Abstract
Definite clinical diagnosis of microvascular angina is not possible with the existing knowledge. Resting electrocardiogram may be normal, and exercise electrocardiogram may be unremarkable. Echocardiography usually does not show regional wall motion abnormalities. Transthoracic Doppler echocardiography can satisfactorily evaluate only left anterior descending coronary artery and that too in some patients. Radio-isotope imaging can detect only severe localized disease. Noninvasive diagnosis needs high index of suspicion. At present, definite diagnosis is based on documentation of normal epicardial coronaries, coronary flow reserve less than 2.5 on adenosine induced hyperemia, and absence of spasm of epicardial coronaries on acetylcholine provocation. Invasive evaluation is costly, needs sophisticated equipments and expertise. Therapeutic and prognostic implications of various parameters remains to be evaluated. At present invasive evaluation is recommended only for patients with intractable symptoms with unconfirmed diagnosis, requiring repeated hospitalization and evaluation with failure of empirical therapy.
Keywords: Angina, Coronary microcirculation, Ischemic heart disease
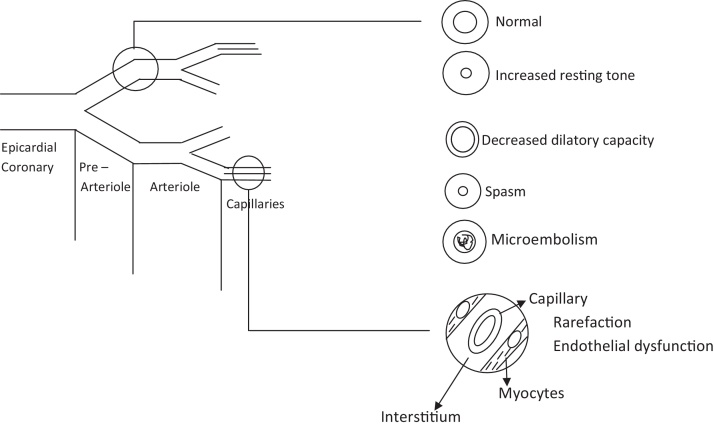
Significant number of patients of angina have coronary microvascular dysfunction alone or in combination with stenosis and/or spasm of epicardial coronary arteries. Several pathophysiology could be responsible. Common possibilities are shown in Fig. 1. Diagnosis of microvascular dysfunction is important because of therapeutic and prognostic implications.
Fig. 1.
Pathophysiology of microvascular angina.
1. Clinical profile
1.1. Chronic stable angina
Microvascular angina can present as chronic stable angina. With the available information, it is not possible to clinically differentiate isolated microvascular angina from angina due to isolated epicardial coronary artery disease.1, 2 It is still more difficult to suspect component of microvascular dysfunction in patients with established epicardial coronary artery disease.3 Persistence of symptoms after successful intervention/surgery can be a clue. Symptoms disproportionate to angiographic findings can be another clue. Absence of quick and/or sufficient relief with nitroglycerine or after cessation of effort has been suggested as another clue. However, patients with critical stenosis of epicardial coronaries especially those with triple vessel disease may also respond slowly. Comparative evaluation of documented cases of isolated epicardial coronary artery stenosis versus those with documented isolated microvascular dysfunction may give some clue to specific clinical features.
1.2. Rest angina/acute coronary syndrome
It is felt that coronary microvascular dysfunction can present as rest angina/acute coronary syndrome.1 Clinical differentiation from epicardial coronary artery disease is difficult. Some clues have been suggested in literature. Patients with microvascular coronary spasm have been shown to have symptoms more frequently at rest, more often at night and in early morning.5 Ong et al.6 observed that compared with patients of primary epicardial spasm, patients with microvascular spasm presented more frequently with ischemic ECG changes during noninvasive testing, exertional dyspnea, and intermittent rest angina in addition to effort angina. Kaski et al.7 observed that distinct from patients with Prinzmetal's angina, ST segment elevation is extremely rare in patients with microvascular angina. Kanatsuka et al.4 observed that nitroglycerine may not provide quick and/or sufficient chest pain control compared with Prinzmetal's angina. Persistence of effort angina despite full control of coronary spasm with vasodilatory therapy also suggests combination of variant and microvascular angina.8 Esophageal motility disorder is frequent comorbidity in patients with syndrome X.9 Differentiation may be challenging. Concomitant effort breathlessness and changes in resting and stress ECG support possibility of microvascular angina. Most of these studies have however, evaluated patients of syndrome X rather than cases of documented microvascular angina. Further, the observed differences are only relative. Definite clinical diagnosis of coronary microvascular dysfunction as a cause of rest angina/acute coronary syndrome is difficult. Evaluation of larger number of well documented cases is needed.
Cardiac syndrome Y (named because of possible causal role of neuropeptide Y) also presents as rest angina. Hemodynamically, it is characterized by an abnormally high microvascular resistance at rest but a normal vasodilatory response to direct vasodilators and pacing.10 Pain may worsen with nitroglycerine if there is significant fall in aortic perfusion pressure. Angiographically, it presents as coronary slow flow.11 It may be transient and ECG stress test response is usually normal.
1.3. Angina equivalents
Patients with “angina equivalent” symptoms have not been evaluated in any study. This area needs to be explored. Similarly patients with atypical chest pain have not been evaluated for microvascular dysfunction. Atypical presentation of angina is seen more frequently in women, diabetics, and elderly. These group of patients are also more likely to have coronary microvascular dysfunction. Some of the patients, who are initially diagnosed to have “nonischemic” discomfort develop classical ischemic syndrome in following few months. It is possible that some of these cases initially have microvascular dysfunction.
