Abstract
PURPOSE: The incidence of sensorineural hearing loss (SNHL) after treatment with combination of intensity-modulated radiation therapy (IMRT) and cisplatin-based chemotherapy in nasopharyngeal carcinoma (NPC) patients was evaluated, and relationships of SNHL with host factors, treatment-related factors, and radiation dosimetric parameters were investigated. METHODS: Fifty-one NPC patients treated with IMRT from 2004 to 2009 were analyzed. All patients received neoadjuvant, concurrent, or adjuvant use of cisplatin. Pure tone audiometry was performed during the follow-up period with a median time of 60 months, ranging from 28 to 84 months. Correlation of SNHL at low frequencies (pure tone average, 0.5-2 kHz) with a series of factors was analyzed. RESULTS: Among 102 ears, 12.7% had low-frequency SNHL and 42.2% had high-frequency (4 kHz) SNHL. The incidence of low-frequency SNHL was greater in patients with age > 40, with T-stage 4, or who received cumulative cisplatin dose (CCD) > 200 mg/m2 (P = .034, .011, and .003, respectively) and in ears with secretory otitis media (SOM) (P = .002). Several dosimetric parameters were found to be correlated with SNHL. Univariate analysis showed that the minimum radiation dose to 0.1 ml highest dose volume (D0.1 ml) of the cochlea was the best radiation-related predictive parameter. Multivariate analysis indicated that CCD, SOM, and D0.1 ml of cochlea (P = .035, .012, and .022, respectively) were the factors associated with SNHL. CONCLUSION: For NPC patients treated with IMRT and chemotherapy, the incidence of treatment-related SNHL was associated with CCD, D0.1 ml of cochlea, and SOM.
Introduction
Nasopharyngeal carcinoma (NPC) has been considered as one of the rarer neoplasms globally, yet it is relatively frequent in some regions, including parts of South-Eastern Asia and a number of provinces in South-Eastern China [1]. Radiotherapy (RT) is the primary modality for the treatment of NPC. High local control and overall survival could be achieved by RT alone or RT in combination with chemotherapy. As survival time increased with improvement in management of this disease, the quality of post-RT life has become a matter of growing concern.
Sensorineural hearing loss (SNHL) has been recognized as an important adverse effect of treatment for patient with NPC [2], [3], [4], [5], [6]. SNHL can occur immediately or in months to years after RT, and plateaus after 18 to 24 months [7], [8]. Radiation damage to hearing apparatus is believed to result in SNHL. In recent years, intensity-modulated radiotherapy (IMRT), which can lead to reduced dose to the hearing apparatus [9], [10], has become a standard RT technique for NPC.
Cisplatin is a cytotoxic agent and radiation sensitizer for the treatment of NPC. The addition of cisplatin-based chemotherapy to RT is recommended for stage III and IVa, or even stage II [11]. However, one of the major side effects of cisplatin is SNHL. Cisplatin and RT might act in synergy and result in an increased risk of SNHL [12], [13], [14], [15], [16]. The relative contribution to SNHL of IMRT or cisplatin chemotherapy remains undefined. It has been reported that radiation dose [2], [17], cisplatin dose used at each cycle [18], or both radiation dose and the concurrent cisplatin dose [19] were useful factors to determine SNHL. However, these reports were based on clinical data with either small patient numbers or short follow-up period, and were not specifically from NPC treatments. It is known that RT for NPC is quite different from that for other head and neck cancers, as the radiation dose to the auditory apparatus is often higher for NPC treatment. Clearly, more mature data from longer follow-up for NPC treatment are needed to identify the relative contribution to SNHL from each parameter. The main purpose of this study was to evaluate the incidence of SNHL with a median follow-up time of 5 years after combined IMRT and cisplatin-based chemotherapy, and to assess various patient-related, treatment-related, and dosimetric parameters associated with SNHL.
Material and Methods
Patient Selection
The Institutional Review Board of Sun Yat-sen University Cancer Center approved the study and consent procedure before starting. Written informed consent was given by each participant for their clinical records to be used in this study. We reviewed the medical records of 51 patients with NPC who were treated between 2004 and 2009. Patients were selected based on the following inclusion criteria: 1) over 18 years old, 2) histologically diagnosed as having NPC stage IIa to IVb according to the American Joint of Cancer Committee (2002), and 3) patients had taken hearing test before treatment. Exclusion criteria included the following: 1) history of hypertension or diabetes, 2) recurrence or metastasis during follow-up, and 3) patients who had middle ear disease or severe hearing impairment before treatment. Whether the patient has secretary otitis media (SOM) was also recorded at the time of follow-up.
Radiation Therapy
All selected patients received curative IMRT as described by Xiao et al. [20]. Briefly, patients were positioned supine and immobilized from head to neck with a thermoplastic mask in both computed tomography simulation and treatment delivery. The planning computed tomography was acquired with a slice thickness of 3 mm and was transferred to a treatment-planning system (CORVUS 3.0/3.2, NOMOS Corp., Cranberry Township, PA) for IMRT planning. The gross tumor volume (GTV) was defined according to diagnostic magnetic resonance imaging, computed tomography and physical examination. The clinical target volume 1 (CTV1) was defined as the nasopharynx GTV plus a 5- to 10-mm margin, whereas CTV2 was the CTV1 plus a 5- to 10-mm margin except 2 to 3 mm posteriorly, including the elective neck area. Planning target volumes for all gross tumor volumes and CTVs were generated automatically after delineation of tumor targets according to the immobilization and localization uncertainties. Organs at risk (OARs) were delineated from the planning computed tomography. In particular, the inner ear, cochlea, and internal auditory canal (IAC) were delineated using bone window (window width = 2000 HU, window level = 400 HU). Figure 1 shows an example of these contours. IMRT plans were generated with a prescribed dose of 68 Gy in 30 fractions to the GTV, 60 to 64 Gy to CTV1 and 50 to 54 Gy to CTV2. The doses to OARs were minimized without sacrificing target coverage.
Figure 1.
