Abstract
Conclusions about the cognitive and neural requirements of saccade control may differ as a result of stimulus presentation method. This issue was examined in the current study by evaluating behavioral differences in pro- and anti-saccade responses among 12 healthy young adults as a function of task presentation method, length of cue-to-target interval, and previous trial type. A one sec cue-to-target interval fostered goal neglect, indicated by an increase in uncorrected errors and reaction times for “error” saccades. There was also a strong relationship between speed of visual orienting (prosaccade latencies) and failed inhibition (antisaccade errors) for the simultaneous condition. Interestingly, only the simultaneous condition produced task switch costs (on saccade latencies and error response percentages). Saccadic task presentation method, therefore, can influence conclusions about the cognitive operations supporting successful performance.
Saccade paradigms are useful for assessing the cognitive and neural correlates of the flexible implementation of behavior (Opris & Bruce, 2005; Munoz & Everling, 2004; Everling & DeSouza, 2005). Saccadic eye movements are generally classified into two basic categories: visually guided (pro-) saccades and more complex volitional saccades, of which antisaccades are an example. Prosaccades require a rapid redirection of gaze toward a peripheral visual stimulus. Antisaccades are more difficult because they require the planning and execution of an eye movement to the mirror image location (in the opposite visual field) of a peripheral stimulus (Hallett, 1978; Hallett & Adams, 1980).
In the eye movement literature, saccades are elicited by stimuli with characteristics that vary across laboratories and paradigms, and which may be determined in part by recording methods. For instance, in the behavioral eye movement laboratory and for event-related brain imaging studies (fMRI, EEG, MEG), participants may perform pro- and anti-saccades either in separate runs or randomly interleaved within the same run. Because of requirements specific to fMRI, however, such studies frequently use “blocked designs” that typically follow one of two patterns: (i) alternating blocks of fixation periods (e.g. 30 secs) and blocks of pro- or anti-trials (e.g. 30 secs or around 8-10 trials each) with only one task type being presented within one run; (ii) alternating blocks of pro- and anti-saccades within a run (e.g., 30 sec of pro- followed by 30 sec of anti-saccades for 5 min). It is often tacitly assumed that the method of task presentation makes little difference to either performance or neural activations associated with performance, although there is some evidence mounting to the contrary (i.e. Cherkasova et al., 2002; Dyckman et al., 2007).
In order to explore the frequency of the various types of trial design, studies of healthy humans participating in antisaccades tasks were surveyed via a PubMed (www.pubmed.gov) search in Fall 2008, revealing 102 relevant articles. A large proportion of these studies used a blocked-type design (63%), interleaved designs accounted for 29% of the studies, while 8% of the papers used both designs. Excepting manuscripts in the task switching literature, the varying methods for stimulus presentation are often overlooked as meaningful sources of performance variance. Indeed, recent work indicates that behavioral and brain imaging conclusions may differ markedly as a function of task demands. For instance, work on pro/anti task-switch costs between single-task blocks and interleaved mixed-task runs (e.g., Cherkasova et al. 2002; Hodgson et al. 2004) indicate significant disparities in antisaccade performance as a function of presentation method, which may be unique to saccadic behavior (Hunt et al., 2006). In addition, Dyckman et al. (2007) demonstrated that differences in task presentation methods may account for variable conclusions regarding brain regions that specifically support antisaccade performance. Without information about how different presentation methods can influence behavior and brain function, it will be difficult to advance cognitive neuroscience fields associated with motor control, context effects, executive control operations, and the neural correlates of psychopathologies, to name a few relevant areas.
The current study addressed this issue by examining behavioral differences in pro- and anti-saccade responses (correct response percentages and correct and error response latencies) as a function of variations in task presentation method and type of preceding trial in interleaved runs (e.g., Hodgson et al., 2004). Using a within-subjects design, analyses evaluated the effects of task presentation method (blocked versus interleaved), length of cue to target interval in interleaved designs (1 sec lead time versus simultaneous presentation), and previous trial type (pro- versus anti-saccade). Data from this study provide information about the extent to which task presentation method should be considered an important source of variance on response characteristics in studies using saccadic responses as dependent variables.
Methods
Participants
Twelve right-handed normal participants (Median age=23.5 years; 25th-75th %tiles=19-31; 33% female) drawn from the Psychology Department research pool completed the study. All participants had normal or corrected-to-normal vision, and no neurological, psychiatric, or substance use disorders (by self-report). Written informed consent was given prior to participation. All procedures in this study were approved by the Institutional Review Board at the University of Georgia.
