Abstract
The enterotoxigenic Escherichia coli strains lead to diarrhoea in humans due to heat-labile and heat-stable (STa) enterotoxins. STa increases Cl-release in intestinal cells, including the human colonic carcinoma T84 cell line, involving increased cGMP and membrane alkalization due to reduced Na+/H+ exchangers (NHEs) activity. Since NHEs modulate intracellular pH (pHi), and NHE1, NHE2, and NHE4 are expressed in T84 cells, we characterized the STa role as modulator of these exchangers. pHi was assayed by the NH4Cl pulse technique and measured by fluorescence microscopy in BCECF–preloaded cells. pHi recovery rate (dpHi/dt) was determined in the absence or presence of 0.25 μmol/L STa (30 minutes), 25 μmol/L HOE-694 (concentration inhibiting NHE1 and NHE2), 500 μmol/L sodium nitroprusside (SNP, spontaneous nitric oxide donor), 100 μmol/L dibutyryl cyclic GMP (db-cGMP), 100 nmol/L H89 (protein kinase A inhibitor), or 10 μmol/L forskolin (adenylyl cyclase activator). cGMP and cAMP were measured in cell extracts by radioimmunoassay, and buffering capacity (ßi) and H+ efflux (J H +) was determined. NHE4 protein abundance was determined by western blotting. STa and HOE-694 caused comparable reduction in dpHi/dt and J H + (~63%), without altering basal pHi (range 7.144–7.172). STa did not alter ßi value in a range of 1.6 pHi units. The dpHi/dt and J H + was almost abolished (~94% inhibition) by STa + HOE-694. STa effect was unaltered by db-cGMP or SNP. However, STa and forskolin increased cAMP level. STa–decreased dpHi/dt and J H + was mimicked by forskolin, and STa + HOE-694 effect was abolished by H89. Thus, incubation of T84 cells with STa results in reduced NHE4 activity leading to a lower capacity of pHi recovery requiring cAMP, but not cGMP. STa effect results in a causal phenomenon (STa/increased cAMP/increased PKA activity/reduced NHE4 activity) ending with intracellular acidification that could have consequences in the gastrointestinal cells function promoting human diarrhoea.
Introduction
Intestinal colon cells are polarized epithelial cells that express a wide range of plasma membrane transporters for a variety of substrates. Membrane transporters at the apical border of these cells promote absorption and release of nutrients, electrolytes and water from and to the intestinal lumen. However, membrane transporters at the basolateral border maintain cell homeostasis by the release of these and other nutrients to the interstitium. The apical membrane of intestinal colon cells is directly exposed to agents and toxins, including the enterotoxigenic Escherichia coli (ETEC) strains, an intestinal agent leading to diarrhoea in humans [1]. ETEC colonizes host intestines and releases heat-labile and/or heat-stable (STa) enterotoxins. STa causes secretory diarrhoea and is responsible for about half of all ETEC–related diarrhoeal diseases, including traveller’s diarrhoea and epidemic diarrhoea of the newborn [1–5].
STa binds to guanylyl cyclase-C (GC-C) receptors expressed in intestine, kidney, testis and lung, leading to an increase in the intracellular cGMP level [6–8]. STa also increases chloride secretion in a cAMP–dependent manner via the cystic fibrosis transmembrane conductance regulator (CFTR) channels in rat jejunum [9]. In an early study, STa was shown to cause mucosal alkalization due to inhibition of the Na+/H+ exchange in rat duodenum [10,11]. However, there are not reports addressing whether this enterotoxin modulates intracellular pH (pHi), and whether this phenomenon would involve Na+/H+ exchangers (NHEs) activity. Since both cGMP and cAMP decrease NHEs activity [12,13], an increase in the intracellular pH (pHi) in response to STa is expected.
NHEs are key in the modulation of intracellular pH (pHi), and are differentially expressed and regulated in intestine epithelial cells [14–17]. At least 11 isoforms of the NHEs family have been identified, out of which NHE1, 2, 3, and 4 are expressed in gastrointestinal membranes [16,17]. NHE4 is highly expressed in the stomach, renal cortex and medulla, ureter, skeletal muscle, heart, liver, and spleen [18]. NHE4 is involved in gastric secretion [19] and plays a large role in controlling pHi [20]. Indeed, NHE4 was identified in the human colon carcinoma cell line T84 [21] and in human colonic crypts [13]. This exchanger isoform modulates plays a determinant role in maintaining pHi homeostasis; however, nothing is known about the regulation of NHE4 activity in T84 cells by ETEC–released STa. Since T84 cells express the GC-C receptors for STa [22], we hypothesize that STa modulates NHE4 activity and the signalling pathways involved in this phenomenon in this cell type. Our findings suggest that STa decreases NHE4 activity, without altering its protein expression via a mechanism that requires cAMP. This could be determinant in the planning of future therapies for human diarrhoea.
Materials and Methods
Cell culture
The cell line T84 derived from colonic adenocarcinoma of male adult human were purchased from the American Type Culture Collection (ATCC, Rockville, MD, USA) and used for the experiments. T84 cells in culture (5% CO2, 37°C, pH 7.4) were maintained in Dulbecco’s modified Eagle’s medium F12 (DMEM/F12, Gibco, Grand Island, NY, USA) containing low (5 mmol/L) D-glucose and supplemented with 14.5 mmol/L NaHCO3, 3.2 mmol/L D-glutamine, 15 mmol/L HEPES, 5% foetal calf serum (FCS), 100 IU/mL penicillin and 100 mg/mL streptomycin (hereafter referred as primary culture medium (PCM)) as described [21]. Cells were harvested with trypsin/EGTA (0.25/0.2%, 3 minutes, 37°C) and seeded on sterile glass coverslips or 24 well plates for further 72 hours culture until confluence. Cells were then rinsed (3 times) with PCM containing 0.2% FCS (low-FCS/PCM) and cultured in this medium for further 48 hours in order to obtain a cell cycle synchronized culture.
Measurement of pHi
T84 cell monolayers in a glass coverslip were mounted in a thermoregulated chamber on an inverted microscope (Nikon Diaphot-TMD, Tokyoi, Japan). The cells were incubated for 10 minutes at 37°C with the fluorescent pH sensitive probe 2,7-bicarboxyethyl-5,6-carboxyfluorescein acetoxymethyl ester (BCECF-AM, 12 μmol/L) (Molecular Probes, Eugene, OR, USA), as described [21]. Cells were then superfused by gravity at 3 mL/minute (37°C) with the control solutions (CS) ((mmol/L) NaCl 141, KCl 5, CaCl2 1, KH2PO4 0.4, MgCl2 0.5, MgSO4 0.4, Na2HPO4 0.3, HEPES 10, D-glucose 0.6 (pH 7.4, 37°C)) using an electromechanic switching system (Heater and Valve Controller, Yale University Electronics Shop, New Haven, CT, USA). The pHi was calculated from fluorescence ratios measured at excitation of 495/440 nm and emission at 520 nm using a Georgia Instruments PMT-400 photomultiplier system, as described [23]. An area of 260 μm diameter was read, including approximately 200–300 cells. Measurements were performed at 2.5–seconds interval for a period of 300 milliseconds per measurement. The pHi was calibrated using 10 μmol/L nigericin in a calibrating solution ((mmol/L) KCl 130, NaCl 20, CaCl2 1, MgCl2 1, HEPES 5 (pH 6.0, 7.0 and 8.0)) as described [21].
