Abstract
Baleen and sperm whales, belonging to the Order Cetartiodactyla, are the largest and heaviest existent mammals in the world, collectively known as large whales. Large whales have been subjected to a variety of conservation means, which could be better monitored and managed if physiological and pathophysiological information, such as pathogen infections, could already be gathered from free-swimming animals instead of carcasses. Parasitic diseases are increasingly recognized for their profound influences on individual, population, and even ecosystem health. Furthermore, a number of parasite species have gained importance as opportunistic neozoan infections in the marine environment. Nonetheless, traditional approaches to study parasitic diseases have been impractical for large whales, since there is no current routine method for the capture and handling of these large animals and there is presently no practical method to obtain blood samples remotely from free-ranging whales. Therefore, we here not only intend to review the endo- and ectoparasite fauna of large whales but also to provide new insights in current available methods for gathering parasitological data by using non- or minimally invasive sampling techniques. We focus on methods, which will allow detailed parasitological studies to gain a broader knowledge on parasitoses affecting wild, free-swimming large whale populations.
Keywords: Cetaceans, Whales, Neozoan parasites, Entamoeba, Balantidium, Giardia
Graphical abstract
Highlights
-
•
Endo- and ectoparasite fauna of free-ranging large whales.
-
•
Overcoming difficulties in obtaining appropriate parasitological sample collection.
-
•
Non- and minimally-invasive methods.
1. Introduction
Over the last two centuries, most large whale species were subjected to severe overexploitation by commercial whaling to the point of becoming almost extinct (Clapham et al., 1999). Fortunately, since the international moratorium on commercial whaling, that has been in place since 1986, some populations have slowly recovered, although most populations have not yet recuperate their historical numbers, and others are still under threat or seriously endangered (Kraus et al., 2005, Freeman, 2008, Reynolds et al., 2009, IUCN, 2012). Moreover, large whales still experience numerous anthropogenic pressures, which warrant closer investigations from a conservation physiology perspective in order to better understand their resilience and ability to recover from overexploitation. Even with the cessation of commercial whaling in most countries, large whales still face chronic exposure to toxins and pollutants, global climate change, as well as several pathologies including naturally occurring parasite infections but also from water contamination by human activities (Van Bressem et al., 2009, Kleinertz et al., 2014b, Moore, 2014).
Large cetaceans can be affected by a wide range of endo- and ectoparasites, which have been the focus of numerous reports mainly dealing with taxonomy, distribution and ecology. Furthermore, a number of parasite species have gained importance as opportunistic neozoan infections in the marine environment. This review focuses on those organisms which we perceive as pathogenic for baleen and sperm whales (known collectively as large whales) as well as on new emerging and neglected neozoan parasites, mainly protozoan parasites. Nonetheless, traditional approaches to studying parasitic diseases have been impractical for large whales, because there is no current routine method for capturing the largest whale species and there is presently no practical method for obtaining blood samples remotely from free-ranging whales. Thus, here we intend, additionally, to review current available methods for gathering parasitological information by using a variety of non- and minimally-invasive sample collection techniques. The following five types of non-(minimally) invasive sample collections are discussed: faecal samples, vomitus samples, respiratory samples, skin/blubber samples and photograph monitoring.
2. Endoparasites of large whales (Physeteridae, Balaenopteridae)
Sperm whales (Physeter macrocephalus) are the largest of the toothed whales and it is the only living member of the family Physeteridae. Sperm whales are apex predators and have a catholic diet: females preferentially prey upon mesopelagic cephalopods, in addition to cephalopods, and males consume demersal fish species including gadoids, sharks and rays (Whitehead, 2009). A wide range of helminths including mainly cestodes, nematodes, trematodes and acanthocephalan parasite species can parasitize sperm whales (see Table 1).
Table 1.
Groups of endo- and ectoparasite species and their site of infection in large whale species (Balaenopteridae, Physeteridae).
| Parasite | Whale species |
Site of infection | |||
|---|---|---|---|---|---|
| Blue whale | Sei whale | Fin whale | Sperm whale | ||
| Protozoa | |||||
| Giardia | + | + | Small intestine | ||
| Entamoeba | + | + | + | Large intestine | |
| Balantidium | + | Large intestine | |||
| Trematoda | |||||
| Zalophotrema | + | Liver/pancreas | |||
| Ogmogaster | + | + | + | Stomach/intestine | |
| Lecithodesmus | + | + | + | Stomach/intestine | |
| Nasitrema | + | + | + | Nasal sinuses | |
| Cestoda | |||||
| Tetrabothrium | + | + | + | + | Small intestine |
| Tetrabothrius | + | + | + | + | Small intestine |
| Trigonocotyle | + | + | + | + | Small intestine |
| Priapocephalus | + | + | + | + | Small intestine |
| Diplogonoporus | + | + | + | + | Liver/intestine |
| Hexagonoporus | + | + | + | + | Small intestine |
| Phyllobotrium | + | + | + | + | Subdermis |
| Acanthocephala | |||||
| Bolbosoma | + | + | + | + | Stomach/intestine |
| Corynosoma | + | Stomach/intestine | |||
| Nematoda | |||||
| Anisakis | + | + | + | + | Stomach |
| Contracaecum | + | + | + | Stomach | |
| Porrocaecum | + | + | + | Stomach | |
| Placentonema | + | Uterus/kidney | |||
| Crassicauda | + | + | + | Uterus/kidney | |
| Stenurus | + | + | + | Lungs | |
| Arthropoda | |||||
| Coronula | + | + | + | + | Skin/subdermis |
| Xenobalanus | + | + | + | Skin/subdermis | |
| Penella | + | + | + | Skin/subdermis | |
| Cyamus | + | + | + | + | Skin |
| Neocyamus | + | + | + | + | Skin |
| Agnatha (fish) | |||||
| Entosphenus | + | + | + | + | Skin/subdermis |
The cestode fauna of sperm whales consists of species from the Tetrabothriidae, Diphyllobothriidae and Phyllobothriidae: Tetrabothrius curilensis, Tetrabothrium wilsoni, Trigonocotyle sp., Priapocephalus grandis (Rees, 1953), Diplogonoporus sp. (Gubanov, 1951, Rees, 1953, Delyamure, 1955), Hexagonoporus physeteris and Phyllobotrium delphini (Baylis, 1932). Most of the tapeworms belonging to the families Tetrabothriidae and Diphyllobothriidae were found in the small intestine of sperm whales, with the exception of the genus Diplogonoporus, which may also reside in the bile ducts. Infections with mature pseudophyllideans, mainly Diphyllobotrium spp. are usually innocuous (Arundel, 1978), but may also cause debilitation and even death of heavily infected animals. Adult cestodes have been described to occasionally encyst within the intestine mucosa or to block the gut lumen (Cordes and O'Hara, 1979, Geraci and St Aubin, 1987). Moreover, plerocercoids cysts of Phyllobotrium spp. are the most commonly encountered parasites in cetaceans worldwide (Ruhnke and Workman, 2013). The definitive hosts of adult Phyllobotrium cestodes are several sharks as described elsewhere (Ruhnke and Workman, 2013, Kuris et al., 2015).
