Abstract
BACKGROUND: We evaluated both estrogen receptor (ER) and human epidermal growth factor receptor 2 (HER2) status on disseminated tumor cells (DTCs) in the bone marrow of 54 patients with early breast cancer and compared these with the corresponding primary tumor (PT). MATERIALS AND METHODS: Bone marrow aspirates were obtained at the time of first surgery, and ER and HER2 status on DTCs was assessed simultaneously by immunocytochemistry using a triple fluorescence staining method. RESULTS: The median number of DTCs was 13 (range 1-95). The concordance rate between ER status on DTC and PT was 74%. Patients with an ER-positive PT were significantly more likely to have at least one ER-positive DTC (34 out of 42) than patients with an ER-negative PT (6 out of 12; P = .031). Thirty-nine (93%) of the 42 patients with ER-positive PT had at least one ER-negative DTC. The concordance rate between HER2 status on DTC and PT was 52%. The probability of having at least one HER2-positive DTC was not related to the HER2 status of the PT (P = 0.56). Twenty-two (46%) of the 48 patients with a HER2-negative PT had at least one HER2-positive DTC. All the six patients with a HER2-positive PT had at least one HER2-negative DTC. CONCLUSION: Taken together, our study confirms that ER and/or HER2 status may differ between DTC and PT. This discordance could be important for patients lacking ER or HER2 expression on the PT but showing ER-positive or HER2-positive DTC because they might benefit from an endocrine and/or HER2-targeted therapy.
Introduction
Metastases are the leading cause of death in breast cancer (BC). The exact mechanism of metastasis development is still not well understood [1], but it seems that the formation of micrometastasis in the bone marrow (BM) may be one of the key events [2]. Different recent studies reported that 15% to 38% of patients with early breast cancer (EBC) are positive for disseminated tumor cells (DTCs) in the BM as determined by immunocytochemistry [3], [4], [5], [6], [7], [8]. In a pooled analysis of 4703 patients with EBC and a follow-up of 10 years, the presence of DTCs in the BM at the time of first diagnosis has been shown to be an independent prognostic factor with respect to poor disease-free survival (DFS) and overall survival (OS) [4]. In addition, DTCs might be able to escape common adjuvant chemotherapy and persist in a dormant state for years [9], [10]. Indeed, the persistence of DTCs after chemotherapy in EBC patients without clinical manifest metastasis, as well as after neoadjuvant chemotherapy in patients with locally advanced BC, is predictive for DFS, cancer-specific survival, and OS [11], [12]. However, it is still not known which circumstances allow these cells to switch to an active mode and spread out into distant organs to manifest metastases years after the end of adjuvant therapy.
Therefore, one of the key issues in BC research is to phenotype and characterize DTCs to identify patients at higher risk of relapse and to select them for secondary adjuvant anticancer therapy (such as endocrine or targeted therapy). Given that the estrogen receptor (ER) and HER2 status of the primary tumor (PT) is an essential factor influencing treatment decisions, we focused our work on the determination of ER and HER2 status on DTCs as compared with the PT. It is known that both hormone receptor and HER2 status may change during the course of disease and lead to a different expression profile of metastases as compared with the PT [13], [14]. Previous data show that both ER status [15] and HER2 status [16] on DTCs may differ compared with the PT; thus, DTCs (which are thought to be precursor cells of manifest metastases) could serve as early predictors of such phenotype switching between PT and metastases.
Our retrospective pilot study of 54 patients with EBC focused on simultaneously determining ER and HER2 protein expressions on individual DTC [defined as cytokeratin (CK)-positive cells] with an easy-to-use triple fluorescence staining method and to compare them to the corresponding PT.
Materials and Methods
Patients
All patients were treated at the Department of Obstetrics and Gynecology, Ludwig Maximilian University (Munich, Germany), between 2006 and 2009. The BM aspiration was performed during initial BC surgery in general anesthesia from the anterior iliac crest. Informed consent was obtained from all patients. The study was conducted in accordance with the ethical principles stated in the most recent version of the Declaration of Helsinki or the applicable guidelines of the International Conference on Harmonization Good Clinical Practice Guideline 1998, whichever represented the greater protection of the individual. It was approved by the ethical committee of the Ludwig Maximilian University (Project Number 007/02). Patients were eligible if they had invasive BC (stages pT1-4, pN0-3, and M0), no other malignant disease during the last 5 years, and no neoadjuvant therapy before surgery. The tumor node metastasis classification according to the revised American Joint Committee on Cancer was used to categorize the tumor stage at primary diagnosis [17]. Only patients with detection of CK-positive cells (DTC +) and known ER and HER2 status of the PT (n = 54) were selected for this analysis.
Determination of the Phenotype of the PT
The phenotype of the PT was routinely evaluated by immunohistochemical staining and by fluorescence in situ hybridization (FISH) in HER2-positive cases in the Department of Pathology of the Ludwig Maximilian University according to the common criteria for hormone receptor and HER2 determination. ER expression was determined semiquantitatively using the immunoreactive score of Remmele and Stegner (IRS) (score nuclear staining × score intensity of ER staining). Tumors having a score of 2 or more were defined as ER positive [18]. HER2 status of the PT was determined using the semiquantitative HERCEPTM test (DAKO, Denmark). HER2 expression was categorized from 0 to 3 +. 3 + is considered positive. In case of 2 +, HER2 amplification was determined by FISH.
BM Specimens
DTCs, defined as CK-positive cells in BM aspirates, were obtained in a retrospective series of 54 patients with EBC before the start of systemic adjuvant therapy. BM aspiration and tumor cell isolation were performed on the basis of consensus recommendations [19]. In short, 5 ml of BM was extracted, collected in EDTA-treated tubes, washed in Hank’s salt solution (Biochrom, Germany, 175g for 10 minutes at 4°C), and processed within 24 hours. Ficoll density gradient centrifugation with a density of 1.077 g/ml (Biochrom, Germany, 1105g for 20 minutes at 4°C) was used to enrich mononucleated cells and to separate them from other BM cells. Subsequently, the interphase layer containing mononucleated cells (including tumor cells) was washed in PBS (535g for 10 minutes at 4°C), and a cell count was performed using Malassez cell counting chambers. A total of 106 cells were spun down at 150g for 5 minutes at room temperature onto each glass slide. The cytospins were air-dried at room temperature overnight. For storage, they were frozen at − 80°C until further use and staining (within 5 years).