pHi recovery
The pHi recovery was examined by applying the NH4Cl pulse technique [21,23,24]. In brief, BCECF-AM loaded cells were superfused with CS until the basal pHi was stabilized (~15 minutes). T84 cells were preincubated with 0.1, 0.25 or 0.75 μmol/L STa for 30 minutes in the presence of 25 μmol/L HOE-694 (a concentration that inhibits NHE1 and NHE2 activity), as described [21,25,26]. The cells were then exposed (2 minutes) to CS supplemented with NH4Cl (NH4Cl/CS solution) ((mmol/L) NaCl 121, KCl 5.4, CaCl2 1, KH2PO4 0.4, MgCl2 0.5, MgSO4 0.4, Na2HPO4 0.3, HEPES 10, D-glucose 0.6, NH4Cl 20 (pH 7.4, 37°C)). After this incubation period the NH4Cl/CS solution was replaced by rinsing the cells with CS free of NH4Cl, without or with 25 μmol/L HOE-694, 500 μmol/L sodium nitroprusside (SNP, spontaneous nitric oxide donor) [27], 100 μmol/L dibutyryl cyclic GMP (db-cGMP), 100 nmol/L H89 (a protein kinase A inhibitor)) [28] or 10 μmol/L forskolin (an activator of adenylyl cyclase) [29].
Initial rates of pHi recovery (dpHi/dt) were calculated from data collected for the first 60 seconds of the recovery (i.e., after removing the NH4Cl load) and fitted by a first order lineal regression as described [21,24]. The results were expressed in pHi units/minute. The fraction of dpHi/dt mediated by NHE4 (NHE4 dpHi/dt) was estimated by the expression:
where Total dpHi/dt is the dpHi/dt estimated in the absence of HOE-694 (i.e., total initial rate), and HOE dpHi/dt is the dpHi/dt estimated in the presence of HOE-694, i.e., under inhibition of NHE1 and NHE2 [21]. The relative effect of STa on NHE4 dpHi/dt (STa RE) was determined by the expression:
where STa-NHE4 dpHi/dt is NHE4 dpHi/dt measured in the presence of STa.
Intrinsic buffering capacity
The ability of intrinsic cellular components to buffer changes in pHi, i.e., intracellular buffer capacity (ßi), was measured as described [21,24]. After determining the basal pHi the cells were incubated in a 0.5 mmol/L KCl-containing Na+-free CS (0Na+/CS) ((mmol/L) N-methyl-D-glucamine (NMDG) 120, KCl 5, CaCl2 1.8, MgCl2 1, HEPES 30, D-glucose 5 (pH 7.4, 37°C)). Cells were then incubated in the latter solution containing decreasing concentrations of NH4Cl (50, 20, 10, 5, 2.5 or 1 mmol/L). The ßi (Beta(i)) was calculated from the expression:
where the intracellular NH4 + concentration ([NH4 +]i) was obtained from the Henderson-Hasselbalch equation on the assumption that [NH3]i (intracellular NH3) was equivalent to [NH3]o (extracellular NH3), and change (pH i ) is the fraction of change in units of pHi value. Knowing the dpHi/dt and ßi values, the rate of overall transmembrane H+ flux (J H +) was calculated from the following expression:
cAMP and cGMP determination
T84 cells were cultured to confluence in 98-well plates. Cells were first treated for 10 minutes with 1 mmol/L 3-isobutyl-1-methylxanthine (IBMX) (Sigma-Aldrich, St. Louis, MO, USA) and next incubated for another 10 minutes with culture medium containing IBMX or IBMX and STa or forskolin. cAMP and cGMP levels were measured by enzyme immunoassay (cAMP or cGMP Direct Biotrak EIA, GE Healthcare, PA, USA) according to manufacturer's instructions. Values of cAMP or cGMP were normalized to total cell protein per well.
Western blotting
Total protein was obtained from confluent T84 cells rinsed (x2) with ice-cold PBS and harvested in 100 μL of lysis buffer (10% SDS, 20% glycerol, 100 mmol/L dithiothreitol, 2.9 mmol/L Tris (pH 6.8), 0.1% bromophenol blue) (63.7 mmol/L Tris/HCl (pH 6.8), 10% glycerol, 2% sodium dodecylsulphate, 1 mmol/L Na3VO4, 50 mg/mL leupeptin, 5% ß-mercaptoethanol) as described [21,27]. Cells were sonicated (6 cycles, 5 seconds, 100 W, 4°C) and total protein was isolated by centrifugation (13500 g, 15 minutes, 4°C). Proteins (50 μg) were separated by polyacrylamide gel (7.5%) electrophoresis, transferred to Immobilon-P polyvinylidene difluoride membranes (BioRad Laboratories, Hertfordshire, UK) and probed with primary monoclonal rabbit anti-NHE1 (1:1000 dilution, 12 hours, 4°C), primary polyclonal rabbit anti-NHE2 (1:1000 dilution, 12 hours, 4°C) (Abcam, Cambridge, UK), primary rat anti-NHE4 antibody (11H11, amino acids 565–675, ~55 kDa) (kindly donated by Dr Daniel Biemesderfer from Yale School of Medicine, New Haven, CT, USA) (1:1000 dilution, 2 hours, 22°C), or monoclonal mouse anti-ß-actin (1:5000 dilution, internal reference) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) antibodies. The membranes were rinsed in Tris buffer saline-0.1% Tween 20 (TBS-T) and further incubated (1 hour) in TBS-T/0.2% bovine serum albumin (BSA) containing secondary horseradish peroxidase-conjugated goat anti-rat or anti-mouse antibodies (Thermo Scientific, Rockford, IL, USA). Proteins were detected by enhanced chemiluminescence (film exposure time was 1 minute) in a ChemiDoc-It 510 Imagen System (UVP, LCC Upland, CA, USA) and quantified by densitometry [27,30].
Statistical analysis
The values are mean ± S.E.M., where n indicates number of different cell cultures (n = 27 for STa–untreated (i.e., control) and 25 STa–treated cells) with 3–4 replicates per experiment. The normality of the data (i.e., parametric) was confirmed with Kolmogorov-Smirnov’s test. The variances across the control and STa-treated cells under Bartlett’s test were homogeneous. Comparisons between two groups were performed by means of Student’s unpaired t-test. The difference between more than two groups were performed by analysis of variance (ANOVA, one or two-ways). If the ANOVA demonstrated a significant interaction between variables, post hoc analyses were performed by the multiple-comparison Bonferroni test. The experimenter running the assays was blinded to the groups allocation before and during the experiments, and when assessing the outcome (i.e., around 30 days). The statistical software GraphPad InStat 3.0b and GraphPad Prism 7.0a.65 (GraphPad Software Inc., San Diego, CA, USA) were used for data analysis. P<0.05 was considered statistically significant.
