Abstract
The choice of soilless growing medium for plant nutrition, growth and support is crucial for improving the eco-sustainability of the production in horticultural systems. As our current understanding of the functional microbial communities inhabiting this ecosystem is still limited, we examined the microbial community development of the two most important growing media (organic and mineral) used in open soilless horticultural systems. We aimed to identify factors that influence community composition over time, and to compare the distribution of individual taxa across growing media, and their potential functionality. High throughput sequencing analysis revealed a distinctive and stable microbial community in the organic growing medium. Humidity, pH, nitrate-N, ammonium-N and conductivity were uncovered as the main factors associated with the resident bacterial communities. Ammonium-N was correlated with Rhizobiaceae abundance, while potential competitive interactions among both Methylophilaceae and Actinobacteridae with Rhizobiaceae were suggested. Our results revealed that soilless growing media are unique niches for diverse bacterial communities with temporal functional stability, which may possibly impact the resistance to external forces. These differences in communities can be used to develop strategies to move towards a sustainable horticulture with increased productivity and quality.
In the U.S., Canada and Europe, 95% of greenhouse vegetables, particularly tomatoes, are produced in soilless greenhouse plant cultivation systems using horticultural growing media1. Open soilless horticultural systems have advantages over traditional systems in that the nutrients, oxygen and water required for a healthy plant growth are controlled2 and that soil-borne pathogens can be circumvented3,4. In Western Europe, nearly all greenhouse-grown tomatoes are produced on mineral growing medium comprised of inorganic synthetic fibres5. Mineral growing media are produced from diabase, limestone and cokes, which are melted together at 1500 °C and spun into fibres6. In contrast, peat and coconut are the most utilised organic-derived constituents of growing media produced in the EU7. While mineral growing medium has a neutral pH, high air content and low density, organic growing medium is characterised by its high organic matter content and capacity for cation exchange with the water solution irrigating the growing medium. In spite of these differences, the yield and number of tomato fruits (Solanum lycopersicum) was comparable among plants grown on either mineral or organic growing media over several consecutive years8.
The sustainability of soilless greenhouse systems relies heavily on increased yields and on the general efficacy of the growing process4. Disinfection measures are taken at the greenhouse to guarantee final yield and quality4. However, this results in the elimination of not only deleterious microorganisms but also of potentially beneficial microbial taxa for the plant. This may ultimately prevent the community from reaching equilibrium and stability, making these soilless cultivation systems at risk of successful pathogen invasion4. Biodiversity protects ecosystems against declines in their functionality, as a consequence of the functional redundancy through the co-existence of multiple species9,10. This can also lead to increased productivity11, due to positive impact on bacterial respiration, microbial biomass production, and plant nutrient storage. In addition, increased temporal functional stability and resistance to external forces, such as nutrient perturbations and invasive species, have been reported10,12.
The complex plant-associated microbial community, also referred to as the “second genome of the plant”, is crucial for plant health, growth and development13. Previous work investigating microbial communities associated with growing media has mainly focused on the absence of pathogenic bacteria and fungi14. There is limited understanding of the factors that influence community composition over time, the distribution of individual taxa across growing media, and their potential functionality. The lack of effective control strategies aiming at enhancing productivity15 increases our need to closely monitor the rhizosphere, the growing medium and its microbial populations.
In this study, we examined the microbial community development of the two most important growing media used in open soilless horticultural systems: organic (GB) and mineral (RW). We hypothesised that RW and GB growing media develop different community structure with potentially unique functionalities. Much of the fluctuation within the rhizosphere is defined by the plant root and the conditions in the growing medium. Therefore, the rhizosphere and the microbial community associated with the growing medium are strongly connected. Knowledge regarding these differences can be used to develop strategies towards sustainable horticulture, with enhanced productivity and quality, and potentially increased resistance to external forces12. The hairy roots syndrome caused by Agrobacterium rhizogenes infection is a major issue in greenhouse horticulture, because total yield may decrease up to 10% in tomato plants16. In our study, naturally occurring A. rhizogenes infection was detected in some plants growing in the RW medium (RWS). The interactions occurring between the roots, rhizosphere and the growing medium and the potential resistance to external forces, represented in our study by A. rhizogenes, were also assessed.
Results
Identification of the microbial community associated with the growing medium
Chitinophagaceae, Xanthomonadaceae, Flavobacteriaceae, Hypomicrobiaceae, Microbacteriaceae, Comamonadaceae, Enterobacteriaceae, Methylophilaceae, Rhizobiaceae, Pseudomonadaceae, and Sphingobacteriaceae were the bacterial families with highest relative abundances in both growing media (Fig. 1A). Chitinophagaceae, Methylophilaceae and Hypomicrobiaceae were abundant in GB, while Microbacteriaceae were increased in RW. Enterobacteriaceae, Verrucomicrobiaceae and Rhizobiaceae were abundant in RWS and decreased in GB (Tables 1 and 2). Permutational multivariate analysis of variance (PERMANOVA) confirmed that growing media type, but not time point, significantly contributed to the differences in the relative abundances of bacterial families (P < 0.05, Fig. 1B). Analysis of the DGGE profiles showed that RWS samples grouped together regardless of time, while the rest of the samples tended to cluster according to time point (Supplementary Fig. 1). The total number of species was higher in GB (P < 0.05), while diversity and evenness among GB and RW was significantly different across time points (P < 0.05, Supplementary Table 1). RW and RWS showed consistent similarities in their diversity and evenness metrics (Supplementary Table 2).
Figure 1.
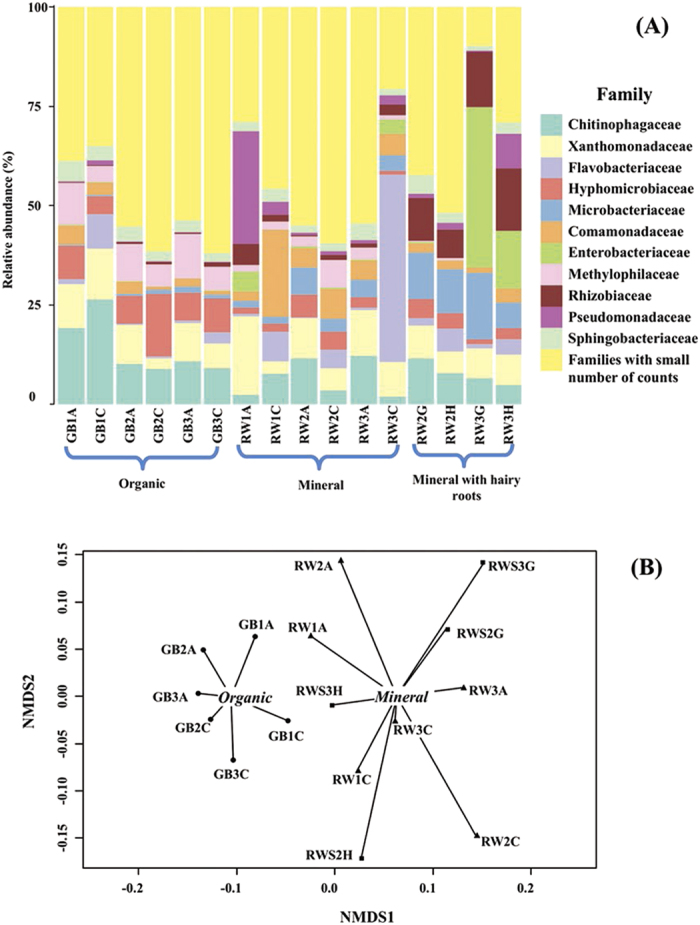
(A) Relative abundances of the bacterial families present in horticultural growing media. Families with the highest sequence count and their corresponding RDP classification are indicated. RW: mineral growing medium; GB: organic growing medium, RWS: mineral medium with hairy roots. Dataset was rarefied to the lowest sequence count; relative abundances were calculated summing the counts of OTUs belonging to the same family. (B) Community structure was significantly different between growing media types. Analysis of multivariate homogeneity of group dispersions (variances) was performed and non-metric multidimensional scaling analysis was used to assess the similarity among bacterial communities. Symbols indicate the growing medium type: circles, organic growing medium (GB); triangles, mineral growing medium (RW); squares, mineral growing medium with hairy roots (RWS). The number in the legend specifies the time point and the letter refers to the sample replicate. For instance, “GB1A” refers to the replicate “A” of organic growing medium, collected at the first time point.
