Abstract
Obesity during pregnancy is associated with an increased risk of short- and long-term metabolic dysfunction in the mother and her offspring. Both higher maternal pregravid body mass index (kg m−2) and excessive gestational weight gain (GWG) have been associated with adverse pregnancy outcomes such as gestational diabetes, preeclampsia and fetal adiposity. Multiple lifestyle intervention trials consisting of weight management using various diets, increased physical activity and behavioral modification techniques have been employed to avoid excessive GWG and improve perinatal outcomes. These randomized controlled trials (RCTs) have achieved modest success in decreasing excessive GWG, although the decrease in GWG was often not within the current Institute of Medicine guidelines. RCTs have generally not had any success with decreasing the risk of maternal gestational diabetes (GDM), preeclampsia or excessive fetal growth often referred to as macrosomia. Although the lack of success for these trials has been attributed to lack of statistical power and poor compliance with study protocols, our own research suggests that maternal pregravid and early pregnancy metabolic condition programs early placenta function and gene expression. These alterations in maternal/placental function occur in the first trimester of pregnancy prior to when most intervention trials are initiated. For example, maternal accrural of adipose tissue relies on prior activation of genes controlling lipogenesis and low-grade inflammation in early pregnancy. These metabolic alterations occur prior to any changes in maternal phenotype. Therefore, trials of lifestyle interventions before pregnancy are needed to demonstrate the safety and efficacy for both the mother and her offspring.
INTRODUCTION
Obesity has become so pervasive that it is now recognized as a major public health concern during pregnancy.1 Obesity is classified as body mass index (BMI), weight in kilograms divided by height in meters squared (kgm−2), using the World Health Organization criteria; underweight BMI < 18.5, normal weight 18.5–24.9, overweight 25.0–29.9, obese class I 30.0–34.9, obese class II 35.0–39.9 and obese class III ≥ 40.2 On the basis of the 2011–2012 National Health and Nutrition Examination Survey (NHANES), the prevalence of obesity in women of reproductive age (20–39 years) in the United States was 31.8% (95% confidence interval (CI) 28.5–35.5) and overweight plus obesity 58.5% (51.4–65.2).3 The prevalence of overweight and obesity is higher in non-Hispanic black and Mexican American women in the United States (Table 1). Although commonly used, the correlation of BMI with body fat within non-pregnant women of reproductive age explains about 50–70% of the variance in fat mass. However, with advancing gestation and the increase in total body water, the correlation becomes progressively less robust.4
Table 1.
Prevalence of obesity by race/ethnicity in women of reproductive age6
| All race/ethnicity groups | Non-Hispanic White | Non-Hispanic Black | Hispanic | Mexican American | |
|---|---|---|---|---|---|
| Body mass index ≥ 30 | |||||
| 20–39 years | 31.9 (28.6–35.5) | 26.9 (23.0–31.3) | 56.2 (44.3–67.5) | 34.4 (30.9–38.2) | 37.8 (33.2–42.7) |
| Body mass index ≥ 25 | |||||
| 20–39 years | 55.8 (49.6–61.9) | 50.7 (43.1–58.2) | 74.2 (65.9–81.1) | 65.4 (59.9–70.5) | 68.8 (62.1–74.8) |
Because obesity during pregnancy has become so prevalent, multiple lifestyle intervention trials (diet, physical activity and behavioral modification) have focused on avoiding excessive gestational weight gain (GWG) as a means to improve maternal metabolic function and perinatal outcomes. Unfortunately, these trials have had only limited success in decreasing perinatal morbidity. Hence, the purpose of this review is to evaluate the maternal and placental metabolism in pregnancy. We plan to demonstrate that lifestyle interventions initiated after the first trimester of pregnancy have minimal effect on placental gene expression and function related to perinatal morbidity.
TRENDS IN MATERNAL AND NEONATAL OBESITY
On the basis of Center for Disease Control and Prevention (CDC) data, there was no significant change in the prevalence of obesity in women of reproductive age from 2003–2004 to 2011–2012.3 From 1999 to 2010, however, there was a trend in the increase in obesity (BMI ≥ 30) in women aged 20–39, that is, 28.4%, (95% CI 24.4–32.4) to 34.0% (95% CI 29.0–39.1) with a higher prevalence in non-Hispanic black and Mexican American women.5 Of greater concern is the increasing prevalence of class II (BMI ≥ 35, 17.2% (95% CI 14.2–20.7)) and class III obesity (BMI ≥ 40, 7.5% (95% CI 5.8–9.7)) in women aged 20–39 in 2009–2010.6
Approximately 17% of US children and adolescents are obese; they have a BMI greater than the CDC 95th percentile for age and gender.7 There has also been an increase in mean term birth weight in developed nations.8,9 In Cleveland, we have reported a significant 116 g increase in mean term birth weight since 1975. This increase in birth weight encompasses weights from the 5th through 95th percentiles.10 Furthermore, high maternal pregravid BMI and not GWG was the strongest correlate for a high birth weight. Although some recent studies have published that the increase in birth weight has reached a plateau,11 we reported that this was secondary to increases in minority populations and earlier gestational age of delivery.12 Of potential greater concern is the increase in the Ponderal Index (weight/length3, an estimate of neonatal adiposity, analogous to BMI in the adult) in our neonatal population over the last decade.12
RECOMMENDATIONS FOR WEIGHT GAIN IN PREGNANT OVERWEIGHT AND OBESE WOMEN
Gestational weight gain recommendations in pregnancy were first published by the Institute of Medicine (IOM) in 1990.13 Since that time, there has been a significant increase in the number of women of childbearing age who are overweight or obese. Women are also becoming pregnant at an older age and with an increasing number of chronic medical conditions such as hypertension and diabetes. In 2009, the IOM revised the gestational weight guidelines taking into account more recent literature and specifically the increased proportion of overweight and obesity in women of reproductive age (Table 2). Although the 2009 IOM recommendations for GWG are not dramatically different from the 1990 guidelines, except for obese women, there were other substantive differences. These included using the WHO criteria for defining pregravid BMI2 and eliminating specific recommendations for certain populations including women of short stature, pregnant adolescents and different racial or ethnic groups.13
Table 2.
Recommendations for total and rate of weight gain during pregnancy by prepregnancy BMI13
| Prepregnancy BMI | BMI+ (kg m−2) (WHO) |
Total weight gain range (lbs) |
Rates of weight Gaina 2nd and 3rd trimester (mean range in lbs per week) |
|---|---|---|---|
| Underweight | < 18.5 | 28–40 | 1 (1–1.3) |
| Normal Weight | 18.5–24.9 | 25–35 | 1 (0.8–1) |
| Overweight | 25.0–29.9 | 15–25 | 0.6 (0.5–0.7) |
| Obese (includes all classes) | ≥30.0 | 11–20 | 0.5 (0.4–0.6) |
Abbreviations: BMI, body mass index; WHO, World Health Organization.
Calculations assume a 0.5–2 kg (1.1–4.4 lbs) weight gain in the first trimester.
The recommendations for obese women were based in part on evidence that there is an inverse relationship between maternal pregravid BMI and GWG.14 Additionally, the IOM considered that there was an obligatory physiologic weight gain during pregnancy necessary for a healthy pregnancy, which consisted of approximately 8 kg of water, 1 kg of protein plus variable amounts (1–6 kg) of adipose tissue.13 Although some authors have suggested that less weight gain for obese women than the current IOM recommendations may improve some perinatal outcomes,15 there may be potential fetal risks relating to inadequate GWG in obese women.16
PRE-PREGNANCY OBESITY OR EXCESSIVE GWG RELATING TO FETAL OVERGROWTH
Thirty-eight percent of normal weight, 63% of overweight and 46% of obese women had gained weight in excess of IOM guidelines.17 These data are based on a general population in the United States obtained from the Pregnancy Risk Assessment Monitoring System (PRAMS) and the Pregnancy Nutrition Surveillance System (PNSS). At our hospital in Cleveland, 59% of overweight and 52% of obese women had excessive GWG. These percentages were higher in African American and Hispanic women. In obese women, excessive GWG was a significant risk factor for cesarean delivery and postpartum weight retention, but was not related to the development of preeclampsia or gestational diabetes (GDM).13 High GWG was associated with a modest increased risk of preterm birth, but was not related to an increased risk of fetal overgrowth or macrosomia.13 Excessive GWG is a primary risk factor for maternal post-partum weight retention, which becomes a significant risk factor for maternal pregravid obesity in a subsequent pregnancy.
