Abstract
Whereas the proform of the nerve growth factor (proNGF) is crucial for eliminating superfluous cells during neuronal development it also promotes apoptosis following brain trauma and neuronal injury. The apoptotic signal is elicited upon formation of a trimeric receptor complex also containing the vps10p domain receptor sortilin and the neurotrophin receptor p75NTR. However, proNGF-induced receptor complex formation has been difficult to directly assess other than by western blotting. We here describe a fluorescence resonance energy transfer (FRET) based fluorescence plate reader assay to monitor the interaction between fluorescently tagged sortilin and p75NTR in live cells. The method is based on a standard fluorescent plate reader found in many biochemical laboratories and the results are evaluated using a microscopy-based quantified sensitized acceptor emission FRET approach making use of a pair of FRET standard constructs. As a result, the effect of proNGF on the interaction between sortilin and p75NTR can be evaluated in live cells allowing for screening and selection of therapeutic compounds interfering with proNGF-induced cell death.
Keywords: FRET-based fluorescence plate reader assay, sortilin, p75NTR, proNGF, high throughput
Introduction
The nerve growth factor (NGF) belongs to the family of growth factors of the neurotrophin family and promotes neuronal survival through the transmembrane neurotrophin receptor p75NTR in conjunction with the tropomyosin receptor kinase A (TrkA) [1,2]. However, NGF is synthesized as a proform (proNGF) with opposing biological activity to that of its mature counterpart as it promotes apoptosis rather than survival [3-5]. This is achieved by engaging in a heterotrimeric receptor complex also containing p75NTR and the neuronal transmembrane receptor sortilin a member of the vps10p domain receptor family [6]. Whereas sortilin engages the prodomain of proNGF, p75NTR targets the mature part of the molecule thereby increasing the affinity for proNGF approximately 100-fold compared to that for each receptor alone [6]. However, sortilin and p75NTR also form a protein complex in the absence of ligand, as they interact through the extracellular juxtamembrane domain of p75NTR [7]. Whereas proNGF promotes neuronal apoptosis during development as demonstrated for postmitotic retinal ganglia cells in the mouse retina [8] proNGF also promotes apoptosis in animal models following neuronal injury such as after kainic acid-induced seizures [9] and following sciatic nerve transection [10]. The proform of other neurotrophins i.e. brain derived neurotropophic factor (proBDNF) and neurotrophin-3 (proNT-3) likewise promote apoptosis by complexing p75NTR and sortilin [11-13].
Using live cells, we aimed at developing a high throughput assay with a standard fluorescent plate reader as detection instrument to monitor the interaction between fluorescently tagged sortilin and p75NTR. FRET occurs when a donor fluorophore transfers energy to an acceptor fluorophore with the FRET efficiency being strongly dependent on the proximity of the two fluorophores, which increases rapidly as the distance between the fluorophores is reduced from 8 to 2 nm. The development of new variants of the green fluorescent proteins has facilitated the use of these proteins in FRET to indicate the proximity of tagged proteins. A well-described FRET pair is the fluorescent proteins cerulean [14] and venus [15], which have better spectral properties in terms of FRET compared to the previously used YFP and CFP [16].
In this paper, we describe the use of a standard fluorescent plate reader in a fluorescent FRET-based assay using live cells expressing the fusion proteins sortilin-cerulean and p75NTR-venus. We show how this system can be used to evaluate the effect of proNGF on the interaction between sortilin and p75NTR. The FRET based assay may provide a new tool for screening potential compounds inhibiting proNGF-induced apoptotic cross-linking of sortilin and p75NTR.
