Abstract
Germline DICER1 mutations have been described in individuals with pleuropulmonary blastoma (PPB), ovarian Sertoli-Leydig cell tumor (SLCT), sarcomas, multinodular goiter, thyroid carcinoma, cystic nephroma and other neoplastic conditions. Early results from the International Ovarian and Testicular Stromal Tumor Registry show germline DICER1 mutations in 48% of girls and women with SLCT. In this report, a young woman presented with ovarian undifferentiated sarcoma. Four years later, she presented with SLCT. She was successfully treated for both malignancies. Sequence results showed a germline intronic mutation in DICER1. This mutation results in an exact duplication of the six bases at the splice site at the intron 23 and exon 24 junction. Predicted improper splicing leads to inclusion of 10 bases of intronic sequence, frameshift and premature truncation of the protein disrupting the RNase IIIb domain. A second individual with SLCT was found to have an identical germline mutation. In each of the ovarian tumors, an additional somatic mutation in the RNase IIIb domain of DICER1 was found. In rare patients, germline intronic mutations in DICER1 that are predicted to cause incorrect splicing can also contribute to the pathogenesis of SLCT.
Keywords: DICER1, Sertoli-Leydig cell tumor, miRNA, ovarian cancer, sarcoma
Introduction
Germline DICER1 mutations were first identified in children with pleuropulmonary blastoma (PPB), a rare but aggressive lung tumor of infancy and early childhood [1, 2]. Conditions which are now known to be associated with germline DICER1 mutations include PPB, Sertoli-Leydig cell tumor (SLCT), cystic nephroma, lung cysts, nodular hyperplasia and/or carcinoma of the thyroid gland, nasal chondromesenchymal hamartomas, embryonal rhabdomyosarcoma of the uterine cervix, ciliary body medulloepithelioma, renal sarcoma, pituitary blastoma, Wilms tumor and pineoblastoma [3–19]. DICER1 encodes an RNase III endonuclease which cleaves precursor microRNAs into active microRNAs. Most tumors in DICER1 syndrome have one allele (often germline) harboring a nonsense or frame-shift mutation predicted to cause full loss of function (LOF) with the other allele harboring a missense mutation in the DICER1 RNase IIIb domain. This combination of mutations in both DICER1 alleles results in improperly cleaved 5p miRNAs from the pre-miRNA hairpin structures, leading to an abnormal ratio of 5p to 3p miRNAs and altering expression of downstream target mRNAs[4]. Data from the International Ovarian and Testicular Stromal Tumor (OTST) Registry indicate that almost half of children and adults with SLCT have germline DICER1 mutations [20]. We have also found biallelic somatic mutations in children with ovarian SLCT in absence of a germline mutation (unpublished OTST Registry data presented at Children’s Oncology Group 2014 Fall Meeting). Here we report two young women with SLCT both of whom have a unique intronic loss of function germline mutation in DICER1 and tumor-specific RNase IIIb missense mutations.
Materials and Methods
Eligible participants with a history of ovarian or testicular stromal tumors were enrolled in the International OTST Registry (www.OTSTregistry.org). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional review board and with the 1964 Helsinki declaration and its later amendments. Informed consent was obtained from all participants or proxy caregivers. Additional written consent for this specific publication was provided by Patient #1 whose unique clinical history poses a potential for identification. This article does not contain any studies with animals performed by any of the authors. The participants described in this report were tested for germline DICER1 mutations using previously described methodology [21]. Initial testing for DICER1 mutations was performed by standard Sanger methods as described previously or by a commercial laboratory (Ambry Genetics, Aliso Viejo, CA). Somatic testing on formalin-fixed paraffin embedded tumor tissue as previously described [21].