Obesity in early pregnancy more than doubles the risk of obesity in the offspring between the ages of 2 and 4.18 Boney et al.19 reported that large for gestational age (LGA) neonates of mothers who were obese or developed GDM were at risk for developing the metabolic syndrome between ages 6 and 11. Maternal obesity and not GDM had a twofold increase in the hazard risk for the metabolic syndrome in the offspring, that is, GDM was not independently correlated with the metabolic syndrome in childhood. Dabelea et al.20 reported that maternal obesity in utero is associated with type 2 diabetes in youth, independent of diabetes during pregnancy. We reported that in overweight/obese women, maternal pregravid BMI and not GWG was the greatest risk for fetal macrosomia, more specifically obesity.21 In our 8-year follow-up studies, maternal pregravid BMI, independent of maternal glucose status or GWG, was the strongest predictor of childhood obesity and metabolic dysfunction.22 In a recent meta-analysis, Phillips et al.23 reported that, although maternal diabetes is associated with increased childhood BMI z-score, this was no longer significant when adjusted for maternal prepregnancy BMI. Therefore, maternal pregravid obesity is not only a risk factor for neonatal adiposity at birth, but also for the long-term risk of obesity and metabolic dysfunction in the offspring independent of maternal GDM or excessive GWG.
APPROACHES TO WEIGHT MANAGEMENT IN PREGNANCY COMPLICATED BY OBESITY
During pregnancy, medications for weight management are not recommended because of safety concerns and side effects.24 The classical anorexiants, such as phentermine, alter the release and reuptake of neurotransmitters that impact on appetite. Other drugs such as Orlistat reduce intestinal fat absorption by inhibiting pancreatic lipase. Metformin, which decreases hepatic glucose production, has been associated with decreased GWG in some studies when used to treat mild GDM.25 Metformin has not been used solely to manage GWG and crosses the placenta in significant amounts.25
The primary weight management strategies during pregnancy are dietary control, exercise and behavior modification. These strategies have been used either alone26,27 or in combination28,29 to avoid excessive GWG. Within each of these strategies, there are variations. For example, with diet, some studies have examined the role of food having a low glycemic index,26 whereas others have employed probiotic interventions.30 Unfortunately, based on a recent Cochrane review, the authors conclude that there is not enough evidence to recommend any specific intervention for preventing excessive weight gain in pregnancy, because of methodological limitations, small sample and effect size.31 In general, nutritional strategies are more useful in avoiding excessive GWG during pregnancy in contrast to increased physical activity.32
LIFESTYLE INTERVENTIONS DURING PREGNANCY: ARE THEY SUCCESSFUL?
There have been numerous prospective trials examining lifestyle intervention for obese women during pregnancy. These studies had outcomes which included avoiding excessive GWG and decreasing adverse perinatal outcomes, specifically macrosomia, GDM and hypertensive disorders. For example, the Low Glycemic Index Diet in a pregnancy study to prevent macrosomia evaluated more than 800 women with a history of delivering a >4000 g or a macrosomic infant.33 Women were randomized to Low Glycemic Index Diet or no intervention at 13 weeks. Despite a decrease in GWG (12.2 vs 13.7 kg) in the intervention group, there was no difference in birth weight, birth weight centile, Ponderal Index or macrosomia between the groups. In 2011, a Danish group reported a randomized control lifestyle intervention trial.28 The intervention consisted of dietary guidance, free membership in a fitness center and personal coaching initiated between 10 and 14 weeks’ gestation. Although there was a decrease in GWG in the intervention group (7.0 vs 8.6 kg, P = 0.01), paradoxically, the infants in the lifestyle intervention group had significantly higher birth weight (3742 vs 3593 g, P = 0.04) compared with controls.
In early 2014, three lifestyle intervention randomized controlled trials during pregnancy were published. Only one of these, the treatment (physical activity) of obese women in pregnancy (TOP), showed a modest (1.38 kg, P = 0.04) effect in a multivariate analysis decreasing GWG. There was no significant effect on either birth weight or LGA newborns.34 The pregnancy and glycemic index outcome study (PREGGIO) was a follow-up randomized controlled trial to a small intensive study of 62 women, showing that a low glycemic index diet in the second and third trimesters of pregnancy reduced fetal birth weight/percentile and Ponderal index. In this study, women were assigned to either a low glycemic index diet or healthy eating according to local standards. Approximately one-third of the subjects in either group were overweight or obese. There were no significant differences in the primary outcomes of fetal growth. In a multivariate analysis, the glycemic load was the only significant predictor of fetal growth, but explained less than 1% of the variance.26 The lifestyle intervention advice for women who are overweight or obese (LIMIT) trial had as the primary objective to determine the effect of antenatal lifestyle interventions on health outcomes in overweight and obese pregnant women. Two thousand and twelve women between 10 and 20 weeks were randomized to a comprehensive dietary and lifestyle intervention and 1104 to standard care according to local standards. There was no significant difference in the incidence of LGA neonates between groups (19 vs 21%, P = 0.24). There were fewer infants who were born greater than 4000 g in the intervention as compared to the standard care group (15% vs 19%, P = 0.04). No information, however, was provided on gestational age of delivery in the two groups, as gestational age is a strong correlate of birth weight at term. There were also no significant differences in GWG or proportion of women whose weight gain were below, within or exceeded the IOM GWG recommendations.29
There have been at least five meta-analyses published in the past 3 years of randomized control trials examining lifestyle intervention during pregnancy. All concluded that lifestyle intervention initiated during pregnancy has limited success in reducing excessive GWG, but not necessarily to within IOM guidelines. The literature contains scant evidence to support further benefits for infant or maternal health (fetal overgrowth, GDM or hypertensive disorders including preeclampsia).32,35–38 A Cochrane Review concluded that results from three randomized controlled trials suggested no significant difference in GDM incidence between women receiving exercise intervention vs routine care.39
In summary, lifestyle intervention initiated during pregnancy may reduce some excessive GWG; however, lifestyle interventions have not been successful in reducing fetal overgrowth, GDM or preeclampsia in obese women. Why have these lifestyle interventions not had greater success? Although there is no obvious answer, the sequence of physiological adaptations which occur in normal pregnancy may provide some clues.
