Abstract
Mating depends on the accurate detection of signals that convey species identity and reproductive state. In African clawed frogs, Xenopus, this information is conveyed by vocal signals that differ in temporal patterns and spectral features between sexes and across species. We characterized spectral sensitivity using auditory evoked potentials (AEPs), commonly known as the auditory brainstem response, in males and females of four Xenopus species. In female X. amieti, X. petersii, and X. laevis, peripheral auditory sensitivity to their species own dyad- two, species-specific dominant frequencies in the male advertisement call – is enhanced relative to males. Males were most sensitive to lower frequencies including those in the male-directed release calls. Frequency sensitivity was influenced by endocrine state; ovariectomized females had male-like auditory tuning while dihydrotesosterone-treated, ovariectomized females maintained female-like tuning. Thus adult, female Xenopus demonstrate an endocrine-dependent sensitivity to the spectral features of conspecific male advertisement calls that could facilitate mating. Xenopus AEPs resemble those of other species in stimulus and level dependence, and in sensitivity to anesthetic (MS-222). AEPs were correlated with body size and sex within some species. A frequency following response, probably encoded by the amphibian papilla, might facilitate dyad source localization via interaural time differences.
Keywords: Xenopus, ABR, matched-filter, sex difference, androgen
Introduction
In many species, acoustic signals serve as the primary means of social communication. Enhanced neural responses to vocal features can be demonstrated in higher auditory regions such as the midbrain in terrestrial frogs (Narins and Capranica, 1980; Edwards et al. 2002; Hoke et al. 2004) and in birds and mammals (Bauer et al. 2002; Klug et al. 2002; Portfors et al. 2009, Woolley and Portfors, 2013). The significance of acoustic features can also differ for males and females accompanied by sex differences in sensitivity of the auditory periphery and midbrain (Narins and Capranica, 1976; 1980; Shen et al. 2011). Matching peripheral auditory processing to informative acoustic features- a hypothesis explored in this study- could facilitate signal reception from a distant source or in noisy environments (Frishkopf et al. 1968; Narins and Capranica 1976; Sisneros et al. 2004; Moreno-Gomez et al. 2013; Simmons 2013).
African clawed frogs (Xenopus) are nocturnal, aquatic anurans whose social communication relies largely on producing and responding to vocal signals (Vigny 1979; Zornik and Kelley 2011). Spectral and temporal features of vocalizations convey species identity, sex and reproductive state (Tobias et al. 2011; 2014). Here we examine frequency-specific neural responses in four species: X. amieti, X. petersii, X. laevis, and X. borealis. In both sexes, the fundamental vocal unit is a sound pulse produced by the larynx (Tobias et al. 2011). The sound pulses in male advertisement calls are composed of spectral dyads: two, simultaneous dominant frequencies (DFs) referred to here as the species own dyad (SOD) (Fig. 1; Wetzel and Kelley, 1983; Tobias et al. 2011). Male advertisement calls are high intensity, long-range signals that attract sexually receptive females (Picker 1983; Yager 1992). In contrast, release calls, evoked when a male or an unreceptive female is clasped by a receptive male, are low intensity and close range (Tobias et al. 2014). Release calls contain a single DF that is similar across species (Tobias et al. 2014), thus spectral cues alone cannot convey information on sex and reproductive state.
Fig. 1.
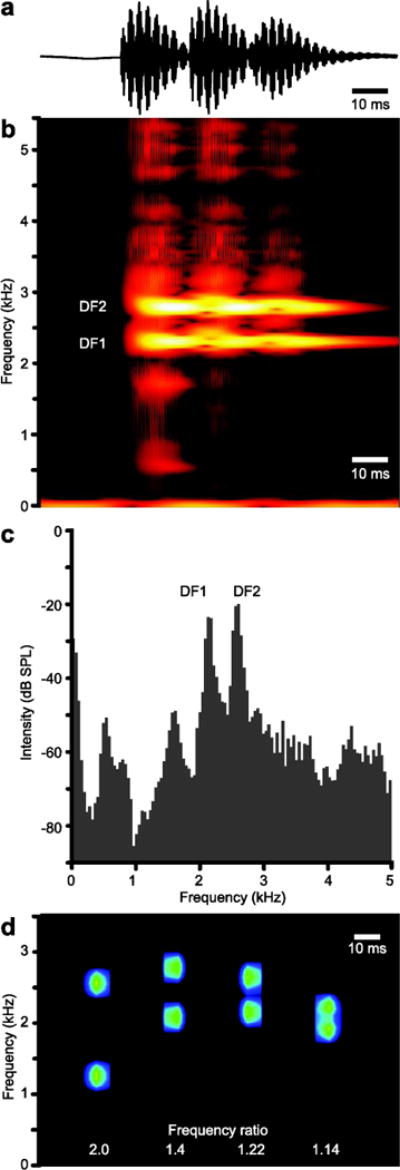
a) An oscillogram of three sound pulses from a male X. petersii call. The spectrogram (b) and frequency power spectrum (c) of the call in (a) illustrates the two, distinct frequencies of high intensity within each pulse. The lower frequency is DF1, the higher DF2. d) A spectrogram of the synthetic SODs for X. borealis, X. amieti, X. petersi, and X.amieti (left to right) with the frequency ratio identified below each sound pulse. In this spectrogram, intensity is coded in blue-green instead of red-yellow (as in b) to distinguish the in vitro and in vivo sound sources.
We examined the sensitivity of the auditory periphery in Xenopus using a non-invasive approach: measuring auditory-evoked potentials (AEPs) from surface electrodes positioned on the head. This approach has been used in several anuran species (Katbamna et al. 2006a; Katbamna et al. 2006b; Brandt et al. 2008; Schrode et al. 2014) and is usually referred to as the auditory brainstem response (ABR). While the timing and quality of the signals suggest that these field potentials represent early stages (VIIIth nerve and hindbrain) in auditory processing (Seaman 1991; Hall 2007), the source of anuran AEPs has not yet been disambiguated in terms of contributions of activity of inner ear hair cells, VIIth nerve afferents and CNS auditory nuclei. We thus adopt the more general term AEP to refer to the sound-induced potentials recorded here.
Previous studies have suggested that the filtering properties of the anuran auditory system are matched to informative spectral features of calls (reviewed in Simmons 2013). In addition, while a variety of evidence suggests that gonadal steroids influence auditory-based social behavior in frogs, little is known about their activational effects on auditory processing (reviewed in Arch and Narins 2009). Frogs also exhibit size-related differences in auditory sensitivity and behavioral responses to sound (Zakon and Wilczynski 1988; Keddy-Hector et al. 1992, Fox 1995; Meenderink et al. 2010), and peripheral auditory sensitivity can be shaped by the mechanical properties of the head and ear (Pinder and Palmer 1983; Smotherman and Narins 2000; Schoffelen 2009). Here, we also compared AEP recordings in the sexes to determine if the larger size of females contributes to auditory responses.
The sound pulses in Xenopus male advertisement calls are dyads composed of two DFs (e.g., Fig. 1). Two tones presented simultaneously interact to generate a beat frequency equal to the higher frequency minus the lower. In mammals, sensitivity to these beat frequencies can be measured in the cochlea (Ruggero 1992) and perception is influenced by the frequencies and amplitudes of the tones used to generate them (Humes 1979). Behavioral (Simmons 1988; Hainfeld 1996) and electrophysiological (Capranica and Moffet 1980; Corwin et al. 1982; Klump et al. 2004) evidence in frogs has shown that many species are sensitive to beat frequencies, envelope modulations, or harmonic structure of acoustic stimuli. In anurans, two sensory epithelia transduce sound: lower frequencies (~0.1 to 1 kHz) are represented in the amphibian papilla and higher frequencies (~1 – 4 kHz) in the basilar papilla (Narins and Capranica 1980; Lewis et al. 1982). In many species of Xenopus, the beat frequencies created by DF2-DF1 of the advertisement call should be less than 1000 Hz, within the typical frequency range of the amphibian papilla (Lewis et al. 1982). We thus also asked if Xenopus are also sensitive to beats by examining the frequency following response (FFR), a phase locked neural response to periodic stimuli thought to be involved in processing complex sounds (Skoe and Kraus 2010). The FFR exhibits plasticity in response to experience with music and speech in humans (Krishnan and Grandour 2009; Krishnan et al. 2010; Bidelman 2013) and might exhibit plasticity in response to endocrine state. We thus asked whether the AEP could contribute to processing harmonic vocal signals in female Xenopus under different hormonal conditions.
Materials and Methods
Animal care and maintenance
Animals were housed and treated in accordance with Columbia University’s IACUC requirements. Frogs were obtained from Xenopus Express (Brooksville, FL, USA), Nasco (Fort Atkinson, WI, USA), or were lab bred. The range and mean of [(male), (female)] body masses in grams were: X. laevis [(32 – 63, mean 50, n = 11), (48 – 177, mean 118, n = 22)]; X. amieti [(3 – 7, mean 5, n = 10), (8 – 14, mean 10, n = 14)]; X. borealis [(14 – 22, mean 18, n = 11), (17 – 45, mean 27, n = 11)]; X. petersii (formerly X. laevis Congo; Furman et al. 2015) [(12 – 18, mean 15, n = 11), (15 – 25, mean 23, n = 14)]. Frogs were housed in polycarbonate aquaria under a 12/12 light-dark cycle and fed Nasco frog brittle. Aquarium water was changed twice weekly.
A group of adult X. laevis females were ovariectomized (OVX; mass range 41–101 g, mean 69 g, n = 15) under tricane methanesulfonate (MS222; Sigma Aldrich, St. Louis, MO, USA) anesthesia. The ovaries and fat bodies were removed through an incision in the abdomen and blood vessels were closed with cautery. A second group (mass range 48 – 98 g, mean 78 g, n = 9) underwent ovariectomy and was implanted with a compressed pellet (Parr Instrument Company Pellet Press; Moline, IL, USA) of 10 mg of dihydrotestosterone (DHT; Sigma Aldrich, St. Louis, MO, USA) in the dorsal lymph sac at the time of surgery. The wound was sutured and the animals recovered for 6 – 8 weeks prior to recording AEPs.
Preparation for Recording
Animals were immobilized with an injection of d-tubocurare (Sigma-Aldrich; St. Louis, MO, USA) into the dorsal lymph sac (3 μg/kg X. amieti; 8 μg/kg X. petersii; 10 μg/kg X. borealis, X. laevis). Frogs were maintained at ambient temperature (22.5 – 24 °C) and were draped with a wet Kimwipe (Kimberly-Clark Global Sales, LLC; Roswell, GA, USA) to maintain skin moisture. Oxygen levels were maintained via cutaneous respiration and air lung reserves. Animals were pre-treated with subcutaneous lidocaine prior to AEP electrode insertion. If an animal regained motility during recording, supplemental curare (3 μg/kg; Sigma-Aldrich; St. Louis, MO, USA) was administered via the dorsal lymph sac followed by a 15 – 30 minute pause in recording. After recording, animals were allowed to recover on a float in their home tank, while draped in a wet Kimwipe to maintain skin moisture.
AEP Procedures and Equipment
We recorded auditory evoked potentials in a sound-attenuated chamber (2 m × 1.6 m × 2 m; Industrial Acoustics Company, Inc., New York, NY, USA). Three, platinum, 30-gauge needle electrodes (Grass Instruments, West Warwick, RI, USA) were inserted beneath the dermis: 1) non-inverting - above the foramen magnum, 2) inverting - posterior to the tympanic disk, 3) ground – dorsal, mid-anterior/posterior. Frogs were stimulated with airborne sound through a closed-field coupler (Christensen-Dalsgaard et al. 2012) that was sealed around the ipsilateral tympanum using ear mold compound (Gold Velvet II; All-American Mold Laboratories, Oklahoma City, OK, USA). The coupler was composed of a speaker (Yuin PK2; Yuin Corporation, China) epoxied to an otoscope tip containing a microphone for calibration (FG-23742-D36; Knowles, Itasca, IL, USA). After sealing the coupler around the tympanic disk, the speaker was calibrated to a 300–7000 Hz frequency sweep using BioSigRZ software (TDT, Gainesville, FL, USA). Microphone sensitivity and calibration were confirmed before and after these experiments using a PCB CAL200 Precision Acoustic Calibrator (Provo, UT, U.S.A.) and model 377B01 condenser microphone (PCB Piezotronics, Inc., Depew, NY, USA). AEPs were acquired using a 10 Hz high-pass filter, a 3000 Hz low-pass filter, and a 60 Hz notch filter.
