Abstract
mRNAs are involved in complicated supramolecular complexes with human 40S and 80S ribosomes responsible for the protein synthesis. In this work, a derivative of nonaribonucleotide pUUCGUAAAA with nitroxide spin labels attached to the 5′-phosphate and to the C8 atom of the adenosine in sixth position (mRNA analog) was used for studying such complexes using double electron-electron resonance/pulsed electron-electron double resonance spectroscopy. The complexes were assembled with participation of tRNAPhe, which targeted triplet UUC of the derivative to the ribosomal peptidyl site and predetermined location of the adjacent GUA triplet coding for Val at the aminoacyl (A) site. The interspin distances were measured between the two labels of mRNA analog attached to the first nucleotide of the peptidyl site bound codon and to the third nucleotide of the A site bound codon, in the absence/presence of second tRNA bound at the A site. The values of the obtained interspin distances agree with those calculated for available near-atomic structures of similar complexes of 40S and 80S ribosomes, showing that neither 60S subunit nor tRNA at the A site have a noticeable effect on arrangement of mRNA at the codon-anticodon interaction area. In addition, the shapes of distance distributions in four studied ribosomal complexes allowed conclusions on conformational flexibility of mRNA in these complexes. Overall, the results of this study are the first, to our knowledge, demonstration of double electron-electron resonance/pulsed electron-electron double resonance application for measurements of intramolecular distances in multicomponent supramolecular complexes involving intricate cellular machineries and for evaluating dynamic properties of ligands bound to these machineries.
Introduction
Electron paramagnetic resonance (EPR) is a powerful tool for studying structural arrangements of biologically important molecules, in particular, of keystone biopolymers, proteins, and nucleic acids (for review see (1, 2, 3, 4, 5, 6)). To apply this approach, biopolymers that bear spin labels (generally, nitroxides) at desired locations are used. In line with this, a number of methods for selective introduction of such labels into proteins and nucleic acids have been elaborated (4, 7, 8, 9, 10, 11). Application of biopolymers with a single-spin label provides structural and dynamic information on the biopolymer fragment containing the label, namely, on accessibility of the label to external paramagnetic probes from the solvent, as well as on polarity of molecular environment and mobility of the label (for review see (3, 4, 10, 12, 13, 14, 15)). Double electron-electron resonance (DEER) or pulse electron-electron double resonance (PELDOR) spectroscopy is widely applied to systems with two spin labels introduced at desired locations of one biopolymer or different biopolymers forming a specific complex. DEER allows measurements of intramolecular and intermolecular interspin distances when their values fall into the range between 2 and 8 nm (16, 17, 18, 19). This approach has been fruitfully used for measurements of interspin distances in proteins and nucleic acids as well as in their complexes (2, 3, 4, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31), but was not yet applied for investigation of multicomponent supramolecular complexes containing proteins and nucleic acids such as ribosomal translational complexes.
Ribosomes are cellular ribonucleoprotein machineries composed of two subunits, small (40S) and large (60S) ones, each containing ribosomal RNA and several dozens of ribosomal proteins. In all organisms from bacteria to humans, ribosomes carry out protein synthesis, during which the genetic information, delivered as the sequences of trinucleotides-codons of mRNAs copied from the DNA, is translated to amino acid sequences of proteins. In the course of translation ribosomes form a number of specific complexes with their ligands, principally important of which are mRNAs containing the genetic information and tRNAs carrying amino acid residues for their incorporation into the nascent polypeptide chain. Spin-labeled mRNA and tRNA derivatives were used for studying ribosomal complexes; however, their application was restricted to a few early works. In one of them the conformational changes in spin-labeled tRNA caused by its binding to the bacterial ribosome were investigated (32). The other one was focused on examination of stoichiometry of binding of spin-labeled poly(U) to mammalian ribosomes and the effect of poly(U) derivatization on its functional competence as mRNA analog (33). Surprisingly, despite the remarkable progress in EPR technologies and spin labeling of RNAs and proteins during following decades, DEER/PELDOR was not yet used for structural studies of ribosomal translational complexes. To date, structural and functional topography of eukaryotic ribosomes has been well studied by means of biochemical approaches such as site-directed cross-linking (for review, see (34)), and structures of several translational complexes have been deciphered at a subatomic level by means of high-resolution cryo-electron microscopy (cryo-EM) (e.g., see (35, 36)) and x-ray crystallography (37). The respective structures deposited at the Protein Data Bank (PDB) allow calculation of intramolecular and intermolecular distances, making eukaryotic ribosomal translational complexes suitable models for testing applicability of DEER/PELDOR for measurements of interspin distances in supramolecular ribonucleoprotein complexes including intricate cellular machineries.
In this study, we applied a nonaribonucleotide derivative bearing simultaneously two nitroxide spin labels as mRNA analog to measure intramolecular distances in complexes of this analog with human 40S and 80S ribosomes. The mRNA analog in these complexes was involved in codon-anticodon interactions with tRNA at the peptidyl (P) site or simultaneously with two tRNAs at the P and aminoacyl (A) sites. The labels were attached via amino-linkers to the 5′-terminal phosphate and to the C8 of adenine in the sixth position. This spin-labeling strategy was chosen because it is known that introduction of foreign chemical groups (in particular, aryl azide cross-linkers) via amino linkers at the 5′-terminal phosphate or at the C8 of adenosines in short mRNAs (oligoribonucleotides) does not affect their ability to form specific complexes with human ribosomes and to participate in codon-anticodon interactions with cognate tRNAs (38, 39). DEER/PELDOR measurements in ribosomal complexes with this mRNA analog provided the mean distances being in agreement with known structural data on the respective ribosomal complexes and led us to conclusions on the extent of conformational flexibility of mRNA in these complexes. Besides, our study clearly demonstrated the applicability of this method for investigating intra- and intermolecular distances in such highly organized ribonucleoproteins as natural supramolecular machineries.