The most prevalent helminths found in sperm whales are gastric species belonging to the family Anisakidae with the species Anisakis ivanizkii (Delyamure, 1955), A. physeteris, A, simplex (Baylis, 1932) and A. skrjabini (Delyamure, 1955) and the large-sized spirurid nematode Placentonema gigantissima (up to 9 m long) which parasitizes the placenta, uterus, mammary gland and subdermis of these marine mammals (Delyamure, 1955) (see Table 1).
Also, intestinal acanthocephalan parasites, belonging to the genera Bolbosoma and Corynosoma, have been reported to occur in the stomach and intestine of sperm whales, including the species Balaenoptera brevicolle, Balaenoptera capitatum, Balaenoptera physeteris and Corynosoma curilensis (Baylis, 1932, Gubanov, 1951, Delyamure, 1955). Nevertheless, the genus Bolbosoma is primarily found in cetaceans, while the genus Corynosoma occurs mainly in pinnipeds (Golvan, 1959). The life cycle of marine acanthocephalans is heteroxenous, involving one or two intermediate hosts, being represented by crustaceans and fishes. Cystacanths of Bolbosoma balaenae were recently found encapsulated in the cephalothorax of the krill species Nyctiphanes couchii from temperate waters in the Northeast Atlantic Ocean (Gregori et al., 2012). It is therefore suggested that large whales become infected either by the oral uptake of Bolbosoma-infected krill species, or by feeding on a second intermediate host (fishes).
The only trematode parasite known so far of sperm whales is the species Zalophotrema curilensis (Delyamure, 1955), which resides in the bile duct and pancreas. Zalophotremosis is associated with parenchymal necrosis, chronic fibrosis and hyperplasia of bile ducts in other cetacean species (Woodard et al., 1969, Geraci and St Aubin, 1987), but very little is known on the pathogenesis of Z. curilensis in sperm whales.
In contrast to sperm whales, the mysticeti [baleen whales; (Balaenopteridae)] are the edentulous whales characterized by having baleen plates for filtering food from water. As a group they feed on a diverse assemblage of schooling planktonic and micronektonic crustaceans and small pelagic schooling fish, but diet composition varies much across species and geographic areas (Bannister, 2009, Groves and Grubb, 2011).
The endoparasite fauna of blue whales (Balaenoptera musculus), sei whales (Balaenoptera borealis) and fin whales (Balaenoptera physalus) resembles in principle the fauna of sperm whales, although they rely on entirely different diets. The gastrointestinal trematode fauna of large baleen whales is richer in species including Ogmogaster antarticus, O. plicatus, Lecithodesmus goliath and L. spinosus, which have been reported to parasitize the stomach as well as the small intestine of large whales (Baylis, 1932). Unfortunately, little is known on the pathogenesis of the genera Ogmogaster and Lecithodesmus in these marine mammals. No hepatic flukes have been described so far in any large baleen whale species.
The spectrum of cestode species found in the small intestine of large baleen whales (blue, fin and sei whales) is rather broad and includes the species Priapocephalus grandis, P. minor, Phyllobotrium delphini, Tetrabothrius affinis, T. arsenyevi, T. wilsoni, T. ruudi, Diphyllobotrium sp., and Diplogonoporus balaenoptera (Baylis, 1932, Delyamure, 1955). Furthermore, several nematode species parasitize other mysticeti, including ascarids such as Anisakis simplex, Contracaecum sp., Porrocaecum decipiens, and giant spirurid nematodes such as Crassicauda crassicauda, C. boopis and C. pacifica. Large baleen whales are known as definitive host of the anthropozoonotic nematode Anisakis sp. with a worldwide distribution (Delyamure, 1955, Klimpel et al., 2004, Nadler et al., 2005, Kleinertz et al., 2014a, Kleinertz et al., 2014b). Anisakis nematodes can be found in the lumen of the stomach or firmly attached, often as clusters, to the gastric mucosa (Geraci and St Aubin, 1987). Mucosal penetration by pre-adult larvae (Young and Lowe, 1969) and adults (McClelland, 1980) can lead to severe ulcers, gastritis and even perforation leading to severe peritonitis (Geraci and St Aubin, 1987). Giant nematodes species (up to 9 m in length) of the subfamily Crassicaudinae (Anderson, 1992), such as C. crassicauda and Crassicauda delamureana, are generally found in the kidneys, renal veins and intrarenal ureters of fin, blue and sei whales in the North Atlantic (Baylis, 1932, Skriabin, 1966, Lambertsen, 1985). The transmission routes of these parasites have not been adequately studied yet, but as typical members of the heteroxenous spirurids, it appears likely that they use arthropods as intermediate hosts promoting infective stage larvae development (Anderson, 1992).
The genus Bolbosoma (with the species B. balaenae, Balaenoptera brevocolle, Balaenoptera hamiltoni, Balaenoptera nipponicum and B. turbinella) is the main acantocephalan and lives in the small intestine of large baleen whales (Baylis, 1932). High parasitic burdens have been associated with mucosal ulceration and even intestinal wall perforation (Morejohn et al., 1975).
