Abstract
Background
Respiratory virus infections are commonly associated with COPD exacerbations, but little is known about the mechanisms linking virus infection to exacerbations. Pathogenic mechanisms in stable COPD include oxidative and nitrosative stress and reduced activity of histone deacetylase-2 (HDAC2), but their roles in COPD exacerbations is unknown. We investigated oxidative and nitrosative stress (O&NS) and HDAC2 in COPD exacerbations using experimental rhinovirus infection.
Methods
Nine subjects with COPD (Global Initiative for Chronic Obstructive Lung Disease stage II), 10 smokers, and 11 nonsmokers were successfully infected with rhinovirus. Markers of O&NS-associated cellular damage, and inflammatory mediators and proteases were measured in sputum, and HDAC2 activity was measured in sputum and bronchoalveolar macrophages. In an in vitro model, monocyte-derived THP-1 cells were infected with rhinovirus and nitrosylation and activity of HDAC2 was measured.
Results
Rhinovirus infection induced significant increases in airways inflammation and markers of O&NS in subjects with COPD. O&NS markers correlated with virus load and inflammatory markers. Macrophage HDAC2 activity was reduced during exacerbation and correlated inversely with virus load, inflammatory markers, and nitrosative stress. Sputum macrophage HDAC2 activity pre-infection was inversely associated with sputum virus load and inflammatory markers during exacerbation. Rhinovirus infection of monocytes induced nitrosylation of HDAC2 and reduced HDAC2 activity; inhibition of O&NS inhibited rhinovirus-induced inflammatory cytokines.
Conclusions
O&NS, airways inflammation, and impaired HDAC2 may be important mechanisms of virus-induced COPD exacerbations. Therapies targeting these mechanisms offer potential new treatments for COPD exacerbations.
Key Words: COPD, host defense, infection, viral disease
Abbreviations: 3-NT, 3-nitrotyrosine; 8-OHdG, 8-hydroxy-2'-deoxyguanosine; GM-CSF, granulocyte-macrophage colony-stimulating factor; GOLD, Global Initiative for Obstructive Lung Disease; HDAC2, histone deacetylase-2; MMP-9, matrix metalloprotease-9; NAC, N-acetylcysteine; O&NS, oxidative and nitrosative stress; TNF-α, tumor necrosis factor-α
COPD is a rapidly growing global epidemic.1 COPD exacerbations cause impaired quality of life, accelerated loss of lung function, and enormous healthcare costs.2 Most exacerbations are a consequence of viral and/or bacterial infections; the most common viruses identified are rhinoviruses.3 The mechanisms of virus-induced exacerbations remain unclear, however.4 Current therapies for exacerbations consist of corticosteroids and antibiotics, but these are not very effective, have frequent adverse effects, and are focused on bacterial exacerbations.5, 6 New treatments are urgently needed and developing these requires a better understanding of the mechanisms of COPD exacerbations.
Pathogenic mechanisms in stable COPD include oxidative and nitrosative stress (O&NS) and reduced expression of the antiinflammatory enzyme histone deacetylase-2 (HDAC2).7, 8, 9 High levels of reactive oxygen species and reactive nitrogen species are generated in COPD, resulting in induction of proinflammatory cytokines and chemokines, mucous hypersecretion, activation of proteases, and damage to cellular components.10 Histone deacetylases remove acetyl groups on histones and influence gene expression. HDAC2 suppresses inflammatory gene expression and is reduced in alveolar macrophages and lung tissue in COPD, and this is believed to be a key mechanism of corticosteroid resistance in COPD.8, 11 Nitrosylation of tyrosine residues on HDAC2 by peroxynitrite results in its inactivation12 and reactive oxygen species activate phosphoinositide 3-kinase, leading to phosphorylation and inactivation of HDAC2.13 Therefore, O&NS exerts proinflammatory effects both through direct induction of inflammatory mediators and reduction of the antiinflammatory action of HDAC2.
The roles of O&NS and HDAC2 in COPD exacerbations are unclear. We have previously demonstrated that experimental rhinovirus infection induces symptoms, airflow obstruction, and airways inflammation in subjects with COPD.14, 15, 16 We hypothesized that O&NS is increased and HDAC2 activity reduced in virus-induced COPD exacerbations.
Methods
Study Design
Ethical approval for the study was obtained from St Mary’s local research ethics committee (study no. 07/H0712/138) and informed consent obtained from all subjects. Subjects with COPD (Global Initiative for Obstructive Lung Disease [GOLD] stage II) and smokers with normal lung function were recruited according the criteria established in our previous study (e-Table 1),15 together with nonsmokers with normal lung function. The patients with COPD were receiving no inhaled corticosteroids, long-acting regular bronchodilators, or oral corticosteroids. Baseline samples of induced sputum and BAL were obtained approximately 14 days prior to infection and subjects were inoculated with 10 50% tissue culture infective doses of rhinovirus 16 on day 0, as described previously.15 Sputum sampling was repeated on subsequent visits on days 3, 5, 9, 12, 15, 21, and 42 postinfection and bronchoscopy on day 7. Respiratory symptoms were recorded daily using diary cards, per our previous studies (e-Table 2). A COPD exacerbation was defined as an increase in the total lower respiratory score of at least 2 points over baseline for at least 2 consecutive days.
Sputum Assessments
A marker of hydroxyl radical damage to DNA, 8-hydroxy-2'-deoxyguanosine (8-OHdG); 8-isoprostane, a marker of lipid peroxidation; and 3-nitrotyrosine (3-NT), a product of tyrosine nitration mediated by peroxynitrite, were measured in sputum supernatants using commercially available enzyme immunoassays (Cayman Chemical Co) according to the manufacturer’s instructions. Sputum nitrite levels were measured using the Griess assay. The Meso Scale Discovery platform (Meso Scale Discovery LLC) was used to measure inflammatory mediators in sputum and BAL supernatants. The mediators measured were the proinflammatory cytokines IL-1β, tumor necrosis factor (TNF)-α, and granulocyte-macrophage colony-stimulating factor (GM-CSF); the neutrophil chemokine CXCL8/IL-8; and the protease matrix metalloprotease-9 (MMP-9). Neutrophil elastase was measured using an enzyme-linked immunosorbent assay according to the manufacturers’ instructions (Immunodiagnostik AG). Further details are provided in the e-Appendix.