Triple Fluorescent Staining of CK, ER, and HER2
The triple fluorescent staining of cytospins to detect tumor cells and determine simultaneously their ER and HER2 status was performed as described in detail before [20]. In short, cytospins were thawed at room temperature and immediately fixed using 3.7% neutral buffered formalin in PBS (Fischer, Saarbrücken, Germany). Subsequently, these were permeabilized for 2 minutes in cold (− 20°C) methanol (Sigma-Aldrich, Steinheim, Germany), washed in PBS, and incubated for 15 minutes in Ultra V Blocking medium (Thermo Scientific, Fremont, CA) to reduce background signals. Cells were then first incubated for 45 minutes with a rabbit monoclonal anti-human ER antibody (dilution 1/100, clone sp-1; Lab Vision, Fremont, CA) and after washing again incubated for 30 minutes with a goat anti-rabbit IgGFab fragment labeled with Cy3 (dilution 1/500, Jackson ImmunoResearch). After another washing step, cells were incubated for 45 minutes with a rabbit polyclonal anti-human HER2 antibody (dilution 1/100, A0485; Dako, Glostrup, Denmark) and with the A45 B/B3 mouse anti-human CK antibody (dilution 1/200, Micromet, Munich, Germany). This is a purified monoclonal pan-CK antibody, which reacts with common epitopes on several CKs including CK8, CK18, and CK19. After washing, the slides were incubated for 30 minutes with a goat anti-rabbit IgG labeled with Coumarin-AMCA (dilution 1/200, Jackson ImmunoResearch) to determine HER2 and a goat anti-mouse IgG labeled with DyLight488 (dilution 1/200, Jackson ImmunoResearch) to detect CK. After a final washing step (PBS) and drying at room temperature, the slides were mounted with Kaiser’s glycerol gelatin (Merck, Darmstadt, Germany).
Cell Culture and Cytospin Preparation
Regarding the human breast adenocarcinoma cell lines, MCF-7 (ATCC HTP-22) was obtained from the European Collection of Cell Cultures (Salisbury, UK), and the SK-BR-3 (ATCC HTB-30) cell line was from the American Type Culture Collection (Rockville, USA). Cryopreservation of cell cultures ranged from passage 1 to 10. Cells were expanded up to a maximum of 20 passages. Cells were grown routinely in Dulbecco’s modified Eagle’s medium (Biochrom, Berlin, Germany) supplemented with 10% fetal bovine serum (PAA, Pasching, Austria).
For cytospin preparation, trypsinized cells were centrifuged (700g, 10 minutes, 4°C) and resuspended in PBS (Biochrom, Berlin, Germany). Then, 106 cells were spread on each cytospin and centrifuged (45g, 5 minutes, room temperature). Cytospins were allowed to dry overnight at room temperature and then stored at − 80°C.
Positive and Negative Control
Control cytospins with CK- and ERα-positive MCF-7 cells as well as CK- and HER2-positive SK-BR-3 cells were prepared, stored, and fixed in the same way to guarantee that ERα negativity or HER2 negativity of a patient's sample was not due to a technical failure. MCF-7 cells were used as positive control for ERα and CK staining and as negative control for HER2. Inversely, SK-BR-3 cells were used as positive control for HER2 and CK staining and as negative control for ERα. One MCF-7 and one SK-BR-3 control slides were systematically evaluated in each batch of patient samples [20].
Cell Detection
Two cytospins (2 × 106 cells) per patient were analyzed. Each slide was inspected by at least two independent investigators and by four in doubtful cases within a maximum of 48 hours after the labeling procedure. The manual analysis was done using a computerized fluorescence microscope Axioskop (Carl Zeiss Micro Imaging GmbH) for phase and fluorescence with × 10 and × 40 magnifications. Each observed DTC was documented with at least one picture with an AxioCam MR camera and an AxioVision software to capture, analyze, and save high-resolution images for the three fluorescence channels (Carl Zeiss Microscopy, Göttingen, Germany).
For identification of DTCs by immunofluorescence, we followed the consensus recommendations for standardized tumor cell detection [19], and for HER2-positive DTCs, we followed the criteria already defined by Solomayer et al. [16]. In general, the cells showed a high nucleus/cytoplasm ratio. CK positivity was defined as the ring-like appearance (green, membrane and cytoplasm staining in periphery) and HER2 overexpression as completely positive membrane staining (blue, moderate or strong). Criteria for ER positivity were a specific staining of the nucleus with a low background, a strong nuclear, and no cytoplasmic or peripheral staining (red).
Statistical Analysis
A patient was classified as having a positive HER2 or ER status of DTCs if at least one HER2-positive or at least one ER-positive DTC was detected. Associations between HER2 status or ER status of DTCs and patient PT characteristics were analyzed using chi-square tests or Fisher's exact tests. The association between the ER status of DTCs (no versus at least one ER-positive DTC) and the IRS of the PT was investigated with the Cochran-Armitage test for trend. All statistical analyses were performed with IBM SPSS Statistics, version 19, and all P values reported are two-sided with P values below .05 being considered as statistically significant.
Results
Patients
Patients and PT characteristics are summarized in Table 1. The mean age was 59.5 years (range 32-85 years). The majority of the 54 patients was postmenopausal and had a ductal invasive tumor. Fifty-seven percent showed an intermediate grading (G2), and 63% had no lymph node involvement. Most of the PTs in the patient population were rather small, with 54% pT1 tumors. Forty-two (78%) patients were diagnosed as ER positive, 6 (11%) were classified as HER2 positive, and 10 patients were classified as triple negative [i.e., negative for ER, progesterone receptor (PR) and HER2].
Table 1.