Results
A 10 year old girl presented with a one month history of dysuria and pelvic pressure. She underwent exploratory laparotomy and left oophorectomy. Pathology showed an undifferentiated sarcoma with limited myogenic phenotype (Fig. 1). The tumor was characterized by moderately atypical spindle cells in a fascicular pattern with a myxoid stroma and areas of cells with anaplasia characterized by enlarged, hyperchromatic nuclei and atypical multipolar mitotic figures. The tumor lacked features of juvenile granulosa cell tumor or microscopically recognizable Sertoli-cells/tubules or Leydig cells. The tumor cells were strongly positive for vimentin and nuclear p53. Rare cells showed weak staining for desmin. Muscle specific actin and MyoD1 were negative. Inhibin was negative. She was treated with vincristine, actinomycin D and cyclophosphamide. Surveillance evaluations showed no evidence of disease until 4 years following her original diagnosis when she presented with amenorrhea and an elevated testosterone. Imaging showed a right sided ovarian mass. Pathology showed an intermediately differentiated SLCT (Fig. 2). Surgical staging showed tumor adherent to the uterus (International Federation of Gynecology and Obstetrics Stage IIA). She was treated with 6 cycles of cisplatin, etoposide and bleomycin chemotherapy. Follow-up radiographic studies showed no evidence of recurrent disease but noted 2 renal cysts. She remains alive and well 15 years following the diagnosis of undifferentiated sarcoma and 12 years following diagnosis of SLCT. Family history is negative for malignancy.
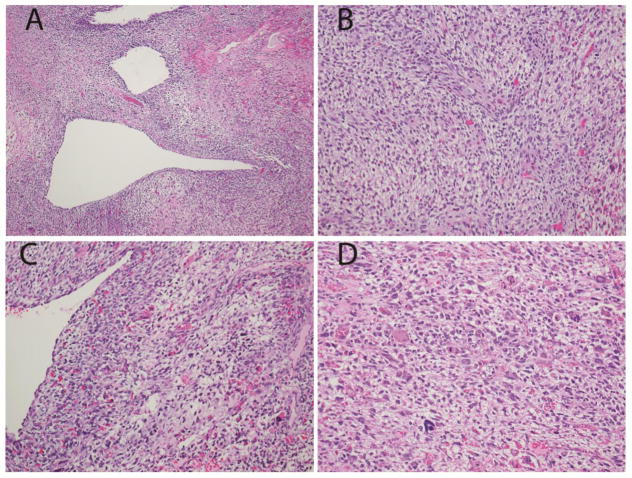
Fig. 1.
Pathologic features of the primary ovarian sarcoma in Patient #1 with DICER1 mutation
a. Low power view shows epithelial lined cysts, solid sarcoma and focal necrosis. b. Higher power view shows spindled and stellate cells in a pale mucoid matrix. c,d. Higher power views highlight areas with significant nuclear pleomorphism. (Hematoxylin and eosin; x100 (a), x200 (b,c,d)).
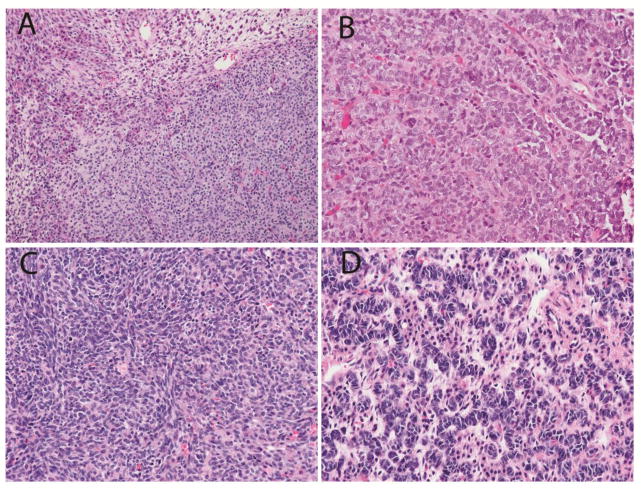
Fig. 2.