HDAC2 Immunoprecipitation and Activity
The HDAC2 isoenzyme was isolated from macrophage pellets obtained from sputum and BAL samples. Immunoprecipitation using a HDAC2 antibody (Insight Biotechnology Ltd) and PureProteome Protein A magnetic beads (EMD Millipore) was performed. HDAC activity was measured using an HDAC activity assay (Cayman Chemicals Co), the details of which are provided in the e-Appendix. Details of the experimental protocols of the in vitro infection of THP-1 cells (a human monocyte cell line) to further investigate the effects of rhinovirus infection on HDAC2 also are provided in the e-Appendix.
Statistical Analysis
Data are presented as either means or median. Changes from baseline were analyzed using repeated measures analysis of variance or the Friedman test, and differences between groups were analyzed using the Holm-Sidak multiple comparisons test or the Kruskal-Wallace test. Correlations between data sets were examined using the Spearman rank correlation coefficient. Correlations were examined using peak postinoculation values as in a dynamic and evolving clinical event, such as a respiratory infection. The temporal relationships between the induction of the different markers was variable between individual subjects, and using set days may miss relationships where timing of events varied. Differences were considered significant for all statistical tests at P values < .05. All reported P values are two-sided. Analysis was performed using GraphPad Prism version 6.00 for Windows (GraphPad Software Inc).
Results
Study Subjects
The clinical characteristics of the subjects successfully infected with rhinovirus 16 are shown in Table 1. Subjects were age and sex matched between the subjects with COPD and both control groups, but the nonsmokers were older than the control subjects who smoked. Cigarette smoke exposure between control subjects who smoked and subjects with COPD was matched for pack-year history.
Table 1.
Baseline Clinical Characteristics of Study Subjects
| Characteristic | Nonsmokers (n = 11) | Smokers (n = 10) | COPD (n = 9) | P Values |
|---|---|---|---|---|
| Age, y | 62.18 ± 1.62 | 52.50 ± 2.23 | 60.44 ± 3.17 | < .05 SMK vs NS |
| Sex, male:female | 4:7 | 4:6 | 6:3 | NS |
| Current smokers | 0 | 10/10 | 8/9 | … |
| Smoking history, pack-years | 0 | 32.1 ± 3.02 | 39.44 ± 3.25 | NS |
| FEV1, L | 3.17 ± 0.17 | 3.24 ± 0.21 | 2.31 ± 0.13 | < .05 COPD vs NS < .01 COPD vs SMK |
| FEV1 % predicted | 102.2 ± 3.34 | 96.60 ± 3.28 | 68.11 ± 1.58 | < .0001 COPD vs SMK and NS |
| FEV1/FVC | 77.62 ± 1.09 | 78.04 ± 2.18 | 61.58 ± 1.80 | < .0001 COPD vs SMK and NS |
Data given as mean ± SEM. NS = nonsmoker; SMK = smoker.
Clinical and Inflammatory Responses
Symptoms and Lung Function
All subjects with COPD experienced an exacerbation following rhinovirus infection (e-Fig 1).15 All exacerbations were level 1, according to the American Thoracic Society (ATS)/European Respiratory Society severity classification.17 FEV1 fell significantly from baseline in the subjects with COPD on days 3, 5 (P < .05), 9 (P < .01), 12 (P < .05), and 15 (P < .01), with a maximum fall of 250 mL (12.92%) on day 15. There were no significant falls from baseline in FEV1 in the control groups (e-Fig 2).
Sputum Inflammatory Cells, Neutrophil Elastase, and Virus Load
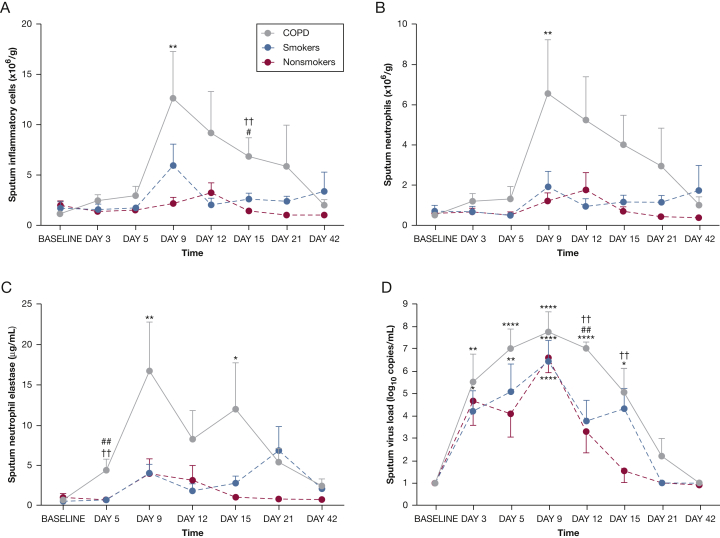
In the subjects with COPD, sputum inflammatory cells were significantly increased over baseline on day 9, and were significantly higher compared with the nonsmokers on days 9 and 15 (Fig 1A and e-Table 3). There was no significant change from baseline in the smokers and the nonsmokers. Sputum neutrophils increased significantly from baseline in the subjects with COPD on day 9 and were significantly higher compared with the smokers on day 12 (Fig 1B). There were no significant changes in numbers of neutrophils in either control group, nor in numbers of macrophages, lymphocytes, or eosinophils in any group. Levels of neutrophil elastase in sputum increased from baseline on days 9 and 15 in the subjects with COPD and were significantly higher in the COPD group compared with control subjects on day 5 (Fig 1C).
Figure 1.
Time course of inflammatory cells, neutrophil elastase, and virus load in sputum during experimental rhinovirus infection. A, Total sputum inflammatory cells (neutrophils, macrophages/monocytes, lymphocytes, eosinophils). B, Sputum neutrophils. C, Sputum neutrophil elastase. D, Sputum virus load. All data are given as mean ± SEM. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 vs baseline. †P < .05; ††P < .01 COPD vs nonsmokers. #P < .01; ##P < .01 COPD vs smokers.
Sputum virus load was increased from baseline on days 3-15 in the subjects with COPD, on days 5 and 9 in the smokers, and on days 3 and 9 in nonsmokers (Fig 1D). Sputum virus load was significantly higher in the COPD group compared with both control groups on day 12 and compared with the nonsmokers on day 15.
