Zds1/Zds2–PP2ACdc55 forms a complex with Rho1 GTPase and specifies Rho1 signaling outcome by regulating Rho1 GAPs in budding yeast.
Abstract
Budding yeast Rho1 guanosine triphosphatase (GTPase) plays an essential role in polarized cell growth by regulating cell wall glucan synthesis and actin organization. Upon cell wall damage, Rho1 blocks polarized cell growth and repairs the wounds by activating the cell wall integrity (CWI) Pkc1–mitogen-activated protein kinase (MAPK) pathway. A fundamental question is how active Rho1 promotes distinct signaling outputs under different conditions. Here we identified the Zds1/Zds2–protein phosphatase 2ACdc55 (PP2ACdc55) complex as a novel Rho1 effector that regulates Rho1 signaling specificity. Zds1/Zds2–PP2ACdc55 promotes polarized growth and cell wall synthesis by inhibiting Rho1 GTPase-activating protein (GAP) Lrg1 but inhibits CWI pathway by stabilizing another Rho1 GAP, Sac7, suggesting that active Rho1 is biased toward cell growth over stress response. Conversely, upon cell wall damage, Pkc1–Mpk1 activity inhibits cortical PP2ACdc55, ensuring that Rho1 preferentially activates the CWI pathway for cell wall repair. We propose that PP2ACdc55 specifies Rho1 signaling output and that reciprocal antagonism between Rho1–PP2ACdc55 and Rho1–Pkc1 explains how only one signaling pathway is robustly activated at a time.
Introduction
Budding yeast Rho1 GTPase is an essential regulator of both cell growth and stress response. Active Rho1 serves as an essential regulatory subunit of the β-1,3-glucan synthase complex that has two redundant catalytic subunits, Fks1 and Fks2 (Fig. 1 A; Drgonová et al., 1996; Mazur and Baginsky, 1996; Qadota et al., 1996). In addition, Rho1 regulates the formin Bni1 (Kohno et al., 1996; Tolliday et al., 2002; Dong et al., 2003) and the exocyst subunit Sec3 (Guo et al., 2001), both of which are essential for polarized cell growth and morphogenesis. Rho1 is also essential for the cell wall integrity (CWI) stress response pathway, where it binds and activates Pkc1 (Nonaka et al., 1995; Kamada et al., 1996; Schmitz et al., 2002). Pkc1 terminates polarized cell growth in part by inducing proteasomal degradation of both Bni1 and Sec3 (Kono et al., 2012). Pkc1 also activates a MAPK cascade that leads to the activation of Mpk1/Slt2 to control the transcription of genes important for stress resistance (Fig. 1 A; Lee and Levin, 1992; Irie et al., 1993; Lee et al., 1993; Martín et al., 1993; Kamada et al., 1995; Nonaka et al., 1995; Schmitz et al., 2002).
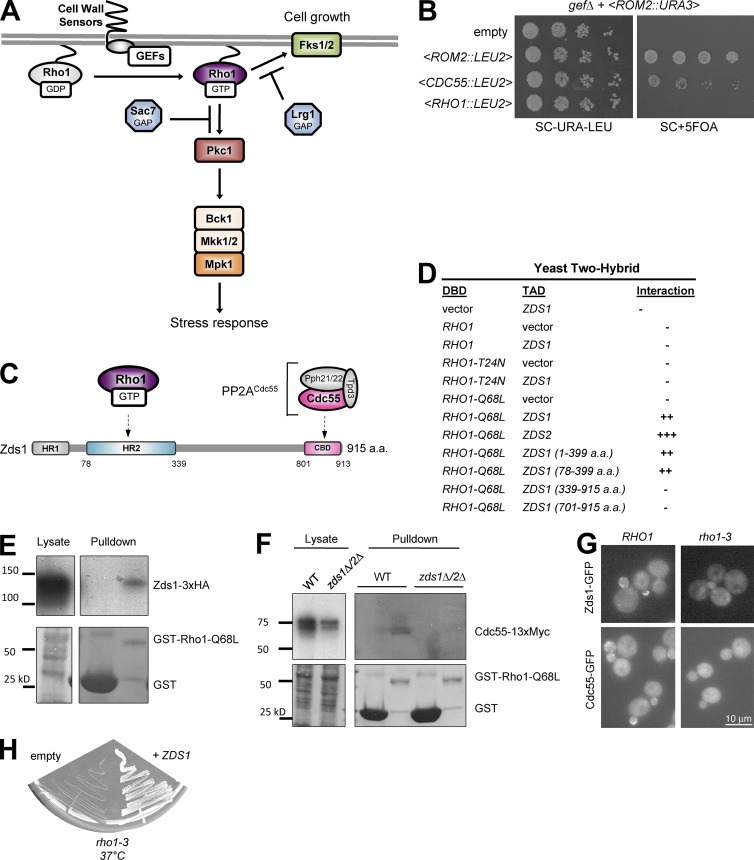
Figure 1.
PP2ACdc55 is a novel Rho1 effector. (A) Model of yeast Rho1 signaling pathways. Activated Rho1 activates either Fks1 for cell growth or Pkc1 for stress response. (B) Overexpression of ROM2 and CDC55 but not wild-type RHO1 from LEU2 harboring plasmids rescued the lethality of gefΔ strain after counterselection of ROM2-URA3 plasmids by 5-FOA. (C) Domain structure of Zds1 and Zds2. Highly conserved region among fungal species (HR1 and HR2) and Cdc55-binding domain (CBD) are boxed. (D) Yeast two-hybrid assay mapped the Rho1 binding site of Zds1 to amino acids 78–339. Strength of the interaction was judged by LacZ assay. DBD, DNA-binding domain; TAD, transcriptional activation domain. (E) In a pull-down assay, Zds1-3xHA can bind to purified GST-Rho1-Q68L. (F) Cdc55-myc physically associates with Rho1-Q68L, and this interaction requires Zds1/Zds2. WT, wild type. (G) The cortical localization of Zds1-GFP and Cdc55-GFP depends on Rho1. Wild-type (RHO1) and rho1-3 mutant cells expressing Zds1-GFP or Cdc55-GFP were incubated at 37°C for 2 h, and GFP signal was imaged. (H) The growth defect of rho1-3 was suppressed by overexpression of ZDS1. Photo was taken after incubation for 2 d at 37°C.
The spatiotemporal regulation of Rho GTPase signaling is governed by the tight control of key regulators that directly affect the GTPase activation cycle, such as guanine nucleotide-exchange factors (GEFs) and GTPase-activating proteins (GAPs). Compared with GEFs, regulation of Rho1 GAP activity is not well understood. There are two major Rho1 GAPs: Lrg1 and Sac7 (Lorberg et al., 2001; Watanabe et al., 2001). Rho1 GAPs have selectivity in downregulating specific Rho1 targets. Specifically, lrg1Δ cells have increased glucan synthesis without affecting Pkc1–Mpk1 activity (Lorberg et al., 2001; Watanabe et al., 2001), and conversely, sac7Δ cells have hyperactivation of Pkc1–Mpk1 without affecting glucan synthesis (Martín et al., 2000; Watanabe et al., 2001; Schmidt et al., 2002). Thus, Lrg1 selectively antagonizes the Rho1–Fks1/Fks2 glucan synthase complex, whereas Sac7 preferentially antagonizes the Rho1–Pkc1 stress response pathway.
In a genetic screen, we identified the regulatory B-subunit of the protein phosphatase 2A (PP2A), Cdc55, and its cortical anchoring proteins Zds1/Zds2 as novel regulators of Rho1 signaling. We show that PP2ACdc55 controls the outcome of Rho1 activation without affecting global Rho1 activity. Cortical Zds1/Zds2–PP2ACdc55 promotes cell wall glucan synthesis through inhibition of the Rho1 GAP, Lrg1. The cortical localization of Zds1/Zds2 and Cdc55 depend on Rho1 activity, which suggests a positive feedback loop that maintains cell wall synthesis throughout bud growth. We also show that Rho1 activation of the Pkc1-Mpk1 cascade results in the loss of cortical Cdc55 and Zds1. Collectively, these data suggest that Rho1 activation of polarized cell growth or stress response signaling is maintained by a mutual antagonism between the Rho1–PP2ACdc55 and Rho1–Pkc1 pathways.