Sertoli-Leydig cell tumor in the contralateral ovary of Patient #1.
a. Low power view showing Leydig cells with abundant pink cytoplasm in upper left adjacent to nests of small Sertoli cells. b. Elongated tubules of Sertoli cells. c. Spindle cell pattern seen focally. d. Ribbons of Sertoli cells with hyperchromatic nuclei. (Hematoxylin and eosin; x100 (a), x400 (b,c,d))
Fourteen years following her original diagnosis, she was tested for germline DICER1 mutation. Sequence results showed an intronic mutation in DICER1 (NM_177438.2:c.5096-12 G>A). DICER1 next generation sequencing was performed on both of her ovarian tumors. The undifferentiated sarcoma showed the germline mutation and a somatic NM_177438.2:c.5125G>A; p.Asp1709Asn DICER1 RNase IIIb mutation. TP53 was also mutated in the sarcoma (NM_000546.4:c.821T>A; p.Val274Asp). Sequencing of the SLCT showed the germline mutation and a somatic RNase IIIb mutation NM_177438.2:c.5439G>T; p.Glu1813Asp. No TP53 mutations were detected in the SLCT.
The same germline intronic mutation (c.5096-12 G>A) was detected in a second adolescent with SLCT (Fig. 3) and 2 other DICER1-related conditions: a lung cyst and thyroid nodule. Sequencing of this individual’s SLCT showed the germline NM_177438.2:c.5096-12G>A and a somatic c.5126A>T; p.Asp1709Val. No TP53 mutations were detected. Family History is notable for hypothyroidism in two first degree relatives and a thyroidectomy for unspecified reasons in a second degree relative. She remains alive and well more than 1 year following diagnosis of SLCT. These two individuals are not known to be related, however, detailed multi-generation pedigrees are not available.
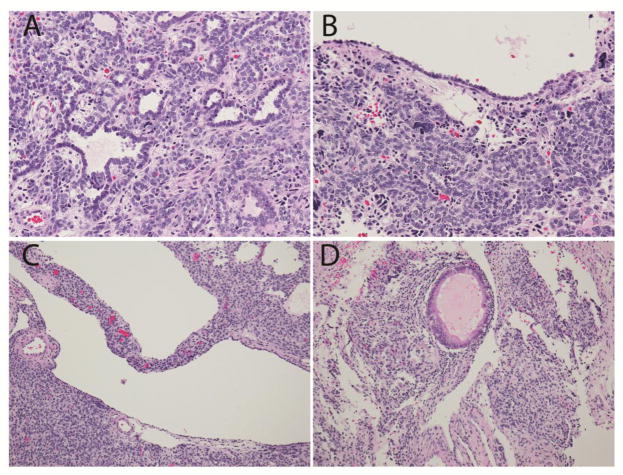
Fig. 3. Pathologic features of the Sertoli-Leydig tumor in patient #2.
a.Tubular structures and nests of Sertoli cells. b. Epithelial lined cyst with nest of Sertoli cells and rare anaplastic cells. c. Cystic structure within the SLCT. d. Focal mucinous gland. (Hematoxylin and eosin; x200 (a,b), x100 (c,d)).
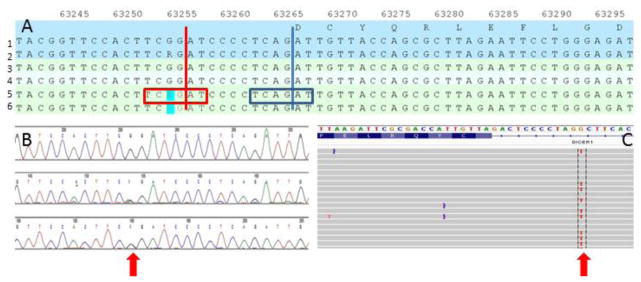
The NM_177438.2:c.5096-12 G>A germline mutation seen in these two patients results in an exact duplication of the six bases at the splice site at the intron 23 and exon 24 junction (Fig. 4). Predicted improper splicing leads to inclusion of 10 bases of intronic sequence, frameshift and premature truncation of the protein (NP803187.1:p.Asp1699AlaFS*8) disrupting the RNase IIIb domain.