We used the closed-field coupler to present auditory stimuli because it reliably delivers specific frequencies directly to the ear. The frequencies used in this study are often distorted when played underwater in the relatively small tanks that are compatible with recording auditory evoked potentials in the laboratory (Akamatsu et al. 2002). Audiograms obtained using the closed-field coupler were consistent with audiograms obtained underwater using behavioral conditioning, including enhanced resolution of frequencies near the DFs of the advertisement call (Elepfandt et al. 2000). The closed-field coupler stimulates the ear and does not mimic the effects of free-field sounds that are influenced by other parts of the frog’s body such as the mouth or lungs (Pinder and Palmer 1984; Aertsen et al. 1986; Narins et al. 1988; Jørgensen et al. 1991). Thus, enhanced sensitivity to frequencies outside of the range of communication vocalizations (3400 Hz; Elepfandt et al. 2000) that have been observed underwater may not be observed with closed coupler stimulation. The spectral sensitivities observed with the closed coupler stimulation technique should also be observed underwater, however.
Stimuli
Stimulus presentation, response acquisition and data storage were conducted with Tucker Davis Technologies (TDT; Gainesville, FL, USA) system III (RZ6 with an RA4PA Medusa preamp with RA4LI headstage) and a PC running SigGenRZ and BioSigRZ software (TDT, Gainesville, FL, USA). AEPs were the average neural response to 512 presentations of each stimulus, alternating in phase by 180 degrees. The inter-stimulus interval was 21 ms.
Clicks
Clicks were square wave pulses, 0.1 ms in duration that produced broadband frequency stimulation up to approximately 60 kHz. Clicks were presented at high intensity (111 dB) to stimulate as many auditory cells as possible. Measuring the amplitude of the click-evoked response (Fig. 2a) allowed us to measure and track general auditory responsiveness across the recording period. Responses to clicks were recorded at four time points over the course of each recording session. In curarized frogs, click amplitudes did not differ significantly from the beginning to the end of the experiment (paired t-tests, all p > 0.05). Across the population, click amplitude decreased by an average of 3% from the beginning to the end of the experiment.
Fig. 2.
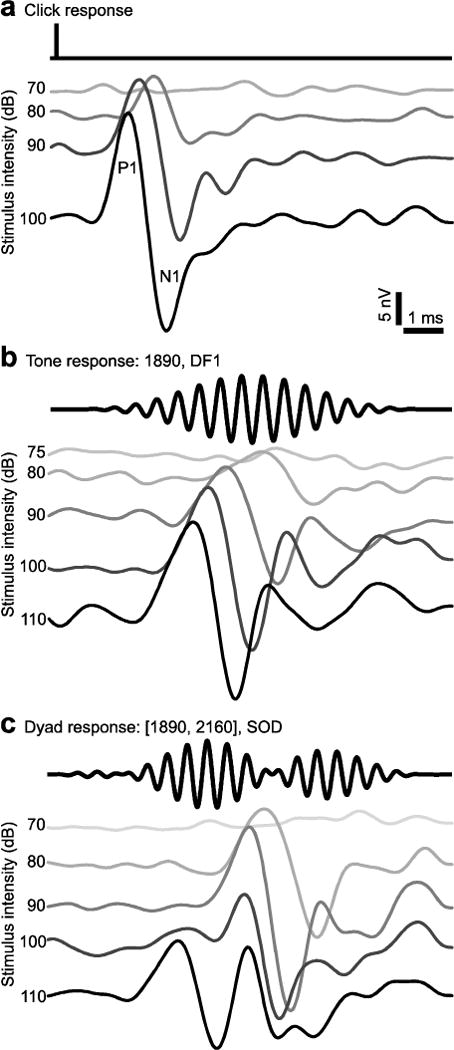
AEPs from a single X. laevis female (DF1: 1890, DF2: 2160, Frequency ratio: 1.14) illustrate the differences between responses to clicks (a), tones (b), and dyads (c). Oscillograms of stimuli are in black above each waterfall plot, stimulus intensities are to the left of each AEP. The first positive (P1) and negative (N1) peaks are indicated in (a).
Tones
Audiograms were generated by recording AEPs to pure tones (10 ms duration, 5 ms rise/fall time, cosine ramp) at approximately 250 Hz steps between 1000 and 4000 Hz, including the DFs of the SOD. To compare frequency sensitivity between sexes, we normalized each audiogram by setting its lowest threshold to 0 dB.
Two-tone dyads
Two-tone (dyad) stimuli (10 ms duration, 5 ms rise/fall time, cosine ramp) were generated and power-matched (root mean square) using customized MATLAB scripts (MathWorks; Natick, MA, USA). Stimuli were 10 ms in duration in order to mimic the sustained portion of the frogs’ sound pulse (Vignal and Kelley 2007) and to allow for adequate frequency information in multi-frequency stimuli. Analysis of spectrograms confirmed well-defined frequency peaks > 50 dB above maximum background levels. Dyads were presented at 100 dB.
Each species has a unique dyad (DF1, the lower frequency and DF2) and DF2:DF1 ratio (Tobias et al. 2011). The SODs and dyad frequency ratios of the species we studied are: X. amieti [2040 Hz, 2710 Hz], 1.33; X. borealis [1250 Hz, 2500 Hz], 2.0; X. petersii [2110 Hz, 2580 Hz], 1.22; X. laevis [1890 Hz, 2160 Hz], 1.14 (Fig. 1d). These values were obtained from in vivo recordings, as described previously (Tobias et al. 2011) and updated with additional calls analyzed by M. Tobias and U. Kwong-Brown.
Two-tone stimuli were presented to frogs while recording AEPs in order to investigate sensitivity to dyad frequencies and frequency ratio. The two tones in our synthetic stimuli were of equal intensity. To test selectivity for frequency, the SOD -[DF1, DF2]- was presented along with dyads in which one of the tones was modified. In one manipulation- [DF1, Variable]- the higher frequency was changed to create dyads with frequency ratios of 1.14, 1.22, 1.33, 1.39, 1.5, 1.6, 1.67, 1.78, 1.88, and 2.0. In the other manipulation- [Variable, DF2]- the lower frequency was changed to create frequency ratios of 1.14, 1.22, 1.33, 1.39, 1.88, 2.0. Frequency ratios (1.14, 1.22, 1.33, 1.5, 2.0) represent those used by various Xenopus species. The additional ratios were from the Western musical scale. More frequency ratios were tested in the first manipulation because DF1 tends to be more prominent in male vocalizations (Tobias et al. 2011).
To examine frequency ratio sensitivity specifically, two additional dyad sets were generated for each species using frequencies not found in male vocalizations. Tones with frequencies near DFs were used as the higher tone: X. amieti, 2250 Hz, 2500 Hz; X. borealis and X. petersii, 2000 Hz, 2250 Hz; X. laevis, 2000 Hz, 2500 Hz. The frequency of the lower tone was changed to create ratios of 1.14, 1.22, 1.33, 1.39, 1.88, and 2.0.
For each species, a single series of all 27 dyads was generated. Stimuli were ordered to maximize the frequency disparity between temporally adjacent dyads. To estimate thresholds, each dyad was presented at three intensities, in 10 dB steps. After initial threshold estimation with AEPs recorded at the 3 intensities, the series order was reversed and additional AEPs were collected in order to measure threshold at a 5 dB intensity resolution. In 30% of frogs, the dyad series order was reversed for the initial collection to test for stimulus order effects. No presentation order-related differences in threshold were observed.
Band-limited masker
Two simultaneous tones produce a beat frequency via amplitude modulation of the stimulus envelope at the rate of the difference between the two frequencies. A beat frequency less than 1000 Hz should specifically stimulate one of the two peripheral auditory organs: the amphibian papilla (Lewis et al. 1982). Since the SOD beat frequency, DF2-DF1, should be less that less than 1000 Hz for most Xenopus (Tobias et al. 2011), dyads might stimulate both the amphibian and the basilar papillae producing to enhanced sensitivity to the SOD. We thus repeated these experiments while playing a band-limited masker to occlude the influence of the amphibian papilla. By playing continuous 100–800 Hz noise during recording, we asynchronously activated cells in the amphibian papillae thus averaging out their effect on the AEP and allowing us to specifically determine the response characteristics of the basilar papilla.
In 5 female X. laevis, a continuous, band-limited masker was played during dyad presentation. The masker was presented to the ipsilateral ear through a second earbud mounted to Y-shaped plastic tube in a modified version of the apparatus described in section 2.2. To set the intensity of the masker, the band was set at 100–5000 Hz and intensity was increased until the AEP peak height to a 111 dB SPL click was decreased by >75%. When played with dyads, the masker was band limited to 100–800 Hz.
AEP analysis
AEPs were acquired using a 10 Hz high-pass filter, a 3000 Hz low-pass filter, and a 60 Hz notch filter. To facilitate quantitative analysis and threshold determination after data collection, an additional 1500 Hz low pass filter was applied to increase the signal to noise ratio. AEP peak to peak frequencies (first positive peak to second positive peak, P1 to P2) ranged from approximately 200–500 Hz near threshold to 1000 Hz at high stimulus intensities, which is consistent with those observed in other studies in Xenopus (Katbamna et al. 2006a; Katbamna et al. 2006b). For peak height assessment, each AEP was frame-shifted on the y-axis so that the average pre-response (0 – 2 ms) value was zero to account for individual variation in offset. Peak latency was measured from the time of stimulus onset; the speaker was, on average, 1.5 cm from the ear resulting in a negligible 0.04 ms delay between stimulus onset and vibration of the tympanic disk.
The response threshold for each stimulus was estimated as the average of the lowest intensity evoking a significant response super-threshold and highest intensity that failed to evoke responses. Significant response waveforms met three criteria: 1) amplitude of the first positive (P1) or negative (N1) peak was at least 2 standard deviations above noise levels measured during the 3 ms pre-response period; P1 occurred from 3 – 8 ms, N1 from 4 – 9 ms; 2) P1 or N1 amplitude exceeded the maximum noise amplitude during the pre-response period; and 3) P1 amplitude exceeded average noise levels over the recording session. Criteria 1 and 3 were adjusted by increasing stringency but held constant for each individual in order to best fit species-typical response amplitude and individual signal-to-noise ratio. Criterion 1 was adjusted by increasing the number of standard deviations between 2 and 3 and Criterion 3 was adjusted by increasing the threshold above average noise level.
Statistical analysis
For each individual in this study, threshold was estimated for all dyads. Thresholds were normally distributed (Shapiro et al. 1968). The multiple frequency manipulations used to generate dyads prevented dyad identity from being treated as a continuous variable. Dyad identity was instead treated as a categorical variable in a repeated measures analysis of variance (ANOVA). A Dunnett’s multiple comparison post hoc test was used to compare the thresholds for all other dyads to that of the SOD.
We reduced the dimensionality of the dyad variable by calculating the absolute frequency difference from SOD for each dyad. To calculate absolute frequency difference from SOD, we summed the absolute value of the minimum differences between a dyad’s frequencies and those of the SOD. For example, the SOD for X. laevis is [1890 Hz, 2160 Hz]. The absolute frequency difference between the SOD and the dyad [2000 Hz, 2280 Hz] would be: abs(1890 Hz – 2000 Hz) + abs(2160 Hz – 2280 Hz) = 230 Hz. Absolute frequency difference as a continuous variable allowed us to examine the relationship between threshold and dyad disparity from SOD but was not used to determine which dyads had significantly higher thresholds than the SOD. Absolute frequency differences were not normally distributed so a Spearman rank order correlation analysis was used.
