Abstract
Background
Cardiopulmonary involvement in systemic sclerosis (SSc) is a poor prognostic factor, due to pulmonary hypertension and right ventricular dysfunction. We assessed the echocardiographic parameters of right ventricular (RV) function in SSc and correlated echocardiographic findings to clinical features of the disease.
Methods
Thirty patients with SSc (cases) and 30 healthy, age-matched subjects (controls) were studied. Echocardiography, including tissue Doppler imaging, was used to evaluate cardiac function.
Results
Pulmonary hypertension could be documented in only 5 cases by Doppler echo, using Bernoulli principle. RV diastolic function was significantly deranged in cases. RV systolic function and left ventricle (LV) diastolic function were also significantly deranged in the cases. RV thickness was increased in patients with SSc. There were no significant differences in the echocardiographic variables between diffuse and limited subtypes of SSc. Myocardial performance index (MPI) of both ventricles were increased in cases. We could demonstrate RV thickness as the single most important predictor of MPI of both ventricles with sensitivity of 82% and specificity of 72% for RV-MPI and 63% for LV-MPI. Diastolic function was not found to be affected by disease duration or Rodnan skin score.
Conclusion
Patients with SSc exhibit abnormal RV and LV diastolic functions as well as abnormal RV systolic function. RV wall thickness was found to be simple and the single best predictor of global myocardial performance. RV dysfunction may be a response to intermittent pulmonary arterial hypertension, lung parenchymal involvement, or secondary to LV diastolic dysfunction in SSc.
Abbreviations: ACE-I, angiotensin converting enzyme inhibitor; DT, deceleration time; DTI, Doppler tissue imaging; E/A ratio, early diastolic/atrial component velocity ratio; ET, ejection time; FVC, forced vital capacity; Hct, hematocrit; HRCT, high-resolution computed tomography; IVCT/ICT, isovolumic contraction time; ILD, interstitial lung disease; IVRT/IRT, isovolumic relaxation time; LV, left ventricle/ventricular; LVEDD, left ventricular end diastolic dimension; LVEDV, left ventricular end diastolic volume; LVEF, left ventricular ejection fraction; LVESD, left ventricular end systolic dimension; LVESV, left ventricular end systolic volume; MPI, myocardial performance index; PAH, pulmonary arterial hypertension; PAP, pulmonary artery pressure; PASP, pulmonary artery systolic pressure; PAT, pulmonary acceleration time; RR, electrocardiographic R–R interval; RVEF, right ventricular ejection fraction; RV, right ventricle/ventricular; SSc, systemic sclerosis
Keywords: Systemic sclerosis, Doppler tissue imaging, Pulmonary hypertension, Tei index
1. Introduction
Systemic sclerosis (SSc) is a multisystem disorder characterized by extensive vascular damage and fibrosis of the skin and internal organs. Cardiac involvement occurs in a significant percentage (≤80%) of these patients.1 Cardiac involvement consists of degeneration of myocardial fibers and irregular areas of interstitial fibrosis that are most prominent around blood vessels.2
Myocardial fibrosis seems to start from the subendocardium in SSc. Therefore, due to the subendocardial location of the longitudinal myofibrils, longitudinal functions and diastolic parameters have particular significance in determining early myocardial involvement. The prognosis for patients with SSc also correlates with the presence of pulmonary hypertension.2
There is a discrepancy between the frequency of clinically evident myocardial disease (25%) and autoptical myocardial fibrosis (81%).3 Invasive and non-invasive diagnostic investigations, as well as necropsy studies, showed that, in SSc, cardiac involvement is one of the most frequent visceral complications that can affect the overall prognosis of the disease.4
The hemodynamic parameters of right ventricular (RV) dysfunction, as measured by cardiac catheterization, are strong predictors of outcome in patients with SSc.5, 6 Denton et al.7 have compared Doppler echocardiography vs. right heart catheterization and found Doppler to be 90% sensitive and 75% specific in detecting pulmonary hypertension as compared to catheterization. However, it may underestimate the true PA (pulmonary artery) pressure in absence of tricuspid regurgitation. In the present study, we have applied the technique of tissue Doppler in order to identify the TDI (tissue Doppler imaging) markers of myocardial function.
2. Methods
2.1. Clinical
Seventy-two patients from Rheumatology Outpatient Clinic with diagnosis of SSc according to American College of Rheumatology criteria8 were screened, and of them, 30 patients were enrolled into the study. Thirty age- and gender-matched healthy volunteers were selected for comparison. Written informed consent was taken from all subjects. The study was conducted for a period of 18 months in Rheumatology clinic of PGIMER. Patients without sinus rhythm, known cardiomyopathies, IHD (ischemic heart disease), significant left-sided valvular disease, CKD (chronic kidney disease) stage III or higher, diabetics, prior cardiac surgery, Bundle branch block on ECG, poor echo window, and those unwilling to give informed consent were excluded.
Detailed history and clinical examination, including Rodnan skin score,9 were done. Disease duration was calculated based on either onset of symptoms of exertional dyspnea, skin thickening, Raynaud's phenomenon, or physician-aided diagnosis of scleroderma. Pulmonary involvement was defined clinically by either dyspnea or bibasilar rales. Renal function tests, LFT (liver function tests), ANA (antinuclear antobody), Chest X-ray, and pulmonary function tests with diffusion capacity of carbon dioxide (DLCO) were done in all patients.