NORMAL PHYSIOLOGICAL ADAPTATIONS DURING PREGNANCY
The changes in body composition during pregnancy are primarily driven by adaptations of maternal metabolic homeostasis. The ultimate goal of pregnancy-induced metabolic alterations is to meet the high energy demands of fetal development. Glucose, the primary oxidative fuel used by feto-placental tissues, needs to be readily available for transplacental transfer, whereas maternal tissues can rely on other energy substrates, such as lipids.40 Changes in insulin secretion and action are occurring over the course of pregnancy. The higher insulin sensitivity of early pregnancy facilitates cellular anabolism through activation of lipogenesis. In contrast, the insulin resistance, which culminates in the third trimester, allows adipose tissue to mobilize the lipids stored earlier and maternal skeletal muscle to utilize less glucose.41 These changes in maternal metabolic homeostasis result in increased circulating levels of insulin and triglycerides in late pregnancy.42,43
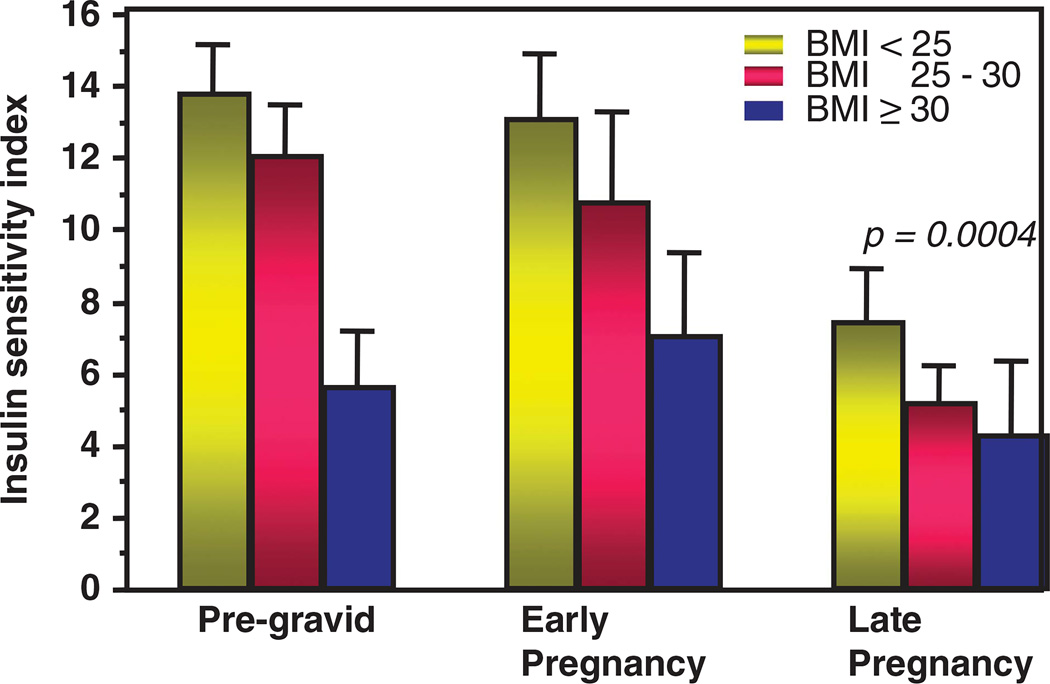
There is a 50–60% decrease in insulin sensitivity with advancing gestation in all pregnant women regardless of pregravid BMI. The decreased insulin sensitivity observed in obese and normal weight women in pregnancy is but a reflection of the maternal pregravid condition.44 The decreased insulin sensitivity in late gestation results in increased nutrient availability, such as glucose and lipids, for the fetus resulting in fetal overgrowth and adiposity (Figures 1 and 2). Because the increased adiposity and decrease in insulin sensitivity in overweight and obese women exists before and during early pregnancy, lifestyle interventions initiated usually in the second trimester are less likely to have any effect on maternal metabolism or metabolic conditions during pregnancy. Not surprisingly, in obese women, maternal pregravid measures of obesity are more strongly correlated with maternal outcome measures such as gestational diabetes, pre-eclampsia and fetal macrosomia as compared with other clinical parameters such as GWG.44 These maternal metabolic alterations during pregnancy are less amenable to lifestyle changes because of the physiological adaptations during pregnancy, the relatively short time between initiation of dietary changes and delivery and the decreased ability to perform significant increased physical activity with advancing gestation.
Figure 1.
The longitudinal changes in insulin sensitivity, pregravid, early pregnancy (12–14 weeks) and late pregnancy (34–36 weeks) as estimated using the hyperinsulinemic–euglycemic clamp in normal weight, overweight and obese women.44
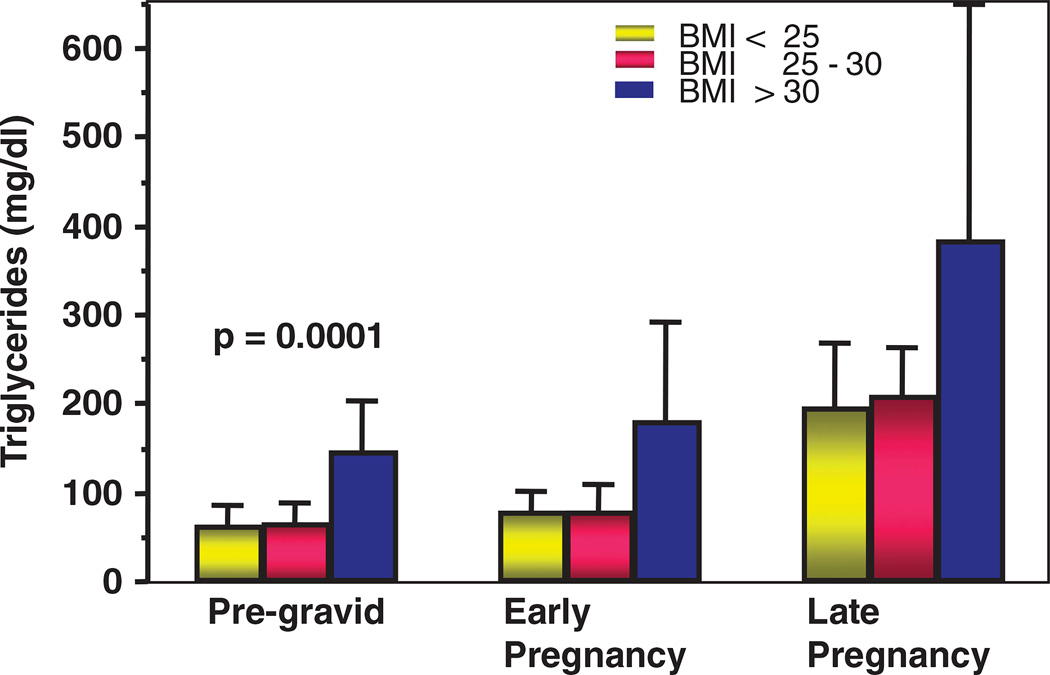
Figure 2.
The longitudinal changes in basal plasma triglyceride concentrations, pregravid, early pregnancy (12–14 weeks) and late pregnancy (34–36 weeks) in normal weight, overweight and obese women.
CELLULAR AND MOLECULAR ADAPTATIONS DURING PREGNANCY
In the pregnant woman, the adaptations of lipid metabolism follow a well-described biphasic pattern. The first half of pregnancy is centered on storing maternal energy as adipose tissue triglycerides, whereas in late pregnancy, the stored lipids are mobilized to be used by peripheral tissues and in preparation for lactation.45 The concentration of all plasma lipids and lipoprotein fractions increase with advancing gestation, with the exception of total non-esterified fatty acids.46 The increase in total cholesterol and trigycerides, very low-density lipoprotein and low-density lipoprotein result in a hyperlipemic environment at the maternal fetal interface.47 This then allows for increased lipid oxidation in maternal liver and subsequent utilization of fatty acids for maternal energy needs. These sequential adaptations are facilitated by modifications in the plasma concentration of steroid hormones with large increases in estradiol and progesterone in healthy pregnancy.48 Estradiol upregulates the secretion of both very low-density lipoprotein hepatic subfractions, resulting in increased plasma trigycerides.46 Maternal obesity is associated with higher plasma concentrations of trigycerides, very low-density lipoprotein-1 and -2 in the first trimester. However, in late pregnancy, plasma trigycerides and trigyceride-rich lipoproteins reach similar elevated concentrations in healthy lean and obese women. Total non-esterified fatty acid concentrations remain unchanged in obese mothers and their fetuses.49 However, there may be subtle differences in the concentration of individual fatty acids, which may be mediated through maternal diet. These data indicate that obese women are less flexible to pregnancy metabolic adaptations, than their normal-weight counterparts.