Sputum Inflammatory Mediators
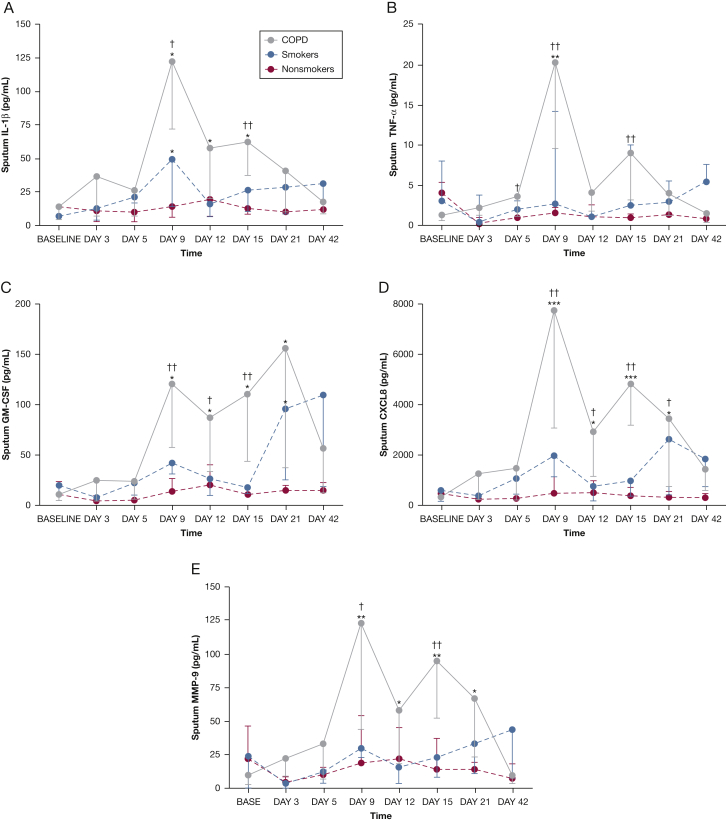
In the study subjects with COPD, there were significant increases from baseline in IL-1β, GM-CSF, CXCL8/IL-8, TNF-α, and MMP-9 in sputum (Fig 2). There was no significant induction of inflammatory mediators in the subjects without COPD apart from IL-1β on day 9 and GM-CSF on day 21 in the smokers. Sputum mediator levels were significantly higher in the subjects with COPD compared with the nonsmokers on days 9 and 15 and for some mediators on days 5, 12, and 21. Levels of inflammatory mediators in BAL both at baseline and postinoculation were generally lower than in sputum and are shown in e-Figure 3 and e-Table 4. Correlations between sputum inflammatory mediators, inflammatory cells, and virus load in the subjects with COPD are shown in Table 2.
Figure 2.
Time course of inflammatory mediators in sputum during experimental rhinovirus infection in COPD. A, Sputum IL-1β. B, Sputum TNF-α. C, Sputum GM-CSF. D, Sputum CXCL8/IL-8. E, Sputum MMP-9. All data are given as median ± IQR. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 vs baseline. †P < .05; ††P < .01 COPD vs nonsmokers. GM-CSF = granulocyte-macrophage colony-stimulating factor; IQR = interquartile range; MMP-9 = matrix metalloprotease-9; TNF-α = tumor necrosis factor-α.
Table 2.
Correlations Between Baseline Sputum HDAC2 Levels and Postinoculation BAL Macrophages HDAC2 Levels and Peak Levels of Sputum Inflammatory Mediators and Virus Load
| Sputum Inflammatory Mediators | Baseline Sputum HDAC2, P Value (r Value) | Postinoculation BAL Macrophages HDAC2 P Value (r Value) |
|---|---|---|
| Peak sputum virus load | .022 (−0.82) | … |
| Peak sputum neutrophil elastase | .022 (−0.81) | .0499 (−0.67) |
| Peak sputum CXCL8/IL-8 | .047 (−0.71) | … |
| Peak sputum TNF-α | .028 (−0.79) | .03 (−0.72) |
| Peak nasal lavage virus load | … | .0096 (−0.8) |
| Peak sputum GM-CSF | … | .0499 (−0.67) |
| Peak sputum nitrite | … | .0125 (−0.78) |
GM-CSF = granulocyte-macrophage colony-stimulating factor; HDAC2 = histone deacetylase-2; TNF-α = tumor necrosis factor-α.
O&NS
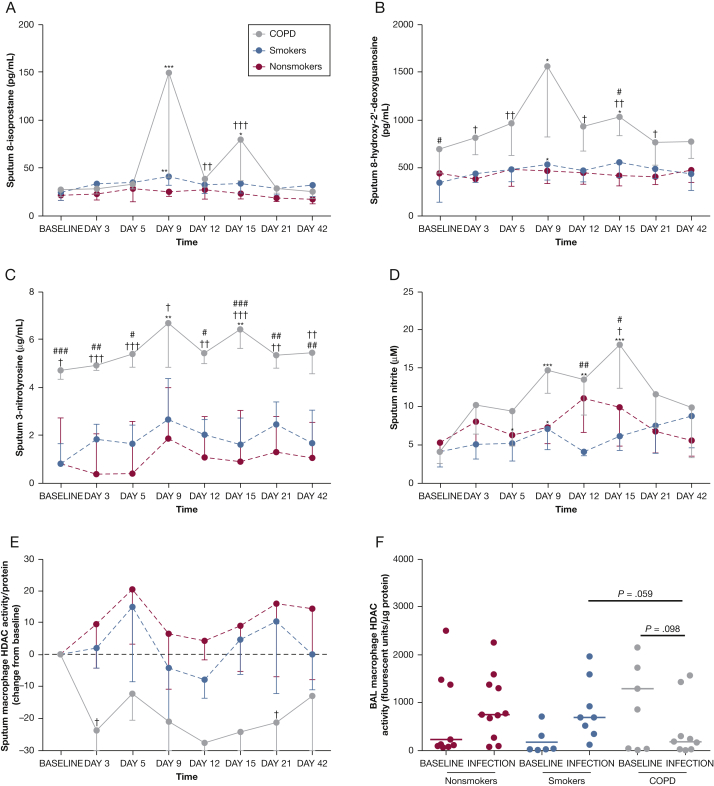
Prior to infection, sputum 8-OHdG levels were higher in the COPD group compared with the smokers (P < .05) and 3-NT levels were higher in the COPD group compared with nonsmokers (P < .05) and smokers (P < .001), with no differences between the groups in baseline levels of nitrite or 8-isoprostane (Fig 3). Markers of O&NS were all significantly induced following rhinovirus infection in subjects with COPD, with much smaller induction seen in the smokers, and were significantly higher in the subjects with COPD compared with the subjects without COPD (Figs 3A-D).
