Abstract
PURPOSE: This study aimed to identify the efficacy and toxicity of the FOLFIRI regimen (fluorouracil, leucovorin, and irinotecan) with irinotecan dose escalation plus bevacizumab as first-line chemotherapy for metastatic colorectal cancer (mCRC) via UGT1A1 genotyping. METHODS: We administered bevacizumab plus FOLFIRI with irinotecan dose escalation to treat 70 mCRC patients. The UGT1A1 *1/*1 and *1/*28 genotypes started with a 180-mg/m2 dose of irinotecan, and UGT1A1 *28/*28 genotype started with a dose of 120 mg/m2. The dose of irinotecan was escalated at increasing intervals of 20 to 30 mg/m2 until grade 3/4 adverse events (AEs) occurred. The clinical response rate, toxicity, and survival were analyzed. RESULTS: The clinical response and disease control rates of mCRC patients treated with FOLFIRI plus bevacizumab were significantly better in patients with UGT1A1 *1/*1 and *1/*28 genotypes than in patients with UGT1A1 *28/*28 (P = .006 and P < .001, respectively). Grade 3/4 AEs were significantly more common in mCRC patients with the UGT1A1 *28/*28 genotype (P < .001). Progression-free survival was significantly higher in UGT1A1 *1/*1 and *1/*28 patients (P = .002). mCRC patients who underwent metastasectomy achieved better overall survival than those who did not undergo metastasectomy (P = .015). CONCLUSIONS: Our study showed that mCRC patients with UGT1A1 *1/*1 and *1/*28 genotypes could receive escalated doses of irinotecan to obtain a more favorable clinical outcome without significant AEs.
Introduction
Colorectal cancer (CRC) is the third most common cause of cancer-related mortality worldwide, with over 1.2 million new cancer cases and 608,700 deaths estimated to have occurred in 2008 [1]. Almost all metastatic CRC (mCRC) patients require systemic treatment to palliate symptoms or downstage tumor status to be eligible for further surgical intervention or metastasectomy. In the past 40 years, 5-fluorouracil (5-FU) has been widely used as an effective chemotherapeutic agent in the treatment of advanced CRC. In the early 1980s, 5-FU combined with leucovorin (LV) was found to increase tumor response rate and the length of time to progression.
New drugs such as irinotecan and oxaliplatin in various combinations with 5-FU/LV have further improved the outcome in many advanced CRC patients [2]. Infusional 5-FU/LV plus irinotecan (FOLFIRI) has emerged as a standard first-line therapeutic option for mCRC patients. Irinotecan is a prodrug that is converted to an active form, 7-ethyl-10-hydroxycamptothecin (SN-38), in vivo where it elicits cytotoxicity via potent inhibition of topoisomerase I. SN-38 is further detoxicated into its inactive metabolite, SN-38G, through glucuronidation by the enzyme uridine diphosphate glucuronosyltransferase (UGT) in the liver [3]. The glucuronidation of SN-38 to SN-38G is a decisive step in the metabolism and detoxification of irinotecan. However, genetic polymorphisms of the UGT1A1 can alter the glucuronidation of SN-38 and as such have a major influence on the pharmacokinetics and toxicity of irinotecan [4]. The number of repeats of TATA box in the UGT1A1 promoter alters UGT1A1 activity [5], with 6TA repeats representing the most common allele of UGT1A1 gene (UGT1A1*1, wild type) and 7TA repeats representing a variant allele (UGT1A1*28, mutant type). Reduced UGT1A1 expression is found in individuals with the UGT1A1*28 variant, and consequently reduced SN-38 glucuronidation and increased irinotecan-related toxicity are well established in patients with this genotype [6].
The recommended dose of irinotecan in FOLFIRI regimen is 180 mg/m2 every 2 weeks. However, this dose is insufficient in patients with the UGT1A1*1 allele and contributes to a poorer clinical outcome [7], [8], [9], [10]. Conversely, 180 mg/m2 of irinotecan in patients with homozygous UGT1A1*28 is associated with a greater number of severe adverse events (AEs) [7], [11]. Increase in the irinotecan dose in a bid to improve the response rate increases the risk of AEs such as neutropenia and diarrhea. To date, the appropriate irinotecan dosage modifications for patients with UGT1A1*1 and UGT1A1*28 alleles are not clearly defined.
Recently, the therapeutic regimen for mCRC has been altered with the introduction of biologic drugs such as bevacizumab and cetuximab [12], [13]. These 2 biologic agents targeting either the vascular endothelial growth factor (bevacizumab) or epidermal growth factor receptor (cetuximab) have been incorporated into previous irinotecan or oxaliplatin-backbone 5-FU/LV chemotherapeutic regimens as first-line intensive therapy for potentially resectable or aggressive tumors [14].
Our study aimed to identify the role of UGT1A1 gene polymorphisms in the efficacy and safety of irinotecan dose escalation in mCRC patients treated with FOLFIRI plus bevacizumab as the first-line setting.
