Abstract
A diastereo- and enantioselective Michael/Henry/ketalization sequence to functionalized tetrahydropyrans is described. The multicomponent cascade reaction uses acetylacetone or β-keto esters, β-nitrostyrenes, and alkynyl aldehydes as substrates affording tetrahydropyrans with five contiguous stereocenters. Employing a bifunctional quinine-based squaramide organocatalyst, the title compounds are obtained in moderate to good yields (27–80%), excellent enantiomeric excesses (93–99% ee), and high diastereomeric ratios (dr > 20:1) after one crystallization.
Over the past years, we have witnessed a strong increase in the number of publications on organocatalysis as the main topic because of their wide applications for the synthesis of valuable chiral entities.1 Today, numerous groups of organocatalysts are known, with the classes of primary2 and secondary amines,3 hydrogen-bonding organocatalysts,4 chiral phosphoric acids,5 as well as N-heterocyclic carbenes6 being used preferentially. The catalytic asymmetric synthesis with these small organic molecules under metal-free conditions now constitutes a rapidly growing research area at the frontier of green chemistry.7 Furthermore, the construction of contiguous stereocenters via cascade reactions from easily available starting materials is one of the main reasons for its exponential increase over the past years.8 Hayashi and co-workers9 reported a cross-aldol reaction of alkynyl aldehydes 4 with other simple aliphatic aldehydes to obtain synthetically useful β-alkynyl-β-hydroxy aldehydes. Similar to the cross-aldol reactions, Henry reactions with α-acidic nitro compounds are possible as well.10 We wanted to combine these methods by incorporating the resulting alcohol functionality in an intramolecular fashion to generate six-membered rings. We envisaged the use of alkynyl aldehydes 4 to facilitate the 1,2-addition to be followed up by a ketalization key step to afford 2-hydroxytetrahydropyrans.11 To the best of our knowledge, an organocascade Michael/Henry/ketalization12 sequence to generate densely functionalized tetrahydropyrans bearing five stereogenic centers including one tetrasubstituted carbon is not known. Moreover, the reported organocatalytic asymmetric Michael/Henry/acetalization sequences require additional base to facilitate the formation of tetrahydropyrans.12b,c The triple bond is a versatile structural element that can be used for several transformations, e.g., cycloadditions or selective reductions to alkenes and as a precursor of ketones. The 2-hydroxy (or rather alkoxy) tetrahydropyran unit is a characteristic structural feature of a huge number of natural products, besides carbohydrates for instance of spiroketals,13 in soraphen A14 and pederin15 as well as in the class of the bryostatins.16
To build up the motif of the tetrahydropyran we planned the addition of a γ-nitro carbonyl compound to an aldehyde.17 Achiral γ-nitro carbonyl compounds were extensively tested, but no good asymmetric inductions could be achieved. After intensive literature research, a method provided by Rawal et al.18 was tested. They developed the synthesis of several Michael adducts between 1,3-diketones with β-nitrostyrenes with a novel squaramide organocatalyst based on cinchonine in excellent yields and enantiomeric excesses. Because of the pseudoenantiomeric nature of the cinchona alkaloids, we decided to employ a quinine-based organocatalyst to prove the diversity of this method by synthesizing the corresponding enantiomer.