Figure 3.
Time course of oxidative and nitrosative stress markers in sputum and HDAC2 activity in sputum and BAL macrophages during experimental rhinovirus infection. A, Sputum 8-isoprostane. B, Sputum 8-hydroxy-2'-deoxyguanosine. C, Sputum 3-nitrotyrosine. D, Sputum nitrite. E, Sputum macrophage HDAC2 activity (change from baseline). F, BAL macrophage HDAC2 activity. All data are given as median ± IQR. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 vs baseline. †P < .05; ††P < .01; †††P < .001 COPD vs nonsmokers. #P < .05; ##P < .01; ###P < .001 COPD vs smokers. HDAC2 = histone deacetylase-2. See Figure 2 legend for expansion of other abbreviation.
Macrophage HDAC2 Activity
At baseline, there were no significant differences between the groups in HDAC2 activity in sputum (data not shown) or BAL macrophages (Fig 3F). Following infection, HDAC2 activity in the smoking control subjects and nonsmoking control subjects did not change significantly from baseline, but tended to increase (Figs 3E, 3F). In the subjects with COPD, there was a trend toward reduced HDAC2 activity in both sputum (P = .064) and BAL macrophages (P = .098) (Figs 3E, 3F). Sputum HDAC2 activity was significantly lower in the subjects with COPD compared with nonsmokers on days 5 and 21 (P < .05), and there was a trend toward lower levels of BAL HDAC2 activity during exacerbation compared with the nonsmokers (P = .095) and smokers (P = .059).
Correlations
Baseline HDAC2
Sputum macrophage HDAC2 activity at baseline correlated inversely with peak postinoculation sputum virus load (P = .022, r = −0.82), sputum neutrophil elastase (P = .022, r = −0.81), CXCL8/IL-8 (P = .047, r = −0.71), and TNF-α (P = .028, r = −0.79) (Table 2). There were no relationships between baseline HDAC2 activity and outcomes following infection in the subjects who did not have COPD.
Oxidative Stress
In the subjects with COPD, peak sputum 8-isoprostane levels and 8-OHdG levels during exacerbations correlated with each other (P = .0068, r = 0.82). 8-OHdG correlated with peak sputum neutrophil elastase (P = .0125, r = 0.78) and sputum 3-NT levels (P = .0016, r = 0.83).
Nitrosative Stress
Peak sputum 3-NT and sputum nitrite levels during exacerbation correlated with each other (P = .0037, r = 0.85). Peak sputum 3-NT level correlated with peak neutrophil elastase level (P = .0009, r = 0.9) and peak sputum nitrite level correlated with peak sputum inflammatory cell numbers (P = .0125, r = 0.778), neutrophil elastase (P = .042, r = 0.68), and neutrophil numbers (P = .0096, r = 0.8) (Table 3).
Table 3.
Correlations Between Peak Levels of Sputum Inflammatory Mediators, Virus Load, Sputum Cell Counts, and Markers of Oxidative and Nitrosative Stress in Subjects With COPD
| Inflammatory Mediators | Virus Load, P Value (r Value) | Inflammatory Cells and Neutrophil Markers, P Value (r Value) |
Oxidative Stress, P Value (r Value) |
Nitrosative Stress, P Value (r Value) |
||||
|---|---|---|---|---|---|---|---|---|
| Total Cell Count | Neutrophils | Neutrophil Elastase | 8-IP | 8-OHdG | 3-NT | Nitrite | ||
| IL-1β | NS | NS | .043 (0.7) | NS | NS | NS | .031 (0.73) | .017 (0.78) |
| TNF-α | NS | .031 (0.73) | .016 (0.77) | .026 (0.75) | NS | NS | .017 (0.78) | .0007 (0.93) |
| GM-CSF | NS | .021 (0.77) | .02 (0.75) | NS | NS | NS | NS | .0053 (0.83) |
| CXCL8/IL-8 | .0096 (0.8) | .0096 (0.8) | .0072 (0.82) | NS | NS | NS | NS | .05 (0.68) |
| MMP-9 | NS | .0031 (0.88) | .0025 (0.87) | NS | NS | NS | .0083 (0.83) | .0004 (0.95) |
8-IP = 8-isoprostane; 8-OHdG = 8-hydroxy-2’-deoxyguanosine; 3-NT = 3-nitrotyrosine; MMP-9 = matrix metalloprotease-9; NS = not significant. See Table 2 legend for expansion of other abbreviations.
HDAC2 Activity
HDAC2 activity in BAL macrophages in subjects with COPD during exacerbations correlated inversely with peak nasal-lavage virus load (P = .0096, r = −0.8), peak sputum GM-CSF (P = .0499, r = −0.67), TNF-α (P = .03, r = −0.72), neutrophil elastase (P = .0499, r = −0.67), and sputum nitrite levels (P = .0125, r = −0.78) (Table 2).