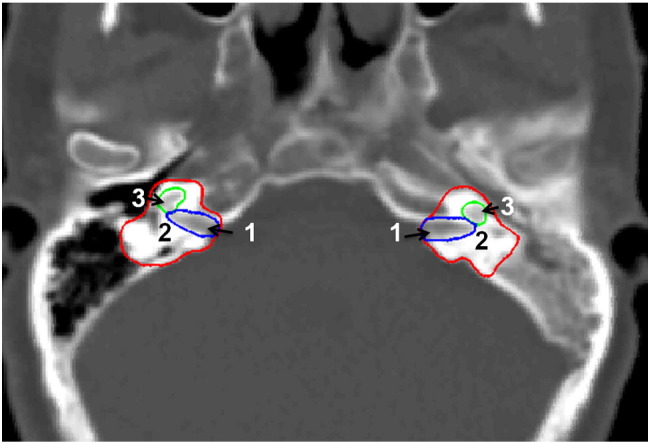
Delineation of (1) internal auditory canal, (2) inner ear, and (3) cochlea.
For this analysis, the dosimetric treatment plans were transferred to the Computational Environment for Radiological Research (CERR 4.0 beta 4) [21] using RTOG file format. To identify factors of being appropriate dosimetric correlation with SNHL, a series of radiation dosimetric parameters, including mean dose, minimum dose, maximum dose, median dose, percentage volume receiving at least x Gy (Vx), and minimum dose to x cubic centimeter of highest dose volume (Dxml) were calculated for the inner ear, cochlea, and IAC from the dosimetric plan for each patient. In addition, the concept of the equivalent uniform dose (EUD) was introduced to consider the overall effect of a nonuniform dose distribution for a given end point [22]. The EUD can be calculated based on dose volume histogram using , where vi is the fractional organ volume receiving a dose Di and a is a tissue-specific parameter that describes the volume effect [23], [24], [25], [26]. This calculation reduces dose volume histogram into a single parameter, EUD. EUD was calculated with a series of “a” values for inner ear, cochlea, and IAC for correlation study with SNHL.
Chemotherapy
Fifty-one patients received combined cisplatin-based chemotherapy, including neoadjuvant, concurrent, and adjuvant use of cisplatin. Cisplatin was given at a dose of 80 mg/m2 every 3 weeks or 40 mg/m2/wk during the course of RT. The mean value of cumulative cisplatin doses was 215.50 ± 107.16 mg/m2 (ranged from 80 to 630 mg/m2, median: 160 mg/m2). Twenty-seven received induction chemotherapy followed by the concurrent chemoradiotherapy. The induction chemotherapy consists of two or three cycles of paclitaxel 135 mg/m2 and carboplatin (area under the curve = 6) every 3 three weeks, or cisplatin 80 mg/m2 on day 1 and 5-fluorouracil 3.5 g/m2 by continuous intravenous infusion on days 2 to 4 every 3 weeks. Two patients received the induction chemotherapy, followed by chemoradiotherapy plus adjuvant chemotherapy. Adjuvant chemotherapy plan included cisplatin 80 mg/m2 on day 1 and 5-fluorouracil 3.5 g/m2 by continuous intravenous infusion on days 2 to 4 every 3 weeks for three to four cycles.
Hearing Assessment
Pure tone air and bone conduction (BC) audiometry, otoscopy, and tympanometry were performed. The air conduction threshold was measured at 0.25 to 8 kHz, and BC threshold was measured at 0.5 to 4 kHz. BC threshold at 4 kHz was selected to represent the high-frequency loss. The pure tone average (PTA), an average of threshold levels at 0.5, 1, and 2 kHz, was chosen to reflect the low-frequency loss. Middle ear function was evaluated by clinical examination, pure tone auditomery (air-bone gap > 10 dB), and tympanometry. Significant SNHL was defined if the increase in hearing threshold was ≥ 15 dB at low frequencies (PTA).
Incidence of SNHL was recorded on per ear basis. To assess the relationship between radiation dose and SNHL, each ear was analyzed independently.
Statistical Analysis
The follow-up time, starting from the completion day of IMRT, ranged from 28 to 84 months (median: 60 months). The chi-square test or Fisher’s exact test (when expected count < 5) was used for comparison of categorical data. To calculate EUD, the value of 1/a was selected from 0.001 to 1. Univariate logistic regression was used to determine the best 1/a value for the EUD calculation. Pearson correlation was used to analyze its correlation with other parameters. Receiver operating characteristic curves were used to calculate sensitivity and specificity at each cutoff value of the dosimetric parameters. The optimal cutoff value is achieved when the sum of the sensitivity and specificity reaches a maximum value. Repeated-measures logistic regression was used to handle the nonindependent data sets. The binary logistic regression analysis was performed to determine the clinical factors and dosimetric parameters for SHNL prediction. P < .10 was used as the cutoff value of statistical significance for variable selection in the univariable modeling. SNHL ear was considered as outcome variable. P value of less than .05 was considered statistically significant. The data processing and statistical analyses were performed using SPSS software (Version 15.0, SPSS Inc., Chicago, IL).
Results
Audiological Assessment and the Incidences of Postradiation SNHL
Fifty-one patients were reviewed in this study, with a median age of 42 years old ranging from 25 to 70, including 1 (2%) stage IIa, 3 (5.9%) stage IIb, 34 (66.7%) stage III, 10 (19.6%) stage IVa, and 3 (5.9%) stage IVb NPCs. Histopathological analysis showed 4 WHO type I, and 47 types II and III.
Thirteen ears (13/102, 12.7%) in eight patients experienced low-frequency SNHL, and 43 ears (43/102, 42.2%) in 31 patients had high-frequency SNHL. The subjective hearing loss was present in 38 (37.3%) ears in 22 patients, and tinnitus was found in 15 (14.7%) ears in 9 patients. Forty-five (44.1%) ears in 34 patients had SOM. Air-bone gaps of > 10 dB were present in 37 (36.2%) ears.
The time interval used for assessing hearing varied from 28 to 84 months after radiotherapy. The median interval between hearing test and the end of the radiochemotherapy was 51 months (range: 28-69 months, 50.2 ± 11.9) and 61 months (range: 28-84 months, 58.7 ± 12.8) for patients with and without SNHL, respectively (P = .027).
