Abstract
While most human Salmonella infections result from exposure to contaminated foods, an estimated 11% of all Salmonella infections are attributed to animal exposures, including both direct animal handling and indirect exposures such as cleaning cages and handling contaminated pet food. This report describes the epidemiologic, environmental and laboratory investigations conducted in the United States as part of the response to an international outbreak of tetracycline-resistant Salmonella enterica serotype I 4,[5],12:i:- infections with over 500 illnesses occurring from 2008 to 2010. This investigation found that illness due to the outbreak strain was significantly associated with exposure to pet reptiles and frozen feeder rodents used as food for pet reptiles. Salmonella isolates indistinguishable from the outbreak strain were isolated from a frozen feeder mice-fed reptile owned by a case patient, as well as from frozen feeder mice and environmental samples collected from a rodent producing facility (Company A). An international voluntary recall of all Company A produced frozen feeder animals sold between May 2009 and July 2010 occurred. Only 13% of cases in our investigation were aware of the association between Salmonella infection and mice or rats. Consumers, the pet industry, healthcare providers and veterinarians need to be aware of the potential health risk posed by feeder rodents, whether live or frozen. Frozen feeder rodent producers, suppliers and distributors should follow the animal food labelling requirements as described in 21 CFR §501.5, and all packages of frozen feeder rodents should include safe handling instructions. Persons should wash their hands thoroughly with soap and water after handling live or frozen feeder rodents, as well as reptiles or anything in the area where the animals live. Continued opportunities exist for public health officials, the pet industry, veterinarians and consumers to work together to prevent salmonellosis associated with pet food, pets and other animals.
Keywords: Salmonella, outbreak, reptiles, frozen feeder rodents, zoonoses, amphibians
Introduction
Salmonella causes an estimated 1.2 million human infections, 23 128 hospitalizations and 452 deaths each year in the United States (Scallan et al., 2011). While most Salmonella infections result from contaminated foods, an estimated 11% of all Salmonella infections are attributed to animal exposure, including direct contact with animals, as well as indirect contact such as cleaning cages or bedding, handling food or food bowls, or handling anything in the area where the animal lives or roams (Hale et al., 2012). Some types of animals, including reptiles and amphibians, (Harris et al., 2009, 2010; Mettee Zarecki et al., 2013), as well as chicks, ducklings and other live poultry (Loharikar et al., 2012; CDC, 2013a,b; Gaffga et al., 2012) have been linked with multiple outbreaks of salmonellosis and pose a higher risk for human Salmonella infections. Colonized animals can appear healthy and clean, but may intermittently shed Salmonella in their faeces (Burnham et al., 1998; Gaffga et al., 2012; Mettee Zarecki et al., 2013). Mice or rats may be fed to carnivorous reptiles and amphibians; some companies raise and sell these ‘feeder rodents’ for use as food for reptiles or amphibians. If the feeder rodents are colonized with Salmonella, the bacteria can be transmitted to reptiles as well as to the humans handling the rodents and reptiles (Goupil et al., 2012). Feeder and pet rodents were previously associated with a multistate, multidrug-resistant Salmonella Typhimurium outbreak in 2004 (Swanson et al., 2007), and vacuum-packed frozen feeder mice from an internet-based supplier were associated with a multistate Salmonella Typhimurium outbreak in 2006 (Fuller et al., 2008).
In December 2008, the United Kingdom’s Health Protection Agency (HPA) detected an increase in human isolates of tetracycline-resistant Salmonella enterica I 4,[5],12:i-, a monophasic variant of serotype Typhimurium (Harker et al., 2011; Hopkins et al., 2012); this pattern had not previously been reported in England or Wales. Investigation by HPA determined 67% of ill persons kept a reptile at home with snakes reported as the most commonly owned reptile; illness was significantly associated with reptile exposure. Additionally, 86% of cases who owned reptiles reported use of frozen feeder mice. Samples of frozen feeder mice from 13 major suppliers, a major share of the supply chain to the United Kingdom, were tested; the outbreak strain was identified in samples from a single supplier (Company A) (Harker et al., 2011). As Company A is located in the United States, HPA notified the Centers for Disease Control and Prevention (CDC) of the investigation in March 2009.
Materials and Methods
Case ascertainment and epidemiologic investigation
Human salmonellosis is a nationally notifiable condition (CSTE, 2012). Clinical laboratories throughout the United States send Salmonella isolates from ill persons to state public health laboratories for serotyping and pulsed-field gel electrophoresis (PFGE) subtyping with the use of standardized methods (Ribot et al., 2006). State public health laboratories and regulatory agencies routinely submit PFGE patterns to PulseNet USA, the national molecular subtyping network for enteric disease surveillance. These PFGE patterns are uploaded to local and national databases which are routinely monitored for temporal and/or geographic case clustering that may represent a common source exposure.
In 2009, upon initial notification by HPA of their ongoing Salmonella I 4,[5],12:i:-outbreak, CDC identified 35 Salmonella I 4,[5],12:i:-isolates with PFGE patterns that were indistinguishable and matched the HPA isolate in the PulseNet USA database (PulseNet USA-defined PFGE XbaI pattern JPXX01.1071). CDC epidemiologists reviewed information from these US cases, but reptile and rodent exposure were only reported by 4 (14%) of the 29 persons interviewed between 1 January and 31 July 2009. Further investigation was not initiated based on this information. By 1 June 2010, PulseNet USA identified an additional 29 isolates matching this PFGE pattern; a review of case patient interviews noted 12 (80%) of the 15 interviewed cases reported reptile exposure. An epidemiologic investigation was initiated, and each respective state health department was asked to confirm the cases enumerated on the PulseNet isolate line list. Due to the increase in reptile exposure in these 2010 cases, an investigation was initiated. Similar to the United Kingdom, the PFGE pattern was rare in the PulseNet USA database, comprising less than 1% of the reported combined Typhimurium/I 4,[5],12:i:- at the time (CDC, unpublished data).
