Abstract
Reports of methamphetamine-related emergency room visits suggest that elevated body temperature is a universal presenting symptom, with lethal overdoses generally associated with extreme hyperthermia. This review summarizes the available information on methamphetamine toxicity as it pertains to elevations in body temperature. First, a brief overview of thermoregulatory mechanisms is presented. Next, central and peripheral targets that have been considered for potential involvement in methamphetamine hyperthermia are discussed. Finally, future areas of investigation are proposed, as further studies are needed to provide greater insight into the mechanisms that mediate the alterations in body temperature elicited by methamphetamine.
Keywords: Autonomic nervous system, Heat production, Hypertension, Hyperthermia, Immune, Methamphetamine, Metabolism, Neurotransmitter, Reactive oxygen species, Stress, Tachycardia, Thermoregulation, Toxicity, Vasoconstriction
1. Introduction
A number of excellent reviews are available outlining the health and societal concerns stemming from methamphetamine (METH) abuse and overdose (Davidson et al., 2001; Cruickshank & Dyer, 2009; Krasnova & Cadet, 2009; Clark et al., 2012; Marshall & O’Dell, 2012), yet there remains a paucity of information related to the hyperthermic effects of METH. In the United States, METH use is responsible for an estimated 94,000 emergency department admissions annually (NIDA, 2011), with elevated body temperature appearing as a universal presenting symptom. METH-induced hyperthermia puts individuals at risk for death and there are few treatment options (Greenblatt & Osterberg, 1961; Schep et al., 2010). Consequently, this review focuses on METH hyperthermia. It covers what is known about the effects of METH on body temperature as well as providing a review of the literature on previously tested hypotheses concerning METH hyperthermia and the outcomes of these studies. Finally, the review suggests directions for future research.
2. Temperature regulation
The regulation of body temperature requires a coordinated effort between central and peripheral mechanisms, with the balance of heat retention and dissipation representing key components of the process. Since pathophysiology results from the disruption of normal physiological functions, understanding how METH may dysregulate body temperature to cause hyperthermia requires a better understanding of how normal temperature regulation occurs, a topic which is briefly reviewed herein. Normal heat loss mechanisms, such as those triggered in response to high ambient temperatures, include: 1) radiation, 2) conduction, 3) convection, and 4) evaporation (Docherty & Green, 2010). The first three processes involve the passive transfer of heat and energy from the body to the colder surrounding environment, while evaporation is an active process that occurs primarily in the form of sweating (or panting in animals).
Normal heat generating mechanisms, such as those triggered in response to cold environments, include: 1) increased metabolic activity of tissues (e.g., increased tissue oxidation), 2) increased muscle activity (e.g., through shivering, exercise), and 3) nonshivering thermogenesis (e.g., through increased lipid and carbohydrate metabolism, brown adipose tissue) (Cannon & Nedergaard, 2004; Docherty & Green, 2010; Morrison & Nakamura, 2011). Additional heat retention strategies include: 1) vasoconstriction (to minimize heat loss by radiation), and 2) insulation (through fat under the skin, piloerection in animals with fur) (Docherty & Green, 2010; Morrison & Nakamura, 2011).
2.1. Anatomy of temperature regulation
Physiological responses used to maintain body temperature are regulated by an integration of central nervous system (CNS) and systemic events, with coordination of these processes primarily controlled in the hypothalamus (Morrison & Nakamura, 2011). Heat and cold are detected by temperature sensors in the body, which are located in both the periphery and CNS. The peripheral sensors are found in the skin and utilize transient receptor potential (TRP) channels on primary sensory afferents to relay information to the CNS, and ultimately the hypothalamus (Morrison & Nakamura, 2011). Once this information reaches the hypothalamus, warm-sensitive neurons in the anterior preoptic area respond to changes in temperature, which are sensed locally in the tissue (Nakayama et al., 1961).
Neurons in the preoptic area of the hypothalamus have synaptic contacts that: 1) activate parasympathetic neurons in the anterior hypothalamus, and 2) inhibit sympathetic neurons in the posterior hypothalamus. Thus, when an increase in temperature is sensed, vasodilation and sweating result due to parasympathetic stimulation and removal of sympathetic tone to blood vessels in the skin (Charkoudian, 2003; Rusyniak & Sprague, 2006). Other physiological responses that occur in an effort to dissipate heat include decreased metabolic and muscle activity (Webb, 1995).
Although the hypothalamus is recognized as the thermoregulatory center that coordinates the information coming in from the periphery via the primary sensory afferents with the out-going responses to the autonomic nervous system, other intervening brain regions may also participate in this coordinated response. These regions include the lateral parabrachial nucleus and the rostral ventromedial medulla (Morrison & Nakamura, 2011).
2.2. Neurochemistry of temperature regulation
The major neurotransmitters involved in thermoregulation are: glutamate (afferents to the hypothalamus and some efferents), γ-aminobutyric acid (GABA; efferents from the hypothalamus), serotonin (brainstem neurons), norepinephrine and acetylcholine (autonomic neurons) (Morrison & Nakamura, 2011). In addition, a number of peptides, hormones, and cytokines can modulate body temperature (Morrison & Nakamura, 2011). The sources of these bioactive molecules are varied and include neurons, glia, myocytes (cardiac and skeletal muscle), endothelial cells, and blood cells (Kiyatkin & Sharma, 2009).
2.3. Body mass scaling
A major challenge for research on hyperthermia and thermoregulatory processes is appropriate extrapolation of data from studies in animals to humans (Gordon, 2007). Most studies involving the hyperthermic effect of METH have been conducted in rodents, with issues related to body mass scaling being of particular concern. The vasomotor index, which quantifies the extent to which an animal can regulate its surface temperature, is positively correlated with body mass (Phillips & Heath, 1995). Smaller animals, such as rodents, have a large surface area:volume ratio compared to humans, and use different strategies to regulate surface temperature. Small animals rely on passive heat dissipation and alterations in metabolic rate to achieve control, whereas large animals, such as humans, have smaller surface area:volume available for heat exchange and depend on sweating and peripheral vasomotor mechanisms to regulate this process, rather than alterations in metabolic rate (Gordon, 2007). A major consequence of these differences is that rodents, with their small size relative to humans, are more vulnerable to fluctuations in ambient temperature than humans.
3. METH and temperature regulation
METH acts as an indirect agonist that can cause the release of monoamines and inhibit their reuptake in both the CNS and periphery (Davidson et al., 2001; Krasnova & Cadet, 2009; Schep et al., 2010). At lower, stimulant doses, METH does not have significant effects on body temperature, but can produce lethal hyperthermia upon exposure to high doses (Albertson et al., 1999; Krasnova & Cadet, 2009). Information on general thermoregulatory mechanisms that are affected by METH are summarized, followed by an overview of environmental factors and disease states that can modulate the effect of METH on body temperature.
3.1. Body temperature dysregulation by METH
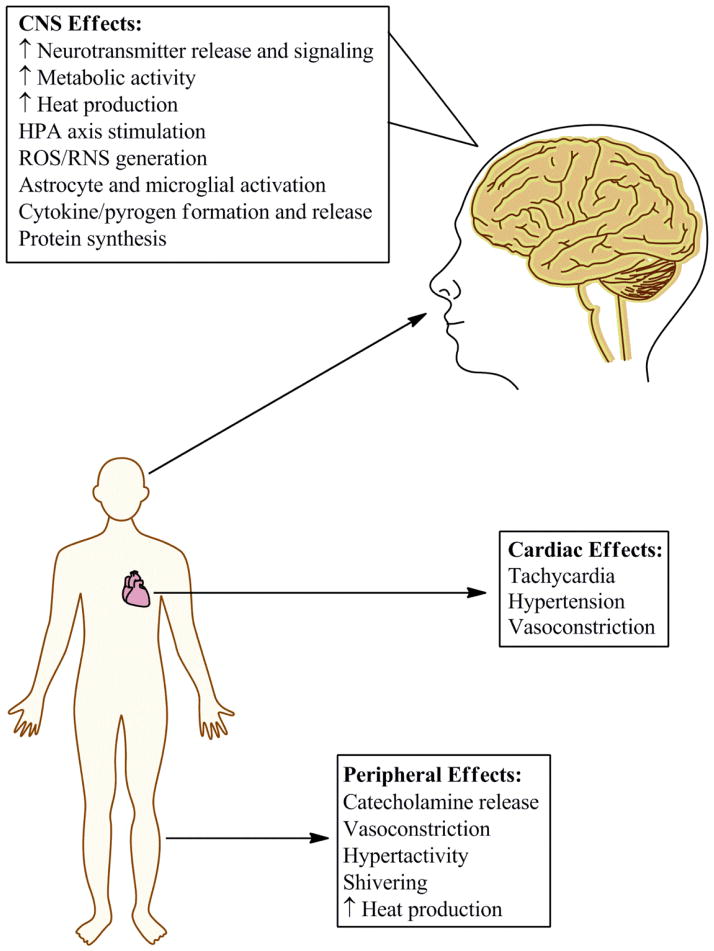
The mechanisms through which METH affects body temperature include both central and peripheral targets (Fig. 1). In general, METH increases body temperature by promoting heat generation and retention, and suppressing responses that would facilitate heat dissipation (Table 1). It should be noted that these effects can vary greatly by species (e.g., rats vs. mice).
Fig. 1.
Methamphetamine causes hyperthermia through a variety of mechanisms involving numerous systems. Methamphetamine causes neurotransmitter release and increased metabolism in the central nervous system (CNS). Additionally, methamphetamine can increase heart rate and result in hyperactivity, leading to increased heat production in the periphery. Vasoconstriction is another consequence of methamphetamine exposure, which results in a decreased ability of the body to rid itself of the increased heat resulting from these effects.
Table 1.
Physiological responses to environmental heat vs. methamphetamine.
| Exposure to high ambient temperature | Exposure to high dose methamphetamine |
|---|---|
| 1) Increase in blood and brain temperature | 1) Increase in brain temperature |
2) Hypothalamic response:
|
2) Hypothalamic response:
|
3) Activation of heat loss mechanisms:
|
3) Activation of heat production mechanisms:
4) Activation of heat loss mechanisms:
|
As a psychomotor stimulant, METH causes increased activation of and metabolism in the CNS and skeletal muscles (Estler, 1975; Makisumi et al., 1998), which may elevate brain and body temperature. In addition, METH causes vasoconstriction (Gordon et al., 1991; Turnipseed et al., 2003; Watts & McCollester, 2006; Haning & Goebert, 2007), which can contribute to the ensuing hyperthermia by preventing heat dissipation in the periphery. In laboratory animals, piloerection may also indicate that heat dissipation mechanisms have become compromised and/or heat retention mechanisms are activated by METH, although it should be cautioned that serotonin release can cause piloerection unrelated to body temperature (Gordon, 1983, 2005). These heat generating processes more often than not overwhelm some efforts by METH-treated laboratory animals to dissipate heat, including pronounced drooling and saliva spreading (Morrison & Nakamura, 2011).
3.2. Modulators of thermoregulation and METH hyperthermia
A number of environmental factors and disease states can affect temperature regulation, with the ones most relevant to the conditions under which METH exposure may occur summarized here. Primary amongst them is ambient temperature, where increasing or decreasing environmental temperature can greatly enhance or reduce METH’s effects on body temperature, respectively (Bowyer et al., 1992, 1994). In METH-treated animals, elevations in brain temperature have also been reported at increased ambient temperatures (Brown & Kiyatkin, 2005; Kiyatkin & Sharma, 2011, 2012). In humans, prolonged exposure to excessive environmental temperatures can cause hyperthermia and death, with 65% of heat-related deaths resulting from exposure to excessive heat (CDC, 2006); this clinical condition would be expected to be greatly exaggerated with co-exposure to METH, as has been shown to be the case for 3,4-methylenedioxymethamphetamine (MDMA) (Green et al., 2004; Kiyatkin & Sharma, 2012). In contrast, cold environments can prevent METH-induced hyperthermia and attenuate resulting METH-induced increases in oxidation products and neurotoxicity (Bowyer et al., 1994; LaVoie & Hastings, 1999; also see below).
Social interaction can also affect body temperature. Earlier studies have reported increases in body and brain temperature following exposure to conspecifics (Kiyatkin et al., 2002). Many studies have shown that exposure of animals to METH when group housed greatly enhances the toxicity and lethality of the drug, a phenomenon also known as “aggregate toxicity” (Greenblatt & Osterberg, 1961). The changes in body temperature may be secondary to arousal-induced neuronal activation since alterations in brain temperature precede and exceed those measured in the body, including head muscles adjacent to the skull (Brown et al., 2003). However, an alternate explanation could relate to the higher energy demands of the brain compared to other tissues, which would be expected to generate heat (Kiyatkin, 2007). Regardless of the underlying mechanisms of the aforementioned, hyperthermia resulting from social interactions has been reported to enhance the toxicity produced by METH by increasing the duration of the hyperthermia (Brown et al., 2003).