According to the protocol of the Rawal group, acetylacetone 1a and β-nitrostyrene 2a reacted smoothly with catalyst A to the Michael adduct 3a, and in the following new step of the one-pot sequence the aldehyde 4a was added to form the hemiketal 5a (Scheme 1). The product was obtained in an excellent enantiomeric excess of 99%, but in a low yield of 30% after column chromatography. To investigate this outcome, we stirred the intermediate Michael product 5a over silica gel and found out that a retro-aldol reaction between C2/C3 occurred. Addition of a small amount of base during chromatography to neutralize the acidity of silica resulted in a noncharacterizable product. To create a stable compound, we investigated several hydroxyl protecting groups, which all had to react under almost neutral or mild conditions. The best result was obtained with the combination of pTSA and HC(OMe)3 in a quantitative yield and with no loss of enantioselectivity. With this knowledge in hand, we added the protecting reagents to the reaction mixture, and thus, we were able to get the desired product in a good yield. Knowing how to overcome the stability problems, we screened for the best reaction conditions. A short temperature screening was conducted with Michael adduct 3a and aldehyde 4a (Table 1). Reducing the reaction temperature from room temperature to −20 °C (entries 1–3) was followed by an increase in yield and diastereoselectivity. Now we focused on the one-pot procedure for the synthesis of the tetrahydropyrans. Following the protocol of the Rawal group, we started with the addition of acetylacetone (1a) and β-nitrostyrene (2a) with 4 mol % of catalyst in DCM at room temperature (Table 2, entry 1). As opposed to the 2 equiv of acetylacetone of the Rawal group, we used a 1:1 ratio because excess acetylacetone would react with aldehyde 4a in an aldol condensation and thus increase the complexity of the final mixture. Reducing the amount of catalyst was not successful. Although the first step occurred quantitatively, we could obtain only 53% yield at 2 mol% catalyst loading, respectively, 11% yield at 0.7 mol % (entries 2 and 3). For the next set of modifications, we changed the amount of aldehyde 4a (Table 2, entry 4–7). With 10 equiv a lower yield as compared to 4 or 2 equiv was obtained, indicating a concentration issue. Further reduction of the amount of the aldehyde led to a small decrease in yield (entries 6 and 7). Extending the reaction time resulted in no increase in yield (entries 8–10). The amount of solvent was reduced to a concentration of 0.5 M, which resulted in an increase of yield (Table 2, entry 11). Having the proper conditions in hand, an extension of the scope was investigated (Table 3). Several different substituents R3 on the aryl moiety of 2 (entries b–d and f) were introduced, giving moderate to good yields of 27–65% and very good enantioselectivities (93–97% ee). Even a heterocyclic group, like the protected N-Boc-indolyl, could be used (Table 3, entry e).
Scheme 1.
Initial Outcome of the Envisaged Domino Sequence
Table 1.
Optimizing the Reaction Temperature for the Henry/Ketalization Sequence

The reaction was performed on a 0.2 mmol scale.
Combined yield of isolated product as a mixture of diastereomers after flash chromatography.
Diastereomeric ratio; major-(2S,3S,4S,5R,6R) vs minor-(2S,3S,4S,5S,6S) diastereomer determined by 1H NMR.
Table 2.
Screening for the Optimal Conditions

| entrya | mol % | 4a (equiv) | timeb (d) | yieldc (%) | drd |
|---|---|---|---|---|---|
| 1 | 4 | 4 | 4 | 62 | >20:1 |
| 2 | 2 | 4 | 5 | 53 | >20:1 |
| 3 | 0.7 | 4 | 5 | 11 | n.d.e |
| 4 | 4 | 10 | 5 | 46 | >20:1 |
| 5 | 4 | 2 | 5 | 61 | >20:1 |
| 6 | 4 | 1.5 | 5 | 52 | >20:1 |
| 7 | 4 | 1.1 | 5 | 50 | >20:1 |
| 8 | 4 | 2 | 6 | 59 | >20:1 |
| 9 | 4 | 2 | 7 | 57 | >20:1 |
| 10 | 4 | 2 | 9 | 59 | >20:1 |
| 11f | 4 | 2 | 5.5 | 79 | >20:1 |
The reaction was performed on a 0.2 mmol scale (0.2 m in DCM).
Sum of reaction time.
Combined yield of isolated product as a mixture of diastereomers after flash chromatography.
Diastereomeric ratio: major-(2S,3S,4S,5R,6R) vs minor-(2S,3S,4S,5S,6S) diastereomer determined by 1H NMR.
Not determined.
Conducted in 0.4 mL of solvent (0.5 M).
Table 3.