Materials and Methods
Ribosomes, tRNAs, and spin label
40S and 60S ribosomal subunits were isolated from unfrozen human placenta according to (40). At the final step of preparation, the subunits were resuspended in D2O up to 60 pmol/μl concentrations and stored in liquid nitrogen in small aliquots, each to be unfrozen only once. Before use, the subunits were reactivated by incubation in binding buffer A (50 mM Tris-HCl, pH 7.5, 100 mM KCl, 13 mM MgCl2, and 0.5 mM EDTA in D2O) at 37°C for 10 min. 80S ribosomes were obtained by association of reactivated 40S and 60S subunits taken in a 40S:60S ratio of 1:1. Activity of the ribosomes in the poly (U)-directed binding of [14C]Phe-tRNAPhe was ∼70%. tRNAPhe and tRNAVal (∼1300 pmol/A260 unit) from Escherichia coli were kindly provided by Dr. V.I. Katunin (B.P. Konstantinov’s St. Petersburg Institute of Nuclear Physics, Gatchina, Russia). Before use for binding with ribosomes, tRNAs were reactivated by incubation in buffer A at 37°C for 5 min. The spin label 3-carboxy-2,2,5,5-tetramethyl-2,5-dihydro-1H-pyrrol-1-oxyl succinimidyl ester (NHS-M2) was prepared according to the procedures described in (41). and used without deuteration.
Spin-labeled mRNA analog
Derivative of nonaribonucleotide UUCGUAAAA bearing simultaneously two spin labels at desired locations was prepared starting from the nonamer derivative bearing an aliphatic ethylene diamine amino linker (EDL) at the atom C8 of adenosine in the sixth position, which was synthesized and purified according to (42). Spin label M2 was introduced at the EDL aliphatic amine moiety by reaction of the nonamer derivative with NHS-M2 in a mixture containing dimethyl sulfoxide (similar procedure was previously described to insert a spin label at the EDL attached to the atom C5 of uridine (7)); the extent of conversion of the EDL-containing nonamer to the spin-labeled one was ∼80%. To introduce second aliphatic amino linker, the obtained oligomer derivative was first phosphorylated at the 5′-terminus by means of incubation of 10 nmol of the oligomer in 50 μl of a standard kinase buffer containing 2 mM ATP and 20 units of polynucleotide kinase at 37°C for 1 h with subsequent high-performance liquid chromatography purification of the phosphorylated oligomer. EDL was attached to the 5′-phosphate of the nonamer derivative via phosphoramide bond by its condensation with ethylene diamine in the presence of triphenylphosphine and dipyridyl disulfide according to the method described earlier (38) with minor modifications (namely, 0.7 M ethylene diamine was taken instead of aromatic 2-chloroethylamine and 1.5 M dimethyl aminopyridine was used instead of N-methyl imidazole). After the reaction, nucleotide material was precipitated by 10 volumes of 2% LiClO4 in acetone and the EDL-containing nonamer derivative was isolated by high-performance liquid chromatography. The extent of EDL-derivatization of 5′-terminal phosphate of the nonamer bearing the spin label at the atom C8 of adenosine was ∼90%. Introduction of the second spin label at the EDL attached to the 5′-terminal phosphate and purification of the obtained nonamer derivative were carried out as described previously. The nonamer derivative containing two spin labels was then dissolved in D2O at a concentration of (1–2) × 10−4 M. Typically, ∼1 nmol of the purified nonamer derivative with two spin labels was finally obtained from 5.5 nmol of the EDL-derivative of UUCGUAAAA taken at the start of the synthesis.
Ribosomal complexes with spin-labeled mRNA analog
40S and 80S ribosomal complexes containing a spin-labeled derivative of nonaribonucleotide as mRNA analog were obtained in buffer A at 20°C. To obtain the ternary complex containing a 40S ribosomal subunit, the mRNA analog and tRNAPhe at the P site, 300 pmol of 40S subunits were first incubated with 400 pmol of tRNAPhe in 6.5 μl of buffer A for 20 min, and then 300 pmol of the mRNA analog dissolved in 1.7 μl of buffer A was added and the mixture was incubated for 1 h. To obtain 40S complex with codon-anticodon interactions at both A and P sites (the quaternary complex), the ternary complex obtained as described previously was mixed with 780 pmol of tRNAVal with subsequent incubation for 1 h. Before EPR analysis, glycerol-d8 was added to the complex up to 40% concentration. Ternary and quaternary 80S ribosomal complexes were obtained by incubation of the respective 40S complexes with 60S subunits taken in 40S:60S ratio of 1:1 for 1 h, and glycerol-d8 was added to the preformed complexes to keep its 40% concentration.