Material and methods
DNA work
A PCR-mediated overlap extension strategy was employed to generate sortilin-cerulean fused via a five amino acids linker (TWQRR). Using sortilinmut/pcDNA3.1/Zeo(-) [7] as template, the primers 5’-CCAGGGGACAAATGCCAGGG-3’ and 5’-ACCATTCTCCGCTGCCAGGTCACAATGAGCACTCCTGCTAC-3’ were used to amplify the upstream fragment. Likewise, using cerulean-C1 [14] as template, the primers 5’-TTGTGACCTGGCAGCGGAGAATGGTGAGCAAGGGCGAGGAG-3’ and 5’-CACACACTTAAGCTACTTGTACAGCTCGTCCATGCC-3’ were used to amplify the downstream fragment. The overlapping PCR products were amplified and inserted into sortilinmut/pcDNA3.1(-)/Zeo, taking advantage of the sortilin luminal BSPEI site and the primer generated AflII site. A similar strategy was used to generate HA-p75NTR-venus. For amplification of the upstream fragment, rat HA-p75NTR/pcDNA3.1/G418 was used as template together with the primers 5’-CACACAGCGGCCGCACCATGTCTGCACTTCTGATC-3’ and 5’-ACCATGCTGTTCCACCTCTTGAAAGCAATATAGGCCACAAG-3’. For the downstream fragment, venus-pcs2 [15] served as template and primers were 5’-AAGAGGTGGAACAGCATGGTGAGCAAGGGCGAGGAG-3’ and 5’-CACACAGAATTCTTACTTGTACAGCTC-3’. The final fragment was inserted into pcDNA3.1(-)/G418 (Invitrogen) using the primer-generated NotI and EcoRI site.
The FRET standard C5V, with cerulean and venus directly tethered by an amino acid linker of five amino acids, has previously been described [17]. The CTV FRET standard, with cerulean and venus separated by 229 amino acids is described in [18].
Cell lines and culturing
Human embryonic kidney cells (HEK293) and rat schwannoma cells (RN22 cells) were cultured and stable cell lines generated as described in [7]. Identification of positive clones was done by western blotting and fluorescent microscopy.
Western blotting
Stably transfected HEK293 cells were processed and subjected to western blotting as previously described [7]. Primary antibodies were anti-NTR-3 (612100) from BD Transduction Laboratories, anti-HA (H6908) and anti-β-actin (A5441) from Sigma.
FRET plate reader data acquisition and analysis
HEK293 cells stably co-expressing sortilin-cerulean and p75NTR-venus or cells expressing one of the two fusion proteins alone as well as untransfected cells (for background fluorescence) were seeded into Corning black 96-well microtiter plates (Sigma) at a density of 20,000/well in triplicates two days prior to the experiment. Immediately before the experiment, medium was exchanged with 200 μl PBS containing 1 mM CaCl2 and MgCl2. Recombinant human furin cleavage-resistant proNGF was then added directly to wells and measurements performed after 30 min at 20°C on a fluorescent plate reader (Victor3, 1420 Multilabel counter, Perkin Elmer). CW lamp energy was set at 10,000 units with continuous lamp control. Counting times were 0.1 sec and a large aperture and readings from the bottom of the well was used. The following filter set was used: cerulean filter set (excitation: 430/15 nm, emission: 460/20 nm); venus filter set (excitation: 485/15, emission 535/15); FRET filter set (430/15 nm, emission 535/15).
Calculation of the apparent FRET efficiency (Eapp%) was performed as follows: Average background values were calculated for each of the three channels and subtracted from the measurements.
The raw FRET signal obtained from the plate reader is contaminated by overlap of the donor emission spectrum with that of the acceptor (donor spectral bleed through (DSBT)) and by direct excitation of the acceptor by the donor excitation light (acceptor spectral bleed trough (ASBT)). This contamination must be quantified and subtracted from the raw FRET signal.
DSBT = CVDexDem × (CDexAem/CDexDem) and
ASBT = CVAexAem × (VDexAem/VAexAem)
Where CV represents cells expressing sortilin-cerulean and p75NTR-venus, C is cells with expression of cerulean, and V is cells with expression of venus. Dex is donor excitation light, Dem is donor emission signal, Aex is acceptor excitation light and Aem is acceptor emission signal.
The corrected FRET signal is:
Fc = raw FRET signal | ASBT | DSBT
Where Fc is the corrected FRET value. The apparent FRET efficiency is calculated as:
Eapp% = 100 × {Fc/(Fc + CVDexDem)}
Confocal laser scanning microscopy and FRET analysis in live cells
HEK293 cells with a stable expression of sortilin-cerulean and p75NTR-venus were seeded two days prior to imaging onto poly-L-Lysine (Sigma) coated coverslips. To assess the effect of proNGF, medium was exchanged with pre-heated PBS (with 1 mM CaCl2 and mgCl2) with and without addition of proNGF and incubated 45 min at 37°C. Imaging was performed on a Zeiss LSM510 confocal microscope equipped with a 40X C-Apochromat objective, N/A 1.2. The laser lines 458 nm and 514 nm were used for excitation. Detection was performed using the Meta-detector with a band pass between 469 -501 nm and 533-576 nm.