2.2. Pulmonary function testing
All the patients underwent pulmonary function testing. FEV1/FVC < 80% was classified as obstructive. In presence of normal FEV1/FVC, FVC was used to classify restrictive pattern as mild (<80–60%), moderate (<60–40%), and severe (<40%), as per our laboratory standards. DLCO was adjusted for alveolar ventilation and hematocrit by the following formula.10
2.3. Cardiological investigations
Resting ECG was performed in all. Echocardiography was done using ultrasound [Acuson Sequoia (C512)] equipped with a 4.0-MHz (V4c; Acuson) transducer and DTI technology. The examination was performed with the subject in the left lateral decubitus position with normal breathing. All tracings were recorded at the end-expiratory phase to reduce respiratory variation. Standard two-dimensional parasternal and apical projections were obtained. All recordings were performed with a simultaneous superimposed phonocardiogram to detect the pulmonary component of the second heart sound (S2) and continuous EKG for pulmonary valve flow recording. All images were recorded at sweep speeds of 50 mm/s and 100 mm/s.
From M-mode echocardiography recordings, measurements were made on consecutive three beats, according to recommendations of the American Society of Echocardiography.11
The left ventricle (LV) mass was calculated using Devereux formula.
where LVID is the internal dimension of LV, PWT is posterior wall thickness, IVST is interventricular septal thickness, and 1.04 = specific gravity of myocardium. All measurements were done in centimeters to get the mass in grams. The mass thus obtained was divided with body surface area to get myocardial mass index. The thickness of RV wall was measured from the subcostal view at the peak of R wave.
2.4. Conventional Doppler echocardiography assessment of global function
From the mitral and tricuspid velocity profiles, E/A ratio, E-wave DT, and isovolumic relaxation time (IVRT) were made according to the recommendations of the American Society of Echocardiography. Velocity of tricuspid regurgitant jet (VTR) was used to measure pulmonary artery systolic pressure. Saline contrast was used to enhance TR jet if no TR jet was seen on conventional Doppler. Pulmonary hypertension was suspected on echocardiography when VTR was >2.8 m/s.
2.5. DTI assessment of regional myocardial RV function
Myocardial systolic and diastolic velocities were recorded using the pulsed-wave DTI technique. Velocities were obtained from the apical four-chamber view. The sample volume was placed at lateral tricuspid annulus and lateral tricuspid annular systolic velocity was measured. Similarly, ventricular septal velocity and lateral mitral annular systolic velocity were taken by placing sample volume at tricuspid septal attachment and mitral lateral annulus, respectively. All measurements were taken from three beats, and the mean value was used.
The measured time intervals from Doppler and tissue Doppler study were corrected for heart rate by dividing with square root of R–R interval and also by another method of the time interval taken as the proportion of R–R interval (expressed as %) for comparison.
2.6. The Tei index
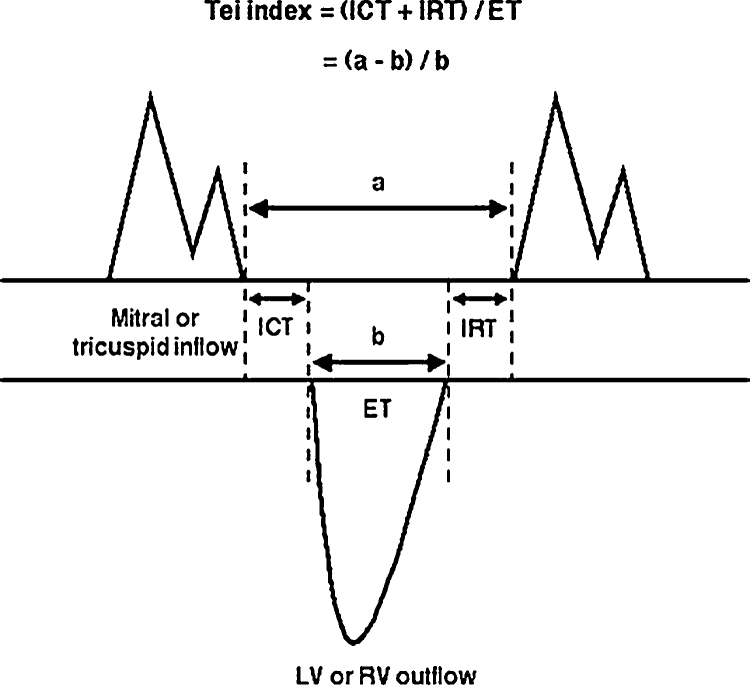
The Tei index12 was used to assess left and right global ventricular function. As originally described by Tei,12 the time intervals used to calculate the index were measured using pulsed-wave Doppler velocity spectra of ventricular inflow and outflow. Mitral or tricuspid flow velocity spectra were obtained by positioning the pulsed-wave Doppler sample volume at the tips of the mitral or tricuspid valve leaflets in the apical four-chamber view. Right ventricular outflow velocity spectra are obtained from the parasternal short-axis view, with the pulsed-wave Doppler sample volume positioned at the pulmonic annulus. Calculation of the Tei index (Fig. 1) involves measuring the time interval a, extending from the cessation of mitral or tricuspid inflow to its subsequent onset; and ejection time b, which is the duration of the left or right ventricular outflow velocity spectrum. As the time intervals include the ICT, ET, and IRT, the Tei index can be expressed as follows:
Fig. 1.
