Abstract
Preoperative serum lactate dehydrogenase (LDH) has been used as a prognostic indicator for patients with hepatocellular carcinoma (HCC) treated with sorafenib or undergoing transcatheter arterial chemoembolization, but its significance in predicting survival of HCC patients who received curative resection remains undefined. A total of 683 patients with histopathologically confirmed HCC were enrolled in this study. The prognostic significance of preoperative serum LDH was determined by Kaplan-Meier analysis and a Cox proportional hazards regression model. The association between the preoperative serum LDH and clinicopathological parameters was evaluated by the χ2 test or linear regression analysis when appropriate. Higher preoperative serum LDH level was associated with worse prognosis. In a multivariate Cox proportional hazards analysis, the preoperative serum LDH level could predict overall survival and recurrence independently. Higher preoperative serum LDH level is associated with the elevated serum alpha-fetoprotein, the presence of hepatitis B surface antigen, larger tumor size, the presence of macrovascular invasion, the advanced tumor–lymph node–metastasis stage, worse tumor differentiation, and Child-Pugh B. Preoperative serum LDH level was an inexpensive, simple, convenient, and routinely measured biomarker exhibiting a potential to select patients at high risk with poor clinical outcome for appropriate treatment strategies.
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common malignancy with an increasing incidence and a dismal survival [1], [2]. It has high heterogeneity and is generally resistant to chemotherapy and radiotherapy. Therefore, resection and liver transplantation still remain the prior curative therapeutic options for patients with HCC [3]. However, the postsurgical recurrence is high, which reaches nearly 50% within 3 years. To date, although risk factors and models associated with postsurgical recurrence have attracted much interest and been widely explored, there is still a long way for these new markers and models to be accepted and applicable in the clinical practice [4]. Hence, it is vital to establish simple and effective means to identify patients at high risk for recurrence and to target intensive clinical follow-up or postsurgical adjuvant therapies in such patients.
Lactate dehydrogenase (LDH) is a metabolic enzyme involved in anaerobic glycolysis and regulated by the PI3K/Akt/mTOR pathways, the c-Myc oncogenic transcription factor, and tumor hypoxia/necrosis [5], [6]. It has been reported that LDHA, comprising tetrameric subunit A of LDH, is not only involved in tumor initiation but also plays an important role in tumor maintenance and progression. LDH is a well-identified prognostic marker in multiple malignancies, including colorectal cancer, breast cancer, lymphoma, melanoma, renal cell carcinoma, and germ cell tumors [7], [8], [9], [10], [11], [12], [13], [14]. Although the role of serum LDH levels in predicting global outcome in HCC patients treated with sorafenib and HCC patients undergoing transcatheter arterial chemoembolization (TACE) has been confirmed, the clinical significance of LDH in HCC patients who received curative resection has not yet been investigated [15], [16].
In the present study, we first determined the best cutoff value of preoperative serum LDH as a prognostic indicator for overall survival (OS) in a group of HCC patients receiving curative resection (the training cohort) and then validated the prognostic significance of LDH with the same cutoff value on the survival in an independent cohort (the validation cohort). We further evaluated the clinicopathological roles of LDH in HCC. In general, our results showed that the preoperative serum LDH is associated with metastasis, HCC progression, and prognosis.
Materials and Methods
Patients
Six hundred and eighty-three patients with pathologically confirmed HCC who underwent curative resection, defined as complete macroscopic removal of the tumor, between January 2008 and June 2012 at the Cancer Center of Sun Yat-sen University in Guangzhou were enrolled in this study. Written informed consent was obtained from all patients before enrollment in the study. This study was performed in strict accordance with the ethical guidelines of the Declaration of Helsinki, and the protocol was approved by the Institutional Review Boards of the Cancer Center.
Each of the patients was absent of any preoperative anticancer treatment and any other malignancies. The clinical stage was determined according to the Union for International Cancer Control/American Joint Committee on Cancer tumor–lymph node–metastasis (TNM) classification system (seventh edition). Tumor differentiation was graded according to the Edmondson-Steiner classification. The clinicopathological features of all patients were summarized in Table 1.
Table 1.