Stimuli
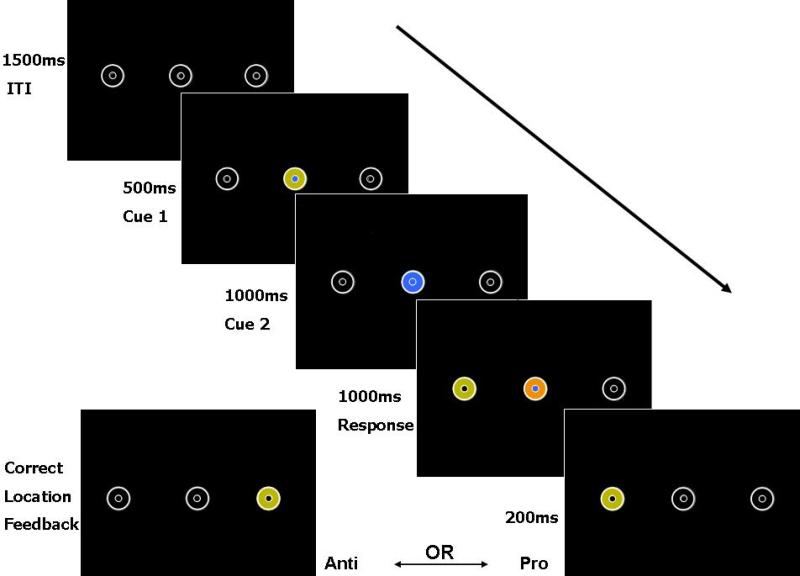
Stimuli were identical for every condition; stimulus events, however, had different meanings as a function of condition. All trials began with a 1500 ms inter-trial interval during which three pairs of concentric circles were outlined in white on a black screen (see Figure 1). Each larger circle (2 deg diameter) contained a smaller circle (0.5 deg in diameter) at its center. The middle pair was at central fixation and the peripheral pairs were located +/−8 deg from central fixation. Subjects were instructed to fixate on the small central circle during this period. Following the inter-trial interval came the cue period, which was divided into two sections: (i) 500 ms during which the outer ring of the central circle was yellow and the inner ring was either blue or orange, followed by (ii) 1000 ms during which the outer ring changed to either blue or orange while the inner circle maintained its previous color. Following the cue period came the response period which was also divided into two sections: (i) 1000 ms during which one of the peripheral large circles turned yellow while the central circles changed to one of four color combinations (blue-blue, blue-orange, orange-blue, orange-orange), and (ii) 200 ms during which the peripheral outer circle at the correct response location was yellow (no change on pro-trials, opposite side on anti-trials) and the central circles turned black. All stimuli at all locations were 5 cd/m2.
Figure 1.
Saccade task stimuli. Note that the stimuli were the same for all conditions; only the instructions changed for each condition (see text for a complete description). All trials started with a 1500 ms inter-trial interval. A subsequent cue period, was divided into two sections: (i) 500 ms during which the outer ring of the central circle was yellow and the inner ring was either blue or orange (blue in the present example), followed by (ii) 1000 ms during which the outer ring changed to either blue or orange (blue in the present case) while the inner circle maintained its previous color (the present example indicates a pro-trial for the long-lead condition). Following the cue period came the response period which was also divided into two sections: (i) 1000 ms during which one of the peripheral large circles turned yellow while the central circles changed to one of four color combinations (blue-blue, blue-orange, orange-blue, orange-orange; the present example indicates an anti-trial during the simultaneous condition), and (ii) 200 ms during which the peripheral outer circle at the correct response location was yellow (no change on pro-trials, opposite side on anti-trials) and the central circles turned black.
There were three different conditions during which pro- and anti-saccade trials (half in each direction) were completed per condition: blocked, long-lead interleaved, and simultaneous interleaved. All subjects completed all conditions in a single session. The order of conditions was randomized across subjects to avoid order effects. Participants were instructed to move their eyes as quickly and accurately as possible to the small central circle at the correct response location (to the peripheral target on pro-trials and to the opposite peripheral location on anti-trials). Participants were given a break after every 50 trials. For the blocked condition, participants knew that they were to generate prosaccades during one run of 200 trials and anti-saccades during a separate run of 200 trials. The only meaningful information from the stimuli, therefore, was the timing and location of peripheral target onset. For the long-lead interleaved condition, the cue period provided the critical information. During the cue period if the inner and outer central circle colors matched (e.g., blue-blue), participants were to generate a prosaccade at the time of peripheral target onset; if those colors were different (e.g., blue-orange), they were to generate an antisaccade at the time of peripheral target onset. Participants had 1000 ms, therefore, during which they knew the response requirement. Four hundred total trials were presented (200 pro- and 200 anti-saccades randomly interleaved). For the simultaneous interleaved condition, the response period provided the critical information. At the beginning of the response period if the inner and outer central circle colors matched they were to generate a prosaccade to the peripheral target; if those colors were different, they were to generate an antisaccade. Four hundred total trials were presented (200 pro- and 200 anti-saccades randomly interleaved). Participants were provided with practice trials until they reported understanding the requirements for each condition (less than 20 trials of practice per condition per participant).
