Abstract
Background:
The role of tumour inflammation and the dysplastic epithelial-stromal interactions on the nature of collagen fibres in the extracellular matrix of dysplastic epithelium is not fully understood. The present study was aimed to evaluate and compare the inflammation and pathological stromal collagen (loosely packed thin disorganized collagen) present in mild, moderate and severe epithelial dysplasias with that of inflammatory fibrous hyperplasias. The basement membrane intactness of epithelial dysplasias was also evaluated to determine if dysplastic epithelial mesenchymal interaction has any role in the integrity of stromal collagen in epithelial dysplasia.
Methods:
Oral epithelial dysplasias, inflammatory fibrous hyperplasia and normal oral mucosal samples were used for the study. Packing, thickness and orientation of collagen fibres in mild, moderate and severe grades of oral epithelial dysplasias (n = 24), inflammatory fibrous hyperplasia (n = 8) and normal oral mucosal samples (n = 8) were analysed based on the polarisation of collagen fibres in picrosirius red polarising stain under polarising microscope.
Results:
All the grades of epithelial dysplasias showed greenish yellow birefringence confirming the presence of loosely arranged pathological collagen in the presence of moderate inflammation. All the cases of inflammatory fibrous hyperplasia showed red polarisation hue and moderate inflammation. A statistically significant difference was found in the packing and orientation of collagen when epithelial dysplasias and inflammatory fibrous hyperplasia were compared (P < 0.01). When the intactness of basement membrane integrity was compared in all the groups of epithelial dysplasia, a statistically significant result was obtained (P < 0.05).
Conclusions:
Presence of significant amount of loosely packed thin disoriented collagen even in mild epithelial dysplasia suggests that tumourigenic factors are released to connective tissue stroma much earlier than expected. Hence we suggest considering the integrity of extracellular matrix collagen, intactness of basement membrane and inflammation associated with dysplasia along with the anaplasia of epithelial cells in the microscopic assessment of dysplastic epithelium.
Keywords: Oral mucosa, Dysplasia, Extracellular matrix, Collagen
INTRODUCTION
Oral squamous cell carcinoma (OSCC) is usually preceded with epithelial dysplasia (ED) which is characterized by the anaplastic changes in stratified squamous epithelial cells. These anaplastic cells gradually degrade the basal lamina (BL) and invade into the underlying connective tissue stroma. The tensile strength or stiffness of the stromal extracellular matrix collagen (EMC) in the connective tissue can regulate the epithelial cell growth and migration in ED and OSCC. However, the dysplastic epithelial-stromal interactions and nature of collagen fibres in the extracellular matrix of dysplastic epithelium is not fully understood.
ED and oral squamous cell carcinoma are usually associated with chronic inflammation. Tumour inflammation is different from the self limiting normal inflammation and other inflammatory lesions. Dysplastic epithelial cells produce tumourigenic cytokines which attract granulocytes and macrophages.1 These innate immune cells can secrete matrix metalloproteinases (MMPs) and interleukins which alter the stromal collagen allowing the invasion of dysplastic epithelial cells.2 Additionally, integrity of stromal collagen can be modulated by lysyloxidases secreted by fibroblasts.3 The tensile strength or stiffness of extracellular matrix can regulate epithelial cell invasion and migration.4 BL is the barrier for the dysplastic epithelial cells from invading into the stroma. It is secreted and synthesised by epithelial cells and connective tissue.5,6 Once the BL is fully or partially degraded, the dysplastic epithelial cells directly interact with the extracellular matrix of connective tissue and invade.7
The collagen fibre thickness as well as packing of collagen can be determined using Picrosirius red staining followed by polarizing microscopy. The examination of collagen fibres by this method helps to differentiate normally packed thick bundles of collagen fibres and pathologic collagen (thin and loosely packed disorganised collagen). Red polarization hue is indicative of thick fibres of closely packed collagen and greenish polarization hue is indicative of loosely packed thin pathologic collagen.8 Picrosirius red is an elongated dye molecule which reacts with collagen and promotes the enhancement of its normal birefringence due to the fact that the dye molecules are aligned parallel with long axis of each collagen molecule.9 The alteration in stromal microenvironment around ED or neoplasm can predict the prognosis of the condition and is also useful in generating new therapeutic targets.
