In asymmetrically dividing C. elegans embryos, the core cortical PAR proteins are required to retain septin and anillin at the anterior cortex away from the contractile ring and to promote normal F-actin levels at the contractile ring and successful cytokinesis.
Abstract
Cytokinesis, the physical division of one cell into two, is thought to be fundamentally similar in most animal cell divisions and driven by the constriction of a contractile ring positioned and controlled solely by the mitotic spindle. During asymmetric cell divisions, the core polarity machinery (partitioning defective [PAR] proteins) controls the unequal inheritance of key cell fate determinants. Here, we show that in asymmetrically dividing Caenorhabditis elegans embryos, the cortical PAR proteins (including the small guanosine triphosphatase CDC-42) have an active role in regulating recruitment of a critical component of the contractile ring, filamentous actin (F-actin). We found that the cortical PAR proteins are required for the retention of anillin and septin in the anterior pole, which are cytokinesis proteins that our genetic data suggest act as inhibitors of F-actin at the contractile ring. Collectively, our results suggest that the cortical PAR proteins coordinate the establishment of cell polarity with the physical process of cytokinesis during asymmetric cell division to ensure the fidelity of daughter cell formation.
Introduction
Cytokinesis, the physical division of one cell into two, occurs trillions of times from fertilization to death, and division failures can have significant consequences, including miscarriage, neurological dysfunction, immunological defects, and cancer (Lacroix and Maddox, 2012; Tormos et al., 2015). Cytokinesis is driven by the constriction of a contractile ring composed of formin-nucleated F-actin and the motor myosin-II, which is primarily positioned and controlled by the mitotic spindle (Green et al., 2012). Divisions in which the daughter cells inherit equal cytoplasmic and cortical components, and thus the same cell fate and size, are referred to as symmetric. In contrast, many cell divisions during embryogenesis and in adult stem cells are asymmetric, resulting in daughter cells with different cell fates and/or cell sizes (Williams and Fuchs, 2013). In metazoans, asymmetric cell division requires G protein–coupled receptors and the partitioning defective (PAR) proteins. The PAR proteins are a conserved set of proteins that segregate to opposing poles of the cell during asymmetric cell division and control the unequal inheritance of cytoplasmic and cortical factors (Macara, 2004; Suzuki and Ohno, 2006; Motegi and Seydoux, 2013).
The Caenorhabditis elegans single-cell zygote has been a seminal system for understanding the molecular regulation of anterior-posterior (A-P) cell polarity and the PAR proteins (Kemphues et al., 1988). In this system, opposing cortical anterior and posterior PAR (aPAR and pPAR, respectively) domains are formed via two genetically and temporally distinct phases: polarity establishment and polarity maintenance (Cuenca et al., 2003). During polarity establishment, the core cortical aPAR proteins (including the scaffolding proteins PAR-3 and PAR-6, the atypical protein kinase PKC-3, and the small GTPase CDC-42) and the core pPAR PAR-2 (a RING finger– and nucleoside triphosphatase–containing protein) are targeted to opposite sides of the cell via anteriorly directed actomyosin-based cortical flows (Hird and White, 1993; Cheeks et al., 2004; Munro et al., 2004). During polarity maintenance, the aPAR proteins and pPAR proteins remain on opposing sides of the cell cortex forming a distinct A-P boundary near the cell equator via mutual inhibition (for extensive reviews of PAR proteins, see Hoege and Hyman, 2013; Motegi and Seydoux, 2013; Rose and Gönczy, 2014).
A role for the cortical PAR proteins in cytokinesis was previously thought to be indirect by controlling spindle length and position (Dechant and Glotzer, 2003). However, emerging evidence suggests that cell polarity has a more active role in cytokinesis, though the underlying molecular mechanisms remain elusive. Perturbations in asymmetrically dividing Drosophila melanogaster neuroblasts revealed that spindle-independent contractile rings can form in association with a G protein–coupled receptor–promoted polarized cap of myosin-II (Cabernard et al., 2010). In C. elegans, depletion of PAR-2 enhances contractile ring constriction defects caused by compromised spindle signaling (Dechant and Glotzer, 2003; Verbrugghe and White, 2007). Moreover, both the aPAR and pPAR proteins “track” with the contractile ring during cytokinesis (Schenk et al., 2010; Pittman and Skop, 2012).
Here, we show that in the asymmetrically dividing C. elegans zygote, cortical A-P polarity protects the cell against cytokinesis failure in the presence of a weakened actomyosin contractile ring. We demonstrate that both the core cortical aPAR (including CDC-42) and pPAR proteins are required for proper F-actin levels at the contractile ring and restrict the localization of two actomyosin-binding and cross-linking proteins, anillinANI-1 and septinUNC-59. We found that anillin and septin have unexpected inhibitory roles in F-actin accumulation at the contractile ring. Thus, we present a model in which cortical PAR proteins protect cytokinesis during asymmetric cell division independent of mitotic spindle length by promoting the anterior retention of anillin and septin away from the contractile ring, thus allowing for robust F-actin accumulation and ring constriction.
Results and discussion
A synergistic interaction between formin and myosin-II mutants during polarity establishment leads to cytokinesis failure
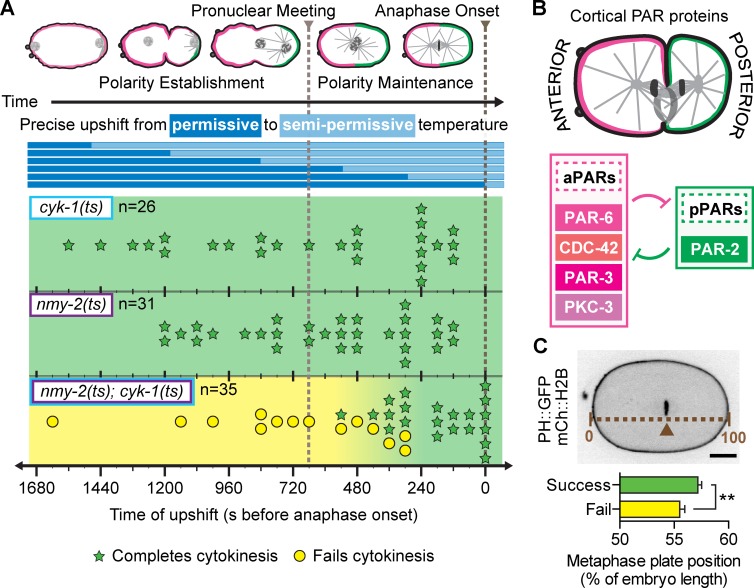
The F-actin nucleator diaphanous-like forminCYK-1 (hereafter formin) and the motor myosin-IINMY-2 (hereafter myosin-II) are known to be critical for cytokinesis (Fig. S1 A; Swan et al., 1998; Shelton et al., 1999; Severson et al., 2002). Using fast-acting temperature-sensitive (ts) mutants with point mutations in the formin dimerization (FH2) domain, required for actin polymerization, and in the myosin-II neck (S2) domain, required for dimerization and head coupling (Liu et al., 2010; Davies et al., 2014), we found that formin and myosin-II are synergistic. At semipermissive temperatures, where the proteins are perturbed but functional enough to allow cytokinesis (Fig. S1 A), 100% of myosin-II(ts) and formin(ts) single mutant embryos divided successfully, whereas ∼40% of myosin-II(ts);formin(ts) double mutant embryos failed to divide, suggesting a synthetic cytokinesis defect (14/35 embryos; Figs. 1 A and S1 B).
Figure 1.
Polarity establishment supports robust cytokinesis. (A) Experimental protocol and rapid temperature upshift results. Each symbol (star or circle) represents a single embryo plotted at the time of upshift. (B) Schematic of cortical PAR protein localization during asymmetric cell division. (C) The metaphase plate (arrowhead) in myosin-II(ts);formin(ts) embryos that complete cytokinesis (n = 21) is positioned more asymmetrically than in those that fail (n = 14; mean ± SD). **, P < 0.005. Bar, 10 µm. cyk-1 = formin; nmy-2 = myosin-II.