Table 1. Effect of time and growing medium type on relative abundance of bacterial families present in horticultural growing media (n = 12).
| Taxonomy | Time point | Growing medium |
P value | Time effect | Time * growing medium interaction | |
|---|---|---|---|---|---|---|
| GB (Mean % ± SEM) | RW (Mean % ± SEM) | |||||
| Acetobacteraceae | 1 | 0.18 ± 0.07 | 0.00 | 0.03 | NS | NS |
| 2 | 0.15 ± 0.07 | 0.00 | ||||
| 3 | 0.13 ± 0.07 | 0.00 | ||||
| Actinobacteridae | 1 | 0.78 ± 0.42 | 0.07 ± 0.42 | 0.04 | NS | NS |
| 2 | 1.81 ± 0.42 | 0.87 ± 0.42 | ||||
| 3 | 1.38 ± 0.42 | 0.28 ± 0.42 | ||||
| Chitinophagaceae | 1 | 19.47 ± 2.89a | 4.42 ± 2.89b | 0.05 | NS | NS |
| 2 | 7.63 ± 2.89 | 6.65 ± 2.89 | ||||
| 3 | 8.16 ± 2.89 | 6.63 ± 2.89 | ||||
| Chromatiaceae | 1 | 0.10 ± 0.14 | 0.02 ± 0.14 | 0.01 | NS | NS |
| 2 | 0.42 ± 0.14 | 0.00 | ||||
| 3 | 0.80 ± 0.14 | 0.00 | ||||
| Conexibacteraceae | 1 | 0.30 ± 0.60 | 0.05 ± 0.60 | 0.03 | 0.01 | NS |
| 2 | 2.11 ± 0.60 | 0.10 ± 0.60 | ||||
| 3 | 1.92 ± 0.60 | 0.09 ± 0.60 | ||||
| Cryomorphaceae | 1 | 0.02 ± 0.48 | 0.84 ± 0.48 | 0.05 | NS | NS |
| 2 | 0.02 ± 0.48a | 1.63 ± 0.48b | ||||
| 3 | 0.00 | 0.52 ± 0.48 | ||||
| Desulfobacteraceae | 1 | 0.08 ± 0.09 | 0.005 ± 0.09 | 0.0001 | 0.003 | 0.003 |
| 2 | 1.18 ± 0.09a | 0.00b | ||||
| 3 | 0.62 ± 0.09a | 0.00b | ||||
| Ectothiorhodospiraceae | 1 | 0.19 ± 0.08 | 0.005 ± 0.08 | 0.05 | NS | NS |
| 2 | 0.22 ± 0.08 | 0.02 ± 0.08 | ||||
| 3 | 0.13 ± 0.08 | 0.00 | ||||
| Erythrobacteraceae | 1 | 0.26 ± 0.03a | 0.05 ± 0.03b | 0.03 | NS | NS |
| 2 | 0.10 ± 0.03 | 0.02 ± 0.03 | ||||
| 3 | 0.18 ± 0.03 | 0.06 ± 0.03 | ||||
| Gemmatimonadaceae | 1 | 1.24 ± 0.19a | 0.03 ± 0.19b | 0.0003 | NS | NS |
| 2 | 1.20 ± 0.19a | 0.19 ± 0.19b | ||||
| 3 | 1.39 ± 0.19a | 0.03 ± 0.19b | ||||
| Hahellaceae | 1 | 0.08 ± 0.06 | 0.00 | 0.04 | NS | NS |
| 2 | 0.18 ± 0.06 | 0.00 | ||||
| 3 | 0.16 ± 0.06 | 0.00 | ||||
| Haliangiaceae | 1 | 0.09 ± 0.08 | 0.005 ± 0.08 | 0.0002 | 0.001 | 0.001 |
| 2 | 0.41 ± 0.08a | 0.02 ± 0.08b | ||||
| 3 | 1.22 ± 0.08a | 0.00b | ||||
| Hyphomicrobiaceae | 1 | 5.69 ± 1.59 | 1.53 ± 1.59 | 0.01 | NS | NS |
| 2 | 8.90 ± 1.59 | 4.47 ± 1.59 | ||||
| 3 | 6.33 ± 1.59 | 1.76 ± 1.59 | ||||
| Hyphomonadaceae | 1 | 0.46 ± 0.21 | 0.07 ± 0.21 | 0.01 | NS | NS |
| 2 | 0.60 ± 0.21 | 0.11 ± 0.21 | ||||
| 3 | 0.95 ± 0.21a | 0.04 ± 0.21b | ||||
| Ignavibacteriaceae | 1 | 0.12 ± 0.04 | 0.00 | 0.02 | NS | NS |
| 2 | 0.06 ± 0.04 | 0.00 | ||||
| 3 | 0.17 ± 0.04 | 0.01 ± 0.04 | ||||
| Methylocystaceae | 1 | 0.14 ± 0.08 | 0.00 | 0.03 | NS | NS |
| 2 | 0.20 ± 0.08 | 0.00 | ||||
| 3 | 0.19 ± 0.08 | 0.00 | ||||
| Methylophilaceae | 1 | 6.34 ± 1.87 | 1.55 ± 1.87 | 0.04 | NS | NS |
| 2 | 5.97 ± 1.87 | 4.09 ± 1.87 | ||||
| 3 | 7.02 ± 1.87 | 1.95 ± 1.87 | ||||
| Microbacteriaceae | 1 | 0.28 ± 0.69 | 1.43 ± 0.69 | 0.003 | NS | NS |
| 2 | 0.67 ± 0.69a | 4.52 ± 0.69b | ||||
| 3 | 1.02 ± 0.69a | 3.94 ± 0.69b | ||||
| Opitutaceae | 1 | 2.98 ± 0.71a | 0.13 ± 0.71b | 0.003 | NS | NS |
| 2 | 4.40 ± 0.71a | 0.79 ± 0.71b | ||||
| 3 | 2.36 ± 0.71 | 0.14 ± 0.71 | ||||
| Phyllobacteriaceae | 1 | 0.48 ± 0.16 | 0.005 ± 0.16 | 0.007 | NS | NS |
| 2 | 0.76 ± 0.16a | 0.22 ± 0.16b | ||||
| 3 | 0.64 ± 0.16a | 0.09 ± 0.16b | ||||
| Prochlorococcaceae | 1 | 0.12 ± 0.11 | 0.07 ± 0.11 | 0.04 | NS | NS |
| 2 | 0.11 ± 0.11 | 0.00 | ||||
| 3 | 0.48 ± 0.11a | 0.00b | ||||
| Rhodobacteraceae | 1 | 0.41 ± 0.05a | 0.12 ± 0.05b | 0.02 | 0.04 | NS |
| 2 | 0.18 ± 0.05 | 0.18 ± 0.05 | ||||
| 3 | 0.16 ± 0.05 | 0.03 ± 0.05 | ||||
| Rhodocyclaceae | 1 | 0.29 ± 0.48 | 0.16 ± 0.48 | 0.04 | 0.02 | NS |
| 2 | 0.69 ± 0.48 | 0.30 ± 0.48 | ||||
| 3 | 3.22 ± 0.48a | 0.73 ± 0.48b | ||||
| Sinobacteraceae | 1 | 0.39 ± 0.11 | 0.02 ± 0.11 | 0.003 | NS | NS |
| 2 | 0.46 ± 0.11 | 0.11 ± 0.11 | ||||
| 3 | 0.65 ± 0.11a | 0.06 ± 0.11b | ||||
| Sorangiineae | 1 | 1.06 ± 0.63 | 0.03 ± 0.63 | 0.0004 | 0.02 | 0.02 |
| 2 | 3.58 ± 0.63a | 0.03 ± 0.63b | ||||
| 3 | 6.18 ± 0.63a | 0.005 ± 0.63b | ||||
| Verrucomicrobiaceae | 1 | 1.23 ± 0.40 | 0.57 ± 0.40 | 0.02 | NS | NS |
| 2 | 2.72 ± 0.40a | 0.98 ± 0.40b | ||||
| 3 | 1.17 ± 0.40 | 0.52 ± 0.40 | ||||
| Vibrionaceae | 1 | 0.12 ± 0.01 | 0.16 ± 0.01 | NS | 0.0002 | NS |
| 2 | 0.03 ± 0.01 | 0.00 | ||||
| 3 | 0.00 | 0.00 | ||||
| Xanthobacteraceae | 1 | 0.75 ± 0.33 | 0.08 ± 0.33 | 0.02 | NS | NS |
| 2 | 1.52 ± 0.33a | 0.15 ± 0.33b | ||||
| 3 | 0.74 ± 0.33 | 0.09 ± 0.33 | ||||
GB, organic growing medium, n = 6. RW, mineral growing medium, n = 6. NS = not significant effect. Different superscripts indicate significantly different means. “0.00” indicates zero relative abundance detected.
