.25.14015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson T, Tremblay F. Hemispheric differences in corticospinal excitability and in transcallosal inhibition in relation to degree of handedness. PLoS ONE. 2013;8:e70286. doi: 10.1371/journal.pone.0070286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davranche K, Tandonnet C, Burle B, Meynier C, Vidal F, Hasbroucq T. The dual nature of time preparation: neural activation and suppression revealed by transcranial magnetic stimulation of the motor cortex. Eur J Neurosci. 2007;25:3766–3774. doi: 10.1111/j.1460-9568.2007.05588.x. [DOI] [PubMed] [Google Scholar]
- Domenech P, Dreher JC. Decision threshold modulation in the human brain. J Neurosci. 2010;30:14305–14317. doi: 10.1523/JNEUROSCI.2371-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Ivry RB. Role of corticospinal suppression during motor preparation. Cereb Cortex. 2009;19:2013–2024. doi: 10.1093/cercor/bhn230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Mazzocchio R, Dambrosia J, Murase N, Olivier E, Cohen LG. Kinematically specific interhemispheric inhibition operating in the process of generation of a voluntary movement. Cereb Cortex. 2005;15:588–593. doi: 10.1093/cercor/bhh160. [DOI] [PubMed] [Google Scholar]
- Duque J, Lew D, Mazzocchio R, Olivier E, Ivry RB. Evidence for two concurrent inhibitory mechanisms during response preparation. J Neurosci. 2010;30:3793–3802. doi: 10.1523/JNEUROSCI.5722-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Labruna L, Verset S, Olivier E, Ivry RB. Dissociating the role of prefrontal and premotor cortices in controlling inhibitory mechanisms during motor preparation. J Neurosci. 2012;32:806–816. doi: 10.1523/JNEUROSCI.4299-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Olivier E, Rushworth M. Top-down inhibitory control exerted by the medial frontal cortex during action selection under conflict. J Cogn Neurosci. 2013;25:1634–1648. doi: 10.1162/jocn_a_00421. [DOI] [PubMed] [Google Scholar]
- Duque J, Labruna L, Cazares C, Ivry RB. Dissociating the influence of response selection and task anticipation on corticospinal suppression during response preparation. Neuropsychologia. 2014;26:269–278. doi: 10.1016/j.neuropsychologia.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Inter-hemispheric inhibition of the human motor cortex. J Physiol. 1992;453:525–546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JI, Shadlen MN. The neural basis of decision making. Annu Rev Neurosci. 2007;30:535–574. doi: 10.1146/annurev.neuro.29.051605.113038. [DOI] [PubMed] [Google Scholar]
- Goldenberg G. Apraxia and the parietal lobes. Neuropsychologia. 2009;47:1449–1459. doi: 10.1016/j.neuropsychologia.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Goldenberg G. Apraxia – the cognitive side of motor control. Cortex. 2014;57:270–274. doi: 10.1016/j.cortex.2013.07.016. [DOI] [PubMed] [Google Scholar]
- Haaland KY. Left hemisphere dominance for movement. Clin Neuropsychol. 2006;20:609–622. doi: 10.1080/13854040590967577. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Elsinger CL, Mayer AR, Durgerian S, Rao SM. Motor sequence complexity and performing hand produce differential patterns of hemispheric lateralization. J Cogn Neurosci. 2004;16:621–636. doi: 10.1162/089892904323057344. [DOI] [PubMed] [Google Scholar]
- Hammond G. Correlates of human handedness in primary motor cortex: a review and hypothesis. Neurosci Biobehav Rev. 2002;26:285–292. doi: 10.1016/s0149-7634(02)00003-9. [DOI] [PubMed] [Google Scholar]
- Hammond G, Faulkner D, Byrnes M, Mastaglia F, Thickbroom G. Transcranial magnetic stimulation reveals asymmetrical efficacy of intracortical circuits in primary motor cortex (Experimentelle Hirnforschung Experimentation cerebrale) Exp Brain Res. 2004;155:19–23. doi: 10.1007/s00221-003-1696-x. [DOI] [PubMed] [Google Scholar]