Characteristics of HCC Patients in the Training and Validation Cohort
| Variables | Training Set |
Validation Set |
|||
|---|---|---|---|---|---|
|
n = 344 |
n = 339 |
P |
|||
| No. | % | No. | % | ||
| Age (years) | .236 | ||||
| ≤ 50 | 164 | 47.7 | 177 | 52.2 | |
| > 50 | 180 | 52.3 | 162 | 47.8 | |
| Gender | .252 | ||||
| Male | 309 | 89.8 | 295 | 87.0 | |
| Female | 35 | 10.2 | 44 | 13.0 | |
| HBsAg | .467 | ||||
| Negative | 47 | 13.7 | 53 | 15.6 | |
| Positive | 297 | 86.3 | 286 | 84.4 | |
| AFP | .483 | ||||
| ≤ 25 ng/ml | 130 | 37.8 | 137 | 40.4 | |
| > 25 ng/ml | 214 | 62.2 | 202 | 59.6 | |
| Tumor number | .245 | ||||
| Single | 293 | 85.2 | 299 | 88.2 | |
| Multiple | 51 | 14.8 | 40 | 11.8 | |
| Tumor size | .504 | ||||
| ≤ 5 cm | 182 | 52.9 | 188 | 55.5 | |
| > 5 cm | 162 | 47.1 | 151 | 44.5 | |
| Macrovascular invasion | .206 | ||||
| Absent | 330 | 95.9 | 331 | 97.6 | |
| Present | 14 | 4.1 | 8 | 2.4 | |
| TNM stage | .055 | ||||
| I-II | 294 | 85.5 | 306 | 90.3 | |
| III-IV | 50 | 14.5 | 33 | 9.7 | |
| Tumor differentiation | .137 | ||||
| I-II | 222 | 64.5 | 202 | 59.6 | |
| III-IV | 118 | 34.3 | 136 | 40.1 | |
| Missing | 4 | 1.2 | 1 | 0.3 | |
| Child-Pugh classification | .830 | ||||
| A | 335 | 97.4 | 331 | 97.6 | |
| B | 9 | 2.6 | 8 | 2.4 | |
Note: The differences in the clinicopathological parameters between the training set and the validation set were evaluated using Mann-Whitney U two-independent-samples tests.
Patients were followed postoperatively with regular surveillance for recurrence basing on the physical examination, the liver function, serum alpha-fetoprotein (AFP) level, abdominal ultrasonography, and chest radiography. When tumor recurrence or metastasis was suspected, further examinations, such as computed tomography and hepatic angiography, were performed. Biopsies were obtained when necessary. Patients with confirmed recurrence received further treatment, including a second liver resection, TACE, radiofrequency ablation, or percutaneous ethanol injection, depending on the location of the tumor and the liver function of the patient [17].
OS was defined as the interval (in months) from the date of surgery to the date of death or from the date of surgery to the last follow-up visit. Disease-free survival (DFS) was defined as the interval between the date of surgery and the diagnosis of recurrence or between the date of surgery and the last observation if no recurrence was observed. This study was censored on 31 December 2014.
Serum LDH Level
Blood samples were collected within one month preoperation. LDH level was measured by spectrophotometric enzyme assay with LDH reagent (Wako Pure Chemical Industries, Ltd., Osaka, Japan) using Hitachi 7600 automated chemistry analyzer (Hitachi, Ltd., Tokyo, Japan) within 2 hours after collection.
Statistical Analyses
The optimal cutoff prognostic value of LDH for OS was estimated by the receiver operating curve analysis, and its prognostic value was confirmed in the validation cohort. The differences in the clinicopathological parameters between the training cohort and the validation cohort were evaluated using Mann-Whitney U two-independent-samples tests.
Correlations between the clinicopathological parameters and LDH level were determined by the χ2 test or linear regression analysis when appropriate. Survival was estimated by the Kaplan-Meier method and compared using the log-rank test. Univariate and multivariate analyses of prognostic factors for OS or DFS were performed using the Cox proportional hazards model. A value of P < .05 was considered statistically significant. Statistical analyses were performed with SPSS software (version 16.0; SPSS Inc., Chicago, IL).
Results
Patient Characteristics
The data of 344 patients from January 2008 to December 2009 were used for the training cohort. The data of 339 patients since January 2010 to June 2012 were enrolled as the validation cohort.
There was no significant difference in age, gender, hepatitis B surface antigen (HBsAg), serum AFP level, tumor number, tumor size, macrovascular invasion, TNM stage, tumor differentiation, and Child-Pugh classification between the training and validation cohorts. The characteristics of the participants in the training and the validation cohorts are shown in Table 1.
The Cutoff Prognostic Value for LDH
The optimal cutoff value of serum LDH for OS was estimated as 188 U/L in the training cohort by the receiver operating curve analysis, with the area under the curve as 0.626 and 95% confidence interval (CI) as 0.564 to 0.689.
Survival Analysis
The median duration of follow-up for the total test set was 41 months (range, 1-86 months). Of the 683 patients examined during the follow-up period, 168 patients (24.6%) died, 326 patients (47.7%) were diagnosed with tumor recurrence, and 333 patients (48.8%) remained alive without recurrence. The median OS and DFS for the whole cohort were 40 and 16 months, respectively.