For audiograms, we examined the relationship between threshold and tone frequency in each species. Tone_frequency could be treated as a continuous variable. However, because of the complex, non-monotonic relationship between threshold and tone frequency, we treated tone_frequency as a categorical variable in a repeated measures ANOVA. Sex and sex*tone_frequency were incorporated into the model. Sex was significant where reported but sex*tone_frequency was not significant for any species.
For comparison of OVX, OVX+DHT, and intact female X. laevis, we used similar methods, replacing treatment with sex. To examine differences in histogram distributions we performed a one way ANOVA on thresholds by treatment.
To examine the effect of sex, species, and size on AEP amplitude we began with a forward selection, stepwise regression analysis. Sex, species, size, and all pairwise comparisons were included in the variables that could have been incorporated into the model. Other tests were used to evaluate hypotheses generated by the full model and explore potential species differences as noted.
Statistical analyses were performed with STATA (v9.0; StataCorp LP, TX, USA) and R (v3.1.3; R foundation, Austria) software.
Results
Stimulus effects on AEP waveform
AEPs in Xenopus were similar in shape and pattern to those reported for other frogs and classes of animals (Corwin et al. 1982; Britton-Powell et al. 2002; Katbamna et al. 2006a; Katbamna et al. 2006b; Schrode et al. 2014; Vélez et al. 2015). The AEP waveform is composed of positive and negative peaks; the shortest latency peaks identified as positive 1 (P1) and negative 1 (N1; Fig. 2). In general, P1 was the most prominent and AEP amplitude increased and latency decreased with increasing stimulus intensity. However, the overall shape of the AEP waveform was different for each stimulus. For example, AEPs evoked by clicks had one or two high amplitude peaks, while responses to tones and dyads were generally more variable in shape, with peaks of smaller amplitudes and longer latencies (Fig. 2b, c). The difference between click and tone AEPs is likely due to differences in the stimulus (Fig. 2). Clicks indiscriminately excite auditory neurons during a brief, 0.1 ms stimulus which leads to a large, brief field potential; tones excite a smaller population of auditory neurons that respond to that frequency and do so over a longer time scale.
We asked whether AEP waveforms were qualitatively different in response to different tone frequencies, particularly the DFs of the species advertisement call. We observed no difference after controlling for threshold. When presented at equal intensities with respect to threshold, DF and non-DF tones produced similar AEP waveforms (not shown).
Sex differences in dyad thresholds
In females, auditory thresholds for the SOD were lower than those of other dyads. Changing either DF1 or DF2 by as little as 200 Hz significantly increased thresholds (Fig. 3). Threshold differences between stimuli that matched the SOD and stimuli that did not match the SOD were significant for the majority of comparisons: 89% in X. amieti, 70% in X. petersii, and 96% in X. laevis (Fig. 3c; Repeated Measures ANOVAs, Dunnett’s multiple comparison post hoc, all p <0.05,). In males, AEP thresholds for the SOD were not lower than thresholds for other dyads (Fig. 3). Frequency shifts away from the SOD had more variable effects on thresholds and only in X. laevis did a frequency shift result in a higher threshold than the SOD (Fig. 3b). In both males and females, dyads composed of higher frequencies had higher thresholds; thresholds increased with the sum of the two frequencies in a dyad (Spearman; ρ = 0.46 males, 0.39 females, all p < 0.001; not shown).
Fig. 3.
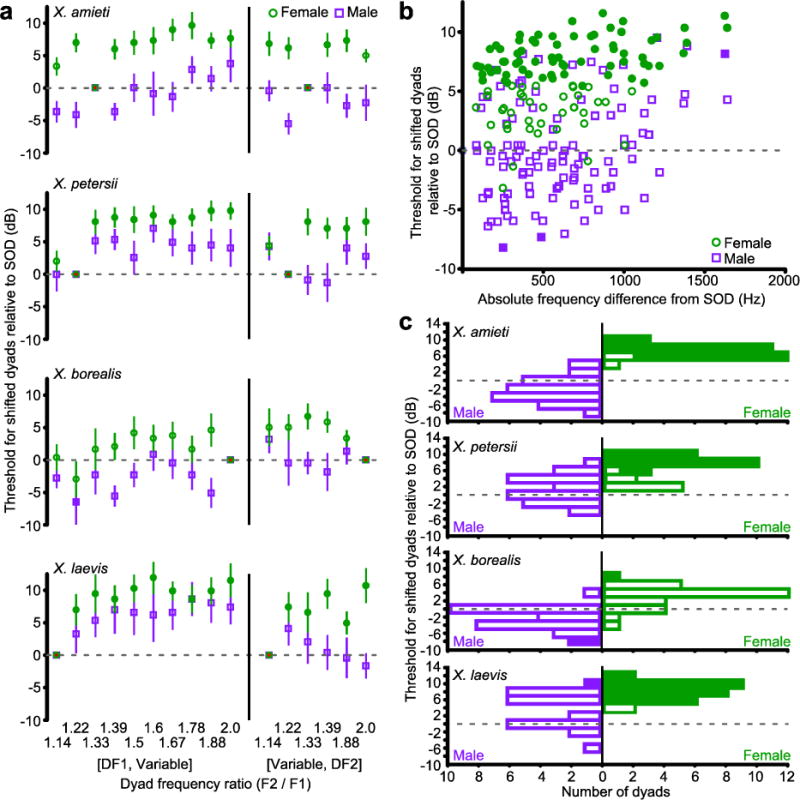
a) AEP thresholds for dyads containing at least one DF from the SOD, in females (green circles) and males (purple squares). Filled symbols indicate thresholds that differ significantly from the SOD threshold (Repeated Measures ANOVAs with Dunnett’s multiple comparisons post hoc, all p < 0.05). The dashed line indicates the threshold for the SOD. Error bars indicate standard error. b) The absolute frequency difference from SOD was calculated as the sum of the absolute differences between each DF and the closest frequency in the altered dyad. All dyads (not just the DF1 or DF2 manipulations shown in (a)) are illustrated and species are grouped using the color conventions in (a). c) The data in (b) presented as a histogram of thresholds for all dyads relative to the threshold for SOD by species. For female Xenopus (green), thresholds for most other dyads are significantly higher (Filled blocks represent dyads with thresholds significantly different from the SOD (dashed line).
We asked if females displayed peripheral auditory sensitivity to the frequency ratio used in the advertisement call of their own species. In female X. amieti, X. laevis, and X. petersii, as the absolute magnitude of the frequency shift away from the SOD increased, thresholds also increased (Fig. 4a; Spearman, all ρ > 0.45, all p < 0.02). Dyads sharing the species-specific ratio were similar to dyads with other ratios (Fig. 4b,c). Al low threshold was only observed for dyad of the species own DF1 and DF2. Thus, female Xenopus are peripherally sensitive to the combination of DF1 and DF2 used in their conspecific male advertisement call but not to the species-typical frequency ratio when produced by non-species specific DFs.
Fig. 4.
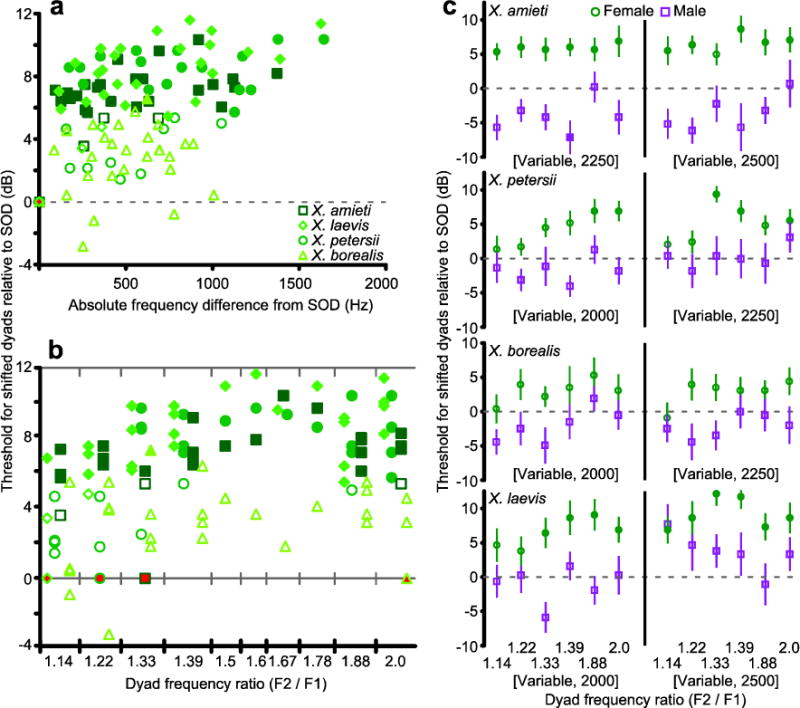
a) Female data from Fig. 3b separated by species: X. amieti (squares), X. laevis (diamonds), X. petersii (circles), and X. borealis (triangles). As in Fig. 3b, filled symbols indicate significantly higher thresholds than the SOD threshold (determined by the same Repeated Measures ANOVA in Fig. 3). b) The dyads in (a) separated by frequency ratio. Dyads with AEP thresholds that differed significantly (same Repeated Measures ANOVA in Fig. 3) from the SOD (filled in red) are filled in green. c) AEP thresholds for dyads composed of arbitrary frequencies that did not contain DF1 or DF2. Filled symbols indicate thresholds that were significantly different (determined by the same Repeated Measures ANOVA in Fig. 3) from the SOD (dashed line). Error bars indicate standard error.
While a similar pattern was observed in X. borealis, females AEPs showed no threshold differences between SOD and non-SOD stimuli (Figs. 3, 4). Because peaks in the frequency spectra of natural sounds at 2:1 ratios contribute to octave equivalence (sounds with 2:1 ratios are perceptually equivalent to each other even in non-human species; Wright et al. 2000), X. borealis females may perceive the SOD stimulus as a single frequency. In other frog species with a 2:1 call frequency ratio, there are minimal sex differences in auditory tuning (Schrode et al. 2014). In frogs that produce calls made up of only one frequency, adding tones to create 2:1 ratios (as well as other ratios used by Xenopus) had no effect on female preference (Gerhardt et al. 1990; Witte et al. 2001; Gerhardt et al. 2007; Gerhardt and Humfeld 2013), suggesting that some frogs are more tuned to frequency than frequency ratio for behavioral decision making.
Sex differences in tone thresholds
Sex- and endocrine-mediated differences in dyad sensitivity could reflect differences in tuning to individual frequencies. We measured thresholds to pure tones within the dyad frequency ranges of all four species, including the DFs of each species’ SOD (Fig. 5). Thresholds for pure tones generally increased with frequency in both sexes and all species (Fig. 5; Spearman, all ρ > 0.61, p < 0.001). In X. laevis, X. amieti, and X. petersii, audiograms differed by sex (Repeated Measures ANOVAs; all p < 0.02). Male threshold minima occurred at or below 1500 Hz, frequencies in release calls (Tobias et al. 2014), and increased above 1500 Hz. Female threshold minima often included DF1 and DF2 of the SOD. Only the X. amieti DF1 threshold was significantly different between sexes (Repeated measures ANOVA with Bonferroni post hoc F(1,22) = 8.86, p = 0.007).
Fig. 5.