EPR experiments
Samples for DEER measurements were prepared at room temperature in glass capillary tubes (OD 1.5 mm, ID 0.9 mm, with the sample volume being ∼10 μl), shock-frozen in liquid nitrogen and investigated at T = 80 K. The data were collected at the Q-band (34 GHz) using a Bruker Elexsys E580 pulse/CW EPR spectrometer equipped with an EN5107D2 resonator and Oxford Instruments temperature control system (maximum available microwave power was limited to 1 W). A standard four-pulse DEER sequence (18) was used with pulse lengths of 20/40 ns for probe (υprobe) and 44 ns for pump (υpump) frequency. The measurements were done at a field position of ∼2.5 mT higher than the maximum of the spectrum, thus using Δυ = (υpump − υprobe) = 70 MHz led to the pump pulse applied at the spectral maximum. In addition to the traditional two-step phase cycle, we implemented cycling of the second pulse at the probe frequency, which gave slightly better results for our experimental setup. The number of scans was 200 for unbound mRNA and complexes 1 and 3 and 400 for complexes 2 and 4. All measurements were reproduced several times. All experimental results were processed using DeerAnalysis2013 (43).
Results and Discussion
Spin-labeled mRNA analog and its complexes with human ribosomes
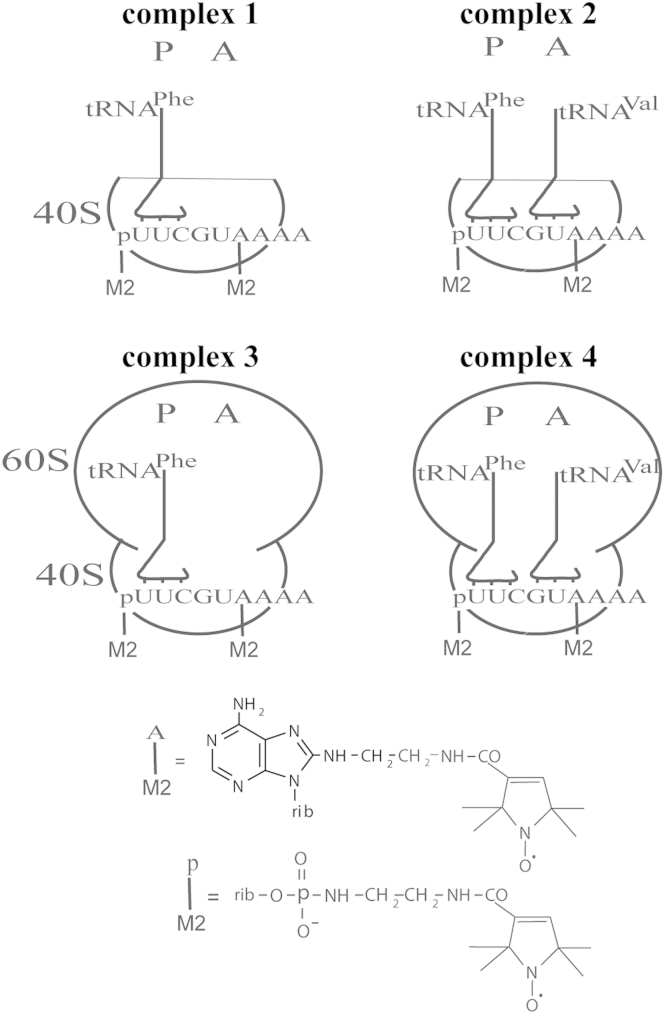
To measure intramolecular distance between different parts of mRNA bound to human 40S or 80S ribosomes, we used a derivative of nonaribonucleotide UUCGUAAAA containing triplets UUC and GUA coding for Phe and Val, respectively. To obtain model complexes of the ribosomes with the mRNA analog, in which these codons are positioned at the ribosomal P and A sites, respectively, we used an approach based on the well-known phenomenon that without translation factors tRNA has maximum affinity to the P site of 80S ribosomes (for review, see (34, 44)). This characteristic of tRNA is displayed as well when tRNA binds to isolated 40S subunits, which are responsible for binding of mRNA and where mRNA codons are recognized by anticodons of tRNAs in the course of the translation process. In the presence of tRNA cognate to one of the mRNA codons, mRNA analog becomes fixed on the ribosome by interaction of the selected codon with the tRNA anticodon at the P site. Such kinds of complexes, in which position of a derivatized nucleotide of mRNA analog on the ribosome was governed by tRNA targeting the desired codon to the P site, were fruitfully used for studying functional topography of human ribosomes with application of mRNA analogs bearing nucleotides with cross-linkers at specific locations (reviewed in (34)). Using a doubly spin-labeled derivative of UUCGUAAAA, we obtained complexes where it was fixed at the P site by interaction of its UUC triplet with tRNAPhe, which predetermined location of the adjacent triplet GUA at the A site (Fig. 1, complexes 1 and 3). The latter made obtaining complexes with two codon-anticodon interactions possible, where tRNAPhe was at the P site and tRNAVal was at the A site (Fig. 1, complexes 2 and 4). We used the same ionic and temperature conditions for the ribosomal complexes formation as in previous studies with short mRNA analogs bearing cross-linkers, but ribosome concentration used in this study was much higher than that applied in the cross-linking studies (39, 45, 46). It is worth noting here that short oligoribonucleotides as mRNA analogs form stable complexes with ribosomes only in the presence of tRNA cognate to one of their codons, and without tRNA they are unable to bind at the ribosomal site corresponding to the area of codon-anticodon interactions (34). Maximum binding (∼0.7–0.8 mol of mRNA analog per mol of 80S ribosomes) was generally achieved in the mentioned cross-linking studies when mRNA analogs were taken in 5- to 10-fold excess over ribosomes. Binding properties of the doubly spin-labeled mRNA analog used in this study were similar to those of the respective unmodified nonaribonucleotide; under conditions used to obtain model complexes for DEER measurements, the ribosomes were mainly (∼70%) saturated with the mRNA analog basepaired with tRNAPhe at the P site (see the Supporting Material), i.e., only minor fraction (∼30%) was free. The ability of the complex 1 to bind tRNAVal at the A site to form complex 2 was also confirmed (see the Supporting Material).