1.4. ST elevation myocardial infarction
It is thought that coronary microvascular dysfunction can cause ST elevation myocardial infarction.1 It is, however, possible that these patients had transient thrombosis followed by microembolization. This could result in myocardial infarction with normal coronary angiogram and microvascular dysfunction. Release of vasoconstrictor molecules and reperfusion injury could also contribute to coronary microvascular dysfunction. It is also possible that myocardial infarction occurred because of transient epicardial coronary artery spasm and subsequently detected coronary microvascular dysfunction was not the actual culprit. Actual role of microvascular dysfunction in ST elevation myocardial infarction is not clear.12
1.5. Stress-induced cardiomyopathy (Apical ballooning syndrome/Takatsubo cardiomyopathy)
Coronary microvascular dysfunction is considered to be involved in genesis of stress-induced cardiomyopathy.13 However, possibility of epicardial coronary spasm as the initial event cannot be excluded by subsequent demonstration of dysfunction and recovery in microvascular function in the affected area. Ischemia itself can also increase microvascular resistance.14 Further, such abnormality is not observed in all cases.15 This hypothesis also cannot explain almost exclusive occurrence in postmenopausal females, occurrence after acute physical stress in some patients, and almost exclusive localization to left ventricular apex in most cases. Prospective studies recruiting subjects with localized microvascular dysfunction and documenting subsequent occurrence of stress cardiomyopathy in the same areas on follow-up can prove etiological relation.
1.6. Chronic diastolic and/or systolic failure
Chronic, diffuse, persistent, and progressive coronary microvascular dysfunction can produce global diastolic and/or systolic dysfunction with normal coronary angiogram. Superficially these cases will resemble idiopathic dilated cardiomyopathy. Coronary microvascular dysfunction has been observed in cases of idiopathic dilated cardiomyopathy.16 Cause/effect relationship remains to be explored.
2. Noninvasive evaluation
2.1. Resting ECG
There is no literature on any specific diagnostic feature in resting electrocardiogram. Resting electrocardiogram may not show ST segment depression even during chest pain episodes.17 Ohba et al.5 have observed that patients with microvascular coronary spasm were likely to have minor borderline ischemic electrocardiogram findings at rest.
2.2. Echocardiography
It has been suggested that chest pain and ischemia like electrocardiographic changes without any wall motion abnormality on echocardiography can suggest microvascular angina.1 However, ischemia was not documented in all these cases. Adenosine and dobutamine can themselves also produce chest pain and ST segment changes. Adenosine has been shown to provoke diastolic dysfunction in cases of microvascular angina.18 Chronic coronary microembolism has also been shown to produce diastolic dysfunction in conscious dogs.19 However, impact of acute embolization in the territory of a coronary artery may not correctly reflect the effect of chronic microvascular dysfunction which is non-uniformly distributed across the microvascular bed20 rather than confined to territory of a large epicardial coronary artery. Microvascular dysfunction may not produce echocardiographically detectable dysfunction even during stress test despite the occurrence of angina, dyspnea, ECC changes, and perfusion abnormalities.21, 22 This has been explained to be due to normally functioning adjacent myocardium. Transient functional disturbance of coronary microvasculature may also escape detection on echocardiography done when the patient is asymptomatic. There can be significant inter-observer variability in interpretation of mild localized hypokinesia. Correlation of site, etiology, extent, and severity of coronary microvascular dysfunction with echocardiographic parameters is not known. Tissue Doppler imaging may detect early long axis diastolic dysfunction in patients with diffuse disease. Adenosine may induce such an abnormality not present on baseline examination.18 Strain rate imaging with speckle tracking may identify focal diastolic and/or systolic dysfunction. Diffuse involvement may produce diffuse diastolic and or systolic dysfunction.
2.3. Exercise ECG
No specific features have been identified in treadmill stress test response. Exercise ECG is usually unremarkable.1 Negative treadmill stress test dose not exclude possibility of intermittent coronary microvascular dysfunction. Patients with coronary microvascular spasm and ‘cardiac syndrome Y’ usually have normal treadmill stress test. It has been observed that angina may not occur even with positive exercise testing. Such a response can be related to impaired cortical processing of neural pain stimuli in patients with microvascular angina.23 It has been suggested that slow recovery or unsatisfactory response to sublingual nitrates may suggest component of microvascular dysfunction.24 Earlier appearance of ECG abnormalities and/or angina during an exercise test performed after sublingual nitrate is also considered to suggest coronary microvascular dysfunction. Comparison of treadmill stress test response of patients with isolated epicardial coronary stenosis with those with documented isolated microvascular dysfunction may give some clue.
2.4. Transthoracic Doppler echocardiography25
Flow in left anterior descending coronary artery can be evaluated by transthoracic Doppler echocardiography. Coronary flow velocity is measured at baseline and again after adenosine induced maximal hyperemia. Difference is taken as representative of coronary flow reserve (CFR). In absence of epicardial coronary artery disease, increased flow is taken as an indirect marker of dilatation of coronary microvasculature in response to adenosine. This method has some limitations. (i) Coronary angiography is needed to exclude epicardial coronary artery disease. (ii) Procedure requires high frequency transducer (7–12 MHz) and highly sensitive and dedicated equipment. (iii) It is significantly dependent on acoustic window. (iv) Usually only left anterior descending coronary artery is accessible for evaluation. Satisfactory assessment of other coronary arteries is either not possible or needs significant angle correction. Even in the left anterior descending coronary artery only mid to distal segment can be evaluated. Dysfunction in the region of branches proximal to the site of sample volume is not evaluated. (v) Only significant difference in coronary flow velocity can be appreciated. Mild coronary microvascular dysfunction is likely to be missed. (vi) The procedure evaluates overall increase in coronary flow. Failure of significant increase in flow is likely only, if most of the branches distal to the site of sample volume are significantly diseased. Microvascular dysfunction can be patchy and heterogeneous.20 Involvement of only some distal vessels may not affect overall impact of adenosine, resulting in false negative response. Compensatory vasodilatation of unaffected distal branches may also contribute to false negative result. (vii) This method evaluates response only to systemic vasodilators. It cannot evaluate resting tone of microcirculation. Further, evaluation of response to vasoconstrictors needs additional invasive procedure.