Patient and PT Characteristics (n = 54)
| Menopausal status | Premenopausal | 15 (27.8%) |
| Postmenopausal | 39 (72.2%) | |
| pT | pT1 | 29 (53.7%) |
| pT2 | 18 (33.3%) | |
| pT3 | 7 (13.0%) | |
| pN | pN0 | 34 (63.0%) |
| pN + | 18 (33.3%) | |
| n/a | 2 (3.7%) | |
| Histological grading | G1 | 5 (9.3%) |
| G2 | 31 (57.4%) | |
| G3 | 18 (33.3%) | |
| Histological type | Ductal | 40 (74.1%) |
| Lobular | 10 (18.5%) | |
| Other | 4 (7.4%) | |
| ER status | Negative | 12 (22.2%) |
| Positive | 42 (77.8%) | |
| PR status | Negative | 20 (37.0%) |
| Positive | 34 (63.0%) | |
| HER2 status | Negative | 48 (88.9%) |
| Positive | 6 (11.1%) |
Enumeration and ER and HER2 Status Determination of the DTCs
Using the MCF-7 and SK-BR-3 control cells, we observed that all 54 patients exhibited at least one CK-positive DTC (Figure 1). Overall, 1082 DTCs were detected in the 54 samples, and the median number of DTCs detected per sample was 13 (range 1-95) DTCs. Figure 2A shows the frequency distribution of the number of DTCs detected in the samples from the 54 patients, illustrating that for most patients only a small number of DTCs were detected. The frequency distribution of the DTCs with regard to their ER and HER2 status is presented in Figure 2B: Of the 1082 DTCs detected, 38 (3.5%) were ER positive/HER2 positive, 142 (13%) were ER positive/HER2 negative, 43 (4.0%) were ER negative/HER2 positive, and 859 (79%) were ER negative/HER2 negative.
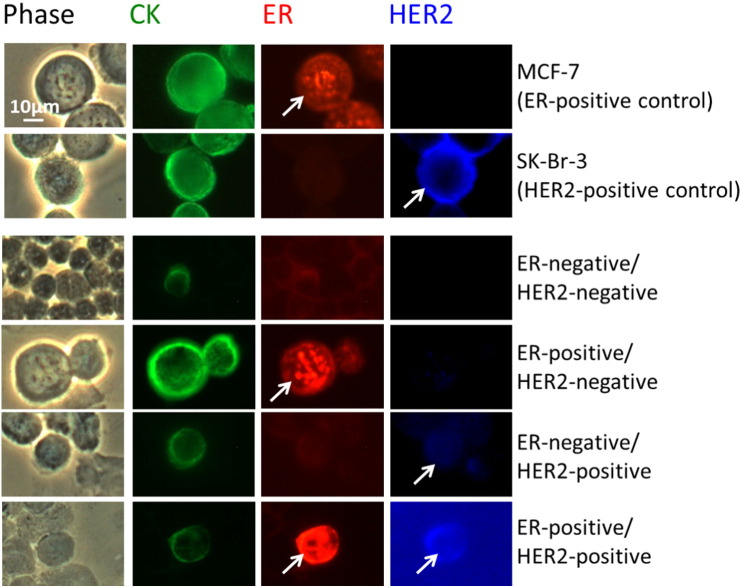
Figure 1.
ER and HER2 status determination in control cells and DTCs.
Phase, CK, ER, and HER2 staining for an ER-positive control (cell line MCF-7), a HER2-positive control (cell line SK-Br-3), and DTCs with four different subtypes (ER negative/HER2 negative, ER positive/HER2 negative, ER negative/HER2 positive, ER positive/HER2 positive). Arrows highlight the ER and HER2 staining: ER with specific staining of the nucleus with a low background, a strong nuclear, and no cytoplasmic or peripheral staining (red); HER2 overexpression as completely positive moderate or strong membrane staining (blue). The triple fluorescence labeling of CK, ER, and HER2 was performed on 106 cells, and phase analysis was performed in parallel. Magnification 40 ×. Scale bar: 10 μm.
Figure 2.
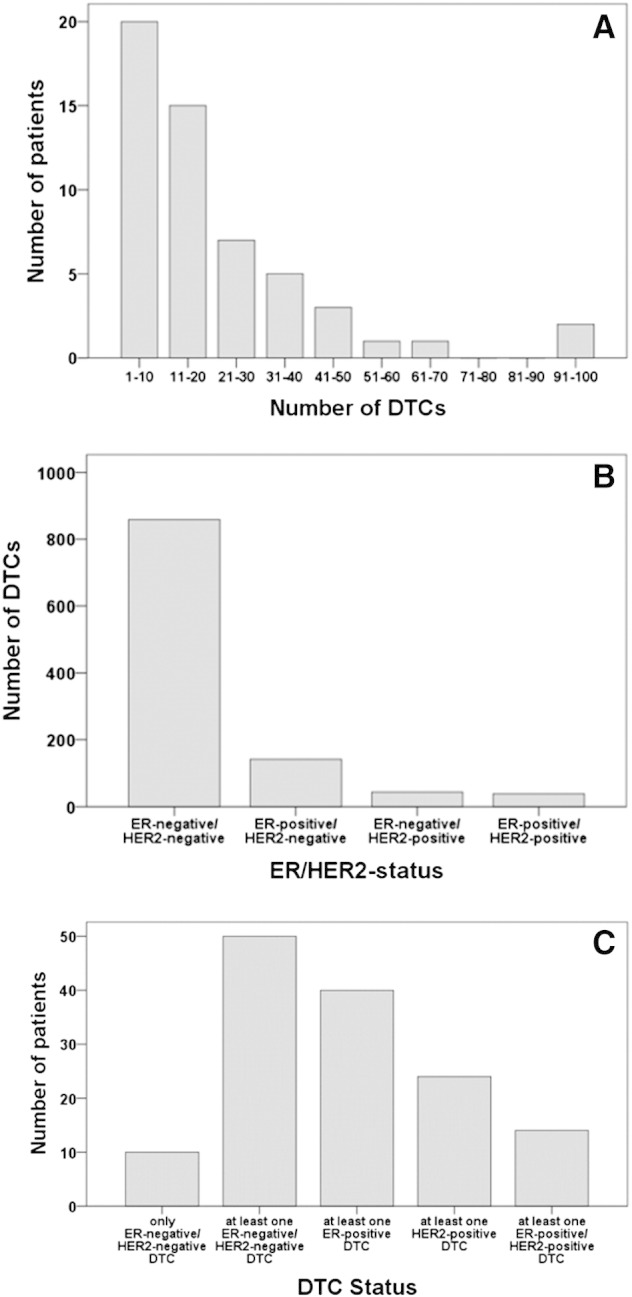
Distribution of DTCs (n = 1082). (A) Number of DTCs detected per patient (n = 54 patients). (B) Number of DTCs according to their ER/HER2 status. (C) Number of patients according to the ER/HER2 status of their DTCs.