In partnership with state and local health departments a case–case study was undertaken to investigate the US portion of this international outbreak. A case was defined as a laboratory-confirmed infection in a person with the outbreak strain of the I 4,[5],12:i:- serotype of Salmonella enterica with an isolation date from 1 January 2010 through 30 July 2010. Potential controls were any identified person with a stool isolate that yielded Salmonella Enteritidis and with an isolate date on or after 1/1/2010. Controls were selected from state enteric databases either retrospectively or prospectively and were matched to case patients by age group (<18 years, or ≥18 years) and state of residence. States attempted to identify two controls for each case. Using a standard questionnaire, cases and controls were queried regarding any reptile and rodent exposures that may have occurred the week before the case’s illness began. When available, feeder rodent purchase information was collected during case interviews and utilized in a traceback investigation to determine the source of the feeder rodents. All case patients and control patients included in this study analysis provided verbal consent; this investigation was part of a public health response to an ongoing outbreak and therefore did not require formal human subjects review.
Environmental investigations
Company A raises and produces frozen feeder mice and rats onsite; the company also sells bags of 25 count frozen chicks which are shipped to them from a hatchery located in the south-eastern United States. The Food and Drug Administration (FDA), the agency responsible for regulating animal feeds that enter interstate commerce, inspected Company A in early July 2010 and collected samples from their facility, including rodent feed, frozen feeder mice and swabs of the environment. During a second inspection in late July– August 2010, samples of frozen rats and frozen feeder chicks were collected, along with additional environmental samples and samples of irradiated frozen mice. During a third inspection in March 2011, samples collected included irradiated frozen feeder mice, non-irradiated frozen rats and swabs of the room where irradiated product is stored prior to shipment. Serotyping and PFGE subtyping of all environmental samples were conducted by FDA laboratories, and results were uploaded to the PulseNet USA database. Samples were collected from patient-owned reptiles by the Wisconsin Division of Public Health per routine procedures.
Anti-microbial susceptibility testing
Human isolates from this outbreak received at CDC through the National Antimicrobial Resistance Monitoring System (CDC-NARMS) program (CDC NARMS, 2010) were tested using broth microdilution (Sensititre® TREK Diagnostic Systems, Cleveland, OH) to determine the minimum inhibitory concentration for each of the 15 anti-microbial agents on the CDC-NARMS panel. Categorical interpretations of susceptible, intermediate or resistant were based on Clinical and Laboratory Standards Institute breakpoints when available (CLSI, 2013). Additional isolates (mouse samples and environmental swabs) obtained from Company A’s production facility in July 2010 were received and tested for resistance to 13 antimicrobials by FDA-NARMS.
Analysis
Data from the case–case study were entered into an Access database (Microsoft Office Access 2007), and statistical analyses were conducted using SAS version 9.2 (SAS Institute, Cary, NC). Descriptive frequencies and medians were calculated. Simple conditional logistic regression models were performed, using Firth penalized likelihood methods, to compute matched odds ratios (mOR) and 95% confidence intervals (Firth, 1993).
Results
Initial case finding and case–control study
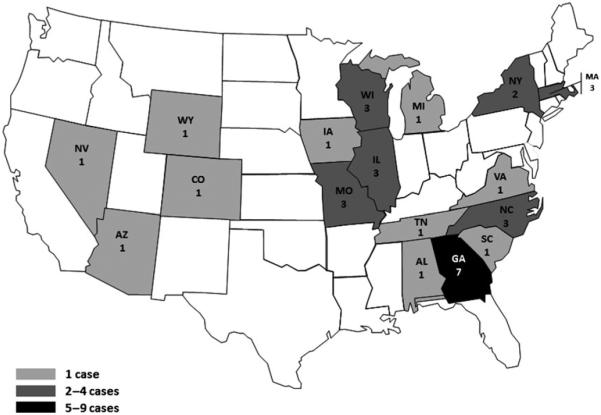
From 1 January 2010 to 30 July 2010, 34 case patients were reported from 17 states in the United States (Fig. 1). Fifteen case patients participated in the case–control study; 29 matched control patients from 10 participating states were enrolled. The median age of case patients was 13 years (range, <1–57) compared with 15 years (range, <1–63) for controls (Table 1). Ten (67%) case patients and 19 (66%) controls were less than 18 years; 6 (40%) case patients and 10 (34%) controls were female (Table 1). Case patients’ illness onset ranged from 24 December 2009 to 9 June 2010 (Fig. 2). All case patients had diarrhoea; four (27%) had bloody diarrhoea, 10 (67%) received antibiotics, and one (7%) was hospitalized overnight (Table 1). Illness lasted a median of 7 days (range, 2–35 days).
Fig. 1.
Geographic Distribution of Outbreak-associated Cases (as of July 30, 2010; only pre-recall cases shown on this map) January – July, 2010 — United States (n = 34).
Table 1.