2.5. Other noninvasive investigations
2.5.1. Contrast stress echocardiography
This modality can detect coronary microvascular dysfunction if significant territory of a coronary artery or a major branch is significantly diseased. Patchy involvement is likely to be missed. Diffuse mild involvement across the myocardium may also fail to produce an area of localized relative diminution in contrast. Studies are needed to define the diagnostic role in cases of microvascular angina.
2.5.2. Stress thallium 201 uptake
It may not reveal localized area of reduced uptake in patients with diffuse mild involvement. Rosano et al.26 have observed relative overall reduction in thallium uptake and reduced washout in their patients with syndrome X compared to control. Overall sensitivity of the test for detecting coronary microvascular dysfunction is low.
2.5.3. 99 Tc-sestamibi imaging
It gives better image quality as compared to thallium. However, lower net extraction by myocardium and greater hepatic and gastrointestinal uptake interfere with proper interpretation of defects specially along the inferior wall.27 Perfusion defects are seen in less than 50% patients of syndrome X.28, 29 Diagnostic significance of positive scan is also not clear. Fragasso et al. performed dobutamine stress echocardiography on 22 patients of syndrome X with stress-induced perfusion defects documented by 99m-Tc-MIBI scintigraphy. Only 12 patients showed wall motion abnormality. In 5 out of 12, the location of dobutamine induced wall motion abnormality did not coincide with site of perfusion defect.30
2.5.4. Cardiovascular magnetic resonance
Adenosine induced reversible subendocardial perfusion defects in presence of normal coronary angiogram can be suggestive of coronary microvascular dysfunction. However, results of previous studies have been controversial.31 Further, the test has low specificity, as significant percentage of patients with subendocardial defects have been shown to have epicardial coronary spasm on intracoronary acetylcholine testing.32 Relatively low resolution, high cost, and scare availability also prevent routine use.
2.5.5. Nuclear magnetic resonance spectroscopy33
It can detect ischemia due to coronary microvascular dysfunction. However, high cost and scare availability prevent routine use. Further, it can explore only anterior myocardium.
Noninvasive tests cannot differentiate between epicardial and microvascular disease and lack sensitivity and specificity for the diagnosis of coronary vasomotor dysfunction.34
3. Invasive evaluation
3.1. Documentation of normal or near normal epicardial coronary arteries
For considering diagnosis of primary microvascular angina, it is necessary to document that epicardial coronary arteries are normal not only in structure but also in function. Normal coronary angiogram does not exclude diffuse atherosclerosis with abnormal epicardial resistance35 and atherosclerosis with positive remodeling. Intravascular ultrasound frequently reveals coronary atherosclerosis in patients with normal coronary angiograms.36 Such epicardial coronary arteries cannot be considered “normal” or “near normal” because even early atherosclerosis produces endothelial dysfunction.5 Endothelial dysfunction even without significant luminal obstruction also carries adverse prognosis. Even intravascular ultrasound cannot exclude such abnormalities in branches of coronary arteries because of practical difficulty in manipulating the transducer in branches. Calcification of epicardial coronaries may also occur without significant luminal obstruction. Such vessels are also likely to have decreased vasodilatation on demand. Normal coronary angiogram also does not exclude possibility of transient thrombosis or spasm of epicardial coronaries. Increased tendency for vasospasm at the site of nonobstructive plaque is well established2 and can contribute to angina in patients with “near normal” coronary angiogram.
Acetylcholine and adenosine respectively cause endothelium dependent and endothelium independent vasodilatation of epicardial coronaries. Normal response to these pharmacological agents does not exclude abnormal response of epicardial coronaries to other stimuli of day-to-day life, e.g. physical or emotional stress. Therefore, epicardial coronaries cannot be considered functionally normal only on the ground of normal response to pharmacological stress.
3.2. Documentation that a lesion in epicardial coronary artery is non flow limiting
Microvascular dysfunction frequently coexists with epicardial coronary artery disease. Microvascular dysfunction can be considered only of epicardial lesion is non-flow limiting.
At present, fractional flow reserve (FFR) is used to assess functional significance of a coronary lesion. FFR is measured as ratio of distal coronary pressure to aortic pressure recorded by pressure pullback recording during maximal hyperemia.37 Value of less than 0.75 is considered to suggest a flow limiting lesion. There may be discordance between FFR and angiographic findings.38 This is frequent with intermediate stenosis specially osteal lesions. Measurement of FFR is also affected by microcirculatory response to adenosine.39 Diseased microcirculation results in low trans-stenotic flow and misleadingly high FFR. On the other hand an exaggerated dilatory response of microcirculation may cause non flow limiting lesions to have low FFR due unusually high hyperemic flow rate. Following measures can be helpful in case of mismatch.
-
-
Hyperemic stenosis resistance index (HSRI)39
It is calculated as hyperemic pressure gradient across a lesion divided by average peak velocity at hyperemia. It requires a dual sensor (Pressure and Doppler Velocity) guide wire. Normal reference value is 0. Value of >0.8 is the threshold for prediction of ischemia. As HSRI incorporates both pressure and flow in the same index it has been shown to be superior to FFR in identifying flow limiting lesson.
-
-
Hemodynamic of a nearby reference vessel without focal epicardial disease39
Although microvascular dysfunction can be heterogeneously distributed across the coronary vascular bed, low value of CFR in a nearby reference vessel strongly suggests diffuse microvascular disease.