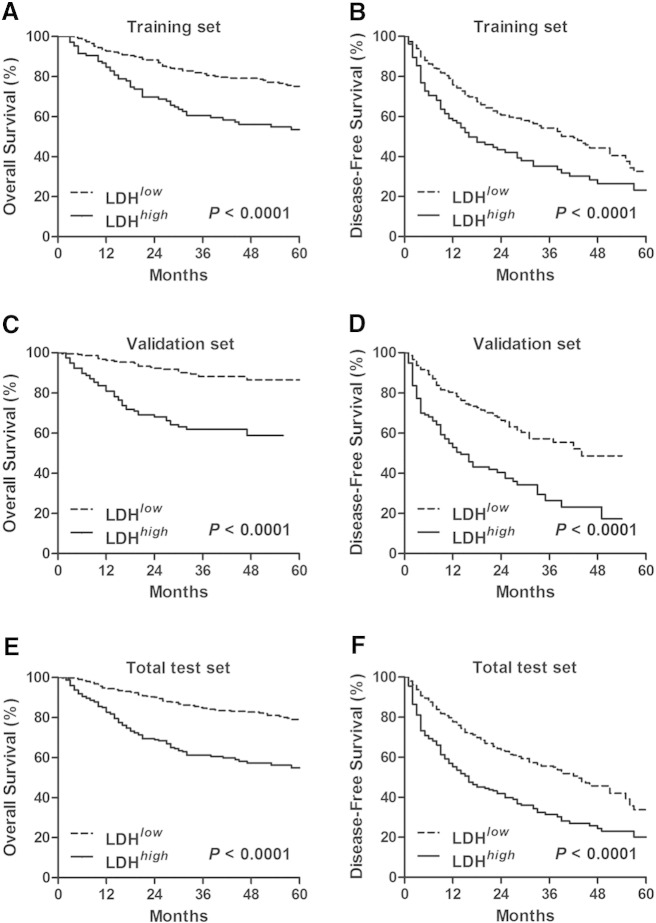
To investigate whether preoperative serum LDH level is associated with the clinical outcome of HCC patients, Kaplan-Meier cumulative survival curves were first plotted in the training set using the log-rank statistic to compare survival rates. As shown in Figure 1A and 1B, survival was profitable in the patients with a lower level of LDH. The OS (median survival, 60 months) and DFS (median survival, 23 months) of patients with a lower level of LDH were prolonged as compared with patients with a higher level of LDH (median survival, 40 months for OS and 10 months for DFS, respectively). Elevated serum LDH level was also associated with worse OS and DFS in the validation set and the total test set (P < .0001, Figure 1).
Figure 1.
Higher preoperative serum LDH level predicted poor survival in HCC patients. The significance of preoperative serum LDH level in predicting OS (A, C, and E) and DFS (B, D, and F) in HCC patients enrolled in the training set (A and B), in the validation set (C and D), and in the total test set (E and F) was estimated by the Kaplan-Meier method and compared by the log-rank test.
Multivariate Cox Proportional Hazards Analysis
To investigate whether preoperative LDH level serves as an independent predictors of OS and DFS, a multivariate Cox proportional hazards analysis was performed, and those variables that were associated with survival by univariate analysis were adopted as covariates (Table 2). In the training set, tumor number, tumor size, Child-Pugh classification, and macrovascular invasion remained independently associated with OS in the multivariate Cox proportional hazards analysis (P = .050, .001, .001, and .022, respectively). The serum LDH level predicted OS independent of these clinical factors [hazard ratio (HR), 1.687; 95% CI, 1.131-2.516; P = .010; Table 2]. HBsAg, tumor number, tumor size, and macrovascular invasion served as independent prognostic factors for DFS in the training set. However, the serum LDH could not independently predict recurrence in this cohort.
Table 2.