Results
Effect of STa on pHi values
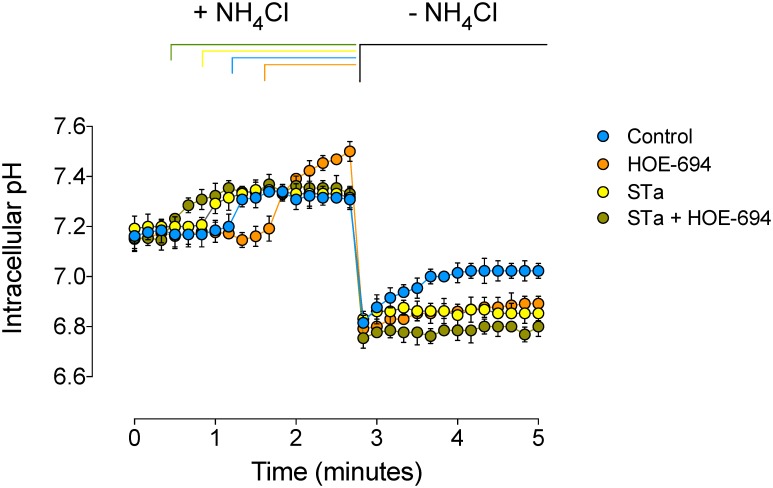
Basal pHi in T84 cells detected in this study was comparable to previous reports in this cell type [21,31,32] and was unaltered in cells preincubated with STa (Table 1, Fig 1). Following the NH4Cl pulse the acidic pHi values detected in the cells exposed to STa or HOE-694 were partially restored (27 ± 3 or 55 ± 6%, respectively) compared with cells in the absence of these agents (Fig 1). When cells were coincubated with STa + HOE-694 the NH4Cl–induced acidic pHi was only minimally restored (9 ± 1%).
Table 1. Modulation of intracellular pH by STa in T84 cells.
| pHi | dpHi/dt | |
|---|---|---|
| Control | 7.170 ± 0.028 | 0.133 ± 0.009 |
| STa | 7.144 ± 0.019 | 0.046 ± 0.009 * |
| HOE-694 | 7.172 ± 0.034 | 0.051 ± 0.010 * |
| HOE-694 + STa | 7.130 ± 0.046 | 0.014 ± 0.001 * † |
| Forskolin | 7.156 ± 0.021 | 0.051 ± 0.010 * |
| Forskolin + STa | 7.171 ± 0.030 | 0.048 ± 0.009 * |
| Forskolin + HOE-694 | 7.161 ± 0.050 | 0.017 ± 0.003 * ‡ |
| Forskolin + HOE-694 + STa | 7.125 ± 0.061 | 0.016 ± 0.002 * ‡ |
| H89 + HOE-694 + STa | 7.143 ± 0.038 | 0.046 ± 0.008 * |
| db-cGMP | 7.21 ± 0.054 | 0.110 ± 0.012 |
| db-cGMP + STa | 7.10 ± 0.021 | 0.050 ± 0.012 * § |
| db-cGMP + HOE-694 | 7.19 ± 0.053 | 0.057 ± 0.002 * § |
| db-cGMP + HOE-694 + STa | 7.14 ± 0.051 | 0.015 ± 0.001 * § ¶ |
| SNP | 7.16 ± 0.026 | 0.123 ± 0.009 |
| SNP + STa | 7.15 ± 0.021 | 0.045 ± 0.011 *& |
| SNP + HOE-694 | 7.11 ± 0.024 | 0.047 ± 0.009 *& |
| SNP + HOE-694 + STa | 7.14 ± 0.052 | 0.015 ± 0.012 *&$ |
The intracellular pH (pHi) was measured in BCECF-AM–preloaded T84 cells as described in Methods. Cells were also subjected to an acid pulse (NH4Cl assay) and the initial rates of pHi recovery (dpHi/dt) was measured in cells in the absence (Control) or presence (30 minutes) of 0.25 μmol/L heat-stable (STa) enterotoxin, 25 μmol/L HOE-694 (Na+/H+ exchangers inhibitor), 10 μmol/L forskolin, 100 nmol/L H89 (protein kinase A inhibitor), 100 μmol/L dibutyryl cyclic GMP (db-cGMP), or 500 μmol/L sodium nitroprusside (SNP). STa at 0.1 and 0.75 μmol/L did not alter pHi values (7.121 ± 0.011 and 7.160 ± 0.014, respectively; P>0.05, n = 4). STa at 0.1 μmol/L partially reduced dpHi/dt value (0.098 ± 0.005 pHi units/minute, P<0.05, n = 4), and inhibition at 0.75 μmol/L (0.056 ± 0.007 pHi units/minute) was similar (P>0.05, n = 4) to 0.25 μmol/L STa (see also Fig 2B).
*P<0.04 versus Control
† P<0.03 versus STa or HOE-694
‡ P<0.03 versus Forskolin, Forskolin + STa, and H89 + HOE-694 + STa,
§ P<0.05 versus db-cGMP
¶ P<0.05 versus db-cGMP + STa and db-cGMP + HOE-694, &P<0.05 versus db-cGMP
$ P<0.03 versus SNP + STa and SNP + HOE-694
Fig 1. Effect of STa on pHi recovery.
T84 cells were preloaded with BCECF-AM in the absence or presence (30 minutes) of 0.25 μmol/L heat-stable (STa) enterotoxin. After transferring the cells into a spectrofluorometer the basal pHi was stabilized and then exposed (1.5–2 minutes) to a control solution containing 20 mmol/L NH4Cl (+ NH4Cl). Cells were then rinsed with a NH4Cl–free solution (–NH4Cl) and left in this medium without (Control) or with 0.25 μmol/L STa, 25 μmol/L HOE-694, or both (STa + HOE-694) (see Methods). Initial rates of pHi recovery were calculated from data collected for the first 60 seconds after removing the NH4Cl load. Values are mean ± S.E.M. (n = 25–27).
Effect of STa on pHi recovery kinetics
Since T84 cells express NHE1, NHE2 and NHE4, but not NHE3 forms [21,33], we assayed which of these forms was involved in STa effect on dpHi/dt. The dpHi/dt values in the presence of STa or HOE-694 were lower (65 ± 7 or 62 ± 6%, respectively) when compared with cells in the absence of these molecules (Table 1). Coincubation of cells with STa + HOE-694 resulted in higher reduction (90 ± 6%) in the dpHi/dt compared with the reduction seen in cells treated with STa or HOE-694 alone.