Electro-oculographic (EOG) recording and analysis
During task performance, participants were seated in a comfortable armchair in a darkened room. They were fitted with EOG sensors at the outer canthi of the two eyes and above and below both eyes for bipolar recording of horizontal and vertical (blinks) eye movements, respectively, using NetAmps amplifiers (Electrical Geodesics; EGI, Eugene, OR). Sensor impedances were kept below 50 kΩ as per manufacturer recommendations. Data were sampled at 250 Hz with an analog filter bandpass of 0.1–200 Hz. Stimuli were presented on a 21 inch high resolution flat panel color monitor located 100 cm from the participant's eyes.
In-house software developed in Matlab (The MathWorks, Natick, MA) was used to analyze blink-free periods of horizontal EOG. Data were initially digitally filtered from 0.5-40 Hz (12db/octave). Saccadic onset and direction were scored using the procedures described in Dyckman and McDowell (2005). Specifically, saccadic onset was based on the increase of the initial saccadic response above position and velocity baselines. Based on the response latency distributions (using single trial data for all subjects, both within and across conditions) saccades on individual trials with latencies less than 140 ms and greater than 660 ms were excluded from the analyses (these extremes accounted for less than 2% of the total number of saccades and constituted outliers in the latency distributions). Responses were sorted into correct and incorrect responses by trial type (pro- or anti-saccade), direction (left or right), and condition (blocked, long-lead interleaved, simultaneous interleaved). There were no significant response direction effects involving trial type or condition on either correct responses, or correct or error response latencies, so the results are presented collapsing over direction.
Results
Percentage of Correct Responses
Percentage of correct responses were analyzed using a condition (blocked, long-lead interleaved, simultaneous interleaved) by trial type (pro, anti) repeated measures ANOVA with Huyhn-Feldt adjusted degrees of freedom. There were significant main effects of condition, F(2,22) = 26.0, p<.001, ε=.770, and trial type, F(1,11) = 15.4, p<.005. Across trial types (see Table 1), the blocked condition had the highest proportion of correct responses, followed by the long-lead interleaved and simultaneous interleaved conditions, with all three conditions differing significantly, t values >3.1, p values <.01. In addition, there were fewer errors on pro- than on anti-trials. There was also a significant condition by trial type interaction, F(2,22) = 4.8, p<.05, ε=.718. This interaction was driven by two features (see Table 1): (1) the percentage of correct anti-responses did not differ significantly between the blocked and long-lead conditions (p>.05), although this same comparison was significant for pro-responses (p<.01); and (2) the percentage of correct pro- and anti-responses did not differ on the long-lead condition (p>.05), while this same difference was significant for both the blocked and simultaneous conditions (p values <.007).
Table 1.
Means and Standard Deviations for Trial Types by Condition
| Correct Trials Latency | Error Trials Latency | Percent Correct | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Blocked Condition | ||||||
| Pro-saccade | 238 ms | 29.7 | N/A | N/A | 98.9 | 1.3 |
| Anti-saccade | 275 ms | 36.2 | 288 ms | 65.8 | 94.8 | 4.9 |
| Long Lead Condition | ||||||
| Pro-saccade | 275 ms | 50.5 | 316 ms | 57.6 | 94.4 | 3.6 |
| Anti-saccade | 301 ms | 59.8 | 301 ms | 50.8 | 91.2 | 5.3 |
| Simultaneous Condition | ||||||
| Pro-saccade | 367 ms | 46.7 | 356 ms | 55.7 | 86.5 | 7.2 |
| Anti-saccade | 378 ms | 50.9 | 336 ms | 32.6 | 78.6 | 10.9 |
Response Latencies
Correct responses
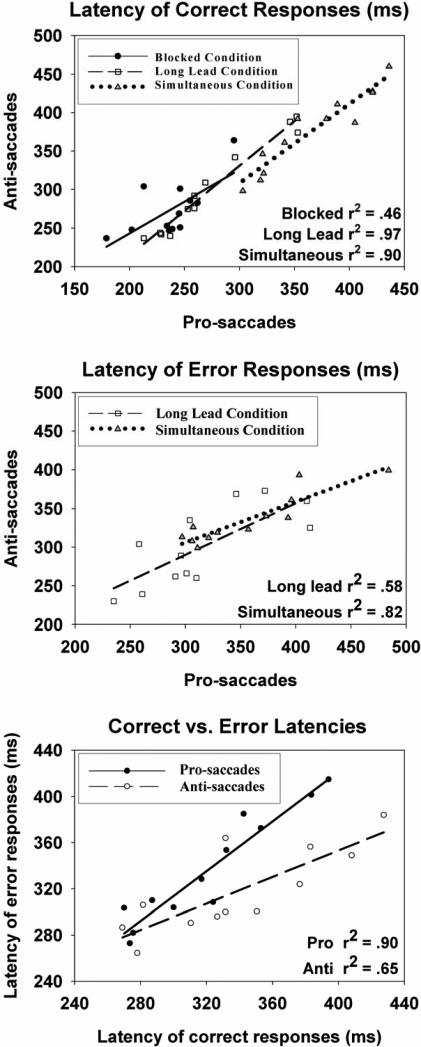
Response latencies on correct trials were analyzed using a condition (blocked, long-lead interleaved, simultaneous interleaved) by trial type (pro, anti) repeated measures ANOVA with Huyhn-Feldt adjusted degrees of freedom. There were significant mains effects of condition, F(2,22)=34.1, p<.001, ε=.883, and trial type, F(1,11)=28.7, p<.001. The interaction was not statistically significant. The blocked condition had the fastest response times followed by the long-lead and simultaneous interleaved conditions (see Table 1), with all three conditions differing significantly, t values >2.9, p values <.015. Across conditions, prosaccades had shorter latencies than antisaccades (see Table 1). There were also strong relationships between pro- and anti-saccade response latencies within conditions (see Figure 2, upper plot). The correlations between pro- and anti-response latencies were higher for the long-lead (r=.98, p<.05) and simultaneous (r=.95, p<.05) conditions than for the blocked condition (r=.68, p<.05). The two interleaved conditions did not differ on the strength of these relationships, but the magnitude of this relationship in the blocked condition was significantly lower than it was for the simultaneous condition (based on Fisher's z, p<.05).