In addition to these metazoans, protozoan parasites have been recently reported to occur in large baleen whales inhabiting the North Atlantic Ocean. Thus, the enteropathogens Entamoeba, Giardia and Balantidium have recently been described for the first time in blue, sei and fin whales (Hermosilla et al., 2015). In the past, particularly protozoan enteropathogens have not obtained sufficient attention in whale investigations, although they endemically occur in the marine environment (Raga et al., 1997, Hughes-Hanks et al., 2005, Raga et al., 2008, Van Bressem et al., 2009, Oliveira et al., 2011, Kleinertz et al., 2014b, Reboredo-Fernandez et al., 2014).
3. Ectoparasites of large whales (Physeteridae, Balaenopteridae)
Referring to marine mammal studies, the term ‘ectoparasite’ has rather loosely been used for any type of organisms (ranging from algae to fish) that somehow clings or attaches to the surface of a marine mammal, and whose mode of attachment, feeding behaviour, or relationship with the definitive host or transport animal (phoresis) are somehow obscure so that a parasitic origin cannot be excluded (Geraci and St Aubin, 1987). What seems undoubted is the fact that many of these organisms have evolved as real ectoparasites of large whales and thus bear the potential to damage their sensitive epidermis. As such, blue whales entering the cold waters of the Antarctic often acquire a yellowish film on their bodies (commonly known as ‘sulfur bottoms’) resulting from diatom infestations, such as Cocconeis ceticola and Navicola spp. (Hart, 1935). These organisms attach firmly to the whale skin surface by sucker-like valves. Occasionally they may penetrate the epidermis thereby becoming saprophytic ectoparasites (Hart, 1935). However, most large whales being infested with diatoms shed these when returning to warmer or subtropical waters (Hart, 1935).
Most ectoparasite infestations are caused by arthropods, which have adapted to the oceanic environment. Such are the epizoic sessile barnacles Coronula diadema, C. reginae and Crytolepas rhachianecti which attach so deeply onto their host skins that a pit remains when being removed or shed (Félix et al., 2006) which eventually results in a scar (Scheffer, 1939). In addition, the closely related aberrant sessile barnacle, Xenobalanus globicipitis, may also cause characteristic star-shaped scars on the epidermis of whales (Bane and Zullo, 1980).
A more serious skin-invader of large whales is Penella balaenoptera (Pennellidae), one of the largest parasitic copepods in the ocean. It actively penetrates the skin of mysticetes and anchors deeply in the blubber, feeding on whale tissue (Clarke, 1966). Sadly, nothing is known on the vector-borne capacity of P. balaenoptera so far, but it can be assumed that it might play a pivotal role in the transmission of invasive pathogens as demonstrated for other marine ectoparasites, i. e. the seal lice Echinophthirius horridus transmitting the heartworm Acanthocheilonema spirocauda (Leidenberger et al., 2007).
Although whale lice (Amphipoda) are grouped into a single compact family (Cyamidae), they are heterogeneous and include at least 11 different species (Leung, 1970). Whale lice species, such as Cyamus catodontis, C. bahamondi, C. ovalis and Neocyamus physeteris parasitizing the skin of sperm whales, are rather considered as euryxenous parasites, whilst the whale lice C. balaenoptera tend to be more host-specific (for review see Leung, 1970). In general, cyamid ectoparasites reside in fissures and crevices of the skin and are often found close to sessile barnacles and on the callosities of the head (Scheffer, 1939, Leung, 1970). The detrimental impact of these organisms are low, nonetheless, in heavily infested animals dermatitis may occur (Leung, 1970).
Primitive fishes (Agnatha), belonging to the lamprey species such as Entosphenus tridentatus, are by far the largest ectoparasites firmly attaching to the skin of large whales. These are polyxenous ectoparasites feeding on cetacean skin as a blood/nutrient source amongst others. Nonetheless, while being firmly attached by the teeth of their sucking disc, the lampreys can cause severe and deep haemorrhagic skin lesions (Pike, 1951). As such, they represent one of the largest and most voracious marine mammal parasites on earth (Pike, 1951, Geraci and St Aubin, 1987).
4. New emerging neozoan parasites within the marine environment
The introduction of opportunistic emerging neozoan infections into the marine ecosystem is of major importance for animal and human welfare (Van Bressem et al., 2009, Kleinertz et al., 2014b, Moore, 2014). In this context, protozoan parasites, such as Toxoplasma gondii, Neospora caninum, Sarcocystis canis, Giardia sp., Cryptosporidium sp., Balantidium sp. and Entamoeba sp., behaved in the past decades as classical neozoan parasites within the marine habitat (Resendes et al., 2002b, Hughes-Hanks et al., 2005, Fujii et al., 2007, Raga et al., 2008, Van Bressem et al., 2009, Kleinertz et al., 2014b, Reichel et al., 2015) and have emerged as devastating pathogens for several pinnipeds (Cabezón et al., 2011), dolphins (Resendes et al., 2002b, Dubey et al., 2008), sea otters (Conrad et al., 2005, Miller et al., 2008) and whales (Inskeep et al., 1990, Omata et al., 2006, Van Bressem et al., 2009, Mazzariol et al., 2012). Apicomplexan-derived diseases such as toxoplasmosis in marine mammals is intriguing and at present indicative of contamination of the oceans and coastal waters with sporulated oocysts (Conrad et al., 2005, Dubey et al., 2008, Dubey, 2009, Reichel et al., 2015). As in the case of terrestrial mammals primary T. gondii-infections in facultative intermediate hosts, congenital toxoplasmosis have also been reported to occur in small-sized cetacean species such as the Indo-Pacific bottlenose dolphin (Tursiops aduncus) (Jardine and Dubey, 2002). Nonetheless, so far nothing is known for large whales with regards to apicomplexan parasite infections. Thus, protozoan parasite infections of humans as well as animals most probably occur by contaminated terrestrial and aquatic sources, emphasizing the need for joint examination of humans, domestic animals and wildlife populations (Resendes et al., 2002a, Kleinertz et al., 2014b) as proposed by the new ‘One Health’ concept (Kahn, 2015).
5. Alternative methods to overcome the challenges in studying large whale ecto- and endoparasites
Given that the direct study of physiological parameters and pathogen infections in free-ranging large cetaceans is necessary to monitor the population health, several methods were established for non-lethal sample assessment (Hunt et al., 2013). We here intend to review non- and minimally-invasive techniques, which can be applied to obtain faecal samples, skin and blubber biopsies, vomitus samples, respiratory vapour samples and photographies.