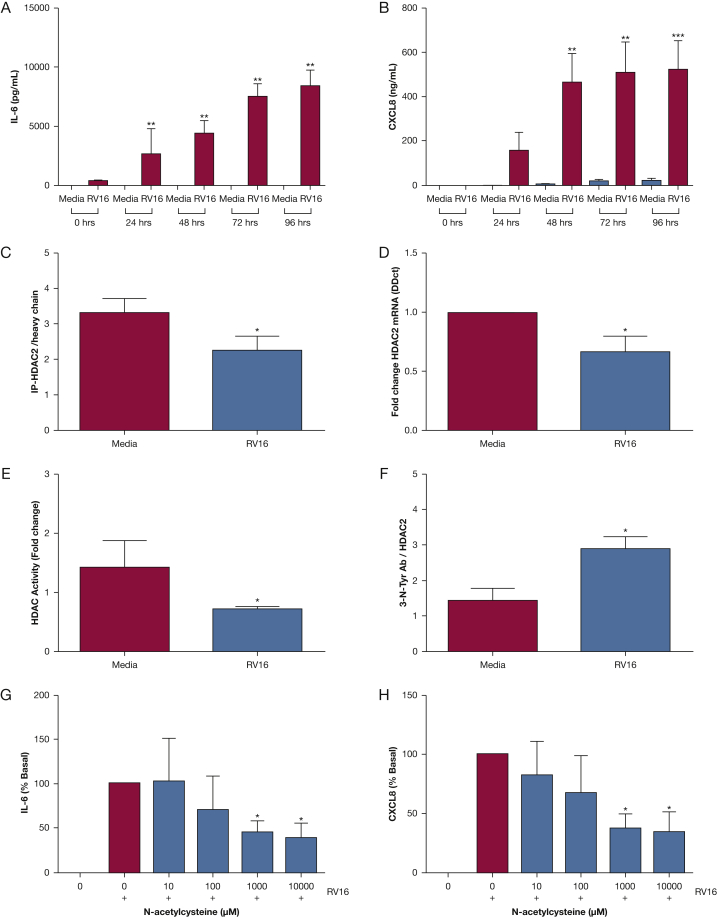
Rhinovirus Infection of Monocytes in vitro
Rhinovirus infection of the monocytic cell line THP-1 resulted in upregulation of the inflammatory cytokines IL-6 and CXCL8/IL-8 (Fig 4). Rhinovirus infection reduced expression of HDAC2 (both mRNA and protein) compared with noninfected cells (Figs 4C, 4D), and reduced HDAC2 activity (Fig 4E). Levels of nitrosylated HDAC2 were increased postinfection compared with noninfected cells (Fig 4F). THP-1 cells were treated with N-acetylcysteine (NAC) to inhibit the actions of peroxynitrate.18, 19, 20 Treatment of the THP-1 cells with NAC led to a dose-dependent reduction in IL-6 and CXCL8/IL-8, with significant reductions in inflammatory mediators observed at doses >1 mM (Figs 4G, 4H).
Figure 4.
Rhinovirus infection of THP-1 cells in vitro. A, Induction of IL-6 by rhinovirus in THP-1 cells. B, Induction of CXCL-8 by rhinovirus in THP-1 cells. C, HDAC2 protein in THP-1 cells infected with rhinovirus. D, HDAC2 RNA in THP-1 cells infected with rhinovirus protein. E, HDAC2 activity in THP-1 cells infected with rhinovirus. F, HDAC2 nitrosylation in THP-1 cells infected with rhinovirus. G, Inhibition of rhinovirus-induced IL-6 by N-acetylcysteine (NAC). H, Inhibition of rhinovirus-induced CXCL8/IL-8 by NAC. Data given as mean ± SE of at least three independent experiments. ∗P < .05.
Discussion
Acute exacerbations are important events in the natural history of COPD, but their pathogenesis remains poorly understood. Prevention of exacerbations remains a key therapeutic goal, but current treatments have only modest benefits and considerable adverse effects. Previous work from our group has demonstrated that experimental rhinovirus infection in subjects with COPD induces respiratory symptoms, airflow obstruction, and neutrophilic inflammation.15 In the current study, we provide novel evidence of the mechanisms of virus-induced COPD exacerbations and identify new potential therapeutic targets.
O&NS, airways inflammation, and reduced HDAC activity are well described in stable COPD, but their roles in COPD exacerbations are unclear. Some studies have reported increased inflammation and oxidative stress in exacerbations,3, 21, 22, 23, 24 whereas others have reported no change compared with the stable state.25, 26, 27, 28 Increased numbers of 3-NT positive cells have been reported in COPD exacerbations,21 and two studies reported that HDAC activity is unchanged in exacerbated COPD.28, 29 In these studies of naturally occurring exacerbations, there are numerous sources of variability that likely account for their discrepant results, including variations in exacerbation etiology, timing of presentation/sampling after exacerbation onset, different treatments, and so forth, that are difficult to eliminate. Understanding the molecular pathways in COPD exacerbations is critical to developing new, more effective preventive and therapeutic strategies. Our model of COPD exacerbation permits repeated lower airway sampling in treatment-naïve subjects in a manner not possible with naturally occurring exacerbations, thereby reducing variability and offering unique insights into the mechanisms of COPD exacerbations.15, 16, 30, 31 Moreover, use of a human model, as opposed to an animal model, provides data that are more likely to be rapidly translated into new therapies. Using this model, we measured markers of inflammation, O&NS, and HDAC activity prior to and following rhinovirus infection.
We previously reported that virus-induced exacerbations are associated with increased neutrophils, and neutrophil elastase, and CXCL8/IL-8 levels.15 In the current study, we replicated these findings in a new cohort of subjects and, in addition, demonstrated that virus infection induces significant increases in the proinflammatory cytokines GM-CSF, TNF-α, and IL-1β, and the protease MMP-9. Levels of 8-isoprostane (a marker of lipid peroxidation), 8-OHdG (a marker of hydroxyl radical damage to DNA), 3-NT (a product of tyrosine nitration mediated by peroxynitrite), and nitrite were significantly increased in sputum in the subjects with COPD, with only small, transient increases in smokers without COPD. There were positive correlations between markers of nitrosative stress and inflammatory mediators in sputum. These data provide new evidence of a pathogenic role of O&NS in COPD exacerbations. Exacerbations are associated with accelerated loss of lung function,32, 33, 34 and O&NS,35, 36 neutrophil elastase,37 and MMP-938 are all associated with airway remodeling. Therefore, these are potential mechanisms linking exacerbations with loss of lung function in COPD.
HDAC2 levels at baseline were not lower in the subjects with COPD subjects, as has been reported previously in patients with COPD GOLD stages II and III.8 Reduced HDAC2 is most pronounced in severe COPD and, therefore, may not be present in subjects with GOLD stage II disease. Following rhinovirus infection, there was a trend toward reduced HDAC2 activity in sputum and BAL macrophages in subjects with COPD, and HDAC activity correlated inversely with airways inflammation, virus load, and nitrite levels. Nitrosylation of HDAC, followed by HDAC degradation, is a mechanism of reduced HDAC2 activity,39 and our in vitro data are the first report, to our knowledge, that virus infection induces nitrosylation of HDAC2. The inverse relationship between airway HDAC2 activity and nitrite levels following virus infection suggests this is relevant in vivo also. Therefore, nitrosylation of HDAC2 may link virus infection, O&NS, impaired HDAC2 activity, and enhanced inflammation in COPD exacerbations.