Effect of STa on ßi and J H +
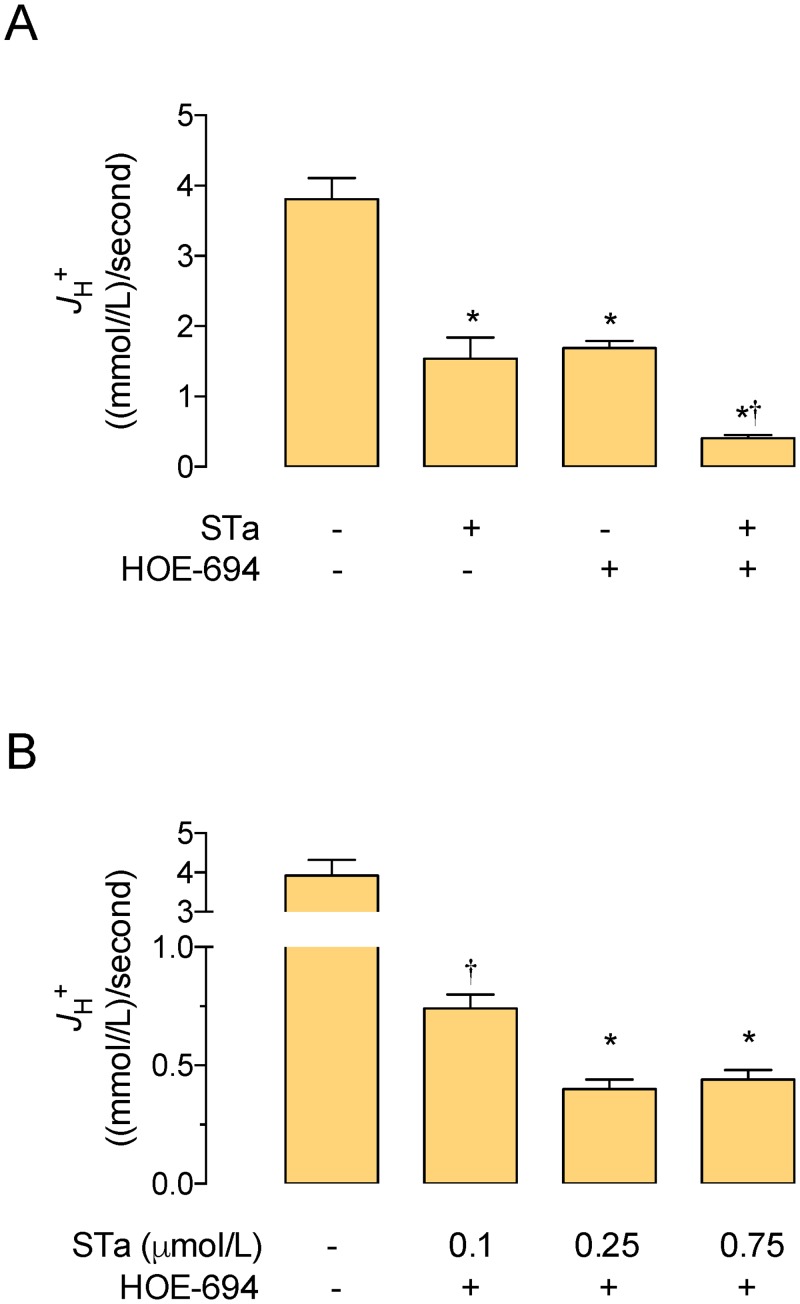
The ßi value detected in T84 cells in the absence of STa (31.1 ± 2.5 (mmol/L)/ intracellular pH units) was similar to that previously reported for this cell type under the same culture and measurement conditions (~31 (mmol/L)/intracellular pH units) [21]. Change in ßi value was not significantly altered by 0.25 μmol/L STa in a range of 1.6 pHi units in T84 cells. Parallel assays show that cells treated with STa exhibit decreased J H + (60 ± 7%) compared with cells in the absence of this toxin (Fig 2A). Since maximal inhibitory effect on this parameter was achieved with 0.25 μmol/L STa in the presence of 25 μmol/L HOE-694 (Fig 2B), this concentration was used in all subsequent experiments. HOE-694 caused a decrease in J H + (56 ± 7%) that was similar (P>0.05) to that in cells in the presence of STa. Coincubation of cells with STa + HOE-694 resulted in a decrease in J H + (89 ± 6%) that was higher compared with the effect seen in cells treated with STa or HOE-694 alone.
Fig 2. Effect of STa on J H+.
The overall transmembrane H+ flux rates (J H+) were calculated from initial rates of pHi recovery and the intrinsic buffer capacity (βi) values (see Methods). A, T84 cells were exposed to culture medium without (–, Control) or with (+) 0.25 μmol/L heat-stable (STa) enterotoxin, 25 μmol/L HOE-694, or both (see Methods). B, T84 cells were exposed to increasing concentrations of STa in the presence of 25 μmol/L HOE-694 as in A. In A, *P<0.05 versus Control, †P<0.05 versus STa or HOE-694. In B, *P<0.05 versus Control, †P<0.05 versus other values in STa + HOE-694. Values are mean ± S.E.M. (n = 25–27).
NHE1, NHE2 and NHE4 protein abundance
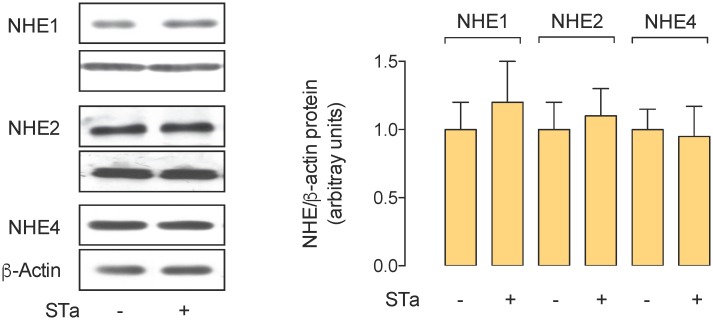
To address whether STa–associated decrease in J H + was due to lower protein abundance of NHE4, or whether this toxin alters NHE1 or NHE2 protein abundance, the protein level of these membrane transporters was assayed. The results show that incubation of T84 cells with STa did not alter NHE1, NHE2 or NHE4 protein abundance (Fig 3).
Fig 3. Effect of STa on NHE4 protein abundance.
Western blot for NHE4 protein abundance in whole extracts of T84 cells exposed for 30 minutes in the absence (Control) or presence (STa) of 0.25 μmol/L heat-stable (STa) enterotoxin. Lower panel: NHE4/β-actin ratio densitometries normalized to 1 in Control. β-Actin is internal reference. Values are mean ± S.E.M. (n = 15).
cGMP and cAMP involvement on NHE4–mediated pHi recovery kinetics
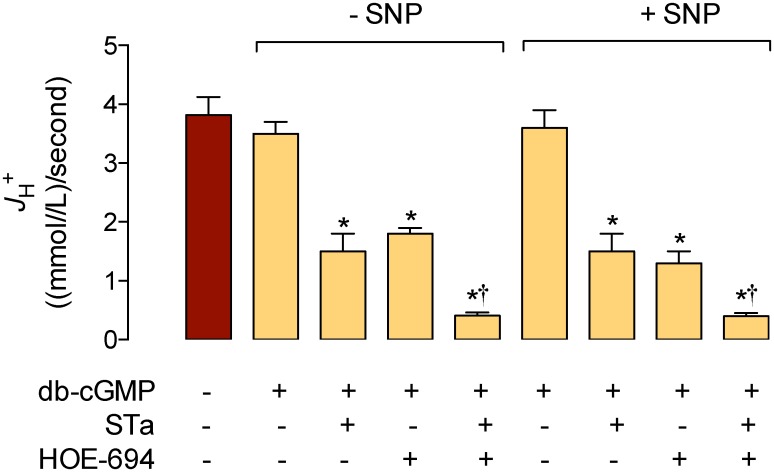
STa is shown to increase the cGMP level in T84 cells [34]; however, the role of cGMP as modulator of NHE4 activity is not addressed [17]. Thus, we next investigated whether STa effect on NHE4–dependent dpHi/dt in this cell type was modulated by direct administration of exogenous cGMP. The results show that dpHi/dt and basal pHi (Table 1), and J H + (Fig 4) were unaltered in T84 cells exposed to db-cGMP in the absence of HOE-694 or STa. However, the reduction in dpHi/dt and J H + seen in response to STa, HOE-694, or STa + HOE-694 was unaltered by db-cGMP. When cells were incubated with SNP (a spontaneous NO donor) [27] the results were similar to those in the presence of db-cGMP (Table 1, Fig 4). Parallel results show that cGMP intracellular level was increased by STa and SNP, confirming previous reports in T84 cells [35] and rat distal colon crypts [36], but it was unaltered by HOE-694 (not shown).