The patient distribution with regard to the presence of ER- and/or HER2-positive DTCs was as follows (Figure 2C): 10 (19%) patients had only ER-negative/HER2-negative DTCs. At least one ER-positive DTC was detected in 40 (74%) patients, at least one HER2-positive DTC was detected in 24 (44%) patients, and 14 (26%) patients had at least one ER-positive/HER2-positive DTC. Overall, 93% of the patients (n = 50) had at least one ER-negative/HER2-negative DTC, and for these 50 patients, ER-negative/HER2-negative DTCs represent on average 80% (range 40%-100%) of all DTCs detected.
Association with Clinicopathological Factors
There was no association between the HER2 status of DTCs (i.e., the presence of at least one HER2-positive DTC) and any of the clinicopathological factors (chi-square test, all P > .4; Table 2). In contrast, the ER status of DTCs was significantly associated with the ER status of the PT (see Table 2 and below), whereas there was no association with any of the other clinicopathological factors (chi-square test, all P > .05; Table 2).
Table 2.
Association of Patient and PT Characteristics with HER2 Status and ER Status of DTCs Detected in BM Samples of 54 BC Patients
| HER2 Status of DTCs |
ER Status of DTCs |
||||||
|---|---|---|---|---|---|---|---|
| At Least One HER2-Positive DTC (n = 24) | No HER2-Positive DTC (n = 30) | P Value | At Least One ER-Positive DTC (n = 40) | No ER-Positive DTC (n = 14) | P Value | ||
| Menopausal status | Premenopausal | 7 | 8 | .839† | 11 | 4 | 1.000⁎ |
| Postmenopausal | 17 | 22 | 29 | 10 | |||
| pT | pT1 | 13 | 16 | .996† | 24 | 5 | .254† |
| pT2 | 8 | 10 | 11 | 7 | |||
| pT3 | 3 | 4 | 5 | 2 | |||
| pN | pN0 | 15 | 19 | .982† | 27 | 7 | .197⁎ |
| pN + | 8 | 10 | 11 | 7 | |||
| n/a | 1 | 1 | 2 | 0 | |||
| Histological grading | G1 | 1 | 4 | .486† | 5 | 0 | .059† |
| G2 | 14 | 17 | 25 | 6 | |||
| G3 | 9 | 9 | 10 | 8 | |||
| Histological type | Ductal | 17 | 23 | .889† | 27 | 13 | .164† |
| Lobular | 5 | 5 | 9 | 1 | |||
| Other | 2 | 2 | 4 | 0 | |||
| ER status | Negative | 5 | 7 | .826† | 6 | 6 | .031† |
| Positive | 19 | 23 | 34 | 8 | |||
| PR status | Negative | 8 | 12 | .614† | 12 | 8 | .070† |
| Positive | 16 | 18 | 28 | 6 | |||
| HER2 status | Negative | 22 | 26 | .682⁎ | 36 | 12 | .643⁎ |
| Positive | 2 | 4 | 4 | 2 | |||
Exact Fisher test.
Chi-square test (without unknowns).
Comparison of ER and HER2 Expression between PT and DTCs
The concordance rate between the ER status of DTCs and PT was 74% (Table 3). Patients with ER-positive PT were significantly more likely to have at least one ER-positive DTC (34 out of 42) than patients with ER-negative PT (6 out of 12; chi-square test, χ2 = 4.66, P = .031; Table 3). Furthermore, 39 (93%) of the 42 patients with ER-positive PT and all of the 12 patients (100%) with ER-negative PT DTCs had at least one ER-negative DTC (not shown in Table 3).
Table 3.
Association between the ER Status of the PT (ER Negative, ER Positive) and the ER Status of DTCs (Only ER-Negative DTCs, At Least One ER-Positive DTC) in 54 Primary BC Patients
| ER Status | DTCs |
Total (%) | ||
|---|---|---|---|---|
| Only ER-Negative DTCs (%) | At Least One ER-Positive DTC (%) | |||
| Tumor | ER negative (%) | 6 (11) | 6 (11) | 12 (22) |
| ER positive (%) | 8 (15) | 34 (63) | 42 (78) | |
| Total (%) | 14 (26) | 40 (74) | 54 (100)⁎ | |
A concordant ER status between PT and DTCs was found in 40 of the 54 (74%) patients.
P = .031 (chi-square test).
The ER status of DTCs (i.e., the probability of having at least one ER-positive DTC) was not associated with the IRS of the PT (Cochran-Armitage test for trend, P = .208).
The concordance rate between HER2 status of DTCs and PT was 52% (Table 4). The probability of having at least one HER2-positive DTC was not significantly related to the HER2 status of the PT (chi-square test, χ2 = 0.34, P = .56). Of the 48 patients with a HER2-negative PT, 22 had at least one HER2-positive DTC, and of the 6 patients with a HER2-positive PT, 2 had at least one HER2-positive DTC (Table 4). Forty-six of the 48 patients with a HER2-negative PT and all of the 6 patients with a HER2-positive PT had at least one HER2-negative DTC. Remarkably, four of the six patients with HER2-positive PT had HER2-negative DTCs only (not shown in Table 4).
Table 4.
Association between the HER2 Status of the PT (HER2 Negative, HER2 Positive) and the HER2 Status of DTCs (Only HER2-Negative DTCs, At Least One HER2-Positive DTC) in 54 Primary BC Patients
| HER2 Status | DTCs |
Total (%) | ||
|---|---|---|---|---|
| Only HER2-Negative DTCs (%) | At Least One HER2-Positive DTC (%) | |||
| Tumor | HER2 negative (%) | 26 (48) | 22 (41) | 48 (89) |
| HER2 positive (%) | 4 (7) | 2 (4) | 6 (11) | |
| Total (%) | 30 (55) | 24 (45) | 54 (100)⁎ | |
A concordant HER2 status between PT and DTCs was found in 28 of the 54 (52%) patients.
P = .56 (chi-square test).