Figure 1.
Model 40S (1 and 2) and 80S (3 and 4) ribosomal complexes with the nonaribonucleotide mRNA analog bearing two spin labels, one at the 5′-terminal phosphate and another at the adenosine in sixth position. P and A are ribosomal tRNA binding sites. In complexes 1 and 3 the mRNA analog is implicated in codon-anticodon interaction with cognate tRNAPhe at the P site, and in complexes 2 and 4 codon-anticodon interactions occur at both the A and the P sites. At the bottom, chemical structures of derivatized adenosine and 5′-terminal phosphate of the mRNA analog are shown.
Measurements of interspin distances in the ribosomal complexes of the spin-labeled mRNA analog
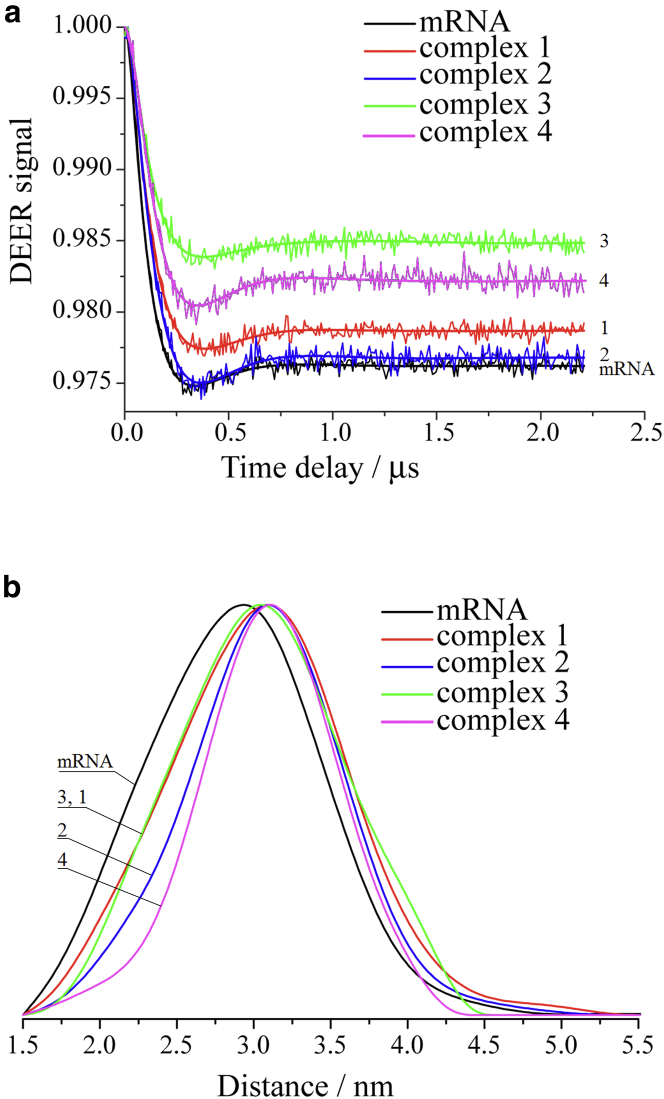
Spin labels attached to the first nucleotide of the P site codon and to the third nucleotide of the A site codon of the mRNA analog (Fig. 1) allowed us to learn whether the distance between the corresponding nucleotide positions is changed upon tRNA-dependent binding of the analog to 40S subunits. Besides, spin labels in these locations enabled monitoring the spin-spin distances depending on 1) interaction of the A site-bound codon with cognate tRNA, and 2) association of the 40S complex with 60S subunits accommodating CCA-ends of tRNAs to the ribosomal catalytic center. Fig. 2 shows the obtained DEER time traces and distance distributions for unbound mRNA and complexes 1–4. The obtained mean distances 〈rDEER〉 and corresponding standard deviation parameters σ are summarized in Table 1. The binding of the mRNA analog to both 40S and 80S ribosomes leads to increased 〈rDEER〉 values compared to the case of unbound analog, which reflects changes of its geometry resulting from this binding. These changes most probably relate to some untwisting of the mRNA analog helical structure caused by its interaction with the ribosomes. As well known, the backbone of single-stranded RNA lacking secondary structure forms a helix where the nucleotide bases are located in stacking, and the unbound mRNA analog should also have such a structure. When mRNA is bound to the ribosome in the region of the codon-anticodon interactions, the angle between its P and A site-bound codons arises independently of the presence of the tRNA at the A site (35, 37, 47). The latter causes disturbance of the stacking between mRNA codons at the P and A sites and thereby apparently leads to increase of the distance between the first nucleotide of the P site codon and the last nucleotide of the A site codon, which is displayed in the growth of the 〈rDEER〉 value.
Figure 2.
Distance measurements in unbound mRNA and complexes 1–4 obtained by Q-band DEER. (a) Background corrected four-pulse DEER traces (intensity is normalized). Lines with noise correspond to the experimental data. Solid lines show best fits obtained using DeerAnalysis2013. (b) Obtained distance distribution for complexes 1–4 after normalization. Regularization parameter is 1000. To see this figure in color, go online.