For quantifying FRET-values, collected images were analyzed using the algorithm implemented in the ImageJ-based PFRET software developed by Professor Periasamy [19]. Using this software, a possible dependence of DSBT and ASBT on donor- and acceptor signal levels are taken into account. All calculations and corrections were performed on background-subtracted images. Lower bounds for signal levels used in ASBT and DSBT correction calculations were set to 15 intensity units. Regions of interest (ROI’s) were chosen automatically on the PFRET image and set to 5X5 pixels. ROI’s were only included in the calculation for the final Eapp% if the signal levels in the donor, acceptor, and FRET channels were above the lower bound value (25 intensity units). PFRET analysis was only performed on the cell surface as a new plug-in was developed for ImageJ, which enabled us to place a mask on each image leaving only the membrane for analysis.
FRET analysis of fixed RN22 cells stained with Alexa 488 and Alexa 555 antibodies
RN22 cells were seeded onto poly-L-Lysine coated coverslips and subsequently incubated with and without proNGF in PBS for 45 min at 20°C. Cells were fixed in 4% paraformaldehyde, blocked in 10% FCS, and incubation with anti-sortilin (#5264) 1:200 (custom made by Dako [20]) and anti-NGFR 1:50 (1157, R&D systems). Secondary antibodies were Alexa-conjugated donkey anti-rabbit 555 (A31572) (1:800) and donkey anti-goat 488 (A11055) (1:500) from Molecular probes. Analysis of cells was done on the Zeiss confocal LSM510 META microscope using a 40x NA 1.2 C-Apochromat. Sensitized acceptor emission FRET measurements and analysis were performed according to the PFRET procedure [19], using the associated ImageJ PFRET plugin. Donor and acceptor signals were detected through 500-530 nm and 565-615 nm emission filters following excitation with 488 nm (donor) or 543 nm (acceptor) using in the range of 10-25 μW and 40-90 μW of laser power, which were kept constant during acquisition of each data set. Using the ImageJ PFRET plugin, a possible dependence of donor- and acceptor spectral bleed throughs (DSBT and ASBT) on donor- and acceptor signal levels are taken into account. Furthermore, it is possible to obtain not only bleed through-corrected FRET-channel images (PFRET images) but also Eapp images as well as Eapp values from ROIs. All calculations and corrections were performed on background-subtracted images. Lower bounds for signal levels used in ASBT and DSBT correction calculations were set to 15 intensity units. ROI’s were chosen automatically on the PFRET image and set to 5X5 pixels. ROI’s were only included if the signal levels in the donor, acceptor, and FRET channels were above the lower bound value (20 intensity units) for more than 80% of the pixels within the ROI.
Statistics
Assuming unequal variances, RN22 cell data were analyzed by a one-way ANOVA followed by Games-Howell’s correction. Furthermore, nonparametric multiple-independent-samples test (Kruskal-Wallis H) was used to confirm the analysis in this study.
Treatment responses in different groups of live cells expressing sortilin-cerulean and p75NTR-venus using the confocal microscope were analyzed by ANOVA after the data were log10 transformed. If the analysis of variance revealed significant group differences, a post-hoc test (Scheffe) was carried out to elucidate the pattern of group differences.
Treatment responses in different groups of cells using the multiplate reader were compared by ANOVA. If the analysis of variance revealed significant group differences, a post-hoc test (Bonferroni and Turkey) was carried out to elucidate the pattern of group differences.