Results and discussion
Cdc55 and Zds1/Zds2 are novel Rho1 effectors
To identify novel regulators of Rho1 signaling, we performed a multicopy suppressor screen using a yeast genomic DNA library for suppressors of the lethality of a yeast strain lacking all three Rho1 GEFs, ROM1, ROM2, and TUS1 (gefΔ; Yoshida et al., 2009). In this screen, we identified CDC55 (Fig. 1 B). CDC55 encodes the regulatory B-subunit of PP2A, which has multiple roles in cell cycle progression and polarized growth (Jiang, 2006). Here, we focused our analysis on Cdc55, because we had previously identified the Cdc55 binding proteins, Zds1 and Zds2, as multicopy suppressors of the temperature-sensitive growth and glucan synthesis defects of the rho1-2 mutant (Sekiya-Kawasaki et al., 2002).
Because PP2ACdc55 and the Cdc55 binding domain of Zds1/Zds2 form a substoichiometric complex in vivo (Wicky et al., 2011), and because Zds1/Zds2 are reported to show two-hybrid interaction with Rho1-GTP (Fig. 1 C; Drees et al., 2001), we first examined if active Rho1, Cdc55, and Zds1/Zds2 could form a complex. We confirmed by two-hybrid assay that both Zds1 and Zds2 specifically interacted with the GTP-locked RHO1-Q68L mutant, but not with type RHO1 or with the nucleotide-free RHO1-T24N mutant (Fig. 1 D). We also found that the highly conserved homology region (HR2) domain (78–339 aa) of Zds1 was sufficient for two-hybrid interaction with RHO1-Q68L (Fig. 1 D). In a GST–pull-down assay, we confirmed the interaction of GAL1-expressed HA-tagged Zds1 in yeast extract with purified GST-Rho1-Q68L (Fig. 1 E). Furthermore, we were able to pull down Cdc55-myc from the yeast cell extract with GST-Rho1-Q68L (Fig. 1 F). The interaction between Rho1 and Cdc55 depended on the presence of Zds1/Zds2, because Cdc55-myc was not associated with GST-Rho1-Q68L in the yeast cell lysates from a zds1Δ zds2Δ double-mutant strain (Fig. 1 F). Thus, Zds1/Zds2 serves as a link between Rho1-GTP and Cdc55.
Because active Rho1, Zds1/Zds2, and Cdc55 all localize to the bud cortex during polarized cell growth (Yamochi et al., 1994; Bi and Pringle, 1996; Rossio and Yoshida, 2011), we also examined their localization dependence. In a temperature-sensitive rho1-3 strain, the cortical localization of Zds1-GFP and Cdc55-GFP was lost at the restrictive temperature, 37°C (Fig. 1 G). Thus, complex formation between active Rho1 and Zds1 may be required for stable localization of PP2ACdc55 at the bud cortex. We found that overexpression of ZDS1 efficiently suppressed the growth defect of the rho1-3 strain at 37°C (Fig. 1 H), suggesting that defects associated with rho1-3 are caused by mislocalization of Zds1. rho1-3 is known to be severely impaired in glucan synthesis, but not in the Pkc1–Mpk1 pathway (Saka et al., 2001), which suggests that Zds1 and Cdc55 function together with Rho1-GTP at the cell cortex to promote cell wall synthesis. Collectively, the GTP-dependent complex formation, localization dependence, and genetic suppression of a rho1 mutant strongly suggest that Cdc55–Zds1/Zds2 is a novel effector of Rho1.
Our attempts to demonstrate direct interaction between bacterially purified Rho1-Q68L and the HR2 domain of Zds1 failed (unpublished data), suggesting that stable interaction between Rho1 and Cdc55-Zds1 may require posttranslational modifications, a hypothesis that is consistent with previous studies that Zds1 is a phosphoprotein (Wicky et al., 2011). Alternatively, Rho1-GTP and Zds1/Zds2 may interact indirectly as part of a larger macromolecular complex, an idea that is suggested by the observation that several other effectors and regulators of Rho GTPases such as Pkc1, Bni1, Gic1/Gic2, Boi1/Boi2, Cla4, and Bem3 were identified as Zds1- or Zds2-binding proteins in the same screen that identified Rho1-GTP (Drees et al., 2001).
PP2ACdc55 is important for glucan synthesis
Because Rho1 has essential roles in cell wall biogenesis and integrity, we next examined the effects of various stresses on cells lacking each subunit of PP2A. Cdc55 is known to function in the cytoplasm and at the bud cortex in a complex with Zds1/Zds2 (Gentry and Hallberg, 2002; Yasutis et al., 2010; Rossio and Yoshida, 2011; Wicky et al., 2011; Rossio et al., 2014). Budding yeast contain two redundant catalytic subunits for PP2A: Pph21 and Pph22. Sit4, a PP6 phosphatase, has been implicated in the regulation of Pkc1-MAPK signaling and is reported to function with Cdc55 (Angeles de la Torre-Ruiz et al., 2002; Jiang, 2006). We found that deletions of CDC55, ZDS1/ZDS2, PPH21/PPH22, and SIT4 were all hypersensitive to the cell wall–damaging reagent SDS as well as low temperature (Fig. 2 A). Although the mechanism of the cold sensitivity phenotype is not well understood, abnormal activation of Pkc1–Mpk1 signaling has been implicated (Schmidt et al., 2002; Córcoles-Sáez et al., 2012; Lockshon et al., 2012).
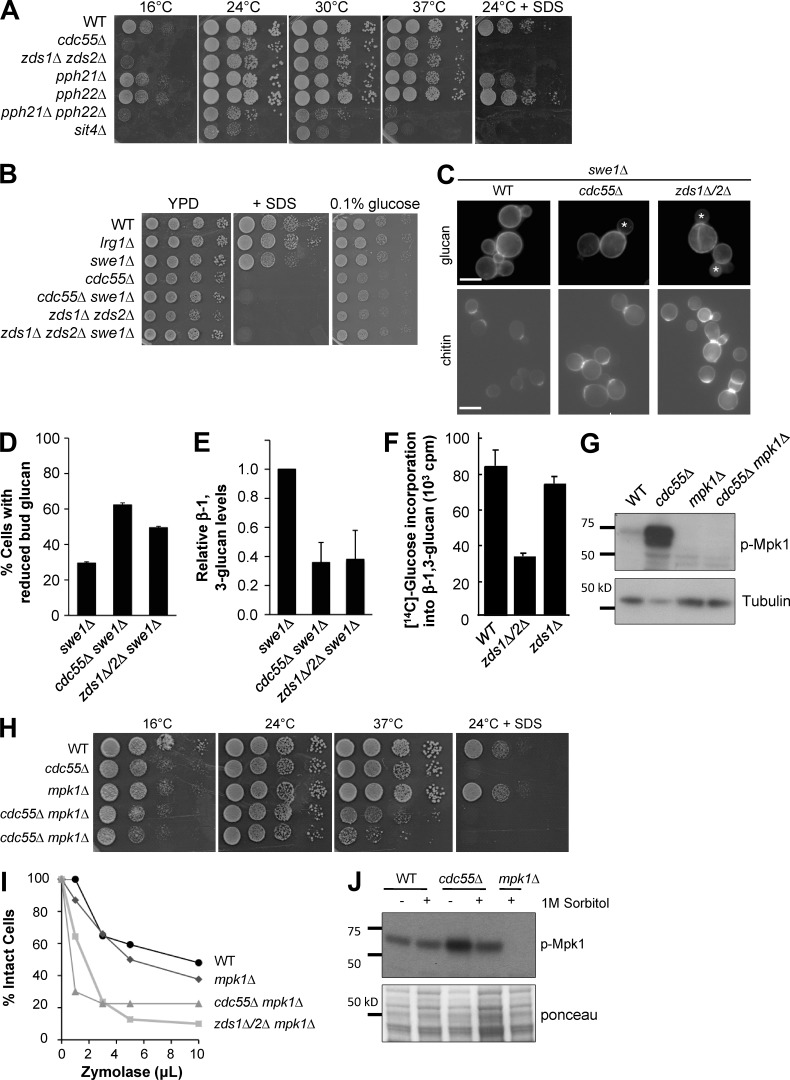
Figure 2.
cdc55Δ cells have reduced glucan synthesis and require Mpk1 activity for cell integrity. (A) Serial dilutions of yeast strains deleted for PP2A subunits are spotted on YPD with or without 0.03% SDS plates and grown at different temperatures. (B) Deletion of SWE1 did not rescue the stress sensitivity of PP2A mutants. (C) Abnormal cell wall in cdc55Δ and zds1Δ zds2Δ cells grown at 25°C. β-1,3-Glucan and chitin were stained with aniline blue and calcofluor white, respectively. Note that this and the following experiments were performed in swe1Δ strains. (D) Quantification of small budded cells with reduced anilin blue staining in the bud. (E) Quantification of the total amount of alkaline-soluble glucan synthesized in low-glucose media. (F) zds1Δ zds2Δ cells are defective for incorporation of glucose into β-1,3-glucan. Cells were incubated at 25°C, labeled with [14C]glucose for 2 h, and measured for incorporation of glucose into β-1,3-glucan. (G) Mpk1 is hyperphosphorylated in cdc55Δ cells grown at 25°C in YPD. (H) cdc55Δ cells require Mpk1 for growth under cell wall–stressing conditions (37°C and SDS). (I) In the absence of Mpk1, cdc55Δ and zds1Δ zds2Δ cells exhibit a severe cell lysis defect when challenged with Zymolyase. Loss of OD600 in the presence of Zymolyase for 60 min at 37°C was monitored as an indicator of cell lysis. (J) Osmotic stabilization by addition of 1 M sorbitol partially reduced the high phospho-Mpk1 levels of cdc55Δ cells. Ponceau staining is shown as a loading control. WT, wild type.