3.3. Evaluation of coronary microvascular function
3.3.1. Slow coronary flow
In absence of epicardial coronary artery disease, rate of runoff of contrast from coronary arteries gives rough impression of rate of flow through microcirculation. Corrected thrombolysis in myocardial infarction (TIMI) frame count is also used for quantitative assessment of coronary blood flow.40 Slow coronary flow is likely to be present only when there is microvascular dysfunction involving major portion of a coronary artery. It is, therefore, likely to represent diffuse involvement of microvasculature and may be positive only in advanced stage of disease.41 It can also occur due to diffuse spasm or increased basal coronary vascular tone. Slow flow confined to one coronary artery can also be due to transient thrombosis followed by thrombolysis and microembolization. Persistence of slow flow is likely to be due to some structural alteration.41
3.3.2. Myocardial blush
Myocardial opacification during coronary angiography allows an approximate visualization of microcirculation.42 However, it is subjective and has low sensitivity.
3.3.3. Detection of increased metabolic markers of ischemia in coronary sinus
Results of previous studies have been controversial even in patients with ischemic appearing ST segment depression during stress tests.43 It has been hypothesized that release of ischemic metabolites by the small ischemic foci may remain undetected because of their dilution in the larger flow from normally perfused myocardium. However, most of these studies have been performed on patients of syndrome X rather than cases documented to have coronary microvascular dysfunction. Studies of documented cases with analysis of blood in the respective cardiac vein can give correct impression. Invasive nature and technical difficulties preclude routine use.
3.3.4. Measurement of CFR
It is measured as ratio of coronary flow at maximal dilation to flow at baseline measured by a Doppler flow wire. Value of less than 2.5 is considered abnormal.37 It interrogates flow in epicardial coronary as well as microcirculation, Therefore, it cannot be used for evaluation of coronary microvascular function in presence of lesions in epicardial coronaries. It also has several other limitations including dependence on baseline blood flow, lower reproducibility, and technical problems.
3.3.5. Calculation of index of microcirculatory resistance (IMR)
A coronary pressure wire is placed in distal coronary artery to measure distal coronary pressure. Using a specific software, the same wire tip is used as distal thermistor, while the shaft of the wire serves as a proximal thermistor. Mean transit time of room temperature saline injected down the coronary artery is derived from coronary thermodilution curve. IMR is calculated as distal coronary pressure multiplied by the hyperemic mean transit time. Using this method in an animal model, Fearon et al.44 concluded that IMR increased after disruption of microcirculation with embolized microspheres and the change was independent of the status of epicardial coronary artery. There are some limitations in transpolating these conclusions to humans.
-
(1)
Application in patients with concomitant stenosis of epicardial coronaries
Epicardial stenosis created by an occluder does not truly represent pathology of coronary artery disease in humans. In humans the lesions can affect different or multiple sites, can have several morphologies and different severity. Further, epicardial coronaries of patients of syndrome X have been shown to have diffuse atherosclerosis and endothelial dysfunction.35
In presence of flow limiting epicardial stenosis, the distal coronary artery is likely to have less adenosine and, therefore less vasodilatation than the segment proximal to obstruction. This will affect the gradient, flow rate, transit time, and distal pressure. In the study of Fearon et al.44 also IMR was higher (p < 0.03) after epicardial stenosis.
In humans, significant epicardial coronary stenosis is usually associated with recruitment of collaterals. Significant collateral circulation is likely to affect the distal pressure, gradient, and transit time. It results in overestimation of IMR.45 Collaterals arising from proximal segment of the same vessel may significantly reduce pressure proximal to obstruction and resultant gradient.
Fearon et al.44 applied the occluder on left anterior descending coronary artery before any branch. It can be considered equivalent to osteal stenosis. In humans, stenosis may be after major proximal branches. Adenosine induced dilatation of these branches and resulting ‘steal phenomenon’ is likely to affect the gradient and calculation of IMR.
In view of above-mentioned limitations, it is clear that IMR may not be reliable in presence of significant stenosis of epicardial coronary artery. It remains a challenge to be solved.
-
(2)
Application in patients with coronary microvascular dysfunction
Fearon et al.44 used microsphere embolization for disrupting the microcirculation. This may correlate only with acute microembolization that follows thrombolytic therapy or coronary intervention. In humans there are several other chronic/intermittent, structural and/or functional alterations that produce coronary microvascular dysfunction.46
Microembolization produces non-homogeneous involvement of microcirculation. Some micro-vessels are likely to receive more emboli and others are likely to receive less or no emboli. This is expected to result in inhomogeneous distribution of adenosine in different vessels. Dilatation of nonobstructed vessels is likely to produce ‘steal phenomenon’ and affect distal pressure.
Calculation of IMR is based on the assumption that at peak hyperemia, the variability of vascular tone and hemodynamic will be eliminated and the minimum microvascular resistance will be achieved. This assumption does not consider inter-individual variability in the effect of adenosine. Failure to achieve maximum hyperemia may result in overestimation of IMR.45 Intracoronary injection of adenosine or nicorandil has been shown to be more effective.47
It is therefore, clear that results of microembolization in animal studies may not apply to coronary microvascular dysfunction in humans.
-
(3)
Other limitations in use of IMR
Distance the sensor is placed down a vessel is an important determinant of the mean transit time. Variability of IMR depending on the distance of the wire down the vessel needs evaluation.45 It is important because it may not be possible to keep the sensor in the ‘distal third’ of the vessel in each patient specially when the distal part is hypoplastic. When the distal one third of the vessel is small in caliber, the sensing wire may have some occluding effect.