To identify the time of requirement for this synthetic interaction, we used a fluidic device (Davies et al., 2014) to rapidly upshift (<20 s) myosin-II(ts);formin(ts) double mutant embryos to this semipermissive temperature at precise time points during cell division. Unexpectedly, we found that this synthetic interaction and cytokinesis failure occur only when myosin-II and formin are both disrupted well before cytokinesis, during establishment and maintenance of A-P polarity (Fig. 1 A). Upshift during polarity establishment caused cytokinesis failure in all myosin-II(ts);formin(ts) double mutants (7/7). Upshift during polarity maintenance caused an intermediate failure rate (7/22), with all failures seen in earlier upshifts. Upshift after the polarity maintenance phase did not cause cytokinesis failure in any double mutants (0/6). Thus, the synthetic effect is strictly limited to the time period correlated with polarity establishment and maintenance and not during cytokinesis directly.
Polarity establishment is dependent on myosin-II but independent of formin activity (Fig. S1 C; Cuenca et al., 2003; Munro et al., 2004; Velarde et al., 2007; Liu et al., 2010). Thus, we hypothesized that synthetic cytokinesis failure is caused by myosin-II function as a polarity protein, synergizing with an independent formin function. Consistent with this hypothesis, we found that the myosin-II(ts);formin(ts) double mutant embryos that failed in cytokinesis were also less polarized, with more centrally located metaphase plates relative to those that succeeded in cytokinesis (Fig. 1 C). It is unlikely that this early interaction represents a delay in functional inhibition, as both ts mutants are very fast acting and show a full loss of function phenotype within seconds upon upshift to restrictive temperature (Davies et al., 2014). Furthermore, cytokinesis completed successfully when embryos were upshifted to restrictive temperature during polarity establishment then downshifted to permissive temperature before anaphase onset (Fig. S1 D). Collectively, these results suggest that establishment of A-P polarity (very early in the cell cycle, before nuclear envelope breakdown) is required for efficient contractile ring constriction during cytokinesis, a significantly later cell cycle event.
Core cortical PAR proteins are required for cytokinesis when formin activity is weakened
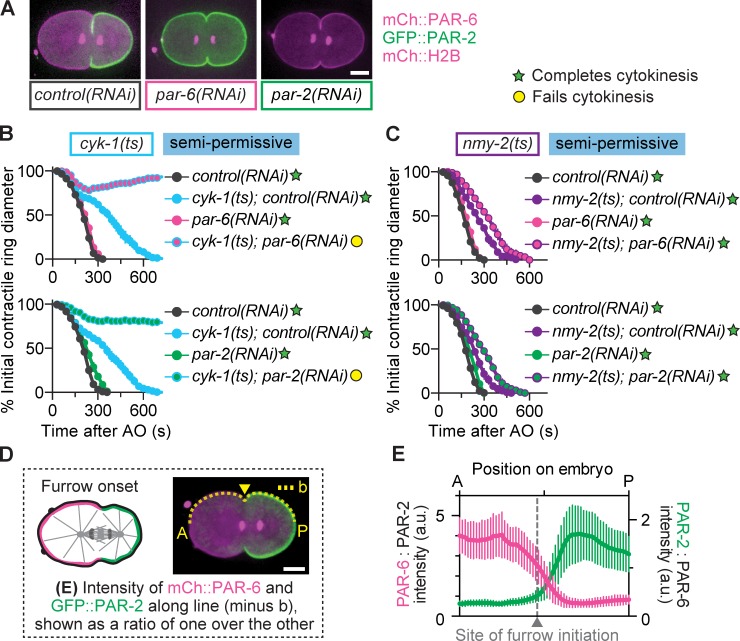
Establishment of A-P polarity requires both core cortical aPAR and pPAR proteins (Figs. 1 B and 2 A). Myosin-II itself is a cortical aPAR, required for polarity establishment and maintenance and localizing to the anterior cortex (Guo and Kemphues, 1996; Cuenca et al., 2003; Liu et al., 2010). To directly test a role for A-P polarity in cytokinesis, we RNAi depleted five different cortical PAR proteins in either formin(ts) or myosin-II(ts) mutants to assess these synthetic interactions for cytokinesis function. Depletion of the aPAR proteins (PAR-6, PAR-3, PKC-3, or CDC-42) or pPAR (PAR-2) caused cytokinesis failure in formin(ts) embryos at semipermissive temperature, with little to no contractile ring constriction (Figs. 2 B and S3 B). In contrast, PAR depletion slowed but did not block cytokinesis in myosin-II(ts) embryos at semipermissive temperature (Figs. 2 C and S3 B). Depletion of each PAR alone, in the absence of another mutation, did not cause cytokinesis failure (Fig. 2, B and C; and Fig. S3 B), consistent with a synthetic effect. Successful PAR depletion was confirmed by four hallmarks of loss of polarity (see Materials and methods sections RNAi and Image analysis; Fig. 2 A; Fig. S2, A–D; and Fig. S3 A). These data suggest that myosin-II and the core cortical PAR proteins act together during A-P polarity establishment to regulate cytokinesis synergistically with formin. That is, the cortical PAR and formin pathways each contribute to cytokinesis, and weakening either one alone is not sufficient to cause cytokinesis failure, but weakening both pathways prevents cytokinesis.
Figure 2.
PAR proteins protect against cytokinesis failure when formin activity is reduced. (A) RNAi depletion of cortical PAR proteins disrupts A-P polarity. (B and C) Depletion of cortical PAR proteins leads to cytokinesis failure in formin(ts) (B) but not myosin-II(ts) mutant embryos (C) at semipermissive temperature (n = 10, all conditions; mean ± SEM). (D) Schematic of cortical line scan used for analysis in E. The arrowhead points to the site of initial furrow formation. (E) The A-P polarity boundary, where PAR-2 levels start to increase relative to PAR-6 levels, coincides with the site of furrow onset (gray line; n = 10; mean ± SD). Bars, 10 µm. AO, anaphase onset; a.u., arbitrary units; b, background. cyk-1 = formin; nmy-2 = myosin-II.
Why are both aPAR proteins and pPAR proteins required for robust cytokinesis when formin activity is weakened? One possibility is that cytokinesis in asymmetrically dividing cells requires opposing cortical PAR domains or the A-P polarity boundary, maintained by mutual aPAR and pPAR exclusion. If so, the A-P polarity boundary might act as a special site that facilitates contractile ring assembly and constriction. Indeed, we found that furrow initiation occurred near the A-P polarity boundary (where PAR-6 levels are decreasing and PAR-2 levels begin to increase), suggesting a possible association between the A-P boundary and the initial site of contractile ring assembly (Fig. 2, D and E).