Univariate and Multivariate Analyses of Variables Associated with Survival and Recurrence in HCC Patients
| OS |
DFS |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Univariate |
Multivariate |
Univariate |
Multivariate |
|||||||
| Variables |
P | HR | 95% CI |
P | P | HR |
95% CI |
P | ||
| Low | High | Low | High | |||||||
| Training set | ||||||||||
| Age: > 50 vs ≤ 50 years | .044 | 1.353 | 0.902 | 2.03 | .144 | .495 | NA | |||
| Gender: male vs female | .775 | NA | .105 | NA | ||||||
| HBsAg: positive vs negative | .239 | NA | .046 | 1.636 | 1.020 | 2.626 | .041 | |||
| AFP: > 25 vs ≤ 25 ng/ml | .008 | 1.447 | 0.929 | 2.254 | .102 | .014 | 1.311 | 0.962 | 1.788 | .087 |
| Tumor number: multiple vs single | .028 | 1.628 | 0.999 | 2.653 | .050 | .000 | 1.929 | 1.331 | 2.796 | .001 |
| Tumor size: > 5 vs ≤ 5 cm | .000 | 2.003 | 1.312 | 3.057 | .001 | .000 | 1.689 | 1.231 | 2.316 | .001 |
| Tumor differentiation: III-IV vs I-II | .130 | NA | .278 | NA | ||||||
| Child-Pugh classification: B vs A | .000 | 3.452 | 1.608 | 7.41 | .001 | .254 | NA | |||
| Macrovascular invasion: present vs absent | .000 | 2.321 | 1.131 | 4.766 | .022 | .000 | 2.226 | 1.109 | 4.467 | .024 |
| LDH: > 188 vs ≤ 188 U/L | .000 | 1.687 | 1.131 | 2.516 | .010 | .001 | 1.263 | 0.916 | 1.742 | .154 |
| Validation set | ||||||||||
| Age: > 50 vs ≤ 50 years | .804 | NA | .151 | NA | ||||||
| Gender: male vs female | .236 | NA | .584 | NA | ||||||
| HBsAg: positive vs negative | .092 | NA | .023 | 1.595 | 0.900 | 2.826 | .109 | |||
| AFP: > 25 vs ≤ 25 ng/ml | .029 | 1.379 | 0.802 | 2.372 | .245 | .005 | 1.366 | 0.946 | 1.971 | .096 |
| Tumor number: multiple vs single | .000 | 2.880 | 1.649 | 5.028 | .000 | .000 | 2.729 | 1.788 | 4.166 | .000 |
| Tumor size: > 5 vs ≤ 5 cm | .000 | 3.404 | 1.863 | 6.217 | .000 | .000 | 1.989 | 1.369 | 2.890 | .000 |
| Tumor differentiation: III-IV vs I-II | .030 | 1.432 | 0.870 | 2.357 | .157 | .004 | 1.259 | 0.886 | 1.789 | .198 |
| Child-Pugh classification: B vs A | .024 | 1.031 | 0.365 | 2.911 | .954 | .000 | 2.989 | 1.271 | 7.030 | .012 |
| Macrovascular invasion: present vs absent | .000 | 3.246 | 1.253 | 8.405 | .015 | .000 | 2.338 | 0.993 | 5.502 | .052 |
| LDH: > 188 vs ≤ 188 U/L | .000 | 2.553 | 1.489 | 4.377 | .001 | .000 | 1.711 | 1.621 | 3.699 | .005 |
| Total test set | ||||||||||
| Age: > 50 vs ≤ 50 years | .148 | NA | .671 | NA | ||||||
| Gender: male vs female | .606 | NA | .118 | NA | ||||||
| HBsAg: positive vs negative | .041 | 1.407 | 0.840 | 2.358 | .195 | .003 | 1.654 | 1.144 | 2.390 | .007 |
| AFP: > 25 vs ≤ 25 ng/ml | .001 | 1.347 | 0.952 | 1.907 | .092 | .000 | 1.285 | 1.008 | 1.640 | .043 |
| Tumor number: multiple vs single | .000 | 1.996 | 1.383 | 2.881 | .000 | .000 | 2.212 | 1.673 | 2.923 | .000 |
| Tumor size: > 5 vs ≤ 5 cm | .000 | 2.445 | 1.727 | 3.459 | .000 | .000 | 1.778 | 1.400 | 2.259 | .000 |
| Tumor differentiation: III-IV vs I-II | .013 | 1.227 | 0.896 | 1.679 | .202 | .005 | 1.169 | 0.924 | 1.479 | .193 |
| Child-Pugh classification: B vs A | .000 | 2.281 | 1.249 | 4.165 | .007 | .002 | 1.993 | 1.105 | 3.594 | .022 |
| Macrovascular invasion: present vs absent | .000 | 2.586 | 1.462 | 4.575 | .001 | .000 | 2.258 | 1.323 | 3.852 | .003 |
| LDH: > 188 vs ≤ 188 U/L | .000 | 1.865 | 1.359 | 2.561 | .000 | .000 | 1.446 | 1.138 | 1.837 | .003 |
Note: Cox proportional hazards regression model; variables associated with survival by univariate analysis were adopted as covariates in multivariate analyses. NA, not applicable.
In the validation set, the multivariate Cox proportional hazards regression analysis also demonstrated that the serum LDH level could predict OS independent of tumor number, tumor size, and macrovascular invasion (HR, 2.553; 95% CI, 1.489-4.337; P = .001; Table 2) and predict recurrence independent of tumor number, tumor size, and Child-Pugh classification (HR, 1.711; 95% CI, 1.621-3.699; P = .005; Table 2).