6. Faecal samples
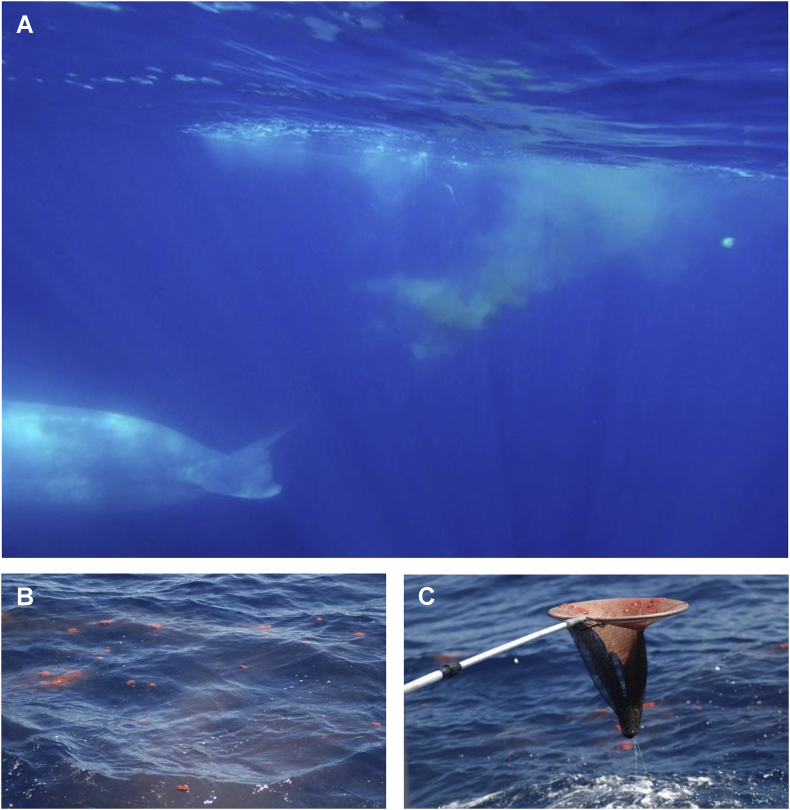
Large whale faecal consistency varies with the species, season and diet, ranging from well-formed floating semi-solid clumps (see Fig. 1) to a more fluid and dispersed plume. Floating semi-solid scat samples can be collected from the water surface (see Fig. 1B) using a fine-mesh nylon net fixed on a telescope (see Fig. 1C). More fluid faeces may be collected in plastic containers. Even heavier and rapidly sinking cetacean faecal material can be collected successfully by divers using plastic tubes as described elsewhere (Kleinertz et al., 2014b).
Fig. 1.
Non-invasive sampling method. Faecal sample collection from free-ranging whales in the North Atlantic Ocean, Portugal: (A) blue whale (Balaenoptera musculus) defecating, (B) floating blue whale (B. musculus) faeces, (C) telescope fixed net with collected blue whale faeces.
Several large whale species defecate on the ocean's surface prior to diving (Fig. 1). Consequently, faecal samples can be collected opportunistically during any large whale monitoring surveys. Faecal samples have been collected by researchers diving in close proximity to cetaceans (Kleinertz et al., 2014b). Moreover, large whale faeces can be identified on the water surface area by trained dogs, as recently demonstrated for North Atlantic right whales and killer whales (Rolland et al., 2007, Hunt et al., 2013).
7. Skin biopsies and blubber samples
Since the early 1990s, the ‘minimally invasive’ dart-based biopsy method has become one of the most common skin sampling techniques for free-swimming large whale species (Barrett-Lennard et al., 1996). Therefore, either a crossbow (see Fig. 2) or a pneumatic rifle with modified dart tips are used (Lambertsen, 1987, Mathews et al., 1988). Additionally, it might be feasible to collect sloughed skin samples at the water surface after vigorous whale activities, such as mating, diving (e. g. sperm whales) or parturition. Such samples have been successfully used before (Whitehead et al., 1990, Valsecchi et al., 1998, Pierszalowski et al., 2013), but their potential for parasitological analyses has not been clarified so far.
Fig. 2.
Minimally-invasive sampling method. Dart-based skin biopsy sampling using a crossbow from a free-ranging sperm whale (Physeter macrocephalus) in the North Atlantic Ocean, Portugal.
Especially polyxenous apicomplexan parasites such as T. gondii and N. caninum, being capable of parasitizing all type of nucleated host cells may be detected in host epithelial cells, fibroblasts, keratinocytes or even adipocytes. In summary, skin/blubber samples bear a wide spectrum of invaluable physiological information, ranging from epidermal lipidomics, proteomics, transcriptomics and hormones (Hunt et al., 2013), but in addition are also useful for the detection of ectoparasitic and dermal microbial diseases.
8. Vomitus samples
Although vomitus has seldom been reported to occur in large cetaceans and it is most probably happening particularly in marine mammals suffering acute oesophagitis or gastritis, it can be investigated. The option of sampling vomitus content is not a new one, since in recent years it has been realized that vomitus might represent a valuable, entirely non-invasive, physiopathological sample that could be collected with relative ease. In this context, a recent study on the gastrointestinal parasite fauna of free-ranging Indo-Pacific bottlenose dolphins (T. aduncus), demonstrated the usefulness of vomitus contents in addition to faeces (Kleinertz et al., 2014b). Consequently, large whale vomitus sampling should be included in the near future to efficiently monitor the animal infection status.
9. Respiratory samples (‘blow cloud’)
Large whales produce clouds of ‘blow’, which consist of exhaled droplets of condensed respiratory vapour when they exhale at the water surface. Whales usually blow several times during a single surfacing interval, and they can often be approached closely by floating boats during this event. In recent years, it has been realized that ‘blow clouds’ may represent valuable, entirely non-invasive samples, since studies on humans and other terrestrial animals showed that breath contains a varying mixture of volatile compounds in the gaseous phase (see Hunt et al., 2013). Furthermore, breath samples may also be used to detect the presence of bacterial, fungal, viral and parasitic infections. There is no doubt that particularly pseudaliid nematodes (Metastrongylidae) can cause severe pneumonia in whales. Cetaceans may act as definitive hosts for the lungworms Pseudalis inflexus, Stenurus minor, Torynurus convolutus and Halocercus invaginatus, all of them residing in the main-stem bronchi (Arnold and Gaskin, 1975, Geraci and St Aubin, 1987). Thus the ‘blow cloud’ might be used for the detection of either lungworm larvae or eggs as described by Woodard et al. (1969). ‘Blow cloud’ droplets have been collected from large whales using a variety of sampling devices being attached to elongated poles and being positioned over the blowholes (Hogg et al., 2009, Schroeder et al., 2009, Hunt et al., 2012).