Calculation of Tei index.
Time intervals were measured at a sweep speed of 100 mm/s and are averaged over at least 3–5 cardiac cycles.
All the echocardiographic measurements were done by one investigator, Dr Manoj Kumar Rohit, who was blinded for patient profile.
2.7. Statistical analysis
A commercially available statistical program (SPSS 15.0 for windows; SPSS; Chicago, IL) was used. All data are presented as mean ± SD. An unpaired Student t-test was used to compare data from patients and control subjects. Pearson correlations were used to compare associations between indexes. Univariate analysis was done to adjust for known confounding variables of ventricular function. Linear and binary logistic regression model was used to determine relationship between different indices, and p < 0.05 was considered statistically significant.
3. Results
3.1. Clinical profile
Clinical profile of age- and sex-matched subjects are presented in Table 1.
Table 1.
Patient characteristics and hemodynamic data.
| Characteristics | Case (n = 30) | Controls (n = 30) | p-value |
|---|---|---|---|
| Age, yrs | 38.87 ± 10.41 | 39.23 ± 10.31 | 0.891 |
| Female/male gender (Number) | 23/7 | 23/7 | |
| Height, cm | 160.97 ± 5.53 | 163.67 ± 6.86 | 0.077 |
| Weight, kg | 55.63 ± 8.42 | 55.9 ± 8.99 | 0.906 |
| BSA, m2 | 1.57 ± 0.12 | 1.59 ± 0.14 | 0.471 |
| HR, beats/min | 90 ± 12 | 72 ± 5 | <0.001* |
| SBP, mmHg | 125 ± 20 | 122 ± 10 | 0.542 |
| DBP, mmHg | 80 ± 11 | 77 ± 8 | 0.224 |
| Median duration of symptoms (in months) | 48 (IQR 21–84) | – | – |
| Creatinine clearance (ml/min/1.73 m2) | 104 ± 21 | – | – |
Data presented as mean ± SD, unless specified otherwise.
Statistically significant.
3.2. Pulmonary function tests and HRCT
Clinical pulmonary involvement was present in 57%. Pulmonary function tests results are in Table 2.
Table 2.
PFT findings of cases.
| Frequency (%) | |
|---|---|
| Normal | 23.3 |
| Restriction | |
| Mild | 23.3 |
| Moderate | 43.3 |
| Severe | 3.3 |
| Obstruction | |
| Mild | 6.7 |
Mean DLCO was low in patient group (mean = 54.7 ml/min/mmHg); however, when corrected for alveolar ventilation and hematocrit, the mean was 103.7, suggesting that the decrease was mainly due to reduced ventilation.
HRCT was available in 18 out of 30 patients, of which 17 was suggestive of ILD.
3.3. LA and LV dimensions and functions
LA and LV size and LV systolic function were similar in both groups (Table 3). However, there were significant differences in the diastolic function parameters and Tei index, which is a measure of global LV function (Table 4). LV ejection fraction was normal in both.
Table 3.
LV systolic function.
| Characteristics | Case (n = 30) | Controls (n = 30) | p-value |
|---|---|---|---|
| 2D and M Mode parameters | |||
| LV end systolic dimension, mm/m2 | 16.3 ± 4.16 | 15.6 ± 2.2 | 0.441 |
| LV end diastolic dimensions, mm/m2 | 27 ± 4 | 28.4 ± 3.2 | 0.127 |
| Fractional shortening, % | 40 ± 11 | 44.2 ± 9 | 0.094 |
| LV end systolic volume index, ml/m2 | 15.7 ± 8.6 | 15 ± 3.7 | 0.687 |
| LV end diastolic volume index, ml/m2 | 44.8 ± 14 | 47.4 ± 9.5 | 0.416 |
| LV ejection fraction, % | 66 ± 10 | 68 ± 4 | 0.208 |
| LV myocardial mass index, g/m2 | 85 ± 26 | 91 ± 12 | 0.346 |
| Conventional Doppler echocardiographic measurements (global function) | |||
| Isovolumic contraction time, ms | 62.4 ± 22 | 52 ± 10.5 | 0.024* |
| Isovolumic contraction time/R–R interval, % | 9.5 ± 3.7 | 6.2 ± 1.3 | <0.001* |
| Ejection time, ms | 227 ± 50.4 | 284 ± 31.2 | <0.001* |
| Tissue Doppler imaging (regional function) | |||
| Mitral annular systolic velocity, cm/s | 22.8 ± 4.7 | 33 ± 2.5 | 0.792 |
| Septal velocity, cm/s | 20.3 ± 3.7 | 19.7 ± 2.9 | 0.512 |
| Isovolumic contraction time, ms | 55 ± 18 | 59 ± 8 | 0.286 |
| Isovolumic contraction time/R–R interval, % | 8.2 ± 2.8 | 7.1 ± 1.4 | 0.061 |
| Modified Simpsons | |||
| LVEF, % | 63 ± 9.5 | 69.4 ± 7.4 | 0.006* |
Statistically significant.
Table 4.