Exercise can also elevate body temperature, by increasing cardiac and skeletal muscle activity (Wendt et al., 2007). In humans, brain and body core temperature rise in parallel in response to exercise (Nybo, 2012). Further increases in body temperature that occur in association with METH can thus exacerbate exercise-induced heat stress on the body. These observations have practical relevance as well in situations where the amphetamines are used clinically. Children and adults with attention deficit hyperactivity disorder (ADHD) are treated with a mixture of amphetamine salts (e.g., Adderall) and may therefore be prone to more difficulty in extreme temperature situations (e.g., participating in sports during the hot summer months).
Stressor exposures in combination with METH can also modulate body temperature in rats and mice. For example, unpredictable stress in rats enhances the hyperthermia produced by METH (Tata et al., 2007). METH-induced extracellular dopamine concentration in stressed vs. unstressed rats does not differ significantly, suggesting the contribution of non-dopaminergic systems in these effects (Tata et al., 2007). In contrast, serotonergic function appears important because the enhancement of METH-induced hyperthermia by stress can be prevented by the 5-HT2 antagonist ketanserin (Doyle & Yamamoto, 2010). This is consistent with the well-established up-regulation of 5-HT2 receptor expression induced by various forms of stress (Davis et al., 1995; Takao et al., 1995; Ossowska et al., 2001; Matuszewich & Yamamoto, 2003). This stress-induced alteration would be expected to enhance the hyperthermic effects mediated through METH-induced release of serotonin (Doyle & Yamamoto, 2010). In contrast to these data obtained in rats, mice exposed to restraint stress in combination with METH show markedly decreased body temperature and resistance to METH-induced dopaminergic neurotoxicity (Miller & O’Callaghan, 1994).
In addition to the aforementioned environmental factors, a number of disease states have been documented to increase the risk of developing hyperthermia. These include: cardiovascular disease (e.g., chronic ischemic heart disease); endocrine, nutritional, and metabolic disorders (e.g., diabetes); infection and psychiatric disorders (CDC, 2006). The extent to which they may be extrapolated to METH-induced hyperthermia remains under investigation.
All of these factors are important to keep in mind because METH hyperthermia in humans generally involves an interaction between exposure to the drug and one or more of these exacerbating factors.
4. Clinical pharmacology and considerations related to METH hyperthermia
An overview of the pharmacokinetics of METH that are relevant for understanding its effects on body temperature are reviewed. In addition, the clinical management of METH hyperthermia is presented, along with select clinical consequences that can result from the hyperthermic effects of METH.
4.1. Pharmacokinetic parameters of METH
As dose to target is an important component of toxicity, the pharmacokinetics of METH are important to understand because different routes of administration can affect the amount of METH getting to the target, thereby affecting the magnitude of effects and their duration of action. METH is used through four primary routes of administration: 1) intravenous, 2) smoking, 3) oral, and 4) intranasal. Route of use dictates bioavailability, with studies demonstrating oral administration resulting in the lowest (67%) and intravenous administration resulting in the highest (100%) absorption (Schep et al., 2010). Intranasal (79%) and smoking (67–90%), depending on technique utilized, fall in between in bioavailability (Cruickshank & Dyer, 2009). Route of administration also affects the time to peak effect. For example, in preclinical studies in rhesus macaques, a similar peak temperature, but delayed onset is observed when METH is administered orally compared to intraperitoneally (Crean et al., 2006, 2007). In humans, intravenous and intranasal dosing results in peak effects within 15 minutes, while smoking has a slightly longer time-to-peak of 18 minutes and lastly, oral uptake results after a significant delay of approximately 3 hours (Cruickshank & Dyer, 2009). Alternative routes of administration, for which little to no pharmacokinetic data exist, have emerged in recent years and require further investigation to elucidate drug uptake. These include body stuffing in which poorly packaged drugs are ingested, usually in an attempt to avoid prosecution by the police; ‘parachuting’ in which the drug is placed into a plastic bag with the seal removed, rolled tightly, and swallowed, theoretically releasing the drug over a prolonged period; and ‘shelving’, also known as intravaginal stuffing (Hendrickson et al., 2006; Kashani & Ruha, 2004; West et al., 2010). Although administration routes of METH that lead to greater bioavailability would be expected to result in increased hyperthermia in clinical situations, it is currently unclear if maximum increases in METH-induced hyperthermia are specifically related to peak plasma concentration, or if increases in body temperature are more related to cumulative exposure.
The metabolism of METH occurs primarily in the liver through three main processes: 1) N-demethylation via cytochrome P450 2D6, 2) aromatic hydroxylation through cytochrome P450 2D6, and 3) β-hydroxylation, producing amphetamine, 4-hydroxymethamphetamine, and norephedrine, respectively (Schep et al., 2010). Some of these metabolites may contribute to sustained hyperthermic effects of METH, with amphetamine reported to produce elevations in body temperature on its own (Levi et al., 2012).
4.2. Treatment of METH hyperthermia
The major presenting symptom in emergency rooms involving a METH overdose is extreme hyperthermia that can be lethal if left untreated (Bowyer et al., 1984; Ito et al., 2008). Other presenting symptoms commonly observed, particularly in high-dose patients, include hypertension, tachycardia, dyspnea, and chest pain, with other features including increased agitation, altered mental status, pupil dilation, shivering, and possible seizure activity (Albertson et al., 1999). Medical interventions focus on strategies to minimize the bioavailability of METH and symptomatic management.
The clinical diagnosis of hyperthermia occurs when the core body temperature exceeds 40° C, at which point normal thermoregulatory mechanisms fail and the body is no longer capable of effectively dissipating heat (Bynum et al., 1978). Currently, no approved pharmacotherapy is available for treating the hyperthermic effects of METH. Administration of ammonium chloride or activated charcoal can assist in resolving METH-induced hyperthermia by increasing the excretion of METH from the body through acidification of the urine or prevention of the absorption of orally ingested METH, respectively (Suchard, 2007). Cooling, which can be accomplished using either external or internal means, however, remains the primary method for the clinical treatment of hyperthermia. External cooling measures can include cool water submersion, misting accompanied by fans, or hypothermia blankets (Eyer & Zilker, 2007; Suchard, 2007). Medical providers must remain vigilant that skin cooling is not performed too quickly as rapid skin cooling produces shivering and vasoconstriction, which would minimize therapeutic benefit. Endovascular cooling, in which the temperature of the patient’s blood is lowered via a cooling catheter, while not generally deemed necessary, is capable of inducing rapid cooling while reducing the impact of shivering and vasoconstriction (Eyer & Zilker, 2007). While these methods have been shown to be beneficial in the treatment of hyperthermia for other disease states, currently it is unknown if these treatments result in better patient outcomes during METH overdose situations and no clear prospective studies have been conducted to determine their effectiveness.
4.3. Clinical consequences of METH hyperthermia
Hyperthermia can promote rhabdomyolysis, multi-organ failure, release of excitotoxic neurotransmitters, increased reactive oxygen species production, and heightened breakdown of cytoskeletal proteins (Eyer & Zilker, 2007; West et al., 2010). Hyperthermia elicited by METH per se has been implicated in a number of these effects and are reviewed below.
One of the major organs especially sensitive to the hyperthermic effects of METH is the liver. An early analysis on the clinical presentations of acute METH toxic reactions reported hepatitis as the second most common presenting complaint (Smith & Fischer, 1970); and more recently, a clinical report showed acute liver damage can occur following METH use even in patients without viral hepatitis (Kamijo et al., 2002). In a rat model of binge METH exposure, further supporting that METH alone does cause acute liver damage, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were elevated along with hepatic histopathological findings (Halpin & Yamamoto, 2012). With regard to the mechanisms involved in METH-induced liver toxicity, an increase in formation of reactive oxygen species (ROS) and subsequent lipid peroxidation, reduced glutathione (GSH) depletion and mitochondrial dysfunction were observed in freshly isolated rat hepatocytes (Eskandari, 2014). Previous studies have shown that an increase in body temperature by itself can also instigate hepatotoxic effects, namely those related to oxidative stress and resulting lipid peroxidation and membrane damage (Skibba et al., 1990, 1991, 1997), and thus most likely contributes to the liver damage observed after METH exposure. Of note, exogenous increases in temperature significantly exacerbate METH-induced hepatotoxicity in vitro (da Silva et al., 2013). In addition, METH-induced hyperthermia has been shown to play a prominent role in facilitating the structural and cellular liver damage and increases in peripheral ammonia produced by METH in vivo (Halpin et al., 2014). Consequently, the hyperthermia appears to dramatically contribute to the severity of METH-induced liver damage. Further investigation, however, is required for more accurate extrapolations and determination of the extent to which METH-induced hyperthermia by itself is responsible for the METH-induced liver toxicity.
METH-induced damage to dopaminergic nerve terminals in rats and mice is also associated with elevated core temperature (Bowyer et al., 1994, 1995; O’Callaghan & Miller, 1994; Miller & O’Callaghan, 1994, 2003; O’Callaghan & Miller, 2002). After administration of METH, increasing core temperature by elevating ambient temperature results in exacerbated dopaminergic neurotoxicity in both mice and rats (Bowyer & Holson, 1995). Decreasing core temperature by pharmacological, genetic and/or physiological means in combination with METH exposure decreases neurotoxicity (Albers & Sonsalla, 1995). For example, in rats, increases in brain levels of METH have no effect on dopaminergic neurotoxicity if body temperature is lowered by reducing ambient temperature (Bowyer et al., 1994, 1995). In essence, exposure to METH confers a poikilothermic effect where any change in ambient temperature appears reflected in corresponding changes in body temperature (see Bowyer et al., 1995). As noted earlier, the mechanistic relationship between METH administration, hyperthermia, neurotoxicity and METH-associated hyperthermia and mortality has not been established. Thus, while elevated temperature appears to be an obligatory component of METH neurotoxicity, the mechanism(s) controlling this are unknown.
5. Pharmacological and molecular biological studies of METH hyperthermia
A number of the monoamines, including norepinephrine, dopamine, and serotonin, appear to be involved in thermoregulation (Lin et al., 1982, 1983; Sprague et al., 2007), and these same neurotransmitters are impacted by METH exposure. Consequently, pharmacological and molecular manipulations involving these systems have long been a focus in characterizing the nature of METH-induced hyperthermia (e.g., Ito et al., 2008; Makisumi et al., 1998; Metzger et al., 2000). Concurrently, the ability of many drugs with no recognized monoaminergic actions (e.g., pentobarbital, MK-801, ethanol, dantrolene) to successfully block METH hyperthermia (Makisumi et al., 1998; Miller & O’Callaghan, 1994) underscores the complexities involved in determining and understanding the mechanisms involved in METH-induced hyperthermia. Accordingly, the following sections summarize data describing the ability of a variety of pharmacological (Table 2) and molecular biological manipulations, targeting monoaminergic and non-monoaminergic systems, to prevent or attenuate METH-induced hyperthermia. In addition, manipulations that have been ineffective against METH-induced body temperature changes are briefly mentioned.
Table 2.
Compounds utilized in preclinical models to mitigate hyperthermia associated with methamphetamine.
| Structure | Compound | Molecular Target |
|---|---|---|
| Norepinephrine/General Monoamine System: | ||