In the total test set, the serum LDH level could predict OS independent of tumor number, tumor size, Child-Pugh classification, and macrovascular invasion (HR 1.865; 95% CI, 1.359-2.561; P < .001; Table 2) and predict recurrence independent of HBsAg, serum AFP level, tumor number, tumor size, Child-Pugh classification, and macrovascular invasion (HR 1.446; 95% CI, 1.138-1.837; P = .003; Table 2). These results showed that the serum LDH level was an independent prognostic factor for both OS and recurrence.
Prognostic Significance of Preoperative LDH in the Low-Risk Subgroups
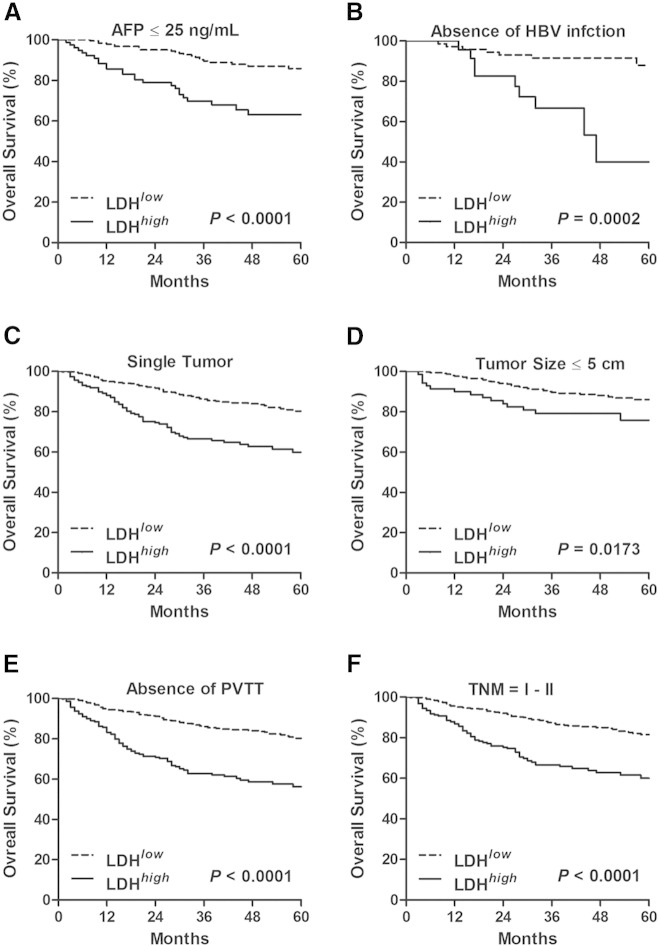
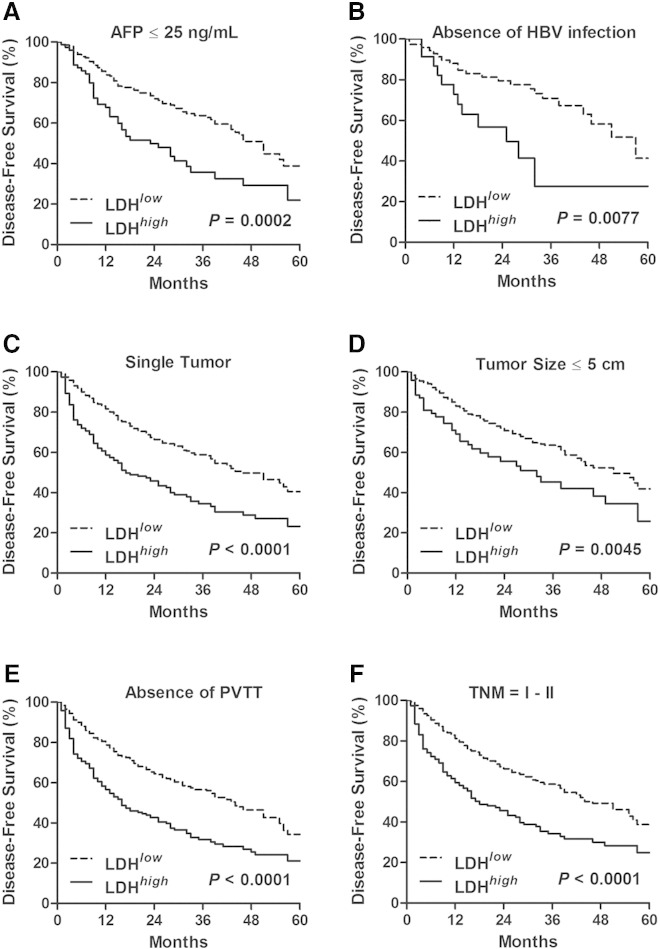
We then assessed the prognostic value of the serum LDH level in various low-risk subgroups in the total test set. As shown in Figures 2A and 3A, in patients with AFP ≤ 25 ng/ml, patients with LDH > 188 U/L had a shorter time to death (median of 36 vs 46 months, P < .0001) and recurrence (median of 15 vs 24 months, P = .0002) than those patients with LDH ≤ 188 U/L. Similar results that high LDH level was associated with worse OS (Figure 2, B–F) and DFS (Figure 3, B–F) were also obtained in the rest of low-risk subgroups, including the cohort of patients without HBsAg, with single tumor, with tumor size ≤ 5 cm, with absence of macrovascular invasion, and with TNM stage I to II. These data demonstrated that serum LDH level served as an effective survival predictor even in the low-risk subgroups.