ROLE OF MATERNAL ADIPOSE TISSUE: METABOLIC AND ENDOCRINE FUNCTIONS
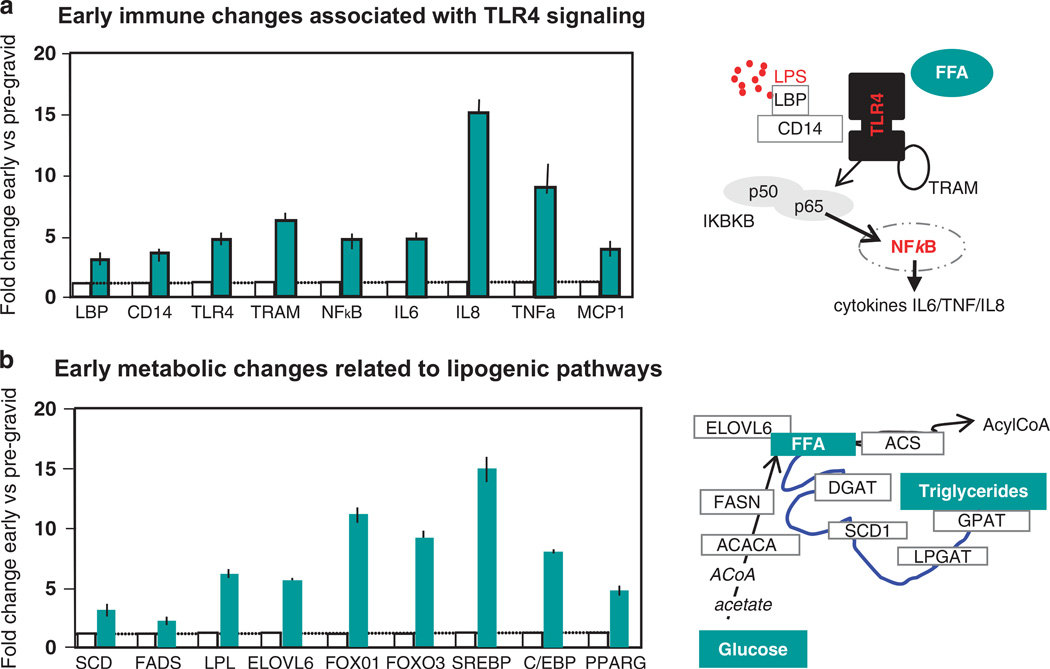
Lean women accrue adipose tissue earlier in pregnancy, whereas, women who are obese are more likely to be insulin-resistant prior to pregnancy and therefore gain less adipose tissue in early gestation.49 Hence, from a population perspective, obese women have less GWG as compared with normal weight women.14 White adipose tissue displays remarkable flexibility with an important and reversible capacity for expansion and contraction throughout adult life. Adaptations of adipose tissue during pregnancy develop in a temporal manner with an array of molecular changes preceding the anthropometric expansion of adipose mass.50 In healthy human pregnancy, early enhancement of adipose tissue and immune response (in comparison with pregravid measures) precedes the appearance of maternal phenotypic changes in body composition and insulin action (Figure 3). This biphasic pattern of early lipogenesis and late pregnancy lipolysis is associated with a sterile low-grade physiological inflammation as an early step towards the later development of physiological insulin resistance which peaks during late pregnancy. A substantial literature has established inflammation as an obligatory component of weight gain and adipose tissue remodeling in obesity and other diseases; low-grade inflammation is also a physiologic adaptation in healthy pregnancy.50
Figure 3.
Adaptations of adipose tissue during pregnancy develop in a temporal manner with an array of molecular changes preceding the anthropometric expansion of adipose mass.51 (a) Longitudinal fold changes in early (12–14 weeks) compared with pregravid measures of immune changes in healthy human adipose tissue during pregnancy. The TLR4 signaling pathway activated by lipopolysaccharide and free fatty acids (FFA) shown on the right of Figure 3a. (b) Longitudinal fold changes in early (12–14 weeks) compared with pregravid measures of lipogenic pathways adipose tissue during pregnancy. The signaling pathways relating to lipogenesis are shown on the right of Figure 3b.
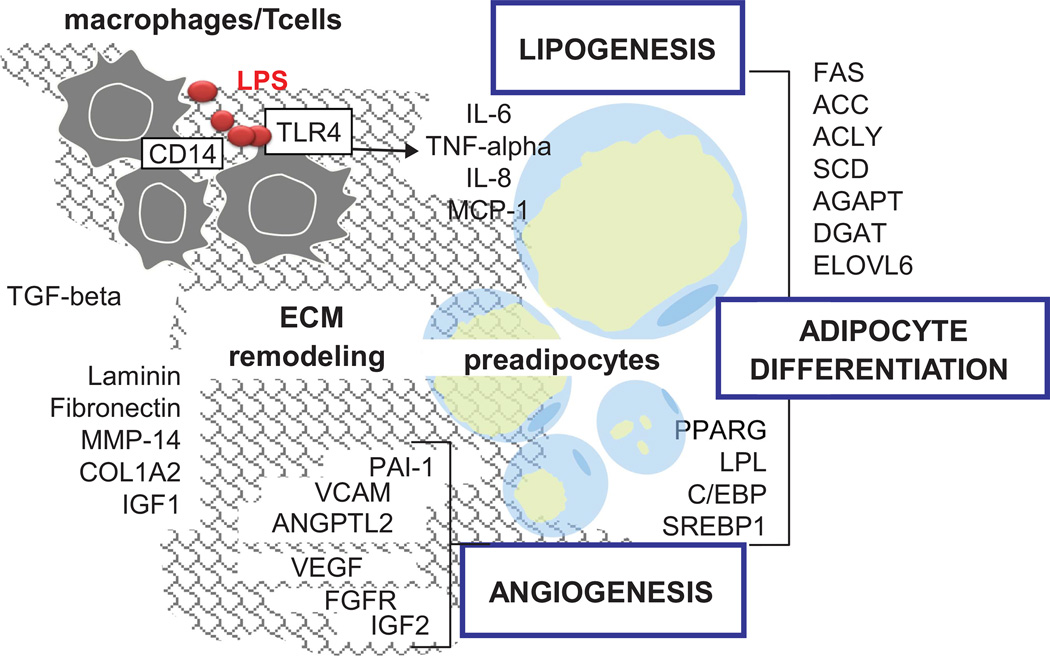
Adipose tissue is an organ demonstrating remarkable plasticity which allows changes in size and structure. Adipose tissue enlargement is a complex process relying on molecular cross-talk between distinct cell types of the stromal-vascular fraction surrounding the adipocytes.51,52 In agreement with this concept, our research suggests that pregnancy-induced adipose tissue expansion involves a combination of cellular mechanisms shared by pre-adipocytes, adipocytes, macrophages and endothelial cells (Figure 4). Different types of cells which are located within the adipose tissue itself contribute to its remodeling during pregnancy. Components of the extracellular matrix, which connect the adipocytes and angiogenic factors, are needed for the vascular growth and differentiation of pre-adipocytes into mature adipocytes which can then store lipids. Lipogenic genes and transcription factors need to be activated to help differentiation and maturation of the small pre-adipocytes into functional adipocytes. The macrophages which infiltrate the stromal cells produce proinflammatory cytokines, interleukin-6 (IL-6), IL-8 and tumor necrosis factor-alpha that may facilitate the development of insulin resistance.
Figure 4.
Pregnancy-related changes in adipose tissue immune network. Depicted are models of cellular networks that contribute to remodeling of adipose tissue in human pregnancy.51 Multiple factors produced by several adjacent cell types cooperate to remodeling of the adipose tissue during pregnancy. Extracellular matrix components and angiogenic factors are needed for vascular and adipocyte growth. Lipogenic genes are required for cell differentiation and lipid storage. Macrophages located outside the adipocytes produce pro-inflammatory cytokines such as Il-6, IL-8 and tumor necrosis factor-alpha that enhance neovascularization and facilitate the development of insulin resistance.
The endocrine function of adipose tissue also evolves during pregnancy. The synthesis and plasma concentration of leptin and adiponectin, two major adipokines, exhibit longitudinal changes parallel to those of insulin sensitivity. Leptin concentrations increase in early pregnancy, and there is a strong positive correlation between plasma leptin and maternal BMI.53 In contrast, adiponectin concentrations decrease with advancing gestation, analogous to decreases in maternal insulin sensitivity.54 Ideally, pregnant women should maintain a positive energy balance to sustain energy requirements for fetal development; therefore, it is not likely that increased leptin concentrations would affect the individual to reduce her food intake as pregnancy progresses. From a pragmatic perspective, it is expected that the rise in maternal leptin levels are not accompanied by the classic central effect in the regulation of food intake.