|
Reserpine | VMAT Inhibitor |

|
α-Methyl-p-tyrosine (AMPT) | Tyrosine Hydroxylase Inhibitor |
| Dopamine System: | ||
|
|
Haloperidol | Dopamine D2 Receptor Antagonist |

|
Sulpiride | Dopamine D2 Receptor Antagonist |

|
Eticlopride | Dopamine D2 Receptor Antagonist |

|
SCH 23390 | Dopamine D1 Receptor Antagonist |

|
Risperidol | D1 and D2 Receptor Antagonist 5-HT2A Receptor Adrenoceptor Blocker |
| Serotonin System: | ||

|
p-Cholorphenyl-alanine (PCPA) | Tryptophan Hydroxylase Inhibitor |

|
NAN-190 | 5-HT1A Receptor Antagonist |

|
Fenfluramine | SERT Inhibitor |
| ROS/RNS System: | ||
|
|
α-Tocopherol | ROS Scavenger |

|
Melatonin | Antioxidant Melatonin Receptor Agonist |
|
|
Deferoxamine | Iron Chelator |

|
7-Nitroindazole | Neuronal Nitric Oxide Synthase (nNOS) Inhibitor |

|
FeTMPγP | Peroxynitrite Catalyst |
| Immune System: | ||
|
Mithramycin | Specificity Protein 1 (SP-1) Inhibitor |
| Miscellaneous Targets: | ||

|
Rottlerin | PKCδ Inhibitor Potassium (BKCa2+) Channels |

|
AC927 | Sigma-1 Receptors Sigma-2 Receptors |
|
|
AZ66 | Sigma-1 Receptors Sigma-2 Receptors |

