Abstract
Background
The HPTN 052 study demonstrated a 96% reduction in HIV transmission in discordant couples using antiretroviral therapy (ART).
Objective
To predict the epidemic impact of treating HIV-discordant couples to prevent transmission.
Design
Mathematical modeling to predict incidence reduction and the number of infections prevented.
Methods
Demographic and epidemiological data from Ghana, Lesotho, Malawi and Rwanda were used to parameterize the model. ART was assumed to be 96% effective in preventing transmission.
Results
Our results show there would be a fairly large reduction in incidence and a substantial number of infections prevented in Malawi. However, in Ghana a large number of infections would be prevented, but only a small reduction in incidence. Notably, the predicted number of infections prevented would be similar (and low) in Lesotho and Rwanda, but incidence reduction would be substantially greater in Lesotho than Rwanda. The higher the proportion of the population in stable partnerships (whether concordant or discordant), the greater the effect of a discordant couple’s intervention on HIV epidemics.
Conclusion
The effectiveness of a discordant couples intervention in reducing incidence will vary among countries due to differences in HIV prevalence and the percentage of couples that are discordant (i.e. degree of discordancy). The number of infections prevented within a country, as a result of an intervention, will depend upon a complex interaction among three factors: population size, HIV prevalence and degree of discordancy. Our model provides a quantitative framework for identifying countries most likely to benefit from treating discordant couples to prevent transmission.
Keywords: antiretroviral therapy, infections prevented, mathematical modeling, prevention of HIV transmission
Introduction
Over the past decade, remarkable progress has been achieved in the scale-up of HIV treatment in low and middle-income countries, particularly in sub-Saharan Africa [1]. However, approximately 2 million individuals globally still acquire HIV infection on an annual basis [1]. In rapid sequence, the results of several clinical trials have re-energized the HIV prevention agenda. Three clinical trials have demonstrated the effectiveness of male circumcision for prevention of HIV acquisition in men [2]. Two recent trials have shown the efficacy of vaginal tenofovir gel and oral tenofovir/emtricitabine for preventing HIV acquisition in women and MSM, respectively [3,4]. Additionally, recent results from the HPTN 052 randomized clinical trial demonstrated that treating HIV-infected partners in discordant couples with antiretroviral therapy (ART) was associated with a 96% decrease in HIV acquisition by the uninfected sexual partners [5]. Earlier evidence from observational studies of HIV discordant couples in sub-Saharan Africa had also demonstrated that the use of ART by the HIV-infected partner was associated with a significant decrease in risk of HIV transmission to the uninfected partner [6,7]. However, the impact of treating discordant couples in reducing HIV epidemics will depend upon the degree to which transmission in these couples contributes to the country-level incidence rate.
The importance of discordant couples in driving an HIV epidemic is a controversial issue. In one study from Rwanda and Zambia, the authors indicated that transmission within discordant married or cohabitating couples could be responsible for up to 60% of new infections [8]. Conflicting findings were reported from Uganda, where transmission of HIV in stable discordant couples accounted for a minority of observed transmissions [9]. Moreover, in a recently published modeling study the complexity of this issue was highlighted by the results, which showed that the contribution of transmission in discordant couples to the overall epidemic is very sensitive to both the overall prevalence of HIV and the proportion of couples that are in stable partnership [10]. Notably, these modeling results could explain the conflicting findings between the empirical studies.
Mathematical modeling is a useful tool to estimate the impact of public health interventions on HIV epidemics. The first modeling analysis of the effect of ART as prevention was published over a decade ago and predicted the impact of treatment in preventing infections in the MSM community in San Francisco [11]. A subsequent modeling study demonstrated that widespread use of treatment could eliminate HIV epidemics [12]. A more recent modeling study has corroborated these earlier results [13].
In this study, we use mathematical modeling to assess the implications of the HPTN 052 study results for the global control of HIV epidemics. Specifically, we predict the potential effect of treating discordant couples to prevent transmission on reducing the annual incidence rate in Lesotho, Malawi, Rwanda and Ghana; we also calculate the annual number of infections that would be prevented in each country.
Methods
To conduct our analyses we used a previously published mathematical model in which individuals in the sexually active population are divided into two groups: one composed of stable couples and the other of individuals in short-term and/or concurrent partnerships [14]. In the model, stable couples include those that are concordant positive, concordant negative or discordant. The model was modified to assess the epidemic-level effect of treating HIV-infected partners in stable discordant couples to prevent transmission. Technical details of the model are given in the Appendix, http://links.lww.com/QAD/A182.