Age, sex, symptom duration and characterization, antibiotic receipt and hospitalization status of cases and age group- and state residence-matched controls – January–July 2010 – United States (n = 44)
| Cases (n = 15) n (%) |
Controlsa (n = 29) n (%) |
|
|---|---|---|
| Median age, years (range) | 13 (<1–57) | 15b (<1–63) |
| Age < 18 years | 10 (67) | 19 (66) |
| Female | 6 (40) | 10 (34) |
| Median duration of symptoms, days (range) |
7b (2–35) | NA |
| Diarrhoea | 15 (100) | NA |
| Bloody diarrhoeab | 4 (27) | NA |
| Received antibioticsb | 10 (67) | NA |
| Spent the night in the hospitalb | 1 (7) | NA |
NA, Not applicable.
Any identified person with a stool isolate that yielded Salmonella Ente-ritidis and with an isolate date on or after 1/1/2010.
More than 5% of data missing.
Fig. 2.
Date of isolation, receipt by state public health department, or upload to PulseNet USA for 74 human isolates of Salmonella I 4,[5],12:i:-United States, December 2009 – March 2011. *Any case isolated, reported, or uploaded during July is counted as pre-recall.
Case patients were significantly more likely than controls to report any reptile or amphibian exposure (73% versus 14%, matched odds ratio [mOR 11.1, 95% confidence interval [CI]: 2.6–103.2) (Table 2). Case patients were also significantly more likely to report being in the same room as a reptile or amphibian (67% versus 14%, mOR 10.4, 95% CI: 2.4–97.4), having contact with the reptile or amphibian habitat (47% versus 7%, mOR 9.0, 95% CI: 1.9–86.0) and feeding a reptile or amphibian (33% versus 3%, mOR 7.3, 95% CI: 1.5–71.5), when compared with controls. Case patients were significantly more likely than controls to report rodent exposure (67% versus 4%, mOR 32.7 , 95% CI: 4.2–4223.1). Case patients were significantly more likely to report ever feeding rodents to reptiles (60% versus 3%, mOR 32.9, 95% CI: 4.1–4248.1) and that the rodents were usually frozen (47% versus 3%, mOR 9.4, 95% CI: 2.0–88.9).
Table 2.
Types of reptile contact, rodent contact and reptile feeding practices, among cases and age group- and state residence-matched controls – January–July 2010 – United States (n = 44)
| Exposure | Cases (n = 15) n (%) |
Controlsa
(n = 29) n (%) |
mORb | 95% CIb |
|---|---|---|---|---|
| Any reptile/amphibian contact |
11 (73) | 4 (14) | 11.1 | 2.6, 103.2 |
| Same room as reptile/amphibian |
10 (67) | 4 (14) | 10.4 | 2.4, 97.4 |
| Contact with animals’ habitat |
7 (47) | 2 (7) | 9.0 | 1.9, 86.0 |
| Touch reptile/amphibian | 6 (40) | 3 (10) | 4.3 | 1.1, 23.6 |
| Fed reptile/amphibian | 5 (33) | 1 (3) | 7.3 | 1.5, 71.5 |
| Any rodent contact | 10 (67) | 1c (4) | 32.7 | 4.2, 4223.1 |
| Fed rodents to reptiles | 9 (60) | 1 (3) | 32.9 | 4.1, 4248.1 |
| Rodents usually bought frozenc |
7 (47) | 1 (3) | 9.4 | 2.0, 88.9 |
Any identified person with a stool isolate that yielded Salmonella Ente-ritidis and with an isolate date on or after January 1 2010.
Matched odds ratios (mOR) and **95% confidence intervals (CI) obtained using Firth penalized likelihood methods.
More than 5% of data missing.
Case patient knowledge and practices
Case patients (n = 15) most frequently reported exposure to snakes (67%), but lizard (13%) and turtle (7%) exposures also occurred (Table 3). Most case patients (60%) owned a reptile and exposures most frequently occurred at school (13%), amusement parks (7%) and friends’ homes (7%). Among those case patients who owned the reptile (n = 9), the median length of ownership was 2 years (range, 0.08–14.8 years), and the median age of the reptile was 5 years (range, 1–15 years). Reptiles were most commonly fed rodents weekly (n=6) or monthly (n = 2). Case patients reported storing frozen rodents in the same freezer with human food (13%) and thawing frozen rodents in their kitchen (20%). Eight (53%) case patients were aware of the association between Salmonella and reptiles or amphibians, while two (13%) were aware of the association between Salmonella and rodents. Case patients were asked about hand hygiene practices; 6 (40%) reported washing their hands after touching a reptile and 3 (20%) reported washing their hands after handling rodents.
Table 3.
Reptile ownership duration, reptile characteristics, location of reptile contact, rodent feeding practices and Salmonella awareness and hand washing practices among cases. January–July 2010 – United States (n = 15)
| Median | Range | |
|---|---|---|
| Duration of ownership, years (n = 8a) | 2 | 0.08–14.8 |
| Age of reptile, years (n = 7a) | 5 | 1.0–15.0 |
| n | % | |
| Owns reptile | 9 | 60 |
| Type of reptileb | ||
| Snake | 10 | 67 |
| Lizard | 2 | 13 |
| Turtle | 1 | 7 |
| Frog/toad | 0 | 0 |
| Location of exposure b | ||
| Home | 9 | 60 |
| School | 2 | 13 |
| Amusement park | 1 | 7 |
| Friends’ homea | 1 | 7 |
| Fed rodents weekly | 6 | 40 |
| Fed rodents monthly | 2 | 13 |
| Frozen rodents stored in freezer with human food |
2 | 13 |
| Frozen rodents thawed in kitchen | 3 | 20 |
| Wash hands after touching reptiles | 6 | 40 |
| Wash hands after touching rodents | 3a | 20 |
| Aware of the connection between Salmonella and reptiles |
8a | 53 |
| Aware of the connection between Salmonella and rodents |
2a | 13 |
More than 5% of data missing.