These results have a number of important implications for our understanding of the pathogenesis of COPD exacerbations and future therapeutic directions. Corticosteroids are used in COPD exacerbations despite modest clinical benefits and frequent adverse effects.5, 40 Reduced HDAC2 may contribute to corticosteroid resistance in stable COPD8 and our results suggest this is also relevant in exacerbated COPD. Thus, enhancing HDAC2 activity through inhibiting virus-induced O&NS has the potential to improve the therapeutic effect of corticosteroids in COPD exacerbations, as well as having a direct antiinflammatory effect itself. This warrants investigation in studies specifically designed to address this issue. The demonstration that O&NS and multiple inflammatory mediators and proteases are elevated in exacerbations may account for the lack of clinical benefit seen with inhibition of single mediators41, 42, 43, 44 and casts doubt on this as a valid therapeutic approach. NAC, both an antioxidant and a peroxynitrite inhibitor, demonstrated an antiinflammatory effect in vitro and may also be effective in vivo.
Reduced baseline HDAC2 activity in sputum macrophages was associated with greater virus loads postinfection; therefore, reduced HDAC2 activity may also be linked to impaired antiviral responses. We observed significantly higher sputum virus loads in subjects with COPD, confirming for the first time, to our knowledge, impaired antiviral immunity in COPD results in more severe lower respiratory virus infection. HDAC activity is required for an effective antiviral response to picornavirus infection45; therefore, reduced HDAC activity may contribute to impaired antiviral host defense and increased severity of clinical illness following virus infections in COPD.
Our study has a number of limitations that are inherent to experimental infection studies. The number of subjects was small and only subjects with moderate COPD were included; therefore, the findings may not be applicable to more severe patients. However, as HDAC2 activity is further reduced8 and exacerbations more frequent in severe COPD,2 we believe these mechanisms are likely to be even more relevant in more severe disease. Secondary bacterial infections did occur in some subjects,30 but the numbers were too small to allow an analysis of the interactions between HDAC2 activity and bacteria (six subjects with bacterial infection, three without). Whether changes in HDAC2 activity affect the ability of macrophages to clear bacteria is a subject of great interest and warrants further investigation. The smokers were significantly younger compared with the nonsmokers, but there were no significant differences in age between the COPD group and the two control groups and, as the key comparisons were between the subjects with COPD and those without, we do not believe this affected the important results. Moreover, Ito et al8 reported that age had no effect on HDAC activity in bronchial biopsies. However, ideally, subsequent studies should use age-matched subjects whenever possible to remove this confounder. We used sputum to measure inflammatory mediators and cells and these do not reflect inflammation in the small airways. The main site of rhinovirus replication is epithelial cells rather than macrophages and, therefore, the significance of the relationship between HDAC2 levels in macrophages and virus load is unclear. The in vitro work was carried out in THP-1 cells rather than alveolar macrophages, as these are difficult to obtain in large enough numbers to perform the necessary experiments. However, the data obtained provide valid mechanistic information as to the potential mechanisms involved in macrophage responses to viral infection in COPD.
In summary, we have demonstrated induction of O&NS, reduced HDAC2 activity, increased inflammatory mediators, and increased virus load in the airways following rhinovirus infection in subjects with COPD. These results provide unique insights into the mechanisms of virus-induced COPD exacerbations and suggest novel therapeutic targets for the development of new targeted therapies in COPD.
Acknowledgments
Author contributions: S. L. J. had full access to all of the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis. J. F, P. M., A. L. D., K. I., P. J. B., S. L. E., O. M. K., W. S. F. W., and I. M. A. contributed to the study design; J. F, P. M., A. L. D., W. E. H., M-B. T-T., A. G. T., A. D. R., C. C., H. Y. P., T. K., J. A., L. S., and S. E. Q. contributed to collecting samples and carrying out experiments; J. F, P. M., and A. L. D. contributed to data analysis; J. F, P. M., A. L. D., K. I., P. J. B., S. L. E., O. M. K., W. S. F. W., and I. M. A. contributed to data interpretation; J. F, P. M., and A. L. D. contributed to writing the manuscript; K. I., P. J. B., S. L. E., O. M. K., W. S. F. W., and I. M. A. contributed to revising the manuscript; and J. F, P. M., A. L. D., W. E. H., M-B. T-T., A. G. T., A. D. R., C. C., H. Y. P., T. K., J. A., L. S., S. E. Q., K. I., P. J. B., S. L. E., O. M. K., W. S. F. W., I. M. A., and S. L. J. approved the final version of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: P. M. has received honoraria and travel grants from GlaxoSmithKline plc. K. I. is an employee of Pulmocide Ltd and has an honorary contract with Imperial College. P. J. B. has served on scientific advisory boards of AstraZeneca plc, Boehringer Ingelheim GmbH, Bespak (Consort Medical plc), Chiesi Pharmaceuticals Inc, Daiichi-Sankyo Co Ltd, Deep Breeze Ltd, GlaxoSmithKline plc, Glenmark Pharmaceuticals, Johnson & Johnson, Merck & Co Inc, Novartis AG, Nycomed International Management GmbH (Takeda Pharmaceutical Co Ltd), Pfizer Inc, Prosonix Ltd, Sun Pharmaceutical Industries Ltd, Teva Pharmaceutical Industries Ltd, and UCB Inc, and has received research funding from Aquinox Pharmaceuticals Inc, AstraZeneca plc, Boehringer Ingelheim GmbH, Chiesi Pharmaceuticals Inc, Daiichi-Sankyo Co Ltd, GlaxoSmithKline plc, Novartis AG, Nycomed International Management GmbH (Takeda Pharmaceutical Co Ltd), Pfizer Inc, Prosonix Ltd, and Sun Pharmaceuticals Industries Ltd. P. J. B. is also a cofounder of RespiVert Ltd (now part of Johnson & Johnson). O. M. K. has received travel grants from Boehringer Ingelheim GmbH. W. S. F. W. has received consultancies from Davos Life Science Pte Ltd. Dr Adcock has received consultancies, honoraria, and travel and research grants from GlaxoSmithKline plc, AstraZeneca plc, Johnson & Johnson, Chiesi Pharmaceuticals Inc, Pfizer Inc, Boehringer Ingelheim GmbH, Novartis AG, and Vectura Group plc. S. L. J. has received consultancies, honoraria, and travel and research grants from GlaxoSmithKline plc, Johnson & Johnson, Sanofi Aventis (Sanofi SA), Chiesi Pharmaceuticals Inc, Pfizer Inc, Boehringer Ingelheim GmbH, AstraZeneca plc, Novartis AG, and Synairgen plc, and has stock options in Synairgen plc. None declared (J. F., A. L. D., W. E. H., M.-B. T.-T., A. G. T., A. D., C. C., H. Y. P., T. K., J. A., L. S., S. E.-Q., S. L. E., I. M. A.)