There were 16.7% (1/6), 22.2% (4/18), 20% (6/30), 0% (0/36), and 16.7% (2/12) ears being diagnosed with SNHL at the time interval of 28 to 36 months, 37 to 48 months, 49 to 60 months, 61 to 72 months, and 73 to 84 months, respectively. There were 54 ears whose hearing was assessed at 28 to 60 months after radiotherapy, 11 of which was found to have SNHL (20.4%). There were 48 ears whose hearing was assessed at 61 to 84 months after radiotherapy, and SNHL was present in 2 ears (4.2%) (odds ratio [OR]: 5.88, 95% confidence interval [CI]: 1.23-28.08, P = .017).
The median treatment durations (for the entire concurrent radiation and chemotherapy) were 43 days (range: 40-50 days, 44.2 ± 3.3) and 44 days (range: 39-57 days, 45.6 ± 4.3) for patients with and without SNHL, respectively (P = .239).
Correlation of Patient- and Treatment-Related Factors and SNHL
Table 1 shows the correlation factors with the incidence of SNHL. The SNHL incidence was significantly greater in patients with age > 40, T-stage 4, and a cumulative cisplatin dose > 200 mg/m2.
Table 1.
Correlation of Patient- and Treatment-Related Factors and SNHL (102 Ears)
| Variables | SNHL in Ears |
SNHL in Patients |
||||||
|---|---|---|---|---|---|---|---|---|
| SNHL/No | OR | 95% CI | P | SNHL/No | OR | 95% CI | P | |
| Age (years) | ||||||||
| > 40 | 11/45 | 5.38 | 1.28-25.67 | .034 | 7/21 | 7.33 | 0.83-64.80 | .059⁎ |
| ≤ 40 | 2/44 | 1 | 1/22 | 1 | ||||
| Gender | ||||||||
| Male | 9/67 | 0.74 | 0.21-2.64 | .735⁎ | 5/33 | 0.51 | 0.10-2.49 | .327⁎ |
| Female | 4/22 | 1 | 3/10 | 1 | ||||
| T stage (UICC 2002) | ||||||||
| 1-3 | 6/72 | 0.20 | 0.06-0.68 | .011⁎ | 4/35 | 0.23 | 0.05-1.12 | .076⁎ |
| 4 | 7/17 | 1 | 4/8 | 1 | ||||
| Stage (UICC 2002) | ||||||||
| IIa-III | 8/68 | 0.31 | 0.15-1.67 | .308⁎ | 5/33 | 0.51 | 0.10-2.49 | .404⁎ |
| IVa-IVb | 5/21 | 1 | 3/10 | 1 | ||||
| Treatment | ||||||||
| CCRT | 2/42 | .015 | 1/21 | .095 | ||||
| IC → CCRT | 9/45 | 6/21 | ||||||
| IC → CCRT → AC | 2/2 | 1/1 | ||||||
| Cumulative cisplatin dose (mg/m2) | ||||||||
| More than 200 | 11/35 | 8.49 | 1.77-40.61 | .003 | 7/16 | 5.67 | 1.05-30.84 | .061⁎ |
| Less than 200 | 2/54 | 1 | 2/26 | 1 | ||||
| Cisplatin prescribed regimen in CCRT | ||||||||
| Weekly cisplatin | 0/16 | .213⁎ | 1/6 | 0.88 | 0.09-8.49 | 1.000⁎ | ||
| Every 3 wk | 13/73 | 7/37 | 1 | |||||
| Carboplatin | ||||||||
| With carboplatin | 3/19 | 1.11 | 0.28-4.42 | 1.000⁎ | 2/9 | 1.26 | 0.22 -7.33 | .557⁎ |
| Without carboplatin | 10/70 | 1 | 6/34 | 1 | ||||
| Treatment time | ||||||||
| Within 6 wk | 7/27 | 2.68 | 0.82-8.72 | .118 | 4/13 | 2.31 | 0.50-10.67 | .416⁎ |
| More than 6 wk | 6/62 | 1 | 4/30 | 1 | ||||
Abbreviations: CT, chemotherapy; CCRT, concurrent chemoradiotherapy; IC → CCRT, induction chemotherapy followed by chemoradiotherapy; IC → CCRT → AC, induction chemotherapy followed by chemoradiotherapy plus adjuvant chemotherapy; UICC, Union for International Cancer Control.
Fisher’s exact test was used for comparison.
Correlation of SOM and SNHL
To assess the relationship between SOM and SNHL, each ear was analyzed independently. It was found that SNHL was present in 11 of the 45 (24.4%) ears with SOM, and in 2 of the 57 (3.5%) ears without SOM (OR: 8.90, 95% CI: 1.86-42.60, P = .002).
Correlation of Radiation Dosimetric Parameters and SNHL
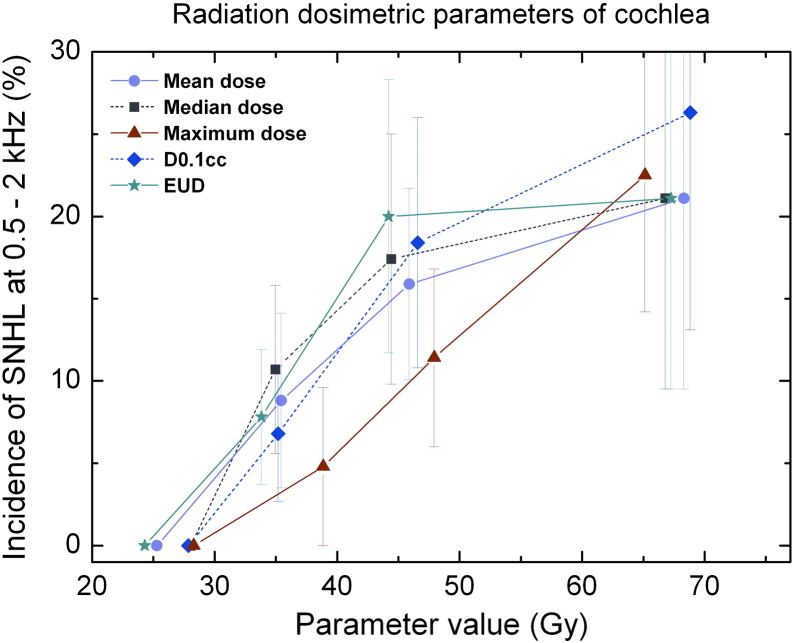
To assess the relationship between radiation dose and SNHL, each ear was analyzed independently. A series of radiation dosimetric parameters for three OARs—cochlea, inner ear, and IAC—that were thought to relate to SNHL is tabulated in Table 2. The correlation analysis suggested that D0.1 ml of cochlea was associated with SNHL (Table 3). Univariate logistic regression show that the EUD of cochlea was mostly significant for determining SNHL when the EUD was calculated with parameter a = 100 (P = .063). The calculated EUD values were found to correlate with the maximum dose, D0.035 ml, median dose, mean dose, and D0.1 ml (r2 = 0.999, 0.984, 0.972, 0.971, and 0.960, respectively, with all Ps < .001). The incident probability of SNHL was plotted against the correlated radiation dosimetric parameters for cochlea in Figure 2.