Cytoplasmic polarity and spindle length are not key regulators of cytokinesis when formin activity is compromised
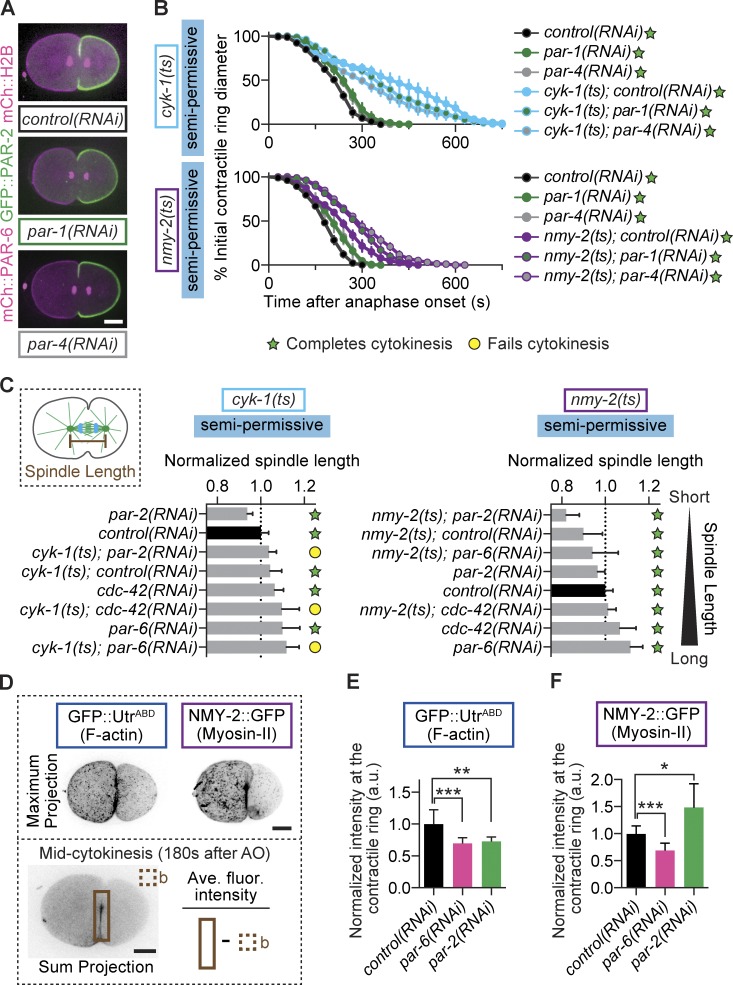
To identify factors downstream of the core cortical PAR proteins during cytokinesis, we first examined PAR-1 and PAR-4, which mediate the asymmetric inheritance of key cytoplasmic components (e.g., RNA granules) and differential daughter cell cycle timing (Fig. S2, E and F) but are dispensable for cortical A-P polarity in the one-cell embryo (Fig. 3 A). PAR-1 localizes to the posterior cell cortex (Boyd et al., 1996); PAR-4 localizes uniformly on the cortex (Watts et al., 2000) and has been shown to regulate myosin-II during cytokinesis (Chartier et al., 2011; Pacquelet et al., 2015). We found that depletion of either PAR-1 or PAR-4, alone or in combination with the formin(ts) or myosin-II(ts) mutation, did not affect cytokinesis (Fig. 3 B), suggesting that this role in cytokinesis is specific to the core cortical PAR proteins.
Figure 3.
The cortical PAR proteins regulate F-actin in the contractile ring during cytokinesis and not via downstream PAR proteins or spindle length. (A) Depletion of neither PAR-1 nor PAR-4 disrupts A-P cortical polarity. (B) RNAi depletion of PAR-1 or PAR-4 does not lead to cytokinesis failure in formin(ts) or myosin-II(ts) mutant embryos at semipermissive temperatures (n = 10, all conditions; mean ± SEM). (C) Spindle lengths for all conditions arranged from shortest to longest at the formin(ts) or myosin-II(ts) semipermissive temperature. Error bars represent SD. (D) Schematic of region and formula used for analysis shown in E and F. The boxed area represents the region used for measurements in the contractile ring. (E) Depletion of PAR-6 or PAR-2 reduces F-actin levels in the contractile ring relative to controls (control(RNAi) n = 13, par-6(RNAi) n = 7, par-2(RNAi) n = 7; mean ± SD). (F) Depletion of PAR-6 or PAR-2 has opposing effects on myosin-II levels in the contractile ring, relative to controls (control(RNAi) n = 13, par-6(RNAi) n = 7, par-2(RNAi) n = 7; mean ± SD). *, P < 0.05; **, P < 0.005; ***, P < 0.0005. Bars, 10 µm. AO, anaphase onset; b, background. cyk-1 = formin; nmy-2 = myosin-II.
It was previously suggested that cortical PAR proteins indirectly regulate cytokinesis by increasing spindle length and astral separation (Dechant and Glotzer, 2003; Lewellyn et al., 2010). We did not find any correlation between reduced spindle length and the success or failure of cytokinesis after cortical PAR depletion (Fig. 3 C). Although PAR-2 depletion decreased spindle length and caused cytokinesis failure in formin(ts) mutants, PAR-6 depletion increased spindle length and also caused cytokinesis failure (Fig. 3 C). Similarly, although PAR-2 depletion in myosin-II(ts) mutants led to very short spindles, cytokinesis did not fail with either PAR-2 or PAR-6 depletion in this mutant (Fig. 3 C). Thus, the effects of core cortical PAR proteins on cytokinesis in this system are not caused by downstream effects on cytoplasmic factors or reduced spindle length.
The core cortical PAR proteins are required for normal F-actin accumulation in the contractile ring
Because the myosin-II–cortical PAR pathway shows a synthetic interaction with the formin pathway during cytokinesis and a major function of formin is actin polymerization, we next tested whether the core cortical PAR proteins regulate F-actin levels in the contractile ring, using GFP-tagged reporters to monitor F-actin and myosin-II (Fig. 3 D). Depletion of either PAR-6 or PAR-2 alone led to a 30% and 27% reduction, respectively, in F-actin levels at the contractile ring (Fig. 3 E), consistent with their synthetic cytokinesis failure phenotypes. Effects on myosin-II levels were inconsistent between PAR-6 and PAR-2, as depletion of PAR-6 led to a 31% decrease and PAR-2 led to a 48% increase in myosin-II levels in the contractile ring (Fig. 3 F), as expected from their opposing effects on global cortical myosin-II levels (Munro et al., 2004). These data suggest that both the cortical aPAR and pPAR proteins promote normal F-actin levels at the contractile ring in asymmetrically dividing cells and that this function is synthetic with compromised formin activity during cytokinesis.
The cortical PAR proteins mediate the anterior retention of anillin and septin and restrict their targeting to the contractile ring
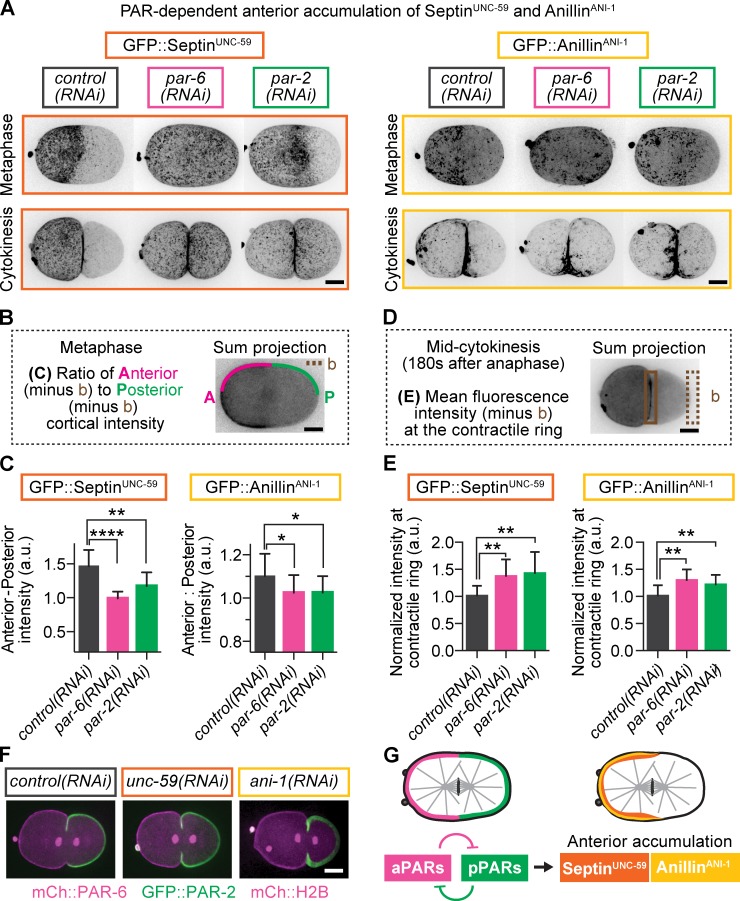
How do the PAR proteins regulate F-actin levels at the contractile ring? The anterior of the early embryo is enriched for several actomyosin-binding and regulatory proteins (Munro et al., 2004). Starting from a list of genes that genetically interact with the cortical PAR proteins (Fievet et al., 2013), we next identified asymmetric (anterior) enrichment of two actomyosin-binding and cross-linking proteins implicated in cytokinesis: septinUNC-59 (hereafter septin) and anillinANI-1 (hereafter anillin; Fig. 4 A–C; and Fig. S3, C and D; Oegema et al., 2000; Field et al., 2005; Straight et al., 2005; Maddox et al., 2007; Bridges and Gladfelter, 2015). We found that this anterior localization of septin and anillin is dependent on the cortical PAR proteins (Fig. 4, A–C; and Fig. S3, C and D). That is, depletion of PAR-6 or PAR-2 led to a decrease in anterior enrichment and increase in posterior enrichment of GFP::septin and GFP::anillin during metaphase (Fig. 4, A–C; and Fig. S3, C and D). During furrow onset, septin and anillin remained polarized in control embryos (Fig. S3, E and F). In contrast, PAR-6 or PAR-2 depletion decreased the polarization of septin and anillin and increased their levels at the contractile ring both at furrow onset (Fig. S3, E and F) and during ring constriction (37% and 42% increases in septin and 29% and 21% increases in anillin after PAR-6 and PAR-2 depletion, respectively; Fig. 4, A, D, and E). Importantly, depletion of neither septin nor anillin disrupted A-P polarity (by PAR-6 and PAR-2 distribution), suggesting that these proteins are downstream of the cortical PAR proteins (Fig. 4 F). Thus, the core cortical PAR proteins are required for the asymmetric anterior localization of both septin and anillin (Fig. 4 G) and to restrict their accumulation at the contractile ring during cytokinesis.