It is known that cdc55Δ and zds1Δ zds2Δ mutants exhibit a hyperelongated bud morphology caused by a cell cycle defect in progression through the G2/M phase (Yang et al., 2000). To exclude possible side effects caused by the G2 delay, we deleted the mitotic inhibitor SWE1 in all cytological analyses that followed. As has been reported, deletion of SWE1 rescued the morphological defects of cdc55Δ and zds1Δ zds2Δ (Yang et al., 2000; Rossio and Yoshida, 2011), but not the SDS sensitivity of these mutants (Fig. 2 B). We also found that the mutants were sensitive to low glucose (0.1%; Fig. 2 B), which suggests a possible problem in cell wall glucan production (Inoue et al., 1999; Douglas, 2001; Roh et al., 2002; Sekiya-Kawasaki et al., 2002).
To closely examine the cell wall defects of cdc55Δ and zds1Δ zds2Δ mutants, we first stained the cell wall glucan and chitin with aniline blue and calcofluor white, respectively. Aniline blue staining of glucan was not severely affected in cdc55Δ and zds1Δ zds2Δ mutants grown in the presence of high glucose (2% in the standard rich media), but we found significantly reduced glucan staining in the bud when the mutants were grown in the medium containing low glucose (0.1%; Fig. 2, C and D). Consistent with these results, the total amount of alkaline-extractable glucan was also significantly reduced in cdc55Δ and zds1Δ zds2Δ mutants (Fig. 2 E). We also noticed that there was a significant accumulation of chitin in the cell wall, possibly through a compensation mechanism for maintaining CWI (Fig. 2 C; Valdivia and Schekman, 2003; Lesage et al., 2004). To directly assess glucan synthesis, we measured the incorporation of 14C-labeled glucose into β-1,3-glucan and found that zds1Δ zds2Δ double-mutant cells exhibited a severe reduction in glucan synthesis (Fig. 2 F). These data suggest that Zds1/Zds2 and Cdc55 are positive regulators for Rho1 in glucan synthesis.
Mpk1 is hyperactivated in the absence of CDC55
We examined Pkc1-MAPK activity, another major downstream target of Rho1, in cdc55Δ cells using a phospho-specific antibody that specifically recognizes the dual phosphorylation of Mpk1 (pT190 and pY192), a hallmark of active Mpk1 (Cobb and Goldsmith, 1995; Kamada et al., 1995; Martín et al., 2000). Under normal growth conditions, Mpk1 was hyperphosphorylated in cdc55Δ cells compared with wild-type cells (Fig. 2 G), which suggests that Cdc55 has a negative role in the Rho1–Pkc1–Mpk1 stress response pathway. Alternatively, it is possible that the hyperactivation of the Rho1–Pkc1–Mpk1 pathway is a consequence of the cell wall defects in cdc55Δ cells. The addition of osmotic stabilizer, 1 M sorbitol, partially suppressed the hyperactivation of Mpk1 in cdc55Δ cells (Fig. 2 J), which suggests that hyperactivation of Mpk1 in these cells was, at least partially, caused by cell wall problems. Indeed, Mpk1 was important for cdc55Δ cell viability at high and low temperatures (Fig. 2 H). We further found that deletion of MPK1 from cdc55Δ and zds1Δ zds2Δ cells caused significant cell wall defects, as these mutants were hypersensitive to the cell wall–digesting enzyme Zymolyase (Fig. 2 I). These results revealed that PP2ACdc55 mutants require the Pkc1–Mpk1 pathway for maintaining cellular integrity but did not necessarily rule out a possible effect of Cdc55 in inhibition of the Pkc1–Mpk1 pathway.
Rho1 activity and localization is not affected in PP2ACdc55 mutants
To understand the mechanism by which PP2ACdc55 regulates Rho1 signaling, we hypothesized that PP2ACdc55 directly regulates either Rho1 or the Rho1 GAPs, because overexpression of CDC55 rescued the gefΔ strain, but not a rho1Δ strain (unpublished data), which suggests that PP2ACdc55 is neither functioning through the known Rho1 GEFs nor bypassing the essential requirement of Rho1. It is unlikely that PP2ACdc55 regulates the only Rho guanosine nucleotide dissociation inhibitor, Rdi1, because deletion of RDI1 failed to rescue the gefΔ strain (Yoshida et al., 2009). We first tested if PP2ACdc55 affected Rho1 activity or localization. To quantify total cellular Rho1 activity, we used the Rho-binding domain of rhotekin in a pull-down assay to measure Rho1-GTP levels (Kono et al., 2008; Yoshida et al., 2009). In this assay, loss of the Rho1 GAP LRG1 resulted in a significant increase in the total amount of Rho1-GTP (Fig. S1 A); however, we were not able to detect a significant effect on Rho1-GTP levels in the absence of CDC55 (Fig. S1 A). The cortical localization of Rho1 is dependent on membrane trafficking and Rdi1 (Abe et al., 2003; Tiedje et al., 2008; Boulter et al., 2010). Because Cdc55 and Rho1 display polarized localization to the bud tip and bud neck, we also examined the localization of GFP-Rho1 and GTP-locked GFP-Rho1-Q68L in cdc55Δ and zds1Δ zds2Δ and did not find a significant change (Fig. S1, B and C). Together, these results suggest that neither activity nor localization of Rho1 is largely affected by PP2ACdc55.
PP2ACdc55 downregulates Lrg1 GAP activity
Previously it was shown that deletion of the Rho1 GAPs, either LRG1 or SAC7, can rescue the gefΔ strain (Yoshida et al., 2009). We hypothesized that Cdc55 may regulate Rho1 GAPs, because overexpression of CDC55 also rescued the gefΔ strain lethality (Fig. 1 B). To monitor the total cellular amount of activated GAP (competent for Rho1-GTP binding), we developed an affinity-based assay by taking advantage of the fact that both Lrg1 and Sac7 bind to the active Rho1-Q68L, but not with wild-type or GDP-locked Rho1, in a two-hybrid assay (Lorberg et al., 2001; Watanabe et al., 2001; Schmidt et al., 2002). Using a constitutively active Rho1 mutant, Rho1-Q68L, we were able to detect robust interaction of Lrg1 and Sac7 GAPs with GST-Rho1-Q68L beads, but not with GST control beads (Fig. 3 A).