10. Imaging techniques for large whales
The visual assessment of the external appearance of an individual animal is essential for the determination of its health and nutritional status. Furthermore, visible aspects, such as skin condition, fresh wounds and ectoparasite loads and distribution (e. g. diatoms, sessile barnacles, whale lice and lampreys) provide additional information on individual and population health. In contrast to small cetaceans, where skin health is assessed by capture and release of the animals as shown elsewhere (Wells et al., 2004), this procedure is not applicable to large whales. Thus, a series of photography-based studies were performed in the last decades using remote observational methods. Most imaging techniques to assess whole body condition and appearance of large whales have been based on photographs taken by airplanes, drones or large-sized ships. In contrast, boat-based photography permits close-up views of the skin. The presence, severity and extent of macroscopic ectoparasite infestations around the head, eyes and the blowholes, and the presence of scars due to past parasite infestations can be easily evaluated by this non-invasive method.
11. Conclusions
In contrast to past parasitological investigations carried out exclusively on killed or stranded large whales, we here review several newly minimal invasive sample collection methods which can be used for future studies on cetacean ecto- and endoparasite fauna. More importantly, these methods will allow us to gain a broader knowledge on the current status of originally terrestrial parasitoses (e. g. neosporosis, toxoplasmosis, giardiasis, cryptospioridiosis, and sarcocystiosis) and also on new emerging anthropozoonotic parasites (e. g. entamoebiasis, balantidiasis) affecting endangered marine species such as large whales. Large whales will continue to be subjected to a variety of natural- and human-related pressures. The constant surveillance of whale health status within their natural habitats via these techniques will be of benefit for monitoring purposes. As a further step, conservation studies on large whales will greatly benefit from cross-disciplinary approaches which may include not only researchers on behavioural ecology but also experts from other fields, i. e. parasitologists, microbiologists, nutritionists, toxicologists, endocrinologist and clinicians.
Conflict of interest
The authors of this review declare that they do not have any conflicts of interest. Furthermore, the authors declare that they have no competing interests.
Acknowledgements
We are deeply thankful to those who contributed to the pictures included in this review: Marta Tobena (Fig. 1B, C) and N. Liebsich (Fig. 2). We further acknowledge funds and support from the Portuguese Fundação para a Ciência e a Tecnologia (FCT), Fundo Regional da Ciência, Tecnologia (FRCT), through research projects TRACE-PTDC/MAR/74071/2006 and MAPCET-M2.1.2/F/012/2011 [FEDER, the Competitiveness Factors Operational (COMPETE), QREN European Social Fund, and Proconvergencia Açores/EU Program]. We acknowledge funds provided by FCT to MARE and by the FRCT – Government of the Azores pluriannual funding. RP is supported by a research grant from the Azores Regional Fund for Science and Technology (M3.1.5/F/115/2012). MAS is supported by FCT through a Program Investigator FCT fellowship (IF/00943/2013).
Contributor Information
Carlos Hermosilla, Email: Carlos.R.Hermosilla@vetmed.uni-giessen.de.
Liliana M.R. Silva, Email: lilianasilva.mv@gmail.com.
Rui Prieto, Email: rcabprieto@gmail.com.
Sonja Kleinertz, Email: sonja.kleinertz@uni-rostock.de.
Anja Taubert, Email: anja.taubert@vetmed.uni-giessen.de.
Monica A. Silva, Email: monica@uac.pt.
References
- Anderson R.C. CABI Pub.; Cambridge: 1992. Nematode Parasites of Vertebrates: Their Development and Transmission. [Google Scholar]
- Arnold P.W., Gaskin D.E. Lungworms (Metastrongyloidea: Pseudaliidae) of harbor porpoise Phocoena phocoena (L. 1758) Can. J. Zool. 1975;53:713–735. doi: 10.1139/z75-087. [DOI] [PubMed] [Google Scholar]
- Arundel J.H. Parasites and parasitic diseases of Australian marina mammals. In: House L., editor. Post-Graduate Committee in Veterinary Science, Proceedings No. 36 of Course for Veterinarians. The University of Sidney; 1978. pp. 323–333. Sidney, Australia. [Google Scholar]
- Bane G.W., Zullo V.A. Observations on a stranded goosebeaked whale (Ziphius cavirostris, Cuvier 1823) and its ectocommensal barnacles (Xenobalanus globicipitis) J. Elisha Mitchell Sci. Soc. 1980;96:1–3. [Google Scholar]
- Bannister J.L. Baleen whales (Mysticetes) In: Perrin W.F., Würsig B., Thewissen J.G.M., editors. Encyclopedia of Marine Mammals. second ed. Academic Press; New York, USA: 2009. pp. 80–89. [Google Scholar]
- Barrett-Lennard L.G., Smith T.G., Ellis G.M. A cetacean biopsy system using lightweight pneumatic darts, and its effect on the behavior of killer whales. Mar. Mammal. Sci. 1996;12:14–27. [Google Scholar]
- Baylis H.A. A list of worms parasitic in Cetacea. Discov. Rep. 1932;6:26. [Google Scholar]