Materials and Methods
Patients
Between June 2009 and October 2012, 70 mCRC patients in the Kaohsiung Medical University Hospital were enrolled according to a retrospective review of medical charts. UGT1A1 genotyping was performed on all patients before initiating bevacizumab plus FOLFIRI chemotherapy. The starting dose of irinotecan depended on the genotyping results and was escalated at increasing intervals of 20 to 30 mg/m2 until grade 3/4 AEs occurred. The 70 patients were divided into 2 groups according to UGT1A1 genotypes. All patients were stage IV mCRC and unrelated ethnic Chinese residing in Taiwan.
Eligibility Criteria
mCRC patients with histologically or radiologically proven metastatic lesions were eligible for this study. Eligibility criteria also included sufficient renal, hepatic, and bone marrow function; an Eastern Cooperative Oncology Group performance status of 0 to 2; no central nervous system metastases; no uncontrolled or serious concurrent medical illnesses; no active infections; no other primary malignancies; age > 18 years; and life expectancy > 3 months. All of enrolled patients have normal liver function tests including total bilirubin level. Patients with other malignant diseases in their medical history were excluded.
Groups and Treatment
Group 1 included patients with the UGT1A1 *1/*1 and *1/*28 genotype. Group 2 included patients with the UGT1A1 *28/*28 genotype. According to our previous reports [15], [16], the treatment regimen comprised bevacizumab (Avastin, Roche Pharmaceuticals, Basel, Switzerland) (5 mg/kg; 120-minute IV infusion) on day 1, followed by irinotecan (starting dose: 180 mg/m2 for group 1 and 120 mg/m2 for group 2; 120-minute IV infusion), LV (200 mg/m2; IV infusion over 2 hours), and 5-FU (400 mg/m2; IV bolus infusion followed by 2400-mg/m2 IV infusion over 46 hourrs), every 2 weeks. The maximal administration dose of irinotecan will be 260, 240, and 210 mg/m2 for UGT1A1 *1/*1, *1/*28, and *28/*28 genotype.
After the first two treatment cycles, hematological and nonhematological AEs (including neutropenia, diarrhea and nausea/vomiting etc.) were assessed. The severity of adverse effects was evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0 (http://ctep.cancer.gov/reporting/ctc.html; accessed in October 2015). If AEs were of less than grade 3, we gradually escalated the dose by 20 to 30 mg/m2 every 2 cycles. Dose escalation was stopped if grade 3 or 4 AEs occurred, and when such grade 3/4 AEs did occur, the patients were subsequently treated with the highest dose of irinotecan that they were previously able to tolerate.
Genotyping
Constitutional gene polymorphisms were analyzed by DNA extraction from 4 ml of peripheral blood using PUREGENE DNA Isolation Kit (Gentra Systems Inc., Minneapolis, MN, USA). All genomic DNA from the patients were analyzed using direct sequencing to determine the UGT1A1 promoter region genotype. Primers used in this study were designed using primer 3 free software (http://primer3.wi.mit.edu). The sequences of the forward and reverse primers were 5ʹ-AGTCACGTGACACAGTCAAACA-3ʹ and 5ʹ-CTTTGCTCCTGCCAGAGGTT-3ʹ, respectively. The PCR volume was 40 μl, and the PCR conditions were as follows: 94°C for 5 minutes; 30 cycles of denaturation for 30 seconds at 94°C, annealing for 20 seconds at 67.5°C, and primer extension for 20 seconds at 72°C; and final extension for 10 minutes at 72°C. Genotypes were verified by fragment analysis of the PCR product using the automated capillary electrophoresis on the ABI PRISM 310 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA) and analyzed using GeneScan and Genotyper software (Applied Biosystems).
Postchemotherapeutic Surveillance
The response to treatment was assessed radiologically by computed tomography, magnetic resonance imaging, or positron emission tomography, and the best response was recorded. The first response assessment was usually after the fourth or sixth cycle in patients who received bevacizumab combined with FOLFIRI chemotherapy. Patients’ responses were classified according to the Response Evaluation Criteria in Solid Tumors (version 1.1) [17]. Complete response (CR) was defined as the disappearance of all target lesions; partial response (PR) was defined as at least a 30% decrease in the sum of the longest diameter from baseline. Progressive disease (PD) was defined as at least a 20% increase in the sum of the longest diameter of target lesions, with the smallest sum of the longest diameters recorded before treatment as reference. PD was also defined as the identification of one or more new lesions. Stable disease (SD) was defined as neither sufficient shrinkage to quality for PR nor sufficient increase to qualify for PD. The best response was defined as the best result recorded by the investigators because the confirmatory imaging evidence of response obtained after four to six cycles of chemotherapy was not consistently available. The median follow-up period was 22 months (range, 6-34 months). This study conformed to the Helsinki Declaration and was approved by the Institutional Review Board of Kaohsiung Medical University Hospital. The written informed consents were collected from all participated patients. For liver/lung metastatic lesions, metastasectomy was performed after a multidisciplinary team meeting. The primary end points were response rate and progression-free survival (PFS); the secondary endpoints were toxicity and overall survival (OS).