Table 2.
Summary of Dosimetric Parameters (102 Ears)
| Parameter | Inner Ear |
Cochlea |
IAC |
|||
|---|---|---|---|---|---|---|
| SNHL | Non-SNHL | SNHL | Non-SNHL | SNHL | Non-SNHL | |
| Volume (ml) | 3.11 ± 1.05 | 2.78 ± 0.87 | 0.33 ± 0.08† | 0.27 ± 0.07 | 0.29 ± 0.09 | 0.26 ± 0.07 |
| Mean dose (Gy) | 39.42 ± 10.12 | 37.90 ± 11.39 | 44.51 ± 13.52 | 42.81 ± 12.76 | 41.66 ± 11.58 | 41.43 ± 13.11 |
| Minimum dose (Gy) | 22.39 ± 7.86 | 21.47 ± 7.21 | 35.55 ± 13.17 | 34.65 ± 12.86 | 33.13 ± 12.40 | 33.89 ± 13.10 |
| Maximum dose (Gy) | 60.38 ± 8.19 | 58.56 ± 10.68 | 54.93 ± 10.65 | 52.08 ± 11.83 | 51.38 ± 10.68 | 49.55 ± 12.57 |
| Median dose (Gy) | 40.98 ± 8.46 | 38.23 ± 9.53 | 44.93 ± 11.48 | 43.06 ± 12.37 | 41.63 ± 11.56 | 41.41 ± 12.99 |
| EUD (Gy)⁎ | 49.13 ± 12.22 | 47.22 ± 10.69 | 52.58 ± 10.40 | 49.90 ± 11.63 | - | - |
| D0.1 ml (Gy) | 53.92 ± 11.25 | 53.22 ± 11.25 | 48.72 ± 13.53 | 44.37 ± 13.03 | 43.72 ± 13.16 | 41.29 ± 15.29 |
| D0.035 ml (Gy) | 56.46 ± 10.91 | 55.78 ± 11.43 | 52.52 ± 12.62 | 48.13 ± 12.51 | 47.44 ± 12.92 | 45.42 ± 11.19 |
| V30 (%) | 68.99 ± 20.43 | 61.66 ± 25.16 | 91.40 ± 11.26 | 84.63 ± 24.17 | 86.26 ± 17.23 | 81.74 ± 26.62 |
| V40 (%) | 43.64 ± 30.26 | 34.87 ± 30.33 | 52.99 ± 36.90 | 46.71 ± 38.63 | 42.10 ± 41.16 | 40.11 ± 39.50 |
| V50 (%) | 22.44 ± 29.89 | 17.92 ± 27.99 | 29.67 ± 41.38 | 23.92 ± 38.62 | 25.95 ± 41.75 | 21.30 ± 38.41 |
| V60 (%) | 9.18 ± 18.85 | 9.16 ± 19.57 | 17.35 ± 34.73 | 16.29 ± 35.25 | 11.54 ± 28.31 | 14.85 ± 34.58 |
| V70 (%) | 1.34 ± 3.34 | 3.24 ± 8.78 | 1.53 ± 5.18 | 4.80 ± 16.59 | 0.57 ± 2.07 | 5.11 ± 16.95 |
†Two-sided P < .05.
EUD was calculated using a = 100 for cochlea and a = 16.7 for inner ear.
Table 3.
Correlation of Radiation Dosimetric Parameters of Cochlea with SNHL (102 Ears)
| Parameters | Cutoff | Incidence Above Cutoff | Incidence Under Cutoff | P | OR | 95% CI |
|---|---|---|---|---|---|---|
| V30 | 69.0% | 15.7% (13/83) | 0.0% (0/19) | .120⁎ | - | - |
| V40 | 32.0% | 15.1% (8/53) | 10.2% (5/49) | .559 | 1.56 | 0.48-5.15 |
| V50 | 14.0% | 23.1% (7/37) | 9.0% (6/65) | .217 | 3.05 | 0.71-7.45 |
| V60 | 35.0% | 21.1% (3/18) | 12.6% (10/84) | .696⁎ | 1.48 | 0.36-6.03 |
| V70 | 1.0% | 15.4% (2/13) | 12.4% (11/89) | .670⁎ | 1.29 | 0.25-6.60 |
| Mean dose | 38Gy | 17.3% (9/52) | 8.0% (4/50) | .236 | 2.41 | 0.69-8.39 |
| Dmin | 32.2Gy | 15.4% (6/39) | 11.1% (7/63) | .553 | 2.11 | 0.45-4.70 |
| Dmax | 54.0Gy | 18.4% (7/38) | 9.4% (6/64) | .225 | 2.18 | 0.68-7.06 |
| Median dose | 34.0Gy | 17.9% (13/82) | 0.0% (0/20) | .067⁎ | - | - |
| EUD (a= 100) | 52.0Gy | 21.6% (7/37) | 7.7% (6/65) | .063⁎ | 3.30 | 1.00-11.00 |
| D0.100 ml | 39.8Gy | 20.0% (11/55) | 4.3% (2/47) | .017 | 5.63 | 1.18-26.85 |
| D0.035 ml | 50.0Gy | 23.1% (8/38) | 9.0% (5/64) | .053 | 3.15 | 0.95-10.45 |
Abbreviations: Dmin: minimum dose; Dmax: maximum dose.