Fig. 4. Unique intronic mutation in DICER1 resulting in duplication of splice site.
a. Germline DICER1 sequencing results from Patient #1 (Lanes 5 and 6) compared with reference genome (Lanes 1–4). Intron-exon boundary indicated by vertical blue line with six base pairs of the splice site in the blue box. Mutated base NM_177438.2:c.5096-12 G>A in blue highlight. Putative new intron-exon boundary indicated by vertical red line. b. Sanger sequence chromatogram from Patient 1. Red arrow indicates heterozygous mutation site. c. Next generation sequence data as displayed with Integrative Genome Viewer [27, 28]. Red arrow highlights the mutated base.
Discussion
Identification of germline DICER1 mutations has significant clinical importance, both for the individual with the mutation indicating risk for other sites of disease, and for relatives and potential offspring who may also carry the mutation. The most urgent clinical relevance is for probands and relatives under the age of 7 years who are at risk for PPB, the most lethal tumor seen within the spectrum of DICER1 related disorders. Early detection and treatment of cystic PPB before transformation into a solid multipatterned sarcoma may positively influence survival and reduce complications of high dose chemotherapy [22].
The finding of an underlying DICER1 mutation also has implications for surveillance for the primary ovarian tumor. In many centers, girls and women with SLCT are followed for 2–5 years following surgery or adjuvant therapy. After that time period, they are presumed to be at low risk for recurrence. Girls and women with DICER1 mutations remain at risk for metachronous ovarian tumors after the usual risk period for recurrence and should be followed with surveillance imaging, usually ultrasound, after the usual period for recurrence has ended. Additional screening recommendations are available at genereviews.org [6]. Genetic counseling is recommended to ensure that options for familial testing and ongoing surveillance are explored.
Intronic mutations have been described in other familial cancer predisposition syndromes including defects in mismatch repair genes and Gorlin syndrome [23–25]. Intronic mutations may in some cases create a new splice site resulting in incorporation of additional bases with or without shifting of the reading frame. In this example, a new exact replica of the splice site was created 10 bases proximal to the normal splice site. This is a unique finding in our cohort of PPB and OTST Registries. Only 7 of 90 germline mutations in children with PPB were intronic and each of these were within 2 base pairs of the splice site, the so-called canonical splice site [26]. One caveat to this study is that 12 of 124 patients with PPB did not have detectable germline DICER1 mutations. While we predict that most of those 12 children will have tumor-specific biallelic mutations, we cannot rule out a deep intronic mutation not detected by our sequencing assay. For patients with ovarian SLCT, only preliminary analysis is available at this time. Twenty individuals have tested positive for germline DICER1 mutations. Of these, 5 have base substitutions involving splice sites; 2 presented here and 3 others with mutations in the canonical splice site.
Conclusion
In this report, we describe the finding of a unique intronic mutation in two patients with SLCT, each with additional DICER1-related conditions. This mutation, which affects the splice site was not detected in SNP databases, 1000 genomes or any of the previously reported series or in other patient samples available in the International OTST or PPB Registries. In the ovarian tumors described in this report, an additional somatic mutation was found in the RNase IIIb domain.
Germline DICER1 mutations should be suspected in individuals with a personal or family history of SLCT but also in patients with juvenile granulosa cell tumor and gynandroblastoma or any ovarian stromal tumor or sarcoma and a personal or family history of nodular thyroid disease or thyroid carcinoma or other DICER1 related conditions. Somatic testing of tumor tissue is the most sensitive way to detect DICER1 mutations which may be germline or tumor-specific. When analyzing DICER1 mutations, it is necessary to consider the role of intronic mutations creating nonfunctional DICER1 protein.
Acknowledgments
The authors wish to thank the patients discussed in this manuscript and their families for their support of DICER1- related research. In addition we are grateful to the many patients, families, genetic counselors and treating physicians who participate in the International OTST Registry and to the Pine Tree Apple Tennis Classic, St. Baldrick’s Foundation, Randy Shaver Community Cancer Fund and Hyundai Hope on Wheels for their ongoing support of cancer research.
Source of Funding This work is supported by National Institutes of Health grant NCI R01CA143167 (DAH,YM, KAS) and The Parson’s Foundation (DAH). The International Ovarian and Testicular Stromal Tumor Registry is supported by St. Baldrick’s Foundation, the Pine Tree Apple Tennis Classic, Hyundai Hope on Wheels and the Randy Shaver Community Cancer Fund.