Statistical Analysis
All data were analyzed using Statistical Package for Social Sciences, Version 14.0, software (SPSS Inc., Chicago, IL, USA). A P value of < .05 was considered statistically significant. Univariate analysis of clinicopathologic features between the two groups (6/6 and 6/7 group vs 7/7 group) was compared using the Pearson chi-square or Fisher exact t test. PFS was defined as the time from the beginning of chemotherapy until the first documentation of progression regardless of the patient’s treatment status. OS was defined as the time between the beginning of chemotherapy and death from any cause. PFS and OS rates were calculated using the Kaplan-Meier method, and the differences in survival rates were analyzed using the log-rank test. A probability of less than .05 was considered statistically significant.
Results
The average age of the patients was 69.0 years (range, 40-78 years; Table 1). Fifty-one patients presented with colon cancer (72.9%) and 19 with rectal cancer (27.1%). Analysis of histological type showed that 6 (8.6%) of the tumors were well differentiated, 54 (77.1%) were moderately differentiated, and 10 (14.3%) were poorly differentiated. With regard to clinicopathologic features, 25 (35.7%) patients had vascular invasion and 44 (62.9%) had perineural invasion. The response and disease control rates were 72.9% (51/70) and 90% (63/70), respectively. Eighteen mCRC patients (25.7%) were suitable for metastasectomy after initial bevacizumab plus FOLFIRI chemotherapy. Grade 3/4 AEs occurred in 7 mCRC patients (10%). Table 2 shows the correlation between UGT1A1 genotypes and clinicopathologic features of the 70 mCRC patients receiving bevacizumab plus FOLFIRI. On univariate analysis, the clinical response and disease control rates were significantly higher in the UGT1A1 *1/*1 and *1/*28 group than in the *28/*28 group (P = .006 and P < .001, respectively). Grade 3/4 AEs were significantly less frequent in the UGT1A1 *1/*1 and *1/*28 group than in the *28/*28 group (P < .001).
Table 1.
Clinicopathologic Features of 70 Metastatic Colorectal Cancer Patients Receiving FOLFIRI Plus Bevacizumab Chemotherapy
| Variables | Number (%) |
|---|---|
| Gender | |
| Male/female | 42 (60.0)/28 (40.0) |
| Age (years) (mean ± SD) | 69.0 ± 12.3 |
| Maximum tumor size (cm) | |
| < 5/≥ 5 | 42 (60.0)/28 (40.0) |
| Location | |
| Colon/rectum | 51 (72.9)/19 (27.1) |
| Depth of invasion | |
| T1+ T2/T3+ T4 | 7 (10.0)/63 (90.0) |
| Lymph node metastasis | |
| N(−)/N(+) | 12 (17.1)/58 (82.9) |
| Vascular invasion | |
| Yes/no | 25 (35.7)/45 (64.3) |
| Perineural invasion | |
| Yes/no | 44 (62.9)/26 (37.1) |
| Histology | |
| WD/MD/PD | 6 (8.6)/54 (77.1)/10 (14.3) |
| Response | |
| CR+PR/SD+PD | 51 (72.9)/19 (27.1) |
| Disease control rate | |
| CR+PR+SD/PD | 63 (90.0)/7 (10.0) |
| Metastasectomy | |
| Yes/no | 18 (25.7)/52 (74.3) |
| AE grade 3/4 | |
| Yes/no | 7 (10.0)/63 (90.0) |
WD: well differentiated; MD: moderately differentiated; PD: poorly differentiated.
Table 2.
Correlation between Different UGT1A1 Genotypes and Clinicopathologic Features by Univariate Analysis
| Genotype *1/*1 and *1/*28 (N = 65) (%) | Genotype *28/*28 (N = 5) (%) | Pa | |
|---|---|---|---|
| Gender | |||
| Male/female | 39 (60.0)/26 (40.0) | 3 (60.0)/2 (40.0) 1.000 | |
| Age (years) | |||
| < 70/≥ 70 | 15 (23.1)/50 (76.9) | 1 (20.0)/4 (80.0) | .875 |
| Maximum size (cm) | |||
| < 5/≥ 5 | 38 (58.5)/27 (41.5) | 4 (80.0)/1 (20.0) | .343 |
| Location | |||
| Colon/rectum | 48 (73.8)/17 (26.2) | 3 (60.0)/2 (40.0) | .502 |
| Depth of invasion | |||
| T1 + T2/T3 + T4 | 6 (9.2)/59 (90.8) | 1 (20.0)/4 (80.0) | .360 |
| Lymph node metastasis | |||
| N(−)/N(+) | 11 (16.9)/54 (83.1) | 1 (20.0)/4 (80.0) | .886 |
| Vascular invasion | |||
| Yes/no | 25 (38.5)/40 (61.5) | 0 (0.0)/5 (100.0) | .097 |
| Perineural invasion | |||
| Yes/no | 40 (61.5)/25 (38.5) | 4 (80.0)/1 (20.0) | .457 |
| Histology | |||
| WD/MD/PD | 6 (9.2)/49 (75.4)/10 (15.4) | 0 (0.0)/5 (100.0)/0 (0.0) | .528 |
| Response | |||
| CR+PR/SD+PD | 50 (76.9)/15 (23.1) | 1 (20.0)/4 (80.0) | .006 |
| Disease control rate | |||
| CR+PR+SD/PD | 61 (93.8)/4 (6.2) | 2 (40.0)/3 (60.0) | < .001 |
| AE grade 3/4 | |||
| Yes/no | 4 (6.2)/61 (93.8) | 3 (60.0)/2 (40.0) | < .001 |
Chi-square test.