MATERIALS AND METHODS
The study group consisted of 40 samples of formalin fixed paraffin-embedded tissue blocks retrieved from the Department of Oral Pathology and Microbiology, Mar Baselios Dental College, Kerala, India. Ethical clearance was obtained prior to this study from the concerned ethical committee (Mar Baselios Medical Mission).
1. Picrosirius red polarising staining
EDs were classified into mild, moderate and severe according to the international histological classification of tumours. Eight samples of each grade of ED and eight samples of inflammatory fibrous hyperplasia along with eight normal oral mucosal samples were used for the study. Paraffin-embedded tissue blocks were sectioned at 5 μm thickness; the sections were floated onto the slides. Sections were incubated at 60°C on the slide warmer for the proper adhesion of sections to the slides. Sections were then deparaffinised, hydrated and stained with Picrosirius red stain (Sigma-Aldrich, St. Louis, MO, USA) for collagen and viewed under polarising microscope. picrosirius red stained slides were evaluated to determine the nature of collagen. Collagen fibres showed polarizing colours varying from orangish red (OR), yellowish orange (YO) and greenish yellow (GY). The percentage of OR, YO, GY fibres were assessed using Lawrence and Mayo grid eye piece in polarising microscope at 10 focal areas. The polarizing colours were assessed in subepithelial zone, intermediate zone and deep zone of the connective tissue stroma.
2. Periodic acid–schiff staining
Paraffin embedded sections of the same tissue blocks were sectioned at 5 μm, deparaffinised, hydrated and stained with periodic acid–Schiff (PAS) (DAKO, Carpinteria, CA, USA) to determine the basement membrane integrity in all the grades of EDs. The PAS basement membrane staining was graded as patchy or intact. The haematoxylin and eosin stained sections of the same slides were used to assess the amount of inflammation in each sample.
3. Statistical analysis
The data obtained was subjected to statistical analysis and basic variation statistical values were calculated. Chi-square test was used for comparison at significance level P < 0.05 with SPSS ver. 14 (SPSS Inc., Chicago, IL, USA).
RESULTS
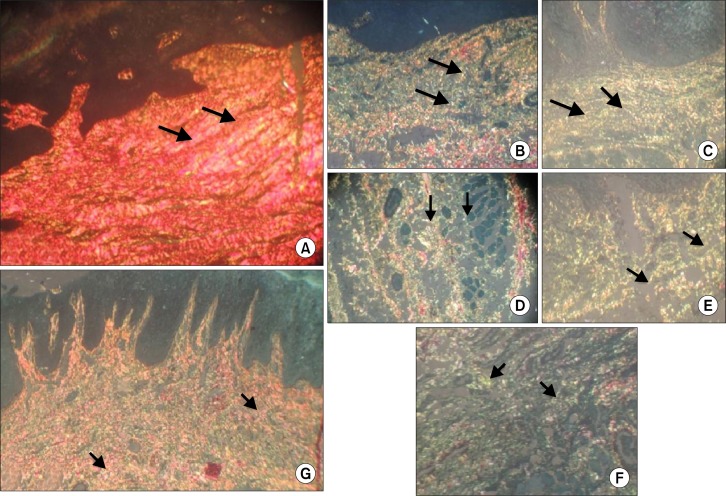
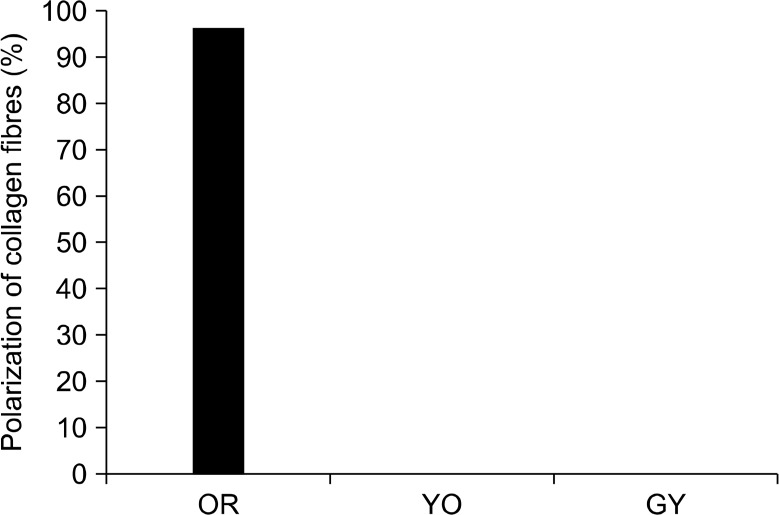
In the present study, 8 controls were stained with picrosirius red stain and they showed predominantly reddish orange bifringence in the lamina propria even in the presence of negligible amount of normal inflammatory cells (Fig. 1A and Fig. 2). All the grades of EDs showed moderate to severe inflammation. Interestingly, mild ED showed predominantly GY birefringence in connective tissue stroma. Some areas showed gradual transition of reddish orange and YO collagen fibres to GY (Fig. 1B). Moderate and severe dysplasia showed GY polarising birefringence predominantly in sub epithelial and deeper connective tissue stroma (Fig. 1C∼1F). On comparison between mild, moderate and severe EDs by chi square, no significant difference in the amount of pathological collagen was observed (P > 0.5) (Table 1).