Fisher’s exact test was used.
Figure 2.
The incidence of SNHL versus radiation dosimetric parameters of cochlea. Significant hearing loss was defined as ≥ 15-dB increase in BC threshold at 0.5 to 2 kHz (PTA).
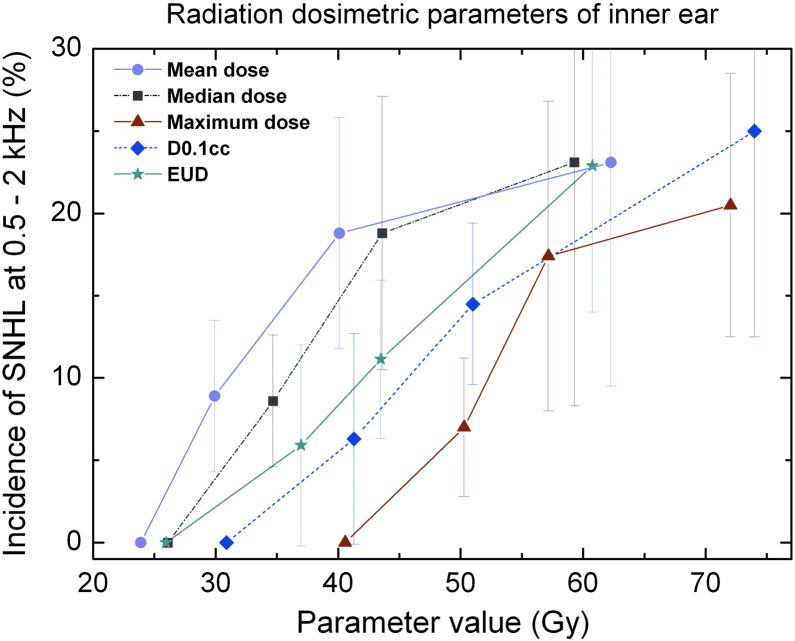
The same correlation analysis was performed for the inner ear. It was found that the parameters D0.1 ml, D0.035 ml, EUD, V50, maximum dose, V40, and median dose of the inner ear were associated with SNHL (Table 4). The probability of SNHL was plotted against these parameters of inner ears in Figure 3.
Table 4.
Correlation of Radiation Dosimetric Parameters of Inner Ear with SNHL (102 Ears)
| Parameters | Cutoff | Incidence Above Cutoff | Incidence Under Cutoff | P | OR | 95% CI |
|---|---|---|---|---|---|---|
| V30 | 62.2% | 17.0% (8/47) | 9.1% (5/55) | .231 | 2.44 | 0.62-6.77 |
| V40 | 22.0% | 18.3% (11/60) | 4.8% (2/42) | .043 | 4.49 | 0.94-21.44 |
| V50 | 2.4% | 19.0% (11/58) | 4.5% (2/44) | .031 | 4.92 | 1.03-23.46 |
| V60 | 0.16% | 15.6% (5/32) | 11.4% (8/70) | .555 | 1.44 | 0.43-4.79 |
| V70 | 6.1% | 10.5% (2/19) | 13.3% (11/83) | 1.000⁎ | 0.77 | 0.16-3.80 |
| Mean dose | 35.0Gy | 17.0% (8/47) | 9.1% (5/55) | .231 | 2.05 | 0.62-6.77 |
| Dmin | 18.8Gy | 16.4% (9/55) | 8.5% (4/47) | .236 | 2.10 | 0.60-7.33 |
| Dmax | 56.8Gy | 19.6% (10/51) | 5.9% (3/51) | .038 | 3.90 | 1.01-15.14 |
| Median dose | 36.8Gy | 20.4% (11/54) | 4.2% (2/48) | .014 | 5.88 | 1.23-28.08 |
| EUD (a= 16.7) | 43.5Gy | 21.1% (12/57) | 2.2% (1/45) | .005 | 11.7 | 1.46-96.01 |
| D0.1 ml | 48.0Gy | 19.4% (12/62) | 2.5% (1/40) | .013 | 9.36 | 1.17-75.11 |
| D0.035 ml | 51.4Gy | 19.6% (11/56) | 4.3% (2/46) | .021 | 5.38 | 1.13-25.67 |
Fisher’s exact test was used.
Figure 3.
The incidence of sensorineural hearing versus radiation dosimetric parameters of inner ear. Significant hearing loss was defined as ≥ 15-dB increase in BC threshold at 0.5 to 2 kHz (PTA).
The correlation analysis performed for IAC indicated that none of the radiation dosimetric parameters of IAC had correlated significantly with SNHL (P = .065-.852).
Multivariate Analysis for Factors Associated with SNHL
Multivariate analysis showed that accumulative cisplatin dose (≥ 200 mg/m2), radiation D0.1 ml of cochlea (≥ 39.8Gy), and SOM (P = .035, .022 and .012, respectively) were determining factors for SNHL (Table 5, Cox and Snell R2 = 0.330, Nagelkerke R2 = 0.619.)
Table 5.
Logistic Regression of Radiation Dosimetric Parameters and Clinical Factor on SNHL (102 Ears)
| Parameters | B | SE | Wald | P | Exp(B) | 95% CI |
|---|---|---|---|---|---|---|
| Accumulative cisplatin dose ≥ 200 mg/m2 | 3.148 | 1.491 | 4.458 | .035 | 23.290 | 1.253-432.775 |
| SOM | 3.231 | 1.291 | 6.262 | .012 | 25.293 | 2.014-317.611 |
| D0.1 ml of cochlea≥39.8 | 0.846 | 0.369 | 5.27 | .022 | 2.33 | 1.13-4.80 |
Abbreviation: IE, inner ear.
Discussion
The incidences of SNHL after IMRT for NPC were reported to be 37% to 51.2% at high frequencies and 6% to 22% at low frequencies [9], [17], [19], [27]. They might increase with more widespread use of combined IMRT and cisplatin chemotherapy. In our study, all patients received combined IMRT and cisplatin-based chemotherapy, and the incidence is concordant with other literature [14], [28], [29]. Attention was paid to the low-frequency SNHL in this study, as the patients with low-frequency SNHL may experience subjective hearing loss in their social life, reducing the quality of life.