LV diastolic function.
| Characteristics | Case (n = 30) | Controls (n = 30) | p-value |
|---|---|---|---|
| Conventional Doppler echocardiographic measurements (global function) | |||
| Mitral E velocity, cm/s | 61.13 ± 20.13 | 73.31 ± 12.91 | 0.007* |
| Mitral A velocity, cm/s | 54.83 ± 20.41 | 56.2 ± 10.9 | 0.765 |
| Mitral E/A | 1.17 ± 0.29 | 1.32 ± 0.17 | 0.021* |
| DT, ms | 160 ± 47 | 236 ± 58 | <0.001* |
| IVRT, ms | 74 ± 24 | 53.5 ± 12 | <0.001* |
| IVRT/R–R interval, % | 11.2 ± 4 | 6.4 ± 1.5 | <0.001* |
| , ms | 90.9 ± 31 | 58.5 ± 13 | <0.001* |
| DTI (regional function)-lateral mitral annulus | |||
| IVRT, ms | 60 ± 20 | 58.4 ± 7 | 0.794 |
| IVRT/R–R interval, % | 8.9 ± 3.1 | 7 ± 1.2 | 0.004* |
| , ms | 72.5 ± 24 | 64 ± 8.7 | 0.078* |
| Global LV myocardial performance index | |||
| LV Tei index | 0.42 ± 0.15 | 0.40 ± 0.07 | 0.474 |
Statistically significant.
3.4. RV systolic function
There was significant increase in the right ventricular free wall thickness among patients. Both blood flow derived and myocardium derived isovolumic contraction time (IVCT) were prolonged in patients after correction for heart rate. Pulmonary acceleration time was also significantly reduced in the patient group (Table 5).
Table 5.
comparison of RV systolic function parameters in cases and controls.
| M MODE | Case (n = 30) | Controls (n = 30) | p-value |
|---|---|---|---|
| RV thickness | 6.7 ± 1.3 | 5.3 ± 0.7 | <0.001 |
| Conventional Doppler echocardiographic measurements (global function) | |||
| PAT, ms | 110 ± 21 | 136 ± 35 | 0.001* |
| IVCT, ms | 60 ± 23 | 48 ± 12 | 0.017* |
| IVCT/R–R interval (%) | 8 ± 4 | 5.8 ± 1.8 | 0.009* |
| DTI (regional function)-lateral tricuspid annulus | |||
| Lateral tricuspid annular systolic velocity, cm/s | 21.8 ± 4.2 | 21.2 ± 2.7 | 0.53 |
| IVCT, ms | 60 ± 17.5 | 55 ± 8.3 | 0.176 |
| IVCT/R–R interval (%) | 9 ± 2.9 | 6.6 ± 1.2 | <0.001* |
Statistically significant.
3.5. RV diastolic function
Tricuspid regurgitation could be documented only in 7 cases; out of which, 5 cases were found to have pulmonary hypertension. By conventional Doppler measurement, tricuspid A velocity was found to be significantly raised in patients with corresponding decrease of E/A velocity. Both blood flow derived and myocardium derived IVRT were prolonged in the patient group even after adjustment for heart rate (Table 6). This difference persisted across various age groups in subgroup analysis (Table 7). There was no significant difference in DTI-derived RV-IVRTc among cases when categorized by disease duration.
Table 6.
RV diastolic function.
| Conventional Doppler echocardiographic measurements (global function) | |||
| Tricuspid E velocity, cm/s | 49.8 ± 11.4 | 49.6 ± 6.3 | 0.935 |
| Tricuspid A velocity, cm/s | 48.7 ± 10.6 | 38 ± 7.3 | <0.001* |
| Tricuspid E/A | 1.04 ± 0.2 | 1.32 ± 0.19 | <0.001* |
| DT, ms | 174 ± 67 | 232 ± 67 | 0.002* |
| IVRT, ms | 68.5 ± 21.1 | 42.6 ± 5.8 | <0.001* |
| IVRT/R–R interval, % | 9.3 ± 4.7 | 5.1 ± 0.7 | <0.001* |
| DTI (regional function)-RV free wall | |||
| IVRT, ms | 67.1 ± 21.3 | 46.6 ± 12.2 | <0.001* |
| IVRT/R–R interval, % | 10.1 ± 3.5 | 5.6 ± 1.7 | <0.001* |
| Tei index | |||
| RV Tei index | 0.54 ± 0.26 | 0.35 ±0.07 | <0.001* |
Statistically significant.
Table 7.
Mean lateral tricuspid IVRT/RR (%) in cases and controls.
| Age groups | Cases | Controls | Mean difference | p-value |
|---|---|---|---|---|
| <35 yrs | 9.5 ± 4.23 (n = 11) | 5.44 ± 1.9 (n = 8) | 4.01 | 0.023* |
| 35–44 yrs | 9.74 ± 3.52 (n = 8) | 5.6 ± 1.4 (n = 13) | 4.15 | 0.001* |
| >45 yrs | 11 ± 2.7 (n = 11) | 5.8 ± 2 (n = 9) | 5.2 | <0.001* |
Statistically significant.
3.6. Global right ventricular performance
The MPI was significantly raised in the patient group (0.54 + 0.26 vs. 0.35 + 0.07, p < 0.001), suggesting overall diminished right ventricular performance in patients.