Figure 1.
Picrosirius red polarising staining. (A) Normal buccal mucosa showing predominantly reddish orange bifringence in the lamina propria. Arrows showing bundles of collagen fibres with reddish orange polarising bifringence (×40). (B) Mild epithelial dysplasia with stromal collagen showing thin loosely packed and separated greenish yellow polarising collagen fibres. Arrows showing greenish yellow polarising collagen fibres and Areas of transition of yellowish orange and reddish orange collagen fibres to greenish yellow fibres (×40). (C) Moderate epithelial dysplasia showing greenish yellow polarising collagen fibres. Arrows indicating greenish yellow collagen fibres (×40). (D) Moderate epithelial dysplasia with deeper areas of connective tissue collagen showing greenish yellow bifringence and arrows indicating greenish yellow collagen fibres and areas of transition of yellowish orange and reddish orange collagen fibres to greenish yellow (×40). (E) Severe epithelail dysplasia with collagen fibers showing greenish yellow birefringence. Arrows indicating greenish yellow collagen fibres (×40). (F) Severe epithelial dysplasia with deeper areas of connective tissue stroma with collagen fibres showing greenish yellow birefringence. Arrowsindicating greenish yellow collagen fibres (×40). (G) Inflammatory fibrous hyperplasia showing reddish orange polarising birefringence predominantly. Arrows showing reddish orange collagen fibres (×10).
Figure 2.
Normal buccal mucosa showing orangish red (OR) hue in the presence of normal negligible inflammation. YO, yellowish orange; GY, greenish yellow.
Table 1.
Comparison of polarization colors of collagen fibers between various grades of epithelial dysplasias
| Group | GY predominant | OR predominant | YO predominant | Total |
|---|---|---|---|---|
| Mild dyspalsia | 7 (83.3) | 0 | 1 (12.5) | 8 |
| Moderate dysplasia | 8 (100) | 0 | 0 | 8 |
| Severe dysplasia | 8 (100) | 0 | 0 | 8 |
| Total | 23 | 0 | 1 | 24 |
Values are presented as number (%) or number only. GY, greenish yellow; OR, orangish red; YO, yellowish orange.
All the samples of inflammatory fibrous hyperplasia showed red polarising birefringence and moderate to severe inflammation (Fig. 1G). Red polarising hue is suggestive of closely packed normally oriented collagen fibres. Statistically significant result was found by chi square when the polarisation of collagen of ED and inflammatory fibrous hyperplasia was compared (P <0.01) (Table 2). All the grades of EDs and inflammatory fibrous hyperplasia showed moderate amount of chronic inflammation. However, in the case of ED loosely packed GY disoriented collagen was found in the presence of moderate and severe inflammation (Table 3). Importantly, collagen fibres present in inflammatory fibrous hyperplasia showed reddish polarising bifringence in the presence of inflammation (Table 4).
Table 2.