Categories not mutually exclusive.
Environmental investigation
Concurrent with the epidemiologic investigation, environmental investigations were undertaken. An outbreak-associated case patient in Wisconsin reported ownership of four bearded dragons (Pogona vitticeps). Health officials in Wisconsin obtained samples of droppings from the reptiles; a culture of one of the reptile’s droppings yielded Salmonella I 4,[5],12:i:- with a PFGE pattern indistinguishable from the outbreak strain. The bearded dragons were fed mice sourced from three stores; one store procured mice from a distributor who purchased frozen mice in bulk from Company A. The distributor repackaged the mice into smaller quantities for resale by the pet store.
Salmonella I 4,[5],12:i:- isolates with a PFGE pattern indistinguishable from the outbreak strain were obtained from all the frozen feeder mice (n = 13) and environmental samples (n = 19) collected during FDA’s initial inspection of Company A in early July 2010. These findings prompted an international voluntary recall of all frozen feeder animals (mice, rats and chicks) produced by Company A between May 2009 and July 2010; an estimated 6 million frozen feeder rodents were subject to the recall.
In late July 2010, Company A began irradiating all frozen feeder mice at a certified food irradiation facility. Samples of irradiated mice collected during the late July–August inspection did not yield Salmonella, but swabs taken in the room where irradiated product is stored prior to distribution, to include the table used for packing irradiated product for shipment, yielded the outbreak strain of Salmonella. Cultures of the rats and frozen feeder chicks collected from Company A did not yield Salmonella.
The irradiated frozen feeder mice samples collected during the March 2011 follow-up inspection by FDA again did not yield Salmonella; rats collected at this time also did not yield Salmonella. However, environmental samples obtained from the irradiated finished product storage area continued to yield the outbreak strain of Salmonella.
Antibiotic susceptibility testing
CDC-NARMS testing of three human isolates from the outbreak identified resistance to tetracycline (3/3 isolates), ceftriaxone (1 of 3 isolates) and ampicillin (1 of 3 isolates). Among the samples collected during the July 2010 inspection, tetracycline resistance was demonstrated in 12 of the 13 Salmonella isolates obtained from frozen mice and all 19 isolates obtained from environmental sampling. An additional environmental isolate collected during the March 2011 follow-up inspection also demonstrated resistance to tetracycline. None of the environmental samples demonstrated resistance to ceftriaxone or ampicillin.
Follow-up case finding
Thirty-six human isolates with onset or estimated onset dates between 1 December 2009 and 30 July 2010 were uploaded to PulseNet USA. Following this investigation, continued surveillance by PulseNet USA identified reports of Salmonella I 4,[5],12:i:- isolates with a PFGE pattern indistinguishable from the outbreak strain after the recall and irradiation intervention had occurred. Following the July 2010 recall, 38 human isolates with onset or estimated onset dates between 1 August 2010 and 31 March 2011 were reported to PulseNet USA (Fig. 2). A full calendar year after the recall, isolates of the outbreak strain continued to be reported to PulseNet USA; between 29 August 2011 and 2 February 2012, 46 new cases of Salmonella I 4, [5],12:i:- were reported from 22 states (CDC, 2012a,b). During that investigation, 27 of these 46 cases were interviewed. Twenty cases (74%) reported reptile or amphibian exposure and 15 (56%) reported feeder rodent exposure. Although tracing the source of the mice was difficult during that investigation because of limited records and complex supplier networks, case patients had purchased rodents from pet stores supplied by breeders who had received mice from Company A.
Discussion
This report summarizes the US investigation into an international outbreak of tetracycline-resistant Salmonella I 4, [5],12:i:- infections with over 500 cases occurring from 2008 to 2010 throughout the United States, the United Kingdom and Canada. Four Canadian provinces identified 22 cases that matched the outbreak strain and had reptile exposure (J. Tataryn, personal communication). In the United Kingdom, 543 total cases had been reported as of 26 February 2013 (C. Lane, unpublished data). As in the United Kingdom, US cases were significantly associated with exposure to reptiles and feeder rodents. Although Company A recalled all frozen feeder animals produced during the time period and initiated irradiation of frozen feeder mice prior to sale, cases with this PFGE pattern continued to be reported in all three affected countries throughout 2012.
In 2011, the American Pet Products Manufacturer’s Association estimated that 4.6 million households own a pet reptile in the United States, an increase from 1.2 million households in 2008 (Shepherd, 2008), suggesting that reptiles have become increasingly popular pets. Reptiles are a well-established source of human salmonellosis and may intermittently shed Salmonella for years (Pfleger et al., 2003). Reptile or amphibian contact has been associated with an estimated 6% of all laboratory-confirmed, sporadic human Salmonella infections in the United States, and 11% of infections among persons aged less than 21 years (Mermin et al., 2004). Reptile-associated Salmonella infections more commonly lead to hospitalization and more fre quently involve infants than do other Salmonella infections (Ackman et al., 1995; Mermin et al., 2004). Once a reptile is infected with Salmonella, it can potentially transmit the infection to persons who handle the reptile or who are exposed to the reptile’s habitat or general environment (Mermin et al., 2004). Isolates obtained from a bearded dragon lizard in Wisconsin as part of this investigation matched the outbreak strain; the owner reported routinely feeding frozen feeder mice to the reptile, suggesting this as a source of colonization with the outbreak strain.