- Hasbroucq T, Kaneko H, Akamatsu M, Possamai CA. Preparatory inhibition of cortico-spinal excitability: a transcranial magnetic stimulation study in man. Brain Res Cogn Brain Res. 1997;5:185–192. doi: 10.1016/s0926-6410(96)00069-9. [DOI] [PubMed] [Google Scholar]
- Hasbroucq T, Osman A, Possamai CA, Burle B, Carron S, et al. Cortico-spinal inhibition reflects time but not event preparation: neural mechanisms of preparation dissociated by transcranial magnetic stimulation. Acta Psychol. 1999;101:243–266. doi: 10.1016/s0001-6918(99)00007-4. [DOI] [PubMed] [Google Scholar]
- Hayashi MJ, Saito DN, Aramaki Y, Asai T, Fujibayashi Y, Sadato N. Hemispheric asymmetry of frequency-dependent suppression in the ipsilateral primary motor cortex during finger movement: a functional magnetic resonance imaging study. Cereb Cortex. 2008;18:2932–2940. doi: 10.1093/cercor/bhn053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herve PY, Leonard G, Perron M, Pike B, Pitiot A, et al. Handedness, motor skills and maturation of the corticospinal tract in the adolescent brain. Hum Brain Mapp. 2009;30:3151–3162. doi: 10.1002/hbm.20734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herve PY, Zago L, Petit L, Mazoyer B, Tzourio-Mazoyer N. Revisiting human hemispheric specialization with neuroimaging. Trends Cogn Sci. 2013;17:69–80. doi: 10.1016/j.tics.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Johnson-Frey SH, Newman-Norlund R, Grafton ST. A distributed left hemisphere network active during planning of everyday tool use skills. Cereb Cortex. 2005;15:681–695. doi: 10.1093/cercor/bhh169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Basso MA. A probabilistic strategy for understanding action selection. J Neurosci. 2010;30:2340–2355. doi: 10.1523/JNEUROSCI.1730-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein PA, Olivier E, Duque J. Influence of reward on corticospinal excitability during movement preparation. J Neurosci. 2012;32:18124–18136. doi: 10.1523/JNEUROSCI.1701-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein PA, Petitjean C, Olivier E, Duque J. Top-down suppression of incompatible motor activations during response selection under conflict. NeuroImage. 2014;86:138–149. doi: 10.1016/j.neuroimage.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Leocani L, Cohen LG, Wassermann EM, Ikoma K, Hallett M. Human corticospinal excitability evaluated with transcranial magnetic stimulation during different reaction time paradigms. Brain. 2000;123(Pt 6):1161–1173. doi: 10.1093/brain/123.6.1161. [DOI] [PubMed] [Google Scholar]
- Macdonell RA, Shapiro BE, Chiappa KH, Helmers SL, Cros D, et al. Hemispheric threshold differences for motor evoked potentials produced by magnetic coil stimulation. Neurology. 1991;41:1441–1444. doi: 10.1212/wnl.41.9.1441. [DOI] [PubMed] [Google Scholar]
- Matsunami K, Hamada I. Effects of stimulation of corpus callosum on precentral neuron activity in the awake monkey. J Neurophysiol. 1984;52:676–691. doi: 10.1152/jn.1984.52.4.676. [DOI] [PubMed] [Google Scholar]
- Michelet T, Duncan GH, Cisek P. Response competition in the primary motor cortex: corticospinal excitability reflects response replacement during simple decisions. J Neurophysiol. 2010;104:119–127. doi: 10.1152/jn.00819.2009. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Opie GM, Semmler JG. Modulation of short- and long-interval intracortical inhibition with increasing motor evoked potential amplitude in a human hand muscle. Clin Neurophysiol. 2014;125:1440–1450. doi: 10.1016/j.clinph.2013.11.015. [DOI] [PubMed] [Google Scholar]