Fig 4. Involvement of cGMP on STa modulation of J H+.
T84 cells were exposed for 30 minutes in the absence (–SNP) or presence (+ SNP) of 500 μmol/L sodium nitroprusside (SNP). The overall transmembrane H+ flux rates (J H+) were calculated from initial rates of pHi recovery and the intrinsic buffer capacity (βi) values (see Methods). Cells were exposed to culture medium without (–, Control, red bar) or with (+) 100 μmol/L dibutyryl cyclic GMP (db-cGMP), 0.25 μmol/L STa, and/or 25 μmol/L HOE-694 (see Methods). *P<0.05 versus Control or corresponding db-CGMP, †P<0.05 versus corresponding STa or HOE-694 in the presence of db-cGMP. Values are mean ± S.E.M. (n = 25–27).
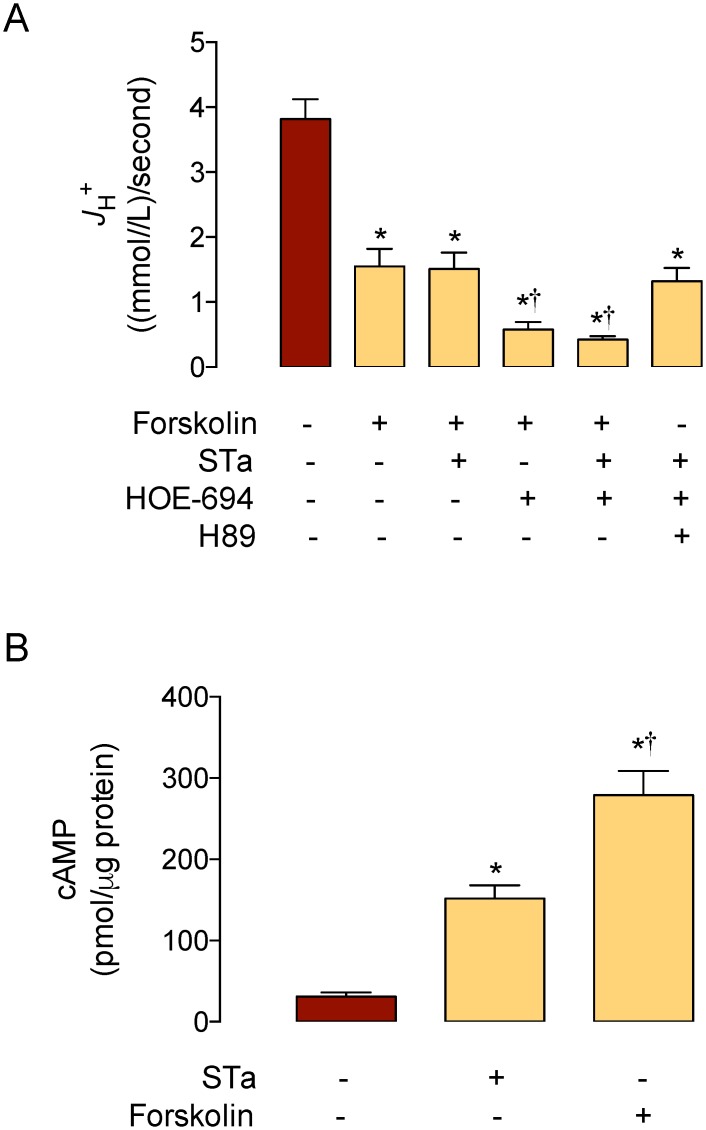
We next assayed whether cAMP was involved in the response of T84 cells to STa–reduced NHE4–mediated pHi recovery kinetics. Cells incubated with forskolin (adenylyl cyclase activator) [29] in the absence of HOE-694 resulted in a decrease in dpHi/dt (Table 1) and J H + (Fig 5A) that was of a similar magnitude to the decrease seen in cells incubated with STa in the absence or presence of this activator. However, in the presence of HOE-694 or STa + HOE-694, forskolin caused a reduction in these parameters that was similar to that seen in cells coincubated with STa + HOE-694 in the absence of this activator. Parallel results show that intracellular level of cAMP increased by STa (4.9 ± 0.5 fold) and forskolin (8.9 ± 1.5 fold) (Fig 5B). Additionally, preincubation of cells with H89 (inhibitor of PKA) [28] reversed the decrease in dpHi/dt and J H + caused by STa + HOE-694 to values that are comparable to STa or HOE-694 alone.
Fig 5. Involvement of cAMP and PKA on STa modulation of J H+.
A, The overall transmembrane H+ flux rates (J H+) were calculated from initial rates of pHi recovery and the intrinsic buffer capacity (βi) values (see Methods). Cells were exposed to culture medium without (–, Control, red bar) or with (+) 10 μmol/L forskolin, 0.25 μmol/L heat-stable (STa) enterotoxin, 25 μmol/L HOE-694, and/or 100 nmol/L H89 (see Methods). B, cAMP levels in cells in the absence (Control) or presence of STa or forskolin as in A. In A, *P<0.05 versus Control, †P<0.05 versus STa, HOE-694, or STa + HOE-694. In B, *P<0.05 versus Control, †P<0.05 versus STa. Values are mean ± S.E.M. (n = 25–27).
Discussion
This study shows that the enterotoxigenic Escherichia coli (ETEC) released heat-stable (STa) enterotoxin decreases the pHi recovery kinetics in the human colocarcinoma T84 cell line. This phenomenon results from a lower activity of NHE4 without altering its protein expression. STa effect depends on the level of cAMP, but not cGMP, and PKA activation. These findings represent a novel mechanism of pHi homeostasis by STa that could have consequences in the physiology of gastrointestinal cells leading to human diarrhoea.
STa modulation of NHEs activity
STa is an enterotoxin that causes gastrointestinal electrolyte imbalance characterized by a higher Cl-release to the gastrointestinal lumen, a phenomenon that ends in diarrhoea in humans [1,3–5]. One of the potential mechanisms for these adverse effects of STa is a mucosal alkalization due to lower activity of plasma membrane mechanisms involved in maintaining transmembrane distribution of H+, including NHEs activity [10,11]. Our results show that STa caused a decrease in NHEs activity resulting in lower H+ efflux (i.e., J H +). This phenomenon may be responsible for the observed reduction in the capacity to restore the pHi recovery kinetics after an acid pulse. This possibility is supported by the findings showing that STa caused a similar reduction in dpHi/dt and J H + (reduction in dpHi/dt / reduction in J H + = 1.1), thus, making possible that alterations in the pHi recovery rate caused by STa was due to reduced H+ efflux kinetics. In addition, since the intrinsic buffering capacity (ßi) values were unaltered by STa (ßi with STa/ßi without STa = 1), it is unlikely that these alterations were the result of an altered ßi in T84 cells. Indeed, in cells incubated with STa the pHi value was not significantly altered (pHi with STa/ pHi without STa = 0.996) compared with cells in the absence of this toxin.