|
CM156 | Sigma-1 Receptors Sigma-2 Receptors |

|
SN79 | Sigma-1 Receptors Sigma-2 Receptors |

|
Phenytoin | Anti-convulsant |
A wide variety of molecular targets and chemical structures have been used to study the hyperthermic effects of methamphetamine. Some of these represent potential options for pharmacotherapies to treat the clinical complications of methamphetamine, including hyperthermia encountered during overdose situations. BKCa2+, large conductance potassium channel; PKC, protein kinase C; ROS, reactive oxygen species; SERT, serotonin transporter; VMAT, vesicular monoamine transporter.
5.1. Monoaminergic systems – Norepinephrine, dopamine, and serotonin
The monoaminergic actions of METH in the CNS are well known, have been thoroughly characterized, and are the subject of a number of reviews (e.g., see Bowyer & Holson, 1995). High dose exposure protocols of METH and related compounds cause the release of large amounts of dopamine, serotonin and norepinephrine accompanied by monoamine oxidase inhibition and a subsequent decrease in the monoamines themselves and their metabolites. A variety of methods have been utilized in trying to understand the role of the various monoamine systems in METH-induced hyperthermia and include: 1) direct injection of the monoamines into various brain areas involved in temperature regulation, 2) depletions of monoamines in general or in specific pools, 3) application of agonists or antagonists of the monoamine receptors, and 4) genetically removing the transporters or receptors for monoamines. None of these manipulations have resulted in a clear picture of how monoamines are involved in METH-induced hyperthermia, although some contribution is indicated. To further complicate the issue, most of these studies have not focused on METH hyperthermia per se, but are more concerned with the role of hyperthermia in promoting neurotoxicity (Albers & Sonsalla, 1995; Miller & O’Callaghan, 1994; Metzger et al., 2000). Certainly, the field would be served by a concerted effort to determine the causes of METH hyperthermia separate from the role this effect plays in METH neurotoxicity.
Although less well-studied than its effects on the CNS, METH also affects monoaminergic systems in the periphery. Reserpine, for example, is a vesicular monoamine transporter (VMAT) inhibitor that acts by depleting catecholamines from peripheral sympathetic nerve endings, causing vasodilation and a reduction in heart rate. It also lowers METH-induced elevations in core body temperature (Albers & Sonsalla, 1995; Ares-Santos et al., 2012), even causing hypothermia in some cases (Thomas et al., 2008). Consistent with the recognized actions of METH in the autonomic nervous system, peripheral chemical sympathectomy by 6-hydroxydopamine (OHDA) can completely block METH-induced hyperthermia (Makisumi et al., 1998), further supporting a role for peripheral monoamines in METH hyperthermia.
However, it should be noted that the contribution of catecholamines to METH hyperthermia can vary depending on species and environmental conditions. For example, depletion of catecholamines by pretreatment with α-methyl-p-tyrosine (AMPT), which inhibits tyrosine hydoxylase, the rate limiting enzyme in the biosynthetic pathway for catecholamines (dopamine, norepinephrine), can attenuate the hyperthermic effects of METH in rats, but not mice (Metzger et al., 2000; Sandoval et al., 2000; Thomas et al., 2008). In addition, the protective effects of AMPT in rats are observed at ambient room temperature, but not at an elevated room temperature (28.5° C) (Metzger et al., 2000). This suggests that catecholamines may be important, but just one of the ways for METH to produce its hyperthermic effects.
In the sections that follow, efforts are made to summarize both peripheral and central effects of monoaminergic systems on body temperature changes. The sections are organized to first provide an overview of the effects of the neurotransmitter systems, with a focus on norepinephrine, dopamine, and serotonin, on thermoregulation in general, then followed by information with regard to their potential contributions to METH hyperthermia.
5.1.1 Norepinephrine systems
Activation of the hypothalamic-pituitary-adrenal (HPA) axis triggers the release of numerous hormones and neurotransmitters, including norepinephrine (Makisumi et al., 1998; Del Rios et al., 2005). In the CNS, the presence of α1-adrenoceptors on thermosensitive neurons in the medial preoptic anterior hypothalamus is noteworthy (Mallick et al., 2002). β-Adrenoceptors are also located in the hypothalamus (Reznikoff et al., 1986; Grimm et al., 1992) and have been implicated in thermoregulation (Docherty & Green, 2010). In the periphery, norepinephrine release triggers vasoconstriction and impaired heat dissipation via actions on adrenoceptors and also by uncoupling metabolism in thermogenic tissues (Morrison & Nakamura, 2011), further worsening hyperthermic events.
Pharmacological interventions that target specific aspects of adrenergic function in the context of METH-induced hyperthermia have received limited attention. The β-adrenoceptor antagonist, propranolol, is one of the few that has been reported to prevent METH-induced hyperthermia in laboratory mice (Albers & Sonsalla, 1995). Although the effects of antagonists for α1, α2A, and β3 receptors have been studied in the context of MDMA (Docherty & Green, 2010, Hysek et al., 2013), similar investigations with METH have yet to be performed.
Most of the manipulations that affect METH-induced noradrenergic function and body temperature have been relatively nonspecific. METH-induced increases in body temperature are prevented by 6-OHDA sympathectomy or adrenalectomy (Makisumi et al., 1998) by inhibiting the presumed release of norepinephine by METH. In adrenalectomized rats treated with dexamethasone, METH elicits hyperthermia, demonstrating modulation by glucocorticoids (Makisumi et al., 1998). Dantrolene, an inhibitor of sarcoplasmic reticulum calcium release in muscle, also prevents METH-induced hyperthermia (Makisumi et al., 1998). Together, the data suggest that METH stimulates norepinephrine release from sympathetic nerve terminals, thereby enhancing thermogenesis in skeletal muscles under the permissive control of glucocorticoids (Makisumi et al., 1998). The adrenoceptor subtypes that mediate these effects remain to be determined.
5.1.2. Dopamine systems
Early studies have shown that direct injection of dopamine or the dopamine agonist, apomorphine, into the anterior preoptic area of the hypothalamus, caudate-putamen, or globus pallidus elicits hypothermia, whereas similar microinjections of dopamine antagonists (e.g., haloperidol, pimozide) produce hyperthermia (Lin et al., 1982). These dopamine-related changes in body temperature appear mediated through alterations in metabolism and cutaneous blood flow, but not respiratory evaporative processes (Lin et al., 1982).
METH can readily enter cells and facilitate the release of dopamine while simultaneously preventing reuptake, resulting in increased dopamine in the synaptic cleft (Davidson et al., 2001; Krasnova & Cadet, 2009). However, in contrast to locally administered dopamine or a dopamine agonist into specific brain regions (Lin et al., 1982), METH-induced release of dopamine causes profound hyperthermia. These differences in response between direct application of dopamine or its agonists/antagonists to a given brain area and the METH-induced hyperthermia suggest that the METH-induced changes in body temperature do not result from dopamine release in the specific areas of the brain characterized in the microinjections studies (e.g., anterior preoptic area of the hypothalamus, caudate-putamen, globus pallidus). Nevertheless, the activation of dopamine receptors (presumably in other regions of the body) appears to be a significant mediator of the hyperthermic effects of METH (Albers & Sonsalla, 1995; Funahashi et al., 1990; He et al., 2004; Broening et al., 2005), as ample evidence summarized below suggests these effects can be mitigated through antagonism or knockout of dopamine receptors.
Numerous studies have shown, for example, that attenuation of dopamine D2 function using pharmacological or molecular biological tools can reduce the hyperthermic effects of METH. METH fails to elicit hyperthermia in dopamine D2 knockout mice (Ito et al., 2008; Granado et al., 2011). Likewise, the dopamine D2 antagonists, haloperidol and sulpiride, attenuate METH-induced hyperthermia in mice (Funahashi et al., 1990; Albers & Sonsalla, 1995). The selective dopamine D2 antagonist, eticlopride, also mitigates the hyperthermic effects of METH at normal ambient temperature, but loses its protective effects at a higher ambient temperature (33° C) (Metzger et al., 2000; Broening et al., 2005); this suggests that D2 receptors alone are not sufficient for mediating heat dissipation under extreme conditions.
Although the magnitude of the effects does not appear as pronounced as for the D2 receptor, D1 receptors may also play an important role. The antipsychotic drug, risperidol, can mitigate the hyperthermia produced by METH when given as either a pre-treatment or post-treatment; this effect is thought to be mediated through interactions with dopamine D1 receptors (Shioda et al., 2010). It should be noted, however, that risperidol also has 5-HT2A receptor and anti-adrenergic activity, both of which may further assist in reducing the hyperthermic effects of METH (Shioda et al., 2010; Cohen, 1994). Further supporting the involvement of D1 receptors in the hyperthermic actions of METH, there is a significant reduction in the extent to which METH produces hyperthermia in mice lacking D1 receptors (Ito et al., 2008; Ares-Santos et al., 2012). Moreover, the D1 antagonist, SCH 23390, attenuates METH-induced hyperthermia (Broening et al., 2005). Similar to the effects seen with D2 antagonists, knockout and antagonism of D1 receptors loses their protective effects at higher ambient temperatures (29° C and 33° C, respectively) (Ares-Santos et al., 2012; Broening et al., 2005). One reason that elevated environmental temperatures may overcome the protective effects conveyed by dopamine antagonists at normal ambient temperatures may be that it can overcome the ability of the antagonists to dissipate heat through peripheral mechanisms under these conditions.
Although there is general support for the involvement of dopamine D1 and D2 receptors in the hyperthermic effects of METH, exceptions have been reported. Raclopride (1 mg/kg) and SCH 23390 (0.1 mg/kg), D2 and D1 antagonists, respectively, did not attenuate the elevated body temperatures produced by a bolus dose of METH (30 mg/kg) in ICR mice; body temperature measurements were taken each hour and the selected doses were ones that protected against other neurotoxic endpoints of METH (Xu et al., 2005). The reason for this divergence in results from the overall pattern reported by others remains to be determined.
The hyperthermic effects of METH are believed to contribute to an increased risk of death. Therefore, it is noteworthy that in dopamine D1 and D2 knockout mice, there is also a reduction in lethality in addition to a reduction in hyperthermia produced by METH (Ito et al., 2008). Moreover, in both the knockout and wild-type groups, the METH-treated mice that survive have lower body temperatures than those that die (Ito et al., 2008).
In contrast to dopamine receptors, the dopamine transporter (DAT) appears to play a more limited role in mediating METH-induced changes in body temperature. In particular, DAT knockout mice show a modest, albeit insignificant, reduction in METH-induced hyperthermia as compared to wild-type controls (Numachi et al., 2007). The compromised ability of METH to fully elicit hyperthermia in the absence of DAT suggests some involvement of an increase in synaptic dopamine by METH in altering body temperature, but indicate that this is most likely not the primary cause of the hyperthermic effects. DAT knockout mice do, however, have a significantly higher LD50 for METH compared to wild-type mice, supporting the importance of dopamine in the lethal effects of METH (Numachi et al., 2007).
Overall, the data indicate that the dopamine releasing actions of METH may contribute to hyperthermia, with dopamine antagonists capable of mitigating the elevations in body temperature. However, the blockade of hyperthermia may be incomplete under some conditions, suggesting the involvement of other contributing systems to the body temperature changes. Moreover, classical dopaminergic brain regions typically implicated in thermogenesis and psychostimulant actions (i.e., hypothalamus and striatum) may not be the principal site of action mediating the dopaminergic component of METH-induced hyperthermia.
5.1.3. Serotonin systems
Serotonergic pathways have been directly implicated in the central control of most forms of thermogenesis (Rothwell, 1994). Previous data have shown that changes in neuronal serotonin levels correlate with changes in brain and core body temperatures (Schwartz et al., 1995; Salmi & Ahlenius, 1998). Direct injection of serotonin into the anterior preoptic area of the hypothalamus or electrical stimulation of the dorsal raphe to release serotonin from the nerve terminals elicits hypothermia, whereas direct injection of serotonergic antagonists (e.g., methysergide, cyproheptadine) into this region produces hyperthermia; these changes in body temperature result from alterations in metabolism and vasodilation/constriction of the blood vessels in the skin (Lin et al., 1983). Activation of 5-HT1A and 5-HT3 receptors in the brain also elicits hypothermia (Voronova et al., 2011). Since the aforementioned antagonists (methysergide, cyproheptadine) attenuate 5-HT2 receptor signaling, it appears that multiple serotonin receptor subtypes participate in central thermoregulatory processes.