Footnotes
Conflicts of Interest: The authors each state that he/she has no conflicts of interest to disclose.
References
- 1.Hill DA, Ivanovich J, Priest JR, Gurnett CA, Dehner LP, Desruisseau D, Jarzembowski JA, Wikenheiser-Brokamp KA, Suarez BK, Whelan AJ, Williams G, Bracamontes D, Messinger Y, Goodfellow PJ. DICER1 mutations in familial pleuropulmonary blastoma. Science. 2009;325(5943):965–67. doi: 10.1126/science.1174334. 1174334 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dishop MK, Kuruvilla S. Primary and metastatic lung tumors in the pediatric population: a review and 25-year experience at a large children’s hospital. Arch Pathol Lab Med. 2008;132(7):1079–103. doi: 10.1043/1543-2165(2008)132[1079:PAMLTI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 3.Foulkes WD, Bahubeshi A, Hamel N, Pasini B, Asioli S, Baynam G, Choong CS, Charles A, Frieder RP, Dishop MK, Graf N, Ekim M, Bouron-Dal Soglio D, Arseneau J, Young RH, Sabbaghian N, Srivastava A, Tischkowitz MD, Priest JR. Extending the Phenotypes Associated with DICER1 Mutations. Hum Mutat. 2011 doi: 10.1002/humu.21600. [DOI] [PubMed] [Google Scholar]
- 4.Pugh TJ, Yu W, Yang J, Field AL, Ambrogio L, Carter SL, Cibulskis K, Giannikopoulos P, Kiezun A, Kim J, McKenna A, Nickerson E, Getz G, Hoffher S, Messinger YH, Dehner LP, Roberts CW, Rodriguez-Galindo C, Williams GM, Rossi CT, Meyerson M, Hill DA. Exome sequencing of pleuropulmonary blastoma reveals frequent biallelic loss of TP53 and two hits in DICER1 resulting in retention of 5p-derived miRNA hairpin loop sequences. Oncogene. 2014 doi: 10.1038/onc.2014.150. 0(onc2014150 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boman F, Hill DA, Williams GM, Chauvenet A, Fournet JC, Soglio DB, Messinger Y, Priest JR. Familial association of pleuropulmonary blastoma with cystic nephroma and other renal tumors: a report from the International Pleuropulmonary Blastoma Registry. J Pediatr. 2006;149(6):850–854. doi: 10.1016/j.jpeds.2006.08.068. S0022-3476(06)00819-5 [pii] [DOI] [PubMed] [Google Scholar]
- 6.Doros L, Yang J, Dehner L, Rossi CT, Skiver K, Jarzembowski JA, Messinger Y, Schultz KA, Williams G, Andre N, Hill DA. DICER1 mutations in embryonal rhabdomyosarcomas from children with and without familial PPB-tumor predisposition syndrome. Pediatr Blood Cancer. 2012;59(3):558–60. doi: 10.1002/pbc.24020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hill DA, Doros L, Schultz KA, Stewart DR, Bauer AJ, Williams G, Rossi CT, Carr A, Yang J, Dehner LP, Messinger Y. DICER1-related disorders. GeneReviews™ [Internet] 2014 [Google Scholar]
- 8.Priest JR, Williams GM, Manera R, Jenkinson H, Brundler MA, Davis S, Murray TG, Galliani CA, Dehner LP. Ciliary body medulloepithelioma: four cases associated with pleuropulmonary blastoma--a report from the International Pleuropulmonary Blastoma Registry. Br J Ophthalmol. 2011;95(7):1001–5. doi: 10.1136/bjo.2010.189779. bjo.2010.189779 [pii] [DOI] [PubMed] [Google Scholar]