Figure 4.
PAR proteins are required to retain septin and anillin in the cell anterior away from the contractile ring. (A) Representative images of maximum projections of GFP::septinUNC-59 and GFP::anillinANI-1 at metaphase and during cytokinesis, with and without A-P polarity. (B) Schematic of anterior and posterior cortical line scans used for analysis shown in C. (C) Ratio of anterior to posterior cortical fluorescence of septin and anillin with and without PAR-6 and PAR-2 depletion (n ≥ 10, all conditions; mean ± SD). (D) Schematic of region and formula used for analysis shown in E. The boxed area represents the region used for measurement in the contractile ring. (E) Septin and anillin levels in the contractile ring increase a disruption of A-P polarity. Error bars represent SD. (F) Depletion of septin or anillin does not disrupt A-P polarity. (G) Anterior enrichment of septin and anillin is PAR dependent but not vice versa. *, P < 0.05; **, P < 0.005; ****, P < 0.0001. Bars, 10 µm. a.u., arbitrary units; b, background. ani-1 = anillin; unc-59 = septin.
Septin and anillin negatively regulate F-actin levels in the contractile ring
Because we found that the core cortical PAR proteins modulate F-actin levels at the contractile ring and are also required to spatially restrict septin and anillin, we next tested whether septin and anillin also modulate F-actin and/or myosin-II levels in the contractile ring. Unexpectedly, RNAi-mediated depletion of either septin or anillin led to increased F-actin levels in the contractile ring (20% and 19%, respectively; Fig. 5, A and B), suggesting that these two proteins inhibit F-actin accumulation. As previously published, depletion of septin or anillin led to a 24% increase or no change (respectively) in myosin-II levels in the ring (Fig. 5, A and C; Maddox et al., 2007; Lewellyn et al., 2011). These results demonstrate that PAR-6 and PAR-2 have opposite effects on spindle length and myosin-II localization but similar effects on F-actin, septin, and anillin levels in the contractile ring during cytokinesis. These results further suggest that septin and anillin function as negative regulators of F-actin accumulation at the contractile ring during cytokinesis, and their PAR-dependent anterior polar retention allows proper F-actin accumulation at the ring during asymmetric cell division.
Figure 5.
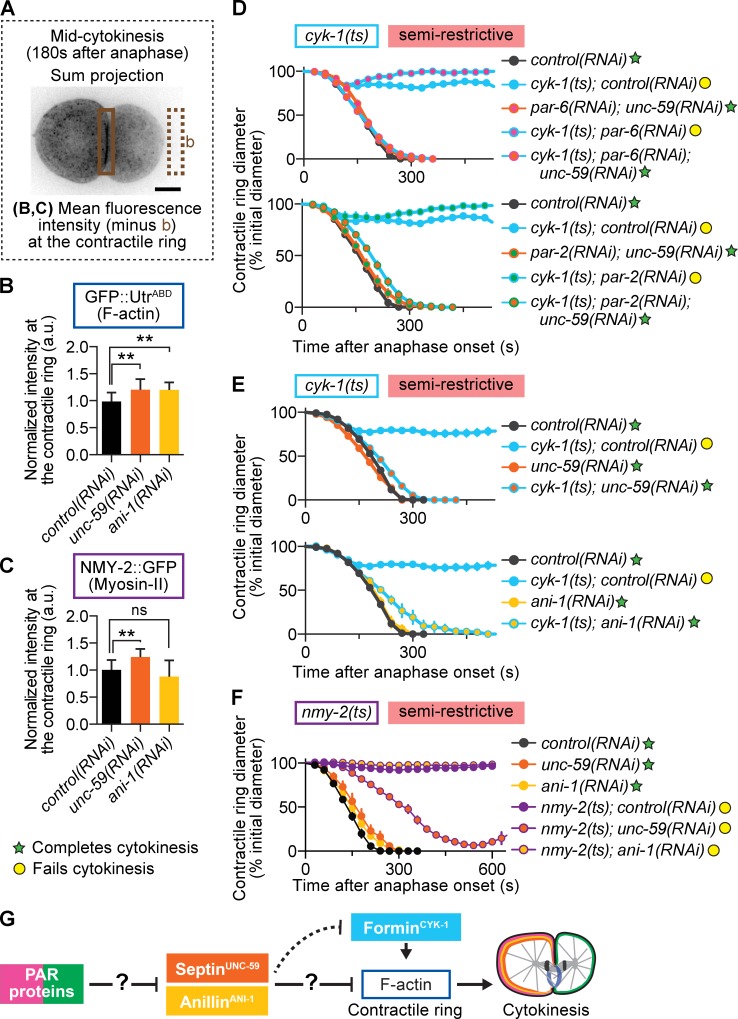
Septin and anillin restrict contractile ring F-actin levels and negatively regulate cytokinesis. (A) Schematic of region and formula used for analysis shown in B and C. The boxed area represents the region used for measurement in the contractile ring. (B and C) Depletion of septin and anillin increased F-actin levels in the contractile ring (B), whereas only septin depletion increased myosin-II levels in the ring (C; n ≥ 10, all conditions; mean ± SD). **, P < 0.005; ns, not significant. (D) Depletion of septin suppresses cytokinesis failure in formin(ts) mutant embryos at semirestrictive temperature, even when A-P polarity is simultaneously disrupted (control(RNAi) n = 9, cyk-1(ts);control(RNAi) n = 11, par-6(RNAi);unc-59(RNAi) n = 11, cyk-1(ts);par-6(RNAi) n = 3, cyk-1(ts);par-6(RNAi);unc-59(RNAi) n = 12, par-2(RNAi);unc-59(RNAi) n = 10, cyk-1(ts);par-2(RNAi) n = 4, cyk-1(ts);par-2(RNAi);unc-59(RNAi) n = 12; mean ± SEM). (E) Depletion of septin and anillin suppresses cytokinesis failure in formin(ts) mutant embryos at semirestrictive temperature (n = 10, all conditions; mean ± SEM). (F) RNAi-mediated depletion of neither anillin nor septin suppresses cytokinesis failure in myosin-II(ts) mutant embryos at semirestrictive temperature (n ≥ 8, all conditions; mean ± SEM). (G) In our genetic model, PAR proteins inhibit septin and anillin localization in the contractile ring by promoting their anterior retention. This prevents septin and anillin from inhibiting F-actin and thereby promotes robust cytokinesis. The question marks reflect that the mechanism of action, whether via a direct interaction or other intermediate players in the pathway, is unknown. Bar, 10 µm. a.u., arbitrary units; b, background. cyk-1 = formin; nmy-2 = myosin-II; ani-1 = anillin; unc-59 = septin.