- Cabezón O., Hall A.J., Vincent C., Pabon M., Garcia-Bocanegra I., Dubey J.P., Almeria S. Seroprevalence of Toxoplasma gondii in North-eastern Atlantic harbor seal (Phoca vitulina vitulina) and grey seal (Halichoerus grypus) Vet. Parasitol. 2011;179:253–256. doi: 10.1016/j.vetpar.2011.01.046. [DOI] [PubMed] [Google Scholar]
- Clapham P., Young S., Brownell R. Baleen whales: conservation issues and the status of the most endangered populations. Mamm. Rev. 1999;29:35–60. [Google Scholar]
- Clarke R. The stalked barnacle Conchoderma, ectoprasitic on whales. Nor. Hvalfangst-Tidene Norwigian Whal. Gaz. 1966;55:153–168. [Google Scholar]
- Conrad P.A., Miller M.A., Kreuder C., James E.R., Mazet J., Dabritz H., Jessup D.A., Gulland F., Grigg M.E. Transmission of Toxoplasma: clues from the study of sea otters as sentinels of Toxoplasma gondii flow into the marine environment. Int. J. Parasitol. 2005;35:1155–1168. doi: 10.1016/j.ijpara.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Cordes D.O., O'Hara P.J. Diseases of captive marine mammals. New Zeal. Vet. J. 1979;27:147–150. doi: 10.1080/00480169.1979.34630. [DOI] [PubMed] [Google Scholar]
- Delyamure S.L. Izdatel'stvo Akiademii Nauk SSSR; Moscow: 1955. Helminthofauna of Marine Mammals (Ecology and Phylogeny) [Google Scholar]
- Dubey J.P. History of the discovery of the life cycle of Toxoplasma gondii. Int. J. Parasitol. 2009;39:877–882. doi: 10.1016/j.ijpara.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Fair P.A., Sundar N., Velmurugan G., Kwok O.C., McFee W.E., Majumdar D., Su C. Isolation of Toxoplasma gondii from bottlenose dolphins (Tursiops truncatus) J. Parasitol. 2008;94:821–823. doi: 10.1645/GE-1444.1. [DOI] [PubMed] [Google Scholar]
- Félix F., Bearson B., Falconí J. Epizoic barnacles removed from the skin of a humpback whale after a period of intense surface activity. Mar. Mammal. Sci. 2006;22:979–984. [Google Scholar]
- Freeman M.M.R. Challenges of assessing cetacean population recovery and conservation status. Endang. Species Res. 2008;6:173–184. [Google Scholar]
- Fujii K., Kakumoto C., Kobayashi M., Saito S., Kariya T., Watanabe Y., Xuan X., Igarashi I., Suzuki M. Seroepidemiology of Toxoplasma gondii and Neospora caninum in seals around Hokkaido. Jpn. J. Vet. Med. Sci. 2007;69:393–398. doi: 10.1292/jvms.69.393. [DOI] [PubMed] [Google Scholar]
- Geraci J.R., St Aubin D.J. Effects of parasites on marine mammals. Int. J. Parasitol. 1987;17:407–414. doi: 10.1016/0020-7519(87)90116-0. [DOI] [PubMed] [Google Scholar]
- Golvan Y.J. A canthocephala of the genus Corynosoma Luhe 1904, mammalian parasites from Alaska & Midway. Ann. Parasit. Hum. Comp. 1959;34:288–321. doi: 10.1051/parasite/1959343288. [DOI] [PubMed] [Google Scholar]
- Gregori M., Aznar F.J., Abollo E., Roura A., Gonzalez A.F., Pascual S. Nyctiphanes couchii as intermediate host for the acanthocephalan Bolbosoma balaenae in temperate waters of the NE Atlantic. Dis. Aquat. Organ. 2012;99:37–47. doi: 10.3354/dao02457. [DOI] [PubMed] [Google Scholar]
- Groves C., Grubb P. JHU Press; 2011. Ungulate Taxonomy. [Google Scholar]
- Gubanov N.M. Giant nematoda from the placenta of Cetacea; Placentonema gigantissima nov. gen., nov. sp. Dokl. Akad. Nauk. SSSR. 1951;77:1123–1125. [PubMed] [Google Scholar]
- Hart T.J. On the diatoms of the skin film of whales, and their possible bearing on problems of whale movements. Discov. Rep. 1935;10:247–282. [Google Scholar]
- Hermosilla C., Silva L.M.R., Kleinertz S., Prieto R., Silva M.A., Taubert A. Endoparasite survey of free-swimming baleen whales (Balaenoptera musculus, B. physalus, B. borealis) and sperm whales (Physeter macrocephalus) using non/minimally-invasive methods. Parasitol. Res. 2015 doi: 10.1007/s00436-015-4835-y. (in press), http://dx.doi.org/10.1007/s00436-015-4835-y. [DOI] [PubMed] [Google Scholar]
- Hogg C.J., Rogers T.L., Shorter A., Barton K., Miller P.J.O., Nowacek D. Determination of steroid hormones in whale blow: it is possible. Mar. Mamm. Sci. 2009;25:605–618. [Google Scholar]
- Hughes-Hanks J.M., Rickard L.G., Panuska C., Saucier J.R., O'Hara T.M., Dehn L., Rolland R.M. Prevalence of Cryptosporidium spp. and Giardia spp. in five marine mammal species. J. Parasitol. 2005;91:1225–1228. doi: 10.1645/GE-545R.1. [DOI] [PubMed] [Google Scholar]
- Hunt K.E., Moore M.J., Rolland R.M., Kellar N.M., Hall A.J., Kershaw J., Raverty S.A., Davis C.E., Yeates L.C., Fauquier D.A., Rowles T.K., Kraus S.D. Overcoming the challenges of studying conservation physiology in large whales: a review of available methods. Conserv. Physiol. 2013;1 doi: 10.1093/conphys/cot006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt K.E., Rolland R., Kraus S. 2012. Development of Respiratory Sampling to Assess Stress Responses in North Atlantic Right Whales. (Annual Report to the US Office of Naval Research, Arlington, VA) [Google Scholar]
- Inskeep W., 2nd, Gardiner C.H., Harris R.K., Dubey J.P., Goldston R.T. Toxoplasmosis in Atlantic bottle-nosed dolphins (Tursiops truncatus) J. Wildl. Dis. 1990;26:377–382. doi: 10.7589/0090-3558-26.3.377. [DOI] [PubMed] [Google Scholar]
- IUCN . 2012. The IUCN Red List of Threatened Specied.www.iucnredlist.org Version 2012.2. [Google Scholar]