Heterogeneity of ER and HER2 Combined Expressions of DTCs
The heterogeneity of the combined ER/HER2 status of DTCs and the association with the ER/HER2 status of the PT are shown in Table 5. The ER/HER2 status of DTCs of a patient was regarded as heterogeneous if the DTCs detected in the BM samples of a patient varied with respect to the ER/HER2 status (there are four different ER/HER2 profiles: ER negative/HER2 negative, ER positive/HER2 negative, ER negative/HER2 positive, ER positive/HER2 positive). A heterogeneous ER/HER2 status on DTCs was found in 40 (83%) of the 48 patients with more than one DTC. Twenty-two (46%) of these patients had DTCs with two different ER/HER2 profiles, 8 (17%) patients had DTCs with three different ER/HER2 profiles, and 10 (21%) patients had DTCs with all four possible ER/HER2 profiles. Seven out of the eight patients with more than one DTC and no heterogeneous ER and HER2 expression (i.e., all DTCs had the same ER/HER2 profile) had exclusively ER-negative/HER2-negative DTCs (range 3-31 DTCs), whereas there was one patient with two ER-positive/HER2-negative DTCs.
Table 5.
Combined ER/HER2 Status of DTCs and Association with the ER/HER2 Status of the PT
| DTCs with One ER/HER2 Profile | DTCs with Two ER/HER2 Profiles | DTCs with Three ER/HER2 Profiles | DTCs with Four ER/HER2 Profiles | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ER/HER2 status of DTCs | ER +/HER2 − | + | + | + | + | + | |||||
| ER +/HER2 + | + | + | + | + | |||||||
| ER −/HER2 − | + | + | + | + | + | + | + | ||||
| ER −/HER2 + | + | + | + | + | |||||||
| ER/HER2 status of PT | ER +/HER2−(n = 38) | 2 | 1 | 5 | 13 | 1 | 2 | 2 | 4 | 8 | |
| ER +/HER2 + (n = 4) | 1 | 2 | 1 | ||||||||
| ER −/HER2 − (n = 10) | 3 | 1 | 3 | 1 | 1 | 1 | |||||
| ER −/HER2 + (n = 2) | 1 | 1 | |||||||||
| Total (n = 54) | 2 | 1 | 10 | 1 | 18 | 1 | 3 | 2 | 6 | 10 | |
Shown are the numbers of patients with a given ER/HER2 status of the PT that have DTCs with ER/HER2 profiles as indicated by the “+” signs above. For example, there were five patients with an ER-positive/HER2-negative PT that had only ER-negative/HER2-negative DTCs, and there were three patients with an ER-negative/HER2-negative PT that had both ER-positive/HER2-negative and ER-negative/HER2-negative DTCs.
Seven out of 10 patients with a triple-negative PT had at least 1 DTC positive for ER, HER2, or both. On the other hand, 9 out of the 10 patients with a triple-negative PT also had at least 1 DTC expressing neither ER nor HER2 (Table 5).
Discussion
In our retrospective study, including 54 patients with EBC and evidence of DTCs in BM, we detected a total of 1082 DTCs, with 17.5% of the DTCs being positive for ER, 7.5% of the DTCs positive for HER2 and 3.5% of the DTCs expressing both ER and HER2 simultaneously. DTCs showing heterogeneity with regard to their combined ER/HER2 status were observed in 83% of patients with more than one DTC. The concordance rate between DTCs and PT regarding ER expression was 74%, whereas the concordance rate for HER2 expression was only 52%. Patients with an ER-positive PT were significantly more likely to have at least one ER-positive DTC compared with patients with an ER-negative PT, whereas the probability of having at least one HER2-positive DTC was not significantly related to the HER2 status of the PT.
The method of simultaneous determination of ER and HER2 expression on tumor cells has been established in our laboratory and was previously evaluated in a study focusing on ER and HER2 status of circulating tumor cells (CTCs) in patients with metastatic BC [20]. The simultaneous determination of ER and HER2 on a single cell with this easy-to-use staining method has several advantages with respect to clinical utility, time, and money and may provide new insights in tumor biology. Furthermore, it may advance DTC detection and therefore improve patient care. Our study is a rather small retrospective study (n = 54) including only patients with evidence of DTCs, which might bias our results. However, to the best of our knowledge, this is the first study that determined ER and HER2 protein status on the same DTCs at the same time. Because of technical reasons, only two important markers (ER and HER2) of BC were analyzed, whereas other potentially informative markers, such as PR, could not be evaluated. We focused on ER as a crucial endocrine marker and HER2 as an essential marker for additional antibody treatment. Further development of the method could extend the spectrum of detectable markers and provide additional information on the phenotype of DTCs. There are, however, several studies that investigated either ER or HER2 expression on DTCs. In a study involving 107 patients with DTCs in the BM, Fehm et al. (2008a) confirmed the findings of some previous smaller studies that the ER status on minimal residual disease does not necessarily mirror the ER status of the PT [15]. In addition, Fehm et al. (2008a) reported that the majority of DTCs tended to be ER negative, which is in accordance with our finding that 902 out of 1082 DTCs were ER negative. However, the concordance rate of the ER status of DTCs compared with the PT in the study by Fehm et al. (2008a) was only 28% and thus considerably lower than the 74% concordance rate observed in our study. Furthermore, the probability of having ER-positive DTCs was not related to the ER status of the PT, which is in contrast to our findings.
Similar to the ER status results, the majority of studies exploring the HER2 status describe some discordance between the HER2 status on DTCs and the corresponding PT. The concordance rate of 52% found in our study is at the lower end of HER2 concordance rates as reported in the previous studies, which ranged from 51% to 77% [3], [6], [16], [21], [22], [23]. An even larger variability seems to exist with respect to the proportion of patients with a HER2-negative PT that have HER2-positive DTCs. Solomayer et al. compared the HER2 status between DTCs and corresponding PT in 46 patients and reported that HER2-positive DTCs were detected in 60% of patients with a HER2-negative PT [16] compared with 51% found in the study by Hartkopf et al. [21] and 46% as observed in our study. Krishnamurthy et al. investigated HER2 gene amplification on DTCs using FISH and showed a discordance rate of HER2 gene amplification between DTCs and PT of 28%, with only 21% of patients with HER2-negative PT having at least one HER2-positive DTC [23]. An even lower proportion was reported by Becker et al. who detected HER2-positive DTCs in 15% to 21% of patients using immunocytochemistry or reverse transcription polymerase chain reaction and observed that only 13% of patients with a HER2-negative PT had HER2-positive DTCs [22]. Clearly, more research and standardization of detection methods are needed to evaluate factors responsible for the large discrepancies with regard to the proportion of patients with a HER2-negative PT that have HER2-positive DTCs.