Table 2. Effect of time and hairy roots presence on relative abundance of bacterial families present in horticultural growing media (n = 10).
| Taxonomy | Time point | Growing medium |
P value | Time effect | Time * hairy roots interaction | |
|---|---|---|---|---|---|---|
| RW (Mean ± SEM) | RWS (Mean ± SEM) | |||||
| Hyphomicrobiaceae | 1 | 1.53 ± 0.57 | 0.00 | NS | 0.01 | NS |
| 2 | 4.47 ± 0.57 | 3.86 ± 0.57 | ||||
| 3 | 1.76 ± 0.57 | 1.68 ± 0.57 | ||||
| Methylophilaceae | 1 | 1.54 ± 0.96 | 0.00 | 0.03 | NS | NS |
| 2 | 4.09 ± 0.96a | 0.35 ± 0.96b | ||||
| 3 | 1.95 ± 0.96 | 0.005 ± 0.96 | ||||
| Planctomycetaceae | 1 | 0.005 ± 0.05 | 0.00 | 0.0003 | 0.0007 | 0.0002 |
| 2 | 0.02 ± 0.05a | 1.06 ± 0.05b | ||||
| 3 | 0.07 ± 0.05 | 0.01 ± 0.05 | ||||
| Rhizobiaceae | 1 | 2.94 ± 0.85 | 0.00 | 0.0001 | 0.03 | 0.05 |
| 2 | 1.02 ± 0.85a | 7.91 ± 0.85b | ||||
| 3 | 2.02 ± 0.8a | 13.02 ± 0.85b | ||||
| Verrucomicrobiaceae | 1 | 0.57 ± 0.45 | 0.00 | 0.02 | 0.02 | 0.2 |
| 2 | 0.98 ± 0.45a | 3.96 ± 0.45b | ||||
| 3 | 0.52 ± 0.45 | 0.53 ± 0.45 | ||||
| Vibrionaceae | 1 | 0.16 ± 0.005 | 0.00 | NS | < 0.0001 | NS |
| 2 | 0.00 | 0.01 ± 0.005 | ||||
| 3 | 0.00 | 0.00 | ||||
RW, mineral growing medium, n = 6. RWS, mineral growing medium with hairy roots, n = 4. NS = not significant effect. Different superscripts indicate significantly different means. “0.00” indicates zero relative abundance detected.
Multiple Factor Analysis (MFA) showed that families associated with GB were represented in Dimension 1 (P < 0.0001, Fig. 2A), accounting for 28% of the variance in relative abundances among all the samples. Gemmatimonadaceae, Sinobacteraceae, Sorangiineae, Opitutaceae, Desulfobacteraceae, Actinobacteridae, Hahellaceae, Gaiellaceae, Hypomicrobiaceae, Methylophilaceae, Acetobacteraceae, Methylocystaceae, Conexibacteriaceae, Xanthobacteraceae and Unclassified Nitrospira were significantly associated with GB. Bacterial families that correlated with RW were represented in Dim. 3 (P < 0.05) and explained 11% of the total variance. Pseudonocardineae, Propionibacterineae, Bacteroidaceae, Commamonadaceae, Incertae Rhizobiales and Cryomorphaceae were associated with this dimension. Rhizobiaceae, Verrucomicrobiaceae, Planctomycetaceae, Simkaniaceae, Piscirickettsiaceae, and Caldilineaceae were families associated with RWS and included in Dim. 5 (P < 0.05, Supplementary Table 3).
Figure 2.
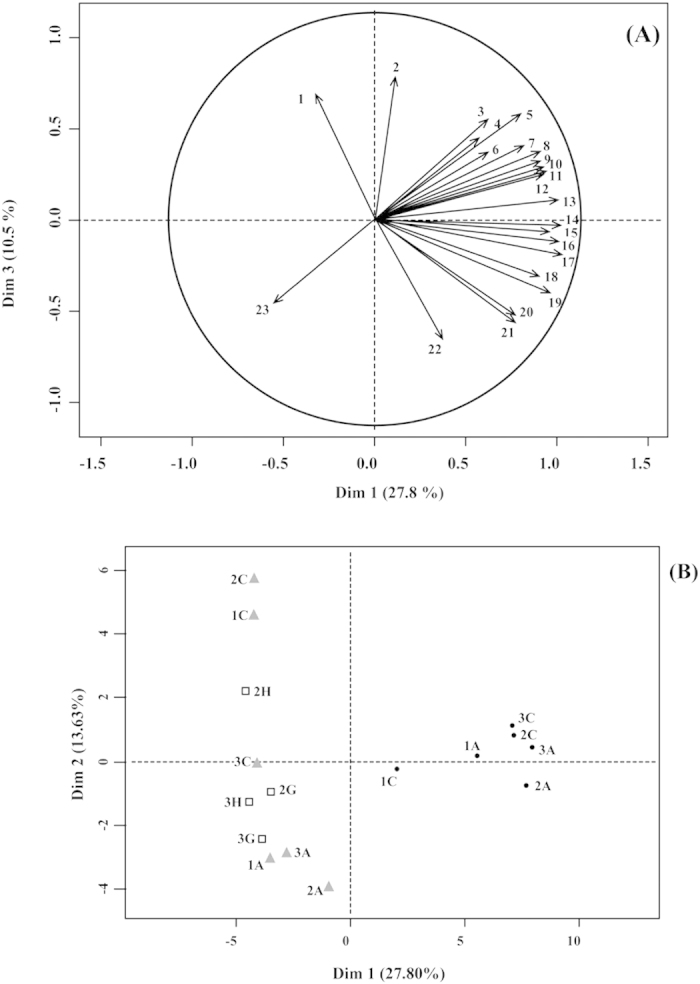
(A) Variations in the abundance of bacterial families in horticultural growing media. According to the correlation circle, the families belonging to the first component of the Multiple Factor Analysis (Dim. 1) are negatively correlated to the abundance of species belonging to Rhizobiaceae (23). Since there are more families in Dim 3., their contribution to the overall variance among samples is smaller. Dimension 3 described the families that were significantly correlated with RW (P < 0.05). 1, Propionibacterineae; 2, Pseudonocardineae; 3, Rhodobacteraceae; 4, Caedibacter; 5, Incertae Rhizobiales; 6, Unclassified Nitrospira; 7, Methylophilaceae; 8, Gaiellaceae; 9, Acetobacteraceae; 10, Actinobacteridae; 11, Xanthobacteraceae; 12, Hahellaceae; 13, Sinobacteraceae; 14, Desulfobacteraceae; 15, Hyphomicrobiaceae; 16, Opitutaceae; 17, Gemmatimonadaceae; 18, Methylocystaceae; 19, Sorangiineae; 20, Hyophomonadaceae; 21, Chromatiaceae; 22, Rhodocyclaceae; 23, Rhizobiaceae. (B) Multiple Factor Analysis map indicated that samples from organic growing medium (GB) displayed similar abundances across time points and differed from those in mineral medium (RW). Bacterial family abundances in samples of RW with hairy roots (RWS) were similar to those in RW. Symbols indicate the growing medium type: black circles for GB, grey triangles for RW and white squares for RWS. The number in the legend specifies the time point and the letter refers to the sample replicate. For instance, the circle labelled as “1A” refers to the replicate “A” of GB, collected at the first time point.
Tukey’s test for pairwise comparisons of group mean dispersions was performed using the vegan package in R. As demonstrated by the diversity and evenness measures (Supplementary Tables 1 and 2), the interaction between time and growing medium type was significant at the third time point (P < 0.05). Based on the relative abundances of the bacterial families and on the measures of alpha diversity and evenness, we validated the presence of distinctive and stable microbial communities associated with each growing medium (Tables 1 and 2 and Fig. 2B).