Depletion of septin and anillin suppresses cytokinesis failure in formin mutants with and without the cortical PAR proteins
We finally predicted that if loss of cortical PAR proteins enhances cytokinesis failure in formin mutants by increasing the levels of septin and anillin in the contractile ring, then depletion of septin and/or anillin should rescue cytokinesis failure in formin(ts) mutants lacking A-P polarity. Consistent with this prediction, we found that depletion of septin completely rescued cytokinesis failure in formin(ts);par-6(RNAi) and formin(ts);par-2(RNAi) zygotes, even at semirestrictive temperature when formin(ts) mutants alone failed in cytokinesis (Figs. 5 D and S1 A). These results suggest that the synthetic interaction between loss of formin activity and loss of PAR proteins is dependent on septin. Thus, the major contribution of the cortical PAR proteins to cytokinesis may be to inhibit septin because in the absence of septin, the PAR proteins are dispensable. Indeed, depletion of septin or anillin rescues cytokinesis failure in formin(ts) mutants but not in myosin-II(ts) mutants (Fig. 5, E and F). Although depletion of septin (but not anillin) led to increased contractile ring constriction in myosin-II(ts) embryos at semirestrictive temperature, cytokinesis ultimately failed in 100% of embryos (Fig. 5 F). This is consistent with the increase in contractile ring myosin-II levels observed after septin (but not anillin) depletion (Fig. 5 C). Collectively, our results suggest a model in which opposing cortical aPAR and pPAR proteins promote robust cytokinesis during asymmetric cell division by mediating the localization of septin and anillin to the cell anterior (Fig. 5 G). Further experiments will be necessary to determine whether this is a direct or indirect effect.
Although we were initially surprised by the inhibitory role for anillin and septin during cytokinesis, there is little consensus for a positive regulatory role for these proteins in cell division. Anillin homologues are not required for contractile ring assembly and constriction in many systems, including budding yeast (Norden et al., 2006), fission yeast (Chang et al., 1996), many C. elegans cell types (Maddox et al., 2007), some cells in Drosophila (Field et al., 2005; O’Farrell and Kylsten, 2008), zebrafish ganglion cell progenitors (Paolini et al., 2015), and even HeLa cells, in which loss of anillin causes cytokinesis failure in only 15–50% of cells (Straight et al., 2005; Piekny and Glotzer, 2008). The septins, originally identified in the asymmetrically dividing budding yeast, are required in that system for mother–daughter cell separation but not for actomyosin contractile ring constriction (Wloka et al., 2011). Septins are also dispensable for most cytokinetic events in fission yeast (Wu et al., 2010), worms (Nguyen et al., 2000; Maddox et al., 2007), some Drosophila cell types (Field et al., 2008), and in many human cell types (Menon and Gaestel, 2015). In fact, ring constriction is faster without the septins in some systems (Lewellyn et al., 2011; Wloka et al., 2011). Anillin and septin have also both been implicated in regulating the asymmetry of contractile ring constriction within the division plane (Maddox et al., 2007). This role is quite different than the PAR-dependent asymmetry in daughter cell fate and size that we describe here. Our data suggest that, at least in asymmetrically dividing C. elegans embryos, anillin and septin function as negative regulators of contractile ring assembly and constriction, perhaps because of their ability to cross-link F-actin and/or myosin-II and thus increase drag or filament severing or reduce filament turnover.
Two classic hallmarks of cancer progression are the loss of cell polarity and defects in cytokinesis (Ganem et al., 2007; Davoli and de Lange, 2011; Halaoui and McCaffrey, 2015). Here, we demonstrate that during asymmetric cell division the PAR proteins actively promote robust contractile ring constriction during cytokinesis. This regulation might help protect against division errors and protect the fidelity of critical asymmetric cell divisions, such as in proliferating stem cells. Our work also suggests that the loss of cell polarity increases the incidence of cytokinesis failure and thus could be a cause, rather than an effect, of cancer development.
Materials and methods
Strain maintenance
C. elegans were maintained on standard nematode growth media plates seeded with OP50 Escherichia coli as previously described (Brenner, 1974). Strain names and genotypes used in this study can be found in Table S1. A. Maddox (University of North Carolina, Chapel Hill, Chapel Hill, NC) provided the strains OD159 [GFP::ANI-1] and OD26 [GFP::UNC-59], the latter of which was crossed with a strain containing the histone marker ltIs37[pAA64; pie-1/mCHERRY::his-58; unc-119 (+)] to generate JCC425. M. Glotzer (University of Chicago, Chicago, IL) provided a strain containing the F-actin marker mgSi3[tb-unc-119(+) GFP::Utrophin], which was crossed with a strain containing the histone marker ltIs37[pAA64; pie-1/mCHERRY::his-58; unc-119 (+)] to generate JCC719.
Temperature control
Control and ts strains were maintained in an incubator (Binder) at a permissive temperature (16.0 ± 0.5°C), except JCC744, JCC719, JCC541, JCC425, and OD159, which were maintained at room temperature to prevent the silencing of fluorescent reporters. Live imaging was performed in a room with homeostatic temperature control set to the desired temperature at least 1 h before the experiment. The temperature of the specimen was continuously monitored using three thermometers attached directly to the objective lens with a glue gun. Where denoted throughout the paper, room temperatures were as follows: formin(ts), semipermissive temperature (18.5 ± 0.5°C); formin(ts), semirestrictive temperature (23.5 ± 0.5°C); myosin-II(ts), semipermissive temperature (22.0 ± 0.5°C); myosin-II(ts), semirestrictive temperature (25.5 ± 0.5°C) and fully restrictive temperature (26.5 ± 0.5°C). During Therminator experiments (Fig. 1 A; and Fig. S1, B and D), the room was maintained at the formin(ts) semipermissive temperature (18.5 ± 0.5°C; Davies et al., 2014).
Rapid temperature shifts
Rapid temperature shifts were performed using a custom-built fluidic system called the Therminator (Bioptechs; Davies et al., 2014) with one water/isopropanol bath maintained at permissive temperature (16.0 ± 0.5°C) and a second bath at the semipermissive temperature (18.5 ± 0.5°C; Figs. 1 A and S1 B) or restrictive temperature (26.5 ± 0.5°C; Fig. S1 D). A switch mechanism determines which water bath supplies liquid for the chamber above the specimen. Forced heat convection from the flow chamber to the glass barrier directly above the specimen chamber rapidly shifts the sample temperature.
Live cell imaging
Young gravid hermaphrodites were dissected in a 16°C M9 buffer (Brenner, 1974) and mounted on a thin 2% agar pad as previously described (Gönczy et al., 1999; Davies et al., 2014). Embryos were filmed using a spinning disc confocal unit (CSU-10; Yokogawa Electric Corporation) with Borealis (Spectral Applied Research) on an inverted microscope (Ti; Nikon) with a 60× 1.4 NA oil-immersion Plan Apochromat objective with 2 × 2 binning on a charge-coupled device camera (Orca-R2; Hamamatsu Photonics). During temperature shift experiments, a 20× 0.75 NA dry objective was used. Z-sectioning was done with a Piezo-driven motorized stage (Applied Scientific Instrumentation), and focus was maintained using Perfect Focus (Nikon) before each z-series acquisition. An acousto-optic tunable filter was used to select the excitation light of two 100-mW lasers for excitation at 491 and 561 nm for eGFP and mCherry, respectively (Spectral Applied Research), and a filter wheel was used for emission wavelength selection (Sutter Instrument). The system was controlled by MetaMorph software (Molecular Devices).
A central differential interference contrast image and a through-cell fluorescent z-series were collected every 30 s to measure contractile ring dynamics (7× 2.0-µm steps; Fig. 2, B and C; Fig. 3 B; Fig. 5, D–F; Fig. S1 A; and Fig. S3 B) or every 60 s for Therminator upshifts (5× 2.0-µm steps; Fig. 1 A; and Fig. S1, B and D) and measurement of fluorescent reporter accumulation in the cell poles and contractile ring (11× 2.5-µm steps; Fig. 2, D and E; Fig. 3, D–F; Fig. 4, B–E; Fig. 5, A–C; and Fig. S3, C–F). 3× 1.0-µm steps were acquired at the cell cortex for Fig. S1 C.