Cisplatin is known to cause SNHL. It has been reported that significant SNHL occurs when cumulative cisplatin dose reaches 200 mg/m2 [30], [31], and a 'plateau' phenomenon is observed when the cisplatin dose reaches 600 mg/m2 [32]. However, the effect of cumulative cisplatin dose on SNHL after combined modality therapy was not seen in some studies [3], [4], [33], [34] where few patients received cisplatin during RT or a low dose was administrated. Low-dose cisplatin to RT might not increase the risk of SNHL. In our study, relatively high doses of cisplatin were administrated to most of the patients. Increased SNHL was observed in patients receiving cumulative cisplatin dose > 200 mg/m2 for the neoadjuvant, concurrent, and adjuvant use of cisplatin. Unlike the data reported for cisplatin, the plateau was not observed in this study.
Carboplatin has been described as an agent with potential to cause SNHL [35]. However, in our study, the patients who received carboplatin chemotherapy before radiotherapy had no increased risk of SNHL. Eleven patients received the carboplatin chemotherapy before radiotherapy. SNHL was present in 2 of them (18.2%) and in 6 of the 40 (15%) patients without carboplatin chemotherapy (P = .557). Sumitsawan and his colleagues also reported that radiation combined with carboplatin did not increase SNHL compared with radiation alone [28], which might support that the carboplatin has less ototoxicity.
Threshold radiation doses for SNHL vary among studies [36]. Most studies only investigated the correlation of mean dose of cochlea, whereas few explored other radiation dosimetric parameters. In this study, we examined a series of radiation dosimetric parameters for three relevant OARs and found that the parameters describing a high dose portion of the distribution, such as V50, D0.1 ml, D0.035 ml, or maximum dose of cochlea and inner ear, were associated with SNHL. Furthermore, the EUD calculation for cochlea indicated that EUD extremely closed to the maximum dose and D0.035 ml, with the best fitting parameter a = 100. This indicated that cochlea might be a serially organized organ; the mean dose for cochlea might not be the best predicting factor for radiation-induced SNHL. As the cochlea is proximal to the NPC target, it usually locates in the high-dose region. This may result in that the dose parameters describing the high-dose portion of the dose distribution for inner ear have a similar correlation with the SNHL as those for the cochlea. None of the radiation dose parameters of IAC was found to significantly correlate to SNHL. This may be because IAC is usually in the low-dose region as it is proximal to the brain stem.
One concern for a small structure such as cochlea is that the radiation dose actually received by cochlea can be sensitive to the daily positioning variation. To address the concern, an effort was made to assess the effect of daily setup variation on the cochlea dose. Considering a daily setup variation of 3 mm, the resulting variation in the daily dose delivered to the cochlea is substantial only when the cochlea is in the region with a sharp dose gradient. For a big portion of the cases studied, the dose gradient across the cochlea was either flat or small, resulting in a small daily variation due to the setup uncertainty. Furthermore, as the daily setup uncertainty is random in nature [37], the effect of the daily dose variation on the accumulative dose would be reduced.
Apart from RT and cisplatin, patient-related factors such as age [3], [4], [33], [38], [39] and SOM [3], [7], [36] have been reported to affect SNHL. The current study also revealed that patients with age > 40 or SOM experienced greater SNHL.
The time interval used for assessing hearing is very wide. It is possible that time might have a role in the severity of SNHL. It is reported that SNHL might be irreversible, and persistent hearing loss would continue to increase with time [36]. However, ears with SNHL had even shorter exposure history than those without SNHL in our study. Perhaps only one (not sequential) hearing test was performed during the long-term follow-up, and pure tone audiometry was performed when the patient came back to follow-up. Selective bias may exist, as the patient who suspected a problem might be more willing to undergo audiometry test. That would partly explain why ears with SNHL had even shorter exposure history.
Another limitation of the current study is that the ear rather than the patient was adopted as the observation unit in our study. Considering that the ears of one patient are not independent and subject to the same therapy procedure, there was intracluster correlation among observations, which would lower statistical power. Furthermore, it is unknown whether there is any confounding effect of SOM. Lastly, for patients with advanced diseases, they may not survive long enough for the observation of SNHL as it is a late effect.
In conclusion, for NPC patients treated with IMRT and cisplatin-based chemotherapy, there might be a significant increase in the incidence of SNHL among patients who received cumulative cisplatin dose > 200 mg/m2, who suffered from SOM, and in whom the minimum radiation dose to 0.1 ml highest dose volume (D0.1 ml) of cochlea was ≥ 39.8 Gy. In addition, various radiation dosimetric parameters for cochlea and those describing high-dose portions of the dose distribution, such as D0.035 ml and D0.1 ml, might correlate with SNHL. The EUD for cochlea might be dominated by the maximum dose, implying that cochlea might be a serially organized organ. The study provides new information on treatment-related SNHL, particularly the new relationships between radiation parameters and SNHL. The information will be useful for future prospective studies.
Footnotes
Competing interests: The authors have declared that no competing interests exist.
Funding: The study is partially supported by grants from the Science and Technology Planning Project of Guangdong Province, 2003, no. 245, and the Medical Scientific Research Foundation of Guangdong Province, no. A2011196.
Contributor Information
Chong Zhao, Email: zhaochong@sysucc.org.cn.
Ming Chen, Email: Chenming@sysucc.org.cn.