Results
Cross-linking of endogenous sortilin and p75NTR can be monitored by FRET
In order to establish a FRET-based fluorescence plate reader assay to monitor sortilin - p75NTR interactions, we took advantage of the rat schwannoma cell line RN22, which express’ endogenous levels of sortilin and p75NTR and which undergoes apoptosis in response to low amounts of proNGF [7,21]. Using fixed RN22 cells, we sought to follow proNGF-induced sortilin - p75NTR complex formation by FRET using fluorescently labelled secondary antibodies. The apparent FRET efficiency (Eapp%) was significantly increased at the cell surface upon addition of 0.05 nM proNGF (~18%) and 0.5 nM (~26%) compared to untreated cells (Figure 1) demonstrating that FRET is a valid method for detection of proNGF-induced sortilin - p75NTR cross-linking at low ligand concentrations. Interestingly, by adding 50 nM proNGF, Eapp% was significantly decreased (~14%) compared to untreated cells whereas 5 nM proNGF had no significant effect.
Figure 1.
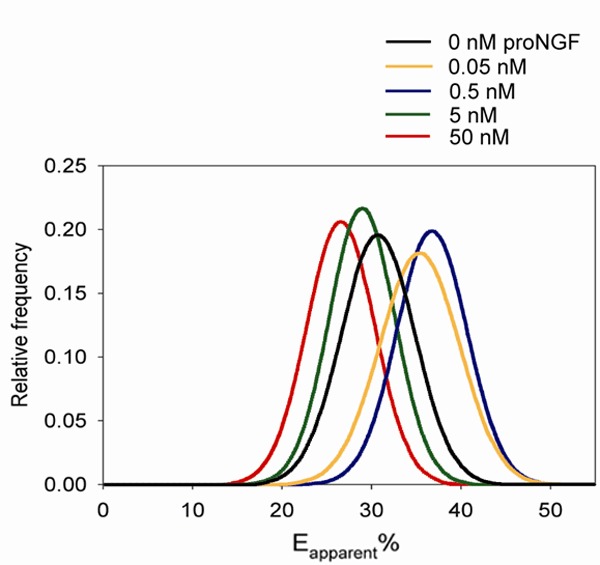
FRET analysis of the sortilin - p75NTR interaction in RN22 cells. RN22 cells with endogenous expression of sortilin and p75NTR were incubated with proNGF followed by fixation and staining with anti-sortilin and anti-p75NTR primary antibodies and fluorescently tagged secondary antibodies. Most groups were significantly different compared to untreated cells (p < 0.001) except cells treated with 5 nM proNGF (n=10 for all concentrations). All data are presented as the mean ± SD.
Monitoring sortilin - p75NTR interactions by FRET using labeled receptor constructs in live cells
Based on these results, we next sought to establish a FRET-based assay for detection of sortilin - p75NTR cross-linking in live cells. As sortilin and p75NTR physically interact through extracellular interactions [7], the intracellular domain of sortilin and p75NTR were substituted for the fluorescent proteins cerulean and venus and attached via a five amino acid polypeptide tether (Figure 2A). The constructs were then stably inserted into HEK293 cells, which do not express p75NTR and only very low amounts of endogenous sortilin. Western blots confirmed expression of both fusion constructs in HEK293 cells and fluorescent images showed expression of both proteins at the cell surface as well as a pool of newly synthesized or internalized proteins from the cell surface located in intracellular vesicles (Figure 2B, 2C). We next analyzed the effect of proNGF on sortilin - p75NTR cross-linking in these cells by use of FRET using confocal microscopy. 48 h post-seeding on coverslips, medium was exchanged to PBS containing proNGF and incubated 45 min before imaging the cells. In order to avoid any FRET signal stemming from receptors within vesicles, which may not be accessible to proNGF, only signals originating from the membrane surface was chosen for analysis (see materials and methods). By adding 0.1 or 0.5 nM proNGF, Eapp% was significantly increased ~40% and ~18% compared to untreated cells (Figure 2D). However, addition of 50 nM proNGF had no significant effect. Thus, our experimental set-up using exogenously expressed fusion proteins in live HEK293 cells allowed detection of sortilin - p75NTR cross-linking induced by physiologically relevant concentrations of proNGF.
Figure 2.