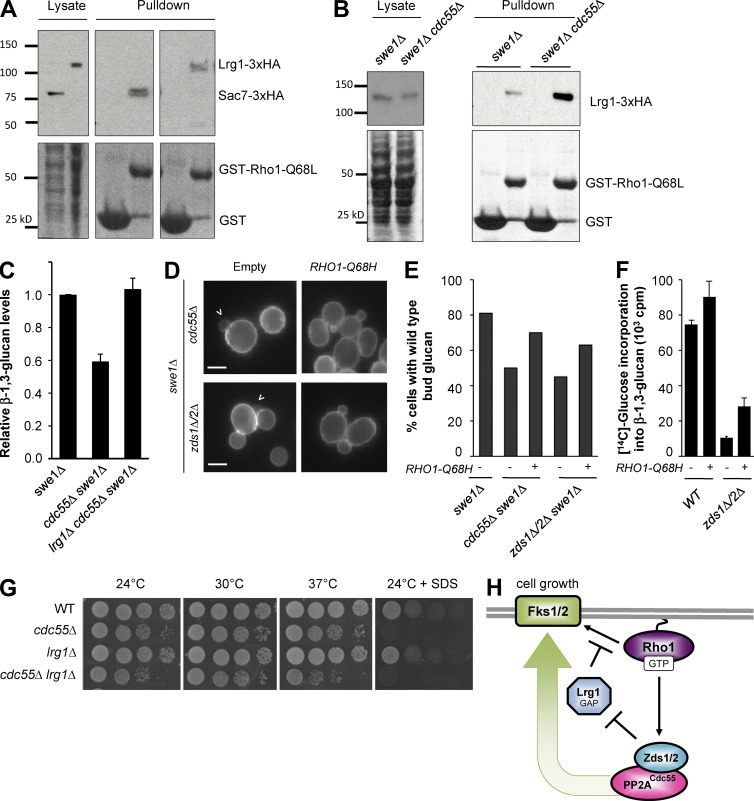
Figure 3.
A Rho1–Zds1/Zds2–PP2ACdc55 positive feedback loop promotes glucan synthesis by inhibition of Lrg1. (A) Pull-down assay for monitoring active Rho1 GAP. GST-Rho1Q68L was incubated with yeast cell lysates expressing 3XHA-tagged Sac7 or Lrg1. Lysates and bead-bound fractions were subjected to SDS-PAGE and Western blotting with anti-HA antibody. (B) In cdc55Δ, more Lrg1-3XHA bound to GST-Rho1Q68L beads compared with wild-type (WT) lysates. (C) Deletion of LRG1 rescued the glucan synthesis defect of cdc55Δ cells. Normalized total glucan synthesis as assayed in Fig. 2 E for indicated strains. (D) Activation of Rho1 can bypass Zds1/Zds2–PP2ACdc55 regulation of glucan synthesis. Arrowhead denotes cells with reduced glucan staining. (E) Quantification of small budded cells with wild-type glucan staining in the bud from D. (F) β-1,3-glucan synthesis defect of zds1Δ zds2Δ is partially rescued by RHO1-Q68H. (G) Deletion of LRG1 did not rescue the SDS sensitivity of cdc55Δ cells. Serial dilutions of yeast strains spotted on YPD with or without 0.03% SDS plates at the indicated temperature. (H) Model for how Zds1/Zds2–PP2ACdc55 promotes glucan synthesis through the inhibition of Rho1 GAP Lrg1.
Having established a method to quantify the amount of activated GAPs from yeast cell lysates, we first tested if loss of CDC55 affected Lrg1 GAP activity, because reduction in glucan synthesis in cdc55Δ implicates hyperactivation of Lrg1. In cells lacking CDC55, Lrg1 was equally expressed in cell lysates (Fig. 3 B) and localized normally to the bud cortex and neck (Fig. S1 D); however, more HA-tagged Lrg1 was bound to GST-Rho1-Q68L beads in cdc55Δ cell lysates compared with wild type (Fig. 3 B). Thus, the total cellular Lrg1 GAP activity was significantly increased in the absence of Cdc55. The glucan synthesis defect of cdc55Δ was indeed caused by hyperactivity of Lrg1. Deletion of LRG1 almost completely rescued the glucan synthesis defect of cdc55Δ (Fig. 3 C). Furthermore, expression of GTP-locked RHO1-Q68H restored glucan synthesis defects of cdc55Δ and zds1Δ zds2Δ (Fig. 3, D–F). These results, together with the physical interaction with Rho1-GTP and localization dependence, strongly suggest that the Zds1/Zds2–PP2ACdc55 complex is a new effector of Rho1 that promotes cell wall biogenesis via inhibition of Lrg1 (Fig. 3 H).
Active Rho1 is harmful to cdc55Δ and zds1Δ zds2Δ
We also examined whether deletion of LRG1 rescues any growth defect associated with cdc55Δ. Contrary to our expectation, SDS sensitivity of cdc55Δ was not rescued by deletion of LRG1; rather, cdc55Δ lrg1Δ exhibited a synthetic growth defect at high temperature (Fig. 3 G). The observed synthetic sickness is likely caused by misregulation of Rho1 activity, because introduction of GTP-locked RHO1-Q68H caused severe growth defects in cdc55Δ (Fig. 4 B) and zds1Δ zds2Δ (Fig. S2). Because the Pkc1–Mpk1 pathway was hyperactivated in cdc55Δ (Fig. 2 G), we suspected that the toxic effect of RHO1-Q68H is caused by abnormal activation of the Rho1–Pkc1 pathway. Indeed, expression of an activated mutant form of Pkc1, PKC1-R398P, caused severe growth defects in cdc55Δ (Fig. 4 B).
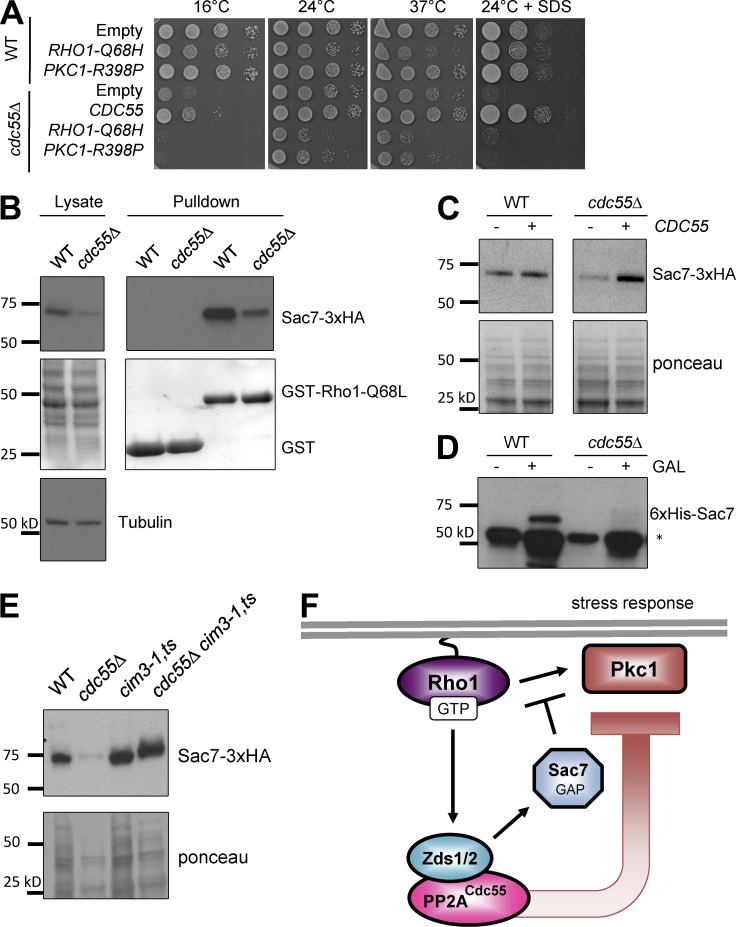
Figure 4.
PP2ACdc55 inhibits Pkc1 activation potentially through stabilization of the Pkc1-specific Rho1 GAP, Sac7. (A) Expression of RHO1-Q68H or PKC1-R398P was toxic to cdc55Δ cells. (B) Sac7-3XHA is less abundant in the cdc55Δ lysates. Lysates and bead-bound fractions were subjected to SDS-PAGE and Western blotting with anti-HA antibody and anti–α-tubulin as a loading control. (C) Expression of CDC55 restored Sac7-3xHA protein levels in cdc55Δ cells to wild-type (WT) levels. (D) The reduction of Sac7 in the cdc55Δ lysates is a posttranscriptional mechanism. Expression of 6xHis-Sac7 from an inducible GAL1 promoter failed to accumulate Sac7 in cdc55Δ cells. *, nonspecific band detected by anti-His antibody (Millipore). (E) Sac7 is degraded by the proteasome. Inactivation of the proteasome by a cim3-1 mutation at the semipermissive temperature of 30°C for 4 h resulted in stabilization of Sac7-3xHA even in the cdc55Δ mutants. (F) Model for how Zds1/Zds2–PP2ACdc55 antagonizes Rho1 activation of Pck1 through the stabilization of the Rho1 GAP Sac7.