Physicochemical and biological environments are unique between the different growing media
Plant yield was determined at the end of the growing season for both the organic and mineral growing medium, and resulted in total accumulated yield (fresh weight) of 59.27 ± 1.52 kg.m−2 and 61.59 ± 0.86 kg.m−2 respectively. Calcium, magnesium, sulphate, nitrate-N, sodium and conductivity were higher in GB than Calcium, magnesium, sulphate, nitrate-N, sodium and conductivity were higher in GB than in RW (P < 0.05). in RW (P < 0.05), while ammonium-N, potassium, iron, and manganese were significantly higher in RW in comparison with GB (P < 0.05, Supplementary Table 4). Ammonium-N, pH, conductivity, potassium, sodium, iron and chloride were the highest in RWS (P < 0.05, Supplementary Table 5). Positive correlations between conductivity and nitrate-N were consistently detected in GB, while ammonium-N was associated with total CFU only at the third time point (P < 0.05, Table 3). In RW, pH was positively correlated with the Agrobacterium sp. CFU, while conductivity, ammonium-N, sulphates and sodium were negatively correlated with the total CFU. Only sulphates were positively correlated with sodium across time points (P < 0.05, Table 4). Positive correlations between calcium and Agrobacterium sp. and total CFU were found in RWS at all times (P < 0.05, Table 5). In contrast, humidity was negatively correlated with Agrobacterium sp. and total CFU when the hairy roots were first detected (P < 0.05). The goal of the MFA was twofold: to discriminate growing media based on the measured variables and to uncover the correlations among the physicochemical and biological characteristics within growing medium. In general, MFA showed that total bacteria, Agrobacterium sp. CFU, humidity, pH, sulphate and conductivity were the traits with highest contribution to the total variance among samples (Fig. 3A). Ammonium-N and Agrobacterium sp. CFU were the two variables with the highest correlation to Dimension 1 (P < 0.0001, Supplementary Table 6), which accounted for 29.8% of the variance. The square correlation ratios measure the degree of association between variables and a particular axis. Thus, the cos2 between the coordinates of the samples and growing medium type revealed that the above were the main characteristics describing the RWS medium on Dim. 1 (cos2 > 0.5). Dim. 2 (26.7% of the total variance) was constructed by the features of GB (sulphate, conductivity, sodium, magnesium, calcium and nitrate-N), while potassium, manganese, iron and humidity were included in Dim. 3 with RW (P < 0.05, 13.6% of the variance). We confirmed that each growing medium was characterised by a unique set of physicochemical and biological variables, which is preserved over time (Fig. 3B).
Table 3. Correlations (r) among physical and chemical characteristics in organic growing medium for cultivating eggplants (GB, n = 15).
| pH | Nitrate-N | P | Ca | Mg | Sulphate | Chloride | Fe | Mn | Total CFU | |
|---|---|---|---|---|---|---|---|---|---|---|
| Humidity | −0.944** (2)0.940** (3) | −0.952**(2) | ||||||||
| pH | −0.881** (3) | |||||||||
| Conductivity | 0.880** (1) 0.996** (2) 0.989** (3) | −0.876** (3) | 0.961** (2) 0.983** (3) | |||||||
| Nitrate-N | 0.978** (2) | −0.886** (3) | 0.967** (2) 0.950** (3) | |||||||
| Ammonium-N | −0.883** (1) | −0.897** (2) | −0.960** (2) | 0.883** (3) | ||||||
| P | −0.889** (1) | 0.961** (1) 0.975** (2) | ||||||||
| K | −0.953** (2) | −0.945** (2) | ||||||||
| Ca | 0.956** (3) | 0.893** (1) | ||||||||
| Mg | 0.920** (3) | −0.929** (1) | 0.883** (2) 0.967** (3) | 0.938** (2) | ||||||
| Na | 0.938** (3) | 0.954** (2) | ||||||||
| Chloride | 0.999*** (2) | 0.962** (3) | ||||||||
| Agrobacterium sp. CFU | 0.959** (3) |
In parenthesis, the time point when the correlation was observed. ***P < 0.0001, **P < 0.05.
Table 4. Correlations (r) among physical and chemical characteristics in mineral growing medium for cultivating eggplants (RW, n = 15).
| pH | Conductivity | Nitrate-N | Ammonium-N | P | K | Ca | Mg | Sulphate | Na | |
|---|---|---|---|---|---|---|---|---|---|---|
| Nitrate-N | 0.915** (1) | |||||||||
| P | 0.933** (3) | 0.894** (2) | 0.984** (2) | −0.954** (1) | ||||||
| K | 0.930** (3) | 0.921** (2) | 0.922** (3) | |||||||
| Ca | 0.894** (1) | 0.897** (2) | 0.951** (2) | −0.941** (1) | 0.998** (1) 0.894** (3) | |||||
| Mg | 0.939** (1) 0.902** (3) | 0.906** (2) | −0.912** (1) | 0.912** (1) 0.958** (3) | 0.907** (3) | 0.925** (1) 0.972** (2) | ||||
| Sulphate | 0.965** (2) | 0.967** (1) | 0.921** (2) | |||||||
| Na | 0.955** (2) | 0.961** (1) | 0.932** (2) | 0.934** (2) 0.887** (3) | 0.955** (2) | 0.996** (1) 0.906** (2) 0.941** (3) | ||||
| Fe | 0.882** (1) | 0.883** (3) | 0.906** (2) | 0.892** (2) | ||||||
| Mn | 0.906** (3) | 0.970** (2) | 0.922** (2) | −0.884** (2) | 0.950** (2) | 0.880** (2) | 0.955** (2) | 0.926** (2) | 0.874** (2) | 0.927** (2) |
| Agrobacterium sp. CFU | 0.891** (2) | −0.945** (1) | ||||||||
| Total CFU | −0.997** (1) | −0.906** (3) | −0.947** (1) | −0.942** (1) |
In parenthesis, the time point when the correlation was observed. ***P < 0.0001, **P < 0.05.
Table 5. Correlations (r) among physical and chemical characteristics in mineral growing medium for cultivating eggplants, with hairy roots syndrome (RWS, n = 10).
| Humidity | pH | Conductivity | Ammonium-N | P | Ca | Mg | Na | Agrobacterium sp. CFU | |
|---|---|---|---|---|---|---|---|---|---|
| Conductivity | 0.949** (3) | ||||||||
| Nitrogen-N | −0.907** (2) | ||||||||
| Ammonium-N | 0.876** (2) | ||||||||
| P | 0.964** (3) | 0.960** (3) | 0.995** (2) | ||||||
| K | 0.887** (3) | −0.899** (2) | 0.893** (2) | ||||||
| Ca | −0.952** (2) | 0.958** (2) | 0.953** (2) | ||||||
| Mg | −0.905** (2) | 0.953** (2) | 0.990** (2) | 0.998*** (2) | 0.934** (2) | ||||
| Sulphate | 0.913** (3) | 0.912** (2) | 0.926** (3) | 0.974** (3) | |||||
| Na | −0.918** (2) | 0.955** (2) | 0.993** (2) | 0.939** (2) | |||||
| Chloride | 0.901** (2) | ||||||||
| Fe | −0.907** (2) | −0.898** (2) | |||||||
| Agrobacterium sp. CFU | −0.883** (2) | 0.995** (2) | 0.953** (2) 0.963** (3) | 0.934** (2) | 0.939** (2) | ||||
| Total CFU | −0.907** (2) | 0.983** (2) | 0.991** (2) 0.915** (3) | 0.991** (2) | 0.994** (2) | 0.971** (2) |
In parenthesis, the time point when the correlation was observed. ***P < 0.0001, **P < 0.05.
Figure 3. Physical and chemical characteristics of the growing media are unique for each environment.
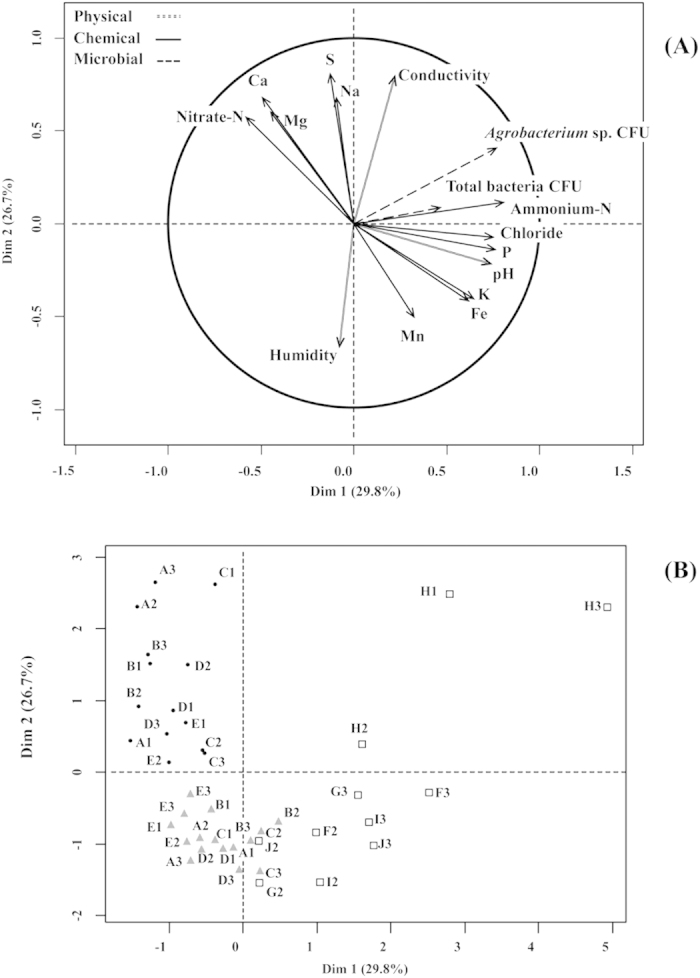
(A) Multiple Factor analysis of the physical and chemical characteristics of horticultural growing media. Correlation circle indicates the contribution of the variables driving the differences among growing media. Long vectors in the same direction indicate positive correlations among variables, whereas long vectors in the opposite direction indicate negative ones. (B) Multiple Factor Analysis highlighted the similarities among growing media samples over time, based on their physical and chemical features. Symbols indicate the growing medium type: black circles for GB, grey triangles for RW and white squares for RWS. The number in the legend specifies the time point and the letter refers to the sample replicate. For instance, the circle labelled as “1A” refers to the replicate “A” of GB, collected at the first time point.