Table 3 shows the different maximal doses of irinotecan tolerated by mCRC patients with variant UGT1A1 genotypes. The UGT1A1 *1/*1 and *1/*28 group started with a 180-mg/m2 dose of irinotecan, which was progressively escalated up to 260 and 240 mg/m2, respectively. The UGT1A1 *28/*28 group started with a dose of 120 mg/m2, but this was progressively escalated up to only 210 mg/m2 in only 1 patient. In addition, 58.4% of patients with the UGT1A1 *1/*1 and *1/*28 genotypes were able to tolerate a maximal irinotecan dose greater than the recommended 180-mg/m2 dose, but only 1 patient with the UGT1A1 *28/*28 genotype (20%) was able to tolerate a dose of > 180 mg/m2 (P < .001, Table 3). However, 1 patient with the UGT1A1 *1/*1 genotype experienced a grade 3 AE, and therefore, the irinotecan dose was reduced to 150 mg/m2.
Table 3.
UGT1A1 Genotyping and Maximal Tolerated Dose of Irinotecan in mCRC Patients Treated with FOLFIRI and Bevacizumab
| UGT1A1 Genotype N (%) |
||||
|---|---|---|---|---|
| Irinotecan Dosage | *1/*1 & *1/*28 | *28/*28 | Total | P |
| 120 mg/m2 | 0 (0%) | 2 (40.0%) | 2 (2.9%) | < .001 |
| 150 mg/m2 | 1 (1.5%) | 1 (20.0%) | 2 (2.9%) | |
| 180 mg/m2 | 26 (40.0%) | 1 (20.0%) | 27 (38.6%) | |
| 210 mg/m2 | 22 (33.8%) | 1 (20.0%) | 23 (32.9%) | |
| 240 mg/m2 | 11 (16.9%) | 0 (0%) | 11 (15.7%) | |
| 260 mg/m2 | 5 (7.7%) | 0 (0%) | 5 (7.1%) | |
| Total | 65 (100%) | 5 (100%) | 70 (100%) | |
Table 4 shows variant UGT1A1 genotypes of mCRC patients and their different clinical response rates to bevacizumab and FOLFIRI. Patients with UGT1A1 *1/*1 and *1/*28 genotypes showed a response rate of 76.9%, compared with a response rate of 20% in patients with the UGT1A1 *28/*28 genotype (P = .001, Table 4). Moreover, grade 3/4 AEs were more common among patients with the UGT1A1 7/7 genotype than among those with the UGT1A1 *1/*1 and *1/*28 genotypes (P < .001, Table 2). However, patients older than 70 years did not show significantly increased rates of grade 3/4 AEs (P = .569, Table 5).
Table 4.
UGT1A1 Genotyping and Response in mCRC Patients Treated with FOLFIRI and Bevacizumab
| UGT1A1 Genotype N (%) |
||||
|---|---|---|---|---|
| Response | *1/*1 &*1/*28 | *28/*28 | Total | P |
| CR | 2 (3.1%) | 0 (0%) | 2 (2.9%) | .001 |
| PR | 48 (73.8%) | 1 (20.0%) | 49 (70.0%) | |
| SD | 11 (16.9%) | 1 (20.0%) | 12 (17.1%) | |
| PD | 4 (6.2%) | 3 (60.0%) | 7 (10.0%) | |
| Total | 65 (100%) | 5 (100%) | 70 (100%) | |
Table 5.