Image analysis
MetaMorph and ImageJ (National Institutes of Health) software were used for all data analysis. Metaphase plate positions were measured 30 s before anaphase onset. In Fig. 1 C, positions are shown relative to the A-P axis with the anterior pole being 0% and the posterior pole being 100%. In Fig. S2 (A and C), the distance between the posterior pole and the metaphase plate was subtracted from the distance between the anterior pole and the metaphase plate. Positions reflect the displacement of the metaphase plate from the cell center (0 µm).
Contractile ring diameter was measured at the cell equator from anaphase onset until, in the case of cytokinesis completion, anaphase of the subsequent cell cycle or, in the case of cytokinesis failure, the contractile ring had completely regressed and the next cell cycle began in the aneuploid cell. The ring diameter was measured as the shortest distance between the furrow tips using a fluorescent marker targeted to the plasma membrane (PH::GFP, pleckstrin homology domain; Audhya et al., 2005). For each time point, the z plane at which the ring diameter was widest was used for this measurement. Contractile ring diameter was plotted as a percentage of the initial diameter (at metaphase) as a function of time. Anaphase onset was determined using a fluorescent histone marker (mCherry::Histone2B; Audhya et al., 2007) and was set as the first time point at which distinct sister chromatids became visible.
Cortical localization of fluorescent markers (GFP::anillinANI-1 and GFP::septinUNC-59; Maddox et al., 2007) was measured at metaphase (one frame before anaphase onset) and furrow onset (one frame before formation of a “double membrane furrow” ∼60 s after anaphase onset). A sum projection of seven central planes was generated, and the mean fluorescence was measured along a line scan tracing the cortex from the anterior to the posterior pole. The mean fluorescence was measured outside of the cell and subtracted from the cortical fluorescence. Values along the cortical line scan were then normalized to the line mean for that cell; that is, a homogeneous cortex would have a value of 1 at all points. In Fig. 2 (D and E), the mean fluorescence of the two polarity markers (mCh::PAR-6 and GFP::PAR-2; Schonegg et al., 2007) was measured similarly but are shown as a ratio of one to the other rather than being normalized to the line mean. Fluorescence values around polar bodies were removed from line scans. Polarized accumulation of GFP::anillinANI-1 and GFP::septinUNC-59 (Fig. 4, B and C) was calculated at metaphase as a ratio of the mean fluorescence in the anterior half to the posterior half of the embryo.
The accumulation of contractile ring markers (GFP::UtrophinABD, myosin-IINMY-2::GFP, GFP::septinUNC-59, and GFP::anillinANI-1; Munro et al., 2004; Burkel et al., 2007; Maddox et al., 2007; Tse et al., 2012) was measured 180 s after anaphase onset from a through-cell z-series sum projection (11 planes encompassing the entire cell). The mean fluorescence was measured in a 65-µm2 region that encompassed the contractile ring. The mean fluorescence of an equivalent background region was measured outside of the cell and subtracted from the mean ring fluorescence. Intensities were then normalized to the contractile ring of control(RNAi) embryos imaged on the same day.
Daughter cell size asymmetry was measured at the time of contractile ring closure (0% initial diameter) and is shown as a ratio of the AB (anterior daughter cell) length to the P1 (posterior daughter cell) length. Cell cycle asymmetry was measured as the difference in time from anaphase onset in AB to the time of anaphase onset in P1. Spindle lengths were measured 150 s after anaphase onset using transmitted light (differential interference contrast) to visualize the spindle poles. Distances were normalized to the spindle length of controls at the same temperature.
RNAi
Exonic sequences from the desired gene were cloned into the multiple cloning site of the L4440 vector using standard cloning techniques and then transformed into HT115 E. coli using CaCl2 transformation as previously described (Timmons et al., 2001). RNAi primers and template DNA for each gene are listed in Table S2. When available, RNAi constructs were obtained directly from the Ahringer RNAi library (Kamath and Ahringer, 2003). For double RNAi constructs (par-6;unc-59 and par-2;unc-59), exonic sequences for either par-6 or par-2 were cloned into the multiple cloning site of pJC55 (L4440 containing unc-59), which was linearized via EcoRI digestion.
RNAi feeding bacteria were grown in Luria broth with ampicillin (100 µg/ml) for 8–16 h at 32°C. 300 µl of this culture was plated on RNAi plates (nematode growth media agar plates [Brenner, 1974] supplemented with 50 µg/ml ampicillin and 1 mM IPTG). These plates were allowed to dry and grow at 32°C for 24–48 h. L1 worms were plated on RNAi plates and then incubated at 16°C for 72 h before filming. Successful RNAi-mediated knockdown of PAR proteins was confirmed using four hallmarks of the loss of polarity (Bossinger and Cowan, 2012): (1) reduced spindle displacement at metaphase, (2) loss of daughter cell size asymmetry after division, (3) high embryonic lethality, and (4) mislocalized fluorescent reporters for the anterior (mCh::PAR-6) and posterior (GFP::PAR-2) domains and/or loss of cell cycle asynchrony in the AB/P1 cell divisions (Fig. S2).
Statistical analysis
Unpaired two-tailed t tests were conducted using Prism (GraphPad Software).
Online supplemental material
Fig. S1 shows that ts mutants allow tuning of specific protein functions that are necessary for cytokinesis. Fig. S2 shows successful RNAi-mediated knockdown of PAR proteins as confirmed by classic hallmarks of the loss of polarity. Fig. S3 shows that cortical PAR proteins regulate cytokinesis and sequester septin and anillin in the cell anterior. Table S1 contains the strain names and genotypes used in the study. Table S2 details the feeding RNAi constructs used, as well as relevant information for their construction when needed. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.201510063/DC1. Additional data are available in the JCB DataViewer at http://dx.doi.org/10.1083/jcb.201510063.dv.
Supplementary Material
Acknowledgments
We thank all members of the Canman, Shirasu-Hiza, and Dumont laboratories for support; Gregg Gundersen, Clare Waterman, Sriramkumar Sundaramoorthy, and Andy Kummel for critical reading; Natalia Spica, Isaiah Thomas, and Vandana Chand for laboratory assistance; Jennifer Waters for microscopy consultation; and Amy Maddox, Michael Glotzer, and the Caenorhabditis Genetics Center for worm strains.
This work is supported by grants ANR-09-RPDOC-005-01 (Agence Nationale de la Recherche) and FRM-AJE201112 (Fondation pour la Recherche Médicale) and the Mairie de Paris Emergence Program to J. Dumont; grants NIH-R01-GM105775 and NIH-R01-AG045842 (National Institutes of Health) to M. Shirasu-Hiza; and grant NIH-DP2-OD008773 (National Institutes of Health) to J.C. Canman.
The authors declare no competing financial interests.
Author contributions: All experiments were conceived by J.C. Canman and S.N. Jordan with input from J. Dumont, T. Davies, M. Shirasu-Hiza, and Y. Zhuravlev. S.N. Jordan did all experiments unless noted otherwise. J.C. Canman and S.N. Jordan made novel strains used in this study. T. Davies and Y. Zhuravlev did some of the PKC-3 and PAR-1/-4 experiments. J.C. Canman, S.N. Jordan, M. Shirasu-Hiza, and J. Dumont wrote the manuscript.