- Jardine J.E., Dubey J.P. Congenital toxoplasmosis in a Indo-Pacific bottlenose dolphin (Tursiops aduncus) J. Parasitol. 2002;88:197–199. doi: 10.1645/0022-3395(2002)088[0197:CTIAIP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Kahn L. One health: a concept for the 21st century. WMA J. 2015;61:2. [Google Scholar]
- Kleinertz S., Damriyasa I.M., Hagen W., Theisen S., Palm H.W. An environmental assessment of the parasite fauna of the reef-associated grouper Epinephelus areolatus from Indonesian waters. J. Helminthol. 2014;88:50–63. doi: 10.1017/S0022149X12000715. [DOI] [PubMed] [Google Scholar]
- Kleinertz S., Hermosilla C., Ziltener A., Kreicker S., Hirzmann J., Abdel-Ghaffar F., Taubert A. Gastrointestinal parasites of free-living Indo-Pacific bottlenose dolphins (Tursiops aduncus) in the Northern Red Sea, Egypt. Parasitol. Res. 2014;113:1405–1415. doi: 10.1007/s00436-014-3781-4. [DOI] [PubMed] [Google Scholar]
- Klimpel S., Palm H.W., Ruckert S., Piatkowski U. The life cycle of Anisakis simplex in the Norwegian Deep (northern North Sea) Parasitol. Res. 2004;94:1–9. doi: 10.1007/s00436-004-1154-0. [DOI] [PubMed] [Google Scholar]
- Kraus S.D., Brown M.W., Caswell H., Clark C.W., Fujiwara M., Hamilton P.K., Kenney R.D., Knowlton A.R., Landry S., Mayo C.A., McLellan W.A., Moore M.J., Nowacek D.P., Pabst D.A., Read A.J., Rolland R.M. Ecology. North Atlantic right whales in crisis. Science. 2005;309:561–562. doi: 10.1126/science.1111200. [DOI] [PubMed] [Google Scholar]
- Kuris A.M., Jaramillo A.G., McLaughlin J.P., Weinstein S.B., Garcia-Vedrenne A.E., Poinar G.O., Jr., Pickering M., Steinauer M.L., Espinoza M., Ashford J.E., Dunn G.L. Monsters of the sea serpent: parasites of an oarfish, Regalecus russellii. J. Parasitol. 2015;101:41–44. doi: 10.1645/14-581.1. [DOI] [PubMed] [Google Scholar]
- Lambertsen R.H. Taxonomy and distribution of a Crassicauda species (Nematoda: Spirurida) infecting the kidney of the common fin whale (Balaenoptera physalus Linne, 1758) J. Parasitol. 1985;71:485–488. [PubMed] [Google Scholar]
- Lambertsen R.H. A biopsy system for large whale and its use for cytogenetics. J. Mammal. 1987;68:443–445. [Google Scholar]
- Leidenberger S., Harding K., Harkonen T. Phocid seals, seal lice and heartworms: a terrestrial host-parasite system conveyed to the marine environment. Dis. Aquat. Organ. 2007;77:235–253. doi: 10.3354/dao01823. [DOI] [PubMed] [Google Scholar]
- Leung Y.M. First record of the whale-louse genus Syncyamus (Cyamidae: Amphipoda) from the western Mediterranean, with notes on the biology of adontocete cyamids. In: Pilleri G., editor. Investigations of Cetacea. Benteli Ag, Berne-Bumpliz, Switzerland. 1970. pp. 243–247. [Google Scholar]
- Mathews E.A., Keller S., Weiner D.B. A method to collect and process skin biopsies for cell-culture from free-ranging gray whales (Eschrichtius robustus) Mar. Mammal. Sci. 1988;4:1–12. [Google Scholar]
- Mazzariol S., Marcer F., Mignone W., Serracca L., Goria M., Marsili L., Di Guardo G., Casalone C. Dolphin Morbillivirus and Toxoplasma gondii coinfection in a Mediterranean fin whale (Balaenoptera physalus) BMC Vet. Res. 2012;8:20. doi: 10.1186/1746-6148-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland G. Phocanema decipiens: pathology in seals. Exp. Parasitol. 1980;49:405–419. doi: 10.1016/0014-4894(80)90075-2. [DOI] [PubMed] [Google Scholar]
- Miller M.A., Miller W.A., Conrad P.A., James E.R., Melli A.C., Leutenegger C.M., Dabritz H.A., Packham A.E., Paradies D., Harris M., Ames J., Jessup D.A., Worcester K., Grigg M.E. Type X Toxoplasma gondii in a wild mussel and terrestrial carnivores from coastal California: new linkages between terrestrial mammals, runoff and toxoplasmosis of sea otters. Int. J. Parasitol. 2008;38:1319–1328. doi: 10.1016/j.ijpara.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Moore M.J. How we all kill whales. ICES J. Mar. Sci. 2014;71:760–763. [Google Scholar]
- Morejohn G.V., Ames J.A., Lewis D.B. Post mortem studies of sea otters, Enhydra lutris L., in California. In: Game C.D.O.F., editor. Marine Resources Technical Report. 1975. p. 82. [Google Scholar]
- Nadler S.A., D'Amelio S., Dailey M.D., Paggi L., Siu S., Sakanari J.A. Molecular phylogenetics and diagnosis of Anisakis, Pseudoterranova, and Contracaecum from northern Pacific marine mammals. J. Parasitol. 2005;91:1413–1429. doi: 10.1645/GE-522R.1. [DOI] [PubMed] [Google Scholar]
- Oliveira J.B., Morales J.A., Gonzalez-Barrientos R.C., Hernandez-Gamboa J., Hernandez-Mora G. Parasites of cetaceans stranded on the Pacific coast of Costa Rica. Vet. Parasitol. 2011;182:319–328. doi: 10.1016/j.vetpar.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Omata Y., Umeshita Y., Watarai M., Tachibana M., Sasaki M., Murata K., Yamada T.K. Investigation for presence of Neospora caninum, Toxoplasma gondii and Brucella-species infection in killer whales (Orcinus orca) mass-stranded on the coast of Shiretoko, Hokkaido. Jpn. J. Vet. Med. Sci. 2006;68:523–526. doi: 10.1292/jvms.68.523. [DOI] [PubMed] [Google Scholar]
- Pierszalowski S.P., Ferrari M., Glockner-Ferrari D., Mizroch S., Clapham P.J., Dickerson B.R. Investigating the feasibility of using DNA from sloughed skin for individual identification and kinship analysis in humpback whales (Megaptera novaeangliae) Mar. Mammal. Sci. 2013;29:533–541. [Google Scholar]