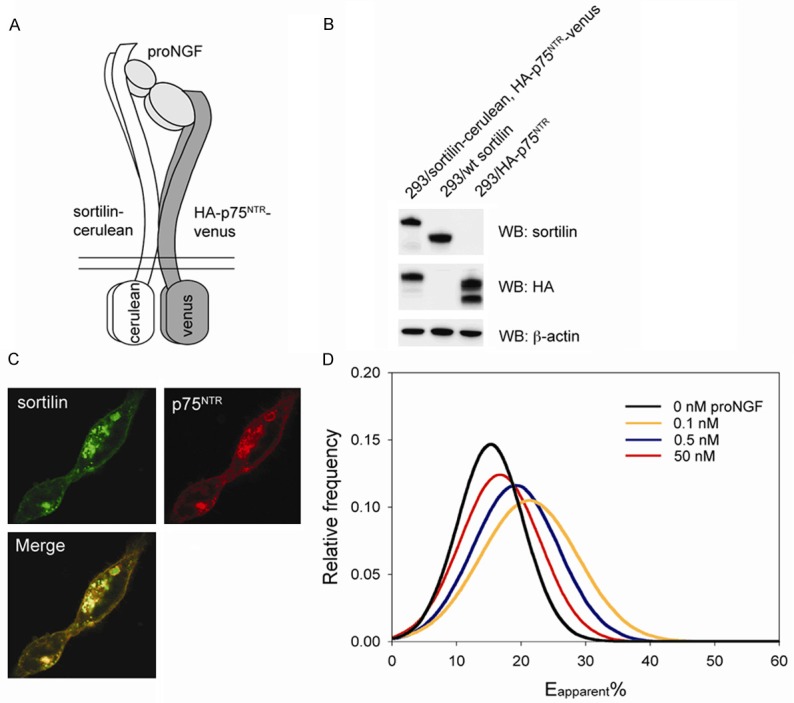
Establishing a FRET-based assay using sortilin-cerulean and p75NTR-venus constructs in HEK293 cells. (A) Sortilin-cerulean and p75NTR-venus constructs were generated by exchanging the sequence of the cytoplasmatic domain of the receptors for the sequence of the fluorescent proteins leaving the binding site for proNGF intact at the extracellular domain. (B and C) Co-expression of fusion proteins in stably transfected cells as revealed by western blotting (B) and confocal microscopy (C). (D) Addition of 0.1 or 0.5 nM proNGF increased FRET between sortilin-cerulean and p75NTR-venus compared to untreated cells. Most groups were significantly different from untreated cells (p < 0.001), however no significant difference could be detected between untreated cells and the cells treated with 50 nM proNGF (n=10-14). All data are presented as the mean ± SD.
Setting up a cellular FRET-based plate reader assay to follow sortilin - p75NTR interactions
These results further encouraged us to establish a high throughput FRET-based assay using the stably transfected HEK293 cell line co-expressing sortilin-cerulean and p75NTR-venus. Cells were seeded into 96-wells plates two days prior to the experiment. Medium was then carefully exchanged with PBS and proNGF added directly to wells and incubated for 30 min. Measurements were done at 20°C using a Victor3, 1420 Multilabel counter fluorescent plate reader from Perkin Elmer. Again, low amounts of proNGF i.e. 0.05, 0.5, 5 nM increased the interaction between sortilin and p75NTR significantly as Eapp% was increased ~34%, ~43%, and ~25% compared to untreated cells. In contrast, high amounts of proNGF i.e. 50 nM had no significant effect (Figure 3A).
Figure 3.
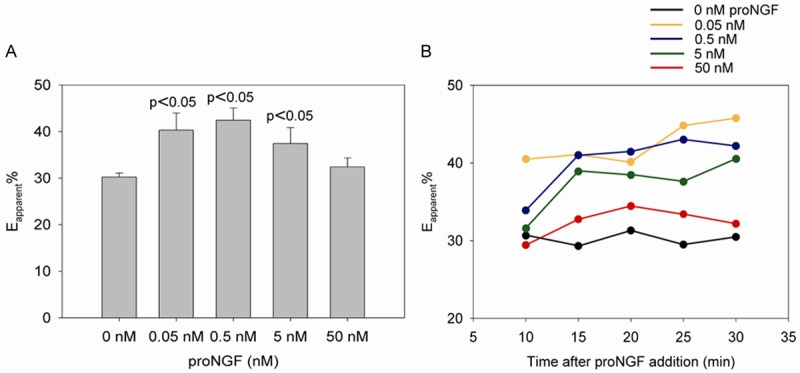
The effect of proNGF on the interaction between sortilin-cerulean and p75NTR-venus using a standard fluorescent plate reader. A. Addition of 0.05, 0.5, or 5 nM proNGF significantly increased the Eapp% by decreasing the distance between the receptors except for cells treated with 50 nM proNGF (n=3). B. During 30 min exposure to low amounts of proNGF, Eapp% increased whereas the FRET-value remained fixed in the absence or in the presence of 50 nM ligand.