Figure 2.
High preoperative serum LDH level predicted poor OS in the low-risk subgroups of HCC. The significance of preoperative serum LDH level in predicting OS in the cohort of HCC patients with AFP ≤ 25 ng/ml (A), with absence of HBsAg (B), with single tumor (C), with tumor size ≤ 5 cm (D), with absence of macrovascular invasion (E), and with TNM stage I to II (F) were estimated by the Kaplan-Meier method and compared by the log-rank test. PVTT, portal vein tumor thrombus.
Figure 3.
High preoperative serum LDH level predicted poor DFS in the low-risk subgroups of HCC. The significance of preoperative serum LDH level in predicting DFS in the cohort of HCC patients with AFP ≤ 25 ng/ml (A), with absence of HBsAg (B), with single tumor (C), with tumor size ≤ 5 cm (D), with absence of macrovascular invasion (E), and with TNM stage I to II (F), were estimated by the Kaplan-Meier method and compared by the log-rank test. PVTT, portal vein tumor thrombus.
Preoperative Serum LDH Level Was Associated with Clinicopathological Features
To determine if there were any significant associations between the clinical characteristics and preoperative serum LDH level, the χ2 analysis was performed. As shown in Table 3, higher preoperative serum LDH level was correlated with the presence of HBsAg (P = .038), tumor size (P = .000), macrovascular invasion (P = .019), TNM stage (P = .001), tumor differentiation (P = .007), and Child-Pugh classification (P = .008). In addition, serum LDH level was positively correlated with serum AFP using linear regression analysis (Figure 4).
Table 3.
Association of Preoperative Serum LDH Level with Clinicopathological Parameters
| Variables | LDH |
P | |
|---|---|---|---|
| Low (≤ 188 U/L) | High (> 188 U/L) | ||
| Age (years) | .168 | ||
| ≤ 50 | 236 | 105 | |
| > 50 | 219 | 123 | |
| Gender | .255 | ||
| Male | 407 | 197 | |
| Female | 48 | 31 | |
| HBsAg | .038 | ||
| Negative | 76 | 24 | |
| Positive | 379 | 204 | |
| Tumor number | .190 | ||
| Single | 400 | 192 | |
| Multiple | 55 | 36 | |
| Tumor size | .000 | ||
| ≤ 5 cm | 299 | 71 | |
| > 5 cm | 156 | 157 | |
| Macrovascular invasion | .019 | ||
| Absent | 446 | 215 | |
| Present | 9 | 13 | |
| TNM stage | .001 | ||
| I-II | 414 | 186 | |
| III-IV | 41 | 42 | |
| Tumor differentiation | .007 | ||
| I-II | 299 | 125 | |
| III-IV | 153 | 101 | |
| Missing | 3 | 2 | |
| Child-Pugh classification | .008 | ||
| A | 449 | 217 | |
| B | 6 | 11 | |
Note: Correlations between the clinicopathological parameters and LDH level were determined by the χ2 test.
Figure 4.
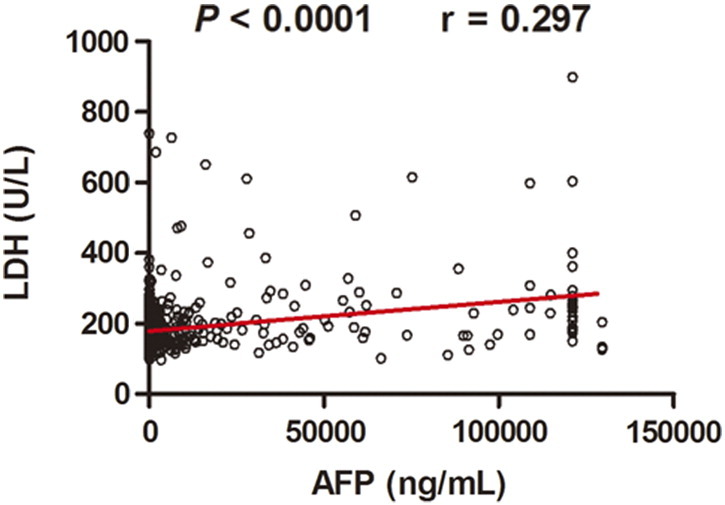
Positive association between preoperative serum LDH and AFP level in HCC patients. Preoperative serum LDH level was plotted against AFP level from the same patient. Linear regression analysis showed significant correlation between the preoperative serum LDH and AFP level.