X. Allen Li, Email: ALi@mcw.edu.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Chen WC, Jackson A, Budnick AS, Pfister DG, Kraus DH, Hunt M.A., Stambuk H., Levegrun S., Wolden S.L. Sensorineural hearing loss in combined modality treatment of nasopharyngeal carcinoma. Cancer. 2006;106(4):820–829. doi: 10.1002/cncr.21683. [DOI] [PubMed] [Google Scholar]
- 3.Ho WK, Wei WI, Kwong DL, Sham JS, Tai PT, Yuen A.P., Au D.K. Long-term sensorineural hearing deficit following radiotherapy in patients suffering from nasopharyngeal carcinoma: a prospective study. Head Neck. 1999;21(6):547–553. doi: 10.1002/(sici)1097-0347(199909)21:6<547::aid-hed8>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 4.Kwong DL, Wei WI, Sham JS, Ho WK, Yuen PW, Chua D.T., Au D.K., Wu P.M., Choy D.T. Sensorineural hearing loss in patients treated for nasopharyngeal carcinoma: a prospective study of the effect of radiation and cisplatin treatment. Int J Radiat Oncol Biol Phys. 1996;36(2):281–289. doi: 10.1016/s0360-3016(96)00302-1. [DOI] [PubMed] [Google Scholar]
- 5.Mujica-Mota MA, Lehnert S, Devic S, Gasbarrino K, Daniel SJ. Mechanisms of radiation-induced sensorineural hearing loss and radioprotection. Hear Res. 2014;312:60–68. doi: 10.1016/j.heares.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Tan PX, Du SS, Ren C, Yao QW, Yuan YW. Radiation-induced Cochlea hair cell death: mechanisms and protection. Asian Pac J Cancer Prev. 2013;14(10):5631–5635. doi: 10.7314/apjcp.2013.14.10.5631. [DOI] [PubMed] [Google Scholar]
- 7.Grau C, Overgaard J. Postirradiation sensorineural hearing loss: a common but ignored late radiation complication. Int J Radiat Oncol Biol Phys. 1996;36(2):515–517. doi: 10.1016/s0360-3016(96)00346-x. [DOI] [PubMed] [Google Scholar]
- 8.Raaijmakers E, Engelen AM. Is sensorineural hearing loss a possible side effect of nasopharyngeal and parotid irradiation? A systematic review of the literature. Radiother Oncol. 2002;65(1):1–7. doi: 10.1016/s0167-8140(02)00211-6. [DOI] [PubMed] [Google Scholar]
- 9.Hsin CH, Chen TH, Young YH, Liu WS. Comparison of otologic complications between intensity-modulated and two-dimensional radiotherapies in nasopharyngeal carcinoma patients. Otolaryngol Head Neck Surg. 2010;143(5):662–668. doi: 10.1016/j.otohns.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Theunissen EA, Zuur CL, Yurda ML, van der Baan S, Kornman AF, de Boer J.P., Balm A.J., Rasch C.R., Dreschler W.A. Cochlea sparing effects of intensity modulated radiation therapy in head and neck cancers patients: a long-term follow-up study. J Otolaryngol Head Neck Surg. 2014;43(1):30. doi: 10.1186/s40463-014-0030-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen QY, Wen YF, Guo L, Liu H, Huang PY, Mo H.Y., Li N.W., Xiang Y.Q., Luo D.H., Qiu F. Concurrent chemoradiotherapy vs radiotherapy alone in stage II nasopharyngeal carcinoma: phase III randomized trial. J Natl Cancer Inst. 2011;103(23):1761–1770. doi: 10.1093/jnci/djr432. [DOI] [PubMed] [Google Scholar]
- 12.Low WK, Toh ST, Wee J, Fook-Chong SM, Wang DY. Sensorineural hearing loss after radiotherapy and chemoradiotherapy: a single, blinded, randomized study. J Clin Oncol. 2006;24(12):1904–1909. doi: 10.1200/JCO.2005.05.0096. [DOI] [PubMed] [Google Scholar]
- 13.Theunissen EA, Dreschler WA, Latenstein MN, Rasch CR, van der Baan S, de Boer J.P., Balm A.J., Zuur C.L. A New Grading System for Ototoxicity in Adults. Ann Otol Rhinol Laryngol. 2014;10:711–718. doi: 10.1177/0003489414534010. [DOI] [PubMed] [Google Scholar]
- 14.Wei Y, Zhou T, Zhu J, Zhang Y, Sun M, Ding X., Wang D., Li H., Li B. Long-term outcome of sensorineural hearing loss in nasopharyngeal carcinoma patients: comparison between treatment with radiotherapy alone and chemoradiotherapy. Cell Biochem Biophys. 2014;69(3):433–437. doi: 10.1007/s12013-014-9814-x. [DOI] [PubMed] [Google Scholar]
- 15.Theunissen EA, Bosma SC, Zuur CL, Spijker R, van der Baan S, Dreschler W.A., de Boer J.P., Balm A.J., Rasch C.R. Sensorineural hearing loss in patients with head and neck cancer after chemoradiotherapy and radiotherapy: a systematic review of the literature. Head Neck. 2015;37(2):281–292. doi: 10.1002/hed.23551. [DOI] [PubMed] [Google Scholar]
- 16.Yasui N, Adachi N, Kato M, Koh K, Asanuma S, Sakata H., Hanada R. Cisplatin-induced hearing loss: the need for a long-term evaluating system. J Pediatr Hematol Oncol. 2014;36(4):e241–e245. doi: 10.1097/MPH.0000000000000028. [DOI] [PubMed] [Google Scholar]
- 17.Petsuksiri J, Sermsree A, Thephamongkhol K, Keskool P, Thongyai K, Chansilpa Y., Pattaranutaporn P. Sensorineural hearing loss after concurrent chemoradiotherapy in nasopharyngeal cancer patients. Radiat Oncol. 2011;6:19. doi: 10.1186/1748-717X-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hitchcock YJ, Tward JD, Szabo A, Bentz BG, Shrieve DC. Relative contributions of radiation and cisplatin-based chemotherapy to sensorineural hearing loss in head-and-neck cancer patients. Int J Radiat Oncol Biol Phys. 2009;73(3):779–788. doi: 10.1016/j.ijrobp.2008.05.040. [DOI] [PubMed] [Google Scholar]
- 19.Chan SH, Ng WT, Kam KL, Lee MCH, Choi CW, Yau T.K., Lee A.W.M. Sensorineural hearing loss after treatment of nasopharyngeal carcinoma: a longitudinal analysis. Int J Radiat Oncol Biol Phys. 2009;73(4):1335–1342. doi: 10.1016/j.ijrobp.2008.07.034. [DOI] [PubMed] [Google Scholar]