We selected four countries in sub-Saharan Africa (Ghana, Lesotho, Malawi and Rwanda) to predict the potential effect of treating discordant couples to prevent transmission. We predicted the effect on reducing the annual incidence rate and we also calculated the number of infections prevented at various coverage levels. The four countries we analyzed differ significantly in population size (Table 1). They also vary in HIV prevalence levels (19.5% in Lesotho, 7.1% in Malawi, 1.7% in Rwanda and 0.9% in Ghana) and in the proportion of couples that are discordant (i.e. discordancy rates: 13.6% in Lesotho, 9.7% in Malawi, 3.1% in Rwanda and 2% in Ghana) [14].
Table 1.
Country-specific population demographics and modeling estimates shown for Lesotho, Malawi, Rwanda and Ghana.
| Lesotho | Malawi | Rwanda | Ghana | |
|---|---|---|---|---|
| Total population sizea | 2 067 000 | 15 263 000 | 9 998 000 | 23 837 000 |
| Proportion 15–49 yearsb | 44.0% | 40.9% | 44.7% | 45.5% |
| Population 15–49 years | 910 000 | 6 243 000 | 4 469 000 | 10 846 000 |
| Number of individuals classified by couple serostatus (×103) | ||||
| Total in couples | 364–637 | 2497–4370 | 1788–3128 | 4338–7592 |
| Concordant negative | 243–426 | 2077–3634 | 1718–3007 | 4158–7276 |
| Concordant postive | 71–124 | 176–309 | 30–53 | 40–69 |
| Discordant | 49–87 | 234–426 | 39–68 | 140–245 |
| Male positive | 33–57 | 141–246 | 25–43 | 73–127 |
| Female positive | 17–29 | 103–180 | 14–25 | 68–118 |
| Total number of HIV infections per year in discordant couples Infections |
2470–4323 | 12 161–21 281 | 1940–3394 | 7006–12 261 |
Estimates for the number of individuals classified by couples serostatus (multiplied by 1000), are calculated using the model and assuming 40% (shown by the low end of the range) or 70% (shown by the high end of the range) of the population are in stable couples; stable couples include concordant positive, concordant negative and discordant.
World Health Organization country profiles (19).
Measure Demographic and Health Surveys (20).
We calibrated the model using country-level prevalence data. To conduct our analyses, we assumed transmission within stable discordant couples would be 10% per year [15], and that (as demonstrated in HPTN 052) ART would be 96% effective in reducing transmission within a discordant couple. Using country-specific data on prevalence, population size and discordancy rates [14], we calculated the number of couples in each of three mutually exclusive categories: concordant positive, concordant negative and discordant (Table 1). We then predicted the potential impact of treating discordant couples to prevent transmission assuming that 40 or 70% of the population are in stable partnerships; stable partnerships include couples that are concordant positive, concordant negative or discordant. We varied treatment coverage (i.e. the percentage of discordant couples that receive ART) from 0 to 100%. Technical details of our analyses are given in the Appendix, http://links.lww.-com/QAD/A182.
Results
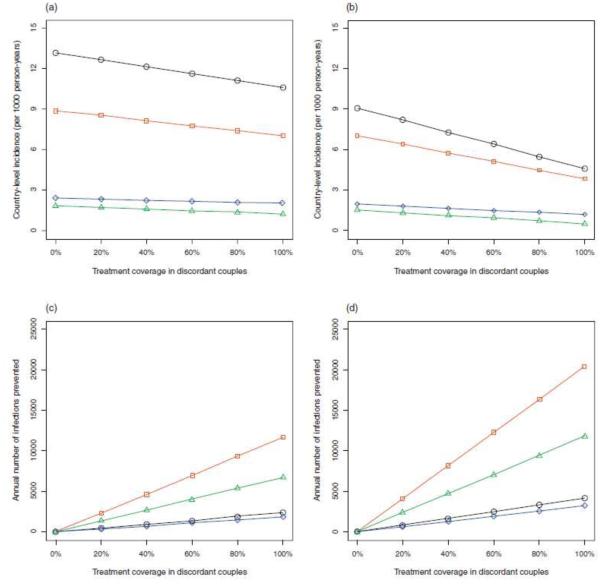
Figure 1a and b show the models’ predictions for the reduction in the annual HIV incidence rate in Ghana, Lesotho, Malawi and Rwanda as a function of increasing treatment coverage in discordant couples. Figure 1a was constructed based on the assumption that 40% of the population are in stable partnerships and Fig. 1b on the assumption that 70% of the population are in stable partnerships. Reduction in the incidence rate per 1000 person-years would be greatest in Lesotho, moderate in Malawi and minimal in Rwanda and Ghana. Even moderate treatment coverage for discordant couples would result in a significant reduction in incidence in Lesotho and Malawi. However, even a very high coverage of treatment would only slightly reduce the incidence rate in Rwanda and Ghana (Fig. 1a and b). The greater the proportion of the population that are in stable partnerships (whether they are concordant positive, concordant negative or discordant) the more effective treatment of discordant couples will be in reducing the annual incidence rate; compare Fig. 1a with Fig. 1b.