DTCs and even HER2 divergence on DTCs [6] may persist after (neo-)adjuvant chemotherapy [9], [24], [25]. Both the HER2 overexpression on DTCs at the time of first diagnosis and the persistence of DTCs after systemic therapy are known independent prognostic factors with respect to poor outcome [11], [12], [21], [26]. For example, Hartkopf et al. [21] reported that mean DFS was significantly shorter in patients with EBC and HER2-positive DTCs compared with patients with only HER2-negative DTCs (n = 151; as determined at the time of first surgery). They showed by using multivariate analysis that the HER2 status on DTCs was an independent predictor of DFS. One current hypothesis is that the spread of tumor cells occurs early in BC history [27] and that these cells may persist in a dormant, nonproliferating state for years [10]. In addition, these cells may demonstrate stem cell characteristics [28] and acquire a more aggressive phenotype during metastatic latency within the BM microenvironment [29]. In line with this, our study shows that 93% of patients with ER-positive PT have ER-negative DTCs and even 46% with HER2-negative PT show HER2-positive DTCs. One possible explanation for the observed discordances is the clonal heterogeneity of the PT, which might result in the dissemination of cells with different phenotype. An additional reason might be that ER-negative and HER2-positive DTCs disseminate more frequently due to their more aggressive phenotype. The fact that patients with ER-positive PT develop ER-negative metastatic lesions [13] and that patients with HER2-negative PT may become HER2 positive [14] during the course of disease is in accordance with this hypothesis and further emphasizes the need of reassessing the current phenotype. For this purpose, DTCs could serve as early predictors of divergences of the phenotype between PT and potential metastases.
Bisphosphonates and trastuzumab have been used to eliminate DTCs and HER2-positive DTCs persisting after adjuvant therapy, respectively [30], [31], [32]. Still, in our study, 79% of the DTCs are negative for both ER and HER2, which may also explain some of the unexpected failures of endocrine or HER2-targeted therapy and define a patient population not suitable for secondary endocrine or HER2-targeted therapy. This underlines the need to develop new targeted agents including stem cell activity. Vincent-Salomon and colleagues suggest that the next step to determine whether eradication of DTCs in the BM after systemic therapy results in longer survival must be large-scale prospective clinical trials [33]. The presence of tumor cells in the BM identifies a population of patients at high risk for recurrence [4], [7], [12], [34]. However, because of issues related to testing, reporting, and lack of standardized protocols, BM aspiration is still not a standard of care and currently not recommended for all patients with BC [7], [19], [23]. Nevertheless, the increasing interest in the examination of BM as a tissue source for the study of micrometastases, including biomarker assessment of detected DTCs, will lead to a better understanding of the biology of metastatic disease [23]. The enumeration and detection of DTCs according to standardized protocols and subsequent phenotypic and molecular characterization of these cells may increase early detection, improve prognostic accuracy, and contribute to an enhanced identification of patients which may benefit from additional systemic anticancer therapy. Further advances in the design of personalized therapies may therefore lead to the stratification of patients to secondary adjuvant therapies. These therapies could include secondary endocrine therapy in case of hormone receptor–negative PT but ER-positive DTCs, or HER2-targeted therapy in patients with HER2-negative BC but evidence of HER2-positive DTCs. Patients might benefit from individualized and personalized treatment options; however, treatment plans and decision making will get even more complex and time consuming, and be a source of mistakes. On the other hand, DTCs lacking ER or HER2 in patients with hormone receptor–positive or HER2-positive PT might explain failures of endocrine or HER2-targeted therapies and open the way for alternative, potentially more appropriate systemic treatments options.
The question of whether a single DTC is representative for the reaction of all occult tumor cells and drug response of the corresponding tumor tissue may only be answered by prospective clinical trials. Furthermore, the suitability of DTC as a surrogate marker to monitor systemic therapy remains to be revealed in future studies [35]. For example, the efficacy of HER2-targeted therapy could be assessed in a clinical trial by sequential detection of HER2-positive DTCs (before and after treatment) [36]. Ongoing trials, for example, focus on personalized targeted therapies according to the phenotype of CTCs in patients with HER2-negative metastatic BC (DETECT III and IV) or on secondary adjuvant HER2-targeted therapy in HER2-negative EBC with persisting CTCs after (neo-)adjuvant chemotherapy.
Conclusion
Differences in ER and HER2 expression on potentially metastases-initiating DTCs or metastases compared with the PT are a known phenomenon in BC and may have clinical implications with respect to targeted treatment approaches. In our study, we showed a high degree of discordance regarding ER and HER2 expression between DTCs and PT by determining both ER and HER2 status simultaneously on the same cell using a triple fluorescent staining method. The presence of ER-negative or HER2-negative DTCs in patients with ER-positive or HER2-positive PT might explain unexpected failures of HER2-targeted or endocrine therapy. The evaluation of ER and HER2 status on DTCs could be especially important for patients lacking ER or HER2 expression on the PT, as patients showing ER-positive or HER2-positive DTCs might benefit from a secondary endocrine or HER2-targeted therapy. Prospective randomized trials are needed to evaluate these possibilities.
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Acknowledgements
We would like to thank all the patients for participating in this study and donating their BM samples for research purposes.