In contrast, systemic administration of a selective 5-HT2A receptor agonist elevates body temperature (Zhang & Tao, 2011). The hyperthermic effect of 5-HT2A agonists can be life threatening and has been attributed at least in some cases to activation of skeletal muscles (Loscher et al., 1990). In addition, peripheral effects of serotonin include the regulation of skin blood flow, which is a major mechanism involved in thermoregulation (Mauer-Spurej, 2005).
As with the other studies investigating the role of monoamine systems in METH-induced hyperthermia, studies investigating the role of the serotonin system have examined how manipulations involving serotonin receptors, transporters and levels of the transmitter influence METH–induced hyperthermia. Commensurate with its actions on the other monoamines, METH facilitates serotonin release from the nerve terminal, inhibits the action of the rate-limiting enzyme, tryptophan hydroxylase, for the production of serotonin, inhibits its transport and causes its long term depletion in some instances (Kuczenski et al., 1995; Fukumura et al., 1998; Ago et al., 2006; Numachi et al., 2008). Reductions in brain serotonin effected by treating mice with p-chlorophenylalanine (PCPA), an irreversible inhibitor of tryptophan hydroxylase, the rate limiting enzyme in the biosynthesis of serotonin, attenuates METH-induced hyperthermia (Thomas et al., 2010). However, treatment of mice with tryptophan hydroxylase does not significantly alter the effects of METH on body temperature, and tryptophan hydroxylase 2 knockout mice which lack brain serotonin in fact exhibit an enhanced hyperthermic response of about 1°C to METH (Thomas et al., 2010). This suggests that when endogenous serotonin levels are reduced, the hyperthermic effects of METH can be inhibited, but if endogenous serotonin is unavailable, then other neurochemical systems may compensate which can result in a paradoxical enhancement of hyperthermia.
Recent studies in rats show that METH also produces significant dose dependent increases in plasma serotonin (Zolkowska et al., 2006; Rothman et al., 2008; Yubero-Lahoz et al., 2012). Because platelets and neurons express the same serotonin transporter (SERT) protein (Lesch et al. 1993) and 99% of the circulating serotonin is stored in platelets (Zolkowska et al., 2006), the elevations in plasma serotonin may well be mediated, at least in part, via interactions of METH with these platelet SERT proteins (Zolkowska et al., 2006; Rothman et al., 2008; Yubero-Lahoz et al., 2012). The association between METH-induced plasma serotonin and body temperature changes was, however, not examined in these studies and remains to be investigated. Nevertheless, while the specific sites in the body that mediate this hyperthermic effect have yet to be determined, peripheral targets are likely involved since central activation of serotonin function tends to cause reductions in body temperature.
It should be noted, however, that SERT per se is unlikely to be a direct contributor to hyperthermia. METH-induced hyperthermia is not altered nor is basal temperature in mice lacking SERT (Numachi et al., 2007). However, in the absence of DAT, SERT can have a compensatory role which permits METH to exert at least some of its hyperthermic effects (Numachi et al., 2007).
Other reports also suggest that blocking the actions of released serotonin can prevent elevations in body temperature (Azzaro & Rutledge, 1973; Ginawi et al., 2005). NAN-190, a 5-HT1A receptor antagonist, blocks METH-induced increases in body temperature, while displaying no effects on its own (Ginawi et al., 2005). In contrast, 8-OH-DPAT, a 5-HT1A receptor agonist that on its own can produce hypothermia (Bristow et al., 1991), fails to attenuate METH-induced hyperthermia (Albers & Sonsalla, 1995).
Paradoxically, fenfluramine, which enhances serotonin function through multiple mechanisms, mitigates the hyperthermic effects of METH (Miller & O’Callaghan, 1994; Albers & Sonsalla, 1995). It, however, remains unclear why this serotonin indirect agonist function would inhibit METH-induced hyperthermia when all other serotonergic compounds that reduce the hyperthermic effects of METH inhibit serotonin function. Moreover, fenfluramine also enhances norepinephrine release (Rothman et al., 2003), suggesting that the protective effects of the drug against METH-induced hyperthermia involve targets other than its classical monoaminergic mechanisms.
In conclusion, the data as a whole suggest that serotonin systems can have a significant modulatory and compensatory role in the hyperthermic effects of METH. However, endogenous serotonin does not appear to be a requirement for METH to elicit hyperthermia.
5.2. Reactive species
In addition to modulating the monoaminergic systems, METH may cause increases in core body temperature through the induction of reactive oxygen and nitrogen species (ROS/RNS) within the CNS. Many studies have thus investigated the effects of antioxidant compounds and inhibitors of enzymes and proteins responsible for the production of these ROS/RNS in the CNS.
Pretreatment of rats with the ROS scavenger, α-tocopherol, attenuates METH-induced hyperthermia (Park et al., 2006). The pineal hormone, melatonin, also acts as a free radical scavenger and prevents METH-induced hyperthermia in mice (Itzhak et al., 1998).
The generation of hydroxyl radicals from H2O2 is catalyzed by iron via the Fenton reaction. The iron chelator, deferoxamine, which inhibits the generation of ROS, also significantly attenuates METH-induced hyperthermia in rats (Park et al., 2006).
Under normal conditions, nitric oxide synthase (NOS) catalyzes the formation of nitric oxide from L-arginine, and has an important role in vasodilation through the relaxation of smooth muscles. Under pathological conditions, NOS can also catalyze the production of the free radical, superoxide. 7-Nitroindazole is a neuronal NOS (nNOS) inhibitor that attenuates METH-induced elevations in body temperature at low ambient temperature (20° C; Callahan & Ricaurte, 1998), but not at normal ambient temperature (Itzhak & Ali, 1996), or at higher ambient temperature (28° C; Callahan & Ricaurte, 1998). Two other nNOS inhibitors, S-methylthiocitrulline and 3-bromo-7-nitroindazole, also cannot block METH-induced hyperthermia at room temperature (Itzhak et al., 2000). However, nNOS knockout mice are resistant to the hyperthermic effects of METH, compared to wild-type mice (Itzhak et al., 1998). Since free radicals are generated very quickly, it is possible that the interventions produce measurable effects against METH-induced hyperthermia only when no catalyzing enzyme is present or the reactions are sufficiently slowed (e.g., by reducing temperature); when the reactions occur at a faster rate at higher temperatures, the intervention attempts may be overcome.
In addition to ROS, studies also suggest the involvement of peroxynitrite radicals in the hyperthermic effects of METH. The peroxynitrite catalyst, 5,10,15,20-tetrakis(N-methyl-4′-pyridyl)porphyrinato iron III (FeTMPγP) protects against METH-induced hyperthermia in rodents (Imam et al., 1999). Moreover, compared to young rats, aged animals are more sensitive to METH-induced formation of peroxynitrite radicals and also exhibit increased sensitivity to the hyperthermic and lethal effects of METH (Imam et al., 2001). In contrast, the radical scavenger, edaravone, blocks peroxynitrite production, but cannot attenuate the hyperthermic effects of METH in mice (Kawasaki et al., 2006), underscoring the complexity of the relationship between METH, the generation of reactive species, and hyperthermia.
5.3. Immune system
Another potential mechanism by which METH is believed to cause hyperthermia is through increases in immune responses, such as cytokine release. These effects appear to be mediated primarily through peripheral, rather than central, mechanisms.
Systemic injections of interferon-γ significantly reduce METH-induced elevations in core body temperature (Hozumi et al., 2008); however, intracerebroventricular treatment has no significant effects on body temperature. These results imply that METH may be modulating immune system responses in the periphery, leading to alterations in body temperature.
Mithramycin is an FDA-approved immunosuppressive antibiotic with antitumor and neuroprotective actions (Gerber & Steinberg, 1976; Sleiman et al., 2011). It also attenuates METH-induced hyperthermia in laboratory animals (Hagiwara et al., 2009). Although the molecular mechanism underlying this protective effect has yet to be determined, mithramycin acts as an inhibitor of the specificity protein 1 (SP-1) family of transcription factors (Ray et al., 1989). Through this mechanism, mithramycin has been reported to abolish the induction of heat shock protein (HSP) 70 (Marinova et al., 2009) which can be activated by METH (Beauvais et al., 2011).
5.4. Miscellaneous mechanisms that affect METH hyperthermia
The activation of protein kinase Cδ (PKCδ), which can result from activation of dopaminergic neurotransmission, also appears to contribute to the hyperthermic effects of METH. Rottlerin, a PKCδ inhibitor, mitigates METH-induced hyperthermia (Shin et al., 2011). It also attenuates the concomitant increases in brain PKCδ produced by METH (Shin et al., 2011). The potential importance of PKCδ in the hyperthermic effects of METH is also supported by the observation that PKCδ knockout mice are resistant to the hyperthermic effects of METH (Shin et al., 2011).
Sigma receptors also appear to play a role in the hyperthermic effects of METH. METH interacts with sigma receptors at micromolar concentrations that can be achieved under physiological conditions (Nguyen et al., 2005). These proteins are found in the hypothalamus, as well as the cardiovascular system (McLean & Weber, 1988; Ela et al., 1994; Zhang & Cuevas, 2002; Tagashira et al., 2010). Pretreatment of mice with selective sigma receptor putative antagonists, including AC927, AZ66, CM156, and SN79, significantly attenuates METH-induced hyperthermia (Matsumoto et al., 2008; Kaushal et al., 2011, 2013; Seminerio et al., 2012, 2013; Robson et al., 2013).
Another miscellaneous compound that has been reported to attenuate METH-induced hyperthermia, without significantly altering body temperature on its own, is phenytoin, an anticonvulsant drug (Albers & Sonsalla, 1995). In addition, the following compounds reduce METH-induced hyperthermia, but also produce hypothermia when administered alone: aminoxyacetic acid (AOAA), a GABA transaminase inhibitor (Albers & Sonsalla, 1995); MK-801, an N-methyl-D-aspartate (NMDA) receptor antagonist (Albers & Sonsalla, 1995); and ibogaine, an alkaloid shown to block many effects of abused substances (Yu et al., 1999).
5.5. Mechanisms that do not affect METH hyperthermia
Many of the drugs that have been previously shown to have no significant effects on METH-induced hyperthermia were initially tested due to their fever-reducing, anti-inflammatory, and/or neuroprotective effects. These compounds and mechanisms are briefly summarized in this section and underscore the differences between the pathophysiology and clinical manifestations of hyperthermia vs. fever (McAllen & Schwartz, 2010). It also serves as a reminder that neuroprotection can be achieved through temperature-independent mechanisms.
Non-steroidal anti-inflammatory drugs (NSAIDs) do not have significant effects on METH-induced hyperthermia. In mice, pretreatment with ibuprofen or aspirin has been confirmed to have no effect on METH-induced hyperthermia (Albers & Sonsalla, 1995; Tsuji et al., 2009).
Similarly, an interleukin-1 (IL-1) receptor antagonist failed to have significant effects on METH-induced hyperthermia in rodents, although it did reduce deaths (Bowyer et al., 1994). Although IL-1 and related family members are known pyrogens (Kluger, 1991; Leon, 2002) whose expression is increased in the hypothalamus following METH (Bandtlow et al., 1990; Yamaguchi et al., 1991; Bowyer et al., 1994), their expression levels are not correlated with the body temperatures of the animals (Seminerio et al., 2012). Moreover, pharmacological intervention is capable of preventing the hyperthermic response to METH in the presence of increases in hypothalamic IL-1β expression (Seminerio et al., 2012).
A number of neuroprotective agents and genetic modifications that were tested primarily to determine their effects on METH-induced neurotoxicity, were shown to lack significant effects on METH-induced hyperthermia. They include: edaravone, a pharmaceutical product available in Japan indicated for ischemia related to acute ischemic stroke (Kawasaki et al., 2006); carnosine, an endogenous antioxidant available in several over-the-counter dietary supplements (Pubill et al., 2002); minocycline, a second generation tetracycline antibiotic that crosses the blood brain barrier to elicit neuroprotective and anti-inflammatory effects in animals models (Zhang et al., 2006); memantine, an NMDA antagonist used in Europe for the treatment of neurodegenerative disorders (Chipana et al., 2008); methylcaconitine, an α7-nicotinic acetylcholine receptor antagonist that inhibits METH-induced ROS production in rat synaptosomes (Escubedo et al., 2005); calcitriol, a vitamin D metabolite and neuroprotective agent (Cass et al., 2006); ketamine, a dissociative anesthetic (Ke et al., 2008); and interleukin-6 knockout mice (Ladenheim et al., 2000).
6. Summary and conclusions
METH elicits elevations in body temperature, with high doses causing hyperthermia, which can be life threatening. Clinically, the hyperthermic effects of METH are treated symptomatically by cooling. No specific pharmacotherapy currently is indicated for treating the hyperthermic effects of METH. This situation is not surprising given that no pharmacological profile exists for protecting against METH-induced hyperthermia because no single mechanism has been identified underlying the hyperthermic actions of METH. Rather, multiple systems appear to contribute to the elevations in body temperature. While there has been an emphasis on drugs affecting monoaminergic systems as a means to lower METH-related hyperthermia, as we note above, this stems from the historical basis of METH actions on monoaminergic neurotransmitters rather than a solid scientific basis for drug interventions targeting a specific monoamine-related temperature mechanism. Indeed, we note that broad classes of pharmacological agents, with no clear mechanistic effects on thermoregulation (e.g. Miller & O’Callaghan, 1994), all may serve as effective countermeasures against METH-induced hyperthermia.