Figure 1. The impact of treating discordant couples to prevent transmission as a function of treatment coverage.
Treatment coverage (i.e., the percentage of discordant couples that receive antiretroviral therapy) ranges from 0 to 100%. Results are shown for Lesotho (black), Malawi (red), Rwanda (blue), and Ghana (green). The effect of treatment coverage on the annual incidence rate (per 1000 person-years) (a) and (b) and on the annual number of infections prevented (c) and (d). Calculations shown in (a) and (c) are based on the assumption that 40% of the population is in stable partnerships; stable partnerships include concordant positive, concordant negative and discordant couples. Calculations shown in (b) and (d) are based on the assumption that 70% of the population is in stable partnerships.
We also used the model to predict the annual number of infections that could be prevented per year in discordant couples, assuming the positive partners are treated with ART. Figure 1c and Fig. 1d show the effect of treatment coverage in discordant couples in Ghana, Lesotho, Malawi and Rwanda. Figure 1c was constructed based on the assumption that 40% of the population are in stable couples and Fig. 1d on the assumption that 70% of the population are in stable couples. These figures show the annual number of infections prevented in discordant couples would be greatest in Malawi, fairly high in Ghana and low in both Lesotho and Rwanda.
Our predictions, for Malawi, demonstrate that there would be a fairly large reduction in annual incidence and a substantial number of infections prevented if HIV treatment in discordant couples was considerably expanded. However, in Ghana there would be a large number of infections prevented, if treatment of discordant couples is expanded, but the reduction in annual incidence would be low. Notably, the predicted annual number of infections prevented is similar (and low) in Lesotho and Rwanda, but the reduction in annual incidence is substantially greater in Lesotho than Rwanda.
Discussion
Country-specific differences in the reduction in HIV incidence due to treatment of discordant couples to prevent transmission depends on two factors: HIV prevalence and the percentage of couples that are discordant. The higher the HIV prevalence and/or the greater the percentage of couples in discordant partnerships the more incidence will be reduced; therefore, the greatest reduction (among the four countries we have analyzed) is anticipated to occur in Lesotho. However, country-specific differences in the number of infections prevented are due to a complex interaction among three factors: population size, HIV prevalence, and the percentage of couples that are discordant. Due to this complex interaction effect it is not obvious which country will benefit the most – in terms of infections prevented – from treating HIV discordant couples to prevent transmission. For example, in our comparison of four countries, our modeling shows that Malawi would benefit the most even though it is neither the country with the greatest population size nor the one with the highest prevalence of HIV or the highest degree of discordancy. Our model provides a framework for quantifying the complex interactions between the demographic and epidemiological factors in order to identify which countries are likely to benefit the most from treating discordant couples to prevent transmission.
The findings from HPTN 052 raise important programmatic, clinical and ethical issues. From a programmatic perspective, to garner the benefits of treatment in discordant couples for prevention, even in countries where the anticipated effect would be substantial will require considerable efforts. Finding such discordant couples will require extensive efforts as their prevalence is loweven in countries with a high overall HIV prevalence. For example, in Lesotho, a country with approximately 20% HIV prevalence, only 14% of couples are estimated to be discordant, whereas approximately 67% are concordant HIV negative and 20% concordant positive [14]. Finding discordant couples will be even more challenging in countries with larger populations such as Ghana, Malawi and Rwanda (Table 1), and in countries where the percentage of couples that are discordant is lower than in Lesotho, for example 10% in Malawi, 3% in Rwanda and 2% in Ghana. Failure to identify discordant couples will result in low treatment coverage. For example, if only 50% of discordant couples in a population are detected and 80% of the positive partners in such couples are initiated on ART to prevent transmission, treatment coverage in discordant couples will only be 40%. In addition, concerted efforts will be needed to rapidly assess such couples, initiate ART for the HIV-infected partner and to provide supportive services to achieve high adherence with long-term maintenance of viral suppression to achieve the prevention potential of ART. At the same time it is unclear whether use of ART for prevention in discordant couples would be associated with risk behavior compensation and a decrease in use of other prevention methods. Thus, optimization of the prevention potential of providing ART for discordant couples will require substantial commitment and the allocation of appropriate resources.