3.7. Relationship between diastolic dysfunction and clinical features
The indices of ventricular diastolic dysfunction for both ventricles IVRT/RR (%) did not correlate to age, duration of disease, skin score, hematocrit-corrected DLCO, or forced vital capacity in the patient group. However, there was significant negative correlation between tricuspid E/A velocity and age [r = (−0.0393), p = 0.043]. RV free wall thickness positively correlated with age (r = 0.444, p = 0.014), duration of disease (r = 0.668, p < 0.001), and negatively correlated with PAT (r = −0.381, p = 0.038) and tricuspid E/A (r = −0.573, p = 0.002).
RV Tei index shows significant correlation only with Hct-corrected DLCO (r = −0.403, p = 0.027) and LV-Tei (r = 0.457, p = 0.011). The diastolic parameter of the two ventricles measured by TDI-derived IVRT/RR (%) also showed good correlation among themselves (r = 0.667, p < 0.001).
4. Discussion
In the present study, we were able to demonstrate that in patients with SSc, diastolic function of both ventricles were significantly deranged.
The RV dysfunction here is clearly evidenced by prolonged global and regional IVRT as well as significantly reduced tricuspid E/A velocity derived from 2-D Doppler; however, significantly reduced tricuspid DT and mean E/A, which is >1, suggest that there is grade II diastolic dysfunction as defined by the Canadian consensus recommendations. The advanced dysfunction is also supported by markers of early systolic dysfunction of RV, which is usually affected late in natural course of SSc.
Two factors are known to cause changes in diastolic function of RV: age and increased heart rate that is usual in SSc due to sympathetic activation. We have matched our cases with controls with respect to age, and all the time intervals were corrected for change in heart rate. Similarly, RV diastolic dysfunction was not correlated with skin score, duration of disease, or any of the PFT markers of abnormal lung function. The only marker significantly affected with hematocrit-corrected DLCO was RV-MPI, a marker of global function of RV (r = −0.403, p = 0.027).
Since the prognosis for patients with SSc correlates well with the presence of pulmonary hypertension,2 and pulmonary hypertension is present in great majority of SSc patients (51.6% in one series involving 31 patients13), measurement of pulmonary arterial pressure seems to be of great value. In our study, there were only 5 cases in which pulmonary hypertension could be predicted by taking TR jet velocity into account. What was interesting is that even when we excluded those 5 cases from our analysis, there were significant differences between cases and control group in the above-mentioned parameters of RV diastolic function, suggesting either pulmonary hypertension is not the only factor responsible for diastolic dysfunction or the methodology applied to detect it is not sensitive enough. With literature search, both of these seem true. It is well known that Doppler echocardiography may underestimate pulmonary artery pressure when obtained from tricuspid regurgitation peak velocity.14, 15, 16 Similarly, apart from pulmonary hypertension, myocardial fibrosis and ischemia, which are the hallmarks of disease, can significantly affect myocardial function in these patients. It has been shown in studies that intermittent coronary vasospasm is prevalent in SSc.17 The pattern of diastolic dysfunction seen in patients can thus be related to myocardial fibrosis and/or ischemia, both of which are known to affect ventricular relaxation and filling. This mechanism of dysfunction is also supported by finding of left ventricular diastolic dysfunction in the present study in the absence of hypertension.
Pulmonary involvement is one of the leading causes of death in SSc. While pulmonary arterial hypertension is commonly seen in long standing limited type of SSc, interstitial pulmonary fibrosis by alveolitis is more common in diffuse type. Since Doppler is not good modality at detecting PAH, mild or early PAH cannot be ruled out in our patient group, and Raynaud's phenomenon in lung is a possibility. This is supported by reduced pulmonary acceleration time, which is documented to be inversely correlated to pulmonary artery systolic pressure in invasive measurements (r = −0.85 with pulmonary artery systolic pressure (PASP))18 and increased RV wall thickness, suggesting increased after load of RV.
There was significant prolongation of IVCT with reduction of ejection time both by Doppler-derived global and TDI-derived regional time intervals, suggesting an early RV systolic dysfunction. RV systolic dysfunction occurs late in the natural course of disease only when disease progresses to involve circular muscle fibers of myocardium. Though RV systolic dysfunction is not very well documented in older literature,19, 20, 21 Hsiao et al.22 found significant RV systolic dysfunction (RVEF < 40%) in 55% of 40 patients studied.
We could also demonstrate significant LV diastolic dysfunction in our patients. In contrast to earlier studies, where the involvement was early with reduction of E/A to <1 and prolongation of deceleration time,23, 24 in our study mitral E/A was >1 with significant reduction of DT. In combination with significantly prolonged global MPI and IVRT (both global and regional), above-mentioned LV time intervals will suggest a pseudo-normalized mitral filling pattern. It has been well known that the diastolic function of the LV is affected by age and hypertension but the difference persisted in the patient group even after adjusting for age and blood pressure. Thirteen patients were using ACE inhibitors at the time of study and there was no significant difference in diastolic time intervals and MPI between those who were on drugs (n = 13) vs. those who were not on drugs (n = 17) (p = 0.216).