However, the increase over pregravid plasma values suggests that leptin may be needed for roles that are different from the hypothalamic regulation of appetite suppression.55 Higher maternal leptin concentrations may reflect a state of leptin resistance that is analogous to that of obese individuals whose elevated leptin levels do not successfully regulate energy homeostasis.56 Another possible explanation for the increase of leptin concentrations in pregnancy is the increase in the bound over free ratio in circulating leptin.55 Leptin can bind to an extracellular (soluble) leptin receptor that is released in the circulation by placental membrane shedding.57 The binding to this soluble isoform may delay the clearance of leptin from the circulation, which results in higher maternal plasma levels.
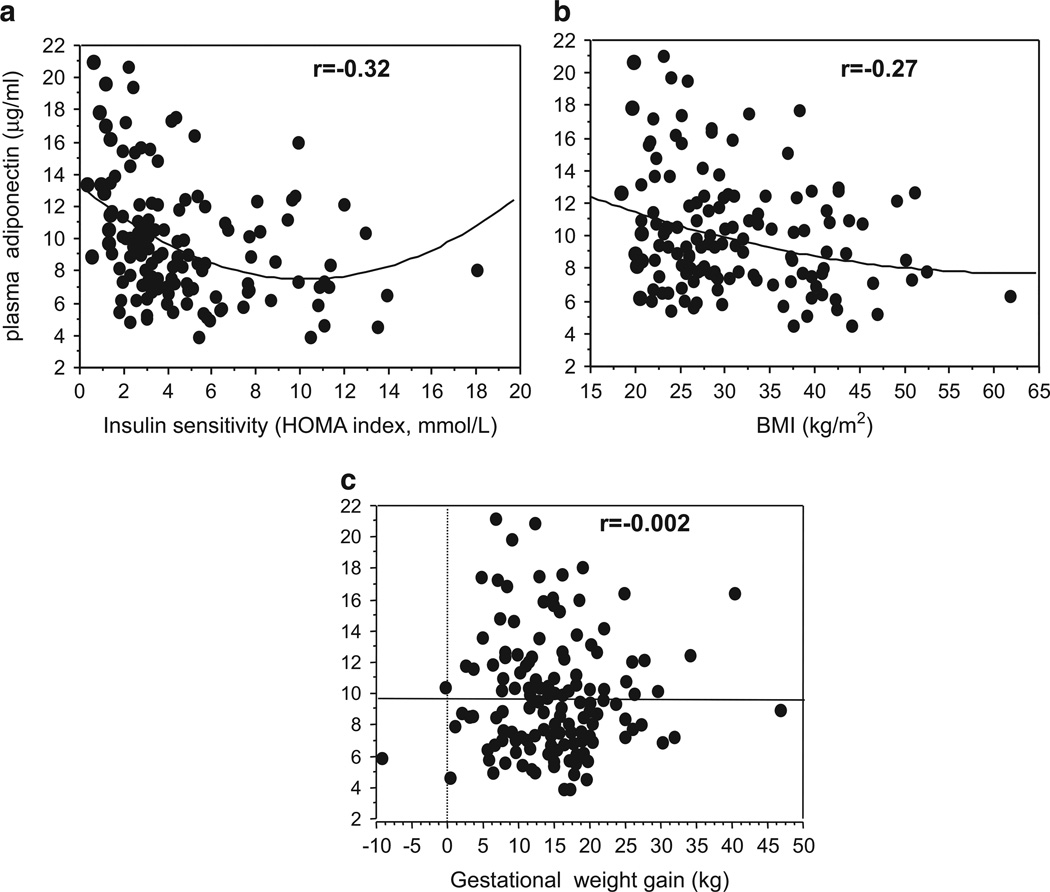
In contrast to leptin, adiponectin plasma concentration decreases in late pregnancy when maternal insulin resistance is highest.54 In contrast with the placenta’s ability to synthesize a large variety of cytokines there is no synthesis of adiponectin in the human placenta. We have proposed that lower maternal plasma adiponectin levels most likely reflect fluctuations in the synthesis and secretion by the maternal white adipose tissue.58 Additionally, inflammatory stress and dietary factors such as a high-fat diet may act as epigenetic regulators of the adiponectin gene downregulating adiponectin synthesis.58 The longitudinal modifications of adipokines in healthy pregnancy are further enhanced in the context of pregnancy associated with diabetes and obesity.59,60 Obesity in late pregnancy is associated with hypo-adiponectinemia which is inversely correlated to maternal insulin sensitivity and BMI but not GWG (Figure 5). The hypoadiponectinemia reflects the decreased expression of adiponectin mRNA and methylation of the adipose adiponectin gene of obese women.58
Figure 5.
The relationship in late pregnancy between maternal plasma adiponectin and maternal: (a) Insulin sensitivity as estimated by Homeostatic Model Assessment-Insulin Resistance (HOMA-IR) r =− 0.32, P=0.27, (b) BMI r =− 0.27, 469 P =0.001 and (c) GWG.
ROLE OF MATERNAL ADIPOSE TISSUE: IMMUNE FUNCTION AND LIPID SIGNALING
Adipose tissue generates a number of signaling molecules which can act within the adipose cells but also systemically. Expansion of the adipose tissue involves the connection and activation of metabolic and immune networks.50 Fatty acids either produced by de novo synthesis or lypolytic degradation of tissue fat play an important role in immune activation. Normal pregnancy is associated with low-grade inflammation that starts at 7–10 weeks gestation persists throughout gestation.50 Activation of Toll-like receptor 4 (TLR4) pathways by fatty acids translates downstream signals leading to the production of pro-inflammatory cytokines.61 The activation of TLR4 signal transduction has been proposed as a molecular link between diet-induced obesity and increased insulin resistance.62 Adipose tissue TLR4 is activated in adipose tissue of obese as compared with normal weight women. Our group and others have proposed that nutritional changes, through either maternal hyperphagia or dyslipidemia, are potential mechanisms which activate TLR4-induced inflammation through circulating fatty acids.63 TLR4 is also abundantly expressed in the placenta and localized on both syncytiotrophoblast cells facing the maternal blood and perivascular cells in contact with fetal blood. The localization of TLR4 at the maternal–fetal interface suggests that placental TLR4 can be targeted by both maternal and fetal derived lipid signals.
THE MATERNAL–FETAL INTERFACE: ROLE OF THE PLACENTA
The insulin resistance of pregnancy and particularly the mechanisms of insulin action are not well understood. Adiponectin, which has insulin-sensitizing properties, is a potential regulator of insulin action. Hence, mechanisms leading to decrease the expression or secretion of adiponectin, may be particularly relevant during pregnancy.64 The negative correlation of adiponectin with maternal estimates of insulin sensitivity and BMI in late pregnancy but not GWG (Figure 5) supports the concept that pregnancy-related regulation of plasma adiponectin may share similarity with adiponectin-induced regulation in obesity.65
The placenta represents an additional source of systemic adipokines and cytokines during pregnancy.66 Leptin is secreted by adipose tissue and the placenta,55 and the rise in plasma leptin levels in pregnancy is mainly contributed by the placenta.67 In contrast, we and others have shown that the placenta is not a source of adiponectin.58,68 However, the placenta may act as a functional target of maternal plasma adiponectin because there are a large amounts of adiponectin receptors expressed at the maternal placental surface. The metabolic actions of adiponectin are mediated via binding to specific adiponectin receptors.69 Both R1 and R2 adiponectin receptors are expressed in the human placenta on the syncitiotrophoblast cells exposed to maternal blood.55 Furthermore, adiponectin regulates placental amino acid transporters via R2 receptor binding70,71 and decreases the production of human chorionic gonadotropin and progesterone.72 These findings suggest that implementing lifestyle intervention strategies aimed at preventing decreases in maternal adiponectin may impact placental transport of nutrients to the fetus.