Sac7 is unstable in the absence of PP2ACdc55
To understand why Pkc1–Mpk1 is hyperactivated in cdc55Δ, we examined total cellular Sac7 GAP activity in both wild-type and cdc55Δ lysates and found a significant reduction in the amount of active Sac7 GAP (Fig. 4 C). The reduction of active Sac7 in cdc55Δ was largely caused by the reduced amount of total Sac7 protein compared with a loading control, α-tubulin (Fig. 4 B). We confirmed that Sac7 protein level is dependent on CDC55, as expression of CDC55 from a plasmid fully restored Sac7 protein abundance in cdc55Δ (Fig. 4 C). Regulation of Sac7 protein level by Cdc55 was not caused by transcriptional control but rather degradation control, because induction of SAC7 expression from an inducible GAL1 promoter failed to overexpress 6xHis-Sac7 in cdc55Δ (Fig. 4D). In contrast, Sac7 was stabilized in a proteasome mutant cim3-1 even in the absence of CDC55 (Fig. 4 E). These results suggest that Cdc55 prevents Pkc1 activation by stabilizing Sac7 (Fig. 4 F). The molecular mechanisms by which PP2A inactivates Lrg1 and protects Sac7 from proteasomal degradation require further investigation but may involve dephosphorylation by PP2A, because both Lrg1 and Sac7 are phosphoproteins in vivo (Swaney et al., 2013).
Antagonism between PP2A and Pkc1
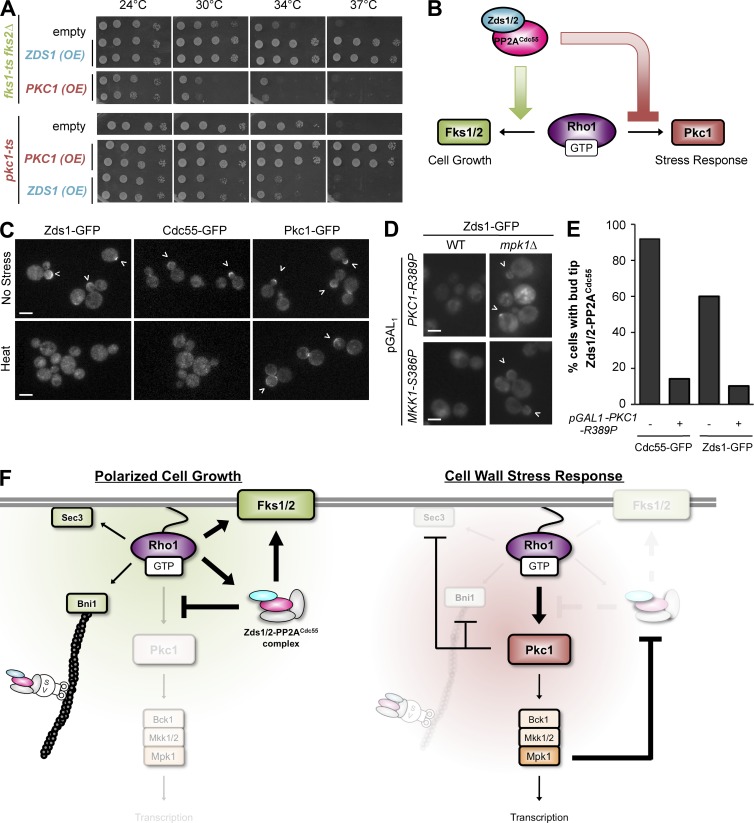
In a complementary approach, we examined the effect of up-regulating PP2ACdc55 activity by overexpressing ZDS1. Consistent with our previous result (Sekiya-Kawasaki et al., 2002), overexpression of ZDS1 efficiently rescued the temperature-sensitive growth defect of the glucan synthase mutant fks1-1154 fks2Δ (Fig. 5 A, top). In contrast, overexpression of ZDS1 was toxic to the temperature-sensitive pkc1-2 mutant at the semipermissive temperatures of 30°C and 34°C (Fig. 5 A, bottom). Interestingly, we also found that overexpression of PKC1 was toxic to fks1-ts fks2Δ, suggesting a possible antagonism between the PP2ACdc55–Zds1/Zds2 and Pkc1–Mpk1 pathways.
Figure 5.
Mutual antagonism between Zds1/Zds2–PP2ACdc55 and Pkc1 controls Rho1 activation of polarized cell growth or stress response. (A) Overexpression of ZDS1 rescued glucan synthase mutant fks1-ts fks2Δ but inhibited growth of pkc1-ts mutant at higher temperature. (B) Working model for Zds1/Zds2–PP2ACdc55 function. Zds1/Zds2–PP2ACdc55 promotes Rho1–Fks1/Fks2 glucan synthesis while inhibiting the Rho1–Pkc1 stress pathway. (C) Cell wall stress removes Zds1/Zds2–PP2ACdc55 complex from the bud cortex. Zds1-GFP and Cdc55-GFP but not Pkc1 are delocalized from the bud cortex (arrowheads) after a heat shock at 39°C. (D) Artificial activation of Pkc1 or Mkk1 is sufficient to remove Zds1 from the cortex in a MPK1-dependent manner. WT, wild type. (E) Quantification of cells with cortically localized Cdc55-GFP or Zds1-GFP before (−) and after (+) GAL induction of the activated PKC1-R389P mutant. More than 50 cells were counted for each strain and condition. (F) Model for the Zds1/Zds2–PP2ACdc55 complex regulation of Rho1 signaling during polarized cell growth (left) and cell wall stress response (right). Two mutually exclusive Rho1 signaling states are maintained by an antagonism between the Zds1/Zds2–PP2ACdc55 complex and Pkc1 kinase.
The inhibitory effects of Zds1 on the Pkc1 pathway predict that PP2ACdc55 needs to be prevented from interacting with Rho1 to allow optimal Pkc1 activation upon cell wall damage (Fig. 5 B). Indeed, both Zds1-GFP and Cdc55-GFP were rapidly delocalized from the bud cortex after cell wall damage by a heat shock (Fig. 5 C). In contrast, Pkc1-GFP was robustly recruited to the cell cortex (Fig. 5 C), as previously described (Andrews and Stark, 2000; Kono et al., 2012). Collectively, these data suggest that Rho1 robustly switches from activation of polarized cell growth effectors to activation of stress response effectors through the removal of cortical polarity factors.
We further found that activation of Pkc1 was sufficient to remove Cdc55 and Zds1 from the bud cortex, even in the absence of cell wall stress. Expression of active Pkc1 (Pkc1-R389P) delocalized Zds1-GFP and Cdc55-GFP from the bud cortex (Fig. 5, D and E). Pkc1-induced delocalization of Zds1 depends on having a functional CWI MAPK cascade, because expression of activated MAPK kinase MKK1-S386P also caused delocalization of Zds1-GFP (Fig. 5 D). Furthermore, the effect of PKC1-R398P and MKK1-S386P on Zds1-GFP delocalization was blocked by deletion of the MAPK MPK1 (Fig. 5 D). Thus, activation of the Pkc1–Mpk1 pathway leads to removal of the PP2ACdc55 complex away from active Rho1 at the cell cortex.
Mutual antagonism between PP2ACdc55 phosphatase and Pkc1 kinase explains how only one signaling pathway is robustly activated at a time
Our genetic and biochemical analysis revealed an interesting role for PP2ACdc55 in Rho1 signaling pathways. Cdc55 promotes cell wall synthesis while antagonizing the activation of Pkc1, without significantly affecting total cellular Rho1 activity. The balance between glucan synthesis and Pkc1 activity is important for cell viability, because simultaneous activation of both pathways by deletion of LRG1 and SAC7 results in lethality (Lorberg et al., 2001). Furthermore, reduced glucan synthesis and impaired Pkc1–Mpk1 signaling also results in lethality (Levin et al., 1990; Mazur et al., 1995; Saka et al., 2001). We propose that PP2ACdc55 regulates this essential balance of Rho1 signaling output, which is consistent with the fact that cdc55Δ cells are hypersensitive to increased dosages of GTP-locked Rho1 (Fig. 4 A).