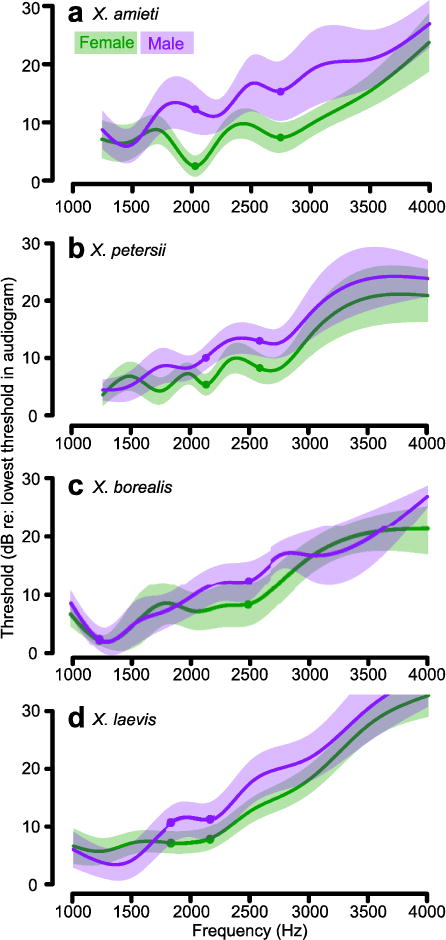
a–d) Female (green) and male (purple) audiograms from four species of Xenopus. Average values and 95% confidence intervals (shaded regions) for each sex and species are shown. Before averaging, individual audiograms were normalized to the frequency of lowest threshold to facilitate comparison of audiogram shape. DF1 and DF2 of the SOD are indicated by filled circles.
Endocrine signals influence female spectral sensitivity
In Xenopus, sexual differentiation of vocal motor circuitry relies on endocrine differences between the sexes (Kelley, 1986; Rhodes et al. 2007; Zornik and Kelley 2011). Ovarian estrogen derives from the aromatization of testosterone (Gruber et al. 2002); androgens are the primary ovarian steroids in Xenopus (Lutz et al. 2001). Because cell bodies in the acoustic ganglion express androgen receptor mRNA (Perez et al. 1996), we tested the hypothesis that female sensitivity to SOD frequencies depends on ovarian steroids. We compared AEP thresholds to tones and frequency dyads in ovariectomized (OVX), intact and OVX + dihydrotestosterone-treated females. Dihydrotestosterone (DHT) is a non-aromatizable androgen.
AEPs in OVX females were less sensitive to the SOD compared to intact controls (ANOVA, F(2,81) = 30.13, P < 0.001, Intact versus OVX: Bonferroni post hoc F = −5.09, p < 0.001), resulting in male-like responses (Fig. 6A). The dyad threshold distribution in OVX females differed significantly from that of OVX+DHT females (OVX versus OVX+DHT: Bonferroni post hoc F = −2.154, p = 0.005) that retained a more female-like response profile (orange, Fig. 6A). The difference in threshold distribution between OVX+DHT and intact females (Intact versus OVX+DHT: ANOVA F(2,81) = 30.13, p <0.001, Bonferroni post hoc F = 2.932, p < 0.001) indicates that the selectivity of OVX+DHT females for the SOD was not as robust as in intact females. Thus DHT only partially maintains their sensitivity to the SOD.
Fig. 6.
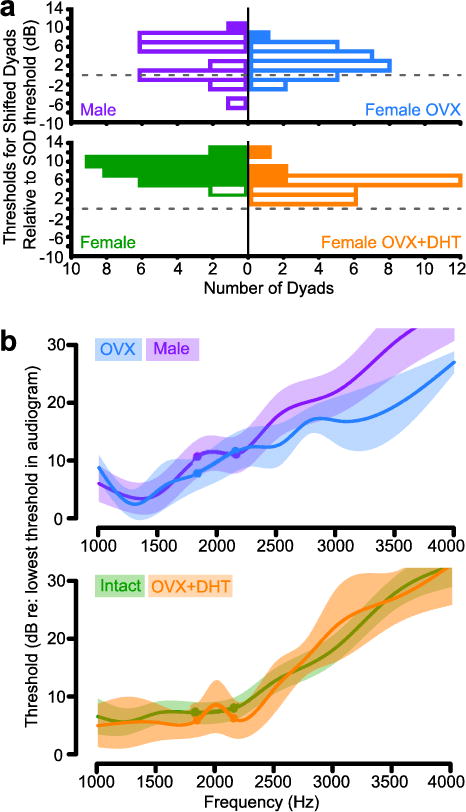
a) Using the conventions in Fig 4a, intact female (green) and male (purple) X. laevis are compared to ovariectomized X. laevis females (OVX, blue) and ovariectomized X. laevis females treated with DHT (OVX+DHT, orange). b) Using conventions from Fig 5, the average OVX audiogram (blue) is compared to that of males (purple) and the average OVX+DHT audiogram (orange) is compared to that of intact females (green).
Audiograms for individual frequencies were male-like in OVX females (Fig. 6B) and differed from audiograms of intact females (Repeated Measures ANOVA, F(1, 350) = 6.36, p = 0.01), with a significant threshold increase at DF2 (Bonferroni, F(1,35) = 4.31, p = 0.04). OVX+DHT female audiograms did not differ from those of intact females (Fig. 6B; Repeated Measures ANOVA). The greatest difference between OVX and OVX+DHT audiograms was the significantly greater sensitivity to DF2 after DHT treatment (Repeated Measures ANOVA, frequency*treatment interaction, F(10,220) = 1.93, p = 0.04; Bonferroni post hoc F(1,22) = 6.65, p = 0.01).
Dyad beat frequencies influenced AEP waveforms
The presentation of two tones simultaneously produces a beat frequency via amplitude modulation of the stimulus envelope at the rate of the difference between the two frequencies (Frequency 2 (F2) – Frequency 1 (F1); Fig. 2c, for example). The specific combination of two frequencies [F1, F2] in a dyad will generate characteristic beat frequencies for each. While not determined specifically for Xenopus, frequencies 1000 Hz and greater are thought to stimulate hair cells in the basilar papilla (Lewis et al. 1982) while those less than 1000 Hz stimulate the amphibian papilla. For most Xenopus, the SOD beat frequency, DF2-DF1, is less than 1000 Hz (Tobias et al. 2011). Thus, a dyad and its beat frequency might stimulate both the amphibian and basilar papillae simultaneously, contributing to enhanced sensitivity to the SOD.
To investigate the effects of beat frequencies, we measured AEPs to dyads played at high intensity (100 dB), where the AEPs to dyads differed from AEPs to tones (Fig. 2b, c). The waterfall plot in Fig. 7a shows the averaged AEP waveforms to selected dyads for X. borealis females. As beat frequency increased from 364 Hz to 630 Hz, the frequency of AEP peaks increased, P1 latency decreased, and P1 peak height increased. At beat frequencies above 630 Hz, these qualities of the AEP waveform appeared to stabilize. There was no qualitative difference between the AEP waveform to the SOD (beat frequency 1250 Hz) and dyads with similar beat frequencies. There was also no effect of endocrine state on the FFR; OVX and OVX+DHT X. laevis females had FFRs that were indistinguishable from intact females and males (data not shown). In all species, males had FFRs similar to females, so the remaining analyses were grouped by species alone.
Fig. 7.
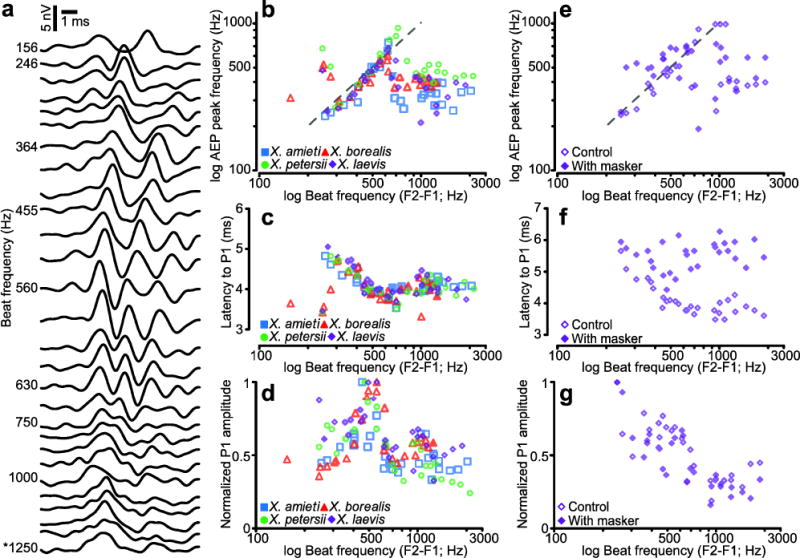
AEP waveforms varied with the beat frequency of dyads. a) A waterfall plot of averaged female X. borealis AEPs with increasing beat frequency (F2–F1) towards the bottom. The asterisk indicates the AEP to the SOD. b) The correlation between the frequency of AEP peaks (P1, P2) and the beat frequency of the dyad from 200–700 Hz. The dashed line is y = x. The correlation between beat frequency and P1 latency (c) and P1 amplitude (d) are also plotted. The SOD for each species is indicated by the filled symbol. e, f, and g plot AEP peak frequency, P1 latency, and amplitude, respectively, using the same conventions as b, c, and d. This subset of animals was exposed to dyads alone (open symbols) and in the presence of a 100–800 Hz masker (filled symbols).
In all four species, the frequency of AEP peaks (P1, P2) followed the beat frequency of the dyad stimulus between approximately 200 and 700 Hz (Fig. 7b). Above beat frequencies of 700 Hz, the AEP peak frequencies were comparatively stable but less than their maximum. The change in peak frequency was correlated with a decrease in latency to P1. As beat frequencies increased to 700 Hz, latencies decreased until they stabilized at approximately 4 ms (Fig. 7c). P1 amplitude increased with beat frequency up to 600 Hz before stabilizing at a sub maximal value (Fig. 7d). Three of the species tested had SODs with beat frequencies less than 700 Hz, X. amieti, X. laevis, and X. petersii (Filled symbols, Fig. 7b–d). Among these, only the X. petersii SOD has a beat frequency (470 Hz) near the optimum for each of these categories, suggesting that the Xenopus frequency following response is not a specialization for communication within species, but is instead a generalized property of their peripheral auditory system.
To test the hypothesis that amphibian papilla is involved in the frequency following response to beat frequencies below 700 Hz, we repeated these experiments while playing a band-limited masker to occlude the influence of the amphibian papilla on the AEP. In 5 X. laevis females, we presented the dyad stimuli at 100 dB, and the dyad stimuli at 100 dB in the presence of a 100–800 Hz, band-limited masker. In this subset of animals, the response to the dyads alone (open symbols) was similar to the population (compare Fig. 7 b to e, c to f, d to g). When compared to the intra-animal controls, the AEPs to dyads with a masker (filled symbols) showed a clear loss of the frequency following response (Fig. 7e) and the latencies were less variable (Fig. 7f), though the relative peak amplitudes were qualitatively similar (Fig. 7g). These results suggest that the sensory organ responsible for transducing low frequency sounds, the amphibian papilla, is involved in the frequency following response.
In humans, the FFR correlates with the perception of consonant and dissonant musical intervals (Bidelman and Krishnan 2009). Our methods were sufficient for observing the FFR, but insufficient for detailed FFR analysis (Skoe and Kraus 2010). However, the frequency ratios of Xenopus calls and the dyads used as test stimuli fit the intervals of the Western musical scale, so we determined whether the consonance or dissonance of a frequency pair contributed to AEP threshold, or P1 peak amplitude and latency at 100 dB. Dyads were ranked in order of consonance based on human psychophysical reporting (Malmberg 1918; Bidelman 2013). There were no correlations between consonance and threshold, peak latency or amplitude either for dyads that contained one common frequency or across all dyads (Pearson’s R, all p > 0.05, data not shown).
Frog species, sex and size influenced AEPs
Frogs can exhibit size-related differences in auditory sensitivity and behavioral responses to sound (Zakon and Wilczynski 1988; Keddy-Hector et al. 1992; Fox 1995; Meenderink and Narins 2010), and across our population sex and species were the greatest contributors to size variation. To investigate whether size, sex, and species contribute to variation in AEP signal amplitude, we used these variables in a linear model predicting the highest amplitude response measured, the P1 in AEPs to clicks presented at 111 dB SPL. Across all the frogs tested, frog mass was the best single predictor of the P1 peak amplitude (Fig. 8, Regression F(1,102) = 28.32, p < 0.0001, R2 = 0.2173) with a negative correlation between mass and P1 peak amplitude.
Fig. 8.