Figure 2.
Response latency plots. Color-coded best fitting linear regression lines are shown (with accompanying r2 values); each dot is the value for an individual subject as a function of condition. Top: Relationships between latencies of correct responses for pro- and anti-saccades. Although the mean latency for each condition differs, the slopes for all conditions are similar. Center: Relationships between latency of error responses for pro- and anti-saccades for long-lead and simultaneous conditions. Error latencies for the blocked condition were not included due to the very low number of errors in that condition. Bottom: Relationships between latencies of correct and error responses for pro- and anti-saccades collapsed over long-lead and simultaneous conditions.
Error responses
There were too few errors in the blocked conditions to reasonably estimate error response latencies on these trials. Response latencies on error trials, therefore, were analyzed using a condition (long-lead interleaved, simultaneous interleaved) by trial type (pro, anti) repeated measures ANOVA. There were significant mains effects of condition, F(1,11)=7.5, p<.05, and trial type, F(1,11)=6.9, p<.05, on error response latencies. The interaction was not statistically significant. The long-lead interleaved condition had faster error response latencies than the simultaneous condition (see Table 1). Across conditions, errors on anti-trials were generated faster than were errors on pro-trials (see Table 1). As with latencies on correct trials, there were strong relationships between pro- and anti-saccade error response latencies within conditions (long-lead r=.76, p<.05; simultaneous r=.91, p<.05; see Figure 2, middle plot); the magnitude of these correlations did not differ significantly (Fisher's z, p>.05).
Relationships between correct and error response latencies
The relationships between correct and error response latencies for the long-lead and simultaneous interleaved conditions (see Figure 2, lower plot) also were investigated. These relationships did not differ as a function of condition, so the results are presented collapsing over conditions. There were strong linear relationships between correct and error response latencies for both pro- (r=.95, p<.05) and anti-responses (r=.81, p<.05), with the magnitude of these correlations being statistically similar (Fisher's z, p > .05). Inspection of the slopes for these relationships (1.1 for pro-trials; 0.6 for anti-trials), and the mean values for correct and error responses, show that participants (1) on pro-trials had slower error responses than correct responses, and (2) on anti-trials had faster error responses than correct responses (see Table 1).
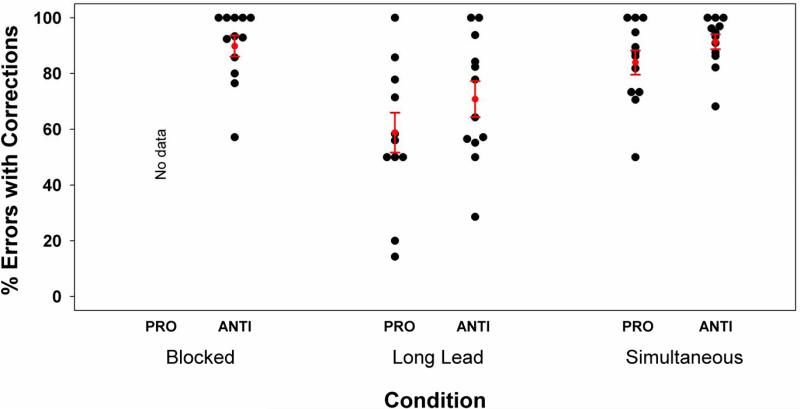
These differences can be explained in part by considering how frequently participants corrected their error responses. Although there were insufficient errors in the blocked pro-saccade condition to obtain meaningful data, when the participants made errors during the blocked anti-saccade condition they corrected there errors over 90% of the time, with most subjects correcting on 100% of error trials (as can be seen in Figure 3 and similar to previous findings (McDowell et al., 1999). A similar pattern was observed for the simultaneous interleaved conditions. During the long-lead interleaved conditions, however, participants corrected their errors on both pro- and anti-trials significantly less frequently than during either the blocked or simultaneous conditions. Within pro-trials this effect was tested using a paired t-test, t(11)=4.5, p<.002. Within anti-trials, this effect was tested using a repeated measures ANOVA with Huyhn-Feldt adjusted degrees of freedom, F(2,22)=9.2, p<.002, ε=.664, with Bonferroni post-hoc tests (the long-lead differing from both the blocked, p<.05, and simultaneous, p<.05, conditions, but the blocked and simultaneous conditions did not differ significantly, p>.90). It would appear, therefore, based on the latency and percent of error corrections data, that when subjects made an error during the long-lead condition they misconstrued it as the proper response.