Similar to reptiles, rodents infected with Salmonella can appear healthy and clean while shedding the organism. Rodents have been previously associated with multistate Salmonella outbreaks (Swanson et al., 2007; Fuller et al., 2008), and Salmonella is frequently isolated from wild and captive rodents (Habermann and Williams, 1958; Umali et al., 2012). Colonized rodents can contaminate cages and bedding, leading to indirect human exposure; direct exposure via handling of the animal can also occur. The process of producing frozen feeder rodents may contribute to external contamination of the rodent with Salmonella. Prior to freezing, groups of the larger rodents are euthanized in a CO2 chamber. During this process, the bowels of the rodents are evacuated. Exposure to the evacuated faeces may further contribute to external contamination of the animals. External contamination of the rodent could increase the risk of human Salmonella exposure, especially if safe handling practices are not followed. In addition, freezing does not kill Salmonella; the organism has been shown to survive freezing for as long as 78 weeks (Escartin et al., 2000; Dominguez and Schaffner, 2009).
Of note, 100% (3/3) of isolates obtained from US human cases and tested by CDC-NARMS demonstrated resistance to tetracycline, while over 400 tetracycline-resistant human isolates were reported from the United Kingdom outbreak (Harker et al., 2011). Additionally, 97% (32/33) of isolates obtained during inspections of Company A’s production facility demonstrated tetracycline resistance. In general, tetracycline resistance is not uncommon in Salmonella; of all Salmonella Typhimurium isolates tested by NARMS in 2010 (n = 366), 29% were resistant to tetracycline (CDC NARMS, 2010). Company A representatives indicated to CDC personnel that Company A had previously used feed containing chlortetracycline and a sulpha antibiotic for non-therapeutic purposes. The exact amounts, durations and dates of administration were not provided, but company officials reported halting the use of these drugs prior to the FDA inspection. As any use of antibiotics can contribute to resistance, it is possible the prior use of antibiotics in the feed by Company A helped select for, or maintain, the tetracycline-resistant strain of Salmonella seen in this outbreak (FDA, 2013a,b,c). Additionally, the administration of non-therapeutic anti-microbials to mice has been shown to lower the infectious dose of resistant Salmonella by a factor of 100 000 and increase the degree of faecal shedding by a factor of 1000. Thus, the use of antibiotics may have facilitated the development and transmission of the tetracycline-resistant Salmonella infections within Company A (Bohnhoff et al., 1954; Latour and Barnum, 1981; Que and Hentges, 1985). While not seen in this outbreak, in general, patients infected with antibiotic-resistant Salmonella have higher hospitalization rates and bloodstream infections than patients infected with susceptible strains (Martin et al., 2004; Varma et al., 2005). Because antimicrobial drug use in both humans and animals can contribute to the development of anti-microbial resistance, it is important to limit medically important drug use to therapeutic indications (CDC, 2013a,b).
Of the 543 cases in the UK, 110 occurred after the recall; 84% of the post-recall cases reported reptile exposure (C. Lane, personal communication). Three of the 22 Canadian cases had illness onset after the recall announcement (J. Tataryn, personal communication). In the United States, a decrease in cases was not reported in the 6 months immediately following the recall. There are many possibilities to explain the protracted nature of this outbreak. Distributors typically purchase frozen rodents in bulk and repackage them in smaller quantities prior to selling them to local stores. Both the initial shipments and the repackaged rodents have minimal package labelling, making traceback of purchases to the distributor or producer challenging. This lack of package labelling could prevent consumers from identifying recalled product in their household or facility. Frozen feeder rodents also have a long shelf life (up to 12 months), raising the possibility that consumers and distributors may have continued to use recalled product for months following the recall. Additionally, infected reptiles may continue to shed Salmonella intermittently for up to 25 weeks (Burnham et al., 1998); a reptile infected prior to the recall could therefore infect their human caretaker months after the recall was initiated. While the irradiation protocol utilized by Company A has not been validated, the irradiation facility ensured a 6-log reduction for Salmonella at the dose range utilized. Mice are shipped from Company A’s production facility to the irradiation facility; after irradiation, they are shipped back to Company A for storage until distributed. Areas in the irradiated finished product storage area, including the table used in packaging irradiated product for shipping, yielded the outbreak strain of Salmonella in both the late July 2010 and March 2011 re-inspections. This finding suggests that the outer surface of the finished product packaging may have become contaminated, contributing to the protracted nature of this outbreak.