30 min time course experiments further showed that the FRET values remained constant for untreated cells whereas Eapp% increased for the proNGF-treated cells except for cells treated with 50 nM proNGF (Figure 3B).
To further characterize and validate our experimental set-up, we used a pair of FRET standards consisting of covalently fused fluorescent proteins cerulean and venus separated by an amino acid linker of variable length [17,18]. For the standard C5V, a linker of five amino acids separates the fluorophores whereas the other standard CTV, contains a linker of 229 amino acids (Figure 4A). As the fluorophores of C5V are in close proximity, high levels of FRET is expected as compared to CTV, which is expected to result in a low FRET index due to the greater distance separating the fluorophores. Non-attached cerulean and venus (C+V) are expected to result in even lower FRET. Following transient transfection of HEK293 cells and using the FRET-based plate reader set-up, we calculated a FRET efficiency value of 5%, 20%, and 32% for cells expressing C+V, CTV and C5V (Figure 4B). Although the Eapp% is somewhat lower than that published by Koushik et al. [17], the result shows the robustness of our assay by being able to detect the expected increase in FRET efficiency as the fluorophores are being brought together.
Figure 4.
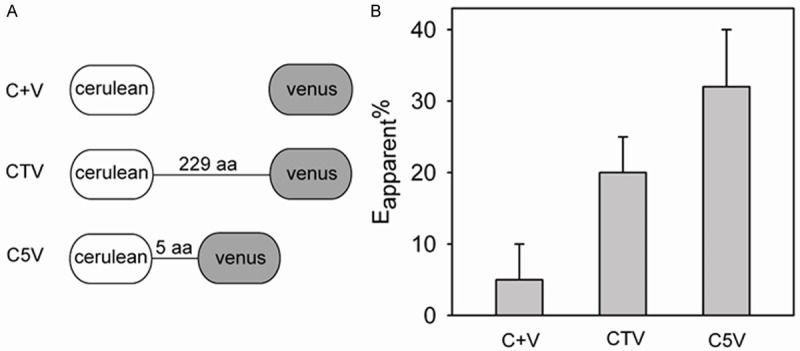
Decreasing the distance between cerulean and venus increases FRET in the plate reader assay. A. Schematic representation of used standard constructs. B. FRET efficiencies obtained using HEK293 cells transiently transfected with CTV, C5V, or co-transfected with plasmids encoding cerulean or venus (C+V) (n=3). All data are presented as the mean ± SD. aa, amino acids.
Discussion
We have used a fluorescent plate reader combined with a FRET-based method to monitor the effect of proNGF on cross-linking of sortilin and p75NTR in live HEK293 cells with a stable expression of the fusion proteins sortilin-cerulean and p75NTR-venus. The assay was tested using a pair of FRET standard constructs cerulean and venus separated by a linker of variable length. The results obtained with the standard constructs suggest that the differences in FRET induced by low levels of proNGF indeed reflect changes in the proximity of sortilin-cerulean and p75NTR-venus. Importantly, the effective concentrations of proNGF used in our FRET-based plate reader assay lies within a physiologically relevant range i.e. 0.05-0.5 nM. This finding also applies to experiments using the confocal microscope for measurements. Interestingly, for all three experimental set-ups, cells treated with higher concentration of proNGF (~5-50 nM) had either no effect or even decreased the interaction rather than increasing it. This might be due to a competitive effect of proNGF, where both sortilin and p75NTR obtain ligand saturation and therefore each interact with a separate molecule of proNGF rather than sharing the ligand, thereby driving the two receptors apart. However, our results highlight the need to determine the optimal concentration of proNGF in the process of establishing the plate reader assay in order to promote maximal cross-linking of sortilin and p75NTR.
FRET analysis was performed using the PFRET algorithm [19], which can be applied to regions of interest with sizes defined by the user, in principle down to the single-pixel level. It should be noted however, that the results acquired from the plate reader is calculated as an average over a population of cells within a well while the results presented from the microscopy measurements represents averages over 5x5 pixel.