Age and Grade 3/4 AEs in mCRC Patients Treated with FOLFIRI and Bevacizumab
| AE Grade 3/4 |
P | |||
|---|---|---|---|---|
| Yes | No | |||
| Age | > 70 | 6 (11.1%) | 48 (88.9%) | |
| < 70 | 1 (6.3%) | 15 (93.8%) | .569 | |
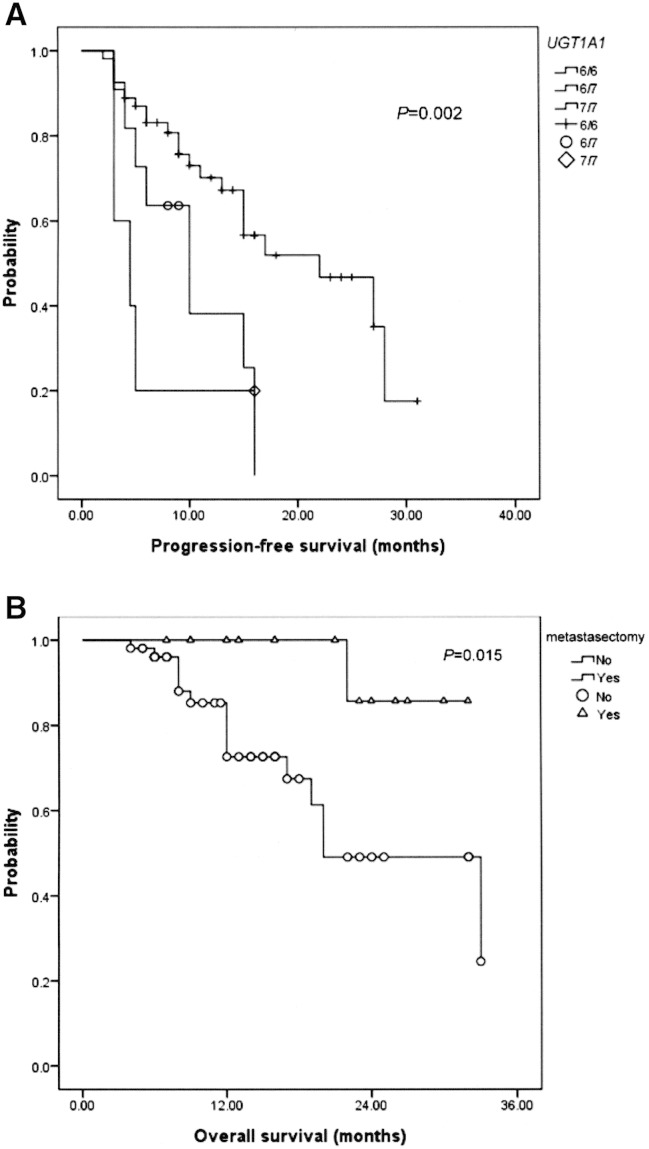
PFS was significantly different for the different UGT1A1 genotypes (P = .002, Figure 1A). Furthermore, the possibility of metastasectomy significantly influenced OS in mCRC patients after bevacizumab plus FOLFIRI chemotherapy (P = .015, Figure 1B).
Figure 1.
(A) Progression-free survival according to the UGT1A1 genotype (P = .002); 6/6, 6/7, and 7/7 means *1/*1, *1/*28, and *28/*28 genotype, respectively. (B) Overall survival in mCRC patients with or without metastasectomy (P = .015).
Discussion
Several trials have used bevacizumab in diverse combinations with irinotecan- or oxaliplatin-based 5-FU/LV regimens as first-line treatment for mCRC, but the results are inconsistent for different regimens [18]. These divergent results can be partially explained by pharmacogenetic studies that have shown that UGT1A1 genetic polymorphisms can result in inefficient irinotecan metabolism. Genotyping of mCRC patients therefore represents an opportunity to enhance the efficacy of irinotecan treatment. We can use individual genotype analysis to predict the severity of irinotecan toxicities and increase the dose of irinotecan to as high levels as are tolerable to achieve maximal benefit for tumor treatment. In this study, approximately 60% of patients that harbored UGT1A1 *1/*1 and *1/*28 genotypes were able to tolerate irinotecan doses greater than the recommended dose of 180 mg/m2 (Table 3). By using genotyping as a guide, patient-specific irinotecan dose optimization represents a way of individualizing cancer therapy [19].
Among newly diagnosed CRC cases, 20% to 25% present with metastatic disease [20], [21]. Surgical resection of metastatic lesions offers a potentially curative approach for CRC patients with locally confined metastases. For mCRC patients with initially unresectable lesions, neoadjuvant chemotherapy combined with targeted agents is favored to downstage disease status to be resectable and improve survival. Reported resectability rates after chemotherapy plus bevacizumab vary widely from 11.8% (225/1914) [22] to 51% (61/120) [23] in different studies [24]. Discrepancies among these studies are attributed to selected chemotherapeutic regimens and study designs, making comparison difficult. Our study demonstrates that the FOLFIRI plus bevacizumab regimen can downstage initially unresectable metastases to resectable disease in 18 of 70 (25.7%) mCRC patients (Table 1). Furthermore, mCRC patients undergoing metastasectomy show significantly improved survival compared with patients without metastasectomy (P = .015).