- Pool EM, Rehme AK, Fink GR, Eickhoff SB, Grefkes C. Handedness and effective connectivity of the motor system. NeuroImage. 2014;99:451–460. doi: 10.1016/j.neuroimage.2014.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prut Y, Fetz EE. Primate spinal interneurons show pre-movement instructed delay activity. Nature. 1999;401:590–594. doi: 10.1038/44145. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Johansen-Berg H, Gobel SM, Devlin JT. The left parietal and premotor cortices: motor attention and selection. NeuroImage. 2003;20(Suppl. 1):S89–S100. doi: 10.1016/j.neuroimage.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Buckley MJ, Behrens TE, Walton ME, Bannerman DM. Functional organization of the medial frontal cortex. Curr Opin Neurobiol. 2007;17:220–227. doi: 10.1016/j.conb.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Sainburg RL. Convergent models of handedness and brain lateralization. Front Psychol. 2014;5:1092. doi: 10.3389/fpsyg.2014.01092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salerno A, Georgesco M. Interhemispheric facilitation and inhibition studied in man with double magnetic stimulation. Electroencephalogr Clin Neurophysiol. 1996;101:395–403. [PubMed] [Google Scholar]
- Sartori L, Cavallo A, Bucchioni G, Castiello U. Corticospinal excitability is specifically modulated by the social dimension of observed actions. Exp Brain Res. 2011;211:557–568. doi: 10.1007/s00221-011-2650-y. [DOI] [PubMed] [Google Scholar]
- Schluter ND, Krams M, Rushworth MF, Passingham RE. Cerebral dominance for action in the human brain: the selection of actions. Neuropsychologia. 2001;39:105–113. doi: 10.1016/s0028-3932(00)00105-6. [DOI] [PubMed] [Google Scholar]
- Serrien DJ, Ivry RB, Swinnen SP. Dynamics of hemispheric specialization and integration in the context of motor control. Nat Rev Neurosci. 2006;7:160–166. doi: 10.1038/nrn1849. [DOI] [PubMed] [Google Scholar]
- Solodkin A, Hlustik P, Noll DC, Small SL. Lateralization of motor circuits and handedness during finger movements. Eur J Neurol. 2001;8:425–434. doi: 10.1046/j.1468-1331.2001.00242.x. [DOI] [PubMed] [Google Scholar]
- Swann N, Tandon N, Canolty R, Ellmore TM, McEvoy LK, et al. Intracranial EEG reveals a time- and frequency-specific role for the right inferior frontal gyrus and primary motor cortex in stopping initiated responses. J Neurosci. 2009;29:12675–12685. doi: 10.1523/JNEUROSCI.3359-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triggs WJ, Subramanium B, Rossi F. Hand preference and transcranial magnetic stimulation asymmetry of cortical motor representation. Brain Res. 1999;835:324–329. doi: 10.1016/s0006-8993(99)01629-7. [DOI] [PubMed] [Google Scholar]
- Usher M, McClelland JL. Loss aversion and inhibition in dynamical models of multialternative choice. Psychol Rev. 2004;111:757–769. doi: 10.1037/0033-295X.111.3.757. [DOI] [PubMed] [Google Scholar]
- Vandermeeren Y, Davare M, Duque J, Olivier E. Reorganization of cortical hand representation in congenital hemiplegia. Eur J Neurosci. 2009;29:845–854. doi: 10.1111/j.1460-9568.2009.06619.x. [DOI] [PubMed] [Google Scholar]
- Verstynen T, Ivry RB. Network dynamics mediating ipsilateral motor cortex activity during unimanual actions. J Cogn Neurosci. 2011;23:2468–2480. doi: 10.1162/jocn.2011.21612. [DOI] [PubMed] [Google Scholar]
- Verstynen T, Diedrichsen J, Albert N, Aparicio P, Ivry RB. Ipsilateral motor cortex activity during unimanual hand movements relates to task complexity. J Neurophysiol. 2005;93:1209–1222. doi: 10.1152/jn.00720.2004. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Hallett M. Hemispheric asymmetry of ipsilateral motor cortex activation during unimanual motor tasks: further evidence for motor dominance. Clin Neurophysiol. 2001;112:107–113. doi: 10.1016/s1388-2457(00)00502-2. [DOI] [PubMed] [Google Scholar]