Figure 3.
Percentage of corrected errors for pro- and anti-trials as a function of condition; each dot is the value for an individual subject. Data were not available for the blocked pro-saccade condition due to the virtual absence of errors. Black squares with error bars indicate group means (±SE).
Relationships Between Response Latencies and Proportion of Correct Responses
Speed-accuracy relationship within trial types
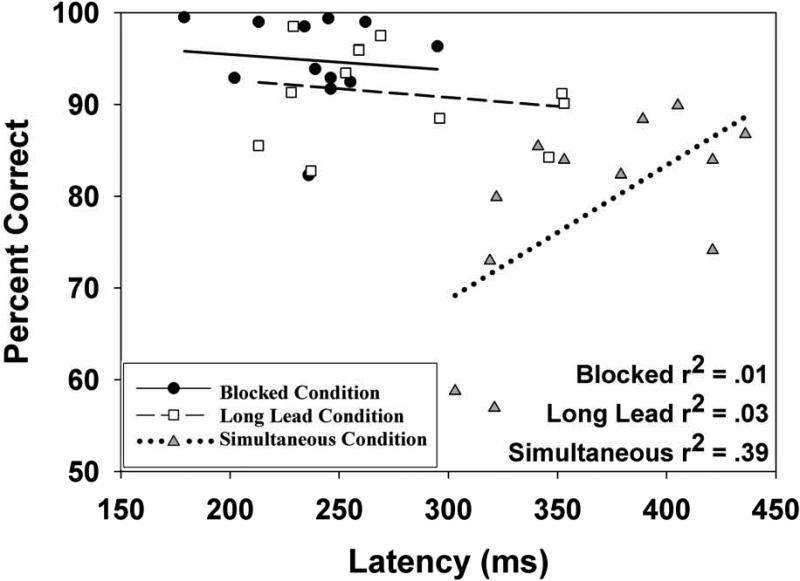
Within conditions, the relationship between correct response latencies and percentage of correct responses were analyzed using regression analyses and were investigated up to quadratic effects (see Figure 4). For both the blocked and long-lead interleaved conditions there were no significant relationships between correct response latencies and percentage of correct trials for either pro- or anti-responses. For the simultaneous condition, both pro- and anti-trials showed a clear positive linear relationship between correct response latency and percentage of correct responses (prosaccade r=.70; antisaccade r=.61, p's<.05), with longer latencies being associated with a higher percentage of correct responses.
Figure 4.
Relationships between correct response latencies and percentage of correct responses for pro- and anti-saccades as a function of condition; each dot is the value for an individual subject. Best-fitting linear regression lines (and accompanying r2 values) are also shown. Graphs are all on the same scale, illustrating the difference in mean latency and percentage of correct responses between conditions.
Prediction of proportion of correct anti-responses from pro-latencies
Recent work has demonstrated that prosaccade latency is an important predictor of antisaccade error rate in schizophrenia (Harris et al., 2006): for blocked presentation of pro- and anti-trials, among schizophrenia patients, but not healthy subjects, faster response latencies during the pro-task predicted more antisaccade errors. This is an interesting and theoretically important finding suggesting that, at least under certain conditions, failed inhibition is associated with speeded visual orienting to an abruptly appearing peripheral visual stimulus. We investigated that same relationship as a function of condition (see Figure 5). The relationship between prosaccade latency and proportion of correct antisaccade responses was only significant during the simultaneous condition (r=.62, p<.05), with the same correlations being small and non-significant for both the blocked (r=.10) and long-lead (r=.17) conditions. Indeed, the magnitude of the relationship and distribution of the data points for the blocked condition is highly similar to those reported by Harris et al. (2006).
Figure 5.
Relationships between percentage of correct anti-responses and pro-saccade response latencies as a function of condition; each dot is the value for an individual subject. Best-fitting linear regression lines (and accompanying r2 values) are also shown.