This study evaluated the dose-limited toxicity and maximal tolerated dose of irinotecan in the FOLFIRI plus bevacizumab regimen, which was used as the first-line treatment for mCRC according to UGT1A1 genotypes. In this dose-escalating trial, we have demonstrated that the recommended dose of 180 mg/m2 for irinotecan is considerably lower than the dose that can be tolerated by patients with the UGT1A1 *1/*1 and *1/*28 genotypes. In fact, some of our patients with UGT1A1 *1/*1 and *1/*28 genotypes can safely tolerate the dose of irinotecan up to 260 and 240 mg/m2, respectively. Our results are consistent with those of previous studies that used the FOLFIRI regimen to treat mCRC patients with irinotecan dose escalation according to UGT1A1 genotypes [7], [8]. For patients with the UGT1A1 *28/*28 genotype, the starting dose of irinotecan should be decreased to diminish the AEs of irinotecan. Stratification of mCRC patients according to genotype results in a significantly higher response rate and PFS in patients with UGT1A1 *1/*1 and *1/*28 genotypes compared with patients with the UGT1A1 *28/*28 genotype. In addition, a response rate was achieved in only 20% of mCRC patients with the UGT1A1 *28/*28 genotype who received FOLFIRI plus bevacizumab therapy. Therefore, it is necessary to consider alternative, more active chemotherapeutic regimens for this subgroup of patients.
Published reports have demonstrated the irinotecan dose-associated toxicities in patients with UGT1A1*28 genotype [25], [26], [27]. The U.S. Food and Drug Administration in 2005 approved the identification of UGT1A1*28 homozygous patients that were recommended with a lower dose of irinotecan administration [28]. Also, the Evaluation of Genomic Application in Practice and Prevention working group has indicated that UGT1A1*28 genotyping will be only clinically useful on the aspect of safety use of irinotecan without compromising the efficacy of this drug [29]. Nevertheless, reduction of dosage might also be associated with reduced tumor therapeutic response and/or increased mortality. The study of Shulman et al. has showed that UGT1A1*28 genotype is strongly associated with severe hematologic toxicity and lower survival of CRC patients in use of irinotecan [30]. Whereas most reports concerning the pretherapeutic check of UGT1A1*28 genotype are for consideration of AEs, our present study emphasizes the encouraging detection of UGT1A1*1 genotype to escalate the dose of irinotecan to achieve the maximal therapeutic effect. Furthermore, the role of irinotecan dose escalation is especially important in Asian races because they inherit more frequency of UGT1A1*1 allele than Caucasians [9], [11].
Besides UGT1A1*28, there are many ethnic differences and frequencies in UGT1A gene, some of which were also reported to influence the efficacy and toxicity of irinotecan via similar interfering hepatic metabolism. Takano et al. [31] reported that the UGT1A1*6 polymorphism is a potential predictor of severe neutropenia derived by irinotecan in Japanese patients. Hazama et al. [32] reported that assessment of UGT1A1*28 and *6 and also UGT1A7*3 and UGT1A9*22 is very important to predict the toxicity of irinotecan in Japanese patients. Hence, the relative contribution of variant UGT1A gene polymorphisms to the prediction of the outcome of FOLFIRI therapy in patients of different races should be determined in the future researches. A limitation of our study is that it is hypothesis generating because of the retrospective design and relatively small study group. Prospective studies involving larger numbers of patients would be planned to confirm our results.
In summary, our findings show that mCRC patients with UGT1A1 *1/*1 and *1/*28 genotypes can tolerate a higher dose of irinotecan to achieve a more favorable therapeutic response while using the FOLFIRI plus bevacizumab regimen. mCRC patients with the UGT1A1 *28/*28 genotype show poorer response with irinotecan-based chemotherapy and may benefit from alternative therapeutic regimens.
Acknowledgements
This work was supported by grants from the Excellence for Cancer Research Center (MOST104-2325-B-037-001); the Ministry of Health and Welfare (MOHW 104-TDU-B-212-124-003), Taiwan, Republic of China; Kaohsiung Medical University Hospital (KMUH100-0M01, KMUH100-0M16, KMUH103-3R16, KMUHS10304, KMUHS10305); the Center for Biomarkers and Biotech Drugs, Kaohsiung Medical University (KMU-TP103C00, KMU-TP103C03, KMU-TP103C07, KMU-TP103H11, KMU-TP104A11); and the Grant of Biosignature in Colorectal Cancers, Academia Sinica, Taiwan.
Footnotes
This work was supported by grants from the Excellence for Cancer Research Center (MOST104-2325-B-037-001); the Ministry of Health and Welfare (MOHW 104-TDU-B-212-124-003), Taiwan, Republic of China; Kaohsiung Medical University Hospital (KMUH100-0M01, KMUH100-0M16, KMUH103-3R16, KMUHS10304, KMUHS10305); the Center for Biomarkers and Biotech Drugs, Kaohsiung Medical University (KMU-TP103C00, KMU-TP103C03, KMU-TP103C07, KMU-TP103H11, KMU-TP104A11); and the Grant of Biosignature in Colorectal Cancers, Academia Sinica, Taiwan.
Disclosure of potential conflicts of interest: The authors have declared no conflicts of interest.
Contributor Information
Meng-Lin Huang, Email: gis1253@gmail.com.