Comparison of polarisation of collagen fibres between epithelial dysplasia and inflammatory fibrous hyperplasia
| Group | GY predominant | OR predominant | YO predominant | Total |
|---|---|---|---|---|
| Epithelial dysplasias | 23 (17.25) [1.92] | 0 (6.00) [6.00] | 1 (0.75) [0.08] | 24 (24.00) [0.00] |
| Fibrous hyperplasia | 0 (5.75) [5.75] | 8 (2.00) [18.00] | 0 (0.25) [0.25] | 8 (8.00) [0.00] |
| Total | 23 | 8 | 1 | 32 |
The chi-square statistic (χ2) is 32. The P-value is < 0.00001. The result is significant at P < 0.01. Values are presented as the observed number (the expected number totals), and [the chi-square statistic for each number]. GY, greenish yellow; OR, orangish red; YO, yellowish orange.
Table 3.
Polarisation of collagen in moderate and severe grades of inflammation in epithelial dysplasia
| Group | GY predominant | OR predominant | YO predominant | Total |
|---|---|---|---|---|
| Moderate infalmmation | 14 (100) | 0 | 1 | 15 |
| Severe infalmmation | 9 (100) | 0 | 0 | 9 |
| Total | 24 | 0 | 0 | 24 |
Values are presented as number (%) or number only. GY, greenish yellow; OR, orangish red; YO, yellowish orange.
Table 4.
Polarisation of collagen in moderate and severe grades of inflammation in inflammatory fibrous hyperplasia
| Group | GY predominant | OR predominant | YO predominant | Total |
|---|---|---|---|---|
| Moderate infalmmation | 0 | 5 (100) | 0 | 5 |
| Severe infalmmation | 0 | 3 (100) | 0 | 3 |
Values are presented as number (%) or number only. GY, greenish yellow; OR, orangish red; YO, yellowish orange.
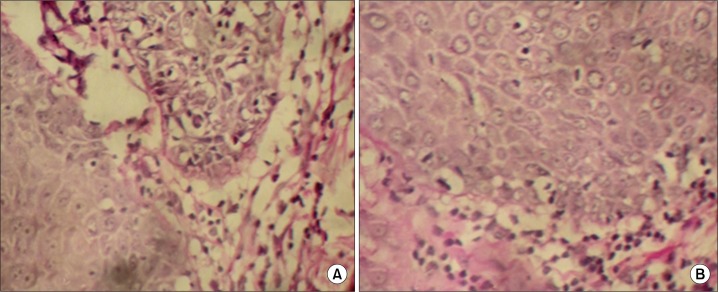
When the integrity of basement membrane was analysed with Per Iodic Acid Schiff staining, all the cases of mild ED showed intact basement membrane characterized by increased and uniform PAS staining. Out of eight cases of moderate dysplasia, four cases showed patchy PAS staining of BL and out of eight cases of severe dysplasia, five cases showed patchy PAS staining of BL (Fig. 3). A statistically significant difference (P < 0.05) was observed on comparison of basal lamina integrity between 3 grades of epithelial dysplasias using chi-square test (Table 5).
Figure 3.
(A) Periodic acid–Schiff (PAS) stained moderate epithelial dysplasia showing patchiness at basement membrane zone (×40). (B) PAS stained severe epithelial dysplasia showing patchiness at basement membrane zone (×40).
Table 5.
Comparison of integrity of BL in various grades of epithelial dysplasias by Per Iodic Acid Schiff method
| Group | Inatact BL | Patchy BL |
|---|---|---|
| Mild dyspalsia | 8 (5.33) [1.33] | 0 (2.67) [2.67] |
| Moderate dysplasia | 6 (5.33) [0.08] | 2 (2.67) [0.17] |
| Severe dysplasia | 2 (5.33) [2.08] | 6 (2.67) [4.17] |
The chi-square statistic (χ2) is 10.5. The P-value is 0.005248. The result is significant at P < 0.05. Values are presented as the observed number, (the expected number totals) and [the chi-square statistic for each number]. BL, basal lamina.