Recently, Codullo et al.25 had reported exercise-induced change in pulmonary artery systolic pressure (ΔPASP > 18 mmHg) as a predictor of development of PAH over a mean follow-up of 3.5 yrs. However, there is heterogeneity of underlying mechanism of ΔPASP and this was shown to be due to rise in pulmonary vascular resistance in only 11% of cases.26 One invasive study involving right heart catheterization has shown the change in PASP is predominantly due to LV diastolic dysfunction than pulmonary arterial vasculopathy.27 Hence, the significance of this new marker too remains uncertain. However, all these studies reinforce the need for assessment of pulmonary hypertension at an early stage.
Enriquez-Sarano found significant negative correlation between pulmonary artery systolic pressure and mitral deceleration time (r = −0.61, p = 0.0001).28 Similarly, Tei index significantly correlated with invasive measurements of ventricular function in porcine model.29 Recently in humans too, a strong positive correlation was found between invasively measured PASP- and DTI-derived IVRTc (r = 0.86), blood pool-derived IVRTc (r = 0.75), and Endothelin-1 levels (r = 0.94); and between DTI-derived IVRTc and ET-1 levels (r = 0.82); p < 0.001 for all correlations.30 As Tei index was found to be a reliable predictor of ventricular function, we tried to find out more simple predictors of Tei index in our cohort.
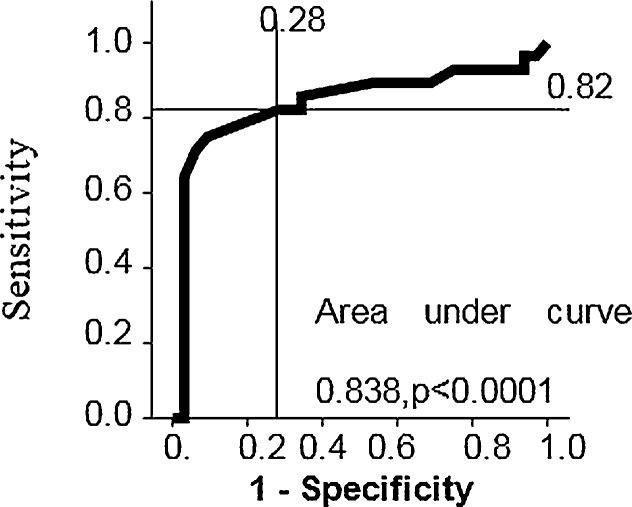
We determined normal cut-offs of Tei index in our population. Upper limit of normal was defined as mean + 2 SE of mean (95% confidence interval). By logistic regression analysis, we found that right ventricular free wall thickness was the single most important predictor for differentiating abnormal from normal Tei index. By ROC curve analysis, it was found that RV thickness of 6 mm or more can predict abnormal RV-MPI with a sensitivity of 82% and specificity of 72% (area under curve 0.838, p < 0.001) (Fig. 2).
Fig. 2.
ROC curve showing sensitivity and specificity of RV wall thickness in predicting RV-MPI.
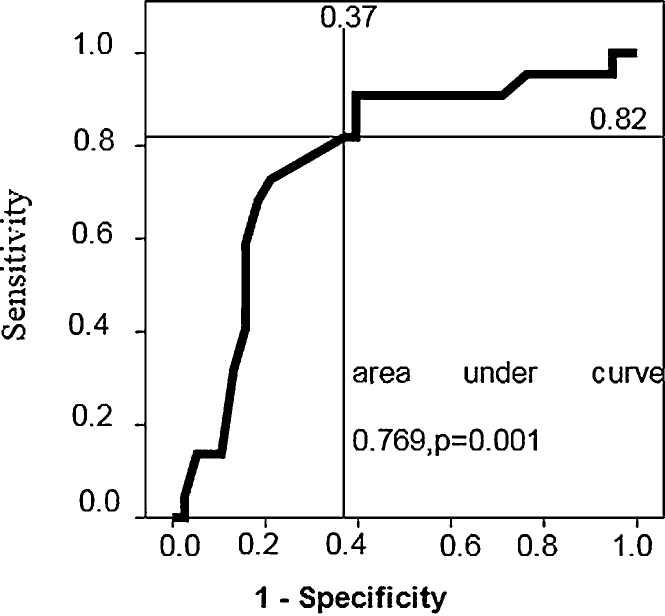
The same cut-off for RV thickness of 6 mm could distinguish abnormal LV-MPI from normal with sensitivity of 82% and specificity of 63% (area under curve 0.769, p = 0.001) (Fig. 3).
Fig. 3.
ROC curve showing trade-off between sensitivity and specificity when RV thickness was used as predictor of LV-MPI.
There are many studies in the past that have studied the heart in SSc and have found significant differences in the echocardiographic parameters in patients with SSc as compared to controls19, 31, 32; most of the studies were having significant proportion of patients with Doppler documented pulmonary hypertension by tricuspid jet peak velocity calculations. Our study differs in that; we showed a significant disturbance in biventricular function in a sample that has very few (16.7%) patients having pulmonary hypertension by conventional Doppler methods, and the significance remained even when this 16.7% was excluded from analysis. This goes in favor of observations made in earlier studies that Doppler may underestimate PASP and demands for need of non-invasive methods for determination of pulmonary artery pressure and ventricular performance. Tissue Doppler seems to be an important step towards achieving this goal and it seems reasonable to assess ventricular function in detail in all patients with SSc.