References
- 1.Pantel K, Woelfle U. Micrometastasis in breast cancer and other solid tumors. J Biol Regul Homeost Agents. 2004;18:120–125. [PubMed] [Google Scholar]
- 2.Chiang AC, Massagué J. Molecular basis of metastasis. N Engl J Med. 2008;359:2814–2823. doi: 10.1056/NEJMra0805239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krishnamurthy S, Cristofanilli M, Singh B, Reuben J, Gao H, Cohen EN, Andreopoulou E., Hall C.S., Lodhi A., Jackson S. Detection of minimal residual disease in blood and bone marrow in early stage breast cancer. Cancer. 2010;116:3330–3337. doi: 10.1002/cncr.25145. [DOI] [PubMed] [Google Scholar]
- 4.Braun S, Vogl FD, Naume B, Janni W, Osborne MP, Coombes RC, Schlimok G., Diel I.J., Gerber B., Gebauer G. A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med. 2005;353:793–802. doi: 10.1056/NEJMoa050434. [DOI] [PubMed] [Google Scholar]
- 5.Falck AK, Bendahl PO, Ingvar C, Isola J, Jönsson PE, Lindblom P, Lövgren K., Rennstam K., Fernö M., Rydén L. Analysis of and prognostic information from disseminated tumour cells in bone marrow in primary breast cancer: a prospective observational study. BMC Cancer. 2012;12:403. doi: 10.1186/1471-2407-12-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krawczyk N, Banys M, Neubauer H, Solomayer EF, Gall C, Hahn M, Becker S., Bachmann R., Wallwiener D., Fehm T. HER2 status on persistent disseminated tumor cells after adjuvant therapy may differ from initial HER2 status on primary tumor. Anticancer Res. 2009;29:4019–4024. [PubMed] [Google Scholar]
- 7.Bidard FC, Vincent-Salomon A, Gomme S, Nos C, de Rycke Y, Thiery JP, Sigal-Zafrani B., Mignot L., Sastre-Garau X., Pierga J.Y. Disseminated tumor cells of breast cancer patients: a strong prognostic factor for distant and local relapse. Clin Cancer Res. 2008;14:3306–3311. doi: 10.1158/1078-0432.CCR-07-4749. [DOI] [PubMed] [Google Scholar]
- 8.Fehm T, Hoffmann O, Aktas B, Becker S, Solomayer EF, Wallwiener D, Kimmig R., Kasimir-Bauer S. Detection and characterization of circulating tumor cells in blood of primary breast cancer patients by RT-PCR and comparison to status of bone marrow disseminated cells. Breast Cancer Res. 2009;11:R59. doi: 10.1186/bcr2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braun S, Kentenich C, Janni W, Hepp F, de Waal J, Willgeroth F, Sommer H., Pantel K. Lack of effect of adjuvant chemotherapy on the elimination of single dormant tumor cells in bone marrow of high-risk breast cancer patients. J Clin Oncol. 2000;18:80–86. doi: 10.1200/JCO.2000.18.1.80. [DOI] [PubMed] [Google Scholar]
- 10.Fehm T, Mueller V, Marches R, Klein G, Gueckel B, Neubauer H, Solomayer E., Becker S. Tumor cell dormancy: implications for the biology and treatment of breast cancer. APMIS. 2008;116:742–753. doi: 10.1111/j.1600-0463.2008.01047.x. [DOI] [PubMed] [Google Scholar]
- 11.Janni W, Vogl FD, Wiedswang G, Synnestvedt M, Fehm T, Juckstock J, Borgen E., Rack B., Braun S., Sommer H. Persistence of disseminated tumor cells in the bone marrow of breast cancer patients predicts increased risk for relapse—a European pooled analysis. Clin Cancer Res. 2011;17:2967–2976. doi: 10.1158/1078-0432.CCR-10-2515. [DOI] [PubMed] [Google Scholar]
- 12.Mathiesen RR, Borgen E, Renolen A, Løkkevik E, Nesland JM, Anker G, Ostenstad B., Lundgren S., Risberg T., Mjaaland I. Persistence of disseminated tumor cells after neoadjuvant treatment for locally advanced breast cancer predicts poor survival. Breast Cancer Res. 2012;14:R117. doi: 10.1186/bcr3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Broom RJ, Tang PA, Simmons C, Bordeleau L, Mulligan AM, O’Malley FP, Miller N., Andrulis I.L., Brenner D.M., Clemons M.J. Changes in estrogen receptor, progesterone receptor and Her-2/neu status with time: discordance rates between primary and metastatic breast cancer. Anticancer Res. 2009;29:1557–1562. [PubMed] [Google Scholar]
- 14.Zidan J, Dashkovsky I, Stayerman C, Basher W, Cozacov C, Hadary A. Comparison of HER-2 overexpression in primary breast cancer and metastatic sites and its effect on biological targeting therapy of metastatic disease. Br J Cancer. 2005;93:552–556. doi: 10.1038/sj.bjc.6602738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fehm T, Krawczyk N, Solomayer EF, Becker-Pergola G, Duerr-Stoerzer S, Neubauer H, Seeger H., Staebler A., Wallwiener D., Becker S. ERalpha-status of disseminated tumour cells in bone marrow of primary breast cancer patients. Breast Cancer Res. 2008;10:R76. doi: 10.1186/bcr2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solomayer EF, Becker S, Pergola-Becker G, Bachmann R, Kramer B, Vogel U, Neubauer H., Wallwiener D., Huober J., Fehm T.N. Comparison of HER2 status between primary tumor and disseminated tumor cells in primary breast cancer patients. Breast Cancer Res Treat. 2006;98:179–184. doi: 10.1007/s10549-005-9147-y. [DOI] [PubMed] [Google Scholar]
- 17.Singletary SE, Allred C, Ashley P, Bassett LW, Berry D, Bland KI, Borgen P.I., Clark G., Edge S.B., Hayes D.F. Revision of the American Joint Committee on Cancer staging system for breast cancer. J Clin Oncol. 2002;20:3628–3636. doi: 10.1200/JCO.2002.02.026. [DOI] [PubMed] [Google Scholar]
- 18.Remmele W, Stegner HE. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe. 1987;8:138–140. [PubMed] [Google Scholar]
- 19.Fehm T, Braun S, Muller V, Janni W, Gebauer G, Marth C, Schindlbeck C., Wallwiener D., Borgen E., Naume B. A concept for the standardized detection of disseminated tumor cells in bone marrow from patients with primary breast cancer and its clinical implementation. Cancer. 2006;107:885–892. doi: 10.1002/cncr.22076. [DOI] [PubMed] [Google Scholar]