When asked about rodents and reptiles as a source of human salmonellosis, only 13% of ill persons reported awareness that rodents can cause human salmonellosis, and 53% were aware that reptiles could cause illness. Consumers, the pet industry, healthcare providers and veterinarians need to be aware of the potential health risks posed by reptile ownership, including the use of live or frozen feeder rodents. CDC recommends that households with children younger than 5 years old or persons with weakened immune systems not own reptiles or rodents (CDC, 2003a,b). CDC additionally recommends that people wash their hands thoroughly with soap and warm water immediately after touching reptiles or rodents, as well as anything in the area where these animals live or roam (CDC, 2003a,b; CDC 2005). Only 20% of cases in our investigation reported hand washing after handling rodents, and only 40% washed their hands after handling their reptile. These low percentages demonstrate a need for improved consumer education on safe handling of both reptiles and their feed, to include safe handling labels on frozen feeder rodent packaging. More detailed recommendations on how to reduce the risk of zoonotic salmonellosis are available on CDC’s website (http://www.cdc.gov/zoonotic/gi/index.html).
This study utilized a case–case method in which other cases of salmonellosis were used as controls. This method assumes that there is no overlap in the causal pathways for the two Salmonella serotypes. This methodology might have been problematic in investigating certain common exposures to Salmonella (i.e. chicken); however, because this study’s aim was to investigate the reptile and rodent exposures which are generally rare among all cases of Salmonella infections, we feel this approach was justified. Another limitation to this study was that there was a relatively small sample size; therefore, the study was not powered to identify small effects. However, despite the limitations of our sample size, we were able to identify the significant effect of reptile and rodent exposures.
As a result of this and other outbreaks, the Pet Industry Joint Advisory Council (PIJAC) led a work group to develop a best management guidance document for the production, distribution and sale of frozen feeder rodents (PIJAC, 2013). Public health officials and the pet industry should work together to develop such guidance aimed at the prevention of future outbreaks associated with frozen feeder rodents. Frozen feeder rodent producers, suppliers and distributors should follow the animal food labelling requirements as described in 21 CFR §501.5 (FDA, 2013a,b, c). Additionally, all packages of frozen feeder rodents should include ‘best by’ dates, lot information and safe handling instructions. The risk of human salmonellosis from frozen feeder rodents is ongoing as demonstrated by a recent outbreak (CDC, 2014). As demonstrated by this outbreak, continued opportunities exist for public health officials, the pet industry, veterinarians and consumers to work together to prevent salmonellosis associated with pet food, pets and other animals.
Impacts.
Exposure to frozen feeder rodents used as food for pet reptiles was epidemiologically associated with illness in an international outbreak of tetracycline-resistant Salmonella infections, demonstrating a need for improved public awareness of the association between Salmonella and frozen feeder rodents.
Adding safe handling instructions to packages of frozen feeder rodents may help reduce the risk of Salmonella infection.
Rodent producers and distributors should keep detailed sales and shipment records and clearly label packages of frozen feeder rodents to allow for more efficient identification and traceback of potentially contaminated products.
Acknowledgements
We would like to thank state and local health departments, the United Kingdom Health Protection Agency, the Public Health Agency of Canada, FDA Center for Veterinary Medicine and CDC National Center for Emerging Zoonotic and Infectious Diseases for their contributions to this investigation.
Funding
This work was supported by the United States Centers for Disease Control and Prevention.
Footnotes
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the Centers for Disease Control and Prevention or the Food and Drug Administration.
Conflict of Interest
There is no conflict of interest.
References
- Ackman DM, Drabkin P, Birkhead G, Cieslak P. Reptile-associated salmonellosis in New York State. Pediatr. Infect. Dis. J. 1995;14:955–959. doi: 10.1097/00006454-199511000-00006. [DOI] [PubMed] [Google Scholar]
- Bohnhoff M, Drake BL, Miller CP. Effect of streptomycin on susceptibility of intestinal tract to experimental Salmonella infection. Proc. Soc. Exp. Biol. Med. 1954;86:132–137. doi: 10.3181/00379727-86-21030. [DOI] [PubMed] [Google Scholar]
- Burnham BR, Atchley DH, DeFusco RP, Ferris KE, Zicarelli JC, Lee JH, Angulo FJ. Prevalence of fecal shedding of Salmonella organisms among captive green iguanas and potential public health implications. J. Am. Vet. Med. Assoc. 1998;213:48–50. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Reptile-associated salmonellosis — selected states, 1998–2002. MMWR Morb. Mortal. Wkly Rep. 2003a;52:1206–1209. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Lymphocytic Choriomeningitis Virus from Pet Rodents. 2003b Available at: http://www.cdc.gov/healthypets/lcmv_rodents.htm (accessed on 20 March 2014)
- Centers for Disease Control and Prevention (CDC) Interim guidance for minimizing risk for human lymphocytic choriomeningitis virus infection associated with rodents. MMWR Morb. Mortal. Wkly Rep. 2005;53:1–3. Dispatch. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). National Antimicrobial Resistance Monitoring System for Enteric Bacteria (NARMS) Human Isolates: Final Report 2010. 2010 Available at: http://www.cdc.gov/narms/reports.html (accessed 20 March 2014)
- Centers for Disease Control and Prevention (CDC) Notes from the Field: Infections with Salmonella I 4,[5], 12:i:- linked to exposure to feeder rodents - United States, August 2011-February 2012. MMWR Morb. Mortal. Wkly Rep. 2012a;61:277. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Notes from the field: outbreak of salmonellosis associated with pet turtle exposures – United States, 2011. MMWR Morb. Mortal. Wkly Rep. 2012b;61:79. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Notes from the Field: Multistate outbreak of Salmonella Infantis, Newport, and Lille infections linked to live poultry from a single mail-order hatchery in Ohio - March-September, 2012. MMWR Morb. Mortal. Wkly Rep. 2013a;62:213. [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Antibiotic Resistance Threats in the United States, 2013. 2013b Available at: http://www.cdc.gov/drugresistance/threat-report-2013 (accessed on 20 March 2014)
- Centers for Disease Control and Prevention (CDC) Multistate Outbreak of Human Salmonella Typhimurium Infections Linked to Frozen Feeder Rodents. 2014 Available at: http://www.cdc.gov/salmonella/typhimurium-rodents-05-14/index.html (accessed on 9 June 2014)
- Clinical and Laboratory Standards Institute, (CLSI) 2013 Available at http://www.clsi.org.