Taken together, higher preoperative serum LDH level was implicated in determining a worse prognosis for both OS and DFS in HCC patients following hepatectomy. This simple, inexpensive, and routinely measured marker exhibits a potential to select patients at high risk with poor clinical outcome for appropriate treatment strategies.
Discussion
In the present study, an elevated serum LDH level was independently associated with poor OS and DFS in a large cohort of 683 HCC patients with hepatectomy, even in the low-risk subgroups. In addition, higher preoperative serum LDH level was also positively correlated with increased serum AFP level. Preoperative serum LDH level is elevated in the cohort of HCC patients with the presence of HBsAg, larger tumor size, presence of macrovascular invasion, advanced TNM stage, worse tumor differentiation, and Child-Pugh B.
Although mounting evidence confirmed LDH as an indirect marker of tumor hypoxia, angiogenesis and worse prognosis, the role of LDH in a large cohort of HCC patients with hepatectomy has never been explored. Scartozzi et al. retrospectively evaluated 114 patients and showed that elevated serum LDH was a poor prognostic factor for HCC patients undergoing TACE. Faloppi et al. demonstrated an independent prognostic significance for elevated serum LDH in 78 patients treated with sorafenib. However, the role of LDH in HCC patients with hepatectomy has never been referred in both studies. In addition, the cases enrolled in both studies were very small. To the best of our knowledge, the present study is the largest one validating the prognostic value of serum LDH level in HCC patients with hepatectomy.
This study demonstrated not only that a preoperative serum LDH level is a prognostic indicator associated with DFS and OS but also that it is an important prognostic indicator for clinical subgroups of patients at low risk of tumor recurrence and tumor-related death, including those patients with AFP ≤ 25 ng/ml, without HBsAg, with single tumor, with tumor size ≤ 5 cm, with absence of macrovascular invasion, and with TNM stage I to II.
To date, the biological link between LDH, hypoxia, and the angiogenesis pathway through the abnormal activation of HIF-1α is well established. In addition, myc and PI3K/Akt/mTOR pathways have also been demonstrated to regulate cellular LDH expression levels at translational and transcriptional levels. Therefore, elevated serum LDH level may not only represent tumor hypoxia and/or angiogenesis but also be present along with abnormal activation of the oncogenic pathways. However, the presence of other systemic diseases should be carefully considered if the serum level of LDH increased because it is also known as a nonspecific marker.
In conclusion, higher preoperative serum LDH level was implicated in determining a worse prognosis for both OS and DFS in HCC patients following hepatectomy. This simple, inexpensive, and routinely measured marker exhibits a potential to select patients at high risk with poor clinical outcome for appropriate treatment strategies.
Footnotes
This study was supported by the National Natural Science Foundation of China (81402252), Sun Yat-Sen University Young Talent Teachers Plan (12ykpy51), and Excellent Young Talent Project of Sun Yat-sen University Cancer Center (04250101).
Conflict of interest statement: none declared.
Contributor Information
Jing-Ping Zhang, Email: zhangjp@sysucc.org.cn.