Interestingly, it was initially shown [31] that T84 cells express mainly NHEs (NHE1, NHE2 and NHE4) [21], in a minor grade Cl-/HCO3 - exchangers and Na+/HCO3 - cotransporters, but not other classical mechanisms of H+ export such as the vacuolar H+-ATPases [37] or H+/K+-ATPases [38]. Out of these membrane transport systems, NHEs play a major role in the removal of intracellular H+ in most cell types maintaining stable pHi and extracellular pH values [14–17,20,37,38].
NHE4 is an isoform of the NHEs family of membrane exchangers whose function results in the modulation of pHi in mammalian cells [14,16,17]. This membrane Na+/H+ exchanger isoform is expressed in the human gastrointestinal tract, and is co-expressed with NHE1 and NHE2, but not NHE3, in T84 cells [21,33], as confirmed in this study. Interestingly, cells exposed to HOE-694 show lower dpHi/dt and J H + most likely via a mechanism involving lower activity of NHE1 and NHE2 isoforms, since the concentration of this inhibitor used in the present study (25 μmol/L) preferentially inhibits these isoforms, but not NHE4 [21,26]. Indeed, cells in the presence of HOE-694 show partial recovery of the pHi value suggesting that not all the pHi recovery is mediated by NHE1 and NHE2, but other mechanism(s) is plausible in this cell type.
Since STa in the presence of HOE-694, i.e., where NHE1 and NHE2 were not functional, almost abolished the dpHi/dt and J H + (both reduced by ~90%), it is likely that NHE4 isoform was inhibited by this enterotoxin in T84 cells. This possibility is supported when we consider that the concentration of STa used in our study is close to the STa half-maximal stimulatory concentration for cGMP accumulation reported in T84 cells [25]. Additionally, the possibility that STa reduces the dpHi/dt and J H + via a mechanism including lower expression of NHE4, or NHE1 or NHE2, is unlikely since the protein abundance for none of these isoforms were altered by the toxin. Thus, STa–reduced H+ efflux seems to be due to a lower activity rather than expression of NHE4 in this cell type. STa effect in the presence of HOE-694 leads a remaining fraction of pHi recovery that accounted for 10% of the total recovery after an acid pulse. This finding could results from other mechanisms than inhibition of NHE1, 2 or 4, such as activity of Cl-/HCO3 - exchangers and/or Na+/HCO3 - cotransporters expressed in T84 cells [31]. Indeed, STa was shown to increase HCO3 - secretion via a higher Na+/HCO3 - activity in duodenal CFRT–/–mice [39]. However, our pHi recovery assays were performed in the absence of extracellular HCO3 - in this cell type making the latter unlikely.
Involvement of cAMP on STa effect
It has been shown that STa increases Cl-secretion in a cAMP–and cGMP–dependent manner via CFTR channels in rat jejunum [9]. Initial reports show that STa–increased cGMP, but unaltered cAMP level in rabbit distal ileum mucosa [40] or reduced cAMP level in mice intestine [41]. Our results show that exposure of T84 cells to STa results in increased cGMP and cAMP levels. Since these nucleotides decrease NHEs activity [12,13], STa–increased levels may have functional consequences on pHi recovery in T84 cells.
Since incubation of cells with exogenous cGMP (db-cGMP) did not alter basal dpHi/dt and J H + in our assays it is likely that this cyclic nucleotide is not involved in the modulation of NHEs activity in T84 cells. Furthermore, the inhibitory effect of STa on dpHi/dt and J H + in the presence of HOE-694 was unaltered by db-cGMP, suggesting that NHE4 inhibition by STa was independent of cGMP. This is supported by the findings showing that dpHi/dt and J H + inhibition by STa or HOE-694 alone was unaltered when cells were coincubated with these molecules and db-cGMP. Additionally, exposure of cells to exogenous NO delivered by SNP, a spontaneous NO donor [27], does not change STa effect in the absence or presence of HOE-694. Since SNP did not alter the reduction in the dpHi/dt and J H + caused by HOE-694 itself, NO in this cell type may not alter this inhibitors’ effectiveness on NHE1 and NHE2.
It was early shown that forskolin, a potent activator of adenylyl cyclase, has a profound effect in T84 transmonolayer net water flux (J W) [29], suggesting that cAMP could be involved in this phenomenon. Unfortunately, the cAMP level was not determined in the latter study. Additionally, incubation of T84 cells with secretagogues whose actions are mediated by cAMP ends with Cl-secretion from this cell type [35,42–44]. However, it is paradoxical that even when the level of cAMP was found unaltered in T84 cells in response to STa, this toxin effect on Cl-secretion closely resembles a cAMP–mediated mechanism in this cell type [35]. Our findings show that cAMP level is increased in T84 cells treated with STa or with forskolin. Since the effect of forskolin alone was to diminish the dpHi/dt and J H + in a same magnitude as STa alone or STa + forskolin, it is likely that a higher cAMP level could be involved in downregulation of NHE4 activity in this cell type. Parallel results suggest that NHE1 and NHE2 may not be under modulation by STa–or forskolin–mediated cAMP increase since the inhibition caused by HOE-694 of dpHi/dt and J H + by itself or in the presence of STa was unaltered by forskolin. Interestingly, since H89, a PKA inhibitor, resulted in restoration of the reduced dpHi/dt and J H + seen in the presence of STa + HOE-694 + forskolin to values that are comparable to those in the presence of these molecules per separate, it is likely that PKA may mediate STa inhibition of NHE4 in T84 cells.
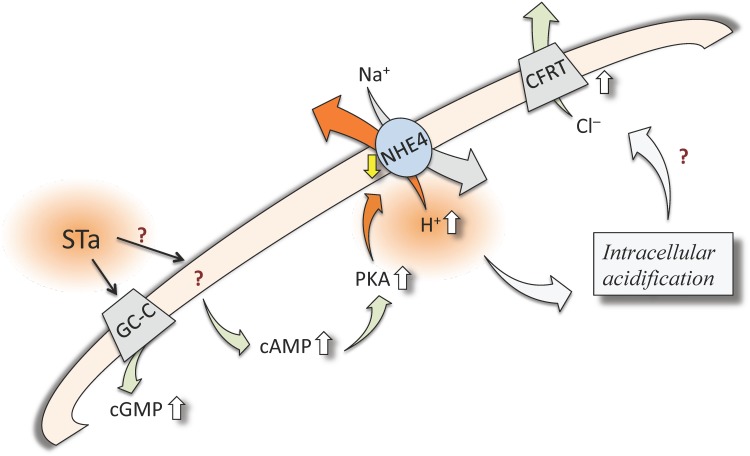
In conclusion, the enterotoxigenic Escherichia coli released STa enterotoxin has a deleterious effect on the normal physiology of T84 cells in vitro. In terms of its association with human diarrhoea this enterotoxin was found to increase not only cGMP levels, but also the cAMP level, perhaps leading to PKA activation in this cell type. It is proposed that STa reduces the capacity of T84 cells to recover the pHi after an acid pulse via a mechanism that includes reduced activity of NHE4, but not NHE1 or NHE2, in this cell type. These findings constitute a novel mechanism of pHi homeostasis by STa in this cell type, and perhaps in the gastrointestinal epithelium, resulting in a deficient recovery rate and H+ efflux after metabolic alterations associated with intracellular acidification. These findings complement the reduced transepithelial electrical resistance caused by STa in T84 cells, indicative of an intestinal barrier dysfunction in addition to STa–induced water secretion [45]. Considering that T84 cells respond with increased Cl-release to STa via cGMP–and cAMP–dependent mechanisms, a role of NHE4 is this phenomenon is proposed. All together the alterations caused by STa in a functional sequence (i.e., STa / increased cAMP / increased PKA activity / decreased NHE4 activity / increased intracellular acidification) (Fig 6) could have consequences in the physiology of gastrointestinal cells promoting human diarrhoea.