Table 1.
Mean distances 〈rDEER〉 and standard deviation parameter σ obtained by EPR for all studied complexes
| Complex Type | 〈rDEER〉 ± σ/nm |
|---|---|
| Unbound mRNA | 2.86 ± 0.51 |
| Complex 1 | 3.03 ± 0.55 |
| Complex 2 | 3.05 ± 0.48 |
| Complex 3 | 3.04 ± 0.54 |
| Complex 4 | 3.09 ± 0.46 |
Note that small differences in 〈rDEER〉 values found for free and bound mRNA analog motivated us to perform a number of additional controls to verify that the binding indeed occurs and that it is specific (see the Supporting Material). First, experiments with radioactively labeled mRNA analog clearly showed that specific binding does occur. Second, the changes of 〈rDEER〉 in the complexes versus free mRNA take place only in the presence of tRNA(s). In addition, electron spin echo envelope modulation on proton/deuteron nuclei was studied. Although electron spin echo envelope modulation results were not as conclusive as those of DEER and data obtained with radioactively labeled mRNA, they generally support formation of specific complexes between spin-labeled mRNA analog and ribosomes. Finally, room temperature CW EPR spectra of spin-labeled mRNA alone, spin-labeled mRNA in the presence of isolated 40S or 60S subunits, and spin-labeled mRNA in the presence of 80S ribosomes and tRNA(s) are all very similar, indicating that the molecular dynamics and conformations of spin labels in corresponding situations should not be significantly different.
The mean 〈rDEER〉 values are found to be similar (3.03–3.09 nm) in all types of the ribosomal complexes studied here, indicating that neither tRNA at the A site nor the 60S subunit have an effect on the arrangement of the mRNA analog at the area of codon-anticodon interactions. The model complex 1 comprising the 40S subunit, mRNA analog, and P site-bound tRNA resembles the 48S preinitiation complex. The latter is formed as the result of scanning of the mRNA by 40S subunit bound to the initiator Met-tRNAiMet and the respective translation initiation factors to reach the start AUG codon. Accordingly, the model 80S complexes 3 and 4 differing by the presence of tRNA at the A site, are similar to 80S elongation complexes. The same means 〈rDEER〉 obtained for complexes 1, 3, and 4 suggest that correct geometry of mRNA in the codon-anticodon interaction area is set by interaction of the P site codon-anticodon duplex with the 40S subunit ensuring proper location of the A site codon, and that this geometry does not change in the course of translation.
Comparison of the measured distances with those calculated from structures of ribosomal complexes deposited in the PDB
The distance between the 5′-phosphate of the first nucleotide of the P site codon and the base of the third nucleotide of the A site codon can also be calculated from the structures of analogous complexes of eukaryotic ribosomes with tRNAs and mRNAs deposited in the PDB (Table 2). The calculations show that this distance indeed slightly depends on the occupation of the A site with tRNA and is the same within similar 40S and 80S ribosomal complexes. The values of ∼3 nm for the distances measured here are rather similar to those calculated from the structures of complexes taken from the PDB. However, all calculated values (Table 2) are somewhat less than those found for complexes 1–4 in this work (Table 1), and the value calculated for the complex with tRNA molecules at the P and A sites is slightly less than those for the complexes where the A site is vacant (Table 2). These differences might be explained by less rigid structure of the ribosomal complexes in glycerol-containing solutions used in our EPR study as compared to those in samples that have been used for x-ray (crystals) and cryo-EM (vitreous state) analyses. More likely, the size of the label and length of the linker between the site of its attachment in mRNA analog and the label are responsible for these small differences. The latter might impede detection of small differences in distances between labels in ribosomal complexes with one and two tRNAs. At the same time, it is worth noting that in two of three structures of ribosomal complexes taken from the PDB for calculation of the previously mentioned distances, the third nucleotide of the A site mRNA codon was uridine, but not adenosine (see Table 2). These dissimilarities might also be the reasons for small differences between the calculated distances (Table 2) and those measured by DEER/PELDOR (Table 1).
Table 2.
Literature-based distances in complexes of eukaryotic ribosomes with tRNAs and mRNAs
| PDB ID (Method of Analysis and Resolution in nm) | Complex | Distance (nm) | Base in 3rd Position of the A Site Codon | Reference |
|---|---|---|---|---|
| 4KZZ (x-ray, 0.70) | 40S • tRNA (P site) • mRNA • eIF1Aa | 2.78 | U | (37) |
| 1VXZ (cryo-EM, 0.63) | 80S • tRNA (P site) • tRNA (E site) • mRNA | 2.74 | A | (36) |
| 4CXB (cryo-EM, 0.69) | 80S • tRNA (P site) • tRNA (A site) • mRNA | 2.58 | U | (35) |
Distances between the P of the 5′-terminal phosphate of mRNA codon at the P site and the C8 of A or C5 of U in the third position of the A site codon calculated from the structures of complexes of eukaryotic ribosomes with tRNAs and mRNAs deposited in the PDB.
eIF1A, eukaryotic translation initiation factor 1A.