- Pike G.C. Lamprey marks on whales. J. Fish. Res. B. Can. 1951;8:275–280. [Google Scholar]
- Raga J.A., Balbuena J.A., Aznar J., Fernandez M. The impact of parasites on marine mammals: a review. Parassitologia. 1997;39:293–296. [PubMed] [Google Scholar]
- Raga J.A., Fernandez M., Balbuena J.A., Aznar F.J. Parasites. In: Perrin W.F., Thewissen H.G.M., Würsing B., editors. Encyclopedia of Marine Mammals. second ed. Academic Press Elsevier Inc.; San Diego: 2008. pp. 821–830. [Google Scholar]
- Reboredo-Fernandez A., Gomez-Couso H., Martinez-Cedeira J.A., Caccio S.M., Ares-Mazas E. Detection and molecular characterization of Giardia and Cryptosporidium in common dolphins (Delphinus delphis) stranded along the Galician coast (Northwest Spain) Vet. Parasitol. 2014;202:132–137. doi: 10.1016/j.vetpar.2014.03.018. [DOI] [PubMed] [Google Scholar]
- Rees G. A record of some parasitic worms from whales in the Ross sea area. Parasitology. 1953;43:27–34. doi: 10.1017/s003118200001831x. [DOI] [PubMed] [Google Scholar]
- Reichel M., Munoz-Caro T., Sanchez Contreras G., Rubio Garcia A., Magdowski G., Gartner U., Taubert A., Hermosilla C. Harbour seal (Phoca vitulina) PMN and monocytes release extracellular traps to capture the apicomplexan parasite Toxoplasma gondii. Dev. Comp. Immunol. 2015;50:106–115. doi: 10.1016/j.dci.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Resendes A.R., Almeria S., Dubey J.P., Obon E., Juan-Salles C., Degollada E., Alegre F., Cabezon O., Pont S., Domingo M. Disseminated toxoplasmosis in a Mediterranean pregnant Risso's dolphin (Grampus griseus) with transplacental fetal infection. J. Parasitol. 2002;88:1029–1032. doi: 10.1645/0022-3395(2002)088[1029:DTIAMP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Resendes A.R., Juan-Salles C., Almeria S., Majo N., Domingo M., Dubey J.P. Hepatic sarcocystosis in a striped dolphin (Stenella coeruleoalba) from the Spanish Mediterranean coast. J. Parasitol. 2002;88:206–209. doi: 10.1645/0022-3395(2002)088[0206:HSIASD]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Reynolds J.E., Marsh H., Ragen T.J. Marine mammal conservation. Endang. Species Res. 2009;7:23–28. [Google Scholar]
- Rolland R.M., Hunt K.E., Doucette G.J., Rickard L.G., Wasser S.K. The inner whale: hormones, biotoxins and parasites. In: Kraus S.D., Rolland R.M., editors. The Urban Whale: North Atlantic Right Whales at the Crossroads. Harvard University Press; Cambridge, MA: 2007. pp. 232–272. [Google Scholar]
- Ruhnke T.R., Workman R.E. Two new species and a new phyllobothriid cestode genus from sharks of the genus Negaprion Whitley (Carcharhiniformes) Syst. Parasitol. 2013;85:37–48. doi: 10.1007/s11230-013-9411-1. [DOI] [PubMed] [Google Scholar]
- Scheffer V.B. Organisms collected from whales in the Aleutian Islands. Murrelet. 1939;20:67. [Google Scholar]
- Schroeder P., Raverty S., Cameron C., Zabek E., Eshghi A., Bain D., Wood B., Rhodes L., Hanson B. National Oceanic and Atmospheric Administration; Seattle, WA, USA: 2009. Investigation into the Microbial Culture and Molecular Screening of Exhaled Breaths of Endagered Southern Resident Killer Whales (SRKW) and Pathogen Screening of the Seasurface Microlayer (SML) in Puget Sound; p. 8. Report to the Northwest Fisheries Science Center. [Google Scholar]
- Skriabin A.S. Naukova Dumka; Kiev: 1966. A New Crassicauda (Crassicauda delamureana n. sp.) -Parasite of the Sea Whale. [In Russ.] [Google Scholar]
- Valsecchi E., Glockner-Ferrari D., Ferrari M., Amos W. Molecular analysis of the efficiency of sloughed skin sampling in whale population genetics. Mol. Ecol. 1998:1419–1422. doi: 10.1046/j.1365-294x.1998.00446.x. [DOI] [PubMed] [Google Scholar]
- Van Bressem M.F., Raga J.A., Di Guardo G., Jepson P.D., Duignan P.J., Siebert U., Barrett T., Santos M.C., Moreno I.B., Siciliano S., Aguilar A., Van Waerebeek K. Emerging infectious diseases in cetaceans worldwide and the possible role of environmental stressors. Dis. Aquat. Organ. 2009;86:143–157. doi: 10.3354/dao02101. [DOI] [PubMed] [Google Scholar]
- Wells R.S., Rhinehart H.L., Hansen L.J., Sweeney J.C., Townsend F.I., Stone R., Casper D.R., Scott M.D., Hohn A.A., Rowles T.K. Bottlenose dolphins as marine ecosystem sentinels: developing a health monitoring system. Ecohealth. 2004;1:246–254. [Google Scholar]
- Whitehead H. Sperm whale Physter macrocephalus. In: Perrin W.F., Würsig B., Thewissen J.G.M., editors. Encyclopedia of Marine Mammals. second ed. Academic Press; New York, USA: 2009. pp. 1091–1097. [Google Scholar]
- Whitehead H., Gordon J., Mathews E.A., Richard K.R. Obtaining skin samples from living sperm whales. Mar. Mammal. Sci. 1990;6:316–326. [Google Scholar]
- Woodard J.C., Zam S.G., Caldwell D.K., Caldwell M.C. Some parasitic diseases of dolphins. Pathol. Vet. 1969;6:257–272. doi: 10.1177/030098586900600307. [DOI] [PubMed] [Google Scholar]
- Young P.C., Lowe D. Larval nematodes from fish of the subfamily Anisakinae and gastro-intestinal lesions in mammals. J. Comp. Pathol. 1969;79:301–313. doi: 10.1016/0021-9975(69)90043-7. [DOI] [PubMed] [Google Scholar]