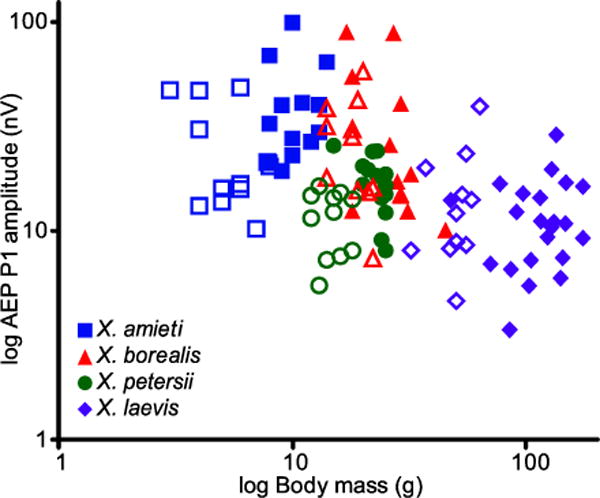
The relationships between AEP amplitude (P1 amplitude of 111 dB click response) and body mass, species (color and shape), and sex (males open, females solid) are plotted.
Incorporating sex as an indicator variable (Male? [0 = No, 1 = Yes]) yielded a negative coefficient and significantly improved the model (Forward selection, F(2,101) = 20.80, p < 0.0001, R2 = 0.2917), suggesting that males had smaller AEP amplitudes than females. Males were smaller than females in all species (mass and snout-vent length, t-tests, all species p < 0.001), so the difference between the sexes ran counter to the general observation that smaller frogs have larger AEP peaks. Frog mass co-varied with species; the largest frogs were always X. laevis and the smallest were X. amieti, but X. borealis and X. petersii were similar in body mass. To disambiguate the role of species and size, we added a categorical variable for X. petersii which significantly improved the model (Forward selection, F(3,100) = 19.36, p < 0.0001, R2 = 0.3675), suggesting that AEP amplitude differed between species, even those with similar mass. Indeed, a model consisting of only categorical variables for species was significant (F(3,100) = 19.17, p < 0.0001, R2 = 0.3651).
To clarify the statistical relationships between AEP amplitude and species, sex, and mass observed in the full model that grouped all species, we examined the relationships between AEP amplitude, sex, and mass within each species. Within species, there were no significant correlations between AEP amplitude and frog mass or snout-vent length (Pearson’s R, all p > 0.05). As in the full model, males tended to produce smaller AEP peaks than females, but this was only significant for X. amieti and X. petersii (Student’s t, p = 0.050 and 0.009, respectively). There was no sex difference in signal amplitude in X. borealis and X. laevis, demonstrating that the sex difference in AEP signal may be species-dependent. Between the two species of similar size, X. borealis and X. petersii (mass, snout-vent length; Student’s t, both p > 0.41), there was a significant difference in AEP signal amplitude (Student’s t, p = 0.001). Therefore, since sex and mass were not strong predictors of AEP amplitude within species, species identity appeared to be the major contributing factor to the relationships observed between sex, mass, and AEP amplitude.
Anesthetic Comparison
Anesthesia and anesthetic state greatly influence the quality of auditory recordings (Katbamna et al. 2006b; Schumacher et al. 2011). To examine the effects of anesthetic state on AEP quality, we compared AEPs from frogs immobilized with d-tubocurare to those anesthetized with tricane methanesulfonate (MS222), a commonly used frog and fish anesthetic. Frogs were immersed in 1% MS222 until deeply anesthetized, determined by lack of response to toe pinch and loss of righting reflex. Exposure to MS222 until the frogs were fully anesthetized resulted in a small AEP that continued to decrease over the first 30–45 m of recording.
To obtain larger, more stable AEPs with MS222, we developed a two-step anesthesia protocol: immersion in 1% MS222 solution for 3 – 7 m, depending on body size, and then transferring the awake frog back to its home tank until the anesthetic had time to take complete effect (5 – 7 m). If ineffective, the frog was the re-immersed in MS222 for 30 – 60 s followed by 5 m in home water. This protocol resulted in shallow anesthesia; there was no righting response, nor response to toe pinch but frogs recovered more rapidly. When necessary, supplemental anesthesia was administered during recording sessions as 5 – 10 drops of 1% MS222 onto the Kimwipe draped over the frog; recording resumed 5 – 10 m later.
Frogs immobilized with curare produced AEPs of greater amplitude that were more stable over time than frogs anesthetized with MS222 (Fig. 9A). Even when the minimum amount of MS222 required to anesthetize a frog for the recording session was delivered, AEP amplitudes to clicks were 63% smaller than those recorded under curare (X. petersii females, MS222 n = 8, curare n = 14; Student’s T, p = 0.01). The effects of MS-222 increased with time (Fig. 9a). For example, in X. petersii females anesthetized with MS222, AEP amplitudes decreased significantly from baseline recordings within an hour (Repeated Measures ANOVA, F(2,7) = 9.083, p = 0.003, Dunnett’s post hocs p < 0.05). When immobilized with curare, the amplitude of the AEP to a 111 dB click did not change over the course of the experiment. Decreases in spectral sensitivity were also observed under MS222; the audiograms of MS222-treated X. amieti females (n = 11) did not display regions of enhanced sensitivity seen in audiograms recorded from curarized X. amieti females (n = 14; Fig. 9b).
Fig. 9.
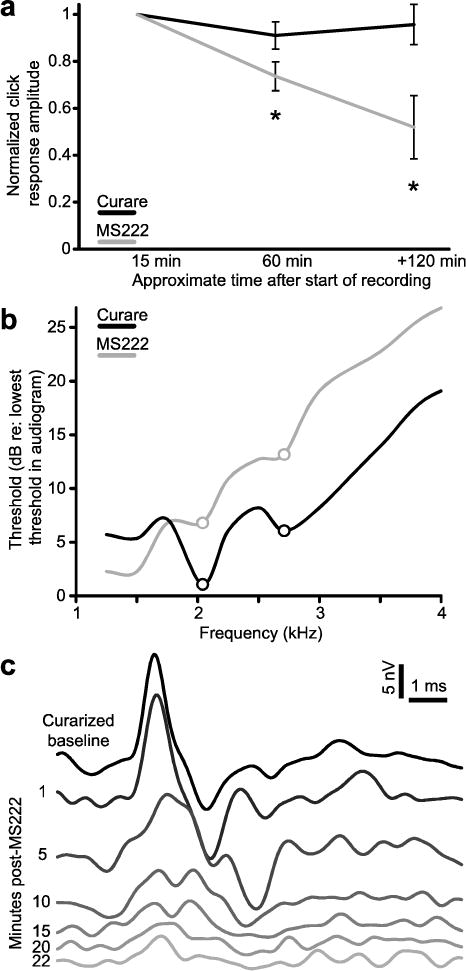
a) The P1 amplitude of AEPs to clicks in frogs treated with MS222 (gray; n = 8 X. petersii females) and curare (black; n = 14 X. petersii females). b) Average audiograms recorded under MS222 anesthesia (gray; n = 11 X. amieti) and when curarized (black, n = 14 X. amieti females). DF1 and DF2 are indicated by circles. c) The AEP of a curarized X. laevis female to a 111 dB click stimulus before and after injection of an anesthetizing dose of MS222 into the dorsal lymph sac.
Decreases in signal quality were also observed when MS222 anesthesia was administered acutely. In the example of Fig. 9c, a curarized X. laevis female was given a 1.5 mL injection of 1% MS222 into the dorsal lymph sac. Over the next 22 minutes, the AEP amplitude to a 111 dB click decreased by 74%. Because of the effects of MS222 on signal quality, all of the results presented outside of this section are from frogs treated only with curare.
Discussion
Our results reveal that Xenopus have sexually dimorphic, endocrine-mediated frequency tuning. Female Xenopus in three of the four species examined were especially sensitive to the spectral features of conspecific male calls. In female X. laevis, the sensitivity to these spectral features was modulated by ovarian signals. We examined factors that could influence AEPs and compared Xenopus AEPs to those of other species. We also discuss potential mechanisms for and the ecological significance of dyad sensitivity.
AEP quality
The AEPs obtained from Xenopus share characteristics with AEPs from other animals, including humans (Corwin et al. 1982; Britton-Powell et al. 2002; Katbamna et al. 2006a; Katbamna et al. 2006b; Hall 2007; Schrode et al. 2014; Vélez et al. 2015). The AEPs demonstrate level dependence; amplitude increases and latency decreases with stimulus intensity. We also observed variations in AEP waveform shape correlated with stimuli. These shape differences were related to differences in threshold to the stimuli presented and the envelope modulation of dyads.
Species identity appeared to make the greatest contribution to AEP amplitude. Larger frogs might be expected to have larger auditory organs and brains yielding higher amplitude AEPs. However, a 4 g X. amieti produces a larger AEP than a 150 g X. laevis. The relationship between body size and AEP amplitude is inverse and appears only across rather than within species. Skull thickness and the size of the ear or auditory nerve contribute to AEP amplitude (Mitchell et al. 1989). The relative position of the recording electrodes was kept constant for all species, but the absolute distance from the AEP source was greater in species with larger skulls and more surrounding tissue. Within each species, females are larger in body size but, at least in X. laevis, the absolute area of the tympanic disk is similar (Mason et al. 2009). Relative to body size, the tympanic disk of females is smaller than that of males (Mason et al. 2009).
These experiments were conducted at ambient temperature, between 22.5 and 24 °C. Changes in neural and hair cell activity has been observed in isolated preparations, even within this narrow temperature range (Smotherman and Narins 1998). How this temperature range influenced our in vivo recordings is not clear but a previous study in Xenopus reported no effect of variations in temperature between 17 and 20 °C on VIIIth nerve and dorsal medullary nucleus activity (Elliott et al. 2007).
One particularly noteworthy finding for future anuran AEP studies is the detrimental impact of MS222 anesthesia. MS222 is widely used in anuran AEP experiments, but anesthesia can diminish auditory sensitivity and recording quality (Katbamna et al. 2006b; Schumacher et al. 2011). Our recordings clearly demonstrate that MS222 anesthesia decreases overall auditory sensitivity and that alternative methods of immobilization should be used in auditory recordings. One potential alternative is benzocaine, which does not appear to have the same detrimental effects on hair cell activity as MS222 (Balak et al. 1990; Meyers et al. 2003) but is also associated with more health risks and mortality (Cakir and Strauch 2005; Cacala et al. 2007). In contrast to curare, a recent paper reports that both benzocaine and MS-222 block sensory nerve activity in Xenopus (Ramlochansingh et al. 2014), again supporting the use of alternative means of immobilization for recordings of neural activity.
Dyad sensitivity
The salience of acoustic features in courtship vocalizations might differ for males and females. The advertisement call of male Xenopus has a spectrally distinctive acoustic feature, a frequency dyad. Female Xenopus are especially sensitive to the dyads that make up their species’ male advertisement call while males tended to be most sensitive to the lower frequencies of male-directed calls.
Small shifts of either DF1 or DF2 away from the SOD abolished the auditory advantage of dyads for females. Females are attracted to male advertisement calls (Picker 1983) and their heightened, peripheral acoustic sensitivity to the SOD could contribute to locating a distant, calling male or to detecting the call when ambient noise levels are high, as would be predicted by the matched filter hypothesis (Frishkopf et al. 1968; Capranica and Moffat 1983; Moreno-Gomez et al. 2013; Simmons 2013). Male advertisement calls are also socially salient for other males; in X. laevis they support vocal dominance and suppress calling in rival males (Tobias et al. 2004). In males, enhanced sensitivity to distant calling males could be disadvantageous by enhancing vocal suppression a factor that might explain why we found that males were not peripherally tuned to the spectral features of the SOD.
Though matched auditory filters in anurans have been studied previously, sex differences in frequency tuning have been reported in only a few species (Narins and Capranica 1976; Wilczynski et al. 1984; Wilczynski et al. 1992; Gerhardt and Schwartz 2001). Seasonal variation associated with the reproductive cycle and the effect of social signals on the auditory system suggest that gonadal hormones influence tuning (Hillery 1984; Penna et al. 1992; Goense and Feng 2005; Arch and Narins 2009; Gall and Wilczynski 2015). The differences between intact and OVX female X. laevis described here support this idea. While there is ample evidence for androgen synthesis within the brains of male and female frogs (Mensah-Nyagan et al. 1996; Bruzzone et al. 2010; Vaudry et al. 2011), in Xenopus our results suggest instead that circulating ovarian hormones generate the female auditory matched filter.