Wan-Li Liu, Email: liuwl@sysucc.org.cn.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Yang JD, Roberts LR. Hepatocellular carcinoma: a global view. Nat Rev Gastroenterol Hepatol. 2010;7:448–458. doi: 10.1038/nrgastro.2010.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poon D, Anderson BO, Chen LT, Tanaka K, Lau WY, Van Cutsem E, Singh H, Chow WC, Ooi LL, Chow P. Management of hepatocellular carcinoma in Asia: consensus statement from the Asian Oncology Summit 2009. Lancet Oncol. 2009;10:1111–1118. doi: 10.1016/S1470-2045(09)70241-4. [DOI] [PubMed] [Google Scholar]
- 4.Villanueva A, Hoshida Y, Toffanin S, Lachenmayer A, Alsinet C, Savic R, Cornella H, Llovet JM. New strategies in hepatocellular carcinoma: genomic prognostic markers. Clin Cancer Res. 2010;16:4688–4694. doi: 10.1158/1078-0432.CCR-09-1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zha X, Wang F, Wang Y, He S, Jing Y, Wu X, Zhang H. Lactate dehydrogenase B is critical for hyperactive mTOR-mediated tumorigenesis. Cancer Res. 2011;71:13–18. doi: 10.1158/0008-5472.CAN-10-1668. [DOI] [PubMed] [Google Scholar]
- 6.Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM, Royer RE, Vander Jagt DL, Semenza GL, Dang CV. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci U S A. 2010;107:2037–2042. doi: 10.1073/pnas.0914433107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Egberts F, Kotthoff EM, Gerdes S, Egberts JH, Weichenthal M, Hauschild A. Comparative study of YKL-40, S-100B and LDH as monitoring tools for stage IV melanoma. Eur J Cancer. 2012;48:695–702. doi: 10.1016/j.ejca.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Weide B, Elsasser M, Buttner P, Pflugfelder A, Leiter U, Eigentler TK, Bauer J, Witte M, Meier F, Garbe C. Serum markers lactate dehydrogenase and S100B predict independently disease outcome in melanoma patients with distant metastasis. Br J Cancer. 2012;107:422–428. doi: 10.1038/bjc.2012.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koukourakis MI, Giatromanolaki A, Sivridis E, Gatter KC, Trarbach T, Folprecht G, Shi MM, Lebwohl D, Jalava T, Laurent D. Prognostic and predictive role of lactate dehydrogenase 5 expression in colorectal cancer patients treated with PTK787/ZK 222584 (vatalanib) antiangiogenic therapy. Clin Cancer Res. 2011;17:4892–4900. doi: 10.1158/1078-0432.CCR-10-2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scartozzi M, Giampieri R, Maccaroni E, Del Prete M, Faloppi L, Bianconi M, Galizia E, Loretelli C, Belvederesi L, Bittoni A. Pre-treatment lactate dehydrogenase levels as predictor of efficacy of first-line bevacizumab-based therapy in metastatic colorectal cancer patients. Br J Cancer. 2012;106:799–804. doi: 10.1038/bjc.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown JE, Cook RJ, Lipton A, Coleman RE. Serum lactate dehydrogenase is prognostic for survival in patients with bone metastases from breast cancer: a retrospective analysis in bisphosphonate-treated patients. Clin Cancer Res. 2012;18:6348–6355. doi: 10.1158/1078-0432.CCR-12-1397. [DOI] [PubMed] [Google Scholar]
- 12.Cairo MS, Sposto R, Gerrard M, Auperin A, Goldman SC, Harrison L, Pinkerton R, Raphael M, McCarthy K, Perkins SL. Advanced stage, increased lactate dehydrogenase, and primary site, but not adolescent age (>/= 15 years), are associated with an increased risk of treatment failure in children and adolescents with mature B-cell non-Hodgkin's lymphoma: results of the FAB LMB 96 study. J Clin Oncol. 2012;30:387–393. doi: 10.1200/JCO.2010.33.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armstrong AJ, George DJ, Halabi S. Serum lactate dehydrogenase predicts for overall survival benefit in patients with metastatic renal cell carcinoma treated with inhibition of mammalian target of rapamycin. J Clin Oncol. 2012;30:3402–3407. doi: 10.1200/JCO.2011.40.9631. [DOI] [PubMed] [Google Scholar]
- 14.Gerlinger M, Wilson P, Powles T, Shamash J. Elevated LDH predicts poor outcome of recurrent germ cell tumours treated with dose dense chemotherapy. Eur J Cancer. 2010;46:2913–2918. doi: 10.1016/j.ejca.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Faloppi L, Scartozzi M, Bianconi M, Svegliati Baroni G, Toniutto P, Giampieri R, Del Prete M, De Minicis S, Bitetto D, Loretelli C. The role of LDH serum levels in predicting global outcome in HCC patients treated with sorafenib: implications for clinical management. BMC Cancer. 2014;14:110. doi: 10.1186/1471-2407-14-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scartozzi M, Faloppi L, Bianconi M, Giampieri R, Maccaroni E, Bittoni A, Del Prete M, Loretelli C, Belvederesi L, Svegliati Baroni G. The role of LDH serum levels in predicting global outcome in HCC patients undergoing TACE: implications for clinical management. PLoS One. 2012;7:e32653. doi: 10.1371/journal.pone.0032653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan ST, Mau Lo C, Poon RT, Yeung C, Leung Liu C, Yuen WK, Ming Lam C, Ng KK, Ching Chan S. Continuous improvement of survival outcomes of resection of hepatocellular carcinoma: a 20-year experience. Ann Surg. 2011;253:745–758. doi: 10.1097/SLA.0b013e3182111195. [DOI] [PubMed] [Google Scholar]