Effect of tRNA molecule at the ribosomal A site on distance distribution widths
We attempted to monitor the effect of tRNA at the A site on the arrangement of mRNA at the codon-anticodon interactions area analyzing the widths of the distance distributions. These widths are contributed by conformational flexibility of spin-labeled mRNA analog and disorder of the rather long linker. For all studied samples the distance distributions widths (σ) are in the range of 0.46–0.55 nm (Fig. 2 b and Table 1). One can see that the σ values displayed by complexes 2 and 4 with tRNAs bound at the P and A sites are smaller than those obtained for complexes 1 and 3 with a vacant A site. For example, for 40S complex 2 with two tRNAs we obtained σ = 0.48 nm, whereas for corresponding complex 1 with single tRNA at the P site σ = 0.55 nm, and this trend of the σ values for the respective complexes was systematically observed. This indicates that conformational flexibility of the spin-labeled mRNA analog in complexes containing tRNAs at the P and A sites is smaller than that in the complexes with the empty A site, as one would expect due to the more rigid fixation of the mRNA by codon-anticodon interactions simultaneously at the P and A sites. Thus, in addition to values of intramolecular distances, the EPR data can provide information on the conformational flexibility of particular mRNA fragments depending on their involvement in interactions with the ribosome and its ligands.
Conclusion
This work demonstrates for the first time, to our knowledge, that DEER/PELDOR spectroscopy can be fruitfully applied to extremely complicated biological systems operating with RNAs. To show this, we have measured a series of spin-spin distances in the doubly spin-labeled mRNA analog involved in different multicomponent ribosomal complexes resembling those formed at the stages of translation initiation and elongation. We have shown that the obtained values agree with the available structural data. In addition, new DEER/PELDOR data allowed characterization of the extent of conformational flexibility of the mRNA analog depending on whether the codon-anticodon interaction took place at the ribosomal A site.
Chemical structure of RNA enables introduction of spin labels at various sites of nucleotides located at different positions in the RNA chain. Therefore, considering the keystone role of RNA molecules in many vitally important biological processes involving multimegadalton complexes, one suggests that the application of DEER/PELDOR spectroscopy in studies of large supramolecular complexes interacting with RNAs is very promising. This approach might be used to study multicomponent structures involving small RNAs (e.g., small nuclear RNAs, small interfering RNAs, small nucleolar RNAs, and microRNAs) formed during pre-mRNA and pre-rRNA processing, RNA interference, transfer of RNAs to exosomes, etc. In such structures, natural RNAs might be replaced by their derivatives bearing spin labels at various positions. For example, labels can be attached at the atoms C8 of adenosine and C5 of uridine of synthetic RNAs via amino linkers, which are easily introduced at the respective positions during automated RNA synthesis yielding RNA derivatives up to 70 nucleotides long (48). EPR data on structure and dynamics of RNAs in biologically significant multicomponent supramolecular complexes might be of great importance, helping to understand the molecular mechanisms of their functioning and opening ways for elaborating novel biomedical applications of these RNAs.
Author Contributions
O.A.K. and M.V.F. performed EPR measurements, analyzed EPR data, and wrote the article; E.G.B. designed research, M.I.M. and A.G.V. synthesized the oligoribonucleotide bearing an amino linker at the C8 of adenosine, A.A.M. phosphorylated the oligomer at the 5′-terminus, introduced amino-linker at the 5′-terminal phosphate of oligomer and coupled spin label to the amino linkers, D.M.G. obtained ribosomal complexes and wrote the article, G.G.K. designed research and wrote the article.
Acknowledgments
This work was supported by the Russian Science Foundation (No. 14-14-00922). We are very thankful to Dr. Igor Kirilyuk (NIOCh SB RAS) for kindly providing us with nitroxide labels.
Editor: Elizabeth Komives.
Footnotes
Supporting Materials and Methods, nine figures, and two tables are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(15)01119-4.
Contributor Information
Matvey V. Fedin, Email: mfedin@tomo.nsc.ru.
Galina G. Karpova, Email: karpova@niboch.nsc.ru.
Elena G. Bagryanskaya, Email: egbagryanskaya@nioch.nsc.ru.
Supporting Material
References
- 1.Schiemann O. Mapping global folds of oligonucleotides by pulsed electron–electron double resonance. In: Daniel H., editor. Methods in Enzymology. Academic Press; San Diego, CA: 2009. pp. 329–351. [DOI] [PubMed] [Google Scholar]
- 2.Schiemann O., Prisner T.F. Long-range distance determinations in biomacromolecules by EPR spectroscopy. Q. Rev. Biophys. 2007;40:1–53. doi: 10.1017/S003358350700460X. [DOI] [PubMed] [Google Scholar]
- 3.Engels J.W., Grünewald C., Wicke L. Site-directed labeling of RNA for distance measurements by EPR. In: Erdmann V.A., Markiewicz W.T., Barciszewski H.J., editors. Chemical Biology of Nucleic Acids: Fundamentals and Clinical Applications. Springer-Verlag, Berlin-Heidelberg; 2014. pp. 385–407. [Google Scholar]