- Council of State and Territorial Epidemiologists, (CSTE) 2012 Available at: http://c.ymcdn.com/sites/www.cste.org/resource/resmgr/PDFs/CSTENotifiableConditionListA.pdf.
- Dominguez SA, Schaffner DW. Survival of Salmonella in processed chicken products during frozen storage. J. Food Prot. 2009;72:2088–2092. doi: 10.4315/0362-028x-72.10.2088. [DOI] [PubMed] [Google Scholar]
- Escartin EF, Saldana Lozano J, Rodriguez Garcia O. Quantitative survival of native Salmonella serovars during storage of frozen raw pork. Int. J. Food Microbiol. 2000;54:19–25. doi: 10.1016/s0168-1605(99)00149-x. [DOI] [PubMed] [Google Scholar]
- Firth D. Bias reduction of maximum likelihood estimates. Biometrika. 1993;80:27–38. [Google Scholar]
- Food and Drug Administration (FDA) 2013a Available at: http://www.gpo.gov/fdsys/pkg/CFR-2012-title21-vol6/pdf/CFR-2012-title21-vol6-sec501-5.pdf (accessed on 20 March 2014)
- Food and Drug Administration (FDA) Guidance for Industry. New Animal Drugs and New Animal Drug Combination Products Administered in or on Medicated Feed or Drinking Water of Food-Producing Animals: Recommendations for Drug Sponsors for Voluntarily Aligning Product Use Conditions with GFI #209. 2013b Available at http://www.fda.gov/downloads/AnimalVeterinary/GuidanceComplianceEnforcement/GuidanceforIndustry/UCM299624.pdf (accessed on 20 March 2014)
- Food and Drug Administration (FDA) Guidance for Industry. The Judicious Use of Medically Important Antimicrobial Drugs in Food-Producing Animals. 2013c Available at http://www.fda.gov/downloads/AnimalVeterinary/GuidanceComplianceEnforcement/GuidanceforIndustry/UCM216936.pdf (accessed on 20 March 2014)
- Fuller CC, Jawahir SL, Leano FT, Bidol SA, Signs K, Davis C, Holmes Y, Morgan J, Teltow G, Jones B, Sexton RB, Davis GL, Braden CR, Patel NJ, Deasy MP. A multi-state Salmonella Typhimurium outbreak associated with frozen vacuum-packed rodents used to feed snakes. Zoonoses Public Health. 2008;55:481–487. doi: 10.1111/j.1863-2378.2008.01118.x. [DOI] [PubMed] [Google Scholar]
- Gaffga NH, Barton Behravesh C, Ettestad PJ, Smelser CB, Rhorer AR, Cronquist AB, Comstock NA, Bidol SA, Patel NJ, Gerner-Smidt P, Keene WE, Gomez TM, Hopkins BA, Sotir MJ, Angulo FJ. Outbreak of salmonellosis linked to live poultry from a mail-order hatchery. N. Engl. J. Med. 2012;366:2065–2073. doi: 10.1056/NEJMoa1111818. [DOI] [PubMed] [Google Scholar]
- Goupil BA, Trent AM, Bender J, Olsen KE, Morningstar BR, Wunschmann A. A longitudinal study of Salmonella from snakes used in a public outreach program. J. Zoo. Wildl. Med. 2012;43:836–841. doi: 10.1638/2011-0281R1.1. [DOI] [PubMed] [Google Scholar]
- Habermann RT, Williams FP., Jr Salmonellosis in laboratory animals. J. Natl Cancer Inst. 1958;20:933–947. [PubMed] [Google Scholar]
- Hale CR, Scallan E, Cronquist AB, Dunn J, Smith K, Robinson T, Lathrop S, Tobin-D’Angelo M, Clogher P. Estimates of enteric illness attributable to contact with animals and their environments in the United States. Clin. Infect. Dis. 2012;54(Suppl 5):S472–S479. doi: 10.1093/cid/cis051. [DOI] [PubMed] [Google Scholar]
- Harker KS, Lane C, De Pinna E, Adak GK. An outbreak of Salmonella Typhimurium DT191a associated with reptile feeder mice. Epidemiol. Infect. 2011;139:1254–1261. doi: 10.1017/S0950268810002281. [DOI] [PubMed] [Google Scholar]
- Harris JR, Bergmire-Sweat D, Schlegel JH, Winpisinger KA, Klos RF, Perry C, Tauxe RV, Sotir MJ. Multistate outbreak of Salmonella infections associated with small turtle exposure, 2007-2008. Pediatrics. 2009;124:1388–1394. doi: 10.1542/peds.2009-0272. [DOI] [PubMed] [Google Scholar]
- Harris JR, Neil KP, Barton Behravesh C, Sotir MJ, Angulo FJ. Recent multistate outbreaks of human Salmonella infections acquired from turtles: a continuing public health challenge. Clin. Infect. Dis. 2010;50:554–559. doi: 10.1086/649932. [DOI] [PubMed] [Google Scholar]
- Hopkins KL, de Pinna E, Wain J. Prevalence of Salmonella enterica serovar 4,[5],12:i:- in England and Wales, 2010. Euro. Surveill. 2012;17:20275. [PubMed] [Google Scholar]