Most of the studies have been performed on left anterior descending coronary artery which is a relatively straight vessel. Left circumflex and right coronary artery have angulations and large branches in middle one third. Studies are needed to find if calculation of IMR will be equally applicable to these vessels.
3.3.6. Coronary microvasculatory spasm provocation test
In patients with history suggestive of vasospastic angina, coronary vasospasm is precipitated by intracoronary injection of acetylcholine. Appearance of angina and ECG changes without spasm of epicardial coronary artery suggests coronary microvascular spasm. However, coronary microvascular spasm frequently coexists with spasm of epicardial coronaries.48 Diagnosis of coronary microvascular spasm is difficult in such cases. Further, absence of epicardial coronary spasm during pharmacological stress does not exclude possibility of vasospastic angina during stresses of day-to-day life, e.g. cold presser or mental stress. It is also important that coronary spasm may be occurring in vessel other than the artery being evaluated. Right coronary artery is most susceptible to vasospasm. Therefore, assessment of coronary microvascular function only in left anterior descending coronary may be insufficient. Pharmacological provocation of coronary spasm also has some risk of arrhythmias.
4. Other issues that need consideration
-
(i)
Most of the data have been developed from patients of syndrome X rather than from patients documented to have microvascular dysfunction.49 Now, we know that there are many causes for chest pain with normal coronary angiogram (Table 1). Future studies should enroll patients documented to have coronary microvascular dysfunction.
-
(ii)
None of the methods can evaluate microcirculatory dysfunction in the territory of the branches proximal to the site of interrogation. Microcirculatory dysfunction localized to the territory of the proximal branches is likely to be missed producing false negative results. It is important because microcirculatory dysfunction may be heterogeneous among different portions of coronary circulation.
-
(iii)
These methods do not evaluate resting tone of microcirculation. Increased resting tone can reduce flow through coronary microcirculation.
-
(iv)
These methods evaluate vasomotor response to acetylcholine and adenosine. Positive as well as negative result to these pharmacological agents may not truly represent response to events of real life resulting in false positive or false negative results.
-
(v)
These tests evaluate functional disturbance of microcirculation. Response in patients with structural defects of microcirculation may be different.
-
(vi)Normal range of values of various criteria for evaluating coronary microvascular function need to be defined in
- -
-
-Different coronary arteries and their branches.52
-
(vii)
Most of the studies have evaluated effect of various pharmacological agents on dilatory capacity of microcirculation. Some persons could have normal vasodilatation but abnormal vasoconstriction. Normal range of vasoconstrictory response to different stress (e.g. cold presser, mental stress) needs to be explored.
-
(viii)Data are also needed to find impact of various factors that may influence the normal range of the parameters of microcirculatory function e.g.
-
-Body mass index.
-
-Left ventricular mass index.
-
-Diameter of epicardial coronaries.
-
-Endothelium dependent and endothelium independent function of epicardial coronary arteries.
-
-Positive remodeling of epicardial coronaries.
-
-Coronary calcification
-
-Coronary risk factors53
-
-
-
(ix)
Inter-individual variability in response to different types of stress and different doses of same pharmacological agents need evaluation.54
-
(x)
Therapeutic and prognostic significance of results of various tests needs evaluation.
-
(xi)
Diagnosis in asymptomatic stage of disease needs evaluation.
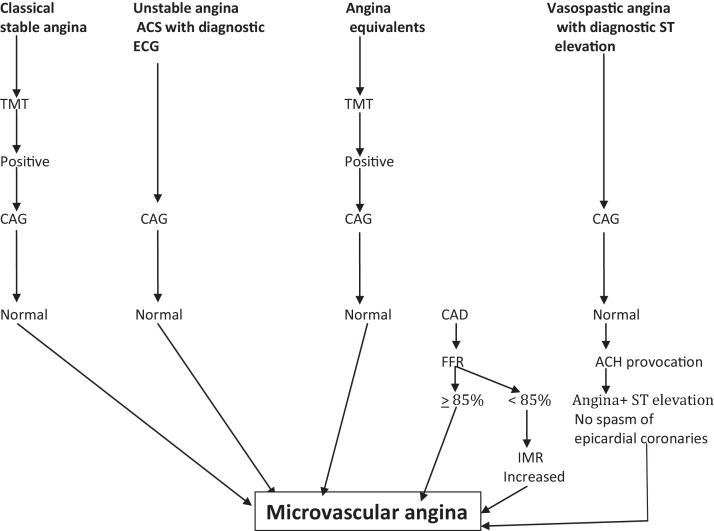
Because of varied etio-patho-physiology, different clinical presentations and scare availability of invasive evaluation, a simple algorhythmic approach is not possible. However, a simple algorithm for clinical working is presented in Fig. 2.
Table 1.
Causes of angina or angina like discomfort with normal coronary angiogram.
| 1. Coronary microvascular dysfunction | ||
| 2. Epicardial coronary artery dysfunction | ||
| (a) | Diffuse atherosclerosis | |
| (b) | Endothelial dysfunction | |
| (c) | Spasm | |
| (d) | Thrombosis followed by thrombolysis | |
| 3. Increased myocardial oxygen demand | ||
| (a) | Systemic hypertension, aortic valve disease. Left ventricular hypertrophy | |
| (b) | Pulmonary hypertension, pulmonary valve disease. Right ventricular hypertrophy | |
| 4. Myocardial diseases | ||
| (a) | Myocarditis | |
| (b) | Dilated cardiomyopathy | |
| (c) | Infiltrative disorders | |
| (d) | Myocyte disorders | |
| 5. Systemic disorders | ||
| (a) | Anemia | |
| (b) | Thyrotoxicosis | |
| 6. Non anginal discomforts | ||
| (a) | Exercise induced bronchospasm | |
| (b) | Esophageal motility disorders, hiatus hernia | |
| (c) | Abnormal pain perception | |
Fig. 2.