Based on these data, we propose the following model for PP2A function in Rho1 signaling (Fig. 5 F). During favorable growth conditions, cortical PP2ACdc55 biases Rho1 to activate polarized cell growth effectors (Bni1, Sec3, and Fks1/Fks2) by inactivating the Rho1 GAP Lrg1. Because Cdc55 is delivered to the bud cortex by polarized secretion (Heger et al., 2011), activation of Bni1, Sec3, and Fks1/Fks2 creates a positive feedback loop of cortical PP2ACdc55 activity that sustains polarized cell growth. At the same time, PP2ACdc55 prevents activation of the Pkc1–Mpk1 pathway by stabilizing the Rho1 GAP Sac7 (Fig. 4, C and D). When a cell is exposed to cell wall stresses, Rho1 rapidly activates Pkc1 and blocks polarized secretion by degradation of Bni1 and Sec3 (Kono et al., 2012). Loss of polarized secretion stops the supply of Cdc55 to the bud cortex. Activation of Pkc1 also triggers a removal of PP2ACdc55 from the cell cortex in a Mpk1-dependent manner.
The mutual antagonism between Zds1/Zds2–PP2ACdc55 phosphatase and Pkc1 kinase activities can form a bistable system in which active Rho1 promotes either polarized cell growth or repair of cell wall wounds. Because small GTPases often regulate multiple signal outputs, we speculate that antagonism between effectors is commonly used to allow robust activation of only one signal outcome at a time.
Materials and methods
Plasmids and strains
Standard methods were used for molecular biology procedures and yeast cell growth. All yeast strains were isogenic to BY4741 (Mata his3Δ0 leu2Δ0 met15Δ0 ura3Δ0 from OpenBiosystems) unless otherwise noted. Yeast strains and plasmids are listed in Tables S1 and S2, respectively. PY strains and PB plasmids were gifts from D. Pellman (Harvard Medical School, Boston, MA). The SWE1-disrupting plasmid was a gift from D. Lew (Duke University, Durham, NC); M. Mizunuma (Hiroshima University, Hiroshima, Japan) provided ZDS1 and ZDS2 expression plasmids; and K. Irie (Tsukuba University, Tsukuba, Japan) provided pNV7-MKK1P386. Gene deletions and modification were constructed by recombination using KANMX6 or HIS3MX6 cassettes provided by J. Pringle (Stanford University, Stanford, CA; Longtine et al., 1998), and accurate integrations were confirmed by PCR. For C-terminal tagging of Lrg1 and Sac7 with 3XHA, a flexible linker (GGSGGS) was introduced between the ORF and tag.
For the yeast two-hybrid β-galactosidase assay, yeast cells were cotransformed with a plasmid containing the LexA DNA binding domain (pBMT116-derived) and a plasmid containing a gene fused to the GAL4 transcriptional activating domain (pACTII-HK derived; Watanabe et al., 2001). For quantitative analysis for β-galactosidase activity, the transformants were cultured in SC-TRP-LEU, and β-galactosidase activity was measured according to the ortho-nitrophenyl-β-galactoside assay method (Guarente, 1983).
Biochemistry
Whole-cell protein extracts were prepared as described previously (Kushnirov, 2000). In brief, cells were pelleted, treated with 0.1 N NaOH, and incubated for 5 min on ice. Afterward, they were pelleted, resuspended in SDS sample buffer (62.5 mM Tris-HCl, pH 6.8, 10% glycerol, 2% SDS, 2% β-mercaptoethanol, and 0.005% bromophenol blue), boiled for 5 min, and pelleted. The supernatants were loaded in a 4–15% mini-gel (Bio-Rad), and Western blotting was performed with indicated antibodies. Commercially available antibodies used were rabbit anti–phospho-p42/44 MAPK (T202/Y204) antibody (Cell Signaling Technology), rat anti–tubulin-α antibody (AbD Serotec), mouse anti–6XHis-HRP antibody (Millipore), and mouse anti–HA 12CA5 antibody (Roche). HRP-conjugated secondary antibodies were obtained from Millipore, and proteins were detected with an enhanced chemiluminescence system (ECL plus; Amersham).
The Rho1-Q68L pull-down assays for Zds1 and Cdc55 interaction and Rho1 GAPs Lrg1 and Sac7 were performed as follows. GST-Rho1-Q68L was purified from Escherichia coli as previously described (García-Mata et al., 2006). Yeast cell pellets from 50-ml cultures were lysed by a glass bead beater method in 400 µl lysis buffer (20 mM Hepes, pH 7.5, 150 mM NaCl, 1 mM EDTA, 5 mM MgCl2, 1% Triton X-100, 1 mM DTT, 1 mM PMSF, and standard protease inhibitors [antipain, leupeptin, pepstatin A, aprotinin, and chymostatin]), and lysates were cleared by low-speed (13,000 rpm) centrifugation for 15 min at 4°C. Supernatants were incubated with a bead-bound GST-Rho1-Q68L fusion for 1 h rotating at 4°C and washed three times, and bound fractions were subjected to SDS-PAGE. Bound Zds1-3XHA or Cdc55-13myc was detected by Western blot analysis with mouse anti–HA 12CA5 antibody (Roche) or mouse anti–myc 9E10 antibody (Millipore).
Quantification of Rho1-GTP by rhotekin pull-down assay was performed as previously described (Yoshida et al., 2006; Kono et al., 2008). Yeast cells were lysed by bead-beating method in a lysis buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 12 mM MgCl2, 1 mM DTT, 1 mM PMSF, 0.6% CHAPS, and standard protease inhibitors), and lysates were cleared by low-speed centrifugation. Cleared supernatants were incubated with commercially available GST-rhotekin beads (Cytoskeleton) for 1 h rotating at 4°C and washed twice, and bound fractions were subjected to SDS-PAGE and Western blotting with a custom made rabbit anti–Rho1 Y1486 antibody.
Fluorescence microscopy
To examine cell wall phenotype, yeast cells were grown overnight in yeast extract peptone dextrose (YPD; 0.5% glucose), refreshed in YPD (0.1% glucose), and grown to early log-phase. Staining of cell wall glucan and chitin was performed as previously described (Watanabe et al., 2001). For glucan staining, yeast cells grown in low-glucose media (0.1% glucose) were washed twice with PBS and incubated with 0.01% aniline blue for 5 min at RT, washed twice with PBS, and imaged. For chitin staining, yeast cells grown in low-glucose media (0.1%) were fixed in 4% formaldehyde for 10 min at RT, washed twice with PBS, incubated with 10 µg/ml calcofluor white for 5 min at RT, washed twice with PBS, and imaged. Images were acquired using a fluorescence microscope (Eclipse E600; Nikon) equipped with a CCD camera (DC350F; Andor) and a 63× (NA 1.4) oil objective. All images were captured and analyzed with NIS-Elements software (Nikon).
Quantification of β-1,3-glucan
Total soluble β-1,3-glucan was quantified in a microtiter-based assay with aniline blue as described in Watanabe et al. (2001). Cells were grown overnight in YPD containing 0.5% glucose, refreshed in YPD containing 0.1% glucose, and allowed to grow for 4 h. Cells were normalized to a final OD600 of 0.2 in 0.5 ml Tris-EDTA buffer, and NaOH was added to a final concentration of 1 N. β-1,3-Glucan was solubilized by incubation for 30 min at 80°C. 2.1-ml aniline blue buffer (0.03% aniline blue, 0.18 N HCl, and 0.5 M glycine/NaOH, pH 9.5) was added, and cells were incubated for 30 min at 50°C. Reactions were allowed to cool for 30 min at RT before measuring fluorescence using an Infinite M200 plate reader (Tecan) at excitation and emission wavelengths of 400 and 460 nm, respectively.
Incorporation of [14C]glucose into β-1,3-glucan was performed as previously described (Sekiya-Kawasaki et al., 2002). Early log-phase cultures were grown to OD600 0.5 in 1 ml of 0.5% glucose media containing 10 µCi [14C]glucose and incubated for 2 h at RT. After labeling, β-1,3-glucan was extracted as described earlier for the microtiter assay.
Online supplemental material
Tables S1 and S2 list yeast strains and plasmids, respectively. Fig. S1 shows Rho1 activity and localization of Rho1 and Lrg1 In cdc55Δ. Fig. S2 shows toxicity of GTP-locked Rho1 in zds1Δ zds2Δ. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.201508119/DC1.
Supplementary Material
Acknowledgments
We thank David Pellman, John Pringle, Daniel Lew, Masaki Mizunuma, Kenji Irie, and the Yeast Genome Resource Center for yeast strains and plasmids and members of Yoshida Laboratory and Keiko Kono for their support. Multicopy suppressor screening for gef∆ was initiated in the Pellman Laboratory with the help of Didem Ilter.
This research was supported by Sprout grant from Brandeis University (E.M. Jonasson and S. Yoshida), an American-Italian Cancer Foundation Postdoctoral fellowship (V. Rossio), and a Massachusetts Life Sciences Center grant (S. Yoshida).