- Latour PB, Barnum DA. Use of ducks as a model to study the effect of antibiotics in feed on the fecal shedding of Salmonella. Am. J. Vet. Res. 1981;42:2105–2108. [PubMed] [Google Scholar]
- Loharikar A, Briere E, Schwensohn C, Weninger S, Wagendorf J, Scheftel J, Garvey A, Warren K, Villamil E, Rudroff JA, Kurkjian K, Levine S, Colby K, Morrison B, May A, Anderson S, Daly E, Marsden-Haug N, Erdman MM, Gomez T, Rhorer A, Castleman J, Adams JK, Theobald L, Lafon P, Trees E, Mitchell J, Sotir MJ, Barton Behravesh C. Four multistate outbreaks of human Salmonella infections associated with live poultry contact, United States. Zoonoses Public Health. 2012;59:347–354. doi: 10.1111/j.1863-2378.2012.01461.x. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Fyfe M, Dore K, Buxton JA, Pollari F, Henry B, Middleton D, Ahmed R, Jamieson F, Ciebin B, McEwen SA, Wilson JB. Increased burden of illness associated with antimicrobial-resistant Salmonella enterica serotype typhimurium infections. J. Infect. Dis. 2004;189:377–384. doi: 10.1086/381270. [DOI] [PubMed] [Google Scholar]
- Mermin J, Hutwagner L, Vugia D, Shallow S, Daily P, Bender J, Koehler J, Marcus R, Angulo RFJ. Reptiles, amphibians, and human Salmonella infection: a population-based, case-control study. Clin. Infect. Dis. 2004;38(Suppl 3):S253–S261. doi: 10.1086/381594. [DOI] [PubMed] [Google Scholar]
- Mettee Zarecki SL, Bennett SD, Hall J, Yaeger J, Lujan K, Mph R, Adams-Cameron M, Winpisinger Quinn K, Brenden R, Biggerstaff G, Hill VR, Sholtes K, Garrett NM, Lafon PC, Barton Behravesh C, Sodha SV. US outbreak of human salmonella infections associated with aquatic frogs, 2008-2011. Pediatrics. 2013;131:724–731. doi: 10.1542/peds.2012-2031. [DOI] [PubMed] [Google Scholar]
- Pet Industry Joint Advisory Council (PIJAC) Best Management Practices for Feeder Rodent Production and Distribution. 2013 Available at http://www.pijac.org/sites/default/files/pdfs/FeederRodentIndustryBMPSept2013.pdf (accessed 20 March 2014)
- Pfleger S, Benyr G, Sommer R, Hassl A. Pattern of Salmonella excretion in amphibians and reptiles in a vivarium. Int. J. Hyg. Environ. Health. 2003;206:53–59. doi: 10.1078/1438-4639-00184. [DOI] [PubMed] [Google Scholar]
- Que JU, Hentges DJ. Effect of streptomycin administration on colonization resistance to Salmonella Typhimurium in mice. Infect. Immun. 1985;48:169–174. doi: 10.1128/iai.48.1.169-174.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribot EM, Fair MA, Gautom R, Cameron DN, Hunter SB, Swaminathan B, Barrett TJ. Standardization of pulsed-field gel electrophoresis protocols for the subtyping of Escherichia coli O157: H7, Salmonella, and Shigella for PulseNet. Foodborne Pathog. Dis. 2006;3:59–67. doi: 10.1089/fpd.2006.3.59. [DOI] [PubMed] [Google Scholar]
- Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM. Foodborne illness acquired in the United States - major pathogens. Emerg. Infect. Dis. 2011;17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd AJ. Results of the 2007 AVMA survey of US pet-owning households regarding use of veterinary services and expenditures. J. Am. Vet. Med. Assoc. 2008;233:727–728. doi: 10.2460/javma.233.5.727. [DOI] [PubMed] [Google Scholar]
- Swanson SJ, Snider C, Braden CR, Boxrud D, Wunschmann A, Rudroff JA, Lockett J, Smith KE. Multi-drug-resistant Salmonella enterica serotype Typhimurium associated with pet rodents. N. Engl. J. Med. 2007;356:21–28. doi: 10.1056/NEJMoa060465. [DOI] [PubMed] [Google Scholar]
- Umali DV, Lapuz RR, Suzuki T, Shirota K, Katoh H. Transmission and shedding patterns of Salmonella in naturally infected captive wild roof rats (Rattus rattus) from a Salmonella-contaminated layer farm. Avian Dis. 2012;56:288–294. doi: 10.1637/9911-090411-Reg.1. [DOI] [PubMed] [Google Scholar]
- Varma JK, Molbak K, Barrett TJ, Beebe JL, Jones TF, Rabatsky-Ehr T, Smith KE, Vugia DJ, Chang HG, Angulo FJ. Antimicrobial-resistant nontyphoidal Salmonella is associated with excess bloodstream infections and hospitalizations. J. Infect. Dis. 2005;191:554–561. doi: 10.1086/427263. [DOI] [PubMed] [Google Scholar]