DISCUSSION
One important hall mark of cancer progression is the degradation of the extracellular matrix which allows the cancer cells to invade the surrounding stroma.10 Dysplastic epithelial cells promote inflammation, produce growth factors, hormones, cytokines and proteinases like MMP.11
In the present study even in mild ED we observed pathological (thin and loosely packed and disorganized) collagen exhibiting GY birefringence in polarising microscopy. In moderate and severe EDs also collagen showed GY birefringence. In our study we could find moderate to severe amount of chronic inflammation in all the grades of EDs and the presence of loosely arranged disoriented collagen fibres. Chronic inflammation associated with ED could be one of the reasons for the disorganisation and loose packing of collagen fibres. Anaplastic cells produce cytokines that attract innate immune cells such as granulocytes and macrophages. These innate immune cells can secrete MMPs and reactive oxygen species which can result in EMC degradation.12 Lymphocyte was found to be more in all the grades of dysplasias. Studies have postulated that T lymphocyte cells could suppress that anti-tumour immune response and intra-tumoural depletion of T cells can lead to the eradication of an established tumour along with development of long-term antitumor memory.13,14 Presence of closely packed collagen fibers with red polarization hue in inflammatory fibrous hyperplasia suggests that tumour inflammation is different from other types of inflammation. This could be because in inflammatory fibrous hyperplasia, the inflammatory cells may induce the liberation of TGF β into the extracellular matrix. TGF β is a potent fibrogenic molecule which plays a synergic role in fibrous hyperplasia.15 All the controls showed OR polarization in extracellular matrix even in the presence of normal negligible inflammation. This indicates that tumour inflammatory cells in ED have the potential to liberate chemokines and extracellular matrix degrading factors whereas normal inflammatory cells in normal mucosa are protective in its function. Therefore, we can suggest that the inflammatory response present in inflammatory reactions is different from tumour inflammation.
Once the BL was fully or partially degraded, the neoplastic cells interacts with connective tissue stroma in which type 1 collagen is disintegrated, facilitating tumour invasion.16,17 In our study groups, moderate and severe dysplasia exhibited patchy staining pattern of PAS at the BL zone and GY thin loosely packed collagen fibres instead of thick reddish fibres in subepithelial zone and deeper zone. Along with tumour associated inflammatory cells, BL disintegration followed by epithelial mesenchymal interaction could be one of the reasons for the disintegration of stromal collagen.
Interaction between tumour cells and extracellular matrix components is essential for tumour growth and metastatic activity.18,19 In our previous study on the expression of tumourogenic factor (inducible nitric oxide synthase enzyme) in all the grades of dysplasia we observed positivity even in mild dysplasia.20 Hence, in the early phase of dysplasia itself tumourogenic factors are released from the cells which could alter stroma and adjacent cells. In the present study the presence of thin loosely packed pathological collagen in mild dysplasia could be due to tumour associated inflammation and the liberation of tumourogenic factors like proteinases in the early phase of dysplasia itself causing collagen disintegration giving a GY bifringence under polarising microscopy. Changes in collagen fibre structure represent a major disorganization of basic skeleton of extracellular matrix.21 This may be the reason for malignant transformation occurring in epithelium associated with lower grades of dysplasia.
In the present study normal collagen is replaced by thinner GY loosely packed collagen in mild, moderate and severe EDs. In the presence of moderate inflammation also, inflammatory fibrous hyperplasia showed thick bundles of normally oriented collagen fibres. Tumour inflammation, tumourogenic factors liberated by anaplastic epithelial cells and anaplastic epithelial mesenchymal interaction could be the reason for extracellular matrix degradation in EDs. In our study, even the mild ED showed loosely packed disorganised collagen fibres. Thus it is logical to assume that oral precancer may undergo carcinogenic changes in extracellular matrix level before exhibiting clinical evidence. This pathological collagen may not be evident in microscopic haematoxylin and eosin stained sections. Hence, we suggest that grading of ED based on the microscopic histology of epithelial cells alone is insufficient for the comprehensive diagnosis, treatment plan and prognosis prediction of oral precancers. Integrity of EMC, intactness of basement memeberane and tumour associated inflammation along with the anaplasia of epithelial cells have to be evaluated in the microscopic diagnosis of ED.