Effect of Preceding Trial Type on Responses
Previous work (Cherkasova et al., 2002; Barton et al., 2006) has demonstrated that preceding trial type can have an effect on latency and accuracy of pro- and anti-responses in interleaved designs. To further examine the relationship between trial types in interleaved tasks, latency of correct trials and percentage of correct responses were compared between trial types (pro versus anti) as functions of preceding trial type (pro versus anti) and condition (long-lead versus simultaneous) in a 3-way repeated-measures ANOVA. For latency, there was a significant main effect of repetition, F(1,11)=14.1, p<.005, with repeated trials (pro-pro and anti-anti) having faster response latencies on the second trial (repeated: M=331 ms, SD=70; opposite: M=345 ms, SD=78). Likewise for proportion of correct responses there was a main effect of repetition, F(1,11)=14.8, p<.005, with repeated trials (pro-pro and anti-anti) having a higher proportion of correct responses (repeated: 91.5%, SD=7.9; opposite: 85.3%, SD=12). There were also two significant effects involving condition: a significant condition by repetition interaction on latency, F(1,11)=7.6, p<.05, and a significant condition by trial type interaction on percentage of correct responses, F(1,11)=17.0, p<.005. For response latency, there was only a switch cost under the simultaneous (repeated M=366 ms, SD=50; opposite M=389 ms, SD=61) compared to the long-lead condition (repeated M=297 ms, SD=71; opposite M=301 ms, SD=68). For proportion of correct responses, the interaction involving condition also was caused by a trial type effect under the simultaneous (pro-trials M=87.3%, SD=11; anti-trials M=78.8%, SD=13) compared to the long-lead condition (pro-trials M=94.2%, SD=4.8; anti-trials M=93.2%, SD=4.2).
Discussion
The present study investigated the effects of commonly used saccade paradigms on basic saccadic response characteristics (percentage of correct responses and correct and error latencies). Method of pro- and anti-saccade stimulus presentations had dramatic effects on behavior. There were two interesting results pertaining to a one sec cue-to-target interval during the long-lead interleaved condition. First, it eliminated the typical prosaccade advantage on the proportion of correct responses. Second, it was sufficient to foster a form of goal neglect, even among these young healthy subjects. This was manifest as an increased percentage of uncorrected errors, apparently because subjects believed that they were making the correct response on those error trials. There were also two interesting results pertaining to the simultaneous interleaved condition. First, a significant correlation between pro-saccade latencies and anti-saccade error rates indicated that for this condition the individual differences in speed of visual orienting was critical for determining correct inhibitory control during antisaccade performance. Second, task switch costs were evident only for this condition, suggesting that, in combination with previous studies, there is an optimal cue-to-target interval time window for observing task switch effects. Differences in response profiles across saccadic task presentation method, therefore, must be considered when evaluating similarities and differences in saccadic performance across research reports. This may be especially true when considering studies of groups with compromised cognitive functioning (e.g., schizophrenia patients, older persons).
Similarities and differences between conditions and trial types on percentage of correct responses and latencies are revealing about the cognitive requirement of the different saccade presentation methods. The blocked condition resulted in the fastest and highest percentages of correct responses, followed by the long-lead and simultaneous interleaved conditions. In addition, and as expected, correct prosaccades were generated more promptly than correct antisaccades independent of condition. To the extent that lower percentages of correct responses provide information about increasing task difficulty, the blocked condition appears to be the easiest and the simultaneous interleaved the most difficult of the tasks. To the extent that longer latencies indicate increased cognitive processing time (Fischer & Weber, 1993), the blocked condition required the shortest and the simultaneous interleaved condition required the longest performance-related computations at the time of peripheral target presentation.
A striking difference between conditions, however, occurred specifically in relation to the long-lead condition where proportion of correct responses for pro- and anti-trials did not differ significantly, which was caused by a relatively larger decrease in percent correct for the long-lead prosaccades as compared to the blocked prosaccades (see Table 1). The highest percentage of anti-errors occurred during the simultaneous interleaved condition, but subjects corrected errors made during this condition at the same rate as during the simpler blocked condition. The high proportion of corrected errors in both the blocked and simultaneous interleaved anti-conditions indicates that subjects were aware when they made an error and followed that error with a corrective saccade in the opposite direction. During the long-lead interleaved condition, however, subjects corrected errors significantly less frequently than during the other two conditions. In addition, the latencies of error responses for both pro- and anti-responses during these trials indicate that subjects believed they were making the correct response. The latency and accuracy data indicate, therefore, that errors made during this presentation method may result from failure to maintain the proper goal state. Nieuwenhuis et al. (2004) also have suggested that some types of saccade errors occur when representation of the goal state has been weakened, and the data presented here provide additional empirical support for this hypothesis.
Another interesting difference between the two interleaved conditions occurred in relation to task-switch costs. A trial different from its preceding trial in type tends to have longer latency and higher tendency for an error than a trial preceding a trial of the same type (Cherkasova et al., 2002., Hodgson et al., 2004.) Cherkasova et al. (2002), however, demonstrated that the latency for anti-saccades preceded by pro-saccades can sometimes elicit faster responses for anti-saccades, a so-called task-switch benefit. Our findings show no task-switch effects during the long-lead condition, but anti-saccade responses in the simultaneous condition showed a task-switch cost. This same effect was also observed by Barton et al. (2006), Greenzang et al. (2007), and Weber (1995), who noted a task-switch cost at short cue to target intervals (200 ms). Studies reporting task-switch benefits for anti-saccades (Barton et al., 2002, 2006; Cherkasova et al, 2002; Manoach et al., 2002) used a considerably longer cue-to- target interval (two sec) than we used here. Interestingly, studies using cue to target intervals ranging from 500 (Hodgson et al., 2004) to 1000 ms (Hunt & Klein, 2002) found no switch costs for either latency or accuracy, mirroring our results for the long-lead condition. The only study to find no task-switch effects at very long cue-to-target intervals (Hallett & Adams, 1980) may indicate that at some point, in this case a 4.5-5.2 sec cue-to-target interval, interleaved tasks require no more cognitive effort than do blocked tasks.