While much is known about the mechanisms that regulate thermal physiology in response to hot and cold ambient environments, the basis for the hyperthermic effects of METH remain largely unknown and relatively unexplored. Most animal studies to date have employed systemic administration of drugs or genetic knockouts to evaluate their effects on METH, making it difficult to conclusively attribute changes to central and/or peripheral sites of action. The few studies that have attempted to do so suggest the importance of peripheral mechanisms, often implicating the ability of the manipulation to affect heat generation within muscle. In that regard, noradrenergic effects in the sympathetic nervous system appear important in mediating METH-induced hyperthermia. Therefore, further investigations into the involvement of specific subtypes of adrenoceptors, particular those that preferentially act in the periphery, would be of value. In addition, studies examining the involvement of peripheral serotonin in mediating the hyperthermic effects of METH are greatly needed.
A wide variety of targets and mechanisms have been implicated in the hyperthermic effects of METH, beyond involvement of a limited number of neurotransmitter systems. These include the blood brain barrier, choroid plexus, meninges-associated vasculature (Kiyatkin & Sharma, 2009; Bowyer et al., 2013) and glial as well as neuronal mechanisms (Kiyatkin & Sharma, 2011). Indeed, as sources of proinflammatory mediators, some potentially involved in temperature regulation, astrocytes and microglia represent overlooked players in METH actions related to hyperthermia.
While assumptions have been made to suggest that METH hyperthermia operates through disruption in known thermoregulatory mechanisms, there is little data to support this notion. Most studies to date have focused on mediators previously implicated in fever with modest success, and those that convey neuroprotective actions have provided only slightly more insight. Given the lack of clear avenues of investigation to pursue to achieve an understanding of METH-induced hyperthermia and its treatment, a more global exploration might be of benefit. In that regard, genome-wide array studies of multiple organs (not just brain) following hyperthermia instigated by elevations in ambient temperature vs. METH-induced hyperthermia might reveal genes and networks involved in mediating elevated temperatures under these two conditions and, hopefully, point to targets for therapeutic intervention.
Health-related complications resulting from METH abuse remains a significant worldwide public health problem. Further studies to better understand the pathophysiology of METH-induced hyperthermia are clearly needed to delineate the molecular mechanisms and biological systems that contribute to it. This information will be critical for developing effective therapeutic interventions to assist in the treatment of this potentially life threatening condition.
Acknowledgments
Some of the studies described herein and the authors were supported by grants from the National Institutes of Health (DA013978, GM081741).
Abbreviations
- AC927
N-phenethylpiperidine oxalate
- ADHD
attention deficit hyperactivity disorder
- ALT
alanine aminotransferase
- AOAA
aminoxyacetic acid
- AST
aspartate aminotransferase
- AZ66
3-(4-(4-cyclohexylpiperizin-1-yl)pentyl)-6-fluorobenzo[d]thiazol-2(3H)one
- AMPT
α-methyl-p-tyrosine
- CM156
3-(4-(4-cyclohexylpiperizin-1-yl)butyl)benzo[d]thiazole-2(3H)-thione
- CNS
central nervous system
- DAT
dopamine transporter
- FeTMPγP
5,10,15,20-tetrakis(N-methyl-4′-pyridyl)porphyrinato iron III
- FDA
Food and Drug Administration
- GABA
γ-aminobutyric acid
- GSH
reduced glutathione
- H2O2
hydrogen peroxide
- HPA
hypothalamic-pituitary-adrenal
- HSP
heat shock protein
- IL-1
interleukin-1
- MDMA
3,4-methylenedioxymethamphetamine
- METH
methamphetamine
- MK-801
(5S,10R)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine hydrogen maleate
- NAN-190
1-(2-methoxyphenyl)-4-(4-[2-phthalimido]butyl)piperazine
- NMDA
N-methyl-D-aspartate
- NOS
nitric oxide synthase
- NSAID
non-steroidal anti-inflammatory drug
- 6-OHDA
6-hydroxydopamine
- 8-OH-DPAT
8-hydroxy-N,N-dipropyl-2-aminotetralin
- PCPA
p-chlorophenylalanine
- PKC
protein kinase C
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SCH 23390
(R)-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride
- SERT
serotonin transporter
- SN79
6-acetyl-3-(4-(4-(4-fluorophenyl)piperazin-1-yl)butyl)benzo[d]oxazol-2(3H)one
- SP-1
specificity protein 1
- TRP
transient receptor potential
- VMAT
vesicular monoamine transporter
Footnotes
Declaration of interest
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ago Y, Nakamura S, Uda M, Kajii Y, Abe M, Baba A, Matsuda T. Attenuation of the 5-HT1A receptor agonist osemozotan of the behavioral effects of single and repeated methamphetamine in mice. Neuropharmacol. 2006;51:914–922. doi: 10.1016/j.neuropharm.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Albers DS, Sonsalla PK. Methamphetamine-induced hyperthermia and dopaminergic neurotoxicity in mice: pharmacological profile of protective and nonprotective agents. J Pharmacol Exp Ther. 1995;275:1104–1114. [PubMed] [Google Scholar]
- Albertson TE, Derlet RW, Van Hoozen BE. Methamphetamine and the expanding complications of amphetamines. West J Med. 1999;170:214–219. [PMC free article] [PubMed] [Google Scholar]
- Ares-Santos S, Granado N, Oliva I, O’Shea E, Martin ED, Colado MI, Moratalla R. Doapmine D1 receptor deletion strongly reduces neurotoxic effects of methamphetamine. Neurobiol Dis. 2012;45:810–820. doi: 10.1016/j.nbd.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Azzaro AJ, Rutledge CO. Selectivity of release of norepinephrine, dopamine, and 5-hydroxytryptamine by amphetamine in various regions of rat brain. Biochem Pharmacol. 1973;22:2801–2813. doi: 10.1016/0006-2952(73)90147-0. [DOI] [PubMed] [Google Scholar]
- Bandtlow CE, Meyer M, Lindholm D, Spranger M, Heumann R, Thoenen H. Regional and cellular codistribution of interleukin 1 beta and nerve growth factor mRNA in the adult rat brain: possible relationship to the regulation of nerve growth factor synthesis. J Cell Biol. 1990;111:1701–1711. doi: 10.1083/jcb.111.4.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavais G, Atwell K, Jaynathi S, Ladenheim B, Cadet JL. Involvement of dopamine receptors in binge methamphetamine-induced activation of endoplasmic reticulum and mitochondrial stress pathways. PLoS One. 2011;6:e28946. doi: 10.1371/journal.pone.0028946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowyer JF, Davies DL, Schmued L, Broening HW, Newport GD, Slikker W, Jr, Holson RR. Further studies of the role of hyperthermia in methamphetamine neurotoxicity. J Pharmacol Exp Ther. 1994;268:1571–1580. [PubMed] [Google Scholar]
- Bowyer JF, Holson RR. Methamphetamine and amphetamine neurotoxicity. In: Chang LW, Dyer RS, editors. Handbook of Neurotoxicology. Marcel Dekker, Inc; New York: 1995. pp. 845–870. [Google Scholar]
- Bowyer JF, Patterson TA, Saini UT, Hanig JP, Thomas M, Camacho L, George NI, Chen JJ. Comparison of the global gene expression of choroid plexus and meninges and associated vasculature under control conditions and after pronounced hyperthermia or amphetamine toxicity. BMC Genomics. 2013;14:147. doi: 10.1186/1471-2164-14-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowyer JF, Tank AW, Newport GD, Slikker W, Jr, Ali SF, Holson RR. The influence of environmental temperature on the transient effects of methamphetamine on dopamine levels and dopamine release in rat striatum. J Pharmacol Exp Ther. 1992;260:817–824. [PubMed] [Google Scholar]
- Bristow LJ, Baucutt L, Thorn L, Hutson PH, Noble A, Beer M, Middlemiss DN, Tricklebank MD. Behvioural and biochemical evidence of the interaction of the putative antipsychotic agent, BMY 14802 with the 5-HT1A receptor. Eur J Pharmacol. 1991;204:21–28. doi: 10.1016/0014-2999(91)90830-j. [DOI] [PubMed] [Google Scholar]
- Broening HW, Morford LL, Vorhees CV. Interactions of dopamine D1 and D2 receptor antagonists with D-methamphetamine-induced hyperthermia and striatal dopamine and serotonin reductions. Synapse. 2005;56:84–93. doi: 10.1002/syn.20130. [DOI] [PubMed] [Google Scholar]
- Brown PL, Kiyatkin EA. Fatal intra-brain heat accumulation induced by methamphetamine at normothermic conditions in rats. Int J Neuroprot Neuroregener. 2005;1:86–90. [Google Scholar]
- Brown PL, Wise RA, Kiyatkin EA. Brain hyperthermia is induced by methamphetamine and exacerbated by social interaction. J Neurosci. 2003;23:3924–3929. doi: 10.1523/JNEUROSCI.23-09-03924.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bynum GD, Pandolf KB, Schuette WH, Goldman RF, Lees DE, Whang-Peng J, Atkinson ER, Bull JM. Induced hyperthermia in sedated humans and the concept of critical thermal maximum. Am J Physiol Regul Integr Comp Physiol. 1978;235:R228–R236. doi: 10.1152/ajpregu.1978.235.5.R228. [DOI] [PubMed] [Google Scholar]
- Callahan BT, Ricaurte GA. Effect of 7-nitroindazole on body temperature and methamphetamine-induced dopamine toxicity. NeuroReport. 1998;9:2691–2695. doi: 10.1097/00001756-199808240-00001. [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Cass WA, Smith MP, Peters LE. Calcitriol protects against the dopamine- and serotonin-depleting effects of neurotoxic doses of methamphetamine. Ann N Y Acad Sci. 2006;1074:261–271. doi: 10.1196/annals.1369.023. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Heat-related deaths – United States 1999–2003. MMWR Morb Mortal Wkly Rep. 2006;55:796–798. [PubMed] [Google Scholar]
- Charkoudian N. Skin blood flow in adult human thermogenesis: how it works, when it does not, and why. Mayo Clin Proc. 2003;78:603–612. doi: 10.4065/78.5.603. [DOI] [PubMed] [Google Scholar]
- Chipana C, Torres I, Camarasa J, Pubill D, Escubedo E. Memantine protects against amphetamine derivatives-induced neurotoxic damage in rodents. Neuropharmacol. 2008;54:1254–1263. doi: 10.1016/j.neuropharm.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Clark KH, Wiley CA, Bradberry CW. Psychostimulant abuse and neuroinflammation: emerging evidence of their interconnection. Neurotox Res. 2012 doi: 10.1007/s12640-012-9334-7. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Cohen LJ. Risperidone. Pharmacotherapy. 1994;14:253–265. [PubMed] [Google Scholar]
- Crean RD, Davis SA, Von Huben SN, Lay CC, Katner SN, Taffe MA. Effects of (+/−)3,4-methylenedioxymethamphetamine, (+/−)3,4-methylenedioxyamphetamine and methamphetamine on temperature and activity in rhesus macaques. Neurosci. 2006;142:515–525. doi: 10.1016/j.neuroscience.2006.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crean RD, Davis SA, Taffe MA. Oral administration of (+/−)3,4-methylenedioxymethamphetamine and (+)methamphetamine alters temperature and activity in rhesus macaques. Pharmacol Biochem Behav. 2007;87:11–19. doi: 10.1016/j.pbb.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruickshank CC, Dyer KR. A review of the clinical pharmacology of methamphetamine. Addiction. 2009;104:1085–1099. doi: 10.1111/j.1360-0443.2009.02564.x. [DOI] [PubMed] [Google Scholar]
- da Silva DD, Silva E, Carmo H. Combination effects of amphetamines under hyperthermia – the role played by oxidative stress. J Appl Toxicol. 2013 doi: 10.1002/jat.2889. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Davidson C, Gow AJ, Lee TH, Ellinwood EH. Methamphetamine neurotoxicity: necrotic and apoptotic mechanisms and relevance to human abuse and treatment. Brain Res Brain Res Rev. 2001;36:1–22. doi: 10.1016/s0165-0173(01)00054-6. [DOI] [PubMed] [Google Scholar]
- Davis S, Heal DJ, Stanford SC. Long-lasting effects of an acute stress on the neurochemistry and function of 5-hydroxytryptaminergic neurons in the mouse brain. Psychopharmacol. 1995;118:267–272. doi: 10.1007/BF02245954. [DOI] [PubMed] [Google Scholar]
- Del Rios M, Lanigan M, Zayas V. Drugs of abuse: providing the best in evidence-based care to “self-medicated” patients. Emerg Med Prac. 2005;7:1–24. [Google Scholar]
- Docherty JR, Green AR. The role of monoamines in the changes in body temperature induced by 3,4-methylenedioxymehtamphetamine (MDMA, ecstasy) and its derivatives. Br J Pharmacol. 2010;160:1029–1044. doi: 10.1111/j.1476-5381.2010.00722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle JR, Yamamoto BK. Serotonin 2 receptor modulation of hyperthermia, corticosterone, and hippocampal serotonin depletion following serial exposure to chronic stress and methamphetamine. Psychoneuroendocrinol. 2010;35:629–633. doi: 10.1016/j.psyneuen.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ela C, Barg J, Vogel Z, Hasin Y, Eilam Y. Sigma receptor ligands modulate contractility, Ca++ influx and beating rate in cultured cardiac myocytes. J Pharmacol Exp Ther. 1994;269:1300–1309. [PubMed] [Google Scholar]
- Escubedo E, Chipana C, Perez-Sanchez M, Camarasa J, Pubill D. Methyllycaconitine prevents methamphetamine-induced effects in mouse striatum: involvement of alpha7 nicotinic receptors. J Pharmacol Exp Ther. 2005;315:658–667. doi: 10.1124/jpet.105.089748. [DOI] [PubMed] [Google Scholar]
- Eskandari MR, Rahmati M, Khajeamiri AR, Kobarfard F, Noubarani M, Heidari H. A new approach on methamphetamine-induced hepatotoxicity: involvement of mitochondrial dysfunction. Xenobiotica. 2014;44:70–76. doi: 10.3109/00498254.2013.807958. [DOI] [PubMed] [Google Scholar]
- Estler CJ. Dependence on age of methamphetamine-produced changes in thermoregulation and metabolism. Experientia. 1975;31:1436–1437. doi: 10.1007/BF01923231. [DOI] [PubMed] [Google Scholar]