The advantage of using the fluorescent plate reader for FRET measurements is that it easily and quickly delivers results and therefore is very useful for high troughput. Furthermore, a plate reader is typically available in most standard biochemical laboratories and will therefore not require investment in new expensive hardware.
It should be noted that the use of cells expressing sortilin and p75NTR fused to cerulean and venus results in a pool of intracellular located proteins, which are most likely not partaking in the interaction with proNGF. As the intracellular located proteins add to the fluorescent signal detected by the plate reader, the effect of proNGF could therefore be expected to be underestimated. It is therefore important to test the plate reader result on a confocal microscope where the intracellular contribution to the fluorescent signal subsequently can be subtracted. However, by using cells stably co-transfected with sortilin-cerulean and p75NTR-venus, the very same cell line can be used for several different FRET-based techniques based on a plate reader, microscope, and flow cytometer.
In conclusion, this paper describes the establishment of a FRET-based fluorescent plate reader assay using live cells to monitor sortilin - p75NTR cross-linking and the influence of proNGF on this interaction. The experimental set-up may provide a useful tool for identifying compounds interfering with cross-linking of sortilin - p75NTR. Using this set-up, we have now initiated work aimed at identifying compounds inhibiting sortilin - p75NTR cross-linking induced by proNGF.
Acknowledgements
Annette Larsen is gratefully thanked for technical assistance. The vectors cereulean-C1 and venus-pcs2 were kindly provided by Professor Dave W. Piston (Vanderbilt University School of Medicine) and Professor Atsushi Miyawaki (RIKEN Brain Science Institute). ProNGF was provided by Professor Elisabeth Schwarz (Institute for Biotechnology, Martin-Luther-Universität, Halle-Wittenberg). The MIND Center is supported by The Lundbeck Foundation. Centre for Stochastic Geometry and Advanced Bioimaging (CSGB) is supported by the Villum Foundation. S. Alwasel would like to extend his sincere appreciation to the Deanship of Scientific Research at King Saud University for its support, RGP- VPP-254.
Disclosure of conflict of interest
None.
Abbreviations
- ASBT
acceptor spectral bleed trough
- DSBT
donor spectral bleed through
- Eapp%
apparent FRET efficiency
- FCS
fetal calf serum
- FRET
fluorescence resonance energy transfer
- HEK-293 cells
Human embryonic kidney 293 cells
- NGF
nerve growth factor
- p75NTR
p75 neurotrophin receptor
- RN22 cells
rat schwannoma cells
- ROI
region of interest
References
- 1.Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- 2.Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beattie MS, Harrington AW, Lee R, Kim JY, Boyce SL, Longo FM, Bresnahan JC, Hempstead BL, Yoon SO. ProNGF induces p75-mediated death of oligodendrocytes following spinal cord injury. Neuron. 2002;36:375–386. doi: 10.1016/s0896-6273(02)01005-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- 5.Harrington AW, Leiner B, Blechschmitt C, Arevalo JC, Lee R, Morl K, Meyer M, Hempstead BL, Yoon SO, Giehl KM. Secreted proNGF is a pathophysiological death-inducing ligand after adult CNS injury. Proc Natl Acad Sci U S A. 2004;101:6226–6230. doi: 10.1073/pnas.0305755101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nykjaer A, Lee R, Teng KK, Jansen P, Madsen P, Nielsen MS, Jacobsen C, Kliemannel M, Schwarz E, Willnow TE, Hempstead BL, Petersen CM. Sortilin is essential for proNGF-induced neuronal cell death. Nature. 2004;427:843–848. doi: 10.1038/nature02319. [DOI] [PubMed] [Google Scholar]