Ability to properly perform both pro- and anti-saccade trials, and even the causative mechanisms for errors, may be a function of task presentation method. On the one hand, blocked presentation may allow for the least direct comparison between correct pro- and anti-trials. For instance, as opposed to the interleaved conditions, correct pro- and anti-saccade latencies had a more modest relationship perhaps indicating a lower correspondence between the neural mechanisms supporting these responses. On the other hand, blocked presentation of anti-trials may most directly measure inhibitory abilities without requiring cognitive demands other than maintaining the same response set over the entire block.
Evidence from this experiment strongly suggests that stimulus presentation method significantly impacts the percent correct and latencies. Two considerations, however, could benefit further explorations of this phenomenon. First, during the long-lead condition, there was a constant cue period of one second which may have contributed to predictability in response planning over the course of hundreds of trials. Future studies could usefully incorporate an unpredictable parametric exploration of cue length and its effects on correct responding and reaction time during a long-lead condition. Second, visible peripheral outlines of possible target locations were used in this study (+/− 8 deg from central fixation). These stimuli were selected precisely because they allowed for the investigation of target selection without the need for co-ordinate calculation (which typically differs in difficulty between pro- and anti-saccade tasks). This does differentiate the task used in the current study, however, from those used in other antisaccade studies which typically required movement to a location with no visual stimulus (i.e. opposite of the visible cue).
When testing for differences between healthy persons and groups with known/suspected dysfunction of brain systems thought to affect executive control processes (e.g., Clementz et al., 1994; Curtis et al., 2001; McDowell et al., 1999; Sweeney et al., 2001), choice of antisaccade presentation method may be a critically important issue. For instance, differences in error rates between-groups during long-lead interleaved presentation methods will lead to difficulties concluding which cognitive/neural system dysfunctions are accounting for increased percentages of error responses. Even more problematic, subgroups of persons in an executive control dysfunction group could have high error rates for markedly different reasons (e.g., inhibitory problems, goal neglect, working memory deficits). This possibility would be a particular difficulty when using antisaccade errors as an indicator of constitutional liability for illness (as in schizophrenia; McDowell et al., 1999), where it is crucially important the causative mechanisms underlying the abnormality are simple and have the possibility for indexing genetic heterogeneity (Gottesman & Gould, 2003). Such measures must provide more direct clues to genetics than the clinical syndromes (Berrettini, 2005; Doyle et al., 2005; Waldman, 2005); if not, they will be of questionable utility delineating the genetic risk to disease expression.
Acknowledgments
This research was funded by the United States Public Health Service (NIH Grant MH57886 to Dr. Clementz).
References
- Barton JJ, Cherkasova MV, Lindgren K, Goff DC, Intriligator JM, Manoach DS. Antisaccades and task switching: studies of control processes in saccadic function in normal subjects and schizophrenic patients. Ann N Y Acad Sci. 2002;956:250–263. doi: 10.1111/j.1749-6632.2002.tb02824.x. [DOI] [PubMed] [Google Scholar]
- Barton JJ, Greenzang C, Hefter R, Edelman J, Manoach DS. Switching, plasticity, and prediction in a saccadic task-switch paradigm. Exp Brain Res. 2006;168(1-2):76–87. doi: 10.1007/s00221-005-0091-1. [DOI] [PubMed] [Google Scholar]
- Berrettini WH. Genetic bases for endophenotypes in psychiatric disorders. Dialogues Clin Neurosci. 2005;7(2):95–101. doi: 10.31887/DCNS.2005.7.2/wberrettini. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkasova MV, Manoach DS, Intriligator JM, Barton JJ. Antisaccades and task-switching: interactions in controlled processing. Exp Brain Res. 2002;144(4):528–537. doi: 10.1007/s00221-002-1075-z. [DOI] [PubMed] [Google Scholar]
- Clementz BA, McDowell JE, Zisook S. Saccadic system functioning among schizophrenia patients and their first-degree biological relatives. J Abnorm Psychol. 1994;103(2):277–287. [PubMed] [Google Scholar]