- 4.Sahu I.D., McCarrick R.M., Lorigan G.A. Use of electron paramagnetic resonance to solve biochemical problems. Biochemistry. 2013;52:5967–5984. doi: 10.1021/bi400834a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krstic I., Endeward B., Prisner T.F. Structure and dynamics of nucleic acids. In: Drescher M., Jeschke G., editors. Vol. 321. Springer-Verlag, Berlin-Heidelberg; 2012. pp. 159–198. (EPR Spectroscopy: Applications in Chemistry and Biology). [DOI] [PubMed] [Google Scholar]
- 6.Qin P.Z., Dieckmann T. Application of NMR and EPR methods to the study of RNA. Curr. Opin. Struct. Biol. 2004;14:350–359. doi: 10.1016/j.sbi.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Babaylova E.S., Ivanov A.V., Bagryanskaya E.G. A versatile approach for site-directed spin labeling and structural EPR studies of RNAs. Org. Biomol. Chem. 2014;12:3129–3136. doi: 10.1039/c3ob42154f. [DOI] [PubMed] [Google Scholar]
- 8.Fielding A.J., Concilio M.G., Hollas M.A. New developments in spin labels for pulsed dipolar EPR. Molecules. 2014;19:16998–17025. doi: 10.3390/molecules191016998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hubbell W.L., López C.J., Yang Z. Technological advances in site-directed spin labeling of proteins. Curr. Opin. Struct. Biol. 2013;23:725–733. doi: 10.1016/j.sbi.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klare J.P., Steinhoff H.J. Spin labeling EPR. Photosynth. Res. 2009;102:377–390. doi: 10.1007/s11120-009-9490-7. [DOI] [PubMed] [Google Scholar]
- 11.Shelke S.A., Sigurdsson S.T. Site-directed spin labelling of nucleic acids. Eur. J. Org. Chem. 2012;2012:2291–2301. [Google Scholar]
- 12.Edwards T.E., Sigurdsson S.T. Electron paramagnetic resonance dynamic signatures of TAR RNA-small molecule complexes provide insight into RNA structure and recognition. Biochemistry. 2002;41:14843–14847. doi: 10.1021/bi026299a. [DOI] [PubMed] [Google Scholar]
- 13.Ding Y., Zhang X., Qin P.Z. Experimental mapping of DNA duplex shape enabled by global lineshape analyses of a nucleotide-independent nitroxide probe. Nucleic Acids Res. 2014;42:e140. doi: 10.1093/nar/gku695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Popova A.M., Kálai T., Qin P.Z. Site-specific DNA structural and dynamic features revealed by nucleotide-independent nitroxide probes. Biochemistry. 2009;48:8540–8550. doi: 10.1021/bi900860w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X., Dantas Machado A.C., Qin P.Z. Conformations of p53 response elements in solution deduced using site-directed spin labeling and Monte Carlo sampling. Nucleic Acids Res. 2014;42:2789–2797. doi: 10.1093/nar/gkt1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milov A.D., Ponomarev A.B., Tsvetkov Y.D. Electron-electron double resonance in electron spin echo: model biradical systems and the sensitized photolysis of decalin. Chem. Phys. Lett. 1984;110:67–72. [Google Scholar]
- 17.Milov A.D., Salikohov K.M., Shirov M.D. Application of ENDOR in electron-spin echo for paramagnetic center space distribution in solids. Fiz. Tverd. Tela. 1981;23:975–982. [Google Scholar]
- 18.Pannier M., Veit S., Spiess H.W. Dead-time free measurement of dipole-dipole interactions between electron spins. J. Magn. Reson. 2000;142:331–340. doi: 10.1006/jmre.1999.1944. [DOI] [PubMed] [Google Scholar]
- 19.Edwards T.E., Sigurdsson S.T. EPR spectroscopic analysis of U7 hammerhead ribozyme dynamics during metal ion induced folding. Biochemistry. 2005;44:12870–12878. doi: 10.1021/bi050549g. [DOI] [PubMed] [Google Scholar]
- 20.Cai Q., Kusnetzow A.K., Qin P.Z. Site-directed spin labeling measurements of nanometer distances in nucleic acids using a sequence-independent nitroxide probe. Nucleic Acids Res. 2006;34:4722–4730. doi: 10.1093/nar/gkl546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grohmann D., Klose D., Werner F. RNA-binding to archaeal RNA polymerase subunits F/E: a DEER and FRET study. J. Am. Chem. Soc. 2010;132:5954–5955. doi: 10.1021/ja101663d. [DOI] [PubMed] [Google Scholar]
- 22.Kim N.K., Bowman M.K., DeRose V.J. Precise mapping of RNA tertiary structure via nanometer distance measurements with double electron-electron resonance spectroscopy. J. Am. Chem. Soc. 2010;132:8882–8884. doi: 10.1021/ja101317g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krstić I., Frolow O., Prisner T.F. PELDOR spectroscopy reveals preorganization of the neomycin-responsive riboswitch tertiary structure. J. Am. Chem. Soc. 2010;132:1454–1455. doi: 10.1021/ja9077914. [DOI] [PubMed] [Google Scholar]
- 24.Schiemann O., Piton N., Prisner T.F. A PELDOR-based nanometer distance ruler for oligonucleotides. J. Am. Chem. Soc. 2004;126:5722–5729. doi: 10.1021/ja0393877. [DOI] [PubMed] [Google Scholar]