- 9.Slade I, Bacchelli C, Davies H, Murray A, Abbaszadeh F, Hanks S, Barfoot R, Burke A, Chisholm J, Hewitt M, Jenkinson H, King D, Morland B, Pizer B, Prescott K, Saggar A, Side L, Traunecker H, Vaidya S, Ward P, Futreal PA, Vujanic G, Nicholson AG, Sebire N, Turnbull C, Priest JR, Pritchard-Jones K, Houlston R, Stiller C, Stratton MR, Douglas J, Rahman N. DICER1 syndrome: clarifying the diagnosis, clinical features and management implications of a pleiotropic tumour predisposition syndrome. J Med Genet. 2011;48(4):273–8. doi: 10.1136/jmg.2010.083790. [DOI] [PubMed] [Google Scholar]
- 10.Stewart DR, Messinger YHM, Williams GM, Doros L, Hill DA, McDermott MB, Dehner LP. Nasal chondromesenchymal hamartomas arise secondary to biallelic inactivation of DICER1. Hum Genet. 2014;133(11):1443–50. doi: 10.1007/s00439-014-1474-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu MK, Sabbaghian N, Xu B, Addidou-Kalucki S, Bernard C, Zou D, Reeve AE, Eccles MR, Cole C, Choong CS, Charles A, Tan TY, Iglesias DM, Goodyer PR, Foulkes WD. Biallelic DICER1 mutations occur in Wilms tumours. J Pathol. 2013;230(2):154–64. doi: 10.1002/path.4196. [DOI] [PubMed] [Google Scholar]
- 12.Delahunt B, Thomson KJ, Ferguson AF, Neale TJ, Meffan PJ, Nacey JN. Familial cystic nephroma and pleuropulmonary blastoma. Cancer. 1993;71(4):1338–42. doi: 10.1002/1097-0142(19930215)71:4<1338::aid-cncr2820710427>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 13.de Kock L, Sabbaghian N, Druker H, Weber E, Hamel N, Miller S, Choong CS, Gottardo NG, Kees UR, Rednam SP, et al. Germ-line and somatic DICER1 mutations in pineoblastoma. Acta Neuropathol. 2014;128:583–595. doi: 10.1007/s00401-014-1318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez-Andreu JA, Ferris J, Esquembre C, Verdeguer A, Gomez J, Castel V. Familial cystic nephroma and pleuropulmonary blastoma. Cancer. 1993;72(9):2792–3. doi: 10.1002/1097-0142(19931101)72:9<2792::aid-cncr2820720943>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 15.Schultze-Florey RE, Graf N, Vorwerk P, Koscielniak E, Schneider DT, Kratz CP. DICER1 syndrome: a new cancer syndrome. Klin Padiatr. 2013;225(3):177–8. doi: 10.1055/s-0033-1337976. [DOI] [PubMed] [Google Scholar]
- 16.Heravi-Moussavi A, Anglesio MS, Cheng SW, Senz J, Yang W, Prentice L, Fejes AP, Chow C, Tone A, Kalloger SE, Hamel N, Roth A, Ha G, Wan AN, Maines-Bandiera S, Salamanca C, Pasini B, Clarke BA, Lee AF, Lee CH, Zhao C, Young RH, Aparicio SA, Sorensen PH, Woo MM, Boyd N, Jones SJ, Hirst M, Marra MA, Gilks B, Shah SP, Foulkes WD, Morin GB, Huntsman DG. Recurrent somatic DICER1 mutations in nonepithelial ovarian cancers. N Engl J Med. 2012;366(3):234–42. doi: 10.1056/NEJMoa1102903. [DOI] [PubMed] [Google Scholar]
- 17.Rio Frio T, Bahubeshi A, Kanellopoulou C, Hamel N, Niedziela M, Sabbaghian N, Pouchet C, Gilbert L, O’Brien PK, Serfas K, Broderick P, Houlston RS, Lesueur F, Bonora E, Muljo S, Schimke RN, Bouron-Dal Soglio D, Arseneau J, Schultz KA, Priest JR, Nguyen VH, Harach HR, Livingston DM, Foulkes WD, Tischkowitz M. DICER1 mutations in familial multinodular goiter with and without ovarian Sertoli-Leydig cell tumors. JAMA. 2011;305(1):68–77. doi: 10.1001/jama.2010.1910. 305/1/68 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schultz KA, Yang J, Doros L, MWG, Harris A, Stewart DR, Messinger Y, Field A, Dehner LP, Hill DA. DICER1-pleuropulmonary blastoma familial tumor predisposition syndrome: a unique constellation of neoplastic conditions. Pathol Case Reviews. 