- Curtis CE, Calkins ME, Grove WM, Feil KJ, Iacono WG. Saccadic disinhibition in patients with acute and remitted schizophrenia and their first-degree biological relatives. Am J Psychiatry. 2001;158(1):100–106. doi: 10.1176/appi.ajp.158.1.100. [DOI] [PubMed] [Google Scholar]
- Doyle AE, Faraone SV, Seidman LJ, Willcut EG, Nigg JT, Waldman ID, et al. Are endophenotypes based on measures of executive functions useful for molecular genetic studies of ADHD? J Child Psychol Psychiatry. 2005;46(7):774–803. doi: 10.1111/j.1469-7610.2005.01476.x. [DOI] [PubMed] [Google Scholar]
- Dyckman KA, Camchong J, Clementz BA, McDowell JE. An effect of context on saccade-related behavior and brain activity. Neuroimage. 2007;36(3):774–784. doi: 10.1016/j.neuroimage.2007.03.023. [DOI] [PubMed] [Google Scholar]
- Dyckman KA, McDowell JE. Behavioral plasticity of antisaccade performance following daily practice. Exp Brain Res. 2005;162(1):63–69. doi: 10.1007/s00221-004-2105-9. [DOI] [PubMed] [Google Scholar]
- Everling S, DeSouza JF. Rule-dependent activity for prosaccades and antisaccades in the primate prefrontal cortex. J Cogn Neurosci. 2005;17(9):1483–1496. doi: 10.1162/0898929054985455. [DOI] [PubMed] [Google Scholar]
- Fischer B, Weber H. Express saccades and visual attention. Behav. Brain Sci. 1993;16(3):553–567. [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Greenzang C, Manoach DS, Goff DC, Barton JJ. Task-switching in schizophrenia: active switching costs and passive carry-over effects in an antisaccade paradigm. Exp Brain Res. 2007;181(3):493–502. doi: 10.1007/s00221-007-0946-8. [DOI] [PubMed] [Google Scholar]
- Hallett PE. Primary and secondary saccades to goals defined by instructions. Vision Res. 1978;18(10):1279–1296. doi: 10.1016/0042-6989(78)90218-3. [DOI] [PubMed] [Google Scholar]
- Hallett PE, Adams BD. The predictability of saccadic latency in a novel voluntary oculomotor task. Vision Res. 1980;20(4):329–339. doi: 10.1016/0042-6989(80)90019-x. [DOI] [PubMed] [Google Scholar]
- Harris MS, Reilly JL, Keshavan MS, Sweeney JA. Longitudinal studies of antisaccades in antipsychotic-naive first-episode schizophrenia. Psychol Med. 2006;36(4):485–494. doi: 10.1017/S0033291705006756. [DOI] [PubMed] [Google Scholar]
- Hodgson TL, Golding C, Molyva D, Rosenthal CR, Kennard C. Reflexive, symbolic, and affective contributions to eye movements during task switching: response selection. J Cogn Neurosci. 2004;16(2):318–330. doi: 10.1162/089892904322984599. [DOI] [PubMed] [Google Scholar]
- Hunt AR, Ishigami Y, Klein RM. Eye movements, not hypercompatible mappings, are critical for eliminating the cost of task set reconfiguration. Psychon Bull Rev. 2006;13(5):923–927. doi: 10.3758/bf03194020. [DOI] [PubMed] [Google Scholar]
- Hunt AR, Klein RM. Eliminating the cost of task set reconfiguration. Mem Cognit. 2002;30(4):529–539. doi: 10.3758/bf03194954. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Lindgren KA, Cherkasova MV, Goff DC, Halpern EF, Intriligator J, et al. Schizophrenic subjects show deficient inhibition but intact task switching on saccadic tasks. Biol Psychiatry. 2002;51(10):816–826. doi: 10.1016/s0006-3223(01)01356-7. [DOI] [PubMed] [Google Scholar]
- McDowell JE, Myles-Worsley M, Coon H, Byerley W, Clementz BA. Measuring liability for schizophrenia using optimized antisaccade stimulus parameters. Psychophysiology. 1999;36(1):138–141. doi: 10.1017/s0048577299980836. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Everling S. Look away: the anti-saccade task and the voluntary control of eye movement. Nat Rev Neurosci. 2004;5(3):218–228. doi: 10.1038/nrn1345. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Broerse A, Nielen MM, de Jong R. A goal activation approach to the study of executive function: an application to antisaccade tasks. Brain Cogn. 2004;56(2):198–214. doi: 10.1016/j.bandc.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Opris I, Bruce CJ. Neural circuitry of judgment and decision mechanisms. Brain Res Brain Res Rev. 2005;48(3):509–526. doi: 10.1016/j.brainresrev.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Rosano C, Berman RA, Luna B. Inhibitory control of attention declines more than working memory during normal aging. Neurobiol Aging. 2001;22(1):39–47. doi: 10.1016/s0197-4580(00)00175-5. [DOI] [PubMed] [Google Scholar]
- Waldman ID. Statistical approaches to complex phenotypes: Evaluating neuropsychological endophenotypes for attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57(11):1347–1356. doi: 10.1016/j.biopsych.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Weber H. Presaccadic processes in the generation of pro and anti saccades in human subjects--a reaction-time study. Perception. 1995;24(11):1265–1280. doi: 10.1068/p241265. [DOI] [PubMed] [Google Scholar]