Fig 6. Potential involvement of cAMP and PKA on STa modulation of J H+.
In T84 cells the enterotoxigenic Escherichia coli (ETEC) released heat-stable enterotoxin (STa) activates guanylyl cyclase-C (GC-C) receptors to generate (green arrow) cyclic GMP (cGMP) increasing (⇧) its intracellular level. STa also increases cyclic AMP (cAMP) level via a mechanism that is not well defined in this cell type (?). Increase in cAMP activates protein kinase A (PKA), which could be responsible of a reduced (⇩) activity of the Na+/H+ exchanger isoform 4 (NHE4). The resulting intracellular accumulation of H+ leads to intracellular acidification, a phenomenon that, via undefined mechanism, could be responsible for the increase in chloride (Cl-) secretion via the cystic fibrosis transmembrane conductance regulator channels (CFRT) reported in this cell type and human diarrhoea.
Acknowledgments
Authors thank research staff at the Cellular Physiology Laboratory of the Biomedical Department, Faculty of Health Sciences, Universidad de Antofagasta, and from the Cellular and Molecular Physiology Laboratory (CMPL) from Pontificia Universidad Católica de Chile.
Data Availability
All relevant data are within the paper.
Funding Statement
Semillero Dirección de Investigación Universidad de Antofagasta (5309, 5313), Chile, http://www.uantof.cl. Fundaçao de Amparo à Pesquisa do Estado de São Paulo – FAPESP, Brazil, http://www.fapesp.br. Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT 1150377, 1150344, 3140516, 11150083), Chile, http://www.conicyt.cl/fondecyt/.
References
- 1. Qadri F, Svennerholm AM, Faruque AS, Sack RB. Enterotoxigenic Escherichia coli in developing countries: epidemiology, microbiology, clinical features, treatment, and prevention. Clin Microbiol Rev. 2005;18: 465–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gross R. Escherichia coli diarrhoea. J Infect. 1983;7: 177–192. [DOI] [PubMed] [Google Scholar]
- 3. Cravioto A, Reyes R, Ortega R, Fernandez G, Hernandez R, Lopez D. Prospective study of diarrhoeal disease in a cohort of rural Mexican children: incidence and isolated pathogens during the first two years of life. Epidemiol Infect. 1988;101: 123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Abu-Elyazeed R, Wierzba A, Mourad L, Peruski B, Kay BA, Rao M, et al. Epidemiology of enterotoxigenic Escherichia coli diarrhea in a pediatric cohort in a periurban area of lower Egypt. J Infect Dis. 1999;179: 382–389. [DOI] [PubMed] [Google Scholar]
- 5. Moon C, Zhang W, Sundaram N, Yarlagadda S, Reddy VS, Arora K, et al. Drug-induced secretory diarrhea: A role for CFTR. Pharmacol Res. 2015;102: 107–112. 10.1016/j.phrs.2015.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Forte LR, Krause WJ, Freeman RH. Escherichia coli enterotoxin receptors: localization in opossum kidney, intestine, and testis. Am J Physiol. 1989;257: F874–F881. [DOI] [PubMed] [Google Scholar]
- 7. Schulz S, Green CK, Yuen PS, Garbers DL. Guanylyl cyclase is a heat-stable enterotoxin receptor. Cell 1990;63: 941–948. [DOI] [PubMed] [Google Scholar]
- 8. Carrithers SL. Diarrhea or colorectal cancer: can bacterial toxins serve as a treatment for colon cancer?. Proc Natl Acad Sci USA. 2003;100: 3018–3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Golin-Bisello F, Bradbury N, Ameen N. STa and cGMP stimulate CFTR translocation to the surface of villus enterocytes in rat jejunum and is regulated by protein kinase G. Am J Physiol. 2005;289: C708–C716. [DOI] [PubMed] [Google Scholar]
- 10. Fawcus K, Gorton VJ, Lucas ML, McEwan GT. Stimulation of three distinct guanylate cyclases induces mucosal surface alkalinisation in rat small intestine in vitro. Comp Biochem Physiol A Physiol. 1997;118: 291–295. [DOI] [PubMed] [Google Scholar]
- 11. Lucas ML. A reconsideration of the evidence for Escherichia coli STa (heat stable) enterotoxin-driven fluid secretion: a new view of STa action and a new paradigm for fluid absorption. J Applied Microbiol. 2001;90: 7–26. [DOI] [PubMed] [Google Scholar]
- 12. Bachmann O, Juric M, Seidler U, Manns M, Yu H. Basolateral ion transporters involved in colonic epithelial electrolyte absorption, anion secretion and cellular homeostasis. Acta Physiol. 2011;201: 33–46. [DOI] [PubMed] [Google Scholar]
- 13. Arena E, Longo W, Roberts K, Geibel P, Nateqi J, Brandstetter M, et al. Functional role of NHE4 as a pH regulator in rat and human colonic crypts. Am J Physiol. 2012;302: C412–C418. [DOI] [PubMed] [Google Scholar]
- 14. Slepkov ER, Rainey JK, Sykes BD, Fliegel L. Structural and functional analysis of the Na+/H+ exchanger. Biochem J. 2007;401: 623–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Orlowski J, Grinstein S. Na+/H+ exchangers. Compr Physiol.2011;1: 2083–2100. 10.1002/cphy.c110020 [DOI] [PubMed] [Google Scholar]
- 16. Provost JJ, Wallert MA. Inside out: targeting NHE1 as an intracellular and extracellular regulator of cancer progression. Chem Biol Drug Des. 2013;81: 85–101. 10.1111/cbdd.12035 [DOI] [PubMed] [Google Scholar]
- 17. Fuster DG, Alexander RT. Traditional and emerging roles for the SLC9 Na+/H+ exchangers. Pflugers Arch. 2014;466: 61–76. 10.1007/s00424-013-1408-8 [DOI] [PubMed] [Google Scholar]
- 18. Pizzonia JH, Biemesderfer D, Abu-Alfa AK, Wu MS, Exner M, Isenring P, et al. Immunochemical characterization of Na+/H+ exchanger isoform NHE4. Am J Physiol. 2013;275: F510–F517. [DOI] [PubMed] [Google Scholar]
- 19. Gawenis LR, Greeb JM, Prasad V, Grisham C, Sanford LP, Doetschman T, et al. Impaired gastric acid secretion in mice with a targeted disruption of the NHE4 Na+/H+ exchanger. J Biol Chem. 2005;280: 12781–12789. [DOI] [PubMed] [Google Scholar]
- 20. Rossmann H, Sonnentag T, Heinzmann A, Seidler B, Bachmann O, Vieillard-Baron D, et al. Differential expression and regulation of Na+/H+ exchanger isoforms in rabbit parietal and mucous cells. Am J Physiol. 2001;281: G447–G458. [DOI] [PubMed] [Google Scholar]