Maternal metabolic homeostasis and pleiotropic functions of the placenta, including endocrine function, are compromised by obesity.73 Structurally and metabolically the placenta is an extremely flexible organ which succeeds to adapt to most maternal metabolic challenges. When exacerbated by maternal obesity, such modifications may eventually regulate lipid fluxes to the fetal circulation through metabolic immune interactions. How the molecular and cellular dysfunctions occurring at the maternal–fetal interface translate into greater fetal adiposity.
SUMMARY
Lifestyle interventions initiated during pregnancy may to some degree reduce excessive GWG, however, these interventions have not been successful in reducing fetal overgrowth, GDM or preeclampsia in obese women. On the basis of our research, we conclude that interventions need to be initiated prior to conception. Just as women with pre-existing diabetes need to normalize glucose levels before pregnancy to decrease the risk of congenital anomalies, obese women must improve metabolic conditioning before pregnancy to decrease complications of fetal overgrowth and gestational diabetes during pregnancy. Because of the increased expression of lipogenic and inflammatory genes in maternal white adipose tissue and placenta of obese women in the first trimester of pregnancy, before any phenotypic changes become clinically apparent, obese women are less amenable to lifestyle changes improving metabolic function and clinical outcomes.
Terms such as ‘Metabolic Rehabilitation’ by Ethan Sims74 and ‘Fuel Mediated Teratogenesis’ by Norbert Freinkel were used to describe the importance of preconceptual metabolic control to avoid later metabolic dysfunction in the mother and her offspring.75
There are benefits of inter-pregnancy weight loss. A report from the research group in Cambridge, UK reported that obese mice fed an obesogenic diet before the first mating, then placed on an exercise regimen before and during a second pregnancy, had decreased placental lipid storage and transfer to the trophoblast.76 The obese exercise group also normalized insulin signaling in the placenta compared with dysregulated signaling in placentas of sedentary obese control mice resulting in improved fetal outcomes. In a recent population-based birth certificate historical cohort of over 10 000 births, mild-to-moderate interpregnancy weight loss in obese women reduced the risk of a subsequent LGA baby, odds ratio 0.61 (95% CI 0.52–0.73), whereas inter-pregnancy weight gain increased the risk of a LGA infant, odds ratio 1.37 (95% CI 1.21–1.54). The inter-pregnancy change in weight was not associated with an increased risk of a small for gestational age infant.77 Therefore, the concept that lifestyle intervention before pregnancy is important in improving placental function and development is gaining traction as a viable paradigm to improve perinatal metabolic outcomes.78 Prospective randomized trials are needed to demonstrate the safety and efficacy of such an intervention for both short and long-term benefits to the mother and her offspring.
ACKNOWLEDGEMENTS
Grant support HD-22965–19 and The CTSC Cleveland at Case Western Reserve University is supported by the National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program, UL1TR000439.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Catalano PM. Increasing maternal obesity and weight gain during pregnancy: the obstetric problems of plentitude. Obstet Gynecol. 2007;110:743–744. doi: 10.1097/01.AOG.0000284990.84982.ba. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Obesity: Preventing and managing the global epidemic. Geneva: World Health Organization; 2000. p. 894. [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindsay CA, Huston L, Amini SB, Catalano PM. Longitudinal changes in the relationship between body mass index and percent body fat in pregnancy. Obstet Gynecol. 1997;39:337–382. doi: 10.1016/S0029-7844(96)00517-0. [DOI] [PubMed] [Google Scholar]
- 5.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 6.FLegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 7.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA. 2012;307:483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ananth CV, Wen SW. Trends in fetal growth among singleton gestations in the United States and Canada, 1985 through 1998. Semin Perinatol. 2002;26:260–267. doi: 10.1053/sper.2002.34772. [DOI] [PubMed] [Google Scholar]
- 9.Surkan PJ, Hsieh CC, Johansson AL, Dickman PW, Cnattingius S. Reasons for increasing trends in large for gestational age births. Obstet Gynecol. 2004;104:720–726. doi: 10.1097/01.AOG.0000141442.59573.cd. [DOI] [PubMed] [Google Scholar]
- 10.Catalano PM. Management of obesity in pregnancy. Obstet Gynecol. 2007;109:419–433. doi: 10.1097/01.AOG.0000253311.44696.85. [DOI] [PubMed] [Google Scholar]
- 11.Donahue SMA, Kleinman KP, Gillman MW, Oken E. Trends in birth weight and gestational length among singleton term births in the United States: 1990–2005. Obstet Gynecol. 2010;115:357–364. doi: 10.1097/AOG.0b013e3181cbd5f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibson KS, Waters T, Catalano PM. Factors relating to national trends in decreasing birth weight in term singleton deliveries. Society for Gynecologic Investigation. 2011;18(Suppl):210A. (abstract F130). [Google Scholar]
- 13.Institute of Medicine. Weight gain during pregnancy: reexamining the guidelines. Washington, DC: National Academy Press; 2009. [PubMed] [Google Scholar]
- 14.Chu SY, Callaghan WM, Bish CL, D’Angelo D. Gestational weight gain by body mass index among US women delivering live births, 2004–2005: fueling future obesity. Am J Obstet Gynecol. 2009;200:271.e1–271.e7. doi: 10.1016/j.ajog.2008.09.879. [DOI] [PubMed] [Google Scholar]
- 15.Artal R, Lockwood CJ, Brown HL. Weight gain recommendations in pregnancy and the obesity epidemic. Obstet Gynecol. 2010;115:152–155. doi: 10.1097/AOG.0b013e3181c51908. [DOI] [PubMed] [Google Scholar]
- 16.Catalano PM, Mele L, Landon MB, Ramin SM, Reddy UM, Casey B, et al. For the Eunice Kennedy Shriver NICHHD Maternal-Fetal Medicine Units Network. Inadequate weight gain in overweight and obese pregnancy women: what is the effect on fetal growth? Am J Obstet Gynecol. 2014;211:137.e1–137.e7. doi: 10.1016/j.ajog.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rasmussen KM, Abrams B, Bodnar LM, Butt NF, Catalano PM, Siega-Riz AM. Recommendations for weight gain during pregnancy in the context of the obesity epidemic. Obstet Gynecol. 2010;116:1191–1195. doi: 10.1097/AOG.0b013e3181f60da7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whitaker RC. Predicting preschooler obesity at birth: the role of maternal obesity in early pregnancy. Pediatrics. 2004;114:e29–e36. doi: 10.1542/peds.114.1.e29. [DOI] [PubMed] [Google Scholar]