Diagnostic algorithm. Abbreviations: Ach, acetylcholine; ACS, acute coronary syndrome; CAD, coronary artery disease; CAG, coronary angiography; IMR, index of microcirculatory resistance; FFR, fractional flow reserve; TMT, treadmill stress test.
5. Conclusion
Microvascular dysfunction is more frequent than appreciated. It may be a primary pathology or may coexist with stenosis and/or spasm of epicardial coronary arteries. Diagnosis is likely to affect management and prognosis. Clinical differentiation from epicardial coronary artery disease is not possible at present. Noninvasive imaging with echocardiography and nuclear perfusion are not sufficiently sensitive. At present the diagnosis is based on combination of normal coronary angiogram, absence of epicardial coronary spasm during acetylcholine infusion and coronary flow reserve less than 2.5 on adenosine induced hyperemia. However, each component of diagnostic criteria has some limitations. Purely dichomatous interpretation of data derived from invasive evaluation should be avoided.39 Clinical profile and results of all investigations should be correlated. It is also important that coronary microvascular dysfunction can be intermittent and cannot be excluded dogmatically if symptoms or laboratory findings do not develop with any given challenge at a given time. However one should also be careful to avoid over diagnosis. There is need to develop a cheap, effective, safe, and widely available noninvasive test for detection of coronary microvascular dysfunction.
Conflicts of interest
The author has none to declare.
References
- 1.Lanza G.A., Crea F. Primary coronary microvascular dysfunction: clinical presentation, pathophysiology and management. Circulation. 2010;121:2317–2325. doi: 10.1161/CIRCULATIONAHA.109.900191. [DOI] [PubMed] [Google Scholar]
- 2.Kothawade K., Merz N.B. Microvascular coronary dysfunction in women: pathophysiology, diagnosis management. Curr Probl Cardiol. 2011;36:291–318. doi: 10.1016/j.cpcardiol.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yilmaz A., Sechtem U. Angina pectoris in patients with normal coronary angiograms: current pathophysiological concepts and therapeutic options. Heart. 2012;98:1020–1029. doi: 10.1136/heartjnl-2011-301352. [DOI] [PubMed] [Google Scholar]
- 4.Kanatsuka H., Eastham C.L., Marcus M.L. Effects of nitroglycerine on the coronary microcirculation in normal and ischemic myocardium. J Cardiovasc Pharmacol. 1992;19:755–763. [PubMed] [Google Scholar]
- 5.Ohba K., Sugiyama S., Sumida H. Microvascular coronary artery spasm presents distinctive clinical features with endothelial dysfunction as non obstructive coronary disease. J Am Heart Assoc. 2012;1:E0002485. doi: 10.1161/JAHA.112.002485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ong P., Athanasiadis A., Borgulya G. High prevalence of a pathological response to acetylcholine testing in patients with angina pectoris and unobstructed coronary arteries. The ACOVA study (Abnormal COronary VAsomation in patients with stable angina and unobstructed coronary arteries) J Am Coll Cardiol. 2012;59:655–662. doi: 10.1016/j.jacc.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 7.Kaski J.C., Aldama G., Cosin-Sales J. Cardiac syndrome X: diagnosis, pathogenesis and management. Am J Cardiovasc Drugs. 2004;4:179–194. doi: 10.2165/00129784-200404030-00005. [DOI] [PubMed] [Google Scholar]
- 8.Infusino F., Lanza G.A., Sestito A. Combination of variant and microvascular angina. Clin Cardiol. 2009;32:E40–E45. doi: 10.1002/clc.20502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cannon R.O., 3rd, Cattau E.L., Jr., Yakshe P.N. Coronary flow reserve, esophageal motility and chest pain in patients with angiographically normal coronary arteries. Am J Med. 1990;88:217–222. doi: 10.1016/0002-9343(90)90145-4. [DOI] [PubMed] [Google Scholar]
- 10.Fineschi M., Gori T. Coronary slow flow: description of a new ‘Cardiac Y’ syndrome. Int J Cardiol. 2009;137:308–310. doi: 10.1016/j.ijcard.2008.05.076. [DOI] [PubMed] [Google Scholar]
- 11.Fragasso G., Chierchia S.L., Arioli F. Coronary slow flow causing transient myocardial hypo perfusion in patients with cardiac syndrome X: long term clinical and functional prognosis. Int J Cardiol. 2009;137:137–144. doi: 10.1016/j.ijcard.2008.06.070. [DOI] [PubMed] [Google Scholar]
- 12.Lerman A., Holmes D.R., Herrmann J. Microcirculatory dysfunction in ST elevation myocardial infarction: cause, consequence or both? Eur Heart J. 2007;28:788–797. doi: 10.1093/eurheartj/ehl501. [DOI] [PubMed] [Google Scholar]
- 13.Galiuto L., De Caterina A.R., Porfidia A. Reversible coronary microvascular dysfunction: a common pathogenetic mechanism in apical ballooning or Taka–Tsubo syndrome. Eur Heart J. 2010;31:1319–1327. doi: 10.1093/eurheartj/ehq039. [DOI] [PubMed] [Google Scholar]
- 14.Marzili M., Sambuceti G., Fedele S. Coronary microcirculatory vasoconstriction during ischemia in patients with unstable angina. J Am Coll Cardiol. 2000;35:327–334. doi: 10.1016/s0735-1097(99)00554-9. [DOI] [PubMed] [Google Scholar]