Tuning differences between intact, OVX and OVX+DHT X. laevis demonstrate plasticity in the adult peripheral auditory system and suggest hormones modulate sensitivity to species-specific DFs. The onset latency of the threshold responses (3 – 5 ms) suggests that they reflect auditory nerve activity. Thus, loci for androgen action could be the hair cells in the auditory periphery (Forlano et al. 2015), or neurons in the acoustic ganglion expressing androgen receptor (Perez et al. 1996). Androgens contribute to feminization or have female-specific effects in other species and are the primary steroid produced in the Xenopus ovary (Lutz et al. 2001). In zebra finches, which also have sexually dimorphic hearing (Phan et al. 2006; Yoder et al. 2015), estradiol has been implicated in the acute and chronic modulation of the auditory system (Remage-Healey et al. 2012; Yoder and Vicario 2012). Though DHT is not aromatized to estradiol, conversion to 5α-androstane-3β,17β-diol might permit interaction with estrogen receptors in the inner ear (Forlano et al. 2015). Estradiol and other ovarian signals absent in males could be necessary for the enhanced auditory sensitivity of females.
In this study we found no influence of sex or endocrine state on the FFR and only a minimal influence on the click-evoked P1 amplitude. Click stimuli contain frequencies that stimulate both the amphibian and basilar papillae. The results of the masking study suggest that the FFR was generated by the amphibian papilla, but the higher frequencies of advertisement calls should instead excite the basilar papilla. Thus, the basilar papilla is a candidate site of plasticity contributing to heightened auditory sensitivity in females.
The FFR in communication
The FFR, when measured with AEP, reflects sustained neural activity that is phase-locked to individual cycles of the stimulus waveform or modulations of the stimulus envelope. Bullfrogs (Bufo americanus), leopard frogs (Rana pipiens), and green tree frogs (Hyla cinerea) are sensitive to envelope modulations and harmonic structure, as demonstrated by behavioral (Gerhardt 1978; Simmons 1988; Hainfeld et al. 1996; Simmons and Bean 2000) and electrophysiological (Capranica and Moffet 1980; Corwin et al. 1982; Klump et al. 2004; Goense and Feng 2005) evidence. While our 10 ms recording window and stimulus duration were shorter than those typically used for recording FFRs, our AEP waveforms show clear correlations between their peak frequency and the beat frequency of the stimulus dyad (Fig. 4) that are level dependent (Fig. 3, Worden and Marsh 1968) and are consistent with the FFR range recorded in other frogs (Corwin et al. 1982; Dunia and Narins 1989; Freedman et al. 1989; Narins and Wagner 1989). Moreover, the 10 ms stimuli we used to evoke the FFRs are similar in length to the sound pulses used in Xenopus communication vocalizations (Vignal and Kelley 2007) and therefore a more biologically relevant stimulus for these species.
By using a band-limited masker, we provide evidence that the FFR was generated by the amphibian papilla. The amphibian papilla contains 250–1200 hair cells, is tonotopically organized, and sensitive to frequencies from 100 – 1000 Hz, depending on species (Lewis et al. 1982; Fox 1995; van Dijk et al. 2011). In contrast, the basilar papilla contains 20 – 200 hair cells, typically has higher thresholds than the amphibian papilla, and is sensitive to frequencies from 1 – 4 kHz, depending on the species (Lewis et al. 1982; Fox 1995; Serrano et al. 2001; van Dijk et al. 2011). The basilar papilla is not tonotopically organized but its cells are still frequency sensitive (Wilczynski et al. 2001; van Dijk et al. 2011). By continuously masking the activity of the amphibian papilla and degrading the FFR, we demonstrated that it was contributing to dyad-evoked AEP waveforms. However, the invariance of the FFR across species, sex, and endocrine state suggests that beat frequencies do not underlie the female sensitivity to their SOD.
All four species and both sexes showed similar FFR patterns, suggesting that the FFR may not be specialized for species-specific communication and does not contribute to sex differences in dyad sensitivity. The call types of X. laevis (trill), X. petersii (trill), X. amieti (burst), and X. borealis (click) vary greatly in their temporal qualities but FFRs up to 700 Hz would not be a limiting factor in processing the temporal qualities of vocalizations; the fastest pulse rate (~140 Hz) is seen in the bursting call of X. amieti (Tobias et al. 2011). However, the beat frequency of X. petersii dyads is near peak frequency, latency, and amplitude optima of the FFR and could facilitate the peripheral processing of vocalizations in this species (Rose and Capranica 1985).
In humans, the FFR is correlated with the perception of speech sounds and music (Krishnan et al. 2010; Bones et al. 2014), and can be influenced by experience, particularly musical training (Chandrasekaran and Kraus 2010; Lerud et al. 2014). The frequency ratios of dyad stimuli in Xenopus advertisement calls form intervals in the Western musical scale. However, we found no correlation between our AEPs and the consonance or dissonance of the dyad intervals. This finding does not preclude the possibility of frequency ratio sensitivity at higher levels in the auditory system (Bidelman and Krishnan 2009; Skoe and Kraus 2010).
Though the FFR to beat frequencies showed no species-specific specializations, it may contribute to the neural coding of sound localization. The interference with the FFR produced by a 100–700 Hz band limited masker suggests that auditory nerve fibers contributing to the FFR respond to the frequency difference F2 – F1, as has been observed in bullfrog (Capranica and Moffatt 1980). At low frequencies the envelope periodicity encoded by these neurons could facilitate sound source localization by interaural time differences (Capranica and Moffatt 1980; Klump et al. 2004). This mechanism could contribute to dyad-mediated mate localization during phonotaxis.
Acknowledgments
This research involved animals that were cared for and treated in accordance with Columbia University’s IACUC requirements.
Funding Sources: Charles H. Revson Foundation (ICH), National Institutes of Health GM103266 (ICH), National Institutes of Health NS28634 (DBK), National Institutes of Health DC009810 (SMNW).
Jakob Christensen-Daalsgard, Catherine Carr, Caitlin Baxter, Hilary Bierman (AEP suggestions and training); Martha Tobias, Ursula Kwong-Brown (Collection and analysis of call features); David Schneider (MATLAB code).
ABBREVIATIONS
- ABR
auditory brainstem response
- AEP
auditory evoked potential
- ANOVA
analysis of variance
- DF
dominant frequency
- DHT
dihydrotestosterone
- F1
frequency one
- F2
frequency two
- FFR
frequency following response
- MS222
tricane methanesulfonate
- N1
first negative AEP peak
- OVX
ovariectomized
- OVX+DHT
ovariectomized and given DHT
- P1
first positive AEP peak
- P2
second positive AEP peak
- SOD
species own dyad
- TDT
Tucker Davis Technologies
Footnotes
The authors declare that they have no conflict of interest.
References
- Aertsen AMHJ, Vlaming MSMG, Eggermont JJ, Johannesma PIM. Directional hearing in the grassfrog (Rana temporaria L.). II. acoustics and modelling of the auditory periphery. Hear Res. 1986;21:17–40. doi: 10.1016/0378-5955(86)90043-2. [DOI] [PubMed] [Google Scholar]
- Akamatsu T, Okumura T, Novarini N, Yan HY. Empirical refinements applicable to the recording of fish sounds in small tanks. J Acoust Soc Am. 2002;112:3073–3083. doi: 10.1121/1.1515799. [DOI] [PubMed] [Google Scholar]
- Arch VS, Narins PM. Sexual hearing: The influence of sex hormones on acoustic communication in frogs. Hear Res. 2009;252:15–20. doi: 10.1016/j.heares.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balak KJ, Corwin JT, Jones JE. Regenerated hair cells can originate from supporting cell progeny: evidence from phototoxicity and laser ablation experiments in the lateral line system. J Neurosci. 1990;10:2502–2512. doi: 10.1523/JNEUROSCI.10-08-02502.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer EE, Klug A, Pollak GD. Spectral determination of responses to species-specific calls in the dorsal nucleus of the lateral lemniscus. J Neurophysiol. 2002;88:1955–1967. doi: 10.1152/jn.2002.88.4.1955. [DOI] [PubMed] [Google Scholar]
- Bidelman GM. The role of the auditory brainstem in processing musically relevant pitch. Front Psychol. 2013;4 doi: 10.3389/fpsyg.2013.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidelman GM, Krishnan A. Neural correlates of consonance, dissonance, and the hierarchy of musical pitch in the human brainstem. J Neurosci. 2009;29:13165–13171. doi: 10.1523/JNEUROSCI.3900-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bones O, Hopkins K, Krishnan A, J PC. Phase locked neural activity in the human brainstem predicts preference for musical consonance. Neurophysologia. 2014;58:23–32. doi: 10.1016/j.neuropsychologia.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt C, Andersen T, Christensen-Dalsgaard J. Demonstration of a portable system for auditory brainstem recordings based on pure tone masking difference. 1st Int Symp on Auditory and Audiological Research. 2008:241–247. [Google Scholar]
- Brittan-Powell EF, Lohr B, Hahn DC, Dooling RJ. Auditory brainstem responses in the eastern screech owl: an estimate of auditory thresholds. J Acoust Soc Am. 2002;118:314–321. doi: 10.1121/1.1928767. [DOI] [PubMed] [Google Scholar]
- Bruzzone F, Do Rego J-L, Luu-The V, Pelletier G, Vallarino M, Vaudry H. Immunohistochemical localization and biological activity of 3β-hydroxysteroid dehydrogenase and 5α-reductase in the brain of the frog, Rana esculenta, during development. J Chem Neuroanat. 2010;39:35–50. doi: 10.1016/j.jchemneu.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Buerkle NP, Schrode KM, Bee MA. Assessing stimulus and subject influences on auditory evoked potentials and their relation to peripheral physiology in green treefrogs (Hyla cinerea) Comp Biochem Physiol A. 2014;178:68–81. doi: 10.1016/j.cbpa.2014.08.005. doi: http://dx.doi.org/10.1016/j.cbpa.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacala KK, Price SJ, Dorcas ME. A comparison of the effectiveness of recommended doses of MS-222 (tricaine methanesulfonate) and orajel ® (benzocaine) for amphibian anesthesia. Herpetological Review. 2007;38:63–66. [Google Scholar]
- Cakir Y, Strauch SM. Tricaine (MS-222) is a safe anesthetic compound compared to benzocaine and pentobarbital to induce anesthesia in leopard frogs (Rana pipiens) Pharmacol Rep. 2005;57:467–474. [PubMed] [Google Scholar]
- Capranica RR, Moffat AJM. Nonlinear properties of the peripheral auditory system in anurans. In: Popper Arthur, Fay Richard., editors. Comparative studies of hearing in vertebrates. Springer-Verlag; New York: 1980. pp. 139–165. Ch. 5. [Google Scholar]