- 7.Skeldal S, Sykes AM, Glerup S, Matusica D, Palstra N, Autio H, Boskovic Z, Madsen P, Castren E, Nykjaer A, Coulson EJ. Mapping of the interaction site between sortilin and the p75 neurotrophin receptor reveals a regulatory role for the sortilin intracellular domain in p75 neurotrophin receptor shedding and apoptosis. J Biol Chem. 2012;287:43798–43809. doi: 10.1074/jbc.M112.374710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jansen P, Giehl K, Nyengaard JR, Teng K, Lioubinski O, Sjoegaard SS, Breiderhoff T, Gotthardt M, Lin F, Eilers A, Petersen CM, Lewin GR, Hempstead BL, Willnow TE, Nykjaer A. Roles for the pro-neurotrophin receptor sortilin in neuronal development, aging and brain injury. Nat Neurosci. 2007;10:1449–1457. doi: 10.1038/nn2000. [DOI] [PubMed] [Google Scholar]
- 9.Volosin M, Song W, Almeida RD, Kaplan DR, Hempstead BL, Friedman WJ. Interaction of survival and death signaling in basal forebrain neurons: roles of neurotrophins and proneurotrophins. J Neurosci. 2006;26:7756–7766. doi: 10.1523/JNEUROSCI.1560-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnett MG, Ryals JM, Wright DE. Pro-NGF, sortilin, and p75NTR: potential mediators of injury-induced apoptosis in the mouse dorsal root ganglion. Brain Res. 2007;1183:32–42. doi: 10.1016/j.brainres.2007.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teng HK, Teng KK, Lee R, Wright S, Tevar S, Almeida RD, Kermani P, Torkin R, Chen ZY, Lee FS, Kraemer RT, Nykjaer A, Hempstead BL. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J Neurosci. 2005;25:5455–5463. doi: 10.1523/JNEUROSCI.5123-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tauris J, Gustafsen C, Christensen EI, Jansen P, Nykjaer A, Nyengaard JR, Teng KK, Schwarz E, Ovesen T, Madsen P, Petersen CM. Proneurotrophin-3 may induce Sortilin-dependent death in inner ear neurons. Eur J Neurosci. 2011;33:622–631. doi: 10.1111/j.1460-9568.2010.07556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yano H, Torkin R, Martin LA, Chao MV, Teng KK. Proneurotrophin-3 is a neuronal apoptotic ligand: evidence for retrograde-directed cell killing. J Neurosci. 2009;29:14790–14802. doi: 10.1523/JNEUROSCI.2059-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rizzo MA, Springer GH, Granada B, Piston DW. An improved cyan fluorescent protein variant useful for FRET. Nat Biotechnol. 2004;22:445–449. doi: 10.1038/nbt945. [DOI] [PubMed] [Google Scholar]
- 15.Nagai T, Ibata K, Park ES, Kubota M, Mikoshiba K, Miyawaki A. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat Biotechnol. 2002;20:87–90. doi: 10.1038/nbt0102-87. [DOI] [PubMed] [Google Scholar]
- 16.Sarkar P, Koushik SV, Vogel SS, Gryczynski I, Gryczynski Z. Photophysical properties of Cerulean and Venus fluorescent proteins. J Biomed Opt. 2009;14:034047. doi: 10.1117/1.3156842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koushik SV, Chen H, Thaler C, Puhl HL 3rd, Vogel SS. Cerulean, Venus, and VenusY67C FRET reference standards. Biophys J. 2006;91:L99–L101. doi: 10.1529/biophysj.106.096206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thaler C, Koushik SV, Blank PS, Vogel SS. Quantitative multiphoton spectral imaging and its use for measuring resonance energy transfer. Biophys J. 2005;89:2736–2749. doi: 10.1529/biophysj.105.061853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wallrabe H, Elangovan M, Burchard A, Periasamy A, Barroso M. Confocal FRET microscopy to measure clustering of ligand-receptor complexes in endocytic membranes. Biophys J. 2003;85:559–571. doi: 10.1016/S0006-3495(03)74500-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munck Petersen C, Nielsen MS, Jacobsen C, Tauris J, Jacobsen L, Gliemann J, Moestrup SK, Madsen P. Propeptide cleavage conditions sortilin/neurotensin receptor-3 for ligand binding. EMBO J. 1999;18:595–604. doi: 10.1093/emboj/18.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pagadala PC, Dvorak LA, Neet KE. Construction of a mutated pro-nerve growth factor resistant to degradation and suitable for biophysical and cellular utilization. Proc Natl Acad Sci U S A. 2006;103:17939–17943. doi: 10.1073/pnas.0604139103. [DOI] [PMC free article] [PubMed] [Google Scholar]