Footnotes
Abbreviations used in this paper:
- A-P
- anterior-posterior
- aPAR
- anterior PAR
- PAR
- partitioning defective
- pPAR
- posterior PAR
- ts
- temperature sensitive
References
- Audhya A., Hyndman F., McLeod I.X., Maddox A.S., Yates J.R. III, Desai A., and Oegema K.. 2005. A complex containing the Sm protein CAR-1 and the RNA helicase CGH-1 is required for embryonic cytokinesis in Caenorhabditis elegans. J. Cell Biol. 171:267–279. 10.1083/jcb.200506124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audhya A., Desai A., and Oegema K.. 2007. A role for Rab5 in structuring the endoplasmic reticulum. J. Cell Biol. 178:43–56. 10.1083/jcb.200701139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossinger O., and Cowan C.R.. 2012. Methods in cell biology: Analysis of cell polarity in C. elegans embryos. Methods Cell Biol. 107:207–238. 10.1016/B978-0-12-394620-1.00007-2 [DOI] [PubMed] [Google Scholar]
- Boyd L., Guo S., Levitan D., Stinchcomb D.T., and Kemphues K.J.. 1996. PAR-2 is asymmetrically distributed and promotes association of P granules and PAR-1 with the cortex in C. elegans embryos. Development. 122:3075–3084. [DOI] [PubMed] [Google Scholar]
- Brenner S. 1974. The genetics of Caenorhabditis elegans. Genetics. 77:71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges A.A., and Gladfelter A.S.. 2015. Septin form and function at the cell cortex. J. Biol. Chem. 290:17173–17180. 10.1074/jbc.R114.634444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkel B.M., von Dassow G., and Bement W.M.. 2007. Versatile fluorescent probes for actin filaments based on the actin-binding domain of utrophin. Cell Motil. Cytoskeleton. 64:822–832. 10.1002/cm.20226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabernard C., Prehoda K.E., and Doe C.Q.. 2010. A spindle-independent cleavage furrow positioning pathway. Nature. 467:91–94. 10.1038/nature09334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang F., Woollard A., and Nurse P.. 1996. Isolation and characterization of fission yeast mutants defective in the assembly and placement of the contractile actin ring. J. Cell Sci. 109:131–142. [DOI] [PubMed] [Google Scholar]
- Chartier N.T., Salazar Ospina D.P., Benkemoun L., Mayer M., Grill S.W., Maddox A.S., and Labbé J.C.. 2011. PAR-4/LKB1 mobilizes nonmuscle myosin through anillin to regulate C. elegans embryonic polarization and cytokinesis. Curr. Biol. 21:259–269. 10.1016/j.cub.2011.01.010 [DOI] [PubMed] [Google Scholar]
- Cheeks R.J., Canman J.C., Gabriel W.N., Meyer N., Strome S., and Goldstein B.. 2004. C. elegans PAR proteins function by mobilizing and stabilizing asymmetrically localized protein complexes. Curr. Biol. 14:851–862. 10.1016/j.cub.2004.05.022 [DOI] [PubMed] [Google Scholar]
- Cuenca A.A., Schetter A., Aceto D., Kemphues K., and Seydoux G.. 2003. Polarization of the C. elegans zygote proceeds via distinct establishment and maintenance phases. Development. 130:1255–1265. 10.1242/dev.00284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies T., Jordan S.N., Chand V., Sees J.A., Laband K., Carvalho A.X., Shirasu-Hiza M., Kovar D.R., Dumont J., and Canman J.C.. 2014. High-resolution temporal analysis reveals a functional timeline for the molecular regulation of cytokinesis. Dev. Cell. 30:209–223. 10.1016/j.devcel.2014.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davoli T., and de Lange T.. 2011. The causes and consequences of polyploidy in normal development and cancer. Annu. Rev. Cell Dev. Biol. 27:585–610. 10.1146/annurev-cellbio-092910-154234 [DOI] [PubMed] [Google Scholar]
- Dechant R., and Glotzer M.. 2003. Centrosome separation and central spindle assembly act in redundant pathways that regulate microtubule density and trigger cleavage furrow formation. Dev. Cell. 4:333–344. 10.1016/S1534-5807(03)00057-1 [DOI] [PubMed] [Google Scholar]
- Field C.M., Coughlin M., Doberstein S., Marty T., and Sullivan W.. 2005. Characterization of anillin mutants reveals essential roles in septin localization and plasma membrane integrity. Development. 132:2849–2860. 10.1242/dev.01843 [DOI] [PubMed] [Google Scholar]
- Field C.M., Maddox A.S., Pringle J.R., and Oegema K.. 2008. Septins in the metazoan model systems Drosophila melanogaster and Caenorhabditis elegans. The Septins. Hall P.A., Russell S.E.H., and Pringle J.R., editors. John Wiley and Sons, Ltd, Chichester, UK: 147–168. [Google Scholar]
- Fievet B.T., Rodriguez J., Naganathan S., Lee C., Zeiser E., Ishidate T., Shirayama M., Grill S., and Ahringer J.. 2013. Systematic genetic interaction screens uncover cell polarity regulators and functional redundancy. Nat. Cell Biol. 15:103–112. 10.1038/ncb2639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganem N.J., Storchova Z., and Pellman D.. 2007. Tetraploidy, aneuploidy and cancer. Curr. Opin. Genet. Dev. 17:157–162. 10.1016/j.gde.2007.02.011 [DOI] [PubMed] [Google Scholar]
- Gönczy P., Schnabel H., Kaletta T., Amores A.D., Hyman T., and Schnabel R.. 1999. Dissection of cell division processes in the one cell stage Caenorhabditis elegans embryo by mutational analysis. J. Cell Biol. 144:927–946. 10.1083/jcb.144.5.927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R.A., Paluch E., and Oegema K.. 2012. Cytokinesis in animal cells. Annu. Rev. Cell Dev. Biol. 28:29–58. 10.1146/annurev-cellbio-101011-155718 [DOI] [PubMed] [Google Scholar]
- Guo S., and Kemphues K.J.. 1996. A non-muscle myosin required for embryonic polarity in Caenorhabditis elegans. Nature. 382:455–458. 10.1038/382455a0 [DOI] [PubMed] [Google Scholar]
- Halaoui R., and McCaffrey L.. 2015. Rewiring cell polarity signaling in cancer. Oncogene. 34:939–950. 10.1038/onc.2014.59 [DOI] [PubMed] [Google Scholar]
- Hird S.N., and White J.G.. 1993. Cortical and cytoplasmic flow polarity in early embryonic cells of Caenorhabditis elegans. J. Cell Biol. 121:1343–1355. 10.1083/jcb.121.6.1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoege C., and Hyman A.A.. 2013. Principles of PAR polarity in Caenorhabditis elegans embryos. Nat. Rev. Mol. Cell Biol. 14:315–322. 10.1038/nrm3558 [DOI] [PubMed] [Google Scholar]
- Kamath R.S., and Ahringer J.. 2003. Genome-wide RNAi screening in Caenorhabditis elegans. Methods. 30:313–321. 10.1016/S1046-2023(03)00050-1 [DOI] [PubMed] [Google Scholar]
- Kemphues K.J., Priess J.R., Morton D.G., and Cheng N.S.. 1988. Identification of genes required for cytoplasmic localization in early C. elegans embryos. Cell. 52:311–320. 10.1016/S0092-8674(88)80024-2 [DOI] [PubMed] [Google Scholar]
- Lacroix B., and Maddox A.S.. 2012. Cytokinesis, ploidy and aneuploidy. J. Pathol. 226:338–351. 10.1002/path.3013 [DOI] [PubMed] [Google Scholar]
- Lewellyn L., Dumont J., Desai A., and Oegema K.. 2010. Analyzing the effects of delaying aster separation on furrow formation during cytokinesis in the Caenorhabditis elegans embryo. Mol. Biol. Cell. 21:50–62. 10.1091/mbc.E09-01-0089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewellyn L., Carvalho A., Desai A., Maddox A.S., and Oegema K.. 2011. The chromosomal passenger complex and centralspindlin independently contribute to contractile ring assembly. J. Cell Biol. 193:155–169. 10.1083/jcb.201008138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Maduzia L.L., Shirayama M., and Mello C.C.. 2010. NMY-2 maintains cellular asymmetry and cell boundaries, and promotes a SRC-dependent asymmetric cell division. Dev. Biol. 339:366–373. 10.1016/j.ydbio.2009.12.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macara I.G. 2004. Par proteins: partners in polarization. Curr. Biol. 14:R160–R162. 10.1016/j.cub.2004.01.048 [DOI] [PubMed] [Google Scholar]