The authors declare no competing financial interests.
Footnotes
Abbreviations used in this paper:
- CWI
- cell wall integrity
- GAP
- GTPase-activating protein
- GEF
- guanine nucleotide-exchange factor
- PP2A
- protein phosphatase 2A
- YPD
- yeast extract peptone dextrose
References
- Abe M., Qadota H., Hirata A., and Ohya Y.. 2003. Lack of GTP-bound Rho1p in secretory vesicles of Saccharomyces cerevisiae. J. Cell Biol. 162:85–97. 10.1083/jcb.200301022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P.D., and Stark M.J.. 2000. Dynamic, Rho1p-dependent localization of Pkc1p to sites of polarized growth. J. Cell Sci. 113:2685–2693. [DOI] [PubMed] [Google Scholar]
- Angeles de la Torre-Ruiz M., Torres J., Arino J., and Herrero E.. 2002. Sit4 is required for proper modulation of the biological functions mediated by Pkc1 and the cell integrity pathway in Saccharomyces cerevisiae. J. Biol. Chem. 277:33468–33476. 10.1074/jbc.M203515200 [DOI] [PubMed] [Google Scholar]
- Bi E., and Pringle J.R.. 1996. ZDS1 and ZDS2, genes whose products may regulate Cdc42p in Saccharomyces cerevisiae. Mol. Cell. Biol. 16:5264–5275. 10.1128/MCB.16.10.5264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulter E., Garcia-Mata R., Guilluy C., Dubash A., Rossi G., Brennwald P.J., and Burridge K.. 2010. Regulation of Rho GTPase crosstalk, degradation and activity by RhoGDI1. Nat. Cell Biol. 12:477–483. 10.1038/ncb2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb M.H., and Goldsmith E.J.. 1995. How MAP kinases are regulated. J. Biol. Chem. 270:14843–14846. 10.1074/jbc.270.25.14843 [DOI] [PubMed] [Google Scholar]
- Córcoles-Sáez I., Ballester-Tomas L., de la Torre-Ruiz M.A., Prieto J.A., and Randez-Gil F.. 2012. Low temperature highlights the functional role of the cell wall integrity pathway in the regulation of growth in Saccharomyces cerevisiae. Biochem. J. 446:477–488. 10.1042/BJ20120634 [DOI] [PubMed] [Google Scholar]
- Dong Y., Pruyne D., and Bretscher A.. 2003. Formin-dependent actin assembly is regulated by distinct modes of Rho signaling in yeast. J. Cell Biol. 161:1081–1092. 10.1083/jcb.200212040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas C.M. 2001. Fungal beta(1,3)-d-glucan synthesis. Med. Mycol. 39:55–66. 10.1080/mmy.39.1.55.66 [DOI] [PubMed] [Google Scholar]
- Drees B.L., Sundin B., Brazeau E., Caviston J.P., Chen G.C., Guo W., Kozminski K.G., Lau M.W., Moskow J.J., Tong A., et al. 2001. A protein interaction map for cell polarity development. J. Cell Biol. 154:549–571. 10.1083/jcb.200104057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drgonová J., Drgon T., Tanaka K., Kollár R., Chen G.C., Ford R.A., Chan C.S., Takai Y., and Cabib E.. 1996. Rho1p, a yeast protein at the interface between cell polarization and morphogenesis. Science. 272:277–279. 10.1126/science.272.5259.277 [DOI] [PubMed] [Google Scholar]
- García-Mata R., Wennerberg K., Arthur W.T., Noren N.K., Ellerbroek S.M., and Burridge K.. 2006. Analysis of activated GAPs and GEFs in cell lysates. Methods Enzymol. 406:425–437. 10.1016/S0076-6879(06)06031-9 [DOI] [PubMed] [Google Scholar]
- Gentry M.S., and Hallberg R.L.. 2002. Localization of Saccharomyces cerevisiae protein phosphatase 2A subunits throughout mitotic cell cycle. Mol. Biol. Cell. 13:3477–3492. 10.1091/mbc.02-05-0065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L. 1983. Yeast promoters and lacZ fusions designed to study expression of cloned genes in yeast. Methods Enzymol. 101:181–191. 10.1016/0076-6879(83)01013-7 [DOI] [PubMed] [Google Scholar]
- Guo W., Tamanoi F., and Novick P.. 2001. Spatial regulation of the exocyst complex by Rho1 GTPase. Nat. Cell Biol. 3:353–360. 10.1038/35070029 [DOI] [PubMed] [Google Scholar]
- Heger C.D., Wrann C.D., and Collins R.N.. 2011. Phosphorylation provides a negative mode of regulation for the yeast Rab GTPase Sec4p. PLoS One. 6:e24332 10.1371/journal.pone.0024332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S.B., Qadota H., Arisawa M., Watanabe T., and Ohya Y.. 1999. Prenylation of Rho1p is required for activation of yeast 1, 3-beta-glucan synthase. J. Biol. Chem. 274:38119–38124. 10.1074/jbc.274.53.38119 [DOI] [PubMed] [Google Scholar]
- Irie K., Takase M., Lee K.S., Levin D.E., Araki H., Matsumoto K., and Oshima Y.. 1993. MKK1 and MKK2, which encode Saccharomyces cerevisiae mitogen-activated protein kinase-kinase homologs, function in the pathway mediated by protein kinase C. Mol. Cell. Biol. 13:3076–3083. 10.1128/MCB.13.5.3076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y. 2006. Regulation of the cell cycle by protein phosphatase 2A in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 70:440–449. 10.1128/MMBR.00049-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada Y., Jung U.S., Piotrowski J., and Levin D.E.. 1995. The protein kinase C-activated MAP kinase pathway of Saccharomyces cerevisiae mediates a novel aspect of the heat shock response. Genes Dev. 9:1559–1571. 10.1101/gad.9.13.1559 [DOI] [PubMed] [Google Scholar]
- Kamada Y., Qadota H., Python C.P., Anraku Y., Ohya Y., and Levin D.E.. 1996. Activation of yeast protein kinase C by Rho1 GTPase. J. Biol. Chem. 271:9193–9196. 10.1074/jbc.271.16.9193 [DOI] [PubMed] [Google Scholar]
- Kohno H., Tanaka K., Mino A., Umikawa M., Imamura H., Fujiwara T., Fujita Y., Hotta K., Qadota H., Watanabe T., et al. 1996. Bni1p implicated in cytoskeletal control is a putative target of Rho1p small GTP binding protein in Saccharomyces cerevisiae. EMBO J. 15:6060–6068. [PMC free article] [PubMed] [Google Scholar]
- Kono K., Nogami S., Abe M., Nishizawa M., Morishita S., Pellman D., and Ohya Y.. 2008. G1/S cyclin-dependent kinase regulates small GTPase Rho1p through phosphorylation of RhoGEF Tus1p in Saccharomyces cerevisiae. Mol. Biol. Cell. 19:1763–1771. 10.1091/mbc.E07-09-0950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono K., Saeki Y., Yoshida S., Tanaka K., and Pellman D.. 2012. Proteasomal degradation resolves competition between cell polarization and cellular wound healing. Cell. 150:151–164. 10.1016/j.cell.2012.05.030 [DOI] [PubMed] [Google Scholar]
- Kushnirov V.V. 2000. Rapid and reliable protein extraction from yeast. Yeast. 16:857–860. [DOI] [PubMed] [Google Scholar]
- Lee K.S., and Levin D.E.. 1992. Dominant mutations in a gene encoding a putative protein kinase (BCK1) bypass the requirement for a Saccharomyces cerevisiae protein kinase C homolog. Mol. Cell. Biol. 12:172–182. 10.1128/MCB.12.1.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.S., Hines L.K., and Levin D.E.. 1993. A pair of functionally redundant yeast genes (PPZ1 and PPZ2) encoding type 1-related protein phosphatases function within the PKC1-mediated pathway. Mol. Cell. Biol. 13:5843–5853. 10.1128/MCB.13.9.5843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage G., Sdicu A.M., Ménard P., Shapiro J., Hussein S., and Bussey H.. 2004. Analysis of beta-1,3-glucan assembly in Saccharomyces cerevisiae using a synthetic interaction network and altered sensitivity to caspofungin. Genetics. 167:35–49. 10.1534/genetics.167.1.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin D.E., Fields F.O., Kunisawa R., Bishop J.M., and Thorner J.. 1990. A candidate protein kinase C gene, PKC1, is required for the S. cerevisiae cell cycle. Cell. 62:213–224. 10.1016/0092-8674(90)90360-Q [DOI] [PubMed] [Google Scholar]