Footnotes
CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
REFERENCES
- 1.Mantovani A. Cancer: inflammation by remote control. Nature. 2005;435:752–3. doi: 10.1038/435752a. [DOI] [PubMed] [Google Scholar]
- 2.Takayama S, Hatori M, Kurihara Y, Kinugasa Y, Shirota T, Shintani S. Inhibition of TGF-beta1 suppresses motility and invasiveness of oral squamous cell carcinoma cell lines via modulation of integrins and down-regulation of matrix-metalloproteinases. Oncol Rep. 2009;21:205–10. [PubMed] [Google Scholar]
- 3.Kagan HM, Li W. Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J Cell Biochem. 2003;88:660–72. doi: 10.1002/jcb.10413. [DOI] [PubMed] [Google Scholar]
- 4.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–54. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Engbring JA, Kleinman HK. The basement membrane matrix in malignancy. J Pathol. 2003;200:465–70. doi: 10.1002/path.1396. [DOI] [PubMed] [Google Scholar]
- 6.Williams HK. Molecular pathogenesis of oral squamous carcinoma. Mol Pathol. 2000;53:165–72. doi: 10.1136/mp.53.4.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 8.de Paula AM, Carvalhais JN, Domingues MG, Barreto DC, Mesquita RA. Cell proliferation markers in the odontogenic keratocyst: effect of inflammation. J Oral Pathol Med. 2000;29:477–82. doi: 10.1034/j.1600-0714.2000.291001.x. [DOI] [PubMed] [Google Scholar]
- 9.Montes GS, Junqueira LC. The use of the Picrosirius-polarization method for the study of the biopathology of collagen. Mem Inst Oswaldo Cruz. 1991;86(Suppl 3):1–11. doi: 10.1590/S0074-02761991000700002. [DOI] [PubMed] [Google Scholar]
- 10.Vilen ST, Salo T, Sorsa T, Nyberg P. Fluctuating roles of matrix metalloproteinase-9 in oral squamous cell carcinoma. Scientific World Journal. 2013;2013:920595. doi: 10.1155/2013/920595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: how hot is the link? Biochem Pharmacol. 2006;72:1605–21. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 12.Ribatti D, Crivellato E. Mast cells, angiogenesis, and tumour growth. Biochim Biophys Acta. 2012;1822:2–8. doi: 10.1016/j.bbadis.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Chakraborty NG, Twardzik DR, Sivanandham M, Ergin MT, Hellstrom KE, Mukherji B. Autologous melanoma-induced activation of regulatory T cells that suppress cytotoxic response. J Immunol. 1990;145:2359–64. [PubMed] [Google Scholar]
- 14.Linehan DC, Goedegebuure PS. CD25+ CD4+ regulatory T-cells in cancer. Immunol Res. 2005;32:155–68. doi: 10.1385/IR:32:1-3:155. [DOI] [PubMed] [Google Scholar]
- 15.Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331:1286–92. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 16.Fenyvesi A. The prognostic significance of type IV collagen expression in colorectal carcinomas. Arch Oncol. 2003;11:65–70. doi: 10.2298/AOO0302065F. [DOI] [Google Scholar]
- 17.Hilska M, Collan Y, Peltonen J, Gullichsen R, Paajanen H, Laato M. The distribution of collagen type-i, type-iii, and type-iv in normal and malignant colorectal mucosa. European Journal of Surgery. 1998;164:457–64. doi: 10.1080/110241598750004274. [DOI] [PubMed] [Google Scholar]
- 18.De Wever O, Mareel M. Role of tissue stroma in cancer cell invasion. J Pathol. 2003;200:429–47. doi: 10.1002/path.1398. [DOI] [PubMed] [Google Scholar]
- 19.Pietras K, Ostman A. Hallmarks of cancer: interactions with the tumor stroma. Exp Cell Res. 2010;316:1324–31. doi: 10.1016/j.yexcr.2010.02.045. [DOI] [PubMed] [Google Scholar]
- 20.Varghese SS, Sunil PM, Madhavan RN. Expression of inducible nitric oxide synthase (iNOS) in oral precancer and oral squamous cell carcinoma: an immunohistochemical study. Cancer Biomark. 2010–2011;8:155–60. doi: 10.3233/CBM-2011-0207. [DOI] [PubMed] [Google Scholar]
- 21.Martins GB, Reis SR, Silva TM. Collagen type I expression in squamous cell carcinoma of the oral cavity. Pesqui Odontol Bras. 2003;17:82–8. doi: 10.1590/S1517-74912003000100016. [DOI] [PubMed] [Google Scholar]