- 20.Bock C, Rack B, Kuhn C, Hofmann S, Finkenzeller C, Jäger B, Jeschke U., Doisneau-Sixou S.F. Heterogeneity of ERα and ErbB2 status in cell lines and circulating tumor cells of metastatic breast cancer patients. Transl Oncol. 2012;5:475–485. doi: 10.1593/tlo.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartkopf AD, Banys M, Meier-Stiegen F, Hahn M, Rohm C, Hoffmann J, Helms G., Taran F.A., Wallwiener M., Walter C. The HER2 status of disseminated tumor cells in the bone marrow of early breast cancer patients is independent from primary tumor and predicts higher risk of relapse. Breast Cancer Res Treat. 2013;138:509–517. doi: 10.1007/s10549-013-2470-9. [DOI] [PubMed] [Google Scholar]
- 22.Becker S, Becker-Pergola G, Fehm T, Wallwiener D, Solomayer E-F. Her2 expression on disseminated tumor cells from bone marrow of breast cancer patients. Anticancer Res. 2005;25:2171–2175. [PubMed] [Google Scholar]
- 23.Krishnamurthy S, Bischoff F, Ann Mayer J, Wong K, Pham T, Kuerer H, Lodhi A., Bhattacharyya A., Hall C., Lucci A. Discordance in HER2 gene amplification in circulating and disseminated tumor cells in patients with operable breast cancer. Cancer Med. 2013;2:226–233. doi: 10.1002/cam4.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fehm T, Becker S, Becker-Pergola G, Sotlar K, Gebauer G, Duerr-Stoerzer S, Neubauer H., Wallwiener D., Solomayer E.F. Presence of apoptotic and nonapoptotic disseminated tumor cells reflects the response to neoadjuvant systemic therapy in breast cancer. Breast Cancer Res. 2006;8:R60. doi: 10.1186/bcr1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quintela-Fandino M, López JM, Hitt R, Gamarra S, Jimeno A, Ayala R, Hornedo J., Guzman C., Gilsanz F., Cortés-Funes H. Breast cancer-specific mRNA transcripts presence in peripheral blood after adjuvant chemotherapy predicts poor survival among high-risk breast cancer patients treated with high-dose chemotherapy with peripheral blood stem cell support. J Clin Oncol. 2006;24:3611–3618. doi: 10.1200/JCO.2005.04.0576. [DOI] [PubMed] [Google Scholar]
- 26.Braun S, Schlimok G, Heumos I, Schaller G, Riethdorf L, Riethmüller G, Pantel K. ErbB2 overexpression on occult metastatic cells in bone marrow predicts poor clinical outcome of stage I-III breast cancer patients. Cancer Res. 2001;61:1890–1895. [PubMed] [Google Scholar]
- 27.Hüsemann Y, Geigl JB, Schubert F, Musiani P, Meyer M, Burghart E, Forni G., Eils R., Fehm T., Riethmüller G. Systemic spread is an early step in breast cancer. Cancer Cell. 2008;13:58–68. doi: 10.1016/j.ccr.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 28.Balic M, Lin H, Young L, Hawes D, Giuliano A, McNamara G, Datar R.H., Cote R.J. Most early disseminated cancer cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotype. Clin Cancer Res. 2006;12:5615–5621. doi: 10.1158/1078-0432.CCR-06-0169. [DOI] [PubMed] [Google Scholar]
- 29.Marsden CG, Wright MJ, Carrier L, Moroz K, Rowan BG. Disseminated breast cancer cells acquire a highly malignant and aggressive metastatic phenotype during metastatic latency in the bone. PLoS One. 2012;7:e47587. doi: 10.1371/journal.pone.0047587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rack B, Jückstock J, Günthner-Biller M, Andergassen U, Neugebauer J, Hepp P, Schoberth A., Mayr D., Zwingers T., Schindlbeck C. Trastuzumab clears HER2/neu-positive isolated tumor cells from bone marrow in primary breast cancer patients. Arch Gynecol Obstet. 2012;285:485–492. doi: 10.1007/s00404-011-1954-2. [DOI] [PubMed] [Google Scholar]
- 31.Rack B, Jückstock J, Genss EM, Schoberth A, Schindlbeck C, Strobl B, Heinrigs M., Rammel G., Zwingers T., Sommer H. Effect of zoledronate on persisting isolated tumour cells in patients with early breast cancer. Anticancer Res. 2010;30:1807–1813. [PubMed] [Google Scholar]
- 32.Bozionellou V, Mavroudis D, Perraki M, Papadopoulos S, Apostolaki S, Stathopoulos E, Stathopoulou A., Lianidou E., Georgoulias V. Trastuzumab administration can effectively target chemotherapy-resistant cytokeratin-19 messenger RNA-positive tumor cells in the peripheral blood and bone marrow of patients with breast cancer. Clin Cancer Res. 2004;10:8185–8194. doi: 10.1158/1078-0432.CCR-03-0094. [DOI] [PubMed] [Google Scholar]
- 33.Vincent-Salomon A, Bidard FC, Pierga JY. Bone marrow micrometastasis in breast cancer: review of detection methods, prognostic impact and biological issues. J Clin Pathol. 2008;61:570–576. doi: 10.1136/jcp.2007.046649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tjensvoll K, Oltedal S, Heikkilä R, Kvaløy JT, Gilje B, Reuben JM, Smaaland R., Nordgård O. Persistent tumor cells in bone marrow of non-metastatic breast cancer patients after primary surgery are associated with inferior outcome. BMC Cancer. 2012;12:190. doi: 10.1186/1471-2407-12-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riethdorf S, Pantel K. Disseminated tumor cells in bone marrow and circulating tumor cells in blood of breast cancer patients: current state of detection and characterization. Pathobiology. 2008;75:140–148. doi: 10.1159/000123852. [DOI] [PubMed] [Google Scholar]
- 36.Vincent-Salomon A, Pierga J-Y, Couturier J, d’Enghien CD, Nos C, Sigal-Zafrani B, Lae M., Fréneaux P., Diéras V., Thiéry P. HER2 status of bone marrow micrometastasis and their corresponding primary tumours in a pilot study of 27 cases: a possible tool for anti-HER2 therapy management? Br J Cancer. 2007;96:654–659. doi: 10.1038/sj.bjc.6603584. [DOI] [PMC free article] [PubMed] [Google Scholar]