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: The authors thank the study participants for their unfailing commitment and enthusiasm; the staff of the Chest and Allergy Clinic and Imperial Clinical Respiratory Research Unit. This article is dedicated to the memory of Dr Joseph Footitt.
Additional information: The e-Appendix, e-Figures, and e-Tables can be found in the Supplemental Materials area of the online article.
Footnotes
Dr Footitt is deceased.
Drs Footitt, Mallia, and Durham contributed equally to this article.
FUNDING/SUPPORT: This study was supported by an Academy of Medical Sciences and Wellcome Trust Starter Grant award to Dr Mallia; Medical Research Council Program [Grant G0600879 to Drs Ito, Barnes, Adcock, and Johnston]; British Medical Association H. C. Roscoe Fellowships to Drs Footitt and Mallia; British Lung Foundation/Severin Wunderman Family Foundation Lung Research Program [Grant P00/2 to Dr Johnston]; by the National Medical Research Council of Singapore [Grant NMRC/CBRG/0027/2012]; Wellcome Trust [Grant 083567/Z/07/Z] for the Centre for Respiratory Infection, Imperial College; and the National Institute for Health Research (NIHR) Biomedical Research Centre funding scheme, NIHR Senior Investigator Award, and the NIHR Clinical Lecturer funding scheme.
Supplementary Data
References
- 1.Mathers C.D., Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vestbo J., Hurd S.S., Agustí A.G. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 3.Papi A., Bellettato C.M., Braccioni F. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006;173(10):1114–1121. doi: 10.1164/rccm.200506-859OC. [DOI] [PubMed] [Google Scholar]
- 4.Singanayagam A., Joshi P.V., Mallia P., Johnston S.L. Viruses exacerbating chronic pulmonary disease: the role of immune modulation. BMC Med. 2012;10:27. doi: 10.1186/1741-7015-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walters J.A., Gibson P.G., Wood-Baker R., Hannay M., Walters E.H. Systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2009;(1):CD001288. doi: 10.1002/14651858.CD001288.pub3. [DOI] [PubMed] [Google Scholar]
- 6.Vollenweider D.J., Jarrett H., Steurer-Stey C.A., Garcia-Aymerich J., Puhan M.A. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;(12):CD010257. doi: 10.1002/14651858.CD010257. [DOI] [PubMed] [Google Scholar]
- 7.Fischer B.M., Pavlisko E., Voynow J.A. Pathogenic triad in COPD: oxidative stress, protease-antiprotease imbalance, and inflammation. Int J Chron Obstruct Pulmon Dis. 2011;6:413–421. doi: 10.2147/COPD.S10770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ito K., Ito M., Elliott W.M. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N Engl J Med. 2005;352(19):1967–1976. doi: 10.1056/NEJMoa041892. [DOI] [PubMed] [Google Scholar]
- 9.Ricciardolo F.L., Caramori G., Ito K. Nitrosative stress in the bronchial mucosa of severe chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2005;116(5):1028–1035. doi: 10.1016/j.jaci.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 10.Chiba T., Chihara J., Furue M. Role of the arylhydrocarbon receptor (AhR) in the pathology of asthma and COPD. J Allergy (Cairo) 2012;2012:372–384. doi: 10.1155/2012/372384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnes P.J. Corticosteroid resistance in patients with asthma and chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2013;131(3):636–645. doi: 10.1016/j.jaci.2012.12.1564. [DOI] [PubMed] [Google Scholar]
- 12.Osoata G.O., Yamamura S., Ito M. Nitration of distinct tyrosine residues causes inactivation of histone deacetylase 2. Biochem Biophys Res Commun. 2009;384(3):366–371. doi: 10.1016/j.bbrc.2009.04.128. [DOI] [PubMed] [Google Scholar]
- 13.To Y., Ito K., Kizawa Y. Targeting phosphoinositide-3-kinase-delta with theophylline reverses corticosteroid insensitivity in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;182(7):897–904. doi: 10.1164/rccm.200906-0937OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mallia P., Message S.D., Contoli M. Lymphocyte subsets in experimental rhinovirus infection in chronic obstructive pulmonary disease. Respir Med. 2014;108(1):78–85. doi: 10.1016/j.rmed.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mallia P., Message S.D., Gielen V. Experimental rhinovirus infection as a human model of chronic obstructive pulmonary disease exacerbation. Am J Respir Crit Care Med. 2011;183(6):734–742. doi: 10.1164/rccm.201006-0833OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mallia P., Message S.D., Kebadze T., Parker H.L., Kon O.M., Johnston S.L. An experimental model of rhinovirus induced chronic obstructive pulmonary disease exacerbations: a pilot study. Respir Res. 2006;7:116. doi: 10.1186/1465-9921-7-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Celli B.R., MacNee W., ATS/ERS Task Force Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 18.Koksel O., Cinel I., Tamer L. N-acetylcysteine inhibits peroxynitrite-mediated damage in oleic acid-induced lung injury. Pulm Pharmacol Ther. 2004;17(5):263–270. doi: 10.1016/j.pupt.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Kanazawa H., Shiraishi S., Okamoto T., Hirata K., Yoshikawa J. Inhibition of bronchoprotective effects of beta2-adrenoceptor agonists by peroxynitrite in guinea pig airways. Am J Respir Crit Care Med. 1999;159(4 pt 1):1272–1276. doi: 10.1164/ajrccm.159.4.9808009. [DOI] [PubMed] [Google Scholar]
- 20.Kondo H., Takahashi M., Niki E. Peroxynitrite-induced hemolysis of human erythrocytes and its inhibition by antioxidants. FEBS Lett. 1997;413(2):236–238. doi: 10.1016/s0014-5793(97)00922-8. [DOI] [PubMed] [Google Scholar]
- 21.Tsoumakidou M., Tzanakis N., Chrysofakis G., Siafakas N.M. Nitrosative stress, heme oxygenase-1 expression and airway inflammation during severe exacerbations of COPD. Chest. 2005;127(6):1911–1918. doi: 10.1378/chest.127.6.1911. [DOI] [PubMed] [Google Scholar]