Correlation of bacterial families with physicochemical and biological characteristics
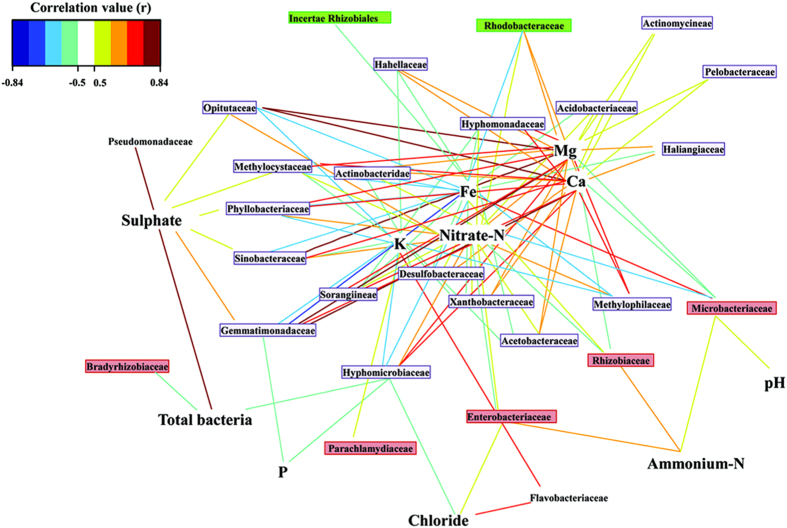
Instead of computing the Pearson correlation coefficients between each pair of variables, bipartite networks were inferred using a pair-wise similarity matrix obtained from the Regularised Canonical Correlation Analysis (Fig. 4). The values in the similarity matrix were computed as the correlation between the relative abundances of bacterial families and the characteristics of the growing medium projected onto the space spanned by the first components retained in the analysis. Three relevant components were obtained setting a threshold to 0.5. In this way, ammonium-N was correlated with Rhizobiaceae abundance. Families associated with RW were correlated with iron (P < 0.05), while potassium, magnesium, calcium and nitrates were associated with GB (P < 0.05). Correspondence Analysis (CA, Supplementary Fig. 2) was used to reveal the association between growing medium (rows) and physicochemical variables and relative abundances (columns). Chi-square statistic indicated strong link between growing medium and both physicochemical variables and relative abundances (P < 0.05). The coordinates of row/column variables represent the number of standard deviations the row/column variables are away from the barycentre17. Therefore, the highest coordinates in Dim.1 belonged to Agrobacterium sp. CFU, Solimonadaceae, Ammonia-N and P (rows) and RWS and time point 3 (columns), all of which explained 92.9% of the variance. Dim. 2 was driven by the relationships among the most abundant families detected in GB at time point 2. The observed interactions confirmed the results of previous correlation analyses (Tables 1 and 2).
Figure 4. Network graph based on the regularised canonical correlations between bacterial family abundance and physicochemical characteristics of growing medium.
Correlations (r) have been filtered for an absolute correlation above 0.5 and are coloured following the key shown. According to the graphing algorithm, stronger correlations are shorter lines, and families with similar abundances within growing medium tend to cluster closely. This representation reveals the relationship between clusters of families linked to the different physical and chemical characteristics of the environment, thus potentially uncovering growing medium-specific populations. In green, bacterial families correlated with RW; in red, bacterial families associated with RWS, in purple, families correlated with GB.
Estimation of the matric potential of the growing media
The water retention curve of the two growing media was determined. Saturated volumetric water content of RW (θs = 0.9834) agreed with previous reports of Wallach18, while the saturated volumetric water content of GB was θs = 0.935. The volumetric water content (θv = 85.3%) of RW corresponds to a matric potential of −0.6 kPa, whereas θv = 83.1% for RWS corresponds to a matric potential of −0.61 kPa. For the organic growing medium, θv = 81.93% represents a matric potential of −0.46 kPa.
Potential resistance to external forces differed between growing media
Seven RW samples (46.7% out of fifteen) were positive for A. rhizogenes biovar 2, whereas samples from GB were negative for any of the tested species of Agrobacterium sp. (Supplementary Table 7). Phytopathogenic Agrobacterium sp. strains harbour the genes required for T-DNA process and transfer in the virulence regions (virC) of the root inducing (pRi) plasmids19,20. Therefore, RW samples positive for A. rhizogenes biovar 2 and all RWS samples were screened for the presence of the virC pathogenicity gene, and all RWS tested positive. Plate counts on the selective medium confirmed the results of the multiplex PCR. Results of the colony PCR in randomly selected wild type isolates validated the presence of the virC gene and of A. rhizogenes biovar 2 in RWS (Supplementary Table 7). Differences in Agrobacterium. sp. CFU between growing media were influenced by time and were lower in RW when compared with RWS (P < 0.05, Supplementary Table 7).
Discussion
Based on the relative abundances of the families associated with each growing medium, we validated the presence of a competitive, distinctive and stable microbial community structure in the organic growing medium. Further, we identified humidity, potassium content, pH and conductivity as the main physicochemical characteristics driving microbial communities in the growing medium.
High-throughput sequencing combined with molecular techniques uncovered the structure of the growing medium-associated microbiota. GB harboured higher bacterial diversity than RW and RWS. Further, GB displayed similar abundances of bacterial families across time points, while both RW and RWS displayed larger variability. These differences could be associated with the different structure and composition of the two types of growing medium, which may provide unique niches for the microbial community21. The density and the biodiversity of the microbial community may be affected by the age of growing medium22. Biodiversity in soilless systems with mineral growing media is low at the start of a crop4, then it increases within weeks23, reaching stability after six weeks of plant growth24. As described in previous reports21, our uncultivated RW medium showed low amounts of nutrients and total bacteria CFU (<102 CFU g−1) in comparison with GB (2.2 × 107 CFU g−1). Quantification of viable cell counts (CFU) from the culturable aerobic microflora colonizing different parts of the system, such as root zone, nutrient solution, growing media and system devices (tubes, gutters, etc.), has been performed25. However, only 1 to 10% of the microflora may be recovered from techniques based on a plate culturing on R2A agar, providing limited information about the entire community present on each growing medium. For this reason, we complemented cultivation studies with molecular characterization of the microbial communities.
The increase in microbial biodiversity observed in the growing medium can be attributed to plant activity. Plants exude up to 21% of their photosynthetically fixed carbon into the root-soil interface26, feeding the microbial communities and influencing their activity and diversity14. Berendsen, et al.27 suggested that plant species can select bacteria through the production of specific root exudates and hence shape the microbiome of the plant. We used eggplants grafted on a tomato root stock known for its high exudation capacity (Solanum lycopersicum L. x Solanum habrochaites). The root exudation in both growing media was estimated to be similar, because cultivated eggplants showed comparable growth characteristics and yield. We found that even after six months, the microbial community in the mineral growing medium (both in RW and RWS) showed high variability across time points. Garbeva, et al.28 hypothesised that in a stable system, each microhabitat is occupied by organisms capable of colonizing niches. A diverse and stable ecosystem at the microhabitat level will resist environmental stresses29 and potentially, pathogen invasion. Mendes, et al.14 suggested that the relative abundance of several bacterial taxa may be an indicator of disease suppression. In this way, increased resistance to pathogen invasion may be related to the total microbial biomass in the growing medium, which competes with pathogens for resources or may cause inhibition through direct antagonism15. Mendes, et al.14 identified Actinobacteria, γ- and β-Proteobacteria (Pseudomonadaceae, Burkholderiaceae, Xanthomonadales) and Firmicutes (Lactobacillaceae) as the most dynamic taxa associated with disease suppression in natural soil. In our study, Rhodocyclaceae and Methylophilaceae (β-Proteobacteria), were correlated to GB, as well as other α-, β and γ-Proteobacteria, such as Hyphomicrobiaceae, Xanthobacteraceae, Phyllobacteriaceae, and Chromatiaceae. Actinobacteria such as Gaiellaceae and Conexibacteraceae were also positively correlated with GB. Furthermore, the abundance of Rhizobiaceae (such as Agrobacterium sp.) was negatively correlated to the abundances of Methylophilaceae and Saprospiraceae in GB. Thus, the relative abundance of several taxa and the stability of a microbial community may be related to the resistance against external invaders, supporting the theory of general suppression. Even though Agrobacterium sp. was detected in both growing media (on average 7.6 × 103 CFU/ml in GB, 2.4 × 104 in RW and 1.0 × 106 CFU/ml in RWS, across time points), samples from GB were negative for the presence of the virC pathogenicity gene. Neither the total CFU nor the presence of particular microbial taxa have been directly associated with resistance to Agrobacterium rhizogenes27.