- 19.Boney CM, Verma A, Tucker R, Wohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115:e290–e296. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- 20.Dabelea D, Mayer-Davis EJ, Lamichhane AP, D'Agostino RB, Jr, Liese AD, Vehik KS, et al. Association of intrauterine exposure to maternal diabetes and obesity with type 2 diabetes in youth. The SEARCH case-control study. Diabetes Care. 2008;31:1422–1426. doi: 10.2337/dc07-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sewell MF, Huston-Presley L, Super DM, Catalano PM. Increased neonatal fat mass, not lean body mass, is associated with maternal obesity. AJOG. 2006;195:1100–1103. doi: 10.1016/j.ajog.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 22.Catalano PM, Farrell K, Thomas A, Huston-Presley L, Mencin P, Hauguel-de Mouzon S, et al. Perinatal risk factors for childhood obesity and metabolic dysregulation. Am J Clin Nutr. 2009;90:1303–1313. doi: 10.3945/ajcn.2008.27416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phillips LH, Santhakumaran S, Gale C, Prior E, Logan KM, Hyde MJ, et al. The diabetic pregnancy and offspring BMI in childhood: a systematic review and meta-analysis. Diabetologia. 2011;54:1957–1966. doi: 10.1007/s00125-011-2180-y. [DOI] [PubMed] [Google Scholar]
- 24.WIN Weight-Control Information Network. Prescription medications for the treatment of obesity. NIDDK. 2013:1–8. [Google Scholar]
- 25.Rowan JA, Hague WM, Gao W, Battin MR, Moore MP. For the MiG Trial Investigators. Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med. 2008;358:2003–2015. doi: 10.1056/NEJMoa0707193. [DOI] [PubMed] [Google Scholar]
- 26.Moses RG, Casey SA, Quinn EG, Cleary JM, Tapsell LC, Milosavljevic M, et al. Pregnancy and glycemic index outcomes study: effects of low glycemic index compared with conventional dietary advice on selected pregnancy outcomes. AM J Clin Nutr. 2014;99:517–523. doi: 10.3945/ajcn.113.074138. [DOI] [PubMed] [Google Scholar]
- 27.Santos IA, Stein R, Fuchs SC, Duncan BB, Ribeiro JP, Droeff LR, et al. Aerobic exercise and submaximal functional capacity in overweight pregnancy women. Obstet Gynecol. 2005;106:243–249. doi: 10.1097/01.AOG.0000171113.36624.86. [DOI] [PubMed] [Google Scholar]
- 28.Vinter CA, Jensen DM, Ovesen P, Beck-Nielsen H, Jørgensen JS. The LiP (Lifestyle in Pregnancy) Study. A randomized controlled trial of lifestyle intervention in 360 obese pregnancy women. Diabetes Care. 2011;34:2502–2507. doi: 10.2337/dc11-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dodd JM, Turnbull D, McPhee A, Deussen AR, Grivell RM, Yelland LN, et al. for the LIMIT Randomised Trial group. Antenatal lifestyle advice for women who are overweight or obese: LIMIT randomised trial. BMJ. 2014;348:g1285. doi: 10.1136/bmj.g1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ilmonen J, Isolauri E, Poussa T, Laitinen K. Impact of dietary counselling and probiotic intervention on maternal anthropometric measurements during and after pregnancy: a randomized placebo-controlled trial. Clin Nutr. 2011;30:156–164. doi: 10.1016/j.clnu.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 31.Muktabhant B, Lumbiganon P, Ngamjarus C, Dowswell T. Interventions for preventing excessive weight gain during pregnancy. Cochrane Database Syst Rev. 2012;4:CD007145. doi: 10.1002/14651858.CD007145.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thangaratinam S, Rogozińska E, Jolly K, Roseboom T, Tomlinson JW, Kunz R, et al. Effects of interventions in pregnancy on maternal weight and obstetric outcomes: metaanalysis of randomized evidence. BMJ. 2012;344:e2088–e2102. doi: 10.1136/bmj.e2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walsh JM, McGowan CA, Mahony R, Foley ME, McAuliffe FM. Low glycemic index diet in pregnancy to prevent macrosomia (ROLO study): randomized control trial. BMJ. 2012;345:e5605. doi: 10.1136/bmj.e5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Renault KM, Norgaard K, Nilas L, Carlsen E, Cortes D, Pryds O, et al. The Treatment of Obese Pregnancy Women (TOP) study: a randomized controlled trial of the effect of physical activity intervention assessed by pedometer with or without dietary intervention in obese pregnant women. Am J Obstet Gynecol. 2014;210:134.e1–134.e9. doi: 10.1016/j.ajog.2013.09.029. [DOI] [PubMed] [Google Scholar]
- 35.Dodd JM, Grivell RM, Crowther CA, Robinson JS. Antenatal interventions for overweight or obese pregnant women: a systematic review of randomized trials. BJOG. 2010;117:1316–1326. doi: 10.1111/j.1471-0528.2010.02540.x. [DOI] [PubMed] [Google Scholar]
- 36.Tanentsapf I, Heitmann BL, Adegboye ARA. Systematic review of clinical trials on dietary interventions to prevent excessive weight gain during pregnancy among normal weight, overweight and obese women. BMC Pregnancy Childbirth. 2011;11:81. doi: 10.1186/1471-2393-11-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quinlivan JA, Julania S, Lam L. Antenatal dietary interventions in obese pregnant women to restrict gestational weight gain to Institute of Medicine recommendations. A metaanalysis. Obstet Gynecol. 2011;118:1395–1401. doi: 10.1097/AOG.0b013e3182396bc6. [DOI] [PubMed] [Google Scholar]
- 38.Thangaratinam S, Jolly K. Obesity in pregnancy: a review of reviews on the effectiveness of interventions. BJOG. 2010;117:1309–1312. doi: 10.1111/j.1471-0528.2010.02670.x. [DOI] [PubMed] [Google Scholar]
- 39.Han S, Middleton P, Crowther CA. Exercise for pregnant women for preventing gestational diabetes mellitus. Cochrane Database Syst Rev. 2012;7:CD009021. doi: 10.1002/14651858.CD009021.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Battaglia FC, Meschia G. Principal substrates of fetal metabolism. Physiol Rev. 1978;58:499–527. doi: 10.1152/physrev.1978.58.2.499. [DOI] [PubMed] [Google Scholar]
- 41.Okereke NC, Huston-Presley L, Amini SB, Kalhan S, Catalano PM. Longitudinal changes in energy expenditure and body composition in obese women with normal and impaired glucose tolerance. Am J Physiol Endocrinol Metab. 2004;287:E472–E479. doi: 10.1152/ajpendo.00589.2003. [DOI] [PubMed] [Google Scholar]
- 42.Ryan EA, O’Sullivan MJ, Skyler JS. Insulin action during pregnancy. Studies with the euglycemic clamp technique. Diabetes. 1985;34:380–389. doi: 10.2337/diab.34.4.380. [DOI] [PubMed] [Google Scholar]
- 43.Knopp RH, Warth MR, Carrol CJ. Lipid metabolism in pregnancy. I. Changes in lipoprotein triglyceride and cholesterol in normal pregnancy and the effects of diabetes mellitus. J Reprod Med. 1975;10:95–101. [PubMed] [Google Scholar]
- 44.Catalano PM, Ehrenberg HM. The short-and long-term implications of maternal and obesity on the mother and her offspring. BJOG. 2006;113:1126–1133. doi: 10.1111/j.1471-0528.2006.00989.x. [DOI] [PubMed] [Google Scholar]
- 45.Catalano PM, Drago NM, Amini SB. Factors affecting fetal growth and body composition. Am J Obstet Gynecol. 1995;172:1459–1463. doi: 10.1016/0002-9378(95)90478-6. [DOI] [PubMed] [Google Scholar]
- 46.Herrera E. Metabolic adaptations in pregnancy and their implications for the availability of substrates to the fetus. Eur J Clin Nutr. 2000;54(Suppl 1):S47–S51. doi: 10.1038/sj.ejcn.1600984. [DOI] [PubMed] [Google Scholar]
- 47.Meyer BJ, Stewart FM, Brown EA, Cooney J, Nilsson S, Olivecrona G, et al. Maternal obesity is associated with the formation of small dense LDL and hypoadiponectinemia in the third trimester. J Clin Endocrinol Metab. 2013;98:643–652. doi: 10.1210/jc.2012-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pepe G, Albrecht E. Glob libr. Women’s Med. Steroid Endocrinology of Pregnancy (ISSN: 1756-2228) 2008 doi: 10.3843/GLOWM.10311. [DOI] [Google Scholar]