- 15.Patel S.M., Lerman A., Lennon R.J. Impaired coronary microvascular reactivity in women with apical ballooning syndrome (Takastsubo stress cardiomyopathy) Eur Heart J Acute Cardiovasc Care. 2013;2:147–152. doi: 10.1177/2048872613475891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rigo F., Cherardia S., Galderisi M. The independent prognostic value of contractile and coronary flow reserve determined by dipyridamole stress echocardiography in patients with idiopathic dilated cardiomyopathy. Am J Cardiol. 2007;99:1154–1158. doi: 10.1016/j.amjcard.2006.11.049. [DOI] [PubMed] [Google Scholar]
- 17.Hurst T., Olson T., Olson L. Cardiac syndrome X and endothelial dysfunction: new concepts in prognosis and treatment. Am J Med. 2006;119:560–566. doi: 10.1016/j.amjmed.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 18.Vinereanu D., Fraser A.G., Robinson M., Lee A., Tweddel A. Adenosine provokes diastolic dysfunction in microvascular angina. Postgrad Med J. 2002;78:40–42. doi: 10.1136/pmj.78.915.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gill R.M., Jones B.D., Corbly A.K. Cardiac diastolic dysfunction in conscious dogs with heart failure induced by chronic coronary microembolization. Am J Physiol Heart Circ Physiol. 2006;291:H3154–H3158. doi: 10.1152/ajpheart.00052.2006. [DOI] [PubMed] [Google Scholar]
- 20.Marroquin O.C., Holubkov R. Heterogenesity of microvascular dysfunction in women with chest pain not attributable to coronary artery disease: implications for clinical practice. Am Heart J. 2003;145:628–635. doi: 10.1067/mhj.2003.95. [DOI] [PubMed] [Google Scholar]
- 21.Nihoyannopoulos P., Kaski J.C., Crake T. Absence of myocardial dysfunction during stress in patients with syndrome X. J Am Coll Cardiol. 1991;18:1463–1470. doi: 10.1016/0735-1097(91)90676-z. [DOI] [PubMed] [Google Scholar]
- 22.Panza J.A., Laurienzo J.M., Curiel R.V. Investigation of the mechanism of chest pain in patients with angiographically normal coronary arteries using transesophageal dobutamine stress echocardiography. J Am Coll Cardiol. 1997;29:293–301. doi: 10.1016/s0735-1097(96)00481-0. [DOI] [PubMed] [Google Scholar]
- 23.Di Franco A., Lanza G.A., Di Monaco A. Coronary microvascular function and cortical pain processing in patients with silent positive exercise testing and normal coronary arteries. Am J Cardiol. 2012;100:1705–1710. doi: 10.1016/j.amjcard.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 24.Lanza G.A., Manzoli A., Bia E. Acute effects of nitrates on exercise testing in patients with syndrome X. Circulation. 1994;90:2695–2700. doi: 10.1161/01.cir.90.6.2695. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe N. Echocardiographic evaluation of coronary blood flow. Approaches and clinical applications. In: Otto C.M., editor. The Practice of Clinical Echocardiography. Saunders; Philadelphia: 2007. pp. 393–402. [Google Scholar]
- 26.Rosano G.M., Peters N.S. Abnormal uptake and washout of thallium 201 in patients with syndrome X and normal appearing scans. Am J Cardiol. 1995;75:400–402. doi: 10.1016/s0002-9149(99)80565-7. [DOI] [PubMed] [Google Scholar]
- 27.Berman D.S., Hayes S.W., Hachamouitch R., Shaw L.J., Germano G. Nuclear cardiology. In: Fuster V., Walsh R.A., Harrington R.A., editors. Hurst's The Heart. McGraw Hill; New York: 2011. pp. 562–598. [Google Scholar]
- 28.Demir H., Kahraman G., Isgorer S., Tan Y.J., Kilic T., Berk F. Evaluation of post stress left ventricular dysfunction and its relationship with perfusion abnormalities using gated SPECT in patients with cardiac syndrome X. Nucl Med Commun. 2008;29:208–214. doi: 10.1097/MNM.0b013e3282f52c49. [DOI] [PubMed] [Google Scholar]
- 29.Langes K., Beuthien-Baumann B., Lubeck M. Impairment of myocardial perfusion reserve in microvascular angina (syndrome X): assessment by 99mTc MIBI SPECT. Nuklearmedizin. 1996;35:193–197. [PubMed] [Google Scholar]
- 30.Fragasso G., Chierchia S.L., Lu C., Babrowski P., Pagnotta P., Rosano G.M. Left ventricular dysfunction during dobutamine stress echocardiography in patients with syndrome X and positive myocardial perfusion scintigraphy. G Ital Cardiol. 1999;29:383–390. [PubMed] [Google Scholar]
- 31.Vermeltfoort I.A., Bondarenka O., Raijmakers P.G. Is subendocardial ischemia present in patients with chest pain and normal coronary angiograms? A cardiovascular MR study. Eur Heart J. 2007;28:1554–1558. doi: 10.1093/eurheartj/ehm088. [DOI] [PubMed] [Google Scholar]
- 32.Yilmaz A., Athanasiadis A., Mahrholdt H. Diagnostic value of perfusion cardiovascular magnetic resonance in patients with angina pectoris but normal coronary angiograms assessed by intracoronary acetylcholine testing. Heart. 2010;96:372–379. doi: 10.1136/hrt.2009.174367. [DOI] [PubMed] [Google Scholar]
- 33.Buchthal S.D., den Hollander J.A., Bairey-Merz C.N. Abnormal myocardial phosphorus – 31 nuclear magnet resonance spectroscopy in women with chest pain but normal coronary angiograms. N Eng J Med. 2000;342:829–835. doi: 10.1056/NEJM200003233421201. [DOI] [PubMed] [Google Scholar]