- Capranica RR, Moffat AJM. Neurobehavioral correlates of sound communication in anurans. In: Ewert JP, Capranica RR, Ingle D, editors. Advances in vertebrate neuroethology. Plenum Press; New York: 1983. pp. 701–730. [DOI] [Google Scholar]
- Chandrasekaran B, Kraus N. The scalp-recorded brainstem response to speech: neural origins and plasticity. Psychophysiology. 2010;47:236–246. doi: 10.1111/j.1469-8986.2009.00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen-Dalsgaard J, Brandt C, Willis KL, Christensen CB, Ketten D, Edds-Walton P, Fay RR, Madsen PT, Carr CE. Specialization for underwater hearing by the tympanic middle ear of the turtle, Trachemys scripta elegans. Proc Biol Soc. 2012;279:2816–2824. doi: 10.1098/rspb.2012.0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin JT, Bullock TH, Schweitzer J. The auditory brainstem response in five vertebrate classes. Electroencephalogr Clin Neurophysiol. 1982;54:629–641. doi: 10.1016/0013-4694(82)90117-1. [DOI] [PubMed] [Google Scholar]
- Dunia R, Narins PM. Temporal resolution in frog auditory-nerve fibers. J Acoust Soc Am. 1989;85:1630–1638. doi: 10.1121/1.397951. [DOI] [PubMed] [Google Scholar]
- Edwards CJ, Alder TB, Rose GJ. Auditory midbrain neurons that count. Nat Neurosci. 2002;5:934–936. doi: 10.1038/nn916. [DOI] [PubMed] [Google Scholar]
- Elepfandt A, Eistetter I, Fleig A, Gunther E, Hainich M, Hepperle S, Traub B. Hearing threshold and frequency discrimination in the purely aquatic frog Xenopus laevis (Pipidae): measurement by means of conditioning. J Exp Biol 203 Pt. 2000;23:3621–3629. doi: 10.1242/jeb.203.23.3621. [DOI] [PubMed] [Google Scholar]
- Elliott TM, Christensen-Dalsgaard J, Kelley DB. Tone and call responses of units in the auditory nerve and dorsal medullary nucleus of Xenopus laevis. J Comp Physiol A. 2007;193:1243–1257. doi: 10.1007/s00359-007-0285-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott TM, Christensen-Dalsgaard J, Kelley DB. Temporally selective processing of communication signals by auditory midbrain neurons. J Neurophysiol. 2011;105:1620–1632. doi: 10.1152/jn.00261.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng AS, Ratnam R. Neural basis of hearing in real-world situations. Annu Rev Psychol. 2000;51:699–725. doi: 10.1146/annurev.psych.51.1.699. [DOI] [PubMed] [Google Scholar]
- Forlano PM, Sisneros JA, Rohmann KN, Bass AH. Neuroendocrine control of seasonal plasticity in the auditory and vocal systems of fish. Front Neuroendocrinol. 2015;37:129–145. doi: 10.1016/j.yfrne.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox JH. Morphological correlates of auditory sensitivity in anuran amphibians. Brain Behav Evol. 1995;45:327–338. doi: 10.1159/000113560. [DOI] [PubMed] [Google Scholar]
- Freedman EG, Ferragamo M, Simmons AM. Masking patterns in the bullfrog (Rana catesbeiana) II: physiological effects. J Acoust Soc Am. 1988;84:2081–2091. doi: 10.1121/1.397053. [DOI] [PubMed] [Google Scholar]
- Frishkopf LS, Capranica RR, Goldstein MH., Jr Neural coding in the bullfrog’s auditory system a teleological approach. Proc IEEE. 1968;56:969–980. doi: 10.1109/PROC.1968.6448.. [DOI] [Google Scholar]
- Furman BLS, Bewick AJ, Harrison TL, Greenbaum E, Gvozdik V, Kusamba C, Evans BJ. Pan-African phylogeography of a model organism, the African clawed frog ‘Xenopus laevis’. Mol Ecol. 2015;24:909–925. doi: 10.1111/mec.13076. [DOI] [PubMed] [Google Scholar]
- Gall MD, Wilczynski W. Hearing conspecific vocal signals alters peripheral auditory sensitivity. Proc R Soc B. 2015;282:1–8. doi: 10.1098/rspb.2015.0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt HC. Mating call recognition in the green treefrog (Hyla cinerea): the significance of some fine-temporal properties. J Exp Biol. 1978;74:59–73. doi: 10.1242/jeb.61.1.229. [DOI] [PubMed] [Google Scholar]
- Gerhardt HC, Allan S, Schwartz JJ. Female green treefrogs (Hyla cinerea) do not selectively respond to signals with a harmonic structure in noise. J Comp Physiol A. 1990;166:791–794. doi: 10.1007/BF00187324. [DOI] [PubMed] [Google Scholar]
- Gerhardt HC, Humfeld SC. Pre-existing sensory biases in the spectral domain in frogs: empirical results and methodological considerations. J Comp Physiol A. 2013;199:151–157. doi: 10.1007/s00359-012-0776-4. [DOI] [PubMed] [Google Scholar]
- Gerhardt HC, Martínez-Rivera CC, Schwartz JJ, Marshall VT, Murphy CG. Preferences based on spectral differences in acoustic signals in four species of treefrogs (Anura: Hylidae) J Exp Biol. 2007;210:2990–2998. doi: 10.1242/jeb.006312. [DOI] [PubMed] [Google Scholar]
- Gerhardt HC, Schwartz JJ. Auditory tuning and frequency preferences in anurans. In: Ryan MJ, editor. Anuran communication. Smithsonian Institution Press; Washington: 2001. pp. 73–85. [Google Scholar]
- Goense JB, Feng AS. Seasonal changes in frequency tuning and temporal processing in single neurons in the frog auditory midbrain. J Neurobiol. 2005;65:22–36. doi: 10.1002/neu.20172. [DOI] [PubMed] [Google Scholar]
- Gruber CJ, Tschugguel W, Schneeberger C, Huber JC. Production and actions of estrogens. N Engl J Med. 2002;346(5):340–52. doi: 10.1056/NEJMra000471. [DOI] [PubMed] [Google Scholar]
- Hainfeld CA, Boatright-Horowitz SL, Boatright-Horowitz SS, Megela Simmons A. Discrimination of phase spectra in complex sounds by the bullfrog (Rana catesbeiana) J Comp Physiol A. 1996;179:75–8. doi: 10.1007/BF00193436. [DOI] [PubMed] [Google Scholar]
- Hall JW. New handbook for auditory evoked responses. Pearson Boston; MA: 2007. [Google Scholar]
- Hillery CM. Seasonality of two midbrain auditory responses in the treefrog, Hyla chrysoscelis. Copeia. 1984;1984:844–852. doi: 10.2307/1445327. [DOI] [Google Scholar]
- Hoke KL, Burmeister SS, Fernald RD, Rand AS, Ryan MJ, Wilczynski W. Functional mapping of the auditory midbrain during mate call reception. J Neurosci. 2004;24:11264–11272. doi: 10.1523/JNEUROSCI.2079-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoke KL, Ryan MJ, Wilczynski W. Social cues shift functional connectivity in the hypothalamus. Proc Natl Acad Sci USA. 2005;102:10712–10717. doi: 10.1073/pnas.0502361102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humes LE. Perception of the simple difference tone (f2–f1) J Acoust Soc Am. 1979;66:1064–1074. doi: 10.1121/1.383325. [DOI] [PubMed] [Google Scholar]
- Jørgensen MB, Schmitz B, Christensen-Dalsgaard J. Biophysics of directional hearing in the frog Eleutherodactylus coqui. J Comp Physiol A. 1991;168:223–232. doi: 10.1007/BF00218414. [DOI] [Google Scholar]
- Katbamna B, Brown JA, Collard M, Ide CF. Auditory brainstem responses to airborne sounds in the aquatic frog Xenopus laevis: correlation with middle ear characteristics. J Comp Physiol A. 2006a;192:381–387. doi: 10.1007/s00359-005-0076-3. [DOI] [PubMed] [Google Scholar]
- Katbamna B, Langerveld AJ, Ide CF. Aroclor 1254 impairs the hearing ability of Xenopus laevis. J Comp Physiol A. 2006b;192:971–983. doi: 10.1007/s00359-006-0134-5. [DOI] [PubMed] [Google Scholar]
- Keddy-Hector AC, Wilczynski W, Ryan MJ. Call patterns and basilar papilla tuning in cricket frogs. II. intrapopulation variation and allometry. Brain Behav Evol. 1992;39:238–246. doi: 10.1159/000114121. [DOI] [PubMed] [Google Scholar]
- Kelley DB. Neuroeffectors for vocalization in Xenopus laevis: hormonal regulation of sexual dimorphism. J Neurobiol. 1986;17:231–248. doi: 10.1002/neu.480170307. [DOI] [PubMed] [Google Scholar]
- Klug A, Bauer EE, Hanson JT, Hurley L, Meitzen J, Pollak GD. Response selectivity for species-specific calls in the inferior colliculus of Mexican free-tailed bats is generated by inhibition. J Neurophysiol. 2002;88:1941–1954. doi: 10.1152/jn.2002.88.4.1941. [DOI] [PubMed] [Google Scholar]
- Klump GM, Benedix JH, Jr, Gerhardt HC, Narins PM. AM representation in green treefrog auditory nerve fibers: neuroethological implications for pattern recognition and sound localization. J Comp Physiol A. 2004;190:1011–1021. doi: 10.1007/s00359-004-0558-8. [DOI] [PubMed] [Google Scholar]
- Krishnan A, Gandour JT. The role of the auditory brainstem in processing linguistically-relevant pitch patterns. Brain Lang. 2009;110:135–148. doi: 10.1016/j.bandl.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A, Gandour JT, Smalt CJ, Bidelman GM. Language-dependent pitch encoding advantage in the brainstem is not limited to acceleration rates that occur in natural speech. Brain Lang. 2010;114:193–198. doi: 10.1016/j.bandl.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerud KD, Almonte FV, Kim JC, Large EW. Mode-locking neurodynamics predict human auditory brainstem responses to musical intervals. Hear Res. 2014;308:41–49. doi: 10.1016/j.heares.2013.09.010. [DOI] [PubMed] [Google Scholar]
- Lewis ER, Baird RA, Leverenz EL, Koyama H. Inner ear: dye injection reveals peripheral origins of specific sensitivities. Science. 1982;215:1641–1643. doi: 10.1126/science.6978525. [DOI] [PubMed] [Google Scholar]
- Lutz LB, Cole LM, Gupta MK, Kwist KW, Auchus RJ, Hammes SR. Evidence that androgens are the primary steroids produced by Xenopus laevis ovaries and may signal through the classical androgen receptor to promote oocyte maturation. Proc Nat Acad Sci U S A. 2001;98:13728–13733. doi: 10.1073/pnas.241471598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmberg CF. The perception of consonance and dissonance. Psychol Monogr. 1918;25:93–133. [Google Scholar]
- Mason M, Wang M, Narins P. Structure and function of the middle ear apparatus of the aquatic frog (Xenopus laevis) Proc Inst Acoust. 2009;31:13–21. [PMC free article] [PubMed] [Google Scholar]
- Mensah-Nyagan AG, Do Rego J-L, Feuilloley M, Marcual A, Lange C, Pelletier G, Vaudry H. In vivo and in vitro evidence for the biosynthesis of testosterone in the telencephalon of the female frog. J Neurochem. 1996;67:413–422. doi: 10.1046/j.1471-4159.1996.67010413.x. [DOI] [PubMed] [Google Scholar]
- Meenderink SWF, Kits M, Narins PM. Frequency matching of vocalizations to inner-ear sensitivity along an altitudinal gradient in the coqui frog. Biol Lett. 2010;6:278–281. doi: 10.1098/rsbl.2009.0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers JR, MacDonald RB, Duggan A, Lenzi D, Standaert DG, Corwin JT, Corey DP. Lighting up the Senses: FM1-43 loading of sensory cells through nonselective ion channels. J Neurosci. 2003;23:4054–4065. doi: 10.1523/JNEUROSCI.23-10-04054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C, Phillips DS, Trune DR. Variables affecting the auditory brainstem response: audiogram, age, gender and head size. Hear Res. 1989;40:75–86. doi: 10.1016/0378-5955(89)90101-9. [DOI] [PubMed] [Google Scholar]
- Moreno-Gomez FN, Sueur J, Soto-Gamboa M, Penna M. Female frog auditory sensitivity, male calls, and background noise: potential influences on the evolution of a peculiar matched filter. Biol J Linn Soc. 2013;110:814–827. doi: 10.1111/bij.12156. [DOI] [Google Scholar]
- Narins PM, Capranica RR. Sexual differences in the auditory system of the tree frog Eleutherodactylus coqui. Science. 1976;192:378–380. doi: 10.1126/science.1257772. [DOI] [PubMed] [Google Scholar]