- 49.Catalano PM, Hauguel-De Mouzon S. Is it time to revisit the Pedersen hypothesis in the face of the obesity epidemic? Am J Obstet Gynecol. 2011;204:479–487. doi: 10.1016/j.ajog.2010.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Resi V, Basu S, Haghiac M, Presley L, Minium J, Kaufman B, et al. Molecular inflammation and adipose tissue matrix remodeling precede physiological adaptations to pregnancy. Am J Physiol Endocrinol Metab. 2012;303:E832–E840. doi: 10.1152/ajpendo.00002.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kirwan JP, Hauguel-De Mouzon S, Lepercq J, Challier JC, Huston-Presley L, Friedman JE, et al. TNF-alpha is a predictor of insulin resistance in human pregnancy. Diabetes. 2002;51:2207–2213. doi: 10.2337/diabetes.51.7.2207. [DOI] [PubMed] [Google Scholar]
- 52.Zhang L, Sugiyama T, Murabayashi N, Umekawa T, Ma N, Kamimoto Y, et al. The inflammatory changes of adipose tissue in late pregnant mice. J Mol Endocrinol. 2011;47:157–165. doi: 10.1530/JME-11-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Highman TJ, Friedman JE, Huston LP, Wong WW, Catalano PM. Longitudinal changes in maternal serum leptin concentrations, body composition, and resting metabolic rate in pregnancy. Am J Obstet Gynecol. 1998;178:1010–1015. doi: 10.1016/s0002-9378(98)70540-x. [DOI] [PubMed] [Google Scholar]
- 54.Catalano PM, Hoegh M, Minium J, Huston-Presley L, Bernard S, Kalhan S, et al. Adiponectin in human pregnancy: implications for regulation of glucose and lipid metabolism. Diabetologia. 2006;49:1677–1685. doi: 10.1007/s00125-006-0264-x. [DOI] [PubMed] [Google Scholar]
- 55.Hauguel-de Mouzon S, Lepercq J, Catalano P. The known and unknown of leptin in pregnancy. Am J Obstet Gynecol. 2006;194:1537–1545. doi: 10.1016/j.ajog.2005.06.064. [DOI] [PubMed] [Google Scholar]
- 56.Considine R, Sinha M, Heiman M. Serum immunoreactive leptin concentrations in normal weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 57.Fruhbeck G, Jebb SA, Prentice AM. Leptin: physiology and pathophysiology. Clin Physiol. 1998;18:399–419. doi: 10.1046/j.1365-2281.1998.00129.x. [DOI] [PubMed] [Google Scholar]
- 58.Haghiac M, Basu S, Presley L, Serre D, Catalano PM, Hauguel-de Mouzon S. Patterns of adiponectin expression in term pregnancy; impact of obesity. J Clin Endorinol Metab. 2014;99:3427–3434. doi: 10.1210/jc.2013-4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Karki S, Chakrabarti P, Huang G, Wang H, Farmer SR, Kandror KV. The multi-level action of fatty acids on adiponectin production by fat cells. PLoS One. 2011;6:e28146. doi: 10.1371/journal.pone.0028146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ranheim T, Haugen F, Staff AC, Braekke K, Harsem NK, Drevon CA. Adiponectin is reduced in gestational diabetes mellitus in normal weight women. Acta Obstet Gynecol Scand. 2004;83:341–347. doi: 10.1111/j.0001-6349.2004.00413.x. [DOI] [PubMed] [Google Scholar]
- 61.Lee JY, Ye J, Gao Z, Youn HS, Lee WH, Zhao L, et al. Reciprocal modulation of Toll-like receptor-4 signaling pathways involving MyD88 and phosphatidylinositol 3-kinase/AKT by saturated and polyunsaturated fatty acids. J Biol Chem. 2003;278:37041–37051. doi: 10.1074/jbc.M305213200. [DOI] [PubMed] [Google Scholar]
- 62.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier J. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jarvie E, Hauguel-de Mouzon S, Nelson SM, Sattar N, Catalano M, Freeman DJ. Lipotoxicity in obese pregnancy and its potential role in adverse pregnancy outcome and obesity in the offspring. Clin Sci (London, Engl) 2010;119:123–129. doi: 10.1042/CS20090640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wong GW, Wang J, Hug C, Tsao TS, Lodish HF. A family ofAcrp30/adiponectin structural and functional paralogs. The exclusive expression and mechanism of action AdpN may be partly influenced by the presence of a distinctive TNFα-like globular domain. Proc Natl Acad Sci USA. 2004;101:10302–10307. doi: 10.1073/pnas.0403760101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lihn AS, Bruun JM, He G, Pedersen SB, Jensen PF, Richelsen B. Lower expression of adiponectin mRNA in visceral adipose tissue in lean and obese subjects. Mol Cell Endocrinol. 2004;219:9–15. doi: 10.1016/j.mce.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 66.Hauguel-de Mouzon S, Guerre-Millo M. The placenta cytokine network and inflammatory signals. Placenta. 2006;27:794–798. doi: 10.1016/j.placenta.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 67.Lepercq J, Cauzac M, Lahlou N, Timsit J, Girard J, Auwerx J, et al. Overexpression of placental leptin in diabetic pregnancy: a critical role for insulin. Diabetes. 1998;47:847850. doi: 10.2337/diabetes.47.5.847. [DOI] [PubMed] [Google Scholar]
- 68.Corbetta S, Bulfamante G, Cortelazzi D, Barresi V, Cetin I, Mantovani G, et al. Diponectin expression in human fetal tissues during mid- and late gestation. J Clin Endocrinol Metab. 2005;90:2397–2402. doi: 10.1210/jc.2004-1553. [DOI] [PubMed] [Google Scholar]
- 69.Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, et al. Targeted disruption of adipoR1 and adipoR2 causes abrogation of adiponectin binding and metabolic actions. Nature Med. 2007;13:332–339. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- 70.Rosario FJ, Schumacher MA, Jiang J, Kanai Y, Powell TL, Jansson T. Chronic maternal infusion of full-length adiponectin in pregnant mice down-regulates placental amino acid transporter activity and expression and decreases fetal growth. J Physiol. 2012;590:1495–1509. doi: 10.1113/jphysiol.2011.226399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jones HN, Jansson T, Powell TL. Full length adiponectin attenuates insulin signaling and inhibits insulin-stimulated amino acid transport in human primary trophoblast cells. Diabetes. 2010;59:1161–1170. doi: 10.2337/db09-0824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McDonald EA, Wolfe MW. Adiponectin attenuation of endocrine function within human term trophoblast cells. Endocrinology. 2009;150:4358–4365. doi: 10.1210/en.2009-0058. [DOI] [PubMed] [Google Scholar]
- 73.Gomes LL, Basu S, Minium J, Hauguel-de Mouzon S, Catalano P. Obesity in pregnancy is associated with decreased placental estradiol biosynthesis. Am J Obstet Gynecol. 2014;210(suppl):S32. [Google Scholar]
- 74.Sims EAH, Horton ES. Endocrine and metabolic adaptation to obesity and starvation. Am J Clin Nutr. 1968;21:1355–1370. doi: 10.1093/ajcn/21.12.1455. [DOI] [PubMed] [Google Scholar]
- 75.Freinkel M, Cockroft DL, Lewis NJ, Gorman L, Akazawa S, Phillips LS, et al. The 1986 McCollum award lecture. Fuel-mediated teratogenesis during early organogenesis: the effects of increased concentrations of glucose, ketones, or somatomedin inhibitor during rat embryo culture. Am J Clin Nutr. 1986;44:986–995. doi: 10.1093/ajcn/44.6.986. [DOI] [PubMed] [Google Scholar]
- 76.Gascoin G, Blackmore H, Musial B, Barnes S, Duque D, Ozanne SE, et al. Early Nutrition. Munich Germany: 2014. Exercise before and during an obese mouse pregnancy restores some placental gene expression and transport function. abstract 56. [Google Scholar]
- 77.Arun JA, Gavard JA, Cantanzaro RB, Artal R, Hopkins SA. The impact of inter-pregnancy weight change on birthweight in obese women. Am J Obstet Gynecol. 2013;208:205.e1–205.e7. doi: 10.1016/j.ajog.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 78.Rono K, Stach-Lempinen B, Klemetti MM, Kaaja RJ, Poyhonen M, Johan JG, et al. Prevention of gestational diabetes through lifestyle intervention: study design and methods of a Finnish randomized controlled trial multicenter trial (RADIEL) BMC Pregnancy Childbirth. 2014;14:70. doi: 10.1186/1471-2393-14-70. [DOI] [PMC free article] [PubMed] [Google Scholar]