- 21. Beltran AR, Ramirez MA, Carraro-Lacroix LR, Hiraki Y, Reboucas NA, Malnic G. NHE1, NHE2, and NHE4 contribute to regulation of cell pH in T84 colon cancer cells. Pflugers Arch. 2008;455: 799–810. [DOI] [PubMed] [Google Scholar]
- 22. Selvaraj NG, Prasad R, Goldstein JL, Rao MC. Evidence for the presence of cGMP-dependent protein kinase-II in human distal colon and in T84, the colonic cell line. Biochim Biophys Acta 2000;1484: 32–43. [DOI] [PubMed] [Google Scholar]
- 23. Fernandez R, Malnic G. H+ ATPase and Cl interaction in regulation of MDCK cell. J Membr Biol. 1998;163: 137–145. [DOI] [PubMed] [Google Scholar]
- 24. Aravena C, Beltran AR, Cornejo M, Torres V, Diaz ES, Guzmán-Gutiérrez, et al. Potential role of sodium-proton exchangers in the low concentration arsenic trioxide-increased intracellular pH and cell proliferation. Plos One 2012;7: e51451 10.1371/journal.pone.0051451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bijvelds MJ, Loos M, Bronsveld I, Hellemans A, Bongartz JP, Ver Donck L, et al. Inhibition of heat-stable toxin-induced intestinal salt and water secretion by a novel class of guanylyl cyclase C inhibitors. J Infect Dis. 2015;212: 1806–1815. 10.1093/infdis/jiv300 [DOI] [PubMed] [Google Scholar]
- 26. Ikuma M, Kashgarian M, Binder HJ, Rajendran VM. Differential regulation of NHE isoforms by sodium depletion in proximal and distal segments of rat colon. Am J Physiol. 1999;276: G539–G549. [DOI] [PubMed] [Google Scholar]
- 27. Pardo F, Silva L, Sáez T, Salsoso R, Gutiérrez J, Sanhueza C, et al. Human supraphysiological gestational weight gain and fetoplacental vascular dysfunction. Int J Obes (Lond). 2015;39: 1264–1273. [DOI] [PubMed] [Google Scholar]
- 28. Ao M, Sarathy J, Domingue J, Alrefai WA, Rao MC. Chenodeoxycholic acid stimulates Cl- secretion via cAMP signaling and increases cystic fibrosis transmembrane conductance regulator phosphorylation in T84 cells. Am J Physiol. 2013;305: C447–C456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Toriano R, Kierbel A, Ramírez MA, Malnic G, Parisi M. Spontaneous water secretion in T84 cells: effects of STa enterotoxin, bumetanide, VIP, forskolin, and A-23187. Am J Physiol. 2001;281: G816–G822. [DOI] [PubMed] [Google Scholar]
- 30. Salsoso R, Guzmán-Gutiérrez E, Sáez T, Bugueño K, Ramírez MA, Farías M, et al. Insulin restores L-arginine transport requiring adenosine receptors activation in umbilical vein endothelium from late-onset preeclampsia. Placenta 2015;36: 287–296. 10.1016/j.placenta.2014.12.007 [DOI] [PubMed] [Google Scholar]
- 31. Ramírez MA, Toriano R, Parisi M, Malnic G. Control of cell pH in the T84 colon cell line. J Membr Biol. 2000;177: 149–157. [DOI] [PubMed] [Google Scholar]
- 32. Musa-Aziz R, Oliveira-Souza M, Mello-Aires M. Signaling pathways in the biphasic effect of ANG II on Na+/H+ exchanger in T84 cells. J Membr Biol. 2005;205: 49–60. [DOI] [PubMed] [Google Scholar]
- 33. Toriano R, Ozu M, Politi MT, Dorr RA, Curto MA, Capurro C. Uroguanylin regulates net fluid secretion via the NHE2 isoform of the Na+/H+ exchanger in an intestinal cellular model. Cell Physiol Biochem. 2011;28: 733–742. 10.1159/000335767 [DOI] [PubMed] [Google Scholar]
- 34. Kots AY, Choi BK, Estrella-Jimenez ME, Warren CA, Gilbertson SR, Guerrant RL, et al. Pyridopyrimidine derivatives as inhibitors of cyclic nucleotide synthesis: Application for treatment of diarrhea. Proc Natl Acad Sci USA. 2008;105: 8440–8445. 10.1073/pnas.0803096105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huott PA, Liu W, McRoberts JA, Giannella RA, Dharmsathaphorn K. Mechanism of action of Escherichia coli heat stable enterotoxin in a human colonic cell line. J Clin Invest. 1988;82: 514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Morel E, Dublineau I, Griffiths NM. Effect of radiation on cAMP, cGMP and Ca2+i pathways and their interactions in rat distal colon. Radiat Res. 2003;160: 263–272. [DOI] [PubMed] [Google Scholar]
- 37. Casey JR, Grinstein S, Orlowski J. Sensors and regulators of intracellular pH. Nat Rev Mol Cell Biol. 2010;11: 50–61. 10.1038/nrm2820 [DOI] [PubMed] [Google Scholar]
- 38. Gillies RJ, Raghunand N, Garcia-Martin ML, Gatenby RA. pH imaging. A review of pH measurement methods and applications in cancers. IEEE Eng Med Biol Mag. 2004;23: 57–64. [DOI] [PubMed] [Google Scholar]
- 39. Sellers ZM, Childs D, Chow JYC, Smith AJ, Hogan DL, Isenberg JI, et al. Heat-stable enterotoxin of Escherichia coli stimulate a non-CFTR-mediated duodenal bicarbonate secretory pathway. Am J Physiol. 2005;288: G654–G663. [DOI] [PubMed] [Google Scholar]
- 40. Field M, Graf LH, Laird WJ, Smith PL. Heat-stable enterotoxin of Escherichia coli: in vitro effects on guanylate cyclase activity, cyclic GMP concentration, and ion transport in small intestine. Proc Natl Acad Sci USA.1978;75: 2800–2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Giannella RA, Drake KW. Effect of purified Escherichia coli heat-stable enterotoxin on intestinal cyclic nucleotide metabolism and fluid secretion. Infect Immun. 1979;24: 19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cartwright CA, McRoberts JA, Mandel KG, Dharmsathaphorn K. Synergistic action of cyclic adenosine monophosphate-and calcium-mediated chloride secretion in a colonic epithelial cell line. J Clin Invest. 1985;76: 1837–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wasserman SI, Barrett KE, Huott PA, Beuerlein G, Kagnoff MF, et al. Immune-related intestinal Cl-secretion. I. Effect of histamine on the T84 cell line. Am J Physiol. 1988;254: C53–C62. [DOI] [PubMed] [Google Scholar]
- 44. Nichols JM, Maiellaro I, Abi-Jaoude J, Curci S, Hofer AM. “Store-operated” cAMP signaling contributes to Ca2+-activated Cl- secretion in T84 colonic cells. Am J Physiol. 2015;309: G670–G679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nakashima R, Kamata Y, Nishikawa Y. Effects of Escherichia coli heat-stable enterotoxin and guanylin on the barrier integrity of intestinal epithelial T84 cells. Vet Immunol Immunopathol. 2013;152: 78–81. 10.1016/j.vetimm.2012.09.026 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.