Scope of the Michael/Henry/Ketalization Sequence To Form Tetrahydropyrans

| 6a | R1 | R2 | R3 | R4 | timeb (d) | yieldc (%) | drd | eee (%) |
|---|---|---|---|---|---|---|---|---|
| a | Me | Me | Ph | Ph | 5.5 | 79 | >20:1 | >99 |
| b | Me | Me | 2-BrC6H4 | Ph | 5.5 | 65 | 13:1 | 93 (99) |
| c | Me | Me | 3-MeOC6H4 | Ph | 6.5 | 45 | 6:1 | 97 (99) |
| d | Me | Me | 3,4–OCHH2OC6H3 | Ph | 5 | 27 | 2:1 | 95 (99) |
| e | Me | Me | 3-(N-Boc-indolyl) | Ph | 6 | 38 | 8:1 | 95 |
| f | Me | Me | 4-NO2C6H4 | Ph | 9 | 46 | 2:1 | 93 |
| g | Me | Me | Ph | 3-FC6H4 | 5.5 | 67 | 5:1 | 94 (99) |
| h | Me | Me | Ph | 4-MeC6H4 | 5.5 | 61 | 7:1 | 97 |
| i | Me | Me | Ph | 2-MeOC6H4 | 5.5 | 80 | 3:1 | 95 (99) |
| j | Me | Me | Ph | cyclopentyl | 5.5 | 68 | 4:1 | 96 |
| k | OMe | Me | Ph | Ph | 5.5 | 60 | 4:1 | 97 (99) |
| l | OMe | Et | Ph | Ph | 5 | 69 | 3:1 | 96 (99) |
| m | OtBu | Me | Ph | Ph | 9 | 34 | 2:1 | 98 |
The reaction was performed on a 0.4 mmol scale (0.5 M in DCM).
Sum of reaction time.
Combined yield of isolated product as a mixture of diastereomers after flash chromatography.
Diastereomeric ratio: major-(2S,3S,4S,5R,6R) vs minor-(2S,3S,4S,5S,6S) diastereomer determined by 1H NMR; after one recrystallization dr > 20:1.
Determined by HPLC analysis on a chiral stationary phase for the major diastereomer; value in parentheses after one recrystallization.
The aromatic part R4 of the aldehyde 4 was substituted with electron-donating and electron-withdrawing groups to yield the cascade product in modest to good yields (61–80%) and in very good enantioselectivities of 94–97% ee (Table 3, entries g–i). Switching to a cyclopentyl moiety (entry j) led to the same range of yield (68%) and enantioselectivity (96% ee). No product was obtained with benzaldehyde or propanal. Desymmetrization of the acetylacetone to the corresponding methyl ester gave 60% yield and 97% ee (Table 3, entry k). Increasing the bulkiness to a tert-butoxy group (entry m) resulted in a drop of obtained product (34% yield) but still impressive 98% ee.
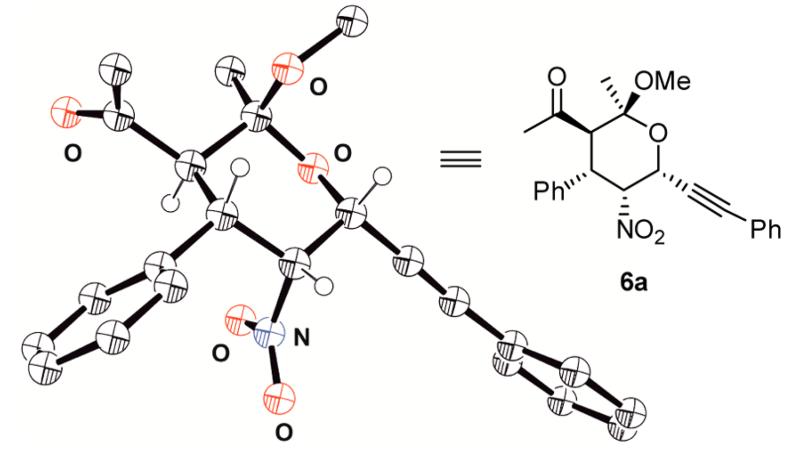
A further domino product was obtained in 69% yield and 96% ee after extending the side chain to an ethyl group (Table 3, entry l). The relative configuration was determined by NOE measurements for compound 6c (Figure 1) as well as the absolute configuration by single-crystal X-ray analysis of compound 6a (Figure 2).19
Figure 1.
Determination of the relative configuration by NOE for compound 6c.
Figure 2.
Determination of the absolute configuration by X-ray crystal structure analysis of compound 6a.