- Eyer F, Zilker T. Bench-to-bedside review: mechanisms and management of hyperthermia due to toxicity. Crit Care. 2007;11:236. doi: 10.1186/cc6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumura M, Cappon GD, Broening HW, Vorhees CV. Methamphetamine-induced dopamine and serotonin reductions in neostriatum are not gender specific n rats with comparable hyperthermic responses. Neurotoxicol Teratol. 1998;20:441–448. doi: 10.1016/s0892-0362(97)00094-9. [DOI] [PubMed] [Google Scholar]
- Funahashi M, Kohda H, Hori O, Hayashida H, Kimura H. Potentiating effect of morphine upon d-methamphetamine-induced hyperthermia in mice. Effects of naloxone and haloperidol. Pharmacol Biochem Behav. 1990;36:345–350. doi: 10.1016/0091-3057(90)90415-e. [DOI] [PubMed] [Google Scholar]
- Gerber NL, Steinberg AD. Clinical use of immunosuppressive drugs: part II. Drugs. 1976;11:90–112. doi: 10.2165/00003495-197611020-00002. [DOI] [PubMed] [Google Scholar]
- Ginawi OT, Al-Majed AA, Al-Suwailem AK. NAN-190, a possible specific antagonist for methamphetamine. Reg Toxicol Pharmacol. 2005;42:122–127. doi: 10.1016/j.yrtph.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Gordon CG, Watkinson WP, O’Callahan JP, Miller DB. Effects of 3,4-methylenedioxymethamphetamine on autonomic thermoregulatory response of the rat. Pharmacol Biochem Behav. 1991;38:339–344. doi: 10.1016/0091-3057(91)90288-d. [DOI] [PubMed] [Google Scholar]
- Gordon CJ. A review of terms and proposed nomenclature for regulated vs. forced changes in body temperature. Life Sci. 1983;32:1285–1295. doi: 10.1016/0024-3205(83)90802-0. [DOI] [PubMed] [Google Scholar]
- Gordon CJ. Temperature and Toxicology: An Integrative, Comparative, and Environmental Approach. CRC Press; Boca Raton, FL: 2005. [Google Scholar]
- Gordon CJ. Thermophysiological responses to hyperthermia drugs: extrapolating from rodent to human. Prog Brain Res. 2007;162:63–79. doi: 10.1016/S0079-6123(06)62005-0. [DOI] [PubMed] [Google Scholar]
- Granado N, Ares-Santos S, Oliva I, O’Shea E, Martin ED, Colado MI, Moratalla R. Dopamine D2-receptor knockout mice are protected against dopaminergic neurotoxicity induced by methamphetamine or MDMA. Neurobiol Dis. 2011;42:391–403. doi: 10.1016/j.nbd.2011.01.033. [DOI] [PubMed] [Google Scholar]
- Green AR, O’Shea E, Colado MR. A review of the mechanisms involved in the acute MDMA (ecstasy)-induced hyperthermic response. Eur J Pharmacol. 2004;500:3–13. doi: 10.1016/j.ejphar.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Greenblatt EN, Osterberg AC. Correlations of activating and lethal effects of excitatory drugs in grouped and isolated mice. J Pharmacol Exp Ther. 1961;131:115–119. [PubMed] [Google Scholar]
- Grimm LJ, Blendy JA, Kellar KJ, Perry DC. Chronic reserpine administration selectively up-regulates beta 1- and alpha 1b-adrenergic receptors in rat brain: an autoradiographic study. Neurosci. 1992;47:77–86. doi: 10.1016/0306-4522(92)90122-i. [DOI] [PubMed] [Google Scholar]
- Hagiwara H, Iyo M, Hashimoto K. Mithramycin protects against dopaminergic neurotoxicity in the mouse brain after administration of methamphetamine. Brain Res. 2009;1301:189–196. doi: 10.1016/j.brainres.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Halpin LE, Northrop NA, Yamamoto BK. Ammonia mediates methamphetamine-induced increases in glutamate and excitotoxicity. Neuropsychopharmacol. 2014;39:1031–1038. doi: 10.1038/npp.2013.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpin LE, Yamamoto BK. Peripheral ammonia as a mediator of methamphetamine neurotoxicity. J Neurosci. 2012;32:13155–13163. doi: 10.1523/JNEUROSCI.2530-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haning W, Goebert D. Electrocardiographic abnormalities in methamphetamine abusers. Addiction. 2007;102(Suppl 1):70–75. doi: 10.1111/j.1360-0443.2006.01776.x. [DOI] [PubMed] [Google Scholar]
- He J, Xu H, Yang Y, Zhang X, Li XM. Neuroprotective effects of olanzapine on methamphetamine-induced neurotoxicity are associated with an inhibition of hyperthermia and prevention of Bcl-2 decrease in rats. Brain Res. 2004;1018:186–192. doi: 10.1016/j.brainres.2004.05.060. [DOI] [PubMed] [Google Scholar]
- Hendrickson RG, Horowitz BZ, Norton RL, Notenboom H. “Parachuting” meth: a novel delivery method for methamphetamine and delayed-onset toxicity from “body stuffing. Clin Toxicol. 2006;44:379–382. doi: 10.1080/15563650600671746. [DOI] [PubMed] [Google Scholar]
- Hozumi H, Asanuma M, Miyazaki I, Fukuoka S, Kikkawa Y, Kimoto N, Kitamura Y, Sendo T, Kita T, Gomita Y. Protective effects of interferon-gamma against methamphetamine-induced neurotoxicity. Toxicol Lett. 2008;177:123–129. doi: 10.1016/j.toxlet.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Hysek CM, Schmid Y, Rickli A, Liechti ME. Carvedilol inhibits cardiostimulant and thermogenic effects of MDMA in humans: Lost in translation. Br J Pharmacol. 2013;170:1273–1275. doi: 10.1111/bph.12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imam SZ, Crow JP, Newport GD, Islam F, Slikker W, Jr, Ali SF. Methamphetamine generates peroxynitrite and produced dopaminergic neurotoxicity in mice: protective effects of peroxynitrite decomposition catalyst. Brain Res. 1999;837:15–21. doi: 10.1016/s0006-8993(99)01663-7. [DOI] [PubMed] [Google Scholar]
- Ito M, Numachi Y, Ohara A, Sora I. Hyperthermic and lethal effects of methamphetamine: Roles of dopamine D1 and D2 receptors. Neurosci Lett. 2008;438:327–329. doi: 10.1016/j.neulet.2008.04.034. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Ali SF. The neuronal nitric oxide synthase inhibitor, 7-nitroindazole, protects against methamphetamine-induced neurotoxicity in vivo. J Neurochem. 1996;67:1770–1773. doi: 10.1046/j.1471-4159.1996.67041770.x. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Gandia C, Huang PL, Ali SF. Resistance of neuronal nitric oxide synthase-deficient mice to methamphetamine-induced doapminergic neurotoxicity. J Pharmacol Exp Ther. 1998;284:1040–1047. [PubMed] [Google Scholar]
- Itzhak Y, Martin JL, Black MD, Ali SF. Effect of melatonin on methamphetamine- and i-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced dopaminergic neurotoxicity and methamphetamine-induced behavioral sensitization. Neuropharmacol. 1998;37:781–791. doi: 10.1016/s0028-3908(98)00067-7. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Martin JL, Ali SF. nNOS inhibitors attenuate methamphetamine-induced dopaminergic neurotoxicity but not hyperthermia in mice. NeuroReport. 2000;11:2943–2946. doi: 10.1097/00001756-200009110-00022. [DOI] [PubMed] [Google Scholar]
- Kamijo Y, Soma K, Nishida M, Namera A, Ohwada T. Acute liver failure following intravenous methamphetamine. Vet Hum Toxicol. 2002;44:216–217. [PubMed] [Google Scholar]
- Kashani J, Ruha AM. Methamphetamine toxicity secondary to inavaginal body stuffing. J Toxicol Clin Toxicol. 2004;42:987–989. doi: 10.1081/clt-200042554. [DOI] [PubMed] [Google Scholar]
- Kaushal N, Seminerio MJ, Shaikh J, Medina MA, Mesangeau C, Wilson LL, McCurdy CR, Matsumoto RR. CM156, a high affinity sigma ligand, attenuates the stimulant and neurotoxic effects of methamphetamine in mice. Neuropharmacol. 2011;61:992–1000. doi: 10.1016/j.neuropharm.2011.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal N, Seminerio MJ, Robson MJ, McCurdy CR, Matsumoto RR. Pharmacological evaluation of SN79, and sigma (σ) receptor ligand, against methamphetamine-induced neurotoxcity in vivo. Eur Neuropsychopharmacol. 2013;23:960–971. doi: 10.1016/j.euroneuro.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T, Ishihara K, Ago Y, Nakamura S, Itoh S, Baba A, Matsuda T. Protective effect of the radical scavenger edaravone against methamphetamine-induced dopaminergic neurotoxicity in mouse striatum. Eur J Pharmacol. 2006;542:92–99. doi: 10.1016/j.ejphar.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Ke JJ, Chen HI, Jen CJ, Kuo YM, Cherng CG, Tsai YP, Ho MC, Tsai CW, Yu L. Mutual enhancement of central neurotoxicity induced by ketamine followed by methamphetamine. Toxicol Appl Pharmacol. 2008;227:239–247. doi: 10.1016/j.taap.2007.10.017. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA. Brain temperature fluctuations during physiological and pathological conditions. Eur J Appl Physiol. 2007;101:3–17. doi: 10.1007/s00421-007-0450-7. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Brown PL, Wise RA. Brain temperature fluctuation: a reflection of functional neural activation. Eur J Neurosci. 2002;17:1–5. doi: 10.1046/j.1460-9568.2002.02066.x. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Sharma HS. Acute methamphetamine intoxication: brain hyperthermia, blood-brain barrier, brain edema, and morphological cell abnormalities. Int Rev Neurobiol. 2009;88:65–100. doi: 10.1016/S0074-7742(09)88004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA, Sharma HS. Expression of heat shock protein (HSP 72 kDa) during acute methamphetamine intoxication depends on brain hyperthermia: neurotoxicity or neuroprotection? J Neural Transm. 2011;118:47–60. doi: 10.1007/s00702-010-0477-5. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kiyatkin EA, Sharma HS. Environmental conditions modulate neurotoxic effects of psychomotor stimulant drugs of abuse. Int Rev Neurobiol. 2012;102:147–171. doi: 10.1016/B978-0-12-386986-9.00006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluger MJ. Fever: role of pyrogens and cryogens. Physiol Rev. 1991;71:93–127. doi: 10.1152/physrev.1991.71.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain Res Rev. 2009;60:379–407. doi: 10.1016/j.brainresrev.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS, Cho AK, Melega W. Hippocampus norepinephrine, caudate dopamine and serotonin, and behavioral responses to the stereoisomers of amphetamine and methamphetamine. J Neurosci. 1995;15:1308–1317. doi: 10.1523/JNEUROSCI.15-02-01308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladenheim B, Krasnova IN, Deng X, Oyler JM, Polettini A, Moran TH, Huestis MA, Cadet JL. Methamphetamine-induced neurotoxicity is attenuated in transgenic mice with a null mutation for interleukin-6. Mol Pharmacol. 2000;58:1247–1256. doi: 10.1124/mol.58.6.1247. [DOI] [PubMed] [Google Scholar]
- LaVoie MJ, Hastings TG. Dopamine quinine formation and protein modification associated with the striatal neurotoxicity of methamphetamine: evidence against a role for extracellular dopamine. J Neurosci. 1999;19:1484–1491. doi: 10.1523/JNEUROSCI.19-04-01484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch KP, Wolozin BL, Murphy DL, Reiderer P. Primary structure of the human platelet serotonin uptake site: identity with the brain serotonin transporter. J Neurochem. 1993;60:2319–2322. doi: 10.1111/j.1471-4159.1993.tb03522.x. [DOI] [PubMed] [Google Scholar]
- Leon LR. Cytokine regulation of fever: studies using gene knockout mice. J Appl Physiol. 2002;92:2648–2655. doi: 10.1152/japplphysiol.01005.2001. [DOI] [PubMed] [Google Scholar]
- Levi MS, Divine B, Hanig JP, Doerge DR, Vanlandingham MM, George NI, Twaddle NC, Bowyer JF. A comparison of methylphenidate-, amphetamine-, and methamphetamine-induced hyperthermia and neurotoxicity in male Sprague-Dawley rats during the waking (lights off) cycle. Neurotoxicol Teratol. 2012;34:253–262. doi: 10.1016/j.ntt.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Lin MT, Chandra A, Tsay BL, Chern YF. Hypothalamic and striatal dopamine activation inhibits heat production in the rat. Am J Physiol Integr Comp Physiol. 1982;242:R471–481. doi: 10.1152/ajpregu.1982.242.5.R471. [DOI] [PubMed] [Google Scholar]
- Lin MT, Wu JJ, Tsay BL. Serotonergic mechanisms in the hypothalamus mediate thermoregulatory responses in rats. Naunyn Schmied Arch Pharmacol. 1983;322:271–278. doi: 10.1007/BF00508342. [DOI] [PubMed] [Google Scholar]
- Loscher W, Witte U, Fredow G, Ganter M, Bickhardt K. Phamacodynamic effects of serotonin (5-HT) receptor ligands in pigs: stimulation of 5-HT2 receptors induces malignant hyperthermia. Naunyn Schmied Arch Pharmacol. 1990;341:483–493. doi: 10.1007/BF00171727. [DOI] [PubMed] [Google Scholar]
- Makisumi T, Yoshida K, Watanabe T, Tan N, Murakami N, Morimoto A. Sympatho-adrenal involvement in methamphetamine-indced hyperthermia through skeletal muscle hypermetabolism. Eur J Pharmacol. 1998;363:107–112. doi: 10.1016/s0014-2999(98)00758-4. [DOI] [PubMed] [Google Scholar]
- Mallick BN, Jha SK, Islam F. Presence of alpha-1 adrenoceptors on thermosensitive neurons in the medial preoptico-anterior hypothalamic area in rats. Neuropharmacol. 2002;42:697–705. doi: 10.1016/s0028-3908(02)00016-3. [DOI] [PubMed] [Google Scholar]
- Marinova Z, Ren M, Wendland JR, Leng Y, Liang MH, Yasuda S, Leeds P, Chuang DM. Valproic acid induces functional heat-shock protein 70 via Class I histone deacetylase inhibition in cortical neurons: a potential role of SP1 acetylation. J Neurochem. 2009;111:976–987. doi: 10.1111/j.1471-4159.2009.06385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JF, O’Dell SJ. Methamphetamine influences on brain and behavior: unsafe at any speed? Trends Neurosci. 2012;35:536–545. doi: 10.1016/j.tins.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto RR, Shaikh J, Wilson LL, Vedam S, Coop A. Attenuation of methamphetamine-induced effects through the antagonism of sigma (σ) receptors: Evidence from in vivo and in vitro studies. Eur Neuropsychopharmacol. 2008;18:871–881. doi: 10.1016/j.euroneuro.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuszewich L, Yamamoto BK. Long-lasting effects of chronic stress on DOI-induced hyperthermia in male rats. Psychopharmacol. 2003;169:169–175. doi: 10.1007/s00213-003-1498-7. [DOI] [PubMed] [Google Scholar]
- Mauer-Spurej E. Circulating serotonin in vertebrates. Cell Mol Life Sci. 2005;62:1881–1889. doi: 10.1007/s00018-005-5149-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllen KJ, Schwartz DR. Adverser drug reactions resulting in hyperthermia in the intensive care unit. Crit Care Med. 2010;38:S244–S252. doi: 10.1097/CCM.0b013e3181dda0d4. [DOI] [PubMed] [Google Scholar]
- McLean S, Weber E. Autoradiographic visualization of haloperidol-sensitive sigma receptors in guinea-pig brain. Neurosci. 1988;25:259–269. doi: 10.1016/0306-4522(88)90024-3. [DOI] [PubMed] [Google Scholar]
- Metzger RR, Haughey HM, Wilkins DG, Gibb JW, Hanson GR, Fleckenstein AE. Methamphetamine-induced rapid decrease in dopamine transporter function: role of dopamine and hyperthermia. J Pharmacol Exp Ther. 2000;295:1077–1085. [PubMed] [Google Scholar]