Jaw-Yuan Wang, Email: cy614112@ms14.hinet.net, jawyuanwang@gmail.com.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Saunders M, Iveson T. Management of advanced colorectal cancer: state of the art. Br J Cancer. 2006;95:131–138. doi: 10.1038/sj.bjc.6603233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mathijssen RH, van Alphen RJ, Verweij J, Loos WJ, Nooter K, Stoter G, Sparreboom A. Clinical pharmacokinetics and metabolism of irinotecan (CPT-11) Clin Cancer Res. 2001;7:2182–2194. [PubMed] [Google Scholar]
- 4.Iyer L, King CD, Whitington PF, Green MD, Roy SK, Tephly TR, Coffman BL, Ratain MJ. Genetic predisposition to the metabolism of irinotecan (CPT-11). Role of uridine diphosphate glucuronosyltransferase isoform 1A1 in the glucuronidation of its active metabolite (SN-38) in human liver microsomes. J Clin Invest. 1998;101:847–854. doi: 10.1172/JCI915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monaghan G, Ryan M, Seddon R, Hume R, Burchell B. Genetic variation in bilirubin UPD-glucuronosyltransferase gene promoter and Gilbert's syndrome. Lancet. 1996;347:578–581. doi: 10.1016/s0140-6736(96)91273-8. [DOI] [PubMed] [Google Scholar]
- 6.Iyer L, Das S, Janisch L, Wen M, Ramirez J, Karrison T, Fleming GF, Vokes EE, Schilsky RL, Ratain MJ. UGT1A1*28 polymorphism as a determinant of irinotecan disposition and toxicity. Pharmacogenomics J. 2002;2:43–47. doi: 10.1038/sj.tpj.6500072. [DOI] [PubMed] [Google Scholar]
- 7.Marcuello E, Paez D, Pare L, Salazar J, Sebio A, del Rio E, Baiget M. A genotype-directed phase I-IV dose-finding study of irinotecan in combination with fluorouracil/leucovorin as first-line treatment in advanced colorectal cancer. Br J Cancer. 2011;105:53–57. doi: 10.1038/bjc.2011.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toffoli G, Cecchin E, Gasparini G, D'Andrea M, Azzarello G, Basso U, Mini E, Pessa S, De Mattia E, Lo Re G. Genotype-driven phase I study of irinotecan administered in combination with fluorouracil/leucovorin in patients with metastatic colorectal cancer. J Clin Oncol. 2010;28:866–871. doi: 10.1200/JCO.2009.23.6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palomaki GE, Bradley LA, Douglas MP, Kolor K, Dotson WD. Can UGT1A1 genotyping reduce morbidity and mortality in patients with metastatic colorectal cancer treated with irinotecan? An evidence-based review. Genet Med. 2009;11:21–34. doi: 10.1097/GIM.0b013e31818efd77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hebbar M, Ychou M, Ducreux M. Current place of high-dose irinotecan chemotherapy in patients with metastatic colorectal cancer. J Cancer Res Clin Oncol. 2009;135:749–752. doi: 10.1007/s00432-009-0580-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsai H-L, Chen C-F, Lu C-Y, Fang W-Y, Wu D-C, Wu I-C, Sheen M-C, Lin S-R, Wang J-Y. Significant Correlation between Polymorphisms of UGT1A1 Gene and Low Irinotecan Toxicity in Colorectal Cancer Patients with FOLFIRI. Open Colorectal Cancer J. 2009;2:21–26. [Google Scholar]
- 12.Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D'Haens G, Pinter T, Lim R, Bodoky G. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 13.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 14.Peeters M, Price T. Biologic therapies in the metastatic colorectal cancer treatment continuum--applying current evidence to clinical practice. Cancer Treat Rev. 2012;38:397–406. doi: 10.1016/j.ctrv.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Yeh YS, Huang ML, Chang SF, Chen CF, Hu HM, Wang JY. FOLFIRI combined with bevacizumab as first-line treatment for metastatic colorectal cancer patients with hyperbilirubinemia after UGT1A1 genotyping. Med Princ Pract. 2014;23:478–481. doi: 10.1159/000358799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu CY, Huang CW, Hu HM, Tsai HL, Huang CM, Yu FJ, Huang MY, Chang SF, Huang ML, Wang JY. Prognostic advantage of irinotecan dose escalation according to uridine diphosphate glucuronosyltransferase 1A1 (UGT1A1) genotyping in patients with metastatic colorectal cancer treated with bevacizumab combined with 5-fluorouracil/leucovorin with irinotecan in a first-line setting. Transl Res. 2014;164:169–176. doi: 10.1016/j.trsl.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Macedo LT, da Costa Lima AB, Sasse AD. Addition of bevacizumab to first-line chemotherapy in advanced colorectal cancer: a systematic review and meta-analysis, with emphasis on chemotherapy subgroups. BMC Cancer. 