- Narins PM, Capranica RR. Neural adaptations for processing the two-note call of the Puerto Rican treefrog, Eleutherodactylus coqui. Brain Behav Evol. 1980;17:48–66. doi: 10.1159/000121790. [DOI] [PubMed] [Google Scholar]
- Narins PM, Ehret G, Tautz J. Accessory pathway for sound transfer in a neotropical frog. Proc Natl Acad Sci U S A. 1988;85:1508–1512. doi: 10.1073/pnas.85.5.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narins PM, Wagner I. Noise susceptibility and immunity of phase locking in amphibian auditory-nerve fibers. J Acoust Soc Am. 1989;85:1255–1265. doi: 10.1121/1.397456. [DOI] [PubMed] [Google Scholar]
- Penna M, Capranica RR, Somers J. Hormone-induced vocal behavior and midbrain auditory sensitivity in the green treefrog, Hyla cinerea. J Comp Physiol A. 1992;170:73–82. doi: 10.1007/BF00190402. [DOI] [PubMed] [Google Scholar]
- Perez J, Cohen MA, Kelley DB. Androgen receptor mRNA expression in Xenopus laevis CNS: sexual dimorphism and regulation in laryngeal motor nucleus. J Neurobiol. 1996;30:556–568. doi: 10.1002/(SICI)1097-4695(199608)30:4<556::AID-NEU10>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Phan ML, Pytte CL, Vicario DS. Early auditory experience generates long-lasting memories that may subserve vocal learning in songbirds. Proc Natl Acad Sci U S A. 2006;103:1088–1093. doi: 10.1073/pnas.0510136103. doi: 1010.1073/pnas.0510136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picker M. Hormonal induction of the aquatic phonotactic response of Xenopus. Behaviour. 1983;84:74–90. doi: 10.1163/156853983X00291. [DOI] [Google Scholar]
- Pinder AC, Palmer AR. Mechanical properties of the frog ear: vibration measurements under free- and closed-field acoustic conditions. Proc R Soc Lond B Biol Sci. 1983;219:371–396. doi: 10.1098/rspb.1983.0079. [DOI] [PubMed] [Google Scholar]
- Portfors CV, Roberts PD, Jonson K. Over-representation of species-specific vocalizations in the awake mouse inferior colliculus. Neuroscience. 2009;162:286–300. doi: 10.1016/j.neuroscience.2009.04.056. [DOI] [PubMed] [Google Scholar]
- Ramlochansingh C, Branoner F, Chagnaud BP, Straka H. Efficacy of tricaine methanesulfonate (MS-222) as an anesthetic agent for blocking sensory-motor responses in Xenopus laevis tadpoles. PlosOne. 2014:e101606. doi: 10.1371/journal.pone.0101606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Dong SM, Chao A, Schlinger BA. Sex-specific, rapid neuroestrogen fluctuations and neurophysiological actions in the songbird auditory forebrain. J Neurophysiol. 2012;107:1621–1631. doi: 10.1152/jn.00749.2011. 1610.1152/jn.00749.02011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes HJ, Yu HJ, Yamaguchi A. Xenopus vocalizations are controlled by a sexually differentiated hindbrain central pattern generator. J Neurosci. 2007;27:1485–1497. doi: 10.1523/JNEUROSCI.4720-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose GJ, Capranica RR. Sensitivity to amplitude modulated sounds in the anuran auditory nervous system. J Neurophysiol. 1985;53:446–465. doi: 10.1152/jn.1985.53.2.446. [DOI] [PubMed] [Google Scholar]
- Ruggero M, Robles L, Rich N, Recio A. Basilar membrane responses to two-tone and broadband stimuli. Philos Trans R Soc Lond B Biol Sci. 1992;336:307–315. doi: 10.1098/rstb.1992.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoffelen RLM, Segenhout JM, van Dijk P. Tuning of the tectorial membrane in the basilar papilla of the northern leopard frog. J Assoc Res Otolaryngol. 2009;10:309–320. doi: 10.1007/s10162-009-0167-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrode KM, Buerkle NP, Brittan-Powell EF, Bee MA. Auditory brainstem responses in Cope’s gray treefrog (Hyla chrysoscelis): effects of frequency, level, sex and size. J Comp Physiol A. 2014;200:221–238. doi: 10.1007/s00359-014-0880-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher JW, Schneider DM, Woolley SMN. Anesthetic state modulates excitability but not spectral tuning or neural discrimination in single auditory midbrain neurons. J Neurophysiol. 2011;106:500–514. doi: 10.1152/jn.01072.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro Samuel S, Wilk Martin B, Chen Hwei J. A comparative study of various tests for normality. J Am Stat Assoc. 1968;63(324):1343–1372. [Google Scholar]
- Seaman RL. Method to record evoked potentials from the frog eighth nerve. Hear Res. 1991;51:301–305. doi: 10.1016/0378-5955(91)90046-C. [DOI] [PubMed] [Google Scholar]
- Simmons AM. Selectivity for harmonic structure in complex sounds by the green treefrog (Hyla cinerea) J Comp Physiol A. 1988;162:397–403. doi: 10.1007/BF00606126. [DOI] [PubMed] [Google Scholar]
- Simmons AM. “To ear is human, to frogive is divine”: Bob Capranica’s legacy to auditory neuroethology. J Comp Physiol A. 2013;199:169–182. doi: 10.1007/s00359-012-0786-2. [DOI] [PubMed] [Google Scholar]
- Simmons AM, Bean ME. Perception of mistuned harmonics in complex sounds by the bullfrog (Rana catesbeiana) J Comp Psych. 2000;114:167–173. doi: 10.1037/0735-7036.114.2.167. [DOI] [PubMed] [Google Scholar]
- Sisneros JA, Forlano PM, Deitcher DL, Bass AH. Steroid-dependent auditory plasticity leads to adaptive coupling of sender and receiver. Science. 2004;205:404–407. doi: 10.1126/science.1097218. [DOI] [PubMed] [Google Scholar]
- Skoe E, Kraus N. Auditory brainstem response to complex sounds: a tutorial. Ear Hear. 2010;31:302–324. doi: 10.1097/AUD.0b013e3181cdb272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J-X, Xu Z-M, Yu Z-L, Wang S, Zheng D-Z, Fan S-C. Ultrasonic frogs show extraordinary sex differences in auditory frequency sensitivity. Nat Commun. 2011;2:1–5. doi: 10.1038/ncomms1339. [DOI] [PubMed] [Google Scholar]
- Smotherman MS, Narins PM. Effect of temperature on electrical resonance in leopard frog saccular hair cells. J Neurophysiol. 1998;79:312–321. doi: 10.1152/jn.1998.79.1.312. [DOI] [PubMed] [Google Scholar]
- Smotherman MS, Narins PM. Hair cells, hearing and hopping: a field guide to hair cell physiology in the frog. J Exp Biol. 2000;203:2237–2246. doi: 10.1242/jeb.203.15.2237. [DOI] [PubMed] [Google Scholar]
- Tobias ML, Barnard C, O’Hagan R, Horng SH, Rand M, Kelley DB. Vocal communication between male Xenopus laevis. Anim Behav. 2004;67:353–365. doi: 10.1016/j.anbehav.2003.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias ML, Corke A, Korsh J, Yin D, Kelley DB. Vocal Competition in male Xenopus laevis frogs. Behav Ecol Sociobiol. 2010;64:1791–1803. doi: 10.1007/s00265-010-0991-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias ML, Evans BJ, Kelley DB. Evolution of advertisement calls in African clawed frogs. Behaviour. 2011;148:519–549. doi: 10.1163/000579511X569435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias ML, Korsh J, Kelley DB. Evolution of male and female release calls in African clawed frogs. Behaviour. 2014;151:1313–1334. doi: 10.1163/1568539X-00003186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk P, Mason MJ, Schoffelen RLM, Narins PM, Meenderink SWF. Mechanics of the frog ear. Hear Res. 2011;273:46–58. doi: 10.1016/j.heares.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudry H, Do Rego J-L, Burel D, Luu-The V, Pelletier G, Vaudry D, Tsutsui K. Neurosteroid biosynthesis in the brain of amphibians. Front Neuroendocrinol. 2011;2:1–9. doi: 10.3389/fendo.2011.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vélez A, Gall MD, Lucas JR. Seasonal plasticity in auditory processing of the envelope and temporal fine structure of sounds in three songbirds. Anim Behav. 2015;103:52–63. doi: 10.1016/j.anbehav.2015.01.036. [DOI] [Google Scholar]
- Vignal C, Kelley DB. Significance of temporal and spectral acoustic cues for sexual recognition in Xenopus laevis. Proc R Soc Lond B Biol Sci. 2007;274:479–488. doi: 10.1098/rspb.2006.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigny C. The mating calls of 12 species and sub-species of the genus Xenopus (Amphibia: Anura) J Zool Lond. 1979;188:103–122. doi: 10.1111/j.1469-7998.1979.tb03394.x. [DOI] [Google Scholar]
- Wetzel DM, Kelley DB. Androgen and gonadotropin effects on male mate calls in South African clawed frogs, Xenopus laevis. Horm Behav. 1983;17:388–404. doi: 10.1016/0018-506X(83)90048-X. [DOI] [PubMed] [Google Scholar]
- Wilczynski W, Zakon HH, Brenowitz EA. Acoustic communication in spring peepers. J Comp Physiol A. 1984;155:577–584. doi: 10.1007/BF00610843. [DOI] [Google Scholar]
- Wilczynski W, Keddy-Hector AC, Ryan MJ. Call patterns and basilar papilla tuning in cricket frogs. I. differences among populations and between sexes. Brain Behav Evol. 1992;39:229–237. doi: 10.1159/000114120. [DOI] [PubMed] [Google Scholar]
- Wilczynski W, Rand AS, Ryan MJ. Evolution of calls and auditory tuning in the Physalaemus pustulosus species group. Brain Behav Evol. 2001;58:137–151. doi: 10.1159/000047268. [DOI] [PubMed] [Google Scholar]
- Witte K, Ryan MJ, Wilczynski W. Changes in the frequency structure of a mating call decrease its attractiveness to females in the cricket frog Acris crepitans blanchardi. Ethology. 2001;107:685–699. [Google Scholar]
- Worden FG, Marsh JT. Frequency-following (microphonic-like) neural responses evoked by sound. Electroencephalogr Clin Neurophysiol. 1968;25:42–52. doi: 10.1016/0013-4694(68)90085-0. [DOI] [PubMed] [Google Scholar]
- Woolley SMN, Portfors CV. Conserved mechanisms of vocalization coding in mammalian and songbird auditory midbrain. Hear Res. 2013;305:45–56. doi: 10.1016/j.heares.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright A, Rivera J, Hulse S, Shyan M, Neiworth J. Music perception and octave generalization in rhesus monkeys. J Exp Psychol Gen. 2000;129:291–307. doi: 10.1037/0096-3445.129.3.291. [DOI] [PubMed] [Google Scholar]
- Yager DD. Underwater acoustic communication in the African pipid frog Xenopus borealis. Bioacoustics. 1992;4:1–24. doi: 10.1080/09524622.1992.9753201. [DOI] [Google Scholar]
- Yoder KM, Phan ML, Lu K, Vicario DS. He hears, she hears: are there sex differences in auditory processing? Dev Neurobiol. 2015;75:302–314. doi: 10.1002/dneu.22231. doi: 310.1002/dneu.22231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder KM, Vicario DS. To modulate and be modulated: estrogenic influences on auditory processing of communication signals within a socio-neuro-endocrine framework. Behav Neurosci. 2012;126:17–28. doi: 10.1037/a0026673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakon HH, Wilczynski W. The physiology of the anuran eighth nerve. In: Fritzsch B, Ryan MJ, Wilczynski W, Hetherington TE, Walkowiak W, editors. The evolution of the amphibian auditory system. John Wiley and Sons; New York: 1988. pp. 125–155. [Google Scholar]
- Zornik E, Kelley DB. A neuroendocrine basis for the hierarchical control of frog courtship vocalizations. Front Neuroendocrinol. 2011;32:353–366. doi: 10.1016/j.yfrne.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