- Miller DB, O’Callaghan JP. Environment-, drug- and stress-induced alterations in body temperature affect the neurotoxicity of substituted amphetamines in the C57BL/6J mouse. J Pharmacol Exp Ther. 1994;270:752–760. [PubMed] [Google Scholar]
- Miller DB, O’Callaghan JP. Elevated environmental temperature and methamphetamine neurotoxicity. Environ Res. 2003;92:48–53. doi: 10.1016/s0013-9351(02)00051-8. [DOI] [PubMed] [Google Scholar]
- Morrison SF, Nakamura K. Central neural pathways fro thermoregulation. Front Biosci. 2011;16:74–104. doi: 10.2741/3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama T, Eisenman JS, Hardy JD. Single unit activity of anterior hypothalamus during local heating. Science. 1961;134:560–561. doi: 10.1126/science.134.3478.560. [DOI] [PubMed] [Google Scholar]
- Nguyen EC, McCracken KA, Pouw B, Matsumoto RR. Involvement of sigma receptors in the actions of methamphetamine: receptor binding and behavioral studies. Neuropharmacol. 2005;49:638–645. doi: 10.1016/j.neuropharm.2005.04.016. [DOI] [PubMed] [Google Scholar]
- NIDA. Drug-related hospital emergency room visits. NIDA Info Facts 2011 [Google Scholar]
- Numachi Y, Ohara A, Yamashita M, Fukushima S, Kobayashi H, Hata H, Watanabe H, Hall FS, Lesch KP, Murphy DL, Uhl GR, Sora I. Methamphetamine-induced hyperthermia and lethal toxicity: role of the dopamine and serotonin transporters. Eur J Pharmacol. 2007;572:120–128. doi: 10.1016/j.ejphar.2007.06.022. [DOI] [PubMed] [Google Scholar]
- Nybo L. Brain temperature and exercise performance. Exp Physiol. 2012;97:333–339. doi: 10.1113/expphysiol.2011.062273. [DOI] [PubMed] [Google Scholar]
- O’Callaghan JP, Miller DB. Neurotoxicity profiles of substituted amphetamines in the C57BL/6J mouse. J Pharmacol Exp Ther. 1994;270:741–751. [PubMed] [Google Scholar]
- O’Callaghan JP, Miller DB. Neurotoxic effects of substituted amphetamines in rats and mice: Challenges to the current dogma. In: Massaro EJ, editor. Handbook of Neurotoxicity. Vol. 2. Humana Press; Totowa, NJ: 2002. pp. 269–301. [Google Scholar]
- Ossowska G, Nowa G, Kata R, Klenk-Majewska B, Danilczuk Z, Zebrowska-Lupina I. Brain monoamine receptors in a chronic unpredictable stress model in rats. J Neural Transm. 2001;108:311–319. doi: 10.1007/s007020170077. [DOI] [PubMed] [Google Scholar]
- Park MJ, Lee SK, Lim MA, Chung HS, Cho SI, Jang CG, Lee SM. Effect of alpha-tocopherol and deferoxamine on methamphetamine-induced neurotoxicity. Brain Res. 2006;1109:176–182. doi: 10.1016/j.brainres.2006.06.030. [DOI] [PubMed] [Google Scholar]
- Phillips PK, Heath JE. Dependency of surface temperature regulation on body size in terrestrial mammals. J Therm Biol. 1995;20:281–289. [Google Scholar]
- Pubill D, Verdaguer E, Sureda FX, Camins A, Pallas M, Camarasa J, Escubedo E. Carnosine prevents methamphetamine-induced gliosis but not dopamine terminal loss in rats. Eur J Pharmacol. 2002;448:165–168. doi: 10.1016/s0014-2999(02)01949-0. [DOI] [PubMed] [Google Scholar]
- Ray R, Snyder RC, Thomas S, Koller CA, Miller DM. Mithramycin blocks protein binding and function of the SV40 early promoter. J Clin Invest. 1989;83:2003–2007. doi: 10.1172/JCI114110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznikoff GA, Manker S, Rhodes CH, Winokur A, Rainbow TC. Localization and quantification of beta-adrenergic receptors in human brain. Neurology. 1986;36:1067–1073. doi: 10.1212/wnl.36.8.1067. [DOI] [PubMed] [Google Scholar]
- Robson MJ, Seminerio MJ, McCurdy CR, Coop A, Matsumoto RR. σ Receptor antagonist attenuation of methamphetamine-induced neurotoxicity is correlated to body temperature modulation. Pharmacol Rep. 2013;65:343–349. doi: 10.1016/s1734-1140(13)71009-0. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Clark RD, Partilla JS, Baumann MH. (+)-Fenfluramine and its major metabolite, (+)-norfenfluramine, are potent substrates for norepinephrine transporters. J Pharmacol Exp Ther. 2003;305:1191–1199. doi: 10.1124/jpet.103.049684. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Zolkowska D, Baumann MH. Serotonin (5-HT) transporter ligands affect plasma 5-HT in rats. Ann NY Acad Sci. 2008;1139:268–284. doi: 10.1196/annals.1432.042. [DOI] [PubMed] [Google Scholar]
- Rothwell NJ. CNS regulation of thermogenesis. Crit Rev Neurobiol. 1994;8:1–10. [PubMed] [Google Scholar]
- Rusyniak DE, Sprague JE. Hyperthermic syndromes induced by toxins. Clin Lab Med. 2006;26:165–184. doi: 10.1016/j.cll.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Salmi P, Ahlenius S. Evidence for functional interactions between 5-HT1A and 5-HT2A receptors in rat thermoregulatory mechanisms. Pharmacol Toxicol. 1998;82:122–127. doi: 10.1111/j.1600-0773.1998.tb01410.x. [DOI] [PubMed] [Google Scholar]
- Sandoval V, Hanson GR, Fleckenstein AE. Methamphetamine decreases mouse striatal dopamine transporter activity: roles of hyperthermia and dopamine. Eur J Pharmacol. 2000;409:265–271. doi: 10.1016/s0014-2999(00)00871-2. [DOI] [PubMed] [Google Scholar]
- Schep LJ, Slaughter RJ, Beasley DM. The clinical toxicology of metamfetamine. Clin Toxicol. 2010;48:675–694. doi: 10.3109/15563650.2010.516752. [DOI] [PubMed] [Google Scholar]
- Schwartz PJ, Wehr TA, Rosenthal NE, Bartko JJ, Oren DA, Luetke C, Murphy DL. Serotonin and thermoregulation. Physiologic and pharmacologic aspects of control revealed by intravenous m-CPP in normal human subjects. Neuropsychopharmacol. 1995;13:105–115. doi: 10.1016/0893-133X(95)00026-A. [DOI] [PubMed] [Google Scholar]
- Seminerio MJ, Kaushal N, Shaikh J, Huber JD, Coop A, Matsumoto RR. Sigma (σ) receptor ligand, AC927 (N-phenethylpiperidine oxalate), attenuates methamphetamine-induced hyperthermia and serotonin damage in mice. Pharmacol Biochem Behav. 2011;98:12–20. doi: 10.1016/j.pbb.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminerio MJ, Hansen R, Kaushal N, Zhang HT, McCurdy CR, Matsumoto RR. The evaluation of AZ66, an optimized sigma receptor antagonist, against methamphetamine-induced dopaminergic neurotoxicity and memory impairment in mice. Int J Neuropsychopharmacol. 2013;16:1033–1044. doi: 10.1017/S1461145712000831. [DOI] [PubMed] [Google Scholar]
- Seminerio MJ, Robson MJ, McCurdy CR, Matsumoto RR. Sigma receptor antagonists attenuate acute methamphetamine-induced hyperthermia by a mechanism independent of IL-1β mRNA expression in the hypothalamus. Eur J Pharmacol. 2012;691:103–109. doi: 10.1016/j.ejphar.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin EJ, Dong CX, Nguyen TX, Bing G, Bach JH, Park DH, Nakayama K, Ali SF, Kanthasamy AG, Cadet JL, Nabeshima T, Kim HC. PKCdelta inhibition enhances tyrosine hydroxylase phosphorylation in mice after methamphetamine treatment. Neurochem Int. 2011;59:39–50. doi: 10.1016/j.neuint.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioda K, Nishijima K, Yoshino T, Kato S. Effect of risperidone on acute methamphetamine-induced hyperthermia in rats. Drug Alcohol Depend. 2010;11:241–249. doi: 10.1016/j.drugalcdep.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Skibba JL, Gwartney EA. Liver hyperthermia and oxidative stress: role of iron and aldehyde production. Int J Hyperthermia. 1997;13:215–226. doi: 10.3109/02656739709012384. [DOI] [PubMed] [Google Scholar]
- Skibba JL, Powers RH, Stadnicka A, Cullinane DW, Almagro UA, Kalbfleisch JH. Oxidative stress as a precursor to the irreversible hepatoceullar injury caused by hyperthermia. Int J Hyperthermia. 1991;7:749–761. doi: 10.3109/02656739109056444. [DOI] [PubMed] [Google Scholar]
- Skibba JL, Powers RH, Stadnicka A, Kalbfleisch JH. Lipid peroxidation caused by hyperthermic perfusion of rat liver. Biochem Pharmacol. 1990;40:1411–1414. doi: 10.1016/0006-2952(90)90411-d. [DOI] [PubMed] [Google Scholar]
- Sleiman SF, Langley BC, Basso M, Berlin J, Xia L, Payappilly JB, Kharel MK, Guo H, Marsh JL, Thompson LM, Mahishi L, Ahuja P, MacLellan WR, Geschwind DH, Coppola G, Rohr J, Ratan RR. Mithramycin is a gene-selective Sp1 inhibitor that identifies a biological intersection between cancer and neurodegeneration. J Neurosci. 2011;31:6858–6870. doi: 10.1523/JNEUROSCI.0710-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DE, Fischer CM. An analysis of 310 cases of acute high-dose methamphetamine toxicity in Haight-Ashbury. Clin Toxicol. 1970;3:117–124. doi: 10.3109/15563657008990106. [DOI] [PubMed] [Google Scholar]
- Sprague JE, Yang X, Sommers J, Gilman TL, Mills EM. Roles of norepinephrine, free fatty acids, thyroid status, and skeletal muscle uncoupling protein 3 expression in sympathomimetic-induced thermogenesis. J Pharmacol Exp Ther. 2007;320:274–280. doi: 10.1124/jpet.106.107755. [DOI] [PubMed] [Google Scholar]
- Suchard JR. Recovery from severe hyperthermia (45 °C) and rhabdomyolysis induced by methamphetamine body-stuffing. West J Emerg Med. 2007;8:93–95. [PMC free article] [PubMed] [Google Scholar]
- Tagashira H, Bhuiyan S, Shioda N, Hasegawa H, Kanai H, Fukunaga K. Sigma receptor stimulation with fluvoxamine ameliorates transverse aortic constriction-induced myocardial hypertrophy and dysfunction in mice. Am J Physiol Heart Circ Physiol. 2010;299:H1535–H1545. doi: 10.1152/ajpheart.00198.2010. [DOI] [PubMed] [Google Scholar]
- Takao K, Nagatani T, Kitamura Y, Kawasaki K, Hayakawa H, Yamawaki S. Chronic forced swim stress of rats increases frontal cortical 5-HT2 receptors and the wet-dog shakes they mediate, but not frontal cortical beta-adrenoceptors. Eur J Pharmacol. 1995;294:721–726. doi: 10.1016/0014-2999(95)00620-6. [DOI] [PubMed] [Google Scholar]
- Tata DA, Raudensky J, Yamamoto BK. Augmentation of methamphetamine-induced toxicity in the rat striatum by unpredictable stress: contribution of enhanced hyperthermia. Eur J Neurosci. 2007;26:739–748. doi: 10.1111/j.1460-9568.2007.05688.x. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Angoa Perez M, Francescutti-Verbeem DM, Shah MM, Kuhn DM. The role of endogenous serotonin in methamphetamine-induced neurotoxicity to dopamine nerve endings of the striatum. J Neurochem. 2010;115:595–605. doi: 10.1111/j.1471-4159.2010.06950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DM, Francescutti-Verbeem DM, Kuhn DM. The newly synthesized pool of dopamine determines the severity of methamphetamine-induced neurotoxicity. J Neurochem. 2008;105:605–616. doi: 10.1111/j.1471-4159.2007.05155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji T, Asanuma M, Miyazaki I, Miyoshi K, Ogawa N. Reduction of nuclear peroxisome proliferation-activated receptor gamma expression in methamphetamine-induced neurotoxicity and neuroprotective effects of ibuprofen. Neurochem Res. 2009;34:764–774. doi: 10.1007/s11064-008-9863-x. [DOI] [PubMed] [Google Scholar]
- Turnipseed SD, Richards JR, Kirk JD, Diercks DB, Anderson EA. Frequency of acute coronary syndrome in patients presenting to the emergency department with chest pain after methamphetamine use. J Emerg Med. 2003;24:369–373. doi: 10.1016/s0736-4679(03)00031-3. [DOI] [PubMed] [Google Scholar]
- Voronova IP, Naumenko VS, Khramova GM, Kozyreva TV, Popova NK. Central 5-HT3 receptor-induced hypothermia is associated with reduced metabolic rate and increased heat loss. Neurosci Lett. 2011;504:209–214. doi: 10.1016/j.neulet.2011.09.024. [DOI] [PubMed] [Google Scholar]
- Watts DJ, McCollester L. Methamphetamine-induced myocardial infarction with elevated troponin I. Am J Emerg Med. 2006;24:132–134. doi: 10.1016/j.ajem.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Webb P. The physiology of heat regulation. Am J Physiol Regul Integr Comp Physiol. 1995;268:R838–R850. doi: 10.1152/ajpregu.1995.268.4.R838. [DOI] [PubMed] [Google Scholar]
- Wendt D, van Loon LJC, van Marken Lichtenbelt WD. Thermoregulation during exercise in the heat. Sports Med. 2007;37:669–682. doi: 10.2165/00007256-200737080-00002. [DOI] [PubMed] [Google Scholar]
- West PL, McKeown NJ, Hendrickson RG. Methamphetamine body stuffers: an observational case series. Ann Emerg Med. 2010;55:190–197. doi: 10.1016/j.annemergmed.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Xu W, Zhu JPQ, Angulo JA. Induction of striatal pre- and postsynaptic damage by methamphetamine requires the dopamine receptors. Synapse. 2005;58:110–121. doi: 10.1002/syn.20185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Kuraishi Y, Minami M, Nakai S, Hirai Y, Satoh M. Methamphetamine-induced expression of interleukin-1 beta mRNA in the rat hypothalamus. Neurosci Lett. 1991;128:90–92. doi: 10.1016/0304-3940(91)90766-m. [DOI] [PubMed] [Google Scholar]
- Yu X, Imam SZ, Newport GD, Slikker W, Jr, Ali SF. Ibogaine blocked methamphetamine-induced hyperthermia and induction of heat shock protein in mice. Brain Res. 1999;823:213–216. doi: 10.1016/s0006-8993(99)01154-3. [DOI] [PubMed] [Google Scholar]
- Yubera-Lahoz S, Ayestas MA, Jr, Blough BE, Partilla JS, Rothman RB, de la Torre R, Baumann MH. Effects of MDMA and related analogs on plasma 5-HT: relevance to 5-HT transporters in blood and brain. Eur J Pharmacol. 2012;674:337–344. doi: 10.1016/j.ejphar.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Tao R. Enhanced responsivity of 5-HT2A receptors at warm ambient temperatures is responsible for the augmentation of the 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI)-induced hyperthermia. Neurosci Lett. 2011;490:68–71. doi: 10.1016/j.neulet.2010.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Cuevas J. Sigma receptors inhibit high-voltage-activated calcium channels in rat sympathetic and parasympathetic neurons. J Neurophysiol. 2002;87:2867–2879. doi: 10.1152/jn.2002.87.6.2867. [DOI] [PubMed] [Google Scholar]
- Zhang L, Kitaichi K, Fujimoto Y, Nakayama H, Shimizu E, Iyo M, Hashimoto K. Protective effects of minocycline on behavioral changes and neurotoxicity in mice after administration of methamphetamine. Prog Neuropsychopharmacol Biol Psychiat. 2006;30:1381–1393. doi: 10.1016/j.pnpbp.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Zolkowska D, Rothman RB, Baumann MH. Amphetamine analogs increase plasma serotonin: implications for cardiac and pulmonary disease. J Pharmacol Exp Ther. 2006;318:604–610. doi: 10.1124/jpet.106.101618. [DOI] [PubMed] [Google Scholar]