2012;12:89–96. doi: 10.1186/1471-2407-12-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McLeod HL, Sargent DJ, Marsh S, Green EM, King CR, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Thibodeau SN. Pharmacogenetic predictors of adverse events and response to chemotherapy in metastatic colorectal cancer: results from North American Gastrointestinal Intergroup Trial N9741. J Clin Oncol. 2010;28:3227–3233. doi: 10.1200/JCO.2009.21.7943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cartwright TH. Treatment decisions after diagnosis of metastatic colorectal cancer. Clin Colorectal Cancer. 2012;11:155–166. doi: 10.1016/j.clcc.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Gill S, Blackstock AW, Goldberg RM. Colorectal cancer. Mayo Clin Proc. 2007;82:114–129. doi: 10.4065/82.1.114. [DOI] [PubMed] [Google Scholar]
- 22.Okines A, Puerto OD, Cunningham D, Chau I, Van Cutsem E, Saltz L, Cassidy J. Surgery with curative-intent in patients treated with first-line chemotherapy plus bevacizumab for metastatic colorectal cancer First BEAT and the randomised phase-III NO16966 trial. Br J Cancer. 2009;101:1033–1038. doi: 10.1038/sj.bjc.6605259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Figueras J, Lopez-Ben S, Alsina M, Soriano J, Hernandez-Yague X, Albiol M, Guardeno R, Codina-Barreras A, Queralt B. Preoperative treatment with bevacizumab in combination with chemotherapy in patients with unresectable metastatic colorectal carcinoma. Clin Transl Oncol. 2013;15:460–466. doi: 10.1007/s12094-012-0952-6. [DOI] [PubMed] [Google Scholar]
- 24.Bruera G, Cannita K, Giuliante F, Lanfiuti Baldi P, Vicentini R, Marchetti P, Nuzzo G, Antonucci A, Ficorella C, Ricevuto E. Effectiveness of liver metastasectomies in patients with metastatic colorectal cancer treated with FIr-B/FOx triplet chemotherapy plus bevacizumab. Clin Colorectal Cancer. 2012;11:119–126. doi: 10.1016/j.clcc.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Hu ZY, Yu Q, Zhao YS. Dose-dependent association between UGT1A1*28 polymorphism and irinotecan-induced diarrhoea: a meta-analysis. Eur J Cancer. 2010;46:1856–1865. doi: 10.1016/j.ejca.2010.02.049. [DOI] [PubMed] [Google Scholar]
- 26.Hu ZY, Yu Q, Pei Q, Guo C. Dose-dependent association between UGT1A1*28 genotype and irinotecan-induced neutropenia: low doses also increase risk. Clin Cancer Res. 2010;16:3832–3842. doi: 10.1158/1078-0432.CCR-10-1122. [DOI] [PubMed] [Google Scholar]
- 27.Hoskins JM, Goldberg RM, Qu P, Ibrahim JG, McLeod HL. UGT1A1*28 genotype and irinotecan-induced neutropenia: dose matters. J Natl Cancer Inst. 2007;99:1290–1295. doi: 10.1093/jnci/djm115. [DOI] [PubMed] [Google Scholar]
- 28.O'Dwyer PJ, Catalano RB. Uridine diphosphate glucuronosyltransferase (UGT) 1A1 and irinotecan: practical pharmacogenomics arrives in cancer therapy. J Clin Oncol. 2006;24:4534–4538. doi: 10.1200/JCO.2006.07.3031. [DOI] [PubMed] [Google Scholar]
- 29.Berg AO, Armstrong K, Botkin J, Calonge N, Haddow J, Hayes M, Kaye C, Phillips KA, Piper M, Richards CS. Recommendations from the EGAPP Working Group: can UGT1A1 genotyping reduce morbidity and mortality in patients with metastatic colorectal cancer treated with irinotecan? Genet Med. 2009;11:15–20. doi: 10.1097/GIM.0b013e31818efd9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shulman K, Cohen I, Barnett-Griness O, Kuten A, Gruber SB, Lejbkowicz F, Rennert G. Clinical implications of UGT1A1*28 genotype testing in colorectal cancer patients. Cancer. 2011;117:3156–3162. doi: 10.1002/cncr.25735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takano M, Kato M, Yoshikawa T, Sasaki N, Hirata J, Furuya K, Takahashi M, Yokota H, Kino N, Horie K. Clinical significance of UDP-glucuronosyltransferase 1A1*6 for toxicities of combination chemotherapy with irinotecan and cisplatin in gynecologic cancers: a prospective multi-institutional study. Oncology. 2009;76:315–321. doi: 10.1159/000209335. [DOI] [PubMed] [Google Scholar]
- 32.Hazama S, Mishima H, Tsunedomi R, Okuyama Y, Kato T, Takahashi K, Nozawa H, Ando H, Kobayashi M, Takemoto H. UGT1A1*6, 1A7*3, and 1A9*22 genotypes predict severe neutropenia in FOLFIRI-treated metastatic colorectal cancer in two prospective studies in Japan. Cancer Sci. 2013;104:1662–1669. doi: 10.1111/cas.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]