- 22.Drost E.M., Skwarski K.M., Sauleda J. Oxidative stress and airway inflammation in severe exacerbations of COPD. Thorax. 2005;60(4):293–300. doi: 10.1136/thx.2004.027946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biernacki W.A., Kharitonov S.A., Barnes P.J. Increased leukotriene B4 and 8-isoprostane in exhaled breath condensate of patients with exacerbations of COPD. Thorax. 2003;58(4):294–298. doi: 10.1136/thorax.58.4.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dekhuijzen P.N., Aben K.K., Dekker I. Increased exhalation of hydrogen peroxide in patients with stable and unstable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1996;154(3 pt 1):813–816. doi: 10.1164/ajrccm.154.3.8810624. [DOI] [PubMed] [Google Scholar]
- 25.Bhowmik A., Seemungal T.A., Sapsford R.J., Wedzicha J.A. Relation of sputum inflammatory markers to symptoms and lung function changes in COPD exacerbations. Thorax. 2000;55(2):114–120. doi: 10.1136/thorax.55.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roland M., Bhowmik A., Sapsford R.J. Sputum and plasma endothelin-1 levels in exacerbations of chronic obstructive pulmonary disease. Thorax. 2001;56(1):30–35. doi: 10.1136/thorax.56.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koutsokera A., Kiropoulos T.S., Nikoulis D.J. Clinical, functional and biochemical changes during recovery from COPD exacerbations. Respir Med. 2009;103(6):919–926. doi: 10.1016/j.rmed.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 28.Kersul A.L., Iglesias A., Ríos Á. Molecular mechanisms of inflammation during exacerbations of chronic obstructive pulmonary disease. Arch Bronconeumol. 2011;47(4):176–183. doi: 10.1016/j.arbres.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Cosio B.G., Iglesias A., Rios A. Low-dose theophylline enhances the anti-inflammatory effects of steroids during exacerbations of COPD. Thorax. 2009;64(5):424–429. doi: 10.1136/thx.2008.103432. [DOI] [PubMed] [Google Scholar]
- 30.Mallia P., Footitt J., Sotero R. Rhinovirus infection induces degradation of antimicrobial peptides and secondary bacterial infection in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(11):1117–1124. doi: 10.1164/rccm.201205-0806OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mallia P., Message S.D., Contoli M. Neutrophil adhesion molecules in experimental rhinovirus infection in COPD. Respir Res. 2013;14:72. doi: 10.1186/1465-9921-14-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Donaldson G.C., Seemungal T.A., Bhowmik A., Wedzicha J.A. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57(10):847–852. doi: 10.1136/thorax.57.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Celli B.R., Thomas N.E., Anderson J.A. Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH study. Am J Respir Crit Care Med. 2008;178(4):332–338. doi: 10.1164/rccm.200712-1869OC. [DOI] [PubMed] [Google Scholar]
- 34.Kanner R.E., Anthonisen N.R., Connett J.E., Lung Health Study Research Group Lower respiratory illnesses promote FEV(1) decline in current smokers but not ex-smokers with mild chronic obstructive pulmonary disease: results from the lung health study. Am J Respir Crit Care Med. 2001;164(3):358–364. doi: 10.1164/ajrccm.164.3.2010017. [DOI] [PubMed] [Google Scholar]
- 35.de Boer W.I., Yao H., Rahman I. Future therapeutic treatment of COPD: struggle between oxidants and cytokines. Int J Chron Obstruct Pulmon Dis. 2007;2(3):205–228. [PMC free article] [PubMed] [Google Scholar]
- 36.Sugiura H., Liu X., Kobayashi T. Reactive nitrogen species augment fibroblast-mediated collagen gel contraction, mediator production, and chemotaxis. Am J Respir Cell Mol Biol. 2006;34(5):592–599. doi: 10.1165/rcmb.2005-0339OC. [DOI] [PubMed] [Google Scholar]
- 37.O’Donnell R., Breen D., Wilson S., Djukanovic R. Inflammatory cells in the airways in COPD. Thorax. 2006;61(5):448–454. doi: 10.1136/thx.2004.024463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Demedts I.K., Brusselle G.G., Bracke K.R., Vermaelen K.Y., Pauwels R.A. Matrix metalloproteinases in asthma and COPD. Curr Opin Pharmacol. 2005;5(3):257–263. doi: 10.1016/j.coph.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 39.Ito K., Hanazawa T., Tomita K., Barnes P.J., Adcock I.M. Oxidative stress reduces histone deacetylase 2 activity and enhances IL-8 gene expression: role of tyrosine nitration. Biochem Biophys Res Commun. 2004;315(1):240–245. doi: 10.1016/j.bbrc.2004.01.046. [DOI] [PubMed] [Google Scholar]
- 40.Bafadhel M., McKenna S., Terry S. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trial. Am J Respir Crit Care Med. 2012;186(1):48–55. doi: 10.1164/rccm.201108-1553OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rennard S.I., Fogarty C., Kelsen S., COPD Investigators The safety and efficacy of infliximab in moderate to severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175(9):926–934. doi: 10.1164/rccm.200607-995OC. [DOI] [PubMed] [Google Scholar]
- 42.Mahler D.A., Huang S., Tabrizi M., Bell G.M. Efficacy and safety of a monoclonal antibody recognizing interleukin-8 in COPD: a pilot study. Chest. 2004;126(3):926–934. doi: 10.1378/chest.126.3.926. [DOI] [PubMed] [Google Scholar]
- 43.Aaron S.D., Vandemheen K.L., Maltais F. TNFα antagonists for acute exacerbations of COPD: a randomised double-blind controlled trial. Thorax. 2013;68(2):142–148. doi: 10.1136/thoraxjnl-2012-202432. [DOI] [PubMed] [Google Scholar]
- 44.Vogelmeier C., Aquino T.O., O’Brien C.D., Perrett J., Gunawardena K.A. A randomised, placebo-controlled, dose-finding study of AZD9668, an oral inhibitor of neutrophil elastase, in patients with chronic obstructive pulmonary disease treated with tiotropium. COPD. 2012;9(2):111–120. doi: 10.3109/15412555.2011.641803. [DOI] [PubMed] [Google Scholar]
- 45.Chang H.M., Paulson M., Holko M. Induction of interferon-stimulated gene expression and antiviral responses require protein deacetylase activity. Proc Natl Acad Sci U S A. 2004;101(26):9578–9583. doi: 10.1073/pnas.0400567101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.