- Maddox A.S., Lewellyn L., Desai A., and Oegema K.. 2007. Anillin and the septins promote asymmetric ingression of the cytokinetic furrow. Dev. Cell. 12:827–835. 10.1016/j.devcel.2007.02.018 [DOI] [PubMed] [Google Scholar]
- Menon M.B., and Gaestel M.. 2015. Sep(t)arate or not – how some cells take septin-independent routes through cytokinesis. J. Cell Sci. 128:1877–1886. 10.1242/jcs.164830 [DOI] [PubMed] [Google Scholar]
- Motegi F., and Seydoux G.. 2013. The PAR network: redundancy and robustness in a symmetry-breaking system. Philos. Trans. R. Soc. Lond. B Biol. Sci. 368:20130010 10.1098/rstb.2013.0010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro E., Nance J., and Priess J.R.. 2004. Cortical flows powered by asymmetrical contraction transport PAR proteins to establish and maintain anterior-posterior polarity in the early C. elegans embryo. Dev. Cell. 7:413–424. 10.1016/j.devcel.2004.08.001 [DOI] [PubMed] [Google Scholar]
- Nguyen T.Q., Sawa H., Okano H., and White J.G.. 2000. The C. elegans septin genes, unc-59 and unc-61, are required for normal postembryonic cytokineses and morphogenesis but have no essential function in embryogenesis. J. Cell Sci. 113:3825–3837. [DOI] [PubMed] [Google Scholar]
- Norden C., Mendoza M., Dobbelaere J., Kotwaliwale C.V., Biggins S., and Barral Y.. 2006. The NoCut pathway links completion of cytokinesis to spindle midzone function to prevent chromosome breakage. Cell. 125:85–98. 10.1016/j.cell.2006.01.045 [DOI] [PubMed] [Google Scholar]
- O’Farrell F., and Kylsten P.. 2008. Drosophila Anillin is unequally required during asymmetric cell divisions of the PNS. Biochem. Biophys. Res. Commun. 369:407–413. 10.1016/j.bbrc.2008.02.060 [DOI] [PubMed] [Google Scholar]
- Oegema K., Savoian M.S., Mitchison T.J., and Field C.M.. 2000. Functional analysis of a human homologue of the Drosophila actin binding protein anillin suggests a role in cytokinesis. J. Cell Biol. 150:539–552. 10.1083/jcb.150.3.539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacquelet A., Uhart P., Tassan J.P., and Michaux G.. 2015. PAR-4 and anillin regulate myosin to coordinate spindle and furrow position during asymmetric division. J. Cell Biol. 210:1085–1099. 10.1083/jcb.201503006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolini A., Duchemin A.L., Albadri S., Patzel E., Bornhorst D., González Avalos P., Lemke S., Machate A., Brand M., Sel S., et al. 2015. Asymmetric inheritance of the apical domain and self-renewal of retinal ganglion cell progenitors depend on Anillin function. Development. 142:832–839. 10.1242/dev.118612 [DOI] [PubMed] [Google Scholar]
- Piekny A.J., and Glotzer M.. 2008. Anillin is a scaffold protein that links RhoA, actin, and myosin during cytokinesis. Curr. Biol. 18:30–36. 10.1016/j.cub.2007.11.068 [DOI] [PubMed] [Google Scholar]
- Pittman K.J., and Skop A.R.. 2012. Anterior PAR proteins function during cytokinesis and maintain DYN-1 at the cleavage furrow in Caenorhabditis elegans. Cytoskeleton (Hoboken). 69:826–839. 10.1002/cm.21053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose L., and Gönczy P.. 2014. Polarity establishment, asymmetric division and segregation of fate determinants in early C. elegans embryos. WormBook. 10.1895/wormbook.1.30.2 [DOI] [PubMed] [Google Scholar]
- Schenk C., Bringmann H., Hyman A.A., and Cowan C.R.. 2010. Cortical domain correction repositions the polarity boundary to match the cytokinesis furrow in C. elegans embryos. Development. 137:1743–1753. 10.1242/dev.040436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonegg S., Constantinescu A.T., Hoege C., and Hyman A.A.. 2007. The Rho GTPase-activating proteins RGA-3 and RGA-4 are required to set the initial size of PAR domains in Caenorhabditis elegans one-cell embryos. Proc. Natl. Acad. Sci. USA. 104:14976–14981. 10.1073/pnas.0706941104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severson A.F., Baillie D.L., and Bowerman B.. 2002. A formin homology protein and a profilin are required for cytokinesis and Arp2/3-independent assembly of cortical microfilaments in C. elegans. Curr. Biol. 12:2066–2075. 10.1016/S0960-9822(02)01355-6 [DOI] [PubMed] [Google Scholar]
- Shelton C.A., Carter J.C., Ellis G.C., and Bowerman B.. 1999. The nonmuscle myosin regulatory light chain gene mlc-4 is required for cytokinesis, anterior-posterior polarity, and body morphology during Caenorhabditis elegans embryogenesis. J. Cell Biol. 146:439–451. 10.1083/jcb.146.2.439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straight A.F., Field C.M., and Mitchison T.J.. 2005. Anillin binds nonmuscle myosin II and regulates the contractile ring. Mol. Biol. Cell. 16:193–201. 10.1091/mbc.E04-08-0758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A., and Ohno S.. 2006. The PAR-aPKC system: lessons in polarity. J. Cell Sci. 119:979–987. 10.1242/jcs.02898 [DOI] [PubMed] [Google Scholar]
- Swan K.A., Severson A.F., Carter J.C., Martin P.R., Schnabel H., Schnabel R., and Bowerman B.. 1998. cyk-1: a C. elegans FH gene required for a late step in embryonic cytokinesis. J. Cell Sci. 111:2017–2027. [DOI] [PubMed] [Google Scholar]
- Timmons L., Court D.L., and Fire A.. 2001. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene. 263:103–112. 10.1016/S0378-1119(00)00579-5 [DOI] [PubMed] [Google Scholar]
- Tormos A.M., Taléns-Visconti R., and Sastre J.. 2015. Regulation of cytokinesis and its clinical significance. Crit. Rev. Clin. Lab. Sci. 52:159–167. [DOI] [PubMed] [Google Scholar]
- Tse Y.C., Werner M., Longhini K.M., Labbe J.C., Goldstein B., and Glotzer M.. 2012. RhoA activation during polarization and cytokinesis of the early Caenorhabditis elegans embryo is differentially dependent on NOP-1 and CYK-4. Mol. Biol. Cell. 23:4020–4031. 10.1091/mbc.E12-04-0268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velarde N., Gunsalus K.C., and Piano F.. 2007. Diverse roles of actin in C. elegans early embryogenesis. BMC Dev. Biol. 7:142 10.1186/1471-213X-7-142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbrugghe K.J., and White J.G.. 2007. Cortical centralspindlin and Gα have parallel roles in furrow initiation in early C. elegans embryos. J. Cell Sci. 120:1772–1778. 10.1242/jcs.03447 [DOI] [PubMed] [Google Scholar]
- Watts J.L., Morton D.G., Bestman J., and Kemphues K.J.. 2000. The C. elegans par-4 gene encodes a putative serine-threonine kinase required for establishing embryonic asymmetry. Development. 127:1467–1475. [DOI] [PubMed] [Google Scholar]
- Williams S.E., and Fuchs E.. 2013. Oriented divisions, fate decisions. Curr. Opin. Cell Biol. 25:749–758. 10.1016/j.ceb.2013.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wloka C., Nishihama R., Onishi M., Oh Y., Hanna J., Pringle J.R., Krauss M., and Bi E.. 2011. Evidence that a septin diffusion barrier is dispensable for cytokinesis in budding yeast. Biol. Chem. 392:813–829. 10.1515/BC.2011.083 [DOI] [PubMed] [Google Scholar]
- Wu J.Q., Ye Y., Wang N., Pollard T.D., and Pringle J.R.. 2010. Cooperation between the septins and the actomyosin ring and role of a cell-integrity pathway during cell division in fission yeast. Genetics. 186:897–915. 10.1534/genetics.110.119842 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.