The plant, as well as the complex biological, chemical and physical interactions in the growing medium influence the microbial communities of the rhizosphere. Previous reports identified pH as the main driver of microbial communities in soil30. Soil moisture is often associated with pH and may have impacted the community composition among GB, RW and RWS. Moreover, GB showed higher relative abundances of Actinobacteridae and α-Proteobacteria, which have been associated with soil pH30. The mobility of rhizobacteria may be limited by the humidity in the growing medium, where water-filled pores may be as large as the bacterial cells31. Although Agrobacterium sp. was detected in GB and RW, the hairy roots were not visually present in GB. Our results indicate that differences in pore size and water distribution between GB and RW may have impacted the mobility of Agrobacterium sp., resulting in decreased disease incidence. High yields of hairy roots indicating A. rhizogenes invasion have been observed when the nitrate-ammonium (NO3−-N/NH4+) ratio was close to 5 with 115 mg NH4+-N/l of soil and 553 mg NO3-−N/l of soil32. In our test, the NO3−-N/NH4+-N ratio was 2.3 for RWS, 31.9 for RW and 147.3 for GB. The low ammonia concentration and the low pH in the GB medium may explain the absence of hairy roots33 and potentially shaped the microbial community composition.
Our methodology provided a comprehensive insight into the complex bacterial interactions in horticultural media, supporting our hypothesis that there are fundamental differences between the bacterial communities associated with each type of horticultural growing medium. Diverse and competitive microbial communities may provide different and unique functionalities. As a consequence, the bacterial community inhabiting the GB medium may have provided functional diversity and temporal stability and resilience to this heterogeneous and fluctuating environment. Ultimately, the interactions in the resident community may also play a role in the resistance to external forces, such as invasive species competing in conventional soilless culture systems. Future alternative control strategies may involve the evaluation of the suppressiveness of microbial groups and the transfer of suppressiveness to conducive soils with 0.1–10% suppressive soil15. The described relationships will also contribute to the understanding of the functional microbial ecology associated with the growing media and the interaction between microbial community and plants. Knowledge regarding these relationships could potentially be used to develop sustainable strategies to increase plant productivity and quality.
Methods
Experimental setting and growing media
The microbial community associated with the different growing media was monitored in a commercial 8.5 ha greenhouse in The Netherlands (51°59’ Latitude and 4°10′ Longitude), cultivating the eggplant Solanum melongena cultivar Jaylo (Rijk Zwaan, The Netherlands), grafted on Solanum lycopersicum L. x Solanum habrochaites Beaufort (De Ruiter, The Netherlands). The two different horticultural growing media were installed at the same time in the greenhouse and the 48 day-old eggplants were planted on top of the two different growing media on the same day. The organic growing medium (GB, Grow Bag, Peltracom, Belgium) was a mixture of white peat (H2-H4 on the von Post scale34 [80% v/v] and coconut fibre [20% v/v]). Slabs of GB and mineral medium (RW, Rock wool, Grotop expert, Grodan, The Netherlands) had the following dimensions: 1.0 m × 0.2 m × 0.085 m and 1.0 m × 0.2 m × 0.075 m, respectively. Both growing media were subjected to identical water and fertilizer treatments during the cultivation period according to standard methods, with standard fertigation solution21. Two eggplants per slab were planted. Each plant was trained to 3 stems, aiming at a plant density of 1.7 plants/m2 resulting in 5.1 stems/m2.
Sample collection
The greenhouse was divided into several blocks each consisting of 6 rows in a randomized block design. Two contiguous blocks were randomly selected and each block contained either RW or GB medium. The two outer rows of each block were not selected, because of possible interactions with the adjacent rows. The eggplants were growing in slabs placed consecutively with an interspacing of 44 cm. One slab was considered an experimental unit. Five slabs from each block were randomly selected from the 4 inner rows and from the two different growing media (GB and RW). Samples of the different experimental units were collected at three time points during the growing season (June, July and August) and at the start of the experiment. Ten subsamples from each experimental unit were collected, pooled, homogenized and treated as a single sample (Supplementary Fig. 3). At each time point, samples were taken from 5 fixed experimental units of each RW and GB, including root material. Each sample of 200 g was divided into 4 homogenous subsamples of 50 g for further analysis: two subsamples (subsample 1 and 2) were used for chemical analyses, one subsample (subsample 3) was stored at 4 °C and used for isolation and identification of Agrobacterium sp. and total CFU, as well as humidity determination, and subsample 4 was immediately stored on dry ice, preserved at −80 °C and used for molecular microbial community analysis. The grower reported previous presence of the hairy roots syndrome, which is caused by the pathogen Agrobacterium rhizogenes. Hence, disease incidence of the hairy roots syndrome was followed up by a monthly visual inspection of the glasshouse. The hairy roots syndrome was detected in one RW slab at the first time point in June. Further visual inspection during July and August revealed increased incidence of the hairy roots syndrome in RW medium. Additional samples of RW from 5 additional slabs showing visual symptoms of the hairy roots syndrome were taken (named RWS). However, no hairy roots were visually identified in the GB throughout the whole experimental period (December 2012 and November 2013).
Physicochemical analysis of the growing medium
The physicochemical characteristics of the different growing media were determined at the start (December 2012) and during the growing season (June, July and August 2013). The chemical analysis was performed as described by Gabriels, et al.35. The humidity (w/w-%) of the growing media was determined according to Verdonck and Gabriels36.
Determination of the hydraulic properties of the growing medium
The soil water retention curve of the RW and the GB media was established using the sand box apparatus37 for pressure potentials between −1 and −10 kPa. For this experiment, 10 replicates of the slab samples were used. The parameters of the van Genuchten equation were estimated and data was fitted38.
Isolation, identification and determination of the Agrobacterium sp. and total cell count
Growing medium was analysed within 48 hours of sample collection. Five grams of the fresh growing medium were mixed with 45 ml of 0.85% NaCl39 and homogenized for 2 minutes, using a Stomacher80 blender (Stomacher, Seward, Worthing, UK). This suspension was used for the determination of the total cell and Agrobacterium sp. count on each medium. For the total cell count, the suspension was plated on R2A agar (Sigma Aldrich, Diegem, Belgium) with cycloheximide (200 mg/l). Agrobacteria colonies were selected and identified following Shams, et al.40. A. rhizogenes was isolated using 2E-Te containing erythritol and 320 mg/l K2TeO3 with cycloheximide. After 5 days of incubation at 28 °C, colony forming units (CFU) were counted for both R2A and 2E-TE medium. The calculation of the CFU was following the procedures outlined by Sutton41, where the detection limit was equal to 1 CFU at the lowest dilution.
DNA extraction
Total DNA was extracted using physical disruption with the bead beating method from Hernandez-Sanabria, et al.42. Cells were lysed in a FastPrep-96 homogeniser (MP Biomedicals, Illkirch, France) and DNA was precipitated with cold ethanol and resuspended in 30 μl of TE buffer (10 mM Tris-HCl, 1 mM EDTA [pH 8.0]). Concentration and quality of DNA were measured based on the absorbance at 260 and 280 nm in a Nanodrop ND 1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA).
Identification of Agrobacterium sp. at strain/biovar level
The potential presence of pathogenic Agrobacterium sp. strains was analysed by multiplex PCR, targeting the 23 S rRNA gene43. Universal forward primer UF and four reverse primers specific for A. tumefaciens (biovar 1), A. rhizogenes (biovar 2), A. vitis, and A. rubi, were used. Conditions of the PCR were described elsewhere43; the primer pairs UF/B1R,UF/B2R, UF/AvR and UF/ArR were employed to amplify fragments of 184, 1066, 478, and 1006 bp length, respectively44. Pathogenic plasmid detection revealed the presence of the virC pathogenicity gene located on the rhizogenic (Ri) plasmid45; PCR conditions for detection of the virC gene followed Kuzmanović, et al.44. Additional confirmation was performed in randomly selected wild type isolates; colony PCR was applied using the protocol described above.