5. Limitations
We were careful to clinically exclude patients with concomitant conditions that would potentially confound interpretation of two-dimensional and Doppler indices. Although vasoactive medications were not discontinued and hypertension was not excluded, our findings were independent of these influences. We did not measure pulmonary venous flow in systole and diastole and neither Valsalva maneuver during the echocardiography, which could have reliably differentiated normal from pseudo-normal diastolic filling pattern; however, the existing data are sufficient to distinguish between the two. We were not able to see the relationship of echo parameters with HRCT findings, as HRCT in all patients was not allowed by local ethics committee and HRCT was available with only 18 out of 30 patients. Correlation with HRCT findings were thus not attempted to avoid selection bias. We did not perform right heart catheterization to verify diastolic function and PA pressures. However, some of the echocardiographic parameters used in the present study are well-validated11. Direct evidence of myocardial fibrosis can only be verified by myocardial biopsy, but an investigation of this kind is not ethical in this group of patients. The sample size was small as many patients were excluded due to poor echo window secondary to skin involvement, and some due to early occurrence of atrial fibrillation, and this has limited the number in all other previously published studies too.
6. Conclusion
In the present study, pulmonary hypertension was detected in only 5 out of 30 patients (16.7%) of SSc by Doppler-defined parameters taking tricuspid regurgitant peak velocity.
Both RV and LV diastolic functions were significantly deranged in patients compared to controls, and the difference persisted even after excluding those 5 patients with Doppler-proven PAH from analysis, suggesting that PASP determination by Doppler alone may underestimate true prevalence of cardiopulmonary involvement.
Tei index has been proven to be a marker of global and regional myocardial performance by invasive studies, and here we could demonstrate RV thickness as a single most important predictor of Tei index of both ventricles.
Conflicts of interest
The authors have none to declare.
Footnotes
Read the Editorial to this manuscript: An Indian-look right into restrictive cardiomyopathies.
References
- 1.D’Angelo W.A., Fries J.F., Masi A.T. Pathological observations in systemic sclerosis (scleroderma): a study of 58 autopsy cases and 58 matched controls. Am J Med. 1969;46:428–440. doi: 10.1016/0002-9343(69)90044-8. [DOI] [PubMed] [Google Scholar]
- 2.Kawut S.M., Taichman D.B., Archer-Chicko C.L. Hemodynamics and survival in patients with pulmonary arterial hypertension related to systemic sclerosis. Chest. 2003;123:344–350. doi: 10.1378/chest.123.2.344. [DOI] [PubMed] [Google Scholar]
- 3.Ferri C., Di Bello V., Martini A. Heart involvement in systemic sclerosis: an ultrasonic tissue characterization study. Lupus. 2005;14:702–707. doi: 10.1136/ard.57.5.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deswal A., Follansbee W.P. Cardiac involvement in scleroderma. Rheum Dis Clin North Am. 1996;22:841–861. doi: 10.1016/s0889-857x(05)70304-5. [DOI] [PubMed] [Google Scholar]
- 5.Mukherjee D., George S.T.D., Coleiro B. Prevalence and outcome in systemic sclerosis associated pulmonary arterial hypertension: application of a registry approach. Ann Rheum Dis. 2003;62:1088–1093. doi: 10.1136/ard.62.11.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Alonzo G.E., Barst R.J., Ayres S.M. Survival in patients with primary pulmonary hypertension. Ann Intern Med. 1991;115:343–349. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 7.Denton C.P., Cailes J.B., Phillips G.D. Comparison of Doppler echocardiography and right heart catheterization to assess pulmonary hypertension in systemic sclerosis. Br J Rheumatol. 1997;36:239–243. doi: 10.1093/rheumatology/36.2.239. [DOI] [PubMed] [Google Scholar]
- 8.Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and therapeutic criteria committee. Arthritis Rheum. 1980;23:581–590. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 9.Steen V.D., Medsger T.A., Rodnan G.P. D-penicilamine therapy in progressive systemic sclerosis (scleroderma): a retrospective analysis. Ann Intern Med. 1982;97:652. doi: 10.7326/0003-4819-97-5-652. [DOI] [PubMed] [Google Scholar]
- 10.Marrades R.M., Diaz O., Roca J. Adjustment of DLCO for hemoglobin concentration. Am J Respir Crit Care Med. 1997;155:236–241. doi: 10.1164/ajrccm.155.1.9001318. [DOI] [PubMed] [Google Scholar]
- 11.Gottdiener J.S., Bednarz J., Devereux R. American Society of Echocardiography recommendations for use of echocardiography in clinical trials: a report from the American society of echocardiography's guidelines and standards committee and the task force on echocardiography in clinical trials. J Am Soc Echocardiogr. 2004;17:1086–1119. doi: 10.1016/j.echo.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 12.Tei C. New non-invasive index for combined systolic and diastolic ventricular function. J Cardiol. 1995;26:135–136. [PubMed] [Google Scholar]