- Lockshon D., Olsen C.P., Brett C.L., Chertov A., Merz A.J., Lorenz D.A., Van Gilst M.R., and Kennedy B.K.. 2012. Rho signaling participates in membrane fluidity homeostasis. PLoS One. 7:e45049 10.1371/journal.pone.0045049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine M.S., McKenzie A. III, Demarini D.J., Shah N.G., Wach A., Brachat A., Philippsen P., and Pringle J.R.. 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 14:953–961. [DOI] [PubMed] [Google Scholar]
- Lorberg A., Schmitz H.P., Jacoby J.J., and Heinisch J.J.. 2001. Lrg1p functions as a putative GTPase-activating protein in the Pkc1p-mediated cell integrity pathway in Saccharomyces cerevisiae. Mol. Genet. Genomics. 266:514–526. 10.1007/s004380100580 [DOI] [PubMed] [Google Scholar]
- Martín H., Arroyo J., Sánchez M., Molina M., and Nombela C.. 1993. Activity of the yeast MAP kinase homologue Slt2 is critically required for cell integrity at 37 degrees C. Mol. Gen. Genet. 241:177–184. 10.1007/BF00280215 [DOI] [PubMed] [Google Scholar]
- Martín H., Rodríguez-Pachón J.M., Ruiz C., Nombela C., and Molina M.. 2000. Regulatory mechanisms for modulation of signaling through the cell integrity Slt2-mediated pathway in Saccharomyces cerevisiae. J. Biol. Chem. 275:1511–1519. 10.1074/jbc.275.2.1511 [DOI] [PubMed] [Google Scholar]
- Mazur P., and Baginsky W.. 1996. In vitro activity of 1,3-β-d-glucan synthase requires the GTP-binding protein Rho1. J. Biol. Chem. 271:14604–14609. 10.1074/jbc.271.24.14604 [DOI] [PubMed] [Google Scholar]
- Mazur P., Morin N., Baginsky W., el-Sherbeini M., Clemas J.A., Nielsen J.B., and Foor F.. 1995. Differential expression and function of two homologous subunits of yeast 1,3-β-d-glucan synthase. Mol. Cell. Biol. 15:5671–5681. 10.1128/MCB.15.10.5671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka H., Tanaka K., Hirano H., Fujiwara T., Kohno H., Umikawa M., Mino A., and Takai Y.. 1995. A downstream target of RHO1 small GTP-binding protein is PKC1, a homolog of protein kinase C, which leads to activation of the MAP kinase cascade in Saccharomyces cerevisiae. EMBO J. 14:5931–5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qadota H., Python C.P., Inoue S.B., Arisawa M., Anraku Y., Zheng Y., Watanabe T., Levin D.E., and Ohya Y.. 1996. Identification of yeast Rho1p GTPase as a regulatory subunit of 1,3-β-glucan synthase. Science. 272:279–281. 10.1126/science.272.5259.279 [DOI] [PubMed] [Google Scholar]
- Roh D.H., Bowers B., Riezman H., and Cabib E.. 2002. Rho1p mutations specific for regulation of beta(1-->3)glucan synthesis and the order of assembly of the yeast cell wall. Mol. Microbiol. 44:1167–1183. 10.1046/j.1365-2958.2002.02955.x [DOI] [PubMed] [Google Scholar]
- Rossio V., and Yoshida S.. 2011. Spatial regulation of Cdc55-PP2A by Zds1/Zds2 controls mitotic entry and mitotic exit in budding yeast. J. Cell Biol. 193:445–454. 10.1083/jcb.201101134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossio V., Kazatskaya A., Hirabayashi M., and Yoshida S.. 2014. Comparative genetic analysis of PP2A-Cdc55 regulators in budding yeast. Cell Cycle. 13:2073–2083. 10.4161/cc.29064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saka A., Abe M., Okano H., Minemura M., Qadota H., Utsugi T., Mino A., Tanaka K., Takai Y., and Ohya Y.. 2001. Complementing yeast rho1 mutation groups with distinct functional defects. J. Biol. Chem. 276:46165–46171. 10.1074/jbc.M103805200 [DOI] [PubMed] [Google Scholar]
- Schmidt A., Schmelzle T., and Hall M.N.. 2002. The RHO1-GAPs SAC7, BEM2 and BAG7 control distinct RHO1 functions in Saccharomyces cerevisiae. Mol. Microbiol. 45:1433–1441. 10.1046/j.1365-2958.2002.03110.x [DOI] [PubMed] [Google Scholar]
- Schmitz H.P., Lorberg A., and Heinisch J.J.. 2002. Regulation of yeast protein kinase C activity by interaction with the small GTPase Rho1p through its amino-terminal HR1 domain. Mol. Microbiol. 44:829–840. 10.1046/j.1365-2958.2002.02925.x [DOI] [PubMed] [Google Scholar]
- Sekiya-Kawasaki M., Abe M., Saka A., Watanabe D., Kono K., Minemura-Asakawa M., Ishihara S., Watanabe T., and Ohya Y.. 2002. Dissection of upstream regulatory components of the Rho1p effector, 1,3-β-glucan synthase, in Saccharomyces cerevisiae. Genetics. 162:663–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaney D.L., Beltrao P., Starita L., Guo A., Rush J., Fields S., Krogan N.J., and Villén J.. 2013. Global analysis of phosphorylation and ubiquitylation cross-talk in protein degradation. Nat. Methods. 10:676–682. 10.1038/nmeth.2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiedje C., Sakwa I., Just U., and Höfken T.. 2008. The Rho GDI Rdi1 regulates Rho GTPases by distinct mechanisms. Mol. Biol. Cell. 19:2885–2896. 10.1091/mbc.E07-11-1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolliday N., VerPlank L., and Li R.. 2002. Rho1 directs formin-mediated actin ring assembly during budding yeast cytokinesis. Curr. Biol. 12:1864–1870. 10.1016/S0960-9822(02)01238-1 [DOI] [PubMed] [Google Scholar]
- Valdivia R.H., and Schekman R.. 2003. The yeasts Rho1p and Pkc1p regulate the transport of chitin synthase III (Chs3p) from internal stores to the plasma membrane. Proc. Natl. Acad. Sci. USA. 100:10287–10292. 10.1073/pnas.1834246100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe D., Abe M., and Ohya Y.. 2001. Yeast Lrg1p acts as a specialized RhoGAP regulating 1,3-β-glucan synthesis. Yeast. 18:943–951. 10.1002/yea.742 [DOI] [PubMed] [Google Scholar]
- Wicky S., Tjandra H., Schieltz D., Yates J. III, and Kellogg D.R.. 2011. The Zds proteins control entry into mitosis and target protein phosphatase 2A to the Cdc25 phosphatase. Mol. Biol. Cell. 22:20–32. 10.1091/mbc.E10-06-0487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamochi W., Tanaka K., Nonaka H., Maeda A., Musha T., and Takai Y.. 1994. Growth site localization of Rho1 small GTP-binding protein and its involvement in bud formation in Saccharomyces cerevisiae. J. Cell Biol. 125:1077–1093. 10.1083/jcb.125.5.1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Jiang W., Gentry M., and Hallberg R.L.. 2000. Loss of a protein phosphatase 2A regulatory subunit (Cdc55p) elicits improper regulation of Swe1p degradation. Mol. Cell. Biol. 20:8143–8156. 10.1128/MCB.20.21.8143-8156.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasutis K., Vignali M., Ryder M., Tameire F., Dighe S.A., Fields S., and Kozminski K.G.. 2010. Zds2p regulates Swe1p-dependent polarized cell growth in Saccharomyces cerevisiae via a novel Cdc55p interaction domain. Mol. Biol. Cell. 21:4373–4386. 10.1091/mbc.E10-04-0326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Kono K., Lowery D.M., Bartolini S., Yaffe M.B., Ohya Y., and Pellman D.. 2006. Polo-like kinase Cdc5 controls the local activation of Rho1 to promote cytokinesis. Science. 313:108–111. 10.1126/science.1126747 [DOI] [PubMed] [Google Scholar]
- Yoshida S., Bartolini S., and Pellman D.. 2009. Mechanisms for concentrating Rho1 during cytokinesis. Genes Dev. 23:810–823. 10.1101/gad.1785209 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.