Community PCR-DGGE analysis
PCR amplifications of the V3 region (~200 bp) of the 16 S rRNA gene of bacteria were performed with universal bacterial primers as described by Øvreås, et al.46. PCR products were purified prior to fingerprinting analysis and DGGE was run on 1 × TAE buffer (AppliChem, Darmstadt, Germany) with a 6% polyacrylamide gel with a 30 to 50% linear denaturing gradient, using the Bio-Rad DCode universal mutation detection system (BioRad, Hercules, CA, USA). Running conditions and analysis using BioNumerics software, version 5.1 (Applied Maths, Sint-Martens Latem, Belgium) were reported by Hernandez-Sanabria, et al.42. New band categories including all the detected bacterial phylotypes on the growing media were created. Frequency of phylotypes exclusively present in samples with hairy roots was determined adapting the methodology of Hernandez-Sanabria, et al.47, for performing Fisher Exact test in R48.
Illumina library generation
The V5-V6 region of the 16 S rRNA gene was amplified using reported primers49. Libraries were prepared by pooling equimolar ratios of amplicons (200 ng of each sample), tagged with a unique barcode50. Resulting libraries were sequenced on a MiSeq (Illumina, Hayward, CA,USA) paired and joined, but only forward reads were selected for the final analysis (140 nt). A quality filter program that runs a sliding window of 10% of the read length, and calculates the local average score based on the Phred quality score of the FASTQ file, was used to trim the 3’-ends of the reads that fell below a quality score of 10. Reads with an N character in their sequence, mismatches within the primers and barcodes or more than 8 homopolymers stretches were discarded. Following primer sequences trimming, sequences were separated based on their barcodes. Number of representative phylotypes were generated using the Uclust algorithm on USEARCH51 by clustering at 97% similarity (1 mismatch), with a confidence level of at least 80, with Cyanobacteria, Eukaryota, and Archaea lineages removed. Filtered database contained only phylotypes present in at least a) one sample at an abundance higher than 1%, b) in 2% of samples at a relative abundance above 0.1%, and 3) in 5% of the samples at any abundance level50. Hence, a total of 475995 reads were obtained. Sequence composition of the dataset was compared using the RDP Classifier tool52 and SILVA database53. After examining read counts, data were randomly rarefied to a chosen maximum depth of 17135 sequences, using the phyloseq package from R54 and rarefaction curves were plotted using the vegan package in R55. Relative abundances of the top twelve taxa, with their deepest possible RDP classification up to the family level were determined and plotted as bar charts56. If any OTU was not classified up to a family level, the consensus sequence was blasted using the NCBI database and taxonomic classification was obtained. Within each sample, total number of species, Fisher’s diversity, Shannon, Simpson and inverse Simpson indices were calculated to assess the alpha diversity. Pielou’s index was used as indicator of evenness in the community. Differences in alpha diversity and evenness measures among horticultural growing medium were compared using a repeated measures mixed model in SAS (version 9.3, SAS Institute, Cary, USA), with growing medium type as a fixed effect and comparing multiple means using Tukey test. Hence, the differences in the diversity measures could be attributed to either time point, growing medium type or to the interaction of time*growing medium type. Chao and Bray-Curtis indices were used to construct dissimilarity matrices of the communities. Therefore, beta diversity of the community was determined, and nMDS was employed to visualise the differences among samples, using the vegan package in R55. Stratified permutational multivariate analysis of variance (PERMANOVA) with 999 permutations was conducted to explore the percentage of variance that could be explained by the differences in beta diversity. ANOVA was applied to uncover whether one of the growing media was more variable than the other55. Differences in relative abundances of bacterial families were compared using a repeated measures mixed model in SAS, with the lsmeans adjustment and Bonferroni correction for multiple comparisons.
Multivariate statistical analysis
Differences in physicochemical characteristics of each horticultural growing medium were compared using a mixed model in SAS. Pearson correlations were used to determine the interactions between the physicochemical characteristics and significance was assumed at P < 0.05. Sixteen variables were included in the analysis (humidity, pH, conductivity, nitrate-N, ammonium-N, phosphorus, potassium, calcium, magnesium, sulphate, sodium, chloride, iron, manganese, CFU of A. rhizogenes sp. and total bacteria). Multiple Factor Analysis (MFA) was employed to detect how the relative abundances of families contributed to the differences between growing media across time points. In addition, MFA was applied to the whole set of variables to assess the correlations among the physical, chemical and microbiological variables detected in both types of growing medium. Each group of variables was weighted and results were explained in a factor map57, where the value of the abundance of each bacterial family (vector) for the corresponding growing medium (factor) was plotted. The function MFA from the FactoMineR package58 was performed in R. Bipartite networks were inferred using a pair-wise similarity matrix obtained from the Regularised Canonical Correlation Analysis59,60. The values in the similarity matrix were computed as the correlation between the relative abundances of bacterial families and the growing medium characteristics projected onto the space spanned by the first components retained in the analysis. Three relevant components were obtained setting a threshold of r ≥ 0.5 and families were disseminated in the plot, in close relation with the variables correlated and with the growing medium where they were more abundant. An additional ordination procedure, Correspondence Analysis (CA), was employed to confirm the relationships among specific bacterial families and the assessed physical and chemical characteristics47.
Additional Information
How to cite this article: Grunert, O. et al. Mineral and organic growing media have distinct community structure, stability and functionality in soilless culture systems. Sci. Rep. 6, 18837; doi: 10.1038/srep18837 (2016).
Supplementary Material
Acknowledgments
This work was supported by the project grant IWT Baekeland mandate 120200 and by the Research Foundation of Flanders (Fonds Wetenschappelijk Onderzoek-Vlaanderen, FWO). The authors acknowledge Guillaume Blanchet and Joris Meys for their statistical support. We thank Iris Plumeier, Silke Kahl for their technical assistance, Tim Lacoere for the artwork in Supplementary Fig. 3, and Frederiek-Maarten Kerckhof, Nicole Hahn, Ruben Props, Amanda K. Luther and Stephen J. Andersen for their advice.
Footnotes
The authors declare no competing financial interests.
Author Contributions Conceived and designed the experiments: O.G., E.H.-S., M.C.V.L., D.R. and N.B. Performed the experiments: O.G., E.H.-S. Illumina libraries processing: R.J. and R.V.-V. Data mining, statistical analysis, results interpretation, figure and table preparation: E.H.-S. and O.G. Contributed reagents/materials/analysis tools: N.B., M.P. and D.H.P. Wrote the paper: O.G. and E.H.-S.
References
- Peet M. & Welles G. Greenhouse tomato production. Crop production science in horticulture 13, 257 (2005). [Google Scholar]
- Raviv M., Lieth J. & Raviv M. Significance of soilless culture in agriculture. Soilless culture theory and practice. Elsevier, Burlington, MA (2008). [Google Scholar]
- Runia W. T. A review of possibilities for disinfection of recirculation water from soilless cultures. In IV International Symposium on Soil and Substrate Infestation and Disinfestation 382. 221–229, http://dx.doi.org/10.17660/ActaHortic.1995.382.25 [Google Scholar]
- Postma J. In Recent Developments in Management of Plant Diseases 133–146 (Springer, 2009). [Google Scholar]
- Islam S. Evaluating performance of ecologically sound organic substrates under different temperature regimes. International Journal of Agriculture and Biology 10, 297–300 (2008). [Google Scholar]
- Pluimers J. An environmental systems analysis of greenhouse horticulture in the Netherlands: the tomato case. (Wageningen Universiteit, 2001). [Google Scholar]
- Altmann M. Socio-economic impact of the peat and growing media industry on horticulture in the EU. Brussel, Epagma (2008). [Google Scholar]
- Grunert O., Perneel M. & Vandaele S. Peat-based organic growbags as a solution to the mineral wool waste problem. Mires and Peat 3, 1–5 (2008). [Google Scholar]
- Yachi S. & Loreau M. Biodiversity and ecosystem productivity in a fluctuating environment: the insurance hypothesis. Proc. Natl. Acad. Sci. 96, 1463–1468 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balvanera P. et al. Quantifying the evidence for biodiversity effects on ecosystem functioning and services. Ecology letters 9, 1146–1156 (2006). [DOI] [PubMed] [Google Scholar]
- McCann K. S. The diversity–stability debate. Nature 405, 228–233 (2000). [DOI] [PubMed] [Google Scholar]
- Wittebolle L. Diversity, stability and functionality of bacterial communities in the nitrogen cycle. Doctoral thesis. Ghent University (2009).