- 13.Karoli N.A., Rebrov A.P. Pulmonary hypertension right and left cardiac cavities in patients with systemic scleroderma. Klin Med (Mosk) 2004;82:47–50. [PubMed] [Google Scholar]
- 14.Brecker S.J., Gibbs J.S., Fox K.M. Comparison of Doppler derived hemodynamic variables and simultaneous high fidelity pressure measurement in severe pulmonary hypertension. Br Heart J. 1994;72:384–389. doi: 10.1136/hrt.72.4.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raeside D.A., Chalmers G., Clelland J. Pulmonary artery pressure variation in patients with connective tissue disease: 24 hour ambulatory pulmonary artery pressure monitoring. Thorax. 1998;53:857–862. doi: 10.1136/thx.53.10.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mininni S., Diricatti G., Vono M.C. Non invasive evaluation of right ventricular systolic pressure during dynamic exercise by saline enhanced Doppler echocardiography in progressive Systemic sclerosis. Angiology. 1996;47:467–474. doi: 10.1177/000331979604700505. [DOI] [PubMed] [Google Scholar]
- 17.Gustafsson R., Mannting F., Kazzam E. Cold induced reversible myocardial ischemia in systemic sclerosis. Lancet. 1989;2:475–479. doi: 10.1016/s0140-6736(89)92088-6. [DOI] [PubMed] [Google Scholar]
- 18.Chan K.L., Currie P.J., Seward J.B. Comparison of three Doppler ultrasound methods in the prediction of pulmonary artery pressure. J Am Coll Cardiol. 1987;9:549–554. doi: 10.1016/s0735-1097(87)80047-5. [DOI] [PubMed] [Google Scholar]
- 19.Lindqvist P., Caidahl K., Neuman-Andersen G. Disturbed right ventricular diastolic function in patients with systemic sclerosis: a Doppler tissue imaging study. Chest. 2005;128:755–763. doi: 10.1378/chest.128.2.755. [DOI] [PubMed] [Google Scholar]
- 20.Kazzam E., Waldenstrom A., Landelius J. Non invasive assessment of left ventricular diastolic function in patients with systemic sclerosis. J Intern Med. 1990;228:183–192. doi: 10.1111/j.1365-2796.1990.tb00214.x. [DOI] [PubMed] [Google Scholar]
- 21.Kazzam E., Caidahl K., Hallgren R. Mitral regurgitation and diastolic flow profile in systemic sclerosis. Int J Cardiol. 1990;29:357–363. doi: 10.1016/0167-5273(90)90126-p. [DOI] [PubMed] [Google Scholar]
- 22.Hsiao S.H., Lee C.Y., Chang S.M. Right heart function in scleroderma: insights from myocardial Doppler tissue imaging. J Am Soc Echocardiogr. 2006;19:507–514. doi: 10.1016/j.echo.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Gullulu S., Kaderli A.A., Ekbul A. Tissue Doppler echocardiography and myocardial performance index in patients with scleroderma. J Int Med Res. 2005;33:417–424. doi: 10.1177/147323000503300407. [DOI] [PubMed] [Google Scholar]
- 24.Armstrong G.P., Whalley G.A., Doughty R.N. Left ventricular function in scleroderma. Br J Rheumat. 1996;35:983–988. doi: 10.1093/rheumatology/35.10.983. [DOI] [PubMed] [Google Scholar]
- 25.Codullo V., Caporali R., Cuomo G. Stress Doppler echocardiography in systemic sclerosis: evidence for a role in the prediction of pulmonary hypertension. Arthritis Rheum. 2013;65:2403–2411. doi: 10.1002/art.38043. [DOI] [PubMed] [Google Scholar]
- 26.Gargani L., Pignone A., Agoston G. Clinical and echocardiographic correlations of exercise-induced pulmonary hypertension in systemic sclerosis: a multicenter study. Am Heart J. 2013;165:200–207. doi: 10.1016/j.ahj.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 27.Ciurzyński M., Bienias P., Irzyk K. Exaggerated increase of exercise-induced pulmonary artery pressure in systemic sclerosis patients predominantly results from left ventricular diastolic dysfunction. Clin Res Cardiol. 2013;102:813–820. doi: 10.1007/s00392-013-0594-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Enriquez-Sarano M., Rossi A., Seward J.B. Determinants of pulmonary hypertension in left ventricular dysfunction. J Am Coll Cardiol. 1997;29:153–159. doi: 10.1016/s0735-1097(96)00436-6. [DOI] [PubMed] [Google Scholar]
- 29.La corte J.C., Cabreriza S.E., Rabkin D.G. Correlation of Tei index with invasive measurements of ventricular function in porcine model. J Am Soc Echocardiogr. 2003;16:442. doi: 10.1016/s0894-7317(03)00110-x. [DOI] [PubMed] [Google Scholar]
- 30.Elnoamany M.F., Dawood A.A. Right ventricular myocardial isovolumic relaxation time as novel method for evaluation of pulmonary hypertension: correlation with endothelin-1 levels. J Am Soc Echocardiogr. 2007;20:462–469. doi: 10.1016/j.echo.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Giunta A., Tirri E., Maione S. Right ventricular diastolic abnormalities in systemic sclerosis: relation to left ventricular involvement and pulmonary hypertension. Ann Rheum Dis. 2000;59:94–98. doi: 10.1136/ard.59.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henein M.Y., Cailes J., O'Sullivan C. Abnormal ventricular long-axis function in systemic sclerosis. Chest. 1995;108:1533–1540. doi: 10.1378/chest.108.6.1533. [DOI] [PubMed] [Google Scholar]