- 20.Xiao WW, Huang SM, Han F, Wu SX, Lu LX, Lin C.G., Deng X.W., Lu T.X., Cui N.J., Zhao C. Local control, survival, and late toxicities of locally advanced nasopharyngeal carcinoma treated by simultaneous modulated accelerated radiotherapy combined with cisplatin concurrent chemotherapy: long-term results of a phase 2 study. Cancer. 2010;117(9):1874–1883. doi: 10.1002/cncr.25754. [DOI] [PubMed] [Google Scholar]
- 21.Deasy JO, Blanco AI, Clark VH. CERR: a computational environment for radiotherapy research. Med Phys. 2003;30(5):979–985. doi: 10.1118/1.1568978. [DOI] [PubMed] [Google Scholar]
- 22.Niemierko A. Reporting and analyzing dose distributions: a concept of equivalent uniform dose. Med Phys. 1997;24(1):103–110. doi: 10.1118/1.598063. [DOI] [PubMed] [Google Scholar]
- 23.Mavroidis P, Lind BK, Brahme A. Biologically effective uniform dose (D) for specification, report and comparison of dose response relations and treatment plans. Phys Med Biol. 2001;46(10):2607–2630. doi: 10.1088/0031-9155/46/10/307. [DOI] [PubMed] [Google Scholar]
- 24.Wang JZ, Li XA. Evaluation of external beam radiotherapy and brachytherapy for localized prostate cancer using equivalent uniform dose. Med Phys. 2003;30(1):34–40. doi: 10.1118/1.1527674. [DOI] [PubMed] [Google Scholar]
- 25.Wu Q, Mohan R, Niemierko A, Schmidt-Ullrich R. Optimization of intensity-modulated radiotherapy plans based on the equivalent uniform dose. Int J Radiat Oncol Biol Phys. 2002;52(1):224–235. doi: 10.1016/s0360-3016(01)02585-8. [DOI] [PubMed] [Google Scholar]
- 26.Ebert MA. Viability of the EUD and TCP concepts as reliable dose indicators. Phys Med Biol. 2000;45(2):441–457. doi: 10.1088/0031-9155/45/2/313. [DOI] [PubMed] [Google Scholar]
- 27.Zuur CL, Simis YJ, Lamers EA, Hart AA, Dreschler WA, Balm A.J., Rasch C.R. Risk factors for hearing loss in patients treated with intensity-modulated radiotherapy for head and neck tumors. Int J Radiat Oncol Biol Phys. 2009;74(2):490–496. doi: 10.1016/j.ijrobp.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 28.Sumitsawan Y, Vaseenon V, Hanprasertpong C, Roongrotwattanasiri K, Chitapanarux I, Isaradisaikul S. High frequency hearing loss following treatment for nasopharyngeal carcinoma. J Med Assoc Thai. 2010;93(3):324–329. [PubMed] [Google Scholar]
- 29.Lin C, Lin SW, Weng SF, Lin YS. Risk of developing sudden sensorineural hearing loss in patients with nasopharyngeal carcinoma: a population-based cohort study. Head Neck. 2014;36(2):203–208. doi: 10.1002/hed.23278. [DOI] [PubMed] [Google Scholar]
- 30.Kopelman J, Budnick AS, Sessions RB, Kramer MB, Wong GY. Ototoxicity of high-dose cisplatin by bolus administration in patients with advanced cancers and normal hearing. Laryngoscope. 1988;98(8 pt 1):858–864. doi: 10.1288/00005537-198808000-00014. [DOI] [PubMed] [Google Scholar]
- 31.Fausti SA, Schechter MA, Rappaport BZ, Frey RH, Mass RE. Early detection of cisplatin ototoxicity. Selected case reports. Cancer. 1984;53(2):224–231. doi: 10.1002/1097-0142(19840115)53:2<224::aid-cncr2820530207>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 32.Skinner R, Pearson AD, Amineddine HA, Mathias DB, Craft AW. Ototoxicity of cisplatinum in children and adolescents. Br J Cancer. 1990;61(6):927–931. doi: 10.1038/bjc.1990.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choy H, Nabid A, Stea B, Scott C, Roa W, Kleinberg L., Ayoub J., Smith C., Souhami L., Hamburg S. Phase II multicenter study of induction chemotherapy followed by concurrent efaproxiral (RSR13) and thoracic radiotherapy for patients with locally advanced non-small-cell lung cancer. J Clin Oncol. 2005;23(25):5918–5928. doi: 10.1200/JCO.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 34.Oh YT, Kim CH, Choi JH, Kang SH, Chun M. Sensory neural hearing loss after concurrent cisplatin and radiation therapy for nasopharyngeal carcinoma. Radiother Oncol. 2004;72(1):79–82. doi: 10.1016/j.radonc.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 35.Bertolini P, Lassalle M, Mercier G, Raquin MA, Izzi G, Corradini N., Hartmann O. Platinum compound-related ototoxicity in children: long-term follow-up reveals continuous worsening of hearing loss. J Pediatr Hematol Oncol. 2004;26(10):649–655. doi: 10.1097/01.mph.0000141348.62532.73. [DOI] [PubMed] [Google Scholar]
- 36.Bhandare N, Jackson A, Eisbruch A, Pan CC, Flickinger JC, Antonelli P., Mendenhall W.M. Radiation therapy and hearing loss. Int J Radiat Oncol Biol Phys. 2010;76(3):S50–S57. doi: 10.1016/j.ijrobp.2009.04.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li XA, Qi XS, Pitterle M, Kalakota K, Mueller K, Erickson B.A., Wang D., Schultz C.J., Firat S.Y., Wilson J.F. Interfractional variations in patient setup and anatomic change assessed by daily computed tomography. Int J Radiat Oncol Biol Phys. 2007;68(2):581–591. doi: 10.1016/j.ijrobp.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 38.Honore HB, Bentzen SM, Moller K, Grau C. Sensori-neural hearing loss after radiotherapy for nasopharyngeal carcinoma: individualized risk estimation. Radiother Oncol. 2002;65(1):9–16. doi: 10.1016/s0167-8140(02)00173-1. [DOI] [PubMed] [Google Scholar]
- 39.Bhandare N, Antonelli PJ, Morris CG, Malayapa RS, Mendenhall WM. Ototoxicity after radiotherapy for head and neck tumors. Int J Radiat Oncol Biol Phys. 2007;67(2):469–479. doi: 10.1016/j.ijrobp.2006.09.017. [DOI] [PubMed] [Google Scholar]