In summary, we have developed an organocatalytic Michael/Henry/ketalization cascade sequence to access highly funtionalized tetrahydropyrans. A hydrogen-bonding organocatalyst on a squaramide basis was used to merge acetylacetone or different β-keto esters with nitroalkenes and ynals. In this manner, tetrahydropyrans bearing five contiguous stereocenters were obtained in moderate to good yields (27–80%), after one recrystallization in high diastereomeric ratios (dr > 20:1) and excellent enantiomeric excesses (93–99% ee).
Supplementary Material
ACKNOWLEDGMENTS
Support from the European Research Council (ERC Advanced Grant “DOMINOCAT”) is gratefully acknowledged.
Footnotes
Experimental procedures and the characterization of all products. This material is available free of charge via the Internet at http://pubs.acs.org.
Notes
The authors declare no competing financial interest.
REFERENCES
- (1).(a) Berkessel A, Groger H. Asymmetric Organocatalysis: From Biomimetic Concepts to Application in Asymmetric Synthesis. Wiley-VCH; Weinheim: 2005. [Google Scholar]; (b) Tsogoeva SB. Eur. J. Org. Chem. 2007:1701. [Google Scholar]; (c) Dondoni A, Massi A. Angew. Chem. 2008;120:4716. [Google Scholar]; Angew. Chem., Int. Ed. 2008;47:4638. [Google Scholar]; (d) MacMillan DWC. Nature. 2008;455:304. doi: 10.1038/nature07367. [DOI] [PubMed] [Google Scholar]; (e) Barbas CF., III Angew. Chem. 2008;120:44. [Google Scholar]; Angew. Chem., Int. Ed. 2008;47:42. [Google Scholar]; (f) Jørgensen KA, Bertelsen S. Chem. Soc. Rev. 2009;38:2178. doi: 10.1039/b903816g. [DOI] [PubMed] [Google Scholar]; (g) Bella M, Gasperi T. Synthesis. 2009:1583. [Google Scholar]; (h) Roca-Lopez D, Sadaba D, Delso I, Herrera RP, Tejero T, Merino P. Tetrahedron: Asymmetry. 2010;21:2561. [Google Scholar]; (i) Merino P, Marquez-Lopez E, Tejero T, Herrera RP. Synthesis. 2010:1. [Google Scholar]; (j) Moyano A, Rios R. Chem. Rev. 2011;111:4703. doi: 10.1021/cr100348t. [DOI] [PubMed] [Google Scholar]; (k) Melchiorre P. Angew. Chem., Int. Ed. 2012;51:9748. doi: 10.1002/anie.201109036. [DOI] [PubMed] [Google Scholar]; (l) Dalko PI. Comprehensive Enantioselective Organocatalysis. Wiley-VCH; Weinheim: 2013. [Google Scholar]; For selected general reviews, see:
- (2).(a) Xu L-W, Luo J, Lu Y. Chem. Commun. 2009:1807. doi: 10.1039/b821070e. [DOI] [PubMed] [Google Scholar]; (b) Cassani C, Martín-Rapún R, Arceo E, Bravo F, Melchiorre P. Nat. Protoc. 2013;8:325. doi: 10.1038/nprot.2012.155. [DOI] [PubMed] [Google Scholar]; (c) Hack D, Enders D. Synthesis. 2013;45:2904. [Google Scholar]; (d) Moran A, Hamilton A, Bo C, Melchiorre P. J. Am. Chem. Soc. 2013;135:9091. doi: 10.1021/ja404784t. [DOI] [PubMed] [Google Scholar]; (e) Kang YK, Kim DY. Adv. Synth. Catal. 2013;355:3131. [Google Scholar]; (f) Duan J, Li P. Catal. Sci. Technol. 2014;4:311. [Google Scholar]; For selected reviews and examples on cinchona alkaloid-derived primary amine catalysis, see:
- (3).(a) Rueping M, Kuenkel A, Tato F, Bats JW. Angew. Chem., Int. Ed. 2009;48:3699. doi: 10.1002/anie.200900754. [DOI] [PubMed] [Google Scholar]; (b) Rueping M, Haack K, Ieasuwan W, Sundén H, Blanco M, Schoepke FR. Chem. Commun. 2011;47:3828. doi: 10.1039/c1cc10245a. [DOI] [PubMed] [Google Scholar]; (c) Enders D, Greb A, Deckers K, Selig P, Merkens C. Chem.—Eur. J. 2012;18:10226. doi: 10.1002/chem.201201493. [DOI] [PubMed] [Google Scholar]; (d) Enders D, Joie C, Deckers K. Chem.—Eur. J. 2013;19:10818. doi: 10.1002/chem.201302127. [DOI] [PubMed] [Google Scholar]; (e) Chatterjee I, Bastida D, Melchiorre P. Adv. Synth. Catal. 2013;355:3124. [Google Scholar]; (f) Zeng X, Ni Q, Raabe G, Enders D. Angew. Chem., Int. Ed. 2013;52:2977. doi: 10.1002/anie.201209581. [DOI] [PubMed] [Google Scholar]; (g) Erdmann N, Philipps AR, Atodiresei I, Enders D. Adv. Synth. Catal. 2013;355:847. [Google Scholar]; (h) Dong L-J, Fan T-T, Wang C, Sun J. Org. Lett. 2013;15:204. doi: 10.1021/ol3032285. [DOI] [PubMed] [Google Scholar]; (i) Wu L, Wang Y, Song H, Tang L, Zhou Z, Tang C. Chem.—Asian J. 2013;8:2204. doi: 10.1002/asia.201300450. [DOI] [PubMed] [Google Scholar]; (j) Alexakis A, Lefranc A, Guénée L. Org. Lett. 2013;15:2172. doi: 10.1021/ol400697n. [DOI] [PubMed] [Google Scholar]; (k) Joie C, Deckers K, Enders D. Synthesis. 2014;46:799. doi: 10.1055/s-0033-1340565. [DOI] [PMC free article] [PubMed] [Google Scholar]; For selected examples on secondary amine organocatalysts, see:
- (4).(a) Schreiner PR. Chem. Soc. Rev. 2003;32:289. doi: 10.1039/b107298f. [DOI] [PubMed] [Google Scholar]; (b) Taylor MS, Jacobsen EN. Angew. Chem. 2006;118:1550. doi: 10.1002/anie.200503132. [DOI] [PubMed] [Google Scholar]; Angew. Chem., Int. Ed. 2006;45:1520. [Google Scholar]; (c) Connon SJ. Chem.—Eur. J. 2006;12:5418. doi: 10.1002/chem.200501076. [DOI] [PubMed] [Google Scholar]; (d) Doyle AG, Jacobsen EN. Chem. Rev. 2007;36:5713. doi: 10.1021/cr068373r. [DOI] [PubMed] [Google Scholar]; (e) Zhang Z, Schreiner PR. Chem. Soc. Rev. 2009;38:1187. doi: 10.1039/b801793j. [DOI] [PubMed] [Google Scholar]; (f) Etzenbach-Effers K, Berkessel A. Top. Curr. Chem. 2009;291:1. doi: 10.1007/978-3-642-02815-1_3. [DOI] [PubMed] [Google Scholar]; (g) Aleman J, Parra A, Jiang H, Jørgensen KA. Chem.—Eur. J. 2011;17:6890. doi: 10.1002/chem.201003694. [DOI] [PubMed] [Google Scholar]; (h) Storer RI, Aciro C, Jones LH. Chem. Soc. Rev. 2011;40:2330. doi: 10.1039/c0cs00200c. [DOI] [PubMed] [Google Scholar]; (i) Albrecht Ł, Dickmeiss G, Acosta FC, Rodriguez-Escrich C, Davis RL, Jørgensen KA. J. Am. Chem. Soc. 2012;134:2543. doi: 10.1021/ja211878x. [DOI] [PubMed] [Google Scholar]; (j) Enders D, Urbanietz G, Cassens-Sasse E, Kees S, Raabe G. Adv. Synth. Catal. 2012;354:1481. [Google Scholar]; (k) Loh CCJ, Hack D, Enders D. Chem. Commun. 2013;49:10230. doi: 10.1039/c3cc46033a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Liu Y, Wang Y, Song H, Zhou Z, Tang C. Adv. Synth. Catal. 