2014;19:90–100. doi: 10.1097/PCR.0000000000000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Witkowski L, Mattina J, Schonberger S, Murray MJ, Huntsman DG, Reis-Filho JS, McCluggage WG, Nicholson JC, Coleman N, Calaminus G, Schneider DT, Arseneau J, Stewart CJ, Foulkes WD. DICER1 hotspot mutations in non-epithelial gonadal tumours. Br J Cancer. 2013;109:2744–50. doi: 10.1038/bjc.2013.637. bjc2013637 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schultz KAP, Harris A, Doros L, Young RH, Dehner LP, Frazier L, Hill DA, Messinger Y. Clinical and genetic aspects of ovarian tumors: Report from the International Ovarian and Testicular Stromal Tumor Registry. J Clin Oncol. 2014;32(15S):5520. [Google Scholar]
- 21.Doros LA, Rossi CT, Yang J, Field A, Williams GM, Messinger Y, Cajaiba MM, Perlman EJ, KAS, Cathro HP, Legallo RD, Lafortune KA, Chikwava KR, Faria P, Geller JI, Dome JS, Mullen EA, Gratias EJ, Dehner LP, Hill DA. DICER1 mutations in childhood cystic nephroma and its relationship to DICER1-renal sarcoma. Mod Pathol. 2014;27:1267–80. doi: 10.1038/modpathol.2013.242. modpathol2013242 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schultz KA, Harris A, Williams GM, Baldinger S, Doros L, Valusek P, Frazier AL, Dehner LP, Messinger Y, Hill DA. Judicious DICER1 testing and surveillance imaging facilitates early diagnosis and cure of pleuropulmonary blastoma. Pediatr Blood Cancer. 2014;61 (9):1695–7. doi: 10.1002/pbc.25092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bianchi F, Raponi M, Piva F, Viel A, Bearzi I, Galizia E, Bracci R, Belvederesi L, Loretelli C, Brugiati C, Corradini F, Baralle D, Cellerino R. An intronic mutation in MLH1 associated with familial colon and breast cancer. Fam Cancer. 2011;10(1):27–35. doi: 10.1007/s10689-010-9371-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bholah Z, Smith MJ, Byers HJ, Miles EK, Evans DG, Newman WG. Intronic splicing mutations in PTCH1 cause Gorlin syndrome. Fam Cancer. 2014;13(3):477–80. doi: 10.1007/s10689-014-9712-9. [DOI] [PubMed] [Google Scholar]
- 25.Petersen SM, Dandanell M, Rasmussen LJ, Gerdes AM, Krogh LN, Bernstein I, Okkels H, Wikman F, Nielsen FC, Hansen TV. Functional examination of MLH1, MSH2, and MSH6 intronic mutations identified in Danish colorectal cancer patients. BMC Med Genet. 2013 Oct;3(14):103. doi: 10.1186/1471-2350-14-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brenneman M, Field A, Yang J, et al. Temporal order of RNase IIIb and loss-of-function mutations during development determines phenotype in DICER1 syndrome: a unique variant of the two-hit tumor suppression mode. F1000Research [v1; ref status: approved with reservations 1. 2015;4:214. doi: 10.12688/f1000research.6746.1. < http://f1000res/5l9>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson JT, Thorvaldsdottir H, Winckler W, Guttman M, Lander ES, Getz G, Mesirov JP. Integrative genomics viewer. Nat Biotechnol. 2011;29(1):24–6. doi: 10.1038/nbt.1754. nbt.1754 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thorvaldsdottir H, Robinson JT, Mesirov JP. Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief Bioinform. 2013;14(2):178–92. doi: 10.1093/bib/bbs017. bbs017 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]