- 25.Schiemann O., Weber A., Sigurdsson S.T. Nanometer distance measurements on RNA using PELDOR. J. Am. Chem. Soc. 2003;125:3434–3435. doi: 10.1021/ja0274610. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X., Tung C.-S., Qin P.Z. Global structure of a three-way junction in a phi29 packaging RNA dimer determined using site-directed spin labeling. J. Am. Chem. Soc. 2012;134:2644–2652. doi: 10.1021/ja2093647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cai Q., Kusnetzow A.K., Qin P.Z. Nanometer distance measurements in RNA using site-directed spin labeling. Biophys. J. 2007;93:2110–2117. doi: 10.1529/biophysj.107.109439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hilger D., Polyhach Y., Jeschke G. Backbone structure of transmembrane domain IX of the Na+/proline transporter PutP of Escherichia coli. Biophys. J. 2009;96:217–225. doi: 10.1016/j.bpj.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyer V., Swanson M.A., Eaton G.R. Room-temperature distance measurements of immobilized spin-labeled protein by DEER/PELDOR. Biophys. J. 2015;108:1213–1219. doi: 10.1016/j.bpj.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duss O., Michel E., Allain F.H.-T. Structural basis of the non-coding RNA RsmZ acting as a protein sponge. Nature. 2014;509:588–592. doi: 10.1038/nature13271. [DOI] [PubMed] [Google Scholar]
- 31.Duss O., Yulikov M., Allain F.H.-T. EPR-aided approach for solution structure determination of large RNAs or protein-RNA complexes. Nat. Commun. 2014;5:3669. doi: 10.1038/ncomms4669. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez A., Tougas G., Dugas H. Interaction of 70 S ribosomes from Escherichia coli with spin-labeled N-Cbz-Phe-tRNAPhe. An electron paramagnetic resonance study. J. Biol. Chem. 1980;255:8116–8120. [PubMed] [Google Scholar]
- 33.Damerau W., Petrov A.I., Bielka H. Probing of mRNA binding sites involved in interactions with rat liver ribosomes using poly(U) spin labeled at the ribose moiety. Biomed. Biochim. Acta. 1986;45:727–736. [PubMed] [Google Scholar]
- 34.Graifer D., Karpova G. Photoactivatable RNA derivatives as tools for studying the structural and functional organization of complex cellular ribonucleoprotein machineries. RSC Adv. 2013;3:2858–2872. [Google Scholar]
- 35.Budkevich T.V., Giesebrecht J., Spahn C.M.T. Regulation of the mammalian elongation cycle by subunit rolling: a eukaryotic-specific ribosome rearrangement. Cell. 2014;158:121–131. doi: 10.1016/j.cell.2014.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Svidritskiy E., Brilot A.F., Korostelev A.A. Structures of yeast 80S ribosome-tRNA complexes in the rotated and nonrotated conformations. Structure. 2014;22:1210–1218. doi: 10.1016/j.str.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lomakin I.B., Steitz T.A. The initiation of mammalian protein synthesis and mRNA scanning mechanism. Nature. 2013;500:307–311. doi: 10.1038/nature12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Graifer D.M., Zenkova M.A., Karpova G.G. Identification of a site on 18 S rRNA of human placenta ribosomes in the region of the mRNA binding center. J. Mol. Biol. 1990;214:121–128. doi: 10.1016/0022-2836(90)90151-B. [DOI] [PubMed] [Google Scholar]
- 39.Molotkov M.V., Graifer D.M., Karpova G.G. mRNA 3′ of the A site bound codon is located close to protein S3 on the human 80S ribosome. RNA Biol. 2006;3:122–129. doi: 10.4161/rna.3.3.3584. [DOI] [PubMed] [Google Scholar]
- 40.Matasova N.B., Myltseva S.V., Karpova G.G. Isolation of ribosomal subunits containing intact rRNA from human placenta: estimation of functional activity of 80S ribosomes. Anal. Biochem. 1991;198:219–223. doi: 10.1016/0003-2697(91)90416-q. [DOI] [PubMed] [Google Scholar]
- 41.Hankovszky H.O., Hideg K., Tigyi J. Nitroxides. Ii. 1-oxyl-2,2,5,5-tetramethylpyrroline-3-carboxylic acid derivatives. Acta Chir. Acad. Sci. Hung. 1978;98:339–348. [Google Scholar]
- 42.Repkova M.N., Ivanova T.M., Venyaminova A.G. H-phosphonate synthesis of oligoribonucleotides containing modified bases. I. Photoactivatable derivatives of oligoribonucleotides with perfluoroarylazide groups in heterocyclic bases. Russian J. Bioorgan. Chem. 1999;25:690–701. [Google Scholar]
- 43.Jeschke G., Chechik V., Jung H. DeerAnalysis2006 - a comprehensive software package for analyzing pulsed ELDOR data. Appl. Magn. Reson. 2006;30:473–498. [Google Scholar]
- 44.Graifer D., Karpova G. Interaction of tRNA with eukaryotic ribosome. Int. J. Mol. Sci. 2015;16:7173–7194. doi: 10.3390/ijms16047173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Demeshkina N., Repkova M., Karpova G. Nucleotides of 18S rRNA surrounding mRNA codons at the human ribosomal A, P, and E sites: a cross-linking study with mRNA analogs carrying an aryl azide group at either the uracil or the guanine residue. RNA. 2000;6:1727–1736. doi: 10.1017/s1355838200000996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Graifer D., Molotkov M., Karpova G. Variable and conserved elements of human ribosomes surrounding the mRNA at the decoding and upstream sites. Nucleic Acids Res. 2004;32:3282–3293. doi: 10.1093/nar/gkh657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lim V., Venclovas C., Muller F. How are tRNAs and mRNA arranged in the ribosome. An attempt to correlate the stereochemistry of the tRNA mRNA interaction with constraints imposed by the ribosomal topography. Nucleic Acids Res. 1992;20:2627–2637. doi: 10.1093/nar/20.11.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Graifer D., Karpova G. General approach for introduction of various chemical labels in specific RNA locations based on insertion of amino linkers. Molecules. 2013;18:14455–14469. doi: 10.3390/molecules181214455. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.