- 34.Cassar A., Chareonthaitawee P., Rihal C.S. Lack of correlation between noninvasive stress tests and invasive coronary vasomotor dysfunction in patients with non-obstructive coronary artery disease. Circ Cardiovasc Interv. 2009;2:237–244. doi: 10.1161/CIRCINTERVENTIONS.108.841056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Bruyne B., Hersbach F., Piyls N.H. Abnormal epicardial coronary resistance in patients with diffuse atherosclerosis but normal coronary angiography. Circulation. 2001;104:2401–2406. doi: 10.1161/hc4501.099316. [DOI] [PubMed] [Google Scholar]
- 36.Khuddus M.A., Pepine C.J., Handberg E.M. An intravascular ultrasound analysis in women experiencing chest pain in the absence of obstructive coronary artery disease: a sub study from National Heart, Lung and Blood Institute Sponsored Women's Ischemic Syndrome Evaluation. J Interv Cardiol. 2010;23:511–519. doi: 10.1111/j.1540-8183.2010.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kern M.J., Lerman A., Bech J.W. Physiological assessment of coronary artery disease in cardiac catheterization laboratory: a scientific statement from the American Heart Association Committee on Diagnostic and Interventional Catheterization, Council on Clinical Cardiology. Circulation. 2006;114:1321–1341. doi: 10.1161/CIRCULATIONAHA.106.177276. [DOI] [PubMed] [Google Scholar]
- 38.Park S.J., Kang S.J., Ahn J.M. Visual-functional mismatch between coronary angiography and fractional flow reserve. Circ Cardiovasc Interv. 2012;5:633–640. doi: 10.1016/j.jcin.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 39.Petraco R., Sen S., Nijjer S. How would I treat? Euro Intervention. 2013;9:160–161. [Google Scholar]
- 40.Gibson C.M., Cannon C.P., Daley W.L. TIMI frame count: a quantitative method of assessing coronary artery flow. Circulation. 1996;93:879–888. doi: 10.1161/01.cir.93.5.879. [DOI] [PubMed] [Google Scholar]
- 41.Bettrame J.F., Limaye S.B., Wuttke R.D. Coronary hemodynamic and metabolic studies of the coronary slow flow phenomenon. Am Heart J. 2003;146:84–90. doi: 10.1016/S0002-8703(03)00124-8. [DOI] [PubMed] [Google Scholar]
- 42.Vant Hof A.W., Liem A., Suryapranata H. Angiographic assessment of myocardial reperfusion in patients treated with primary angioplasty for acute myocardial infarction: myocardial blush grade. Jwolle Myocardial Infarction study group. Circulation. 1998;97:2302–2306. doi: 10.1161/01.cir.97.23.2302. [DOI] [PubMed] [Google Scholar]
- 43.Cannon R.O., III Microvascular angina and the continuing dilemma of chest pain with normal coronary angiograms. J Am Coll Cardiol. 2009;54:877–885. doi: 10.1016/j.jacc.2009.03.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fearon W.F., Balsam L.B., Omar Farouque H.M. Novel index for invasively assessing the coronary microcirculation. Circulation. 2003;107:3129–3132. doi: 10.1161/01.CIR.0000080700.98607.D1. [DOI] [PubMed] [Google Scholar]
- 45.Ng M.K.C., Yeng A.C., Fearon W.F. Invasive assessment of the microcirculation: superior reproducibility and less hemodynamic dependence of index of microcirculatory resistance compared with coronary flow reserve. Circulation. 2006;113:2054–2061. doi: 10.1161/CIRCULATIONAHA.105.603522. [DOI] [PubMed] [Google Scholar]
- 46.Mittal S.R. Microvascular angina – etiopathogenesis and diagnostic strategies. In: Venugopal K., editor. Cardiology Update. Cardiological Society of India; 2013. pp. 208–221. [Google Scholar]
- 47.Koo B.K. How would I treat? Euro Intervention. 2013;9:159. [Google Scholar]
- 48.Teragawa H., Mitsuba N., Ishibashi K. Evaluation of coronary microvascular function in patients with vasospastic angina. World J Cardiol. 2013;5:1–7. doi: 10.4330/wjc.v5.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herrman J., Kaski J.C., Lerman A. Coronary microvascular dysfunction in the clinical setting: from mystery to reality. Eur Heart J. 2012;33:2771–2781. doi: 10.1093/eurheartj/ehs246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Egashira K., Inou T., Hirooka Y. Effect of age on endothelium dependent vasodilatation of resistance coronary artery by acetylcholine in humans. Circulation. 1993;88:77–81. doi: 10.1161/01.cir.88.1.77. [DOI] [PubMed] [Google Scholar]
- 51.Kern M.J., Bach R.G., Mechem C.J. Variations in normal coronary vasodilatory reserve stratified by artery, gender, heart transplantation and coronary artery disease. J Am Coll Cardiol. 1996;28:1154–1160. doi: 10.1016/S0735-1097(96)00327-0. [DOI] [PubMed] [Google Scholar]
- 52.Charenthaitawee P., Kaufman P.A., Rimoldi O. Heterogenesity of resting and hyperemic myocardial blood flow in healthy humans. Cardiovasc Res. 2001;50:151–161. doi: 10.1016/s0008-6363(01)00202-4. [DOI] [PubMed] [Google Scholar]
- 53.Sestito A., Lanza G.A., De Monaco A. Relation between cardiovascular risk factors and coronary microvascular dysfunction in cardiac syndrome X. Cardiovasc Med. 2011;12:322–327. doi: 10.2459/JCM.0b013e3283406479. [DOI] [PubMed] [Google Scholar]
- 54.Murtagh B., Higano S.T., Lennen R. Role of incremental doses of intracoronary adenosine for fractional flow reserve assessment. Am Heart J. 2003;146:99–105. doi: 10.1016/S0002-8703(03)00120-0. [DOI] [PubMed] [Google Scholar]