2013;355:2544. [Google Scholar]; (m) Gosh AK, Zhou B. Tetrahedron Lett. 2013;54:3500. doi: 10.1016/j.tetlet.2013.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]; (n) Sun W, Hong L, Zhu G, Wang Z, Wie X, Ni J, Wang R. Org. Lett. 2014;16:544. doi: 10.1021/ol4034226. [DOI] [PubMed] [Google Scholar]; (o) Zhou E, Liu B, Dong C. Tetrahedron: Asymmetry. 2014;25:181. [Google Scholar]; (p) Han X, Dong C, Zhou H-B. Adv. Synth. Catal. 2014;356:1275. [Google Scholar]; For selected reviews and examples of hydrogen-bonding catalysis, see:
- (5) (a).Akiyama T, Itoh J, Yokota K, Fuchibe K. Angew. Chem. 2004;116:1592. doi: 10.1002/anie.200353240. [DOI] [PubMed] [Google Scholar]; Angew. Chem., Int. Ed. 2004;43:1566. [Google Scholar]; (b) Uraguchi D, Terada M. J. Am. Chem. Soc. 2004;126:5356. doi: 10.1021/ja0491533. [DOI] [PubMed] [Google Scholar]; (c) Rueping M, Azap C. Angew. Chem., Int. Ed. 2006;45:7832. doi: 10.1002/anie.200603199. [DOI] [PubMed] [Google Scholar]; (d) Baudequin C, Zamfir A, Tsogoeva SB. Chem. Commun. 2008:4637. doi: 10.1039/b804477e. [DOI] [PubMed] [Google Scholar]; (e) Evans CG, Gestwicki JE. Org. Lett. 2009;11:2957. doi: 10.1021/ol901114f. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]; (f) Terada M. Synthesis. 2010:1929. [Google Scholar]; (g) Taylor JE, Daniels DSB, Smith AD. Org. Lett. 2013;15:6058. doi: 10.1021/ol402955f. [DOI] [PubMed] [Google Scholar]; (h) Courant T, Kumarn S, He L, Retailleau P, Masson G. Adv. Synth. Catal. 2013;355:836. [Google Scholar]; (i) Izquierdo J, Orue A, Scheidt KA. J. Am. Chem. Soc. 2013;135:10634. doi: 10.1021/ja405833m. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Enders D, Stöckel BA, Rembiak A. Chem. Commun. 2014;50:4489. doi: 10.1039/c4cc00427b. [DOI] [PubMed] [Google Scholar]; (k) Tian X, Hofmann N, Melchiorre P. Angew. Chem., Int. Ed. 2014;53:2997. doi: 10.1002/anie.201310487. [DOI] [PubMed] [Google Scholar]; (l) Mori K, Wakazawa M, Akiyama T. Chem. Sci. 2014;5:1799. [Google Scholar]; (m) Zhong S, Nieger M, Bihlmeier A, Shi M, Bräse S. Org. Biomol. Chem. 2014;12:3265. doi: 10.1039/c4ob00234b. [DOI] [PubMed] [Google Scholar]
- (6).(a) Heitbaum M, Glorius F, Escher I. Angew. Chem., Int. Ed. 2006;45:4732. doi: 10.1002/anie.200504212. [DOI] [PubMed] [Google Scholar]; (b) Enders D, Niemeier O, Henseler A. Chem. Rev. 2007;107:5606. doi: 10.1021/cr068372z. [DOI] [PubMed] [Google Scholar]; (c) Moore JL, Rovis T. Top. Curr. Chem. 2010;291:118. doi: 10.1007/978-3-642-02815-1_18. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Biju AK, Kuhl N, Glorius F. Acc. Chem. Res. 2011;44:1182. doi: 10.1021/ar2000716. [DOI] [PubMed] [Google Scholar]; (e) Grossmann A, Enders D. Angew. Chem. 2012;124:320. doi: 10.1002/anie.201105415. [DOI] [PubMed] [Google Scholar]; Angew. Chem., Int. Ed. 2012;51:314. [Google Scholar]; (f) Izquierdo J, Hutson GE, Cohen DT, Scheidt KA. Angew. Chem. 2012;124:11854. doi: 10.1002/anie.201203704. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem., Int. Ed. 2012;51:11686. [Google Scholar]; (g) Bugaut X, Glorius F. Chem. Soc. Rev. 2012;41:3511. doi: 10.1039/c2cs15333e. [DOI] [PubMed] [Google Scholar]; (h) Ni Q, Zhang H, Grossmann A, Loh CCJ, Merkens C, Enders D. Angew. Chem., Int. Ed. 2013;52:13562. doi: 10.1002/anie.201305957. [DOI] [PubMed] [Google Scholar]; (i) Ryan SJ, Candish L, Lupton DW. Chem. Soc. Rev. 2013;42:4906. doi: 10.1039/c3cs35522e. [DOI] [PubMed] [Google Scholar]; (j) Song X, Ni Q, Grossmann A, Enders D. Chem.—Asian J. 2013;8:2965. doi: 10.1002/asia.201300938. [DOI] [PubMed] [Google Scholar]; For selected reviews and examples on NHC catalysis, see:
- (7)(a).Wende RC, Schreiner PR. Green Chem. 2012;14:1821. [Google Scholar]; (b) Liu DDJ, Chen EY-X. Green Chem. 2014;16:964. [Google Scholar]
- (8).(a) Enders D, Grondal C, Hüttl MRM. Angew. Chem. 2007;119:1590. doi: 10.1002/anie.200603129. [DOI] [PubMed] [Google Scholar]; Angew. Chem., Int. Ed. 2007;46:1570. [Google Scholar]; (b) Walji AM, MacMillan DWC. Synlett. 2007:1477. [Google Scholar]; (c) Grondal C, Jeanty M, Enders D. Nat. Chem. 2010;2:167. doi: 10.1038/nchem.539. [DOI] [PubMed] [Google Scholar]; (d) Albrecht Ł, Jiang H, Jørgensen KA. Angew. Chem., Int. Ed. 2011;50:8492. doi: 10.1002/anie.201102522. [DOI] [PubMed] [Google Scholar]; (e) Lu L-Q, Chen J-R, Xiao W-J. Acc. Chem. Res. 2012;45:1278. doi: 10.1021/ar200338s. [DOI] [PubMed] [Google Scholar]; (f) Pellissier H. Adv. Synth. Catal. 2012;354:237. [Google Scholar]; (g) Goudedranche S, Raimondi W, Bugaut X, Constantieux T, Bonne D, Rodriguez J. Synthesis. 2013;45:1909. [Google Scholar]; (h) Chauhan P, Enders D. Angew. Chem., Int. Ed. 2014;53:1485. doi: 10.1002/anie.201309952. [DOI] [PubMed] [Google Scholar]; (i) Volla CMR, Atodiresei I, Rueping M. Chem. Rev. 2014;114:2390. doi: 10.1021/cr400215u. [DOI] [PubMed] [Google Scholar]; For selected reviews on organocatalytic domino/cascade reactions, see:
- (9).Hayashi Y, Kojima M, Yasui Y, Kanda Y, Mukaiyama T, Shomura H, Nakamura D, Ritmaleni I, Sato I. ChemCatChem. 2013;5:2887. [Google Scholar]
- (10).(a) Xu D-Q, Wang Y-F, Luo S-P, Zhang S, Zhong A-G, Chen H, Xu Z-Y. Adv. Synth. Catal. 2008;350:2610. [Google Scholar]; (b) Tan B, Chua PJ, Li Y, Zhong G. Org. Lett. 2008;10:2437. doi: 10.1021/ol8007183. [DOI] [PubMed] [Google Scholar]; (c) Tsakos M, Elsegood MRJ, Kokotos CG. Chem. Commun. 2013;49:2219. doi: 10.1039/c3cc39165e. [DOI] [PubMed] [Google Scholar]; (d) Qian H, Zhao W, Sung HH-Y, Williams ID, Sun J. Chem. Commun. 2013;49:4361. doi: 10.1039/c2cc37102b. [DOI] [PubMed] [Google Scholar]; (e) Quintavalla A, Lombardo M, Sanap SP, Trombini C. Adv. Synth. Catal. 2013;355:938. [Google Scholar]; (f) Dai Q, Arman H, Zhao JC-G. Chem.—Eur. J. 2013;19:1666. doi: 10.1002/chem.201203104. [DOI] [PubMed] [Google Scholar]; (g) Arai T, Yamamoto Y. Org. Lett. 2014;16:1700. doi: 10.1021/ol500361w. [DOI] [PubMed] [Google Scholar]; For recent selected examples of organocatalytic Michael–Henry domino reactions, see:
- (11).(a) Heumann LV, Keck GE. Org. Lett. 2007;9:1951. doi: 10.1021/ol070573h. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Larrosa I, Romea P, Urpi F. Tetrahedron. 2008;64:2683. [Google Scholar]; (c) Gotoh H, Okamura D, Ishikawa H, Hayashi Y. Org. Lett. 2009;11:4056. doi: 10.1021/ol901483x. [DOI] [PubMed] [Google Scholar]; (d) Chandrasekhar S, Mallikarjun K, Pavankumarreddy G, Rao KV, Jagadeesh B. Chem. Commun. 2009;45:4985. doi: 10.1039/b904662c. [DOI] [PubMed] [Google Scholar]; (e) Olier C, Kaafarani M, Gastaldi S, Bertrand MP. Tetrahedron. 2010;66:413. [Google Scholar]; (f) Wu Y, Lu A, Liu Y, Yu X, Wang Y, Wu G, Song H, Zhou Z, Tang C. Tetrahedron: Asymmetry. 2010;21:2988. [Google Scholar]; (g) Li X, Yang L, Peng C, Xie X, Leng H-J, Wang B, Tang Z-W, He G, Ouyang L, Huang W, Han B. Chem. Commun. 2013;49:8692. doi: 10.1039/c3cc44004d. [DOI] [PubMed] [Google Scholar]; (h) Urbanietz G, Atodiresei I, Enders D. Synthesis. 2014;46:1261. doi: 10.1055/s-0033-1340826. [DOI] [PMC free article] [PubMed] [Google Scholar]; For selected recent examples on tetrahydropyran syntheses, see:
- (12)(a).Posner GH, Crouch RD. Tetrahedron. 1990;46:7509. [Google Scholar]; (b) Ishikawa H, Sawano S, Yasui Y, Shibata Y, Hayashi Y. Angew. Chem., Int. Ed. 2011;50:3774. doi: 10.1002/anie.201005386. [DOI] [PubMed] [Google Scholar]; (c) Uehara H, Imashiro R, Hernandez-Torres G, Barbas CF., III Proc. Natl. Acad. Sci. U.S.A. 2010;107:20672. doi: 10.1073/pnas.1003350107. [DOI] [PMC free article] [PubMed] [Google Scholar]; For Michael/Henry/acetalization examples, see:
- (13)(a).Perron F, Albizati KF. Chem. Rev. 1989;89:1617. [Google Scholar]; (b) Raju BR, Saikia AK. Molecules. 2008;13:1942. doi: 10.3390/molecules13081942. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Favre S, Vogel P, Gerber-Lemaire S. Molecules. 2008;13:2570. doi: 10.3390/molecules13102570. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Sperry J, Liu Y-C, Brimble MA. Org. Biomol. Chem. 2010;8:29. doi: 10.1039/b916041h. [DOI] [PubMed] [Google Scholar]
- (14).Bedorf N, Schomburg D, Gerth K, Reichenbach H, Höfle G. Liebigs Ann. Chem. 1993:1017. [Google Scholar]
- (15)(a).Willson TM, Kocienski P, Jarowicki K, Isaac K, Faller A, Campbell S, Bordner J. Tetrahedron. 1990;46:1757. [Google Scholar]; (b) Willson TM, Kocienski P, Jarowicki K, Isaac K, Faller A, Campbell S, Bordner J. Tetrahedron. 1990;46:1767. [Google Scholar]
- (16).Hale KJ, Hummersone MG, Manaviazar S, Frigerio M. Nat. Prod. Rep. 2002;19:413. doi: 10.1039/b009211h. [DOI] [PubMed] [Google Scholar]
- (17).Enders D, Hahn R, Atodiresei I. Adv. Synth. Catal. 2013;355:1126. [Google Scholar]
- (18).Malerich JP, Hagihara K, Rawal VH. J. Am. Chem. Soc. 2008;130:14416. doi: 10.1021/ja805693p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).CCDC 999310 (6a) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.