The lack of certainty with regards to the individual clinical benefit of ART for the HIV-infected partner in a discordant partnership who otherwise would not be eligible for treatment based on their own HIV disease stage also raises ethical concerns. Findings from HPTN 052 demonstrated a statistically significant, albeit modest benefit of early versus delayed ART in the HIV-infected partners with a statistically significant higher risk of grade 3 or 4 laboratory abnormalities in the early ART group [5]. In addition, offering ART to individuals with earlier HIV disease for the purpose of prevention raises equity concerns, as there are currently millions with advanced HIV disease who urgently need ART for their own health and survival [1].
In summary, we have developed a model that provides a quantitative framework for identifying the characteristics of the countries most likely to benefit from treating discordant couples to prevent transmission. Our modeling shows that treatment of discordant couples is unlikely to be the sole answer for controlling HIV epidemics. However, we have shown that if high treatment coverage levels are reached for discordant couples, this intervention could significantly reduce incidence and prevent a substantial number of infections in certain countries. These insights can assist policy makers and public health practitioners in deciding whether to prioritize the identification and treatment of discordant couples as a key component of their HIV control strategy. Such an approach will enable the findings from HPTN 052 to be appropriately integrated into broader HIV control programs around the world.
Acknowledgements
We acknowledge the following support provided by NIH: Award#: 5RO1A183038-3, RO1AI041935 and R21AI086701.
Footnotes
Conflicts of interest
The authors have no conflicts of interest to disclose.
References
- 1.UNAIDS Global Report 2010 http://www.unaids.org/globalreport/documents/20101123_GlobalReport_full_en.pdf. [Accessed 15 June 2011]
- 2.Siegfried N, Muller M, Deeks JJ, Volmink J. Male circumcision for prevention of heterosexual acquisition of HIV in men. Cochrane Database Syst Rev. 2009;(2):CD003362. doi: 10.1002/14651858.CD003362.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–1174. doi: 10.1126/science.1193748. Epub 2010 Jul 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. Lancet. 2009;373:48–57. [Google Scholar]
- 5.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N. Prevention of HIV-1 Infection with Early Antiretroviral Therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donnell D, Baeten JM, Kiarie J, Thomas KK, Stevens W, Cohen CR, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet. 2010;375:2092–2098. doi: 10.1016/S0140-6736(10)60705-2. Epub 2010 May 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reynolds SJ, Makumbi F, Nakigozi G, Kagaayi J, Gray RH, Wawer M, et al. HIV-1 transmission among HIV-1 discordant couples before and after the introduction of antiretroviral therapy. AIDS. 2011;25:473–477. doi: 10.1097/QAD.0b013e3283437c2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunkle KL, Stephenson R, Karita E, Chomba E, Kayitenkore K, Vwalika C, et al. New heterosexually transmitted HIV infections in married or cohabiting couples in urban Zambia and Rwanda: an analysis of survey and clinical data. Lancet. 2008;371:2183–2191. doi: 10.1016/S0140-6736(08)60953-8. [DOI] [PubMed] [Google Scholar]
- 9.Gray R, Sempiija V, Shelton J, Serwadda D, Nalingoda F, Kagaayi J, et al. The contribution of HIV-discordant relationships to new HIV infections in Rakai, Uganda. AIDS. 2011;25:863–865. doi: 10.1097/QAD.0b013e3283448790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blower SM, Gershengorn HB, Grant RM. A tale of two futures: HIV and antiretroviral therapy in San Francisco. Science. 2000;287:650–654. doi: 10.1126/science.287.5453.650. [DOI] [PubMed] [Google Scholar]
- 11.Velasco-Hernandez JX, Gershengorn HB, Blower SM. Could widespread use of combination antiretroviral therapy eradicate HIV epidemics? Lancet Infect Dis. 2002;2:487–493. doi: 10.1016/s1473-3099(02)00346-8. [DOI] [PubMed] [Google Scholar]
- 12.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV-1 transmission: a mathematical model. Lancet. 2009;373:48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 13.Coburn BJ, Gerberry DJ, Blower S. Quantification of the role of discordant couples in driving incidence of HIV in sub-Saharan Africa. Lancet Infect Dis. 2011;11:263–264. doi: 10.1016/S1473-3099(11)70080-9. [DOI] [PubMed] [Google Scholar]
- 14.Eyawo O, de Walque D, Ford N, Gakii G, Lester RT, Mills EJ. HIV status in discordant couples in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:770–777. doi: 10.1016/S1473-3099(10)70189-4. [DOI] [PubMed] [Google Scholar]
- 15.Guthrie BL, de Bruyn G, Farquhar C. HIV-1-Discordant couples in sub-Saharan Africa: explanations and implications for high rates of discordancy. Curr HIV Res. 2007;5:416–429. doi: 10.2174/157016207781023992. [DOI] [PubMed] [Google Scholar]