- Raaijmakers J. M. In Principles of Plant-Microbe Interactions 411–417 (Springer, 2015). [Google Scholar]
- Mendes R. et al. Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 332, 1097–1100 (2011). [DOI] [PubMed] [Google Scholar]
- Weller D. M., Raaijmakers J. M., Gardener B. B. M. & Thomashow L. S. Microbial populations responsible for specific soil suppressiveness to plant pathogens 1. Annual Review of Phytopathology 40, 309–348 (2002). [DOI] [PubMed] [Google Scholar]
- Van Calenberge B. W. L., Moerkens R., Van Kerckhove S. & Hanssen I. Gekke wortels in Vlaanderen: Stand van zaken. In Proeftuinnieuws. 3 (Flanders, 2013). [Google Scholar]
- Bendixen M. A practical guide to the use of correspondence analysis in marketing research. Marketing Research On-Line 1, 16–36 (1996). [Google Scholar]
- Wallach R. Physical characteristics of soilless media in Soilless culture: theory and practice. Vol. 3, 41–116. Elsevier, London (2008). [Google Scholar]
- Sawada H., Ieki H. & Matsuda I. PCR detection of Ti and Ri plasmids from phytopathogenic Agrobacterium strains. Appl. Environ. Microb. 61, 828–831 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J. et al. The bases of crown gall tumorigenesis. J. Bacteriol. 182, 3885–3895 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonneveld C. & Voogt W. Plant nutrition of greenhouse crops. Vol. 1 (Springer, 2009). [Google Scholar]
- Vallance J. et al. Pathogenic and beneficial microorganisms in soilless cultures. Agronomy for Sustainable Development, Springer Verlag (Germany) 31(1), 191–203 (2011). [Google Scholar]
- Postma J., Willemsen-de Klein M. J. & van Elsas J. D. Effect of the indigenous microflora on the development of root and crown rot caused by Pythium aphanidermatum in cucumber grown on rockwool. Phytopathology 90, 125–133 (2000). [DOI] [PubMed] [Google Scholar]
- Calvo‐Bado L. A. et al. Microbial community responses associated with the development of oomycete plant pathogens on tomato roots in soilless growing systems. J. Appl. Microbiol. 100, 1194–1207 (2006). [DOI] [PubMed] [Google Scholar]
- Strayer R. Dynamics of microorganism populations in recirculating nutrient solutions. Advances in Space Research 14, 357–366 (1994). [DOI] [PubMed] [Google Scholar]
- Marschner H. & Marschner P. Marschner’s mineral nutrition of higher plants. Vol. 89 (Academic press, 2012). [Google Scholar]
- Berendsen R. L., Pieterse C. M. & Bakker P. A. The rhizosphere microbiome and plant health. Trends in plant science 17, 478–486 (2012). [DOI] [PubMed] [Google Scholar]
- Garbeva P., Van Veen J. & Van Elsas J. Microbial diversity in soil: selection of microbial populations by plant and soil type and implications for disease suppressiveness. Annu. Rev. Phytopathol. 42, 243–270 (2004). [DOI] [PubMed] [Google Scholar]
- Loreau M. et al. Biodiversity and ecosystem functioning: current knowledge and future challenges. Science 294, 804–808 (2001). [DOI] [PubMed] [Google Scholar]
- Lauber C. L., Hamady M., Knight R. & Fierer N. Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Appl. Environ. Microb. 75, 5111–5120 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parke J. L. Root colonization by indigenous and introduced microorganisms. In The rhizosphere and plant growth (pp. 33–42). Springer: Netherlands, (1991). [Google Scholar]
- Bensaddek L., Gillet F., Saucedo J. E. N. & Fliniaux M.-A. The effect of nitrate and ammonium concentrations on growth and alkaloid accumulation of Atropa belladonna hairy roots. J. biotechnol. 85. 35–40 (2001). [DOI] [PubMed] [Google Scholar]
- Sevón N., Hiltunen R. & Oksman-Caldentey K. Chitosan increases hyoscyamine content in hairy root cultures of Hyoscyamus muticus. Pharm. Pharmacol. Lett. 2, 96–99 (1992). [Google Scholar]
- von Post L. & Granlund. E. “Soedra Sveriges torvtillgangar. 1. Arsb. 19, Ser. C. no. 335.” Geol. Surv. Sweden, Stockholm (1926). [Google Scholar]
- Gabriels D., Hartmann R., Verplancke H., Cornelis W. & Verschoore P. Werkwijzen voor grondanalyses. Universiteit Gent, Gent, Belgium (1998). [Google Scholar]
- Verdonck O. & Gabriels R. Reference method for the determination of physical properties of plant substrates. II. Reference method for the determination of chemical properties of plant substrates. Acta Hort. 302, 169–179 (1992). [Google Scholar]
- DIN, E. 13041. Soil Improvers and growing media–Determination of physical properties–Dry bulk density, air volume, water volume, shrinkage value and total pore space. German Version prEN, Beuth, Berlin/Cologne (2012). [Google Scholar]
- De Swaef T., Verbist K., Cornelis W. & Steppe K. Tomato sap flow, stem and fruit growth in relation to water availability in rockwool growing medium. Plant Soil 350, 237–252 (2012). [Google Scholar]
- Opelt K. & Berg G. Diversity and antagonistic potential of bacteria associated with bryophytes from nutrient-poor habitats of the Baltic Sea Coast. Appl. Environ. Microb. 70, 6569–6579 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shams M., Lavire C., Muller D., Vial L., Campillo T. & Nesme X. Rapid and efficient methods to isolate, type strains and determine species of Agrobacterium spp. in pure culture and complex environments. INTECH Open Access Publisher (2012). [Google Scholar]
- Sutton S. Accuracy of Plate Counts. Journal of validation technology 17, 42–46. (2011). [Google Scholar]
- Hernandez-Sanabria E., Goonewardene L. A., Wang Z., Durunna O. N. & Moore S. S. Impact of feed efficiency and diet on adaptive variations in the bacterial community in the rumen fluid of cattle. Appl. Environ. Microb. 78, 1203–1214 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puławska J., Willems A. & Sobiczewski P. Rapid and specific identification of four Agrobacterium species and biovars using multiplex PCR. Syst. Appl. Microbiol. 29, 470–479 (2006). [DOI] [PubMed] [Google Scholar]
- Kuzmanović N., Gašić K., Ivanović M., Prokić A. & Obradović A. Identification of Agrobacterium vitis as a causal agent of grapevine crown gall in Serbia. Arch. Biol. Sci. 64, 1487–1494 (2012). [DOI] [PubMed] [Google Scholar]
- Suzaki K., Yoshida K. & Sawada H. Detection of tumorigenic Agrobacterium strains from infected apple saplings by colony PCR with improved PCR primers. J. Gen. Plant Pathol. 70, 342–347 (2004). [Google Scholar]
- Øvreås L., Forney L., Daae F. L. & Torsvik V. Distribution of bacterioplankton in meromictic Lake Saelenvannet, as determined by denaturing gradient gel electrophoresis of PCR-amplified gene fragments coding for 16S rRNA. Appl. Environ. Microb. 63, 3367–3373 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Sanabria E., Goonewardene L. A., Wang Z., Zhou M. & Moore S. S. Influence of sire breed on the interplay among rumen microbial populations inhabiting the rumen liquid of the progeny in beef cattle. PloS One 8, e58461 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2013). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/
- Bohorquez L. C. et al. In-depth characterization via complementing culture-independent approaches of the microbial community in an acidic hot spring of the Colombian Andes. Microb. Ecol. 63, 103–115 (2012). [DOI] [PubMed] [Google Scholar]
- Camarinha‐Silva A. et al. Comparing the anterior nare bacterial community of two discrete human populations using Illumina amplicon sequencing. Environ. Microbiol. 16(9), 2939–2952 (2014). [DOI] [PubMed] [Google Scholar]
- Edgar R. C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26, 2460–2461 (2010). [DOI] [PubMed] [Google Scholar]
- Wang Q., Garrity G. M., Tiedje J. M. & Cole J. R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microb. 73, 5261–5267 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruesse E. et al. SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 35, 7188–7196 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurdie P. J. & Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PloS One 8, e61217 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksanen J., Kindt R., Legendre P., O’Hara B. & Simpson G. L. The vegan package. Community ecology package, 631–637 (2007). [Google Scholar]
- Kerckhof F.-M. et al. Optimized Cryopreservation of Mixed Microbial Communities for Conserved Functionality and Diversity. PloS One 9, e99517 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Tayrac M., Lê S., Aubry M., Mosser J. & Husson F. Simultaneous analysis of distinct Omics data sets with integration of biological knowledge: Multiple Factor Analysis approach. BMC genomics 10, 32 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê S., Josse J. & Husson F. FactoMineR: an R package for multivariate analysis. J. Stat. Softw. 25, 1–18 (2008). [Google Scholar]
- Lê Cao K.-A., González I. & Déjean S. integrOmics: an R package to unravel relationships between two omics datasets. Bioinformatics 25, 2855–2856 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- González I., Lê Cao K.-A., Davis M. J. & Déjean S. Visualising associations between paired ‘